Effects of Land Use and Water Level Fluctuations on Phytoplankton in Mediterranean Reservoirs in Cyprus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling
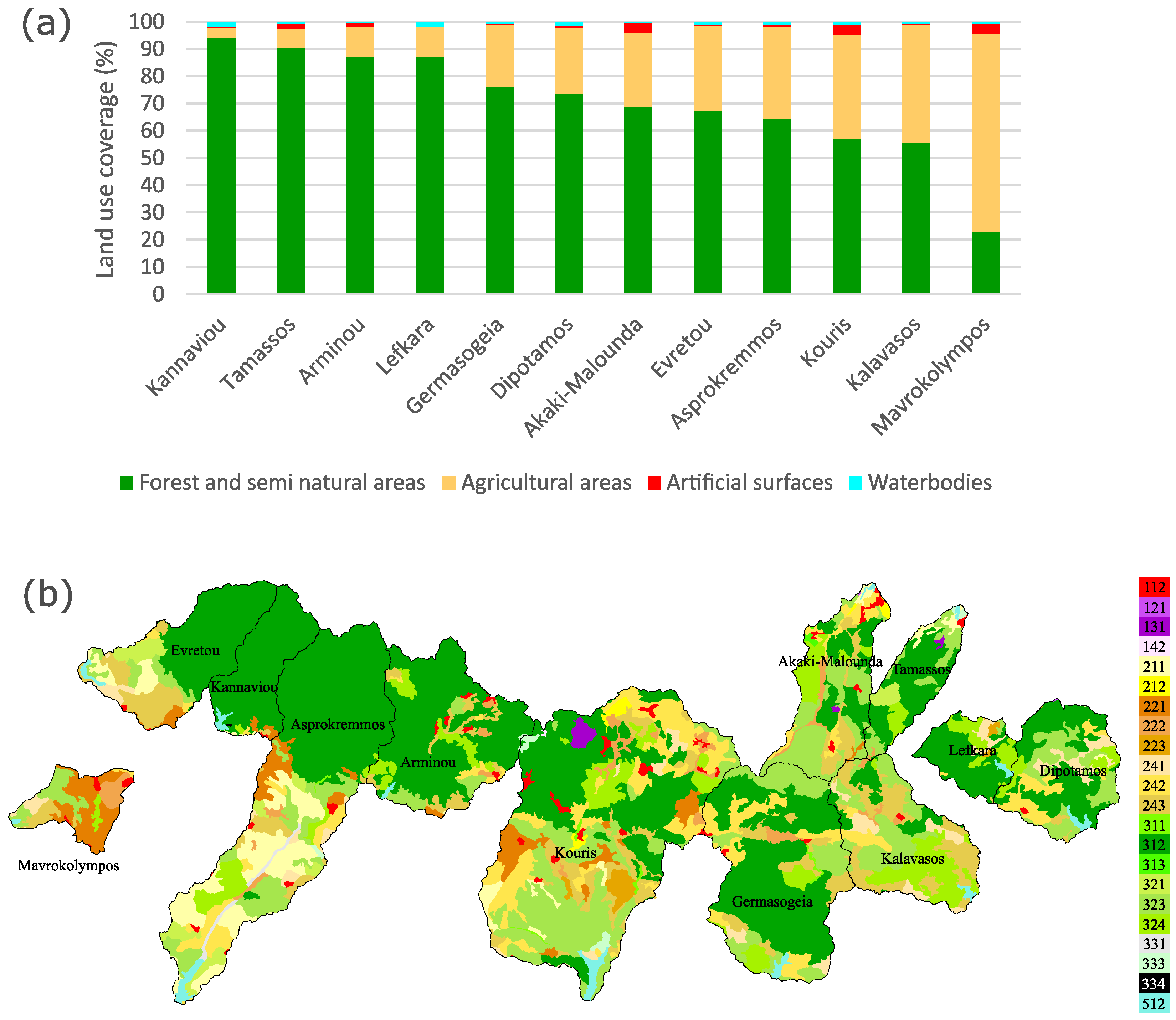
2.2. Land Use Coverage
2.3. Water Level
- X = the observed water level at a given time;
- μ = the mean water level over the period of interest (e.g., the full study period);
- σ = the standard deviation of water level over the same period.
2.4. Microscopy Analysis
2.5. Data Analysis
3. Results
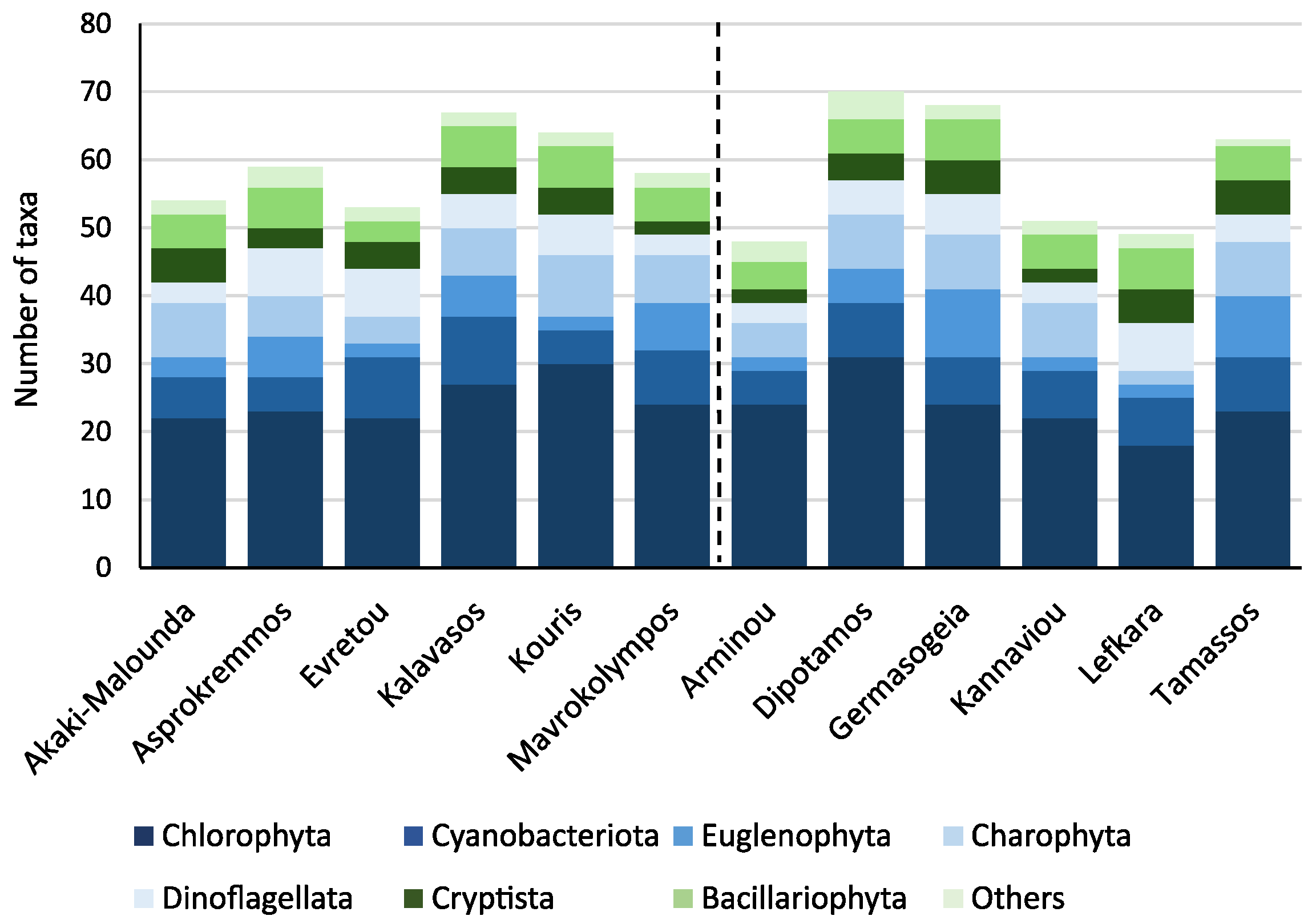
3.1. Catchment Land Use Coverage and Phytoplankton Community Structure
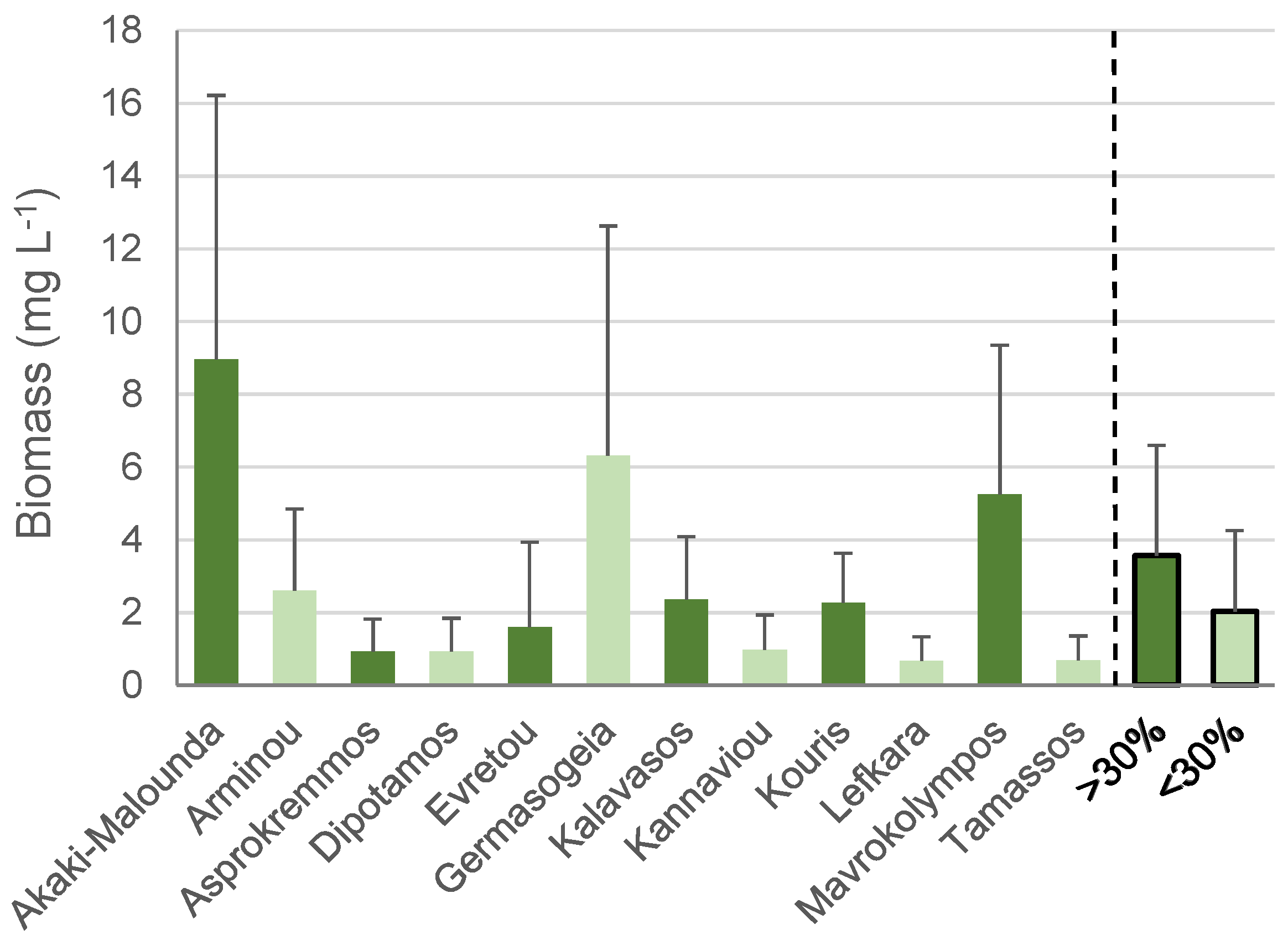
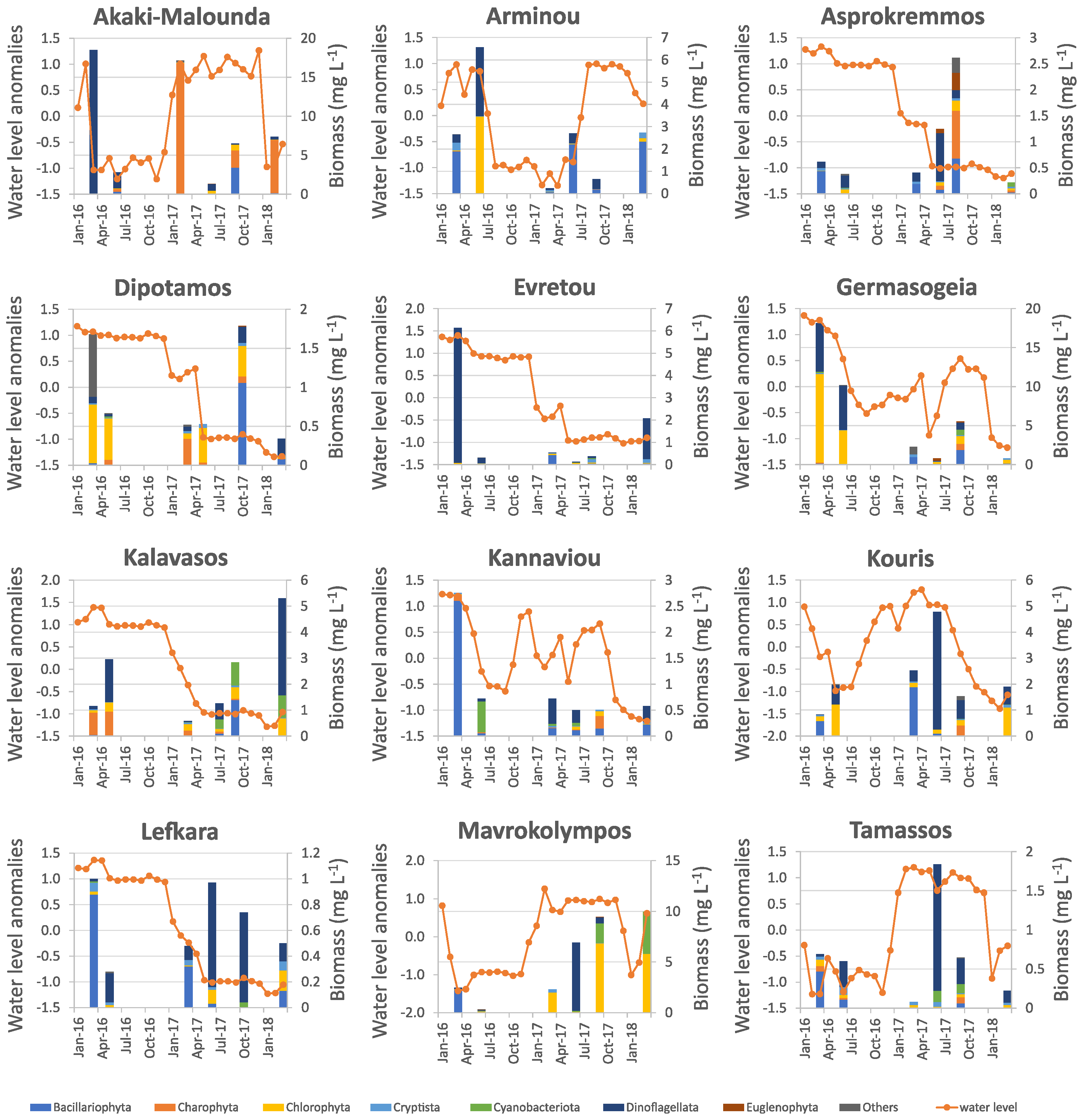
3.2. Water Level Fluctuations
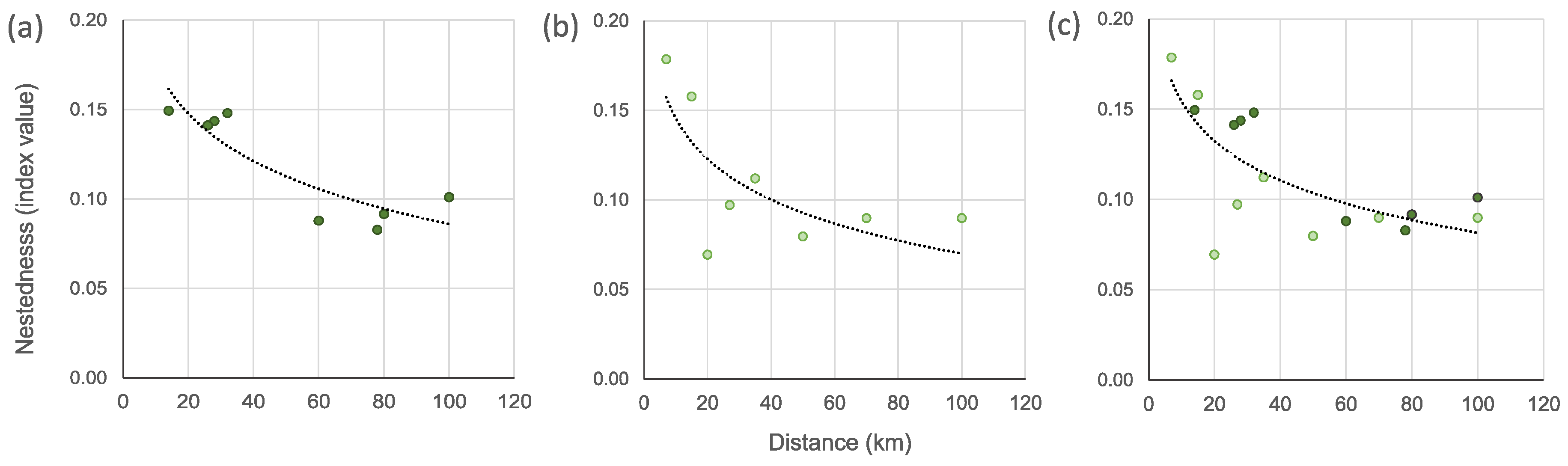
3.3. Spatial Beta Diversity
4. Discussion
4.1. Land Use Types Influence on Phytoplankton Community
4.2. Water Level Fluctuations and Their Effects on Phytoplankton Community
4.3. Geographical Distance and Its Influence on Phytoplankton Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sommer, U. Life Forms of Aquatic Organisms. In Freshwater and Marine Ecology; Springer International Publishing: Cham, Switzerland, 2024; pp. 53–113. [Google Scholar]
- Soininen, J.; Korhonen, J.J.; Karhu, J.; Vetterli, A. Disentangling the spatial patterns in community composition of prokaryotic and eukaryotic lake plankton. Limnol. Oceanogr. 2011, 56, 508–520. [Google Scholar] [CrossRef]
- Heino, J.; Alahuhta, J.; Bini, L.M.; Cai, Y.; Heiskanen, A.S.; Hellsten, S.; Kortelainen, P.; Kotamäki, N.; Tolonen, K.T.; Vihervaara, P.; et al. Lakes in the era of global change: Moving beyond single-lake thinking in maintaining biodiversity and ecosystem services. Biol. Rev. 2021, 96, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Moustaka-Gouni, M.; Sommer, U. Effects of Harmful Blooms of Large-Sized and Colonial Cyanobacteria on Aquatic Food Webs. Water 2020, 12, 1587. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76. [Google Scholar] [CrossRef]
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy; European Commission: Brussels, Belgium, 2000. [Google Scholar]
- Katsiapi, M.; Moustaka-Gouni, M.; Sommer, U. Assessing ecological water quality of freshwaters: PhyCoI—A new phytoplankton community Index. Ecol. Inform. 2016, 31, 22–29. [Google Scholar] [CrossRef]
