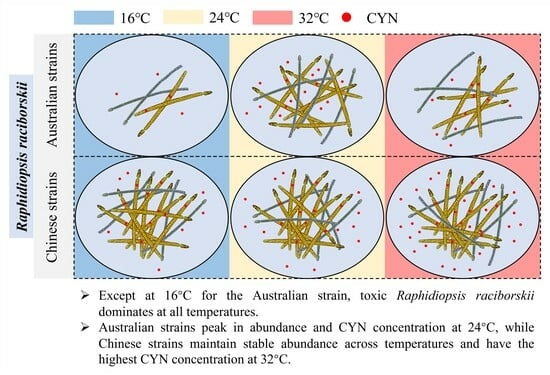
Effects of Temperature on Competition Between Toxic and Non-Toxic Raphidiopsis raciborskii and Cylindrospermopsin Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Raphidiopsis raciborskii Strains and Culture Conditions
2.2. Monoculture Experiments
2.3. Co-Culture Experiments
2.4. DNA Extraction and Real-Time PCR
2.5. CYN Measurement
2.6. Data Analysis and Processing
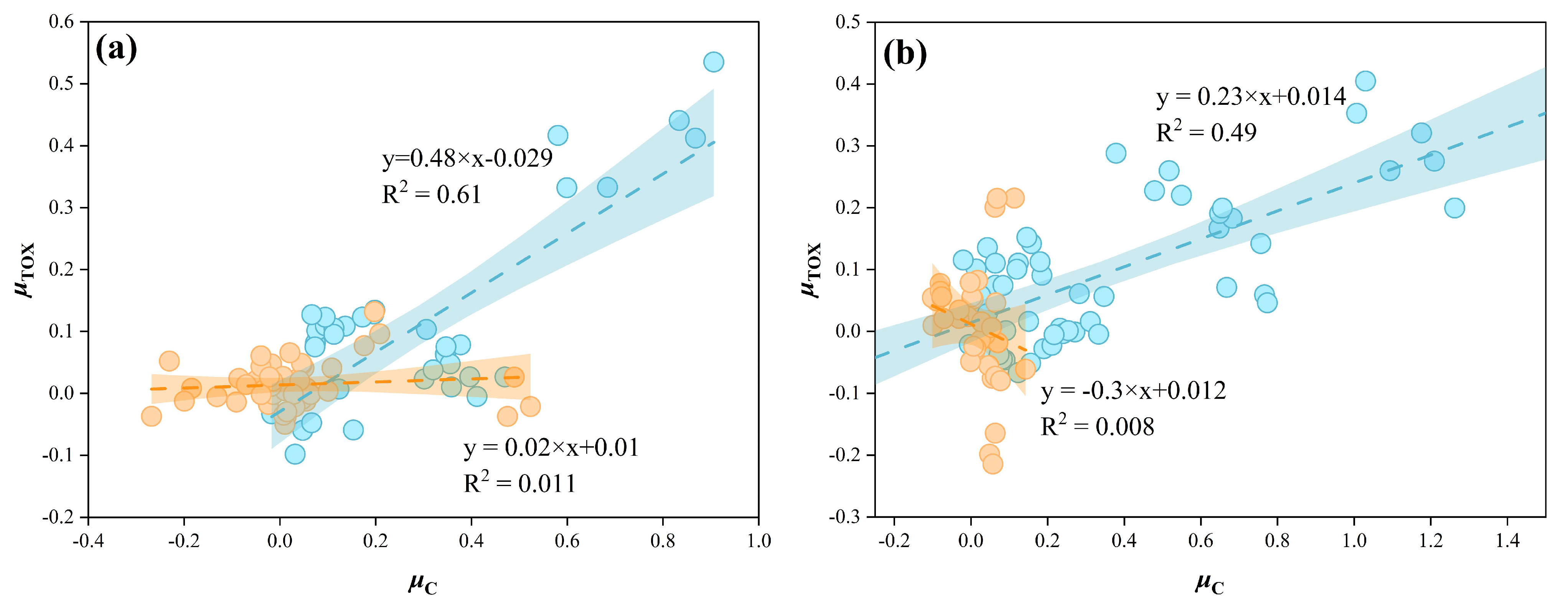
2.6.1. Analysis of the Relationship Between Growth Rate and CYN Production
2.6.2. The Rate of Competitive Displacement
2.6.3. Data Analysis
3. Results
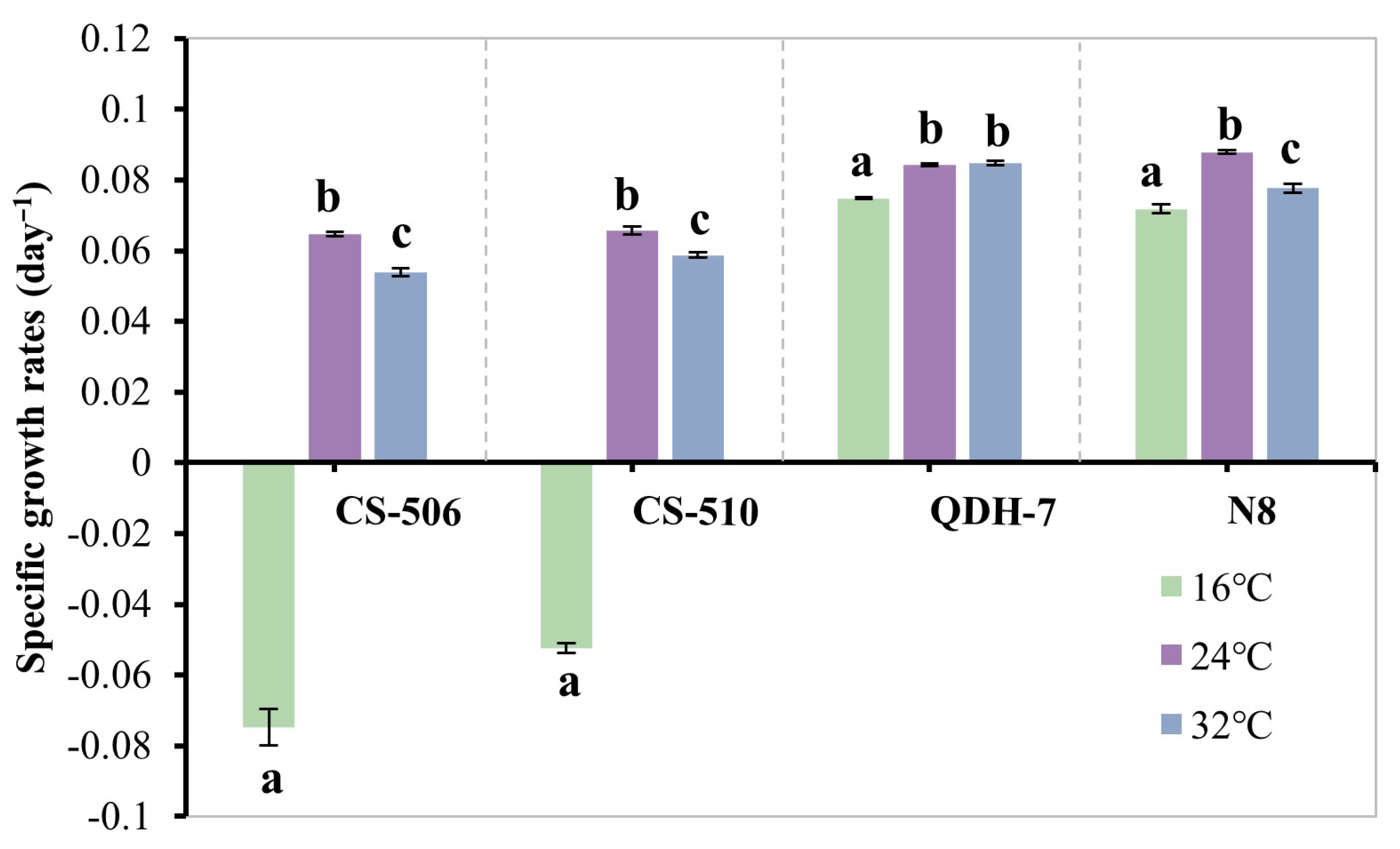
3.1. Specific Growth Rate of R. raciborskii Under Monoculture Conditions
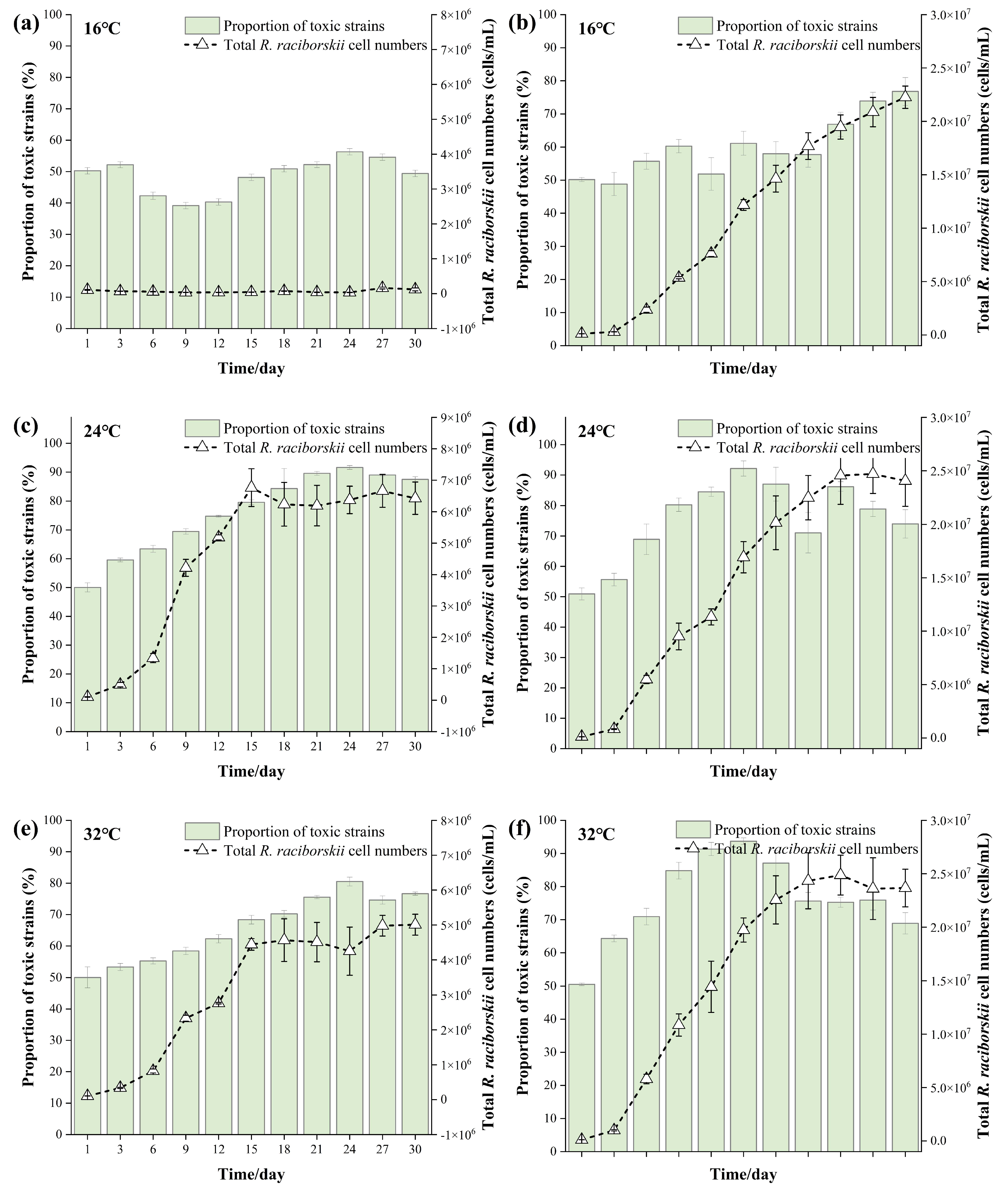
3.2. Changes in Total R. raciborskii Abundance and Proportion of CYN-Producing Strains in the Co-Culture Experiments
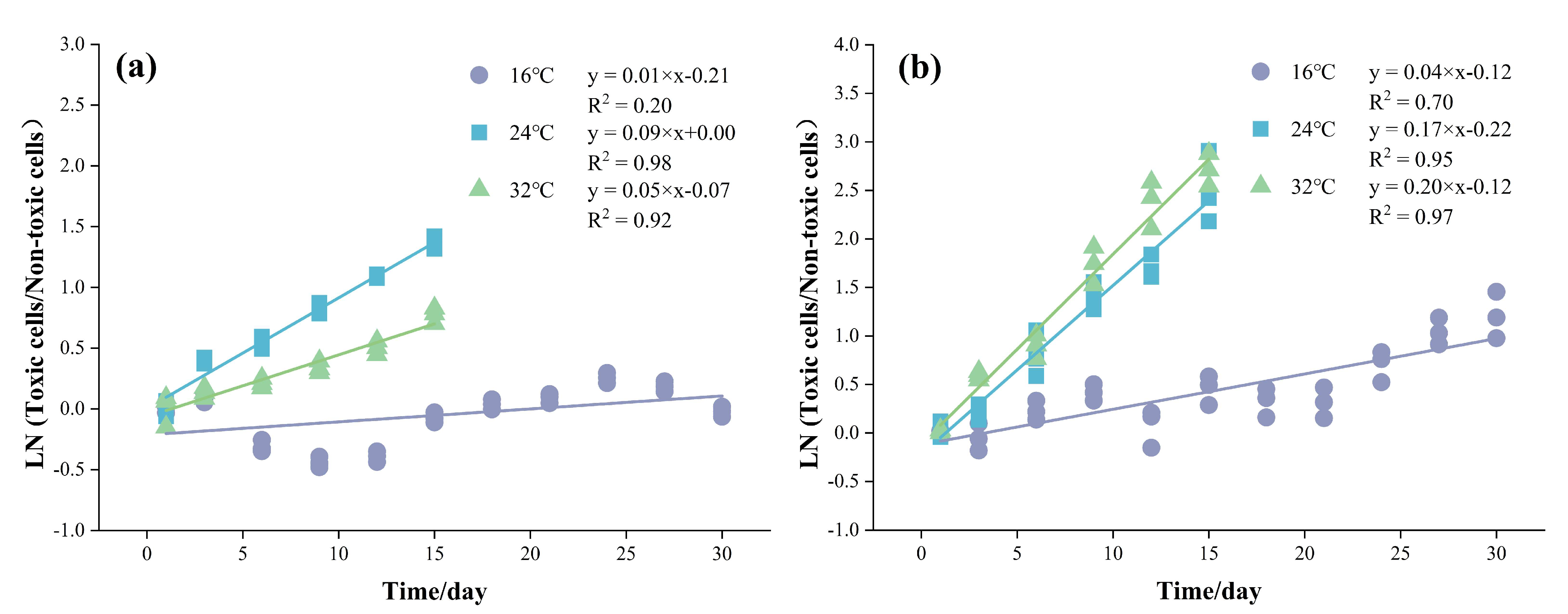
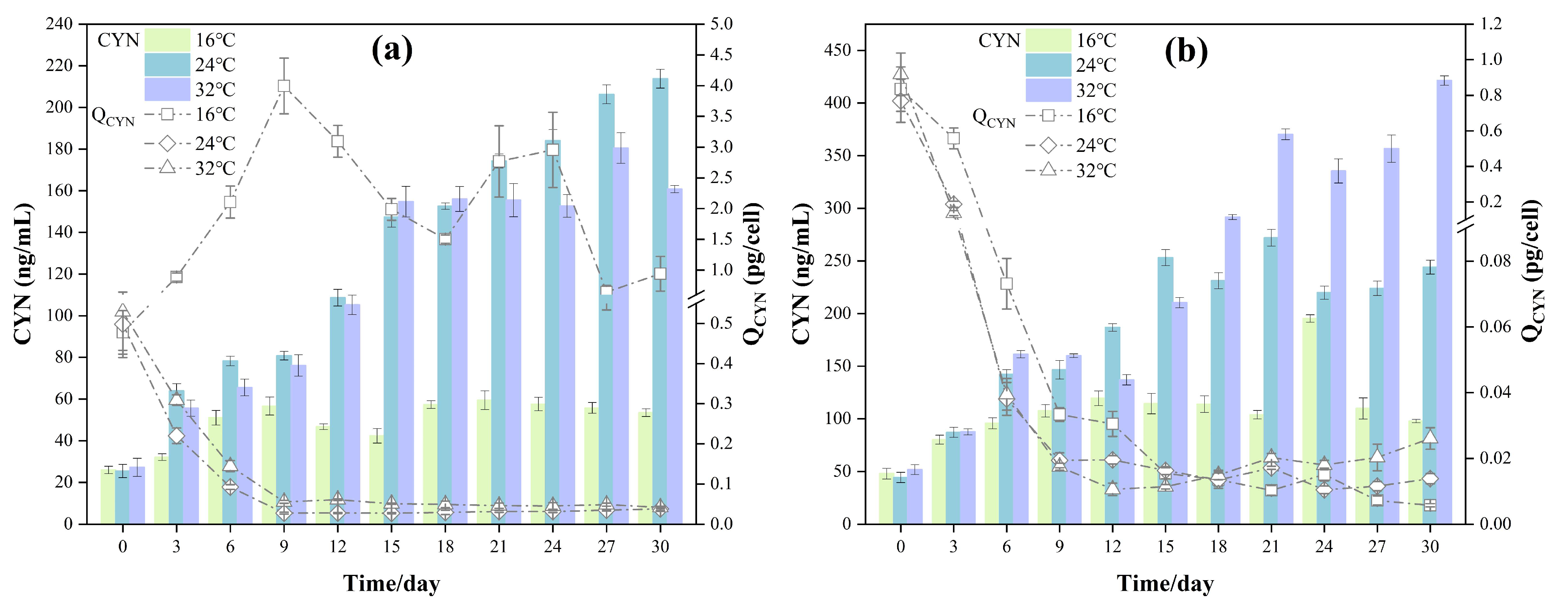
3.3. Changes in CYN Concentration and QCYN in the Co-Culture Experiments
4. Discussion
5. Conclusions and Perspectives
5.1. Conclusions
5.2. Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguilera, A.; Gómez, E.B.; Kastovsky, J.; Echenique, R.O.; Salerno, G.L. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia 2018, 57, 130–146. [Google Scholar] [CrossRef]
- Burford, M.A.; Beardall, J.; Willis, A.; Orr, P.T.; Magalhaes, V.F.; Rangel, L.M.; Azevedo, S.M.F.O.E.; Neilan, B.A. Understanding the winning strategies used by the bloom-forming cyanobacterium Cylindrospermopsis raciborskii. Harmful Algae 2016, 54, 44–53. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, S.; Shi, J. Overview of the distribution and adaptation of a bloom-forming cyanobacterium Raphidiopsis raciborskii: Integrating genomics, toxicity, and ecophysiology. J. Oceanol. Limnol. 2022, 40, 1774–1791. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Y.; Li, R.; Yang, Y.; Jiang, Y. Recent Advances in the Ecology of Bloom-Forming Raphidiopsis (Cylindrospermopsis) raciborskii: Expansion in China, Intraspecific Heterogeneity and Critical Factors for Invasion. Int. J. Environ. Res. Public Health 2023, 20, 1984. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef] [PubMed]
- Byth, S. Palm Island Mystery Disease. Med. J. Aust. 1980, 2, 40–42. [Google Scholar] [CrossRef]
