Ecological Drivers and Community Perceptions: Conservation Challenges for the Critically Endangered Elongated Tortoise (Indotestudo elongata) in Jalthal Forest, Eastern Nepal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Survey
2.3. Covariates Selection
2.4. Questionnaire Survey
2.5. Data Analysis
3. Results
3.1. Factors Affecting the Distribution of Elongated Tortoise
3.2. Demographic Composition of the Local Residents Surveyed
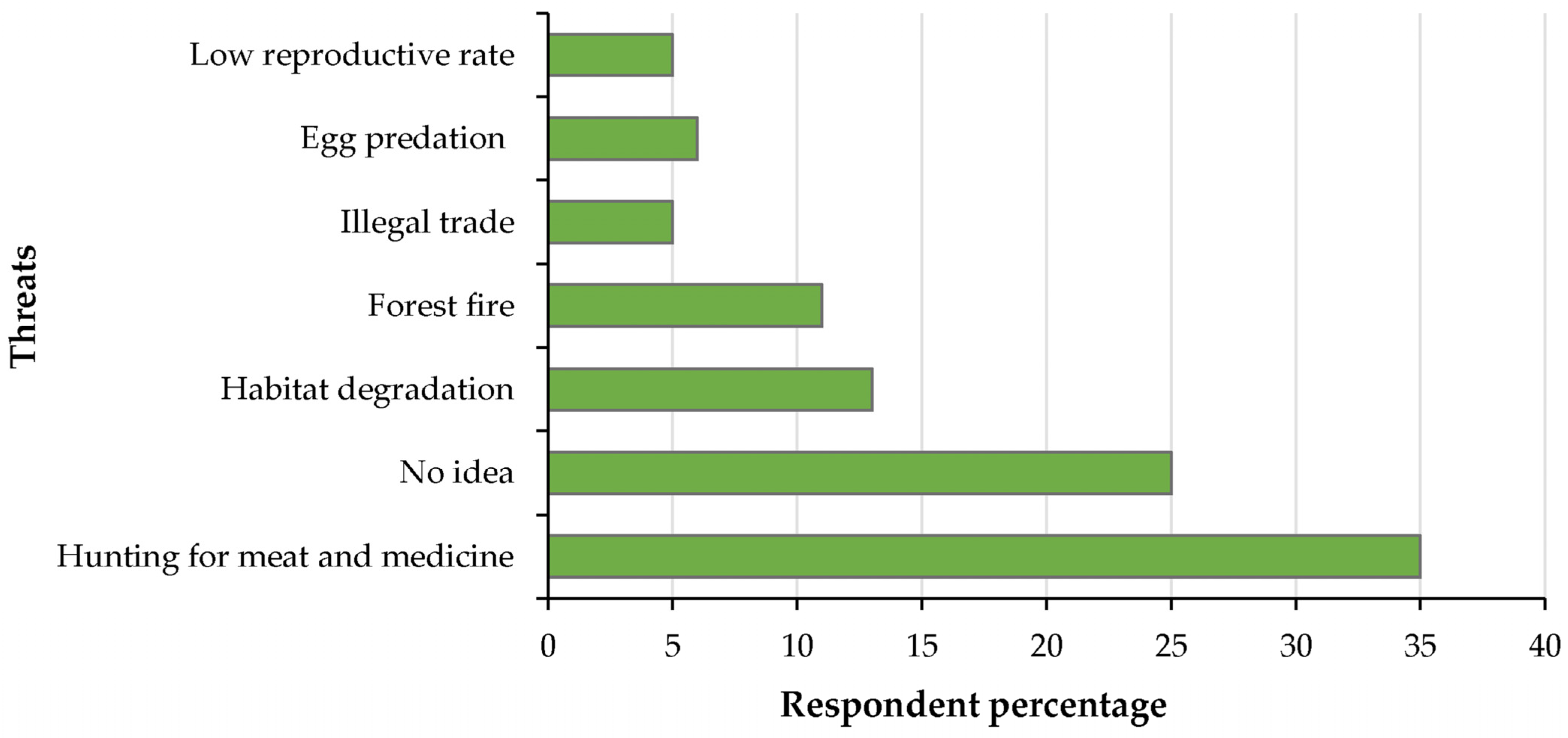
3.3. Respondents’ Attitudes vs. Conservation Challenges to Elongated Tortoises in the Jalthal Forest
3.4. Knowledge of Status and Conservation Approaches for the Elongated Tortoise
4. Discussion
4.1. Factors Affecting the Occurrence of the Elongated Tortoise
4.2. Threats and Local Residents’ Perception Toward the Elongated Tortoise
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDI | Human Disturbance Index |
| CFUG | community forest user group |
| CI | confidence interval |
| GLM | generalized linear model |
| ICC | intra-class correlation coefficient |
| VIF | variance inflation factor |
| IAPS | invasive alien plant species |
References
- Ihlow, F.; Dawson, J.; Hartmann, T.; Som, S. Indotestudo Elongata (Blyth 1854)—Elongated Tortoise, Yellow-Headed Tortoise, Yellow Tortoise. Chelonian Res. Monogr. 2016, 5, 096. [Google Scholar] [CrossRef]
- Rahman, S.; Platt, K.; Das, I.; Choudhury, B.C.; Ahmed, M.F.; Cota, M.; McCormack, T.; Timmins, R.J.; Singh, S. Indotestudo Elongata (Errata Version Published in 2019). IUCN Red List. Threat. Species 2019 2019, 8235, e.T10824A152051190. [Google Scholar]
- Kästle, W.; Khambu, K.R.; Schleich, H. Field Guide to Amphibians and Reptiles of Nepal; ARCO-Nepal: Düsseldorf, Germany, 2013. [Google Scholar]
- Schleich, H.H.; Kästle, W. Amphibian and Reptiles of Nepal; A. R. G. Gantner Verlag Kommanditgesellschaft: Ruggell, Liechtenstein, 2002; ISBN 3904144790. [Google Scholar]
- Jacobsen, R.M.; Sverdrup-Thygeson, A.; Kauserud, H.; Mundra, S.; Birkemoe, T. Exclusion of Invertebrates Influences Saprotrophic Fungal Community and Wood Decay Rate in an Experimental Field Study. Funct. Ecol. 2018, 32, 2571–2582. [Google Scholar] [CrossRef]