- Carney, E. Relative influence of lake age and watershed land use on trophic state and water quality of artificial lakes in Kansas. Lake Reserv. Manag. 2009, 25, 199–207. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Q.; Liu, G. Effects of watershed land use and lake morphometry on the trophic state of Chinese lakes: Implications for eutrophication control. Clean-Soil Air Water 2011, 39, 35–42. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, J.; Zhao, H.; Deng, S.; Lin, M.; Wang, N.; Liu, M.; Hu, S.; Luo, L. Land Use Impact on Water Quality and Phytoplankton Community Structure in Danjiangkou Reservoir. Diversity 2024, 16, 275. [Google Scholar] [CrossRef]
- Katsiapi, M.; Mazaris, A.D.; Charalampous, E.; Moustaka-Gouni, M. Watershed land use types as drivers of freshwater phytoplankton structure. In Phytoplankton Responses to Human Impacts at Different Scales, Developments in Hydrobiology; Springer: Dordrecht, The Netherlands, 2012; Volume 221, pp. 121–131. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Stanley, E.H.; Vander Zanden, M.J. State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 2011, 36, 75–99. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Soranno, P.A.; Cheruvelil, K.S.; Wagner, T.; Webster, K.E.; Bremigan, M.T. Effects of land use on lake nutrients: The importance of scale, hydrologic connectivity, and region. PLoS ONE 2015, 10, e0135454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Gao, J.; Cai, Y. The direct and indirect effects of land use and water quality on phytoplankton communities in an agriculture-dominated basin. Environ. Monit. Assess. 2020, 192, 760. [Google Scholar] [CrossRef] [PubMed]
- Borics, G.; Nagy, L.; Miron, S.; Grigorszky, I.; László-Nagy, Z.; Lukács, B.A.; Toth, L.G.; Várbíró, G. Which factors affect phytoplankton biomass in shallow eutrophic lakes? Hydrobiologia 2013, 714, 93–104. [Google Scholar] [CrossRef]
- Kakouei, K.; Kraemer, B.M.; Anneville, O.; Carvalho, L.; Feuchtmayr, H.; Graham, J.L.; Higgins, S.; Pomati, F.; Rudstam, L.G.; Stockwell, J.D.; et al. Phytoplankton and cyanobacteria abundances in mid-21st century lakes depend strongly on future land use and climate projections. Glob. Change Biol. 2021, 27, 6409–6422. [Google Scholar] [CrossRef]
