Seed Traits and Curculio Weevil Infestation: A Study in Quercus mongolica
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plot Setup and Seed Collection
2.3. Q. mongolica Seed Traits
2.4. Quantitative Assessment of Infestation Parameters
infested by multiple weevil species at the same time/total infested seeds) × 100%
species/total infested seeds) × 100%
weevil species at the same time/total infested seeds) × 100%
species/total number of weevils) × 100%
2.5. Identification of Seed-Infesting Weevils
2.6. Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
3.1. Weevil Identification
3.2. Weevil Infestation
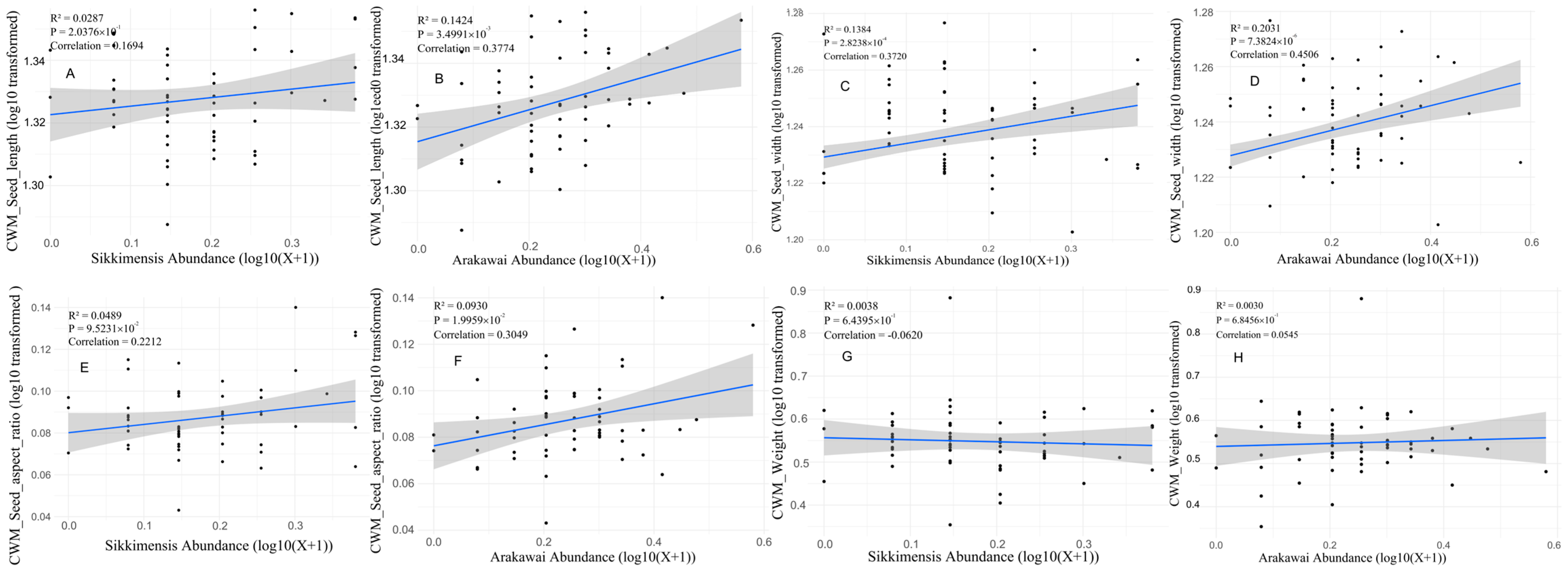
3.3. Seed Traits and Weevil Infestation Preference
4. Discussion
4.1. Acorn Size and Weevil Oviposition Preferences
4.2. Multi-Species Infestation and Larval Competition
4.3. Management Implications and Ecological Insights
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boadi, P.A.N.; Nboyine, J.A.; Kusi, F.; Ibrahim, Y.J.; Lawer, E.A. Rapid assessment of post-dispersal seed removal in an agricultural landscape of semi-arid West Africa. Agrofor. Syst. 2023, 98, 37–46. [Google Scholar] [CrossRef]
- Ohsawa, M. The role of isolated old oak trees in maintaining beetle diversity within larch plantations in the central mountainous region of Japan. For. Ecol. Manag. 2007, 250, 215–226. [Google Scholar] [CrossRef]
- Bartlow, A.W.; Agosta, S.J.; Curtis, R.; Yi, X.; Steele, M.A. Acorn size and tolerance to seed predators: The multiple roles of acorns as food for seed predators, fruit for dispersal and fuel for growth. Integr. Zool. 2018, 13, 251–266. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Reich, P.B.; Hernández, A.; Wright, S.J. Species with greater seed mass are more tolerant of conspecific neighbours: A key driver of early survival and future abundances in a tropical forest. Ecol. Lett. 2016, 19, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Foffová, H.; Ćavar Zeljković, S.; Honěk, A.; Martinková, Z.; Tarkowski, P.; Saska, P. Which seed properties determine the preferences of carabid beetle seed predators? Insects 2020, 11, 757. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, S.M.; Davis, R.B.; Esperk, T.; Gotthard, K.; Mutanen, M.; Valdma, D.; Tammaru, T. Comparative analysis of larval growth in Lepidoptera reveals instar-level constraints. Funct. Ecol. 2020, 34, 1391–1403. [Google Scholar] [CrossRef]
- Fenner, M. Seed size and chemical composition: The allocation of minerals to seeds and their use in early seedling growth. Bot. J. Scotl. 2004, 56, 163–173. [Google Scholar] [CrossRef]
- Dylewski, Ł.; Ortega, Y.K.; Bogdziewicz, M.; Pearson, D.E. Seed size predicts global effects of small mammal seed predation on plant recruitment. Ecol. Lett. 2020, 23, 1024–1033. [Google Scholar] [CrossRef]
- Peguero, G.; Muller-Landau, H.C.; Jansen, P.A.; Wright, S.J. Cascading effects of defaunation on the coexistence of two specialized insect seed predators. J. Anim. Ecol. 2016, 86, 136–146. [Google Scholar] [CrossRef]
- Shoemaker, L.G.; Barner, A.K.; Bittleston, L.S.; Teufel, A.I. Quantifying the relative importance of variation in predation and the environment for species coexistence. Ecol. Lett. 2020, 23, 939–950. [Google Scholar] [CrossRef]
- Reut, M.; Bonal, R.; Chrabąszcz, M.; Moniuszko, H. Cannibalism as competition strategy in larvae of the acorn weevil Curculio glandium (Coleoptera: Curculionidae). Diversity 2023, 15, 145. [Google Scholar] [CrossRef]