- Zheng, B.; He, S.; Zhao, L.; Li, J.; Du, Y.; Li, Y.; Shi, J.; Wu, Z. Does temperature favour the spread of Raphidiopsis raciborskii, an invasive bloom-forming cyanobacterium, by altering cellular trade-offs? Harmful Algae 2023, 124, 102406. [Google Scholar] [CrossRef]
- Sprőber, P.; Shafik, H.M.; Présing, M.; Kovács, A.W.; Herodek, S. Nitrogen uptake and fixation in the cyanobacterium Cylindrospermopsis raciborskii under different nitrogen conditions. Hydrobiologia 2003, 506, 169–174. [Google Scholar] [CrossRef]
- Dixon, R.; Kahn, D. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2004, 2, 621–631. [Google Scholar] [CrossRef]
- Shatwell, T.; Köhler, J. Decreased nitrogen loading controls summer cyanobacterial blooms without promoting nitrogen-fixing taxa: Long-term response of a shallow lake. Limnol. Oceanogr. 2019, 64, S166–S178. [Google Scholar] [CrossRef]
- Xu, H.; McCarthy, M.J.; Paerl, H.W.; Brookes, J.D.; Zhu, G.; Hall, N.S.; Qin, B.; Zhang, Y.; Zhu, M.; Hampel, J.J. Contributions of external nutrient loading and internal cycling to cyanobacterial bloom dynamics in Lake Taihu, China: Implications for nutrient management. Limnol. Oceanogr. 2021, 66, 1492–1509. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, J.; Li, R. Comparative studies on photosynthesis and phosphate metabolism of Cylindrospermopsis raciborskii with Microcystis aeruginosa and Aphanizomenon Flos-aquae. Harmful Algae 2009, 8, 910–915. [Google Scholar] [CrossRef]
- Tokodi, N.; Latkowska, E.; Klimczak, B.; Kłodawska, K.; Malec, P.; Willis, A.; Kokociński, M.; Antosiak, A.; Dziga, D. Toward understanding the mechanisms of Raphidiopsis raciborskii response to chill/light stress. J. Phycol. 2025, 61, 488–508. [Google Scholar] [CrossRef]
- Aubriot, L.; Clivio, F.; Somma, A.; Galvanese, E.; Colombo, S.; Haakonsson, S. Control of cyanobacterial blooms with iron addition can favor stress-tolerant toxic species. Harmful Algae 2025, 142, 102784. [Google Scholar]
- Mohamed Nor, N.H.; Te, S.H.; Mowe, M.A.D.; Gin, K.Y.-H. Environmental factors influence cylindrospermopsin production of Cylindrospermopsis raciborskii (CR12). J. Plankton Res. 2019, 41, 114–126. [Google Scholar] [CrossRef]
- Xiao, M.; Hamilton, D.P.; Chuang, A.; Burford, M.A. Intra-population strain variation in phosphorus storage strategies of the freshwater cyanobacterium Raphidiopsis raciborskii. FEMS Microbiol. Ecol. 2020, 96, fiaa092. [Google Scholar] [CrossRef]