- Burns, C.W.; Galbraith, L.M. Relating planktonic microbial food web structure in lentic freshwater ecosystems to water quality and land use. J. Plankton Res. 2007, 29, 127–139. [Google Scholar] [CrossRef]
- Wang, L.; Cai, Q.; Xu, Y.; Kong, L.; Tan, L.; Zhang, M. Weekly dynamics of phytoplankton functional groups under high water level fluctuations in a subtropical reservoir-bay. Aquat. Ecol. 2011, 45, 197–212. [Google Scholar] [CrossRef]
- Li, J.; Yang, W.; Li, W.; Mu, L.; Jin, Z. Coupled hydrodynamic and water quality simulation of algal bloom in the Three Gorges Reservoir, China. Ecol. Eng. 2018, 119, 97–108. [Google Scholar] [CrossRef]
- Znachor, P.; Nedoma, J.; Hejzlar, J.; Seďa, J.; Komárková, J.; Kolář, V.; Mrkvička, T.; Boukal, D.S. Changing environmental conditions underpin long-term patterns of phytoplankton in a freshwater reservoir. Sci. Total Environ. 2020, 710, 135626. [Google Scholar] [CrossRef]
- Jeppesen, E.; Brucet, S.; Naselli-Flores, L.; Papastergiadou, E.; Stefanidis, K.; Noges, T.; Noges, P.; Attayde, J.L.; Zohary, T.; Coppens, J.; et al. Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and related changes in salinity. Hydrobiologia 2015, 750, 201–227. [Google Scholar] [CrossRef]
- Qian, K.; Liu, X.; Chen, Y. Effects of water level fluctuation on phytoplankton succession in Poyang Lake, China-a five year study. Ecohydrol. Hydrobiol. 2016, 16, 175–184. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Katsiapi, M.; Moustaka, M.; Michaloudi, E.; Kormas, A.K. Phytoplankton and water quality in a Mediterranean drinking-water reservoir (Marathonas Reservoir, Greece). Environ. Monit. Assess. 2011, 185, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xiao, J.; Ou, T.; Han, M.; Wang, J.; Chen, J.; Li, Y.; Salmaso, N. Impact of water level fluctuations on the development of phytoplankton in a large subtropical reservoir: Implications for the management of cyanobacteria. Environ. Sci. Pollut. Res. 2018, 25, 1306–1318. [Google Scholar] [CrossRef]