- Van Dijk, P.P. The Natural History of the Elongated Tortoise Indotestudo Elongata (Blyth, 1853)(Reptilia: Testudines) in a Hill Forest Mosaic in Western Thailand with Notes on Sympatric Turtle Species: Being Also the Scientific Report of Project 307 Resource Partitionin; National University of Ireland: Dublin, Ireland, 1998. [Google Scholar]
- Zou, J.Y.; Cadotte, M.W.; Bässler, C.; Brandl, R.; Baldrian, P.; Borken, W.; Stengel, E.; Luo, Y.H.; Müller, J.; Seibold, S. Wood Decomposition Is Increased by Insect Diversity, Selection Effects, and Interactions between Insects and Microbes. Ecology 2023, 104, e4184. [Google Scholar] [CrossRef]
- Ihlow, F.; Rödder, D.; Bochynek, T.; Sothanin, S.; Handschuh, M.; Böhme, W. Reinforcement as a Conservation Tool—Assessing Site Fidelity and Movement of the Endangered Elongated Tortoise Indotestudo Elongata (Blyth, 1854). J. Nat. Hist. 2014, 48, 2473–2485. [Google Scholar] [CrossRef]
- Platt, S.; Swe, T.; Ko Ko, W.; Platt, K.; Myo Myo, K.; Rainwater, T.; Emmett, D. Geochelone Platynota (Blyth 1863)–Burmese Star Tortoise, Kye Leik. Conserv. Biol. Freshw. Turt. Tortoises 2011, 57.1–57.9. [Google Scholar] [CrossRef]
- Sriprateep, K.; Aranyavalai, V.; Aowphol, A.; Thirakhupt, K. Population Structure and Reproduction of the Elongated Tortoise Indotestudo Elongata (Blyth, 1853) at Ban Kok Village, Northeastern Thailand. Trop. Nat. Hist. 2013, 13, 21–37. [Google Scholar] [CrossRef]
- Senneke, D. Keeping and Breeding Indotestudo Elongata the Elongated Tortoise. Tortoise Trust. Newsl. 2000, 15, 3–4. [Google Scholar]
- Tharapoom, K. Radio-Telemetry Study of Home Range Size and Activities of Elongated Tortoise Indotestudo Elongata (Blyth, 1853) at Huai Kha Khang Wildlife Sanctuary. Master’s Thesis, Chulalongkorn University, Bangkok, Thailand, 1996. [Google Scholar][Green Version]
- Jenkins, M.D. Tortoises and Freshwater Turtles: The Trade in South East Asia. TRAFFIC Int. 1995, iv, 1–49. [Google Scholar]
- Subba, A.; Khanal, L. Threats to the Critically Endangered Yellow-Headed Tortoise Indotestudo Elongata in Jalthal Forest, Eastern Lowland Nepal. Oryx 2024, 58, 129–132. [Google Scholar] [CrossRef]
- Aryal, P.C.; Dhamala, M.K.; Bhurtel, B.P.; Suwal, M.K.; Rijal, B. Turtles of Nepal: A Field Guide for Species Accounts and Distribution; Environmental Graduates in Himalaya (EGH): Kathmandu, Nepal; Resources HimalayaFoundation and Companions for Amphibians and Reptiles of Nepal (CARON): Kathmandu, Nepal, 2010. [Google Scholar][Green Version]