- Reut, M.; Chrabąszcz, M.; Moniuszko, H. Timing is everything. Temporal and spatial niche segregation in Curculio spp. (Coleoptera: Curculionidae) associated with oak trees. Insects 2021, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Muñoz, A.; Bonal, R.; Espelta, J.M. Acorn–weevil interactions in a mixed-oak forest: Outcomes for larval growth and plant recruitment. For. Ecol. Manag. 2014, 322, 98–105. [Google Scholar] [CrossRef]
- Arias-Leclaire, H.; Bonal, R.; García-López, D.; Espelta, J.M. Role of seed size, phenology, oogenesis and host distribution in the specificity and genetic structure of seed weevils (Curculio spp.) in mixed forests. Integr. Zool. 2018, 13, 267–279. [Google Scholar] [CrossRef]
- Ofcarcik, R.P.; Burns, E.E. Chemical and physical properties of selected acorns. J. Food Sci. 1971, 36, 576–578. [Google Scholar] [CrossRef]
- Bonal, R.; Espelta, J.M.; Vogler, A.P. Complex selection on life-history traits and the maintenance of variation in exaggerated rostrum length in acorn weevils. Oecologia 2011, 167, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.B.; Hahn, P.G.; Metcalf, E.C.; Maron, J.L. Seed size of co-occurring forb species predicts rates of predispersal seed loss from insects. Ecosphere 2022, 13, e4032. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Xu, J.; Bai, L.; Gan, X.; Li, F. Study on factors influencing DNA sequencing by automatic genetic analyzer. J. Hyg. Res. 2015, 44, 440–443. [Google Scholar]
- Thermo Fisher Scientific. BigDye™ Terminator v3.1 Cycle Sequencing Kit User Guide; Thermo Fisher Scientific: South San Francisco, CA, USA, 2016. [Google Scholar]
- Kim, Y.J.; Moon, S.R.; Yoon, C.; Kim, G.H. Measurement and comparison of morphology of developmental stages of chestnut weevil, Curculio sikkimensis (Coleoptera: Curculionidae). Korean J. Appl. Entomol. 2010, 49, 11–16. [Google Scholar] [CrossRef]
- Pelsue, F.W., Jr.; Zhang, R. A review of the genus Curculio from China with descriptions of four new taxa. Part V. The Curculio dentipes (Roelofs) group (Coleoptera: Curculionidae: Curculionini). Coleopt. Bull. 2005, 59, 293–303. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zhang, Z.; Zeng, Y.; Hu, H.; Hao, Y.; Huang, S.; Li, B. Common methods for phylogenetic tree construction and their implementation in R. Bioengineering 2024, 11, 480. [Google Scholar] [CrossRef]
- Sakamoto, T.; Ortega, J.M. TaxOnTree: A tool that generates trees annotated with taxonomic information. Cold Spring Harb. Lab. 2020, 12. [Google Scholar] [CrossRef]
- Cuffe, P. Healy: Data Visualization: A Practical Introduction [Book Review]. IEEE Trans. Prof. Commun. 2019, 62, 310–311. [Google Scholar] [CrossRef]
- Fragnière, A.L.; Bacher, S.; Kehrli, P. Identifying candidate host plants for trap crop against Drosophila suzukii in vineyards. J. Pest. Sci. 2024, 97, 1975–1991. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Li, Q. Interspecific variation in seed traits facilitates divergent pre-dispersal seed predation among five stone oak species. Ecol. Process. 2025, 14, 42. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Espelta, J.M.; Bonal, R. Tolerance to seed predation mediated by seed size increases at lower latitudes in a Mediterranean oak. Ann. Bot. 2019, 123, 707–714. [Google Scholar] [CrossRef]
- Szendrei, Z.; Malo, E.; Stelinski, L.; Rodriguez-Saona, C. Response of cranberry weevil (Coleoptera: Curculionidae) to host plant volatiles. Environ. Entomol. 2009, 38, 861–869. [Google Scholar] [CrossRef]
- Kafle, B.D. Olfaction Mediated Host Selection in a Specialist Weevil Used for Biological Control of an Invasive Plant. Ph.D. Dissertation, University of Idaho, Moscow, ID, USA, 2016. [Google Scholar]