- Allende, L.; Tell, G.; Zagarese, H.; Torremorell, A.; Pérez, G.; Bustingorry, J.; Escaray, R.; Izaguirre, I. Phytoplankton and primary production in clear-vegetated, inorganic-turbid, and algal-turbid shallow lakes from the pampa plain (Argentina). Hydrobiologia 2009, 624, 45–60. [Google Scholar] [CrossRef]
- Anderson, M.J.; Crist, T.O.; Chase, J.M.; Vellend, M.; Inouye, B.D.; Freestone, A.L.; Sanders, N.J.; Cornell, H.V.; Comita, L.S.; Davies, K.F.; et al. Navigating the multiple meanings of β diversity: A roadmap for the practicing ecologist. Ecol. Lett. 2011, 14, 19–28. [Google Scholar] [CrossRef]
- Mazaris, A.D.; Moustaka-Gouni, M.; Michaloudi, E.; Bobori, D.C. Biogeographical patterns of freshwater micro-and macroorganisms: A comparison between phytoplankton, zooplankton and fish in the eastern Mediterranean. J. Biogeogr. 2010, 37, 1341–1351. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, B.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Distinct large-scale biogeographic patterns of fungal communities in bulk soil and soybean rhizosphere in China. Sci. Total Environ. 2018, 644, 791–800. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar]
- Jin, L.; Chen, H.; Xue, Y.; Soininen, J.; Yang, J. The scale-dependence of spatial distribution of reservoir plankton communities in subtropical and tropical China. Sci. Total Environ. 2022, 845, 157179. [Google Scholar] [CrossRef]
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Chang, C.; Gao, L.; Wei, J.; Ma, N.; He, Q.; Pan, B.; Li, M. Spatial and environmental factors contributing to phytoplankton biogeography and biodiversity in mountain ponds across a large geographic area. Aquat. Ecol. 2021, 55, 721–735. [Google Scholar] [CrossRef]
- Baselga, A.; Gómez-Rodríguez, C. Assessing the equilibrium between assemblage composition and climate: A directional distance-decay approach. J. Anim. Ecol. 2021, 90, 1906–1918. [Google Scholar] [CrossRef] [PubMed]
- Soininen, J.; McDonald, R.; Hillebrand, H. The distance decay of similarity in ecological communities. Ecography 2007, 30, 3–12. [Google Scholar] [CrossRef]
- Turley, L.; Bréthaut, C.; Pflieger, G. Institutions for reoperating reservoirs in semi-arid regions facing climate change and competing societal water demands: Insights from Colorado. Water Int. 2021, 47, 30–54. [Google Scholar] [CrossRef]