- Rawat, Y.B.; Bhattarai, S.; Poudyal, L.P.; Subedi, N. Herpetofauna of Shuklaphanta National Park, Nepal. J. Threat. Taxa 2020, 12, 15587–15611. [Google Scholar] [CrossRef]
- Sharma, L.N.; Tamang, S.R.; Poudel, Y.B.; Subba, A.; Timsina, S.; Adhikari, B.; Paudel, N.S. Biodiversity beyond Protected Areas: Gaps and Opportunities in Community Forest. J. For. Livelihood 2021, 20, 45–61. [Google Scholar]
- Neupane, B.; Budhathoki, S.; Khatiwoda, B. Human-Elephant Conflict and Mitigation Measures in Jhapa District, Nepal. J. For. Livelihood 2018, 16, 103–112. [Google Scholar] [CrossRef]
- Thapa, N.; Bhatta, G.D.; Khatri, S. Jalthal Forest: A Preliminary Survey. J. Plant Resour. 2003, 2003, 70–75. [Google Scholar]
- Bhattarai, K.P. Invasive Species Management in Nepal; a Pathway to Sustainable Forest Management. J. Plant Resour. 2017, 15, 14–20. [Google Scholar]
- Rai, K.R. Environmental Impacts, Systematics and Distribution of Herpetofauna from East Nepal. Ph.D. Thesis, Tribhuvan University, Kirtipur, Nepal, 2004. [Google Scholar][Green Version]
- Deepak, V.; Vasudevan, K. Factors Influencing the Occurrence and Vulnerability of the Travancore Tortoise Indotestudo Travancorica in Protected Areas in South India. Oryx 2015, 49, 669–676. [Google Scholar] [CrossRef]
- Som, S.; Cottet, M. Rescue and Relocation Programme of Turtles and Tortoises and Elongated Tortoise Monitoring Programme in the Nam Theun 2 Reservoir (Laos). Hydroécologie Appliquée 2016, 19, 383–406. [Google Scholar] [CrossRef]
- Tamang, S.R.; Sharma, L. Invasive Species Management in Nepal; a Pathway to Sustainable Forest Management. In Asia-Pacific Forest Sector Outlook: Innovative Forestry for Sustainable Future. Youth Contributions from Asia and the Pacific; Roshetko, P.N., Meybeck, J.M., Eds.; CGIAR Research Program on Forest, Trees and Agroforestry and Rome, Italy; Food and Agriculture Organization of the United Nations: Bogor, Indonesia, 2021; pp. 107–116. [Google Scholar][Green Version]
- Tichý, L. Field Test of Canopy Cover Estimation by Hemispherical Photographs Taken with a Smartphone. J. Veg. Sci. 2016, 27, 427–435. [Google Scholar] [CrossRef]
- Patrignani, A.; Ochsner, T.E. Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Rein, G.; Huang, X. Smouldering Wildfires in Peatlands, Forests and the Arctic: Challenges and Perspectives. Curr. Opin. Environ. Sci. Health 2021, 24, 100296. [Google Scholar] [CrossRef]