- Gobler, C.J.; Burkholder, J.M.; Davis, T.W.; Harke, M.J.; Johengen, T.; Stow, C.A.; Van de Waal, D.B. The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 2016, 54, 87–97. [Google Scholar] [CrossRef]
- Pearson, L.A.; Dittmann, E.; Mazmouz, R.; Ongley, S.E.; D’Agostino, P.M.; Neilan, B.A. The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria. Harmful Algae 2016, 54, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Barnard, M.A.; Chaffin, J.D.; Plaas, H.E.; Boyer, G.L.; Wei, B.; Wilhelm, S.W.; Rossignol, K.L.; Braddy, J.S.; Bullerjahn, G.S.; Bridgeman, T.B.; et al. Roles of Nutrient Limitation on Western Lake Erie CyanoHAB Toxin Production. Toxins 2021, 13, 47. [Google Scholar] [CrossRef]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Harke, M.J.; Davis, T.W.; Watson, S.B.; Gobler, C.J. Nutrient-Controlled Niche Differentiation of Western Lake Erie Cyanobacterial Populations Revealed via Metatranscriptomic Surveys. Environ. Sci. Technol. 2016, 50, 604–615. [Google Scholar] [CrossRef]
- Lei, L.; Li, C.; Peng, L.; Han, B.-P. Competition between toxic and non-toxic Microcystis aeruginosa and its ecological implication. Ecotoxicology 2015, 24, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Rinta-Kanto, J.M.; Konopko, E.A.; DeBruyn, J.M.; Bourbonniere, R.A.; Boyer, G.L.; Wilhelm, S.W. Lake Erie Microcystis: Relationship between microcystin production, dynamics of genotypes and environmental parameters in a large lake. Harmful Algae 2009, 8, 665–673. [Google Scholar] [CrossRef]
- Yoshida, M.; Yoshida, T.; Takashima, Y.; Hosoda, N.; Hiroishi, S. Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol. Lett. 2007, 266, 49–53. [Google Scholar] [CrossRef]
- Wang, M.; Shi, W.; Chen, Q.; Zhang, J.; Yi, Q.; Hu, L. Effects of nutrient temporal variations on toxic genotype and microcystin concentration in two eutrophic lakes. Ecotoxicol. Environ. Saf. 2018, 166, 192–199. [Google Scholar] [CrossRef]
- Orr, P.T.; Rasmussen, J.P.; Burford, M.A.; Eaglesham, G.K.; Lennox, S.M. Evaluation of quantitative real-time PCR to characterise spatial and temporal variations in cyanobacteria, Cylindrospermopsis raciborskii (Woloszynska) Seenaya et Subba Raju and cylindrospermopsin concentrations in three subtropical Australian reservoirs. Harmful Algae 2011, 10, 234. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Merrick, C.; Ryan, D.; Humpage, A.; Bowling, L.; Neilan, B.A. Community Composition, Toxigenicity, and Environmental Conditions during a Cyanobacterial Bloom Occurring along 1,100 Kilometers of the Murray River. Appl. Environ. Microbiol. 2012, 78, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Burford, M.A.; Davis, T.W.; Orr, P.T.; Sinha, R.; Willis, A.; Neilan, B.A. Nutrient-related changes in the toxicity of field blooms of the cyanobacterium, Cylindrospermopsis raciborskii. FEMS Microbiol. Ecol. 2014, 89, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lou, I.; Ung, W.K.; Kong, Y.; Mok, K.M. Analysis of cylindrospermopsin- and microcystin-producing genotypes and cyanotoxin concentrations in the Macau storage reservoir. Hydrobiologia 2014, 741, 51–68. [Google Scholar] [CrossRef]
- Lei, L.; Lei, M.; Cheng, N.; Chen, Z.; Xiao, L.; Han, B.-P.; Lin, Q. Nutrient Regulation of Relative Dominance of Cylindrospermopsin-Producing and Non-cylindrospermopsin-Producing Raphidiopsis raciborskii. Front. Microbiol. 2021, 12, 793544. [Google Scholar] [CrossRef]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.K.; Litchman, E. Effects of temperature and nitrogen availability on the growth of invasive and native cyanobacteria. Hydrobiologia 2016, 763, 357–369. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef]
- Sinha, R.; Pearson, L.A.; Davis, T.W.; Burford, M.A.; Orr, P.T.; Neilan, B.A. Increased incidence of Cylindrospermopsis raciborskii in temperate zones–Is climate change responsible? Water Res. 2012, 46, 1408–1419. [Google Scholar] [CrossRef]
- Dziallas, C.; Grossart, H.-P. Increasing Oxygen Radicals and Water Temperature Select for Toxic Microcystis sp. PLoS ONE 2011, 6, e25569. [Google Scholar] [CrossRef]
- Meng, H.; Guo, Y.; Zhang, L.; Qi, Y.; Liu, J.; He, H.; Wang, G.; Li, S.; Zhang, L. Effects of Elevated Overwinter Temperature on the Growth Strategies of Microcystis aeruginosa. Freshw. Biol. 2025, 70, e14370. [Google Scholar] [CrossRef]
- Li, X.; Tikhonova, I.V.; Potapov, S.A.; Krasnopeev, A.Y.; Zhuchenko, N.A.; Niao, X.; Wang, L.; Sorokovikova, E.G.; Wang, W.; Belykh, O.I. World’s largest oligotrophic Lake Baikal: Concerns about cyanobacterial blooms and potential microcystin producers along the littoral zone. Harmful Algae 2025, 144, 102841. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Fan, L.; Wu, Y.; Q, W.; Shang, T.; Cheng, N.; Lei, L. Low dominance of toxic genotype within the Cylindrospermopsis raciborskii population and its affecting factors in tropical reservoirs of southern China a case study of Zhuhai city. Acta Hydrobiol. Sin. 2024, 48, 211–221. [Google Scholar]