- Kuzma, S.; Saccoccia, L.; Chertock, M. 25 Countries, Housing One-Quarter of the Population, Face Extremely High-Water Stress; World Resources Institute: Washington, DC, USA, 2023. [Google Scholar]
- Ma, S.; Kassinos, S.C.; Fatta Kassinos, D.; Akylas, E. Effects of selective water withdrawal schemes on thermal stratification in Kouris Dam in Cyprus. Lakes Reserv. Res. Manag. 2008, 13, 51–61. [Google Scholar] [CrossRef]
- Papadaskalopoulou, C.; Giannakopoulos, C.; Lemesios, G.; Zachariou-Dodou, M.; Loizidou, M. Challenges for water resources and their management in light of climate change: The case of Cyprus. Desalination Water Treat. 2015, 53, 3224–3233. [Google Scholar] [CrossRef]
- De, H.C.; Catalan, J.; Dörflinger, G.; Ferreira, J.; Kemitzoglou, D.; Laplace-Treyture, C.; Pahissa, L.J.; Marchetto, A.; Mihail, O.; Morabito, G.; et al. Water Framework Directive Intercalibration Technical Report: Mediterranean Lake Phytoplankton Ecological Assessment Methods; Publications Office of the European Union: Luxembourg, 2014; p. 70. [Google Scholar]
- Polykarpou, P.; Katsiapi, M.; Genitsaris, S.; Stefanidou, N.; Dörflinger, G.; Moustaka-Gouni, M.; Economou-Amilli, A.; Raitsos, D.E. Phytoplankton Diversity and Blooms in Ephemeral Saline Lakes of Cyprus. Diversity 2023, 15, 1204. [Google Scholar] [CrossRef]
- Hadjimitsis, D.G.; Hadjimitsis, M.G.; Clayton, C.; Clarke, B.A. Determination of turbidity in Kourris Dam in Cyprus utilizing Landsat TM remotely sensed data. Water Resour. Manag. 2006, 20, 449–465. [Google Scholar] [CrossRef]
- Hadjimitsis, D.G.; Clayton, C. Assessment of temporal variations of water quality in inland water bodies using atmospheric corrected satellite remotely sensed image data. Environ. Monit. Assess. 2009, 159, 281–292. [Google Scholar] [CrossRef]
- Council of the European Commission. Council directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities Bruss. 1992, 206, 7–49. [Google Scholar]
- European Environmental Agency. Corine Land Cover Technical Quide; Addendum; European Environmental Agency: Copenhagen, Denmark, 2000. [Google Scholar]
- CEN 15204; Water Quality. Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique). European Committee for Standardization, Management Centre: Brussels, Belgium, 2006.