- Pokharel, M.; Subba, A.; Rai, D.; Bhandari, S.; Ghimirey, Y. Fine-Scale Ecological and Anthropogenic Variables Predict the Habitat Use and Detectability of Sloth Bears in the Churia Habitat of East Nepal. Ecol. Evol. 2022, 12, e8512. [Google Scholar] [CrossRef]
- Subba, A.; Tamang, G.; Lama, S.; Limbu, J.H.; Basnet, N.; Kyes, R.C.; Khanal1, L. Habitat Occupancy of the Critically Endangered Chinese Pangolin (Manis Pentadactyla) under Human Disturbance in an Urban Environment: Implications for Conservation. Ecol. Evol. 2024, 14, e70726. [Google Scholar] [CrossRef] [PubMed]
- Ritchey, E.L.; McGrath, J.M.; Gehring, D. Determining Soil Texture by Feel. J. Agric. Educ. 2015, 8, 1–3. [Google Scholar]
- Thein, S. A Flow Diagram for Teaching Texture-by-feel Analysis. J. Agron. Educ. 1979, 8, 54–55. [Google Scholar] [CrossRef]
- Salley, S.W.; Herrick, J.E.; Holmes, C.V.; Karl, J.W.; Levi, M.R.; McCord, S.E.; Van der Waal, C.; Van Zee, J.W. A Comparison of Soil Texture-by-feel Estimates: Implications for the Citizen Soil Scientist. Soil Sci. Soc. Am. J. 2018, 82, 1526–1537. [Google Scholar] [CrossRef]
- Kish, L. An Outline of Probability Selection Methods. In Genetics and the Epidemiology of Chronic Diseases; US Department of Health, Education and Welfare: Washington, DC, USA, 1965; pp. 165–176. [Google Scholar][Green Version]
- Singer, E.; Couper, M.P. Some Methodological Uses of Responses to Open Questions and Other Verbatim Comments in Quantitative Surveys. Methods Data Anal. 2017, 11, 115–134. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: http://www.R-project.org/ (accessed on 16 March 2024).[Green Version]
- Bartoń, K. MuMIn: Multi-Model Inference. R Package Version 1.10.0. 2023. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 16 March 2024).[Green Version]
- Burnham, K.P.; Anderson, D.R.; Burnham, K.P. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar][Green Version]
- Wickham, H. Reshaping Data with the Reshape Package. J. Stat. Softw. 2007, 21, 1–20. [Google Scholar] [CrossRef]
- Araújo, M.B.; Anderson, R.P.; Barbosa, A.M.; Beale, C.M.; Dormann, C.F.; Early, R.; Garcia, R.A.; Guisan, A.; Maiorano, L.; Naimi, B.; et al. Standards for Distribution Models in Biodiversity Assessments. Sci. Adv. 2019, 5, eaat4858. [Google Scholar] [CrossRef]