- Briand, E.; Bormans, M.; Quiblier, C.; Salençon, M.-J.; Humbert, J.-F. Evidence of the Cost of the Production of Microcystins by Microcystis aeruginosa under Differing Light and Nitrate Environmental Conditions. PLoS ONE 2012, 7, e29981. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Lei, L.; Lei, M.; Lu, Y.; Peng, L.; Han, B.-P. Development of real-time PCR for quantification of Cylindrospermopsis raciborskii cells and potential cylindrospermopsin-producing genotypes in subtropicalreservoirs of southern China. J. Appl. Phycol. 2019, 31, 3749–3758. [Google Scholar] [CrossRef]
- Lei, L.; Peng, L.; Yang, Y.; Han, B.-P. Development of Time-Resolved Fluoroimmunoassay for Detection of Cylindrospermopsin Using Its Novel Monoclonal Antibodies. Toxins 2018, 10, 255. [Google Scholar] [CrossRef]
- Orr, P.T.; Willis, A.; Burford, M.A. Application of first order rate kinetics to explain changes in bloom toxicity—The importance of understanding cell toxin quotas. J. Oceanol. Limnol. 2018, 36, 1063–1074. [Google Scholar] [CrossRef]
- Van de Waal, D.B.; Verspagen, J.M.H.; Finke, J.F.; Vournazou, V.; Immers, A.K.; Kardinaal, W.E.A.; Tonk, L.; Becker, S.; Van Donk, E. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J. 2011, 5, 1438–1450. [Google Scholar] [CrossRef] [PubMed]
- Walls, J.T.; Wyatt, K.H.; Doll, J.C.; Rubenstein, E.M.; Rober, A.R. Hot and toxic: Temperature regulates microcystin release from cyanobacteria. Sci. Total Environ. 2018, 610–611, 786–795. [Google Scholar] [CrossRef]
- Godoy, R.F.B.; Trevisan, E.; Battistelli, A.A.; Crisigiovanni, E.L.; do Nascimento, E.A.; da Fonseca Machado, A.L. Does water temperature influence in microcystin production? A case study of Billings Reservoir, São Paulo, Brazil. J. Contam. Hydrol. 2023, 255, 104164. [Google Scholar]
- Briand, J.F.; Leboulanger, C.; Humbert, J.F.; Bernard, C.; Dufour, P. Cylindrospermopsis raciborskii (Cyanobacteria) invasion at mid-latitudes: Selection, wide physiological tolerance, or global warming? J. Phycol. 2004, 40, 231–238. [Google Scholar] [CrossRef]
- dos Santos Silva, R.D.; Chia, M.A.; Barbosa, V.V.; dos Santos Severiano, J.; de Lucena Barbosa, J.E. Synergistic effects of temperature and nutrients on growth and saxitoxin content of the cyanobacterium Raphidiopsis raciborskii. J. Appl. Phycol. 2022, 34, 941–952. [Google Scholar] [CrossRef]