- CEN/EN 16695; Water Quality—Guidance on the Estimation of Microalgal Biovolume. European Committee for Standardization, Management Centre: Brussels, Belgium, 2015.
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik: Mit 1 Tabelle und 15 abbildungen im Text und auf 1 Tafel. Int. Ver. Für Theor. Und Angew. Limnol. Mitteilungen 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Tromboni, F.; Dodds, W. Relationships Between Land Use and Stream Nutrient Concentrations in a Highly Urbanized Tropical Region of Brazil: Thresholds and Riparian Zones. Environ. Manag. 2017, 60, 30–40. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Gallardo-Mayenco, A.; Guerrero, F.; Toja, J. Environmental factors and phytoplankton composition in Mediterranean reservoirs. Limnetica 2009, 28, 35–46. [Google Scholar]
- Borics, G.; Wolfram, G.; Chiriac, G.; Belkinova, D.; Donabaum, K. Intercalibration of the National Classifications of Ecological Status for Eastern Continental Lakes: Biological Quality Element: Phytoplankton; Poikane, S., Ed.; Publications Office of the European Union: Luxembourg, 2018; ISBN 978-92-79-92972-4. EUR 29338 EN; JRC112693. [Google Scholar] [CrossRef]
- Romero, F.; Ruiz, M.; Gálvez-Cloutier, R. Impacts of drought on phytoplankton dynamics in Mediterranean reservoirs. Ecol. Indic. 2018, 85, 759–768. [Google Scholar] [CrossRef]
- European Environment Agency (EEA). Corine Land Cover (CLC) 1990, 2000, 2006, 2012, 2018 Datasets. 2020. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 20 January 2025).
- Pulido-Villena, E.; Reche, I.; Morales-Baquero, R. Significance of atmospheric inputs of phosphorus and nitrogen to Mediterranean lakes. Limnol. Oceanogr. 2006, 51, 830–837. [Google Scholar]
- Qu, Y.; Wu, N.; Guse, B.; Fohrer, N. Distinct indicators of land use and hydrology characterize different aspects of riverine phytoplankton communities. Sci. Total Environ. 2022, 851, 158209. [Google Scholar] [CrossRef]
- Fraterrigo, J.M.; Downing, J.A. The influence of land use on lake nutrients varies with watershed transport capacity. Ecosystems 2008, 11, 1021–1034. [Google Scholar] [CrossRef]
- Tong, S.T.Y.; Chen, W.; Susanna, T.Y. Modeling the relationship between land use and surface water quality. J. Environ. Manag. 2002, 66, 377–393. [Google Scholar] [CrossRef]
- Smith, V.H.; Tilman, G.D.; Nekola, J.C. Eutrophication: Impacts of excess nutrient inputs on freshwater, marine, and terrestrial ecosystems. Environ. Pollut. 1999, 100, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Ostos, E.; Cruz-Pizarro, L.; Basanta, A.; George, D.G. The influence of wind-induced mixing on the horizontal distribution of phytoplankton in a Mediterranean lake. Freshw. Biol. 2008, 53, 435–447. [Google Scholar]
- Moustaka-Gouni, M. Phytoplankton succession and diversity in a warm monomictic, relatively shallow lake: Lake Volvi, Macedonia, Greece. Hydrobiologia 1993, 249, 33–42. [Google Scholar] [CrossRef]
- Albay, M.; Akçaalan, R. Factors influencing the phytoplankton steady state assemblages in a drinking-water reservoir (Ömerli reservoir, Istanbul). In Phytoplankton and Equilibrium Concept: The Ecology of Steady-State Assemblages, Proceedings of the 13th Workshop of the International Association of Phytoplankton Taxonomy and Ecology (IAP), Castelbuono, Italy, 1–8 September 2002; Springer: Dordrecht, The Netherlands, 2003; pp. 85–95. [Google Scholar]
- Santos, J.B.; Silva, L.H.; Branco, C.W.; Huszar, V.L. The roles of environmental conditions and geographical distances on the species turnover of the whole phytoplankton and zooplankton communities and their subsets in tropical reservoirs. Hydrobiologia 2016, 764, 171–186. [Google Scholar] [CrossRef]
- Harris, G.P.; Baxter, G. Interannual variability in phytoplankton biomass and species composition in a subtropical reservoir. Freshw. Biol. 1996, 35, 545–560. [Google Scholar] [CrossRef]