- Joshi, A.; Kale, S.; Chandel, S.; Pal, D. Likert Scale: Explored and Explained. Br. J. Appl. Sci. Technol. 2015, 7, 396–403. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive Habitat Distribution Models in Ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Das, I. A Field Guide to the Reptiles of South-East Asia; New Holland Publishers: Wahroonga, Australia, 2010. [Google Scholar][Green Version]
- Khan, S.; Nathand, A.; Das, A. The Distribution of the Elongated Tortoise (Indotestudo Elongata)on the Indian Subcontinent: Implications for Conservation and Management. Herpetol. Conserv. Biol. 2020, 15, 212–227. [Google Scholar]
- Platt, S.G.; Ko, W.K.; Platt, K.; Myo, K.M.; Soe, M.M.; Rainwater, T.R. Species Inventory and Conservation Status of Chelonians in Natma Taung, National Park, Myanmar. Hamadryad 2012, 36, 1–11. [Google Scholar]
- Platt, S.G.; Khaing, S.T.; Ko, W.K. A Tortoise Survey of Shwe Settaw Wildlife Sanctuary, Myanmar, with Notes on the Ecology of Geochelone Platynota and Indotestudo Elongata. Chelonian Conserv. Biol. 2001, 4, 172–177. [Google Scholar]
- Van Dijk, P.P.; Stuart, B.L.; Rhodin, A.G.J. Asian Turtle Trade. In Proceedings of the a Workshop on Conservation and Trade of Freshwater Turtles and Tortoises in Asia, Phnom Penh, Cambod, 1–4 December 1999; ISBN 0965354016. [Google Scholar][Green Version]
- Luiselli, L.; Starita, A.; Carpaneto, G.M.; Segniagbeto, G.H.; Amori, G. A Short Review of the International Trade of Wild Tortoises and Freshwater Turtles Across the World and Throughout Two Decades. Chelonian Conserv. Biol. 2016, 15, 167–172. [Google Scholar] [CrossRef]
- Le, D.D.; Broad, S. Investigations into Tortoise and Freshwater Turtle Trade in Vietnam; IUCN Species Survival Commission: Gland, Switzerland; Cambridge, UK, 1995. [Google Scholar][Green Version]

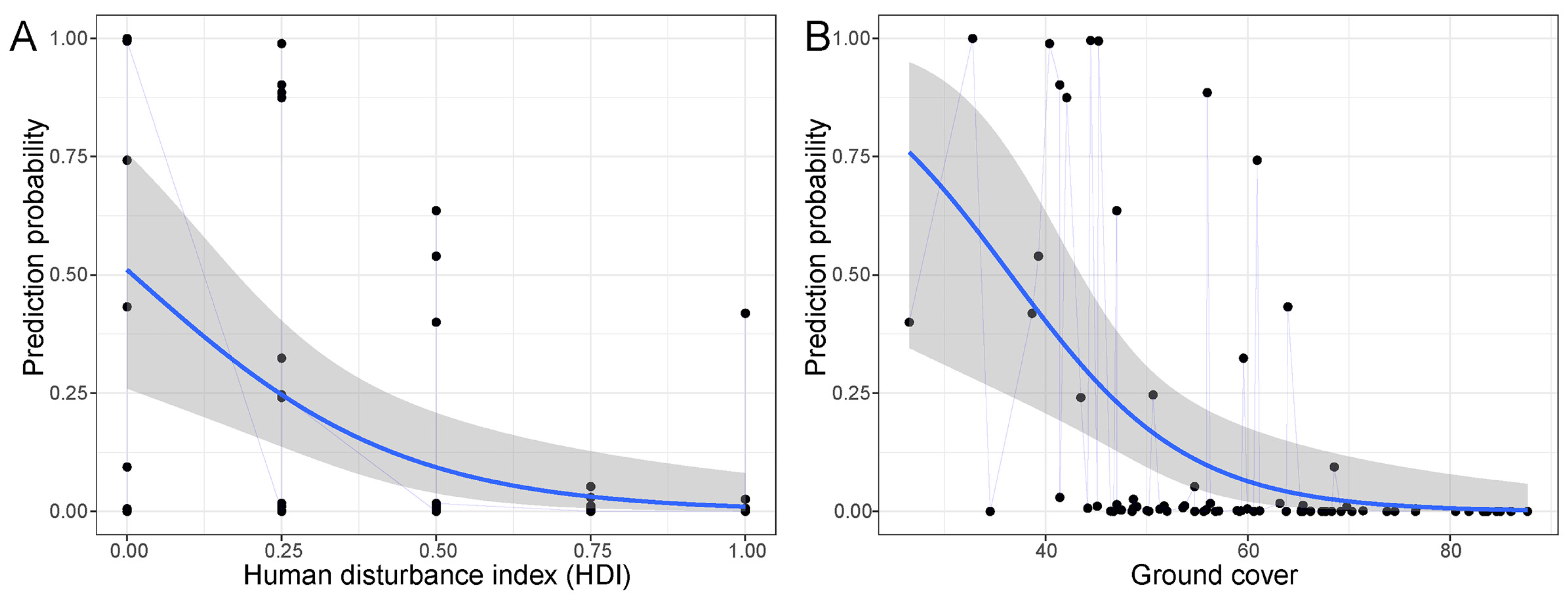

| Variable | Estimate (β) | Std. Error | LCI | UCI | z Value | p-Value |
|---|---|---|---|---|---|---|
| (Intercept) | −20.950 | 4179.6 | 8353 | 83311.1 | 0.005 | 0.9961 |
| Ground cover | −3.4966 | 1.4477 | −6.373 | −0.161 | 2.382 | 0.0172 * |
| HDI | −8.1066 | 3.4036 | −1.487 | −1.33 | 2.348 | 0.0189 * |
| Loamy soil | 21.8501 | 4179.6 | −8.310 | 8353.9 | 0.005 | 0.9959 |
| Sandy soil | 7.2908 | 6787.5 | 13,523.6 | 13,538.2 | 0.001 | 0.9992 |