- Recknagel, F.; Zohary, T.; Rücker, J.; Orr, P.T.; Branco, C.C.; Nixdorf, B. Causal relationships of Raphidiopsis (formerly Cylindrospermopsis) dynamics with water temperature and N:P-ratios: A meta-analysis across lakes with different climates based on inferential modelling. Harmful Algae 2019, 84, 222–232. [Google Scholar] [CrossRef]
- Willis, A.; Chuang, A.W.; Woodhouse, J.N.; Neilan, B.A.; Burford, M.A. Intraspecific variation in growth, morphology and toxin quotas for the cyanobacterium, Cylindrospermopsis raciborskii. Toxicon 2016, 119, 307–310. [Google Scholar] [CrossRef]
- Lu, Y.; Lei, M.; Ye, J.; Lei, L.; Han, B. Intraspecific variation of morphological traits and toxin-producing capacity and phylogenetic analysis for Cylindrospermopsis raciborskii from Qiandenghu Lake, Guangdong Province. J. Lake Sci. 2020, 32, 144–153. [Google Scholar]
- Yu, T.; Dai, J.; Lei, L.; Peng, L. Effects of temperature, irradiance and nitrate on the growth of Cylindrospermopsis raciborskii N8. J. Lake Sci. 2014, 26, 441–446. [Google Scholar]
- Bonilla, S.; Aubriot, L.; Soares, M.C.S.; González-Piana, M.; Fabre, A.; Huszar, V.L.M.; Lürling, M.; Antoniades, D.; Padisák, J.; Kruk, C. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiol. Ecol. 2012, 79, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Wang, Y.; Guan, Y.; Chen, Y.; Li, R.; Yu, G. Occurrence of Raphidiopsis raciborskii blooms in cool waters: Synergistic effects of nitrogen availability and ecotypes with adaptation to low temperature. Environ. Pollut. 2021, 270, 116070. [Google Scholar] [CrossRef]
- Briand, E.; Yéprémian, C.; Humbert, J.F.; Quiblier, C. Competition between microcystin- and non-microcystin-producing Planktothrix agardhii (cyanobacteria) strains under different environmental conditions. Environ. Microbiol. 2008, 10, 3337–3348. [Google Scholar] [CrossRef]
- Giannuzzi, L. Cyanobacteria Growth Kinetics; IntechOpen: London, UK, 2019. [Google Scholar]
- Rzymski, P.; Poniedziałek, B. In search of environmental role of cylindrospermopsin: A review on global distribution and ecology of its producers. Water Res. 2014, 66, 320–337. [Google Scholar] [CrossRef]
- Holland, A.; Kinnear, S. Interpreting the possible ecological role(s) of cyanotoxins: Compounds for competitive advantage and/or physiological aide? Mar. Drugs 2013, 11, 2239–2258. [Google Scholar] [CrossRef]