- Moustaka-Gouni, M.; Vardaka, E.; Michaloudi, E.; Kormas, K.A.; Tryfon, E.; Mihalatou, H.; Gkelis, S.; Lanaras, T. Plankton food web structure in a eutrophic polymictic lake with a history of toxic cyanobacterial blooms. Limnol. Oceanogr. 2006, 51, 715–727. [Google Scholar] [CrossRef]
- Chen, L.; Ge, L.; Wang, D.; Zhong, W.; Zhan, T.; Deng, A. Multi-objective water-sediment optimal operation of cascade reservoirs in the Yellow River Basin. J. Hydrol. 2022, 609, 127744. [Google Scholar] [CrossRef]
- Liao, N.; Zhang, L.; Chen, M.; Li, J.; Wang, H. The influence mechanism of water level operation on algal blooms in canyon reservoirs and bloom prevention. Sci. Total Environ. 2024, 912, 169377. [Google Scholar] [CrossRef]
- Sabater, S. Alterations of the global water cycle and their effects on river structure, function and services. Freshw. Rev. 2008, 1, 75–89. [Google Scholar] [CrossRef]
- Zohary, T.; Ostrovsky, I. Ecological impacts of excessive water level fluctuations in stratified freshwater lakes. Inland Waters 2011, 1, 47–59. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Padisák, J.; Reynolds, C.S.; Sommer, U. Intermediate Disturbance Hypothesis in Phytoplankton Ecology; Developments in Hydrobiology 81; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; Reprinted from Hydrobiologia 249. [Google Scholar]
- Dokulil, M.T.; Teubner, K. Cyanobacterial dominance in lakes. Hydrobiologia 2000, 438, 1–12. [Google Scholar] [CrossRef]
- Sommer, U. Comparison between steady state and non-steady state competition: Experiments with natural phytoplankton. Limnol. Oceanogr. 1985, 30, 335–346. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Huszar, V.; Kruk, C.; Naselli-Flores, L.; Melo, S. Towards a functional classification of the freshwater phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae, Teil 3. Siisswasserjlora von Mitteleuropu, Band 2; Gustav Fischer Verlag: Stuttgart, Germany; New York, NY, USA, 1991. [Google Scholar]
- Wehr, J.D.; Sheath, R.G. Freshwater Algae of North America: Ecology and Classification; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Brook, A.J. The Biology of Desmids; Blackwell Scientific Publications: Boston, MA, USA, 1981. [Google Scholar]
- Fowler, J.; Cohen, L.; Jarvis, P. Practical Statistics for Field Biology; John Wiley & Sons: New York, NJ, USA, 1998. [Google Scholar]
- Naselli-Flores, L.; Barone, R. Fight on plankton! Or, phytoplankton shape and size between nutrient supply and hydrodynamic forces. Hydrobiologia 2011, 668, 19–26. [Google Scholar]
- Romero, J.R.; Antenucci, J.P.; Imberger, J. The effects of lake morphology on the stratification and mixing of reservoirs in Mediterranean climates. Limnetica 2016, 35, 233–250. [Google Scholar]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef]
- Gallardo, B.; García, M.; Cabezas, A.; González, E.; González, M.; Ciancarelli, C.; Comín, F.A. Macroinvertebrate patterns along environmental gradients and hydrological connectivity within a regulated river-floodplain. Aquat. Sci. 2008, 70, 248–258. [Google Scholar] [CrossRef]
- Sugihara, G.; May, R.; Ye, H.; Hsieh, C.; Deyle, E.; Fogarty, M.; Munch, A. Detecting Causality in Complex Ecosystems. Science 2012, 338, 496–500. [Google Scholar] [CrossRef]
- Naselli-Flores, L. Phytoplankton assemblages in twenty-one Sicilian reservoirs: Relationships between species composition and environmental factors. In The Trophic Spectrum Revisited; Developments in Hydrobiology; Reynolds, C.S., Dokulil, M., Padisák, J., Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 150. [Google Scholar] [CrossRef]
- Horner-Devine, M.; Lage, M.; Hughes, J.B.; Bohannan, B.J.M. A taxa–area relationship for bacteria. Nature 2004, 432, 750–753. [Google Scholar] [CrossRef]
- Bell, T. Experimental tests of the bacterial distance–decay relationship. ISME J. 2010, 4, 1357–1365. [Google Scholar] [CrossRef]