| Silty soil | 18.0886 | 4179.6 | −8313.9 | 8350.1 | 0.004 | 0.9966 |
| Canopy cover | 0.7343 | 0.6872 | −0.631 | 2.099 | 1.054 | 0.2919 |
| Settlement distance | −0.8994 | 0.8387 | −2.566 | 0.7673 | 1.058 | 0.2902 |
| Invasive species (presence) | 0.8909 | 1.6712 | −2.441 | 4.222 | 0.524 | 0.6003 |
| Sal forest | 1.3304 | 1.3745 | −1.410 | 4.071 | 0.951 | 0.3414 |
| Wetland | 0.8748 | 3.4994 | −6.102 | 7.852 | 0.246 | 0.8059 |
| Socio-Demographic Status | Category | Total | Percentage (%) |
|---|---|---|---|
| Gender | Male | 76 | 32 |
| Female | 160 | 58 | |
| Age | Young adult (18–39) | 87 | 37 |
| Middle age (40–59) | 113 | 48 | |
| Old age (>60) | 36 | 15 | |
| Education | Illiterate | 90 | 38 |
| Primary | 12 | 5 | |
| Secondary | 54 | 23 | |
| Higher secondary | 56 | 24 | |
| University level | 24 | 10 | |
| Ethnicity | Brahmin | 80 | 34 |
| Chettri | 59 | 25 | |
| Janajati | 79 | 33 | |
| Madhesi | 18 | 8 | |
| Occupation | Agriculture | 184 | 78 |
| Business | 5 | 2 | |
| Employee | 35 | 15 | |
| Student | 12 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limbu, K.; Subba, A.; Limbu, N.; Khanal, L.; Kyes, R.C. Ecological Drivers and Community Perceptions: Conservation Challenges for the Critically Endangered Elongated Tortoise (Indotestudo elongata) in Jalthal Forest, Eastern Nepal. Diversity 2025, 17, 458. https://doi.org/10.3390/d17070458
Limbu K, Subba A, Limbu N, Khanal L, Kyes RC. Ecological Drivers and Community Perceptions: Conservation Challenges for the Critically Endangered Elongated Tortoise (Indotestudo elongata) in Jalthal Forest, Eastern Nepal. Diversity. 2025; 17(7):458. https://doi.org/10.3390/d17070458
Chicago/Turabian StyleLimbu, Kamala, Asmit Subba, Nishan Limbu, Laxman Khanal, and Randall C. Kyes. 2025. "Ecological Drivers and Community Perceptions: Conservation Challenges for the Critically Endangered Elongated Tortoise (Indotestudo elongata) in Jalthal Forest, Eastern Nepal" Diversity 17, no. 7: 458. https://doi.org/10.3390/d17070458
APA StyleLimbu, K., Subba, A., Limbu, N., Khanal, L., & Kyes, R. C. (2025). Ecological Drivers and Community Perceptions: Conservation Challenges for the Critically Endangered Elongated Tortoise (Indotestudo elongata) in Jalthal Forest, Eastern Nepal. Diversity, 17(7), 458. https://doi.org/10.3390/d17070458