- Rzymski, P.; Poniedziałek, B.; Kokociński, M.; Jurczak, T.; Lipski, D.; Wiktorowicz, K. Interspecific allelopathy in cyanobacteria: Cylindrospermopsin and Cylindrospermopsis raciborskii effect on the growth and metabolism of Microcystis aeruginosa. Harmful Algae 2014, 35, 1–8. [Google Scholar] [CrossRef]
- B-Béres, V.; Vasas, G.; Dobronoki, D.; Gonda, S.; Nagy, S.A.; Bácsi, I. Effects of Cylindrospermopsin Producing Cyanobacterium and Its Crude Extracts on a Benthic Green Alga—Competition or Allelopathy? Mar. Drugs 2015, 13, 6703–6722. [Google Scholar] [CrossRef]
- Pinheiro, C.; Azevedo, J.; Campos, A.; Loureiro, S.; Vasconcelos, V. Absence of negative allelopathic effects of cylindrospermopsin and microcystin-LR on selected marine and freshwater phytoplankton species. Hydrobiologia 2013, 705, 27–42. [Google Scholar] [CrossRef]
- Figueredo, C.C.; Giani, A.; Bird, D.F. Does allelopathy contribute to Cylindrospermopsis raciborskii (cyanobacteria) bloom occurrence and geographic expansion? J. Phycol. 2007, 43, 256–265. [Google Scholar] [CrossRef]
- Willis, A.; Adams, M.P.; Chuang, A.W.; Orr, P.T.; O’Brien, K.R.; Burford, M.A. Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Wołoszyńska) Seenayya et Subba Raju). Harmful Algae 2015, 47, 27–34. [Google Scholar] [CrossRef]
- Park, G.; Norton, L.; Avery, D.; Dam, H.G. Grazers modify the dinoflagellate relationship between toxin production and cell growth. Harmful Algae 2023, 126, 102439. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pančić, M.; Andersen, K.H.; Kiørboe, T. The cost of toxin production in phytoplankton: The case of PST producing dinoflagellates. ISME J. 2018, 13, 64–75. [Google Scholar] [CrossRef]
- Lei, L.; Liu, W.; Chen, Z.; Peng, L.; Xiao, L.; Han, B.; Neilan, B.A. Grazer-induced toxin production is energetically costly and significantly reduces growth of cylindrospermopsin-producing cyanobacteria. Limnol. Oceanogr. 2024, 69, 2929–2940. [Google Scholar] [CrossRef]
- Cirés, S.; Wörmer, L.; Timón, J.; Wiedner, C.; Quesada, A. Cylindrospermopsin production and release by the potentially invasive cyanobacterium Aphanizomenon ovalisporum under temperature and light gradients. Harmful Algae 2011, 10, 668–675. [Google Scholar] [CrossRef]
