Biodiversity in Agricultural Landscapes: Inter-Scale Patterns in the Po Plain (Italy)
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Studies
2.2. Floristic and Vegetational Analyses
2.3. Landscape Ecology Analyses
2.4. Inter-Scale Comparisons of Landscape Ecology and Floristic–Vegetational Traits
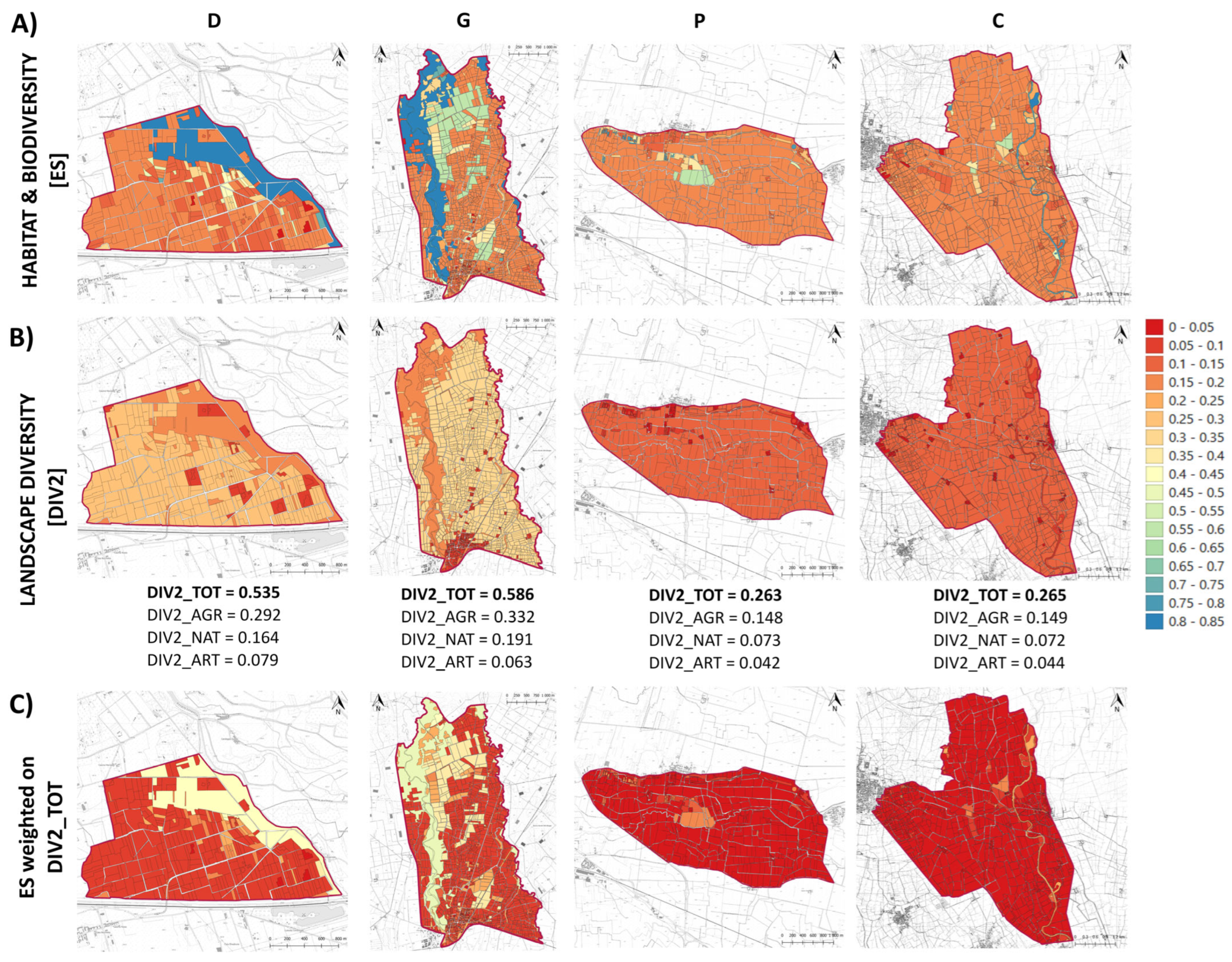
2.5. Mapping Inter-Scale Biodiversity
3. Results
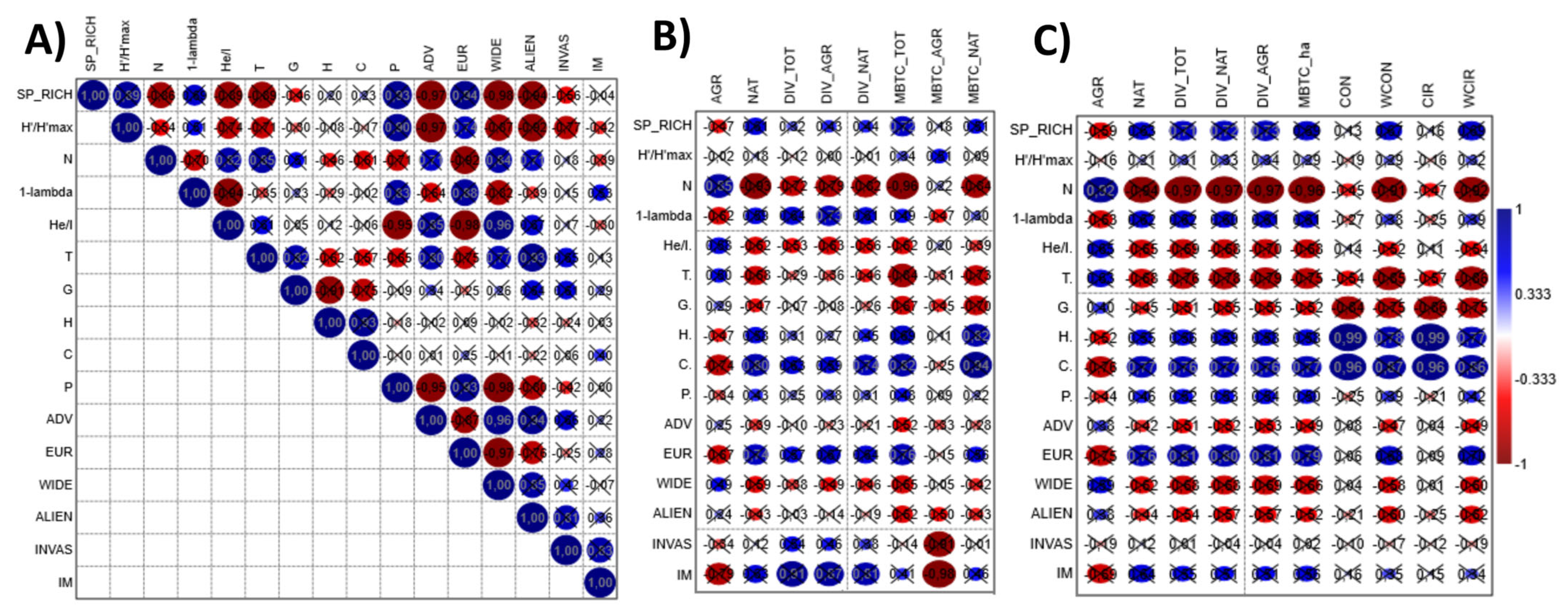
3.1. Dataset Exploration
3.2. Floristic–Vegetational Indicator Correlation Patterns
3.3. Floristic–Vegetational and Extra-Local Landscape Ecology Indicators’ Correlation Patterns
3.4. Floristic–Vegetational and Local Landscape Ecology Indicator Correlation Patterns
3.5. Inter-Scale Biodiversity Maps
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ES | Ecosystem service |
| POLY | Polyculturae |
| SCI | Specific coverage index |
| SP_RICH | Species richness |
| He/I | Helophytes/hydrophytes |
| T | Therophytes |
| G | Geophytes |
| H | Hemicryptophytes |
| C | Chamaephytes |
| P | Phanerophytes |
| ADV | Adventitious |
| EUR | Eurasiatic |
| WIDE | Wide distribution |
| ALIEN | Alien/total species |
| INVAS | Invasive alien/alien species |
| H’/H’max | Shannon equitability index |
| N | Naturalness index |
| 1-lambda | Gini–Simpson diversity index |
| IM | Index of maturity |
| TOT | Total landscape system |
| NAT | Natural landscape subsystem |
| AGR | Agricultural landscape subsystem |
| ART | Artificial landscape subsystem |
| MTX | Matrix |
| DIV | Landscape diversity |
| DIV2 | Normalized landscape diversity |
| CON | Connectivity |
| WCON | Weighted connectivity |
| CIR | Circuitry |
| WCIR | Weighted circuitry |
| MBTC | Mean biological territorial capacity |
Appendix A
| Category | Index | U.o.M. | D | G | P | C | |
|---|---|---|---|---|---|---|---|
| FLORA | Richness | SP_RICH | n. | 129 | 225 | 70 | 156 |
| Biological forms | He/I | % | 0.5 | 0.2 | 0.14 | 5 | |
| T | % | 0.25 | 0.18 | 0.26 | 0.25 | ||
| G | % | 0.13 | 0.9 | 0.11 | 0.14 | ||
| H | % | 0.39 | 0.44 | 0.41 | 0.35 | ||
| C | % | 0.3 | 0.4 | 0.3 | 0.1 | ||
| P | % | 0.15 | 0.23 | 0.4 | 0.21 | ||