- Fleurot, E.; Venner, S.; Pélisson, P.F.; Débias, F.; Bel-Venner, M.C. The morphological allometry of four closely related and coexisting insect species reveals adaptation to the mean and variability of the resource size. Oecologia 2022, 200, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Hu, X.H.; Liu, D.C.; Ouyang, A.; Tong, X.; Wang, Y.J.; Wang, R.; Chen, X.Y. High diversity and strong variation in host specificity of seed parasitic acorn weevils. Insect Conser. Diver. 2021, 14, 367–376. [Google Scholar] [CrossRef]
- Addesso, K.M.; McAuslane, H.J.; Stansly, P.A.; Schuster, D.J. Host-marking by female pepper weevils, Anthonomus eugenii. Entomol. Exp. Appl. 2007, 125, 269–276. [Google Scholar] [CrossRef]
- Evans, K.G.; Neale, Z.R.; Holly, B.; Canizela, C.C.; Juliano, S.A. Survival-larval density relationships in the field and their implications for control of container-dwelling Aedes mosquitoes. Insects 2022, 14, 17. [Google Scholar]
- Than, A.T.; Ponton, F.; Morimoto, J. Integrative developmental ecology: A review of density-dependent effects on life-history traits and host-microbe interactions in non-social holometabolous insects. Evol. Ecol. 2020, 34, 659–680. [Google Scholar] [CrossRef]
- Ishii, Y.; Shimada, M. Competitive exclusion between contest and scramble strategists in Callosobruchus seed–beetle modeling. Popul. Ecol. 2008, 50, 197–205. [Google Scholar] [CrossRef]
- Cope, J.M.; Fox, C.W. Oviposition decisions in the seed beetle, Callosobruchus maculatus (Coleoptera: Bruchidae): Effects of seed size on superparasitism. J. Stored. Prod. Res. 2003, 39, 355–365. [Google Scholar] [CrossRef]
- Bonal, R.; Munoz, A. Seed growth suppression constrains the growth of seed parasites: Premature acorn abscission reduces Curculio elephas larval size. Ecol. Entomol. 2008, 33, 31–36. [Google Scholar] [CrossRef]
- Giga, D.P.; Smith, R.H. Intraspecific competition in the bean weevils Callosobruchus maculatus and Callosobruchus rhodesianus (Coleoptera: Bruchidae). J. Appl. Ecol. 1991, 28, 918–929. [Google Scholar] [CrossRef]
- Govindan, B.N.; Kéry, M.; Swihart, R.K. Host selection and responses to forest fragmentation in acorn weevils: Inferences from dynamic occupancy models. Oikos 2012, 121, 623–633. [Google Scholar] [CrossRef]
- Desouhant, E.; Debouzie, D.; Ploye, H.; Menu, F. Clutch size manipulations in the chestnut weevil, Curculio elephas: Fitness of oviposition strategies. Oecologia 2000, 122, 493–499. [Google Scholar] [CrossRef]
- Kellner, K.F.; Riegel, J.K.; Swihart, R.K. Effects of silvicultural disturbance on acorn infestation and removal. New For. 2014, 45, 265–281. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Hou, L.-M.; Guo, Y.-L.; Xing, M.-E.; Li, H.-Y.; Meng, Q.-F.; Feng, L.-C. Seed Traits and Curculio Weevil Infestation: A Study in Quercus mongolica. Diversity 2025, 17, 421. https://doi.org/10.3390/d17060421
Li S, Hou L-M, Guo Y-L, Xing M-E, Li H-Y, Meng Q-F, Feng L-C. Seed Traits and Curculio Weevil Infestation: A Study in Quercus mongolica. Diversity. 2025; 17(6):421. https://doi.org/10.3390/d17060421
Chicago/Turabian StyleLi, Shuang, Li-Min Hou, Yan-Lin Guo, Meng-En Xing, Hao-Yue Li, Qing-Fan Meng, and Li-Chao Feng. 2025. "Seed Traits and Curculio Weevil Infestation: A Study in Quercus mongolica" Diversity 17, no. 6: 421. https://doi.org/10.3390/d17060421
APA StyleLi, S., Hou, L.-M., Guo, Y.-L., Xing, M.-E., Li, H.-Y., Meng, Q.-F., & Feng, L.-C. (2025). Seed Traits and Curculio Weevil Infestation: A Study in Quercus mongolica. Diversity, 17(6), 421. https://doi.org/10.3390/d17060421