- Martiny, J.B.; Bohannan, B.J.; Brown, J.H.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Horner-Devine, M.C.; Kane, M.; Krumins, J.A.; Kuske, C.R.; et al. Microbial biogeography: Putting microorganisms on the map. Nat. Rev. Microbiol. 2006, 4, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zorzal-Almeida, S.; Bini, L.M.; Bicudo, D.C. Beta diversity of diatoms is driven by environmental heterogeneity, spatial extent and productivity. Hydrobiologia 2017, 800, 7–16. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, R.; Lin, Q.; Hou, J.; Liu, Y.; Han, B.P.; Naselli-Flores, L. Spatial structure and β-diversity of phytoplankton in Tibetan Plateau lakes: Nestedness or replacement? Hydrobiologia 2018, 808, 301–314. [Google Scholar] [CrossRef]
- Katsiapi, M.; Moustaka-Gouni, M.; Vardaka, E.; Kormas, K.A. Different phytoplankton descriptors show asynchronous changes in a shallow urban lake (L. Kastoria, Greece) after sewage diversion. Fundam. Appl. Limnol. 2013, 182, 219–230. [Google Scholar] [CrossRef]
- Chrisostomou, A.; Moustaka-Gouni, M.; Sgardelis, S.; Lanaras, T. Air-dispersed phytoplankton in a Mediterranean river-reservoir system (Aliakmon-Polyphytos, Greece). J. Plankton Res. 2009, 31, 877–884. [Google Scholar] [CrossRef]
- Genitsaris, S.; Moustaka-Gouni, M.; Kormas, K.A. Airborne microeukaryote colonists in experimental water containers: Diversity, succession, life histories and established food webs. Aquat. Microb. Ecol. 2011, 62, 139–152. [Google Scholar] [CrossRef][Green Version]
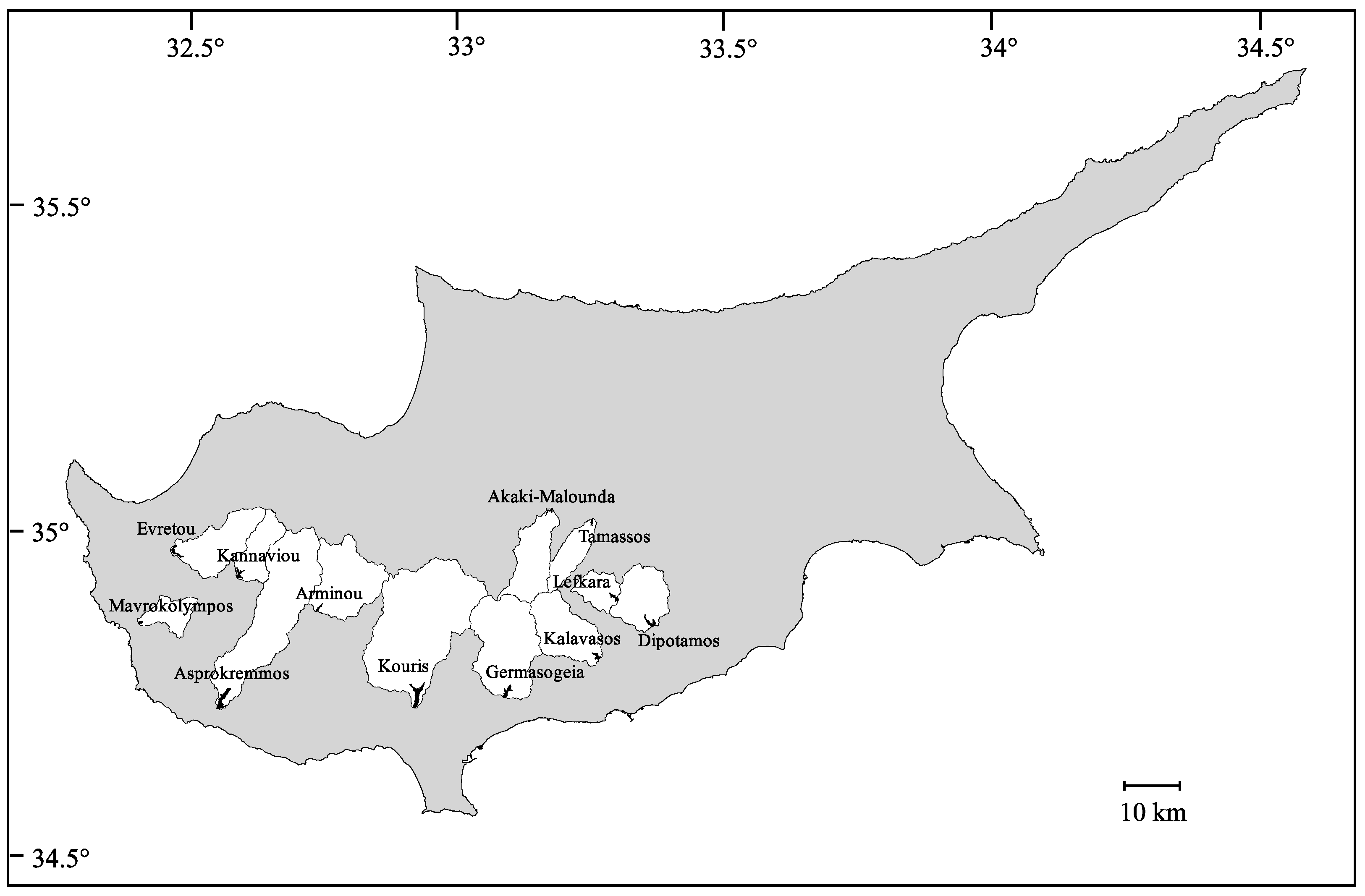

| Reservoir | Altitude (m a.s.l.) | Latitude (N) | Longitude (E) | Surface Area (LA) (km2) | Catchment Area (WA) (km2) | WA:LA | Max Depth, Approx. (m) | Max Storage Capacity (million m3) | Max. Water Level Fluctuation (m) |
|---|---|---|---|---|---|---|---|---|---|
| Akaki-Malounta *▲ | 370 | 35.03 | 33.17 | 0.33 | 84 | 255 | 22 | 2 | 7.4 |
| Arminou *▲ | 404 | 34.88 | 32.74 | 0.42 | 120 | 282 | 32 | 4 | 14.1 |
| Asprokremmos *▲ | 37 | 34.73 | 32.56 | 2.63 | 224 | 85 | 41 | 52 | 18.1 |
| Dipotamos *▲ | 135 | 34.85 | 33.36 | 1.19 | 79 | 65 | 39 | 16 | 13.7 |
| Evretou ▲ | 103 | 34.97 | 32.47 | 0.97 | 91 | 93 | 63 | 24 | 10.0 |
| Germasogeia *▲ | 55 | 34.75 | 33.09 | 1.18 | 163 | 138 | 27 | 14 | 16.1 |
| Kalavasos * | 126 | 34.80 | 33.26 | 0.80 | 97 | 121 | 50 | 17 | 19.0 |
| Kannaviou *▲ | 370 | 34.93 | 32.59 | 0.98 | 55 | 56 | 61 | 18 | 12.4 |
| Kouris *▲ | 156 | 34.73 | 32.92 | 3.06 | 304 | 99 | 87 | 115 | 17.9 |
| Lefkara *▲ | 297 | 34.89 | 33.29 | 0.67 | 38 | 57 | 59 | 14 | 13.9 |
| Mavrokolympos ▲ | 81 | 34.86 | 32.41 | 0.25 | 38 | 151 | 31 | 2 | 12.8 |
| Tamassos * | 384 | 35.02 | 33.25 | 0.32 | 46 | 145 | 21 | 3 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polykarpou, P.; Stefanidou, N.; Katsiapi, M.; Moustaka-Gouni, M.; Genitsaris, S.; Dörflinger, G.; Economou-Amilli, A.; Raitsos, D.E. Effects of Land Use and Water Level Fluctuations on Phytoplankton in Mediterranean Reservoirs in Cyprus. Diversity 2025, 17, 457. https://doi.org/10.3390/d17070457
Polykarpou P, Stefanidou N, Katsiapi M, Moustaka-Gouni M, Genitsaris S, Dörflinger G, Economou-Amilli A, Raitsos DE. Effects of Land Use and Water Level Fluctuations on Phytoplankton in Mediterranean Reservoirs in Cyprus. Diversity. 2025; 17(7):457. https://doi.org/10.3390/d17070457
Chicago/Turabian StylePolykarpou, Polina, Natassa Stefanidou, Matina Katsiapi, Maria Moustaka-Gouni, Savvas Genitsaris, Gerald Dörflinger, Athena Economou-Amilli, and Dionysios E. Raitsos. 2025. "Effects of Land Use and Water Level Fluctuations on Phytoplankton in Mediterranean Reservoirs in Cyprus" Diversity 17, no. 7: 457. https://doi.org/10.3390/d17070457
APA StylePolykarpou, P., Stefanidou, N., Katsiapi, M., Moustaka-Gouni, M., Genitsaris, S., Dörflinger, G., Economou-Amilli, A., & Raitsos, D. E. (2025). Effects of Land Use and Water Level Fluctuations on Phytoplankton in Mediterranean Reservoirs in Cyprus. Diversity, 17(7), 457. https://doi.org/10.3390/d17070457