| Chorotypes | ADV | % | 0.22 | 0.16 | 0.24 | 0.18 | |
| EUR | % | 0.29 | 0.36 | 0.14 | 0.27 | ||
| WIDE | % | 0.33 | 0.27 | 0.40 | 0.31 | ||
| Allochthony | ALIEN | % | 0.27 | 0.20 | 0.28 | 0.24 | |
| INVAS | % | 0.74 | 0.55 | 0.63 | 0.59 | ||
| VEGETATION | Diversity | H’/H’max | - | 0.51 | 0.58 | 0.5 | 0.57 |
| N | - | 0.91 | 0.88 | 0.96 | 0.94 | ||
| 1-lambda | - | 0.85 | 0.82 | 0.67 | 0.82 | ||
| Maturity | IM | - | 3 | 1.1 | 0.7 | 0.6 | |
| Extra-Local | Local | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Index | U.o.M. | D | G | P | C | D | G | P | C | |
| Matrix | AGR | % | 0.55 | 0.60 | 0.87 | 88.56 | 0.70 | 0.69 | 0.93 | 0.93 |
| NAT | % | 0.27 | 0.31 | 0.06 | 0.05 | 0.24 | 0.26 | 0.04 | 0.04 | |
| Diversity | DIV_TOT | - | 2.13 | 1.7 | 1.11 | 1.09 | 1.55 | 1.81 | 0.74 | 0.79 |
| DIV_NAT | - | 0.59 | 0.51 | 0.25 | 0.23 | 0.48 | 0.59 | 0.21 | 0.22 | |
| DIV_AGR | - | 0.92 | 0.8 | 0.56 | 0.6 | 0.84 | 1.03 | 0.42 | 0.45 | |
| Biological territorial capacity | MBTC_TOT | Mcal/ha/yr | 1.88 | 2.39 | 1.26 | 1.17 | 2.22 | 2.56 | 1.16 | 1.18 |
| MBTC_NAT | Mcal/ha/yr | 4.51 | 5.32 | 3.48 | 2.52 | |||||
| MBTC_AGR | Mcal/ha/yr | 1.01 | 1.14 | 1.14 | 1.14 | |||||
| Connectivity & circuitry | CON | - | 0.33 | 0.39 | 0.36 | 0.24 | ||||
| WCON | - | 0.22 | 0.3 | 0.16 | 0.12 | |||||
| CIR | - | −0.01 | 0.08 | 0.03 | −0.14 | |||||
| WCIR | - | −0.18 | −0.05 | −0.28 | −0.32 | |||||
References
- Jackson, L.; Brussaard, L.; Ruiter; Pascual, U.; Perrings, C.; Bawa, K. Agrobiodiversity; Levin, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 126–135. [Google Scholar]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef]
- IPBES. Global Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—EU Biodiversity Strategy for 2030—Bringing Nature Back into Our Lives; 20.5.2020 COM(2020) 380 Final; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- European Union. Biodiversity on Farmland: CAP Contribution Has Not Halted the Decline—Special Report; European Union: Brussels, Belgium, 2020. [Google Scholar]