- Saker, M.L.; Griffiths, D.J. The effect of temperature on growth and cylindrospermopsin content of seven isolates of Cylindrospermopsis raciborskii (Nostocales, Cyanophyceae) from water bodies in northern Australia. Phycologia 2000, 39, 349–354. [Google Scholar] [CrossRef]
- Rücker, J.; Stüken, A.; Nixdorf, B.; Fastner, J.; Chorus, I.; Wiedner, C. Concentrations of particulate and dissolved cylindrospermopsin in 21 Aphanizomenon-dominated temperate lakes. Toxicon 2007, 50, 800–809. [Google Scholar] [CrossRef]
- Wiedner, C.; Rücker, J.; Fastner, J.; Chorus, I.; Nixdorf, B. Seasonal dynamics of cylindrospermopsin and cyanobacteria in two German lakes. Toxicon 2008, 52, 677–686. [Google Scholar] [CrossRef]
- Pierangelini, M.; Sinha, R.; Willis, A.; Burford, M.A.; Orr, P.T.; Beardall, J.; Neilan, B.A. Constitutive Cylindrospermopsin Pool Size in Cylindrospermopsis raciborskii under Different Light and CO2 Partial Pressure Conditions. Appl. Environ. Microbiol. 2015, 81, 3069–3076. [Google Scholar] [CrossRef]
| Gene | Primer and Probe Names | Primer and Probe Sequences | Product Length |
|---|---|---|---|
| rpoC1 | CYL-F | 5′-CGGGTTCGTCATAGAGGTATTG-3′ | 186 bp |
| CYL-R | 5′-GCTACAGGTGCTGCTAACTT-3′ | ||
| CYL-P | 5′(FAM)-TAACAGAATCACGAGTTCGCCGCC-(BHQ1)3′ | ||
| cyrJ | CyrJ-F | 5′-TGATTCGCCAACCCAAAGAA-3′ | 165 bp |
| CyrJ-R | 5′-GATCGTTCAGCAAGTCGTGT-3′ | ||
| CyrJ-P | 5′(FAM)-CGGAGTAATCCCGCCTGTCATAGATGC-(BHQ1)3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Tang, X.; Zhang, S.; Lei, M.; Lei, L. Effects of Temperature on Competition Between Toxic and Non-Toxic Raphidiopsis raciborskii and Cylindrospermopsin Production. Diversity 2025, 17, 450. https://doi.org/10.3390/d17070450
Liu W, Tang X, Zhang S, Lei M, Lei L. Effects of Temperature on Competition Between Toxic and Non-Toxic Raphidiopsis raciborskii and Cylindrospermopsin Production. Diversity. 2025; 17(7):450. https://doi.org/10.3390/d17070450
Chicago/Turabian StyleLiu, Wei, Xin Tang, Sainan Zhang, Mingting Lei, and Lamei Lei. 2025. "Effects of Temperature on Competition Between Toxic and Non-Toxic Raphidiopsis raciborskii and Cylindrospermopsin Production" Diversity 17, no. 7: 450. https://doi.org/10.3390/d17070450
APA StyleLiu, W., Tang, X., Zhang, S., Lei, M., & Lei, L. (2025). Effects of Temperature on Competition Between Toxic and Non-Toxic Raphidiopsis raciborskii and Cylindrospermopsin Production. Diversity, 17(7), 450. https://doi.org/10.3390/d17070450