- IPCC. Climate Change and Land, an IPCC Special Report on Climatechange, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- MEA. Ecosystems and Human Well-Being—Synthesis, Millennium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- EEA. State of Nature in the EU: Results from Reporting Under the Nature Directives 2013–2018; EEA: Luxembourg, 2020; Volume EEA Report 10/2020. [Google Scholar]
- Stoate, C.; Báldi, A.; Beja, P.; Boatman, N.D.; Herzon, I.; van Doorn, A.; de Snoo, G.R.; Rakosy, L.; Ramwell, C. Ecological impacts of early 21st century agricultural change in Europe—A review. J. Environ. Manag. 2009, 91, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Poláková, J.; Tucker, G.M.; Hart, K.; Dwyer, J.; Rayment, M. Addressing Biodiversity and Habitat Preservation Through Measures Applied Under the Common Agricultural Policy; Report Prepared for DG Agriculture and Rural Development; Institute for European Environmental Policy: London, UK, 2011. [Google Scholar]
- Gerstner, K.; Dormann, C.F.; Stein, A.; Manceur, A.M.; Seppelt, R. Effects of land use on plant diversity—A global meta-analysis. J. Appl. Ecol. 2014, 51, 1690–1700. [Google Scholar] [CrossRef]
- Almeida-Rocha, J.M.; Soares, L.A.S.S.; Andrade, E.R.; Gaiotto, F.A.; Cazetta, E. The impact of anthropogenic disturbances on the genetic diversity of terrestrial species: A global meta-analysis. Mol. Ecol. 2020, 29, 4812–4822. [Google Scholar] [CrossRef]
- Matuoka, M.A.; Benchimol, M.; de Almeida-Rocha, J.M.; Morante-Filho, J.C. Effects of anthropogenic disturbances on bird functional diversity: A global meta-analysis. Ecol. Indic. 2020, 116, 106471. [Google Scholar] [CrossRef]
- Battisti, C.; Poeta, G.; Fanelli, G. An Introduction to Disturbance Ecology; Springer: Cham, Switzerland, 2016; p. XIII, 178. [Google Scholar]
- Hailu, F. The role of agrobiodiversity and diverse causes of its losses and methods of conservation: A review. Food Humanit. 2025, 4, 100500. [Google Scholar] [CrossRef]
- Jin, H.; Xu, J.; Peng, Y.; Xin, J.; Peng, N.; Li, Y.; Huang, J.; Zhang, R.; Li, C.; Wu, Y.; et al. Impacts of landscape patterns on plant species diversity at a global scale. Sci. Total Environ. 2023, 896, 165193. [Google Scholar] [CrossRef] [PubMed]
- Lecoq, L.; Mony, C.; Saiz, H.; Marsot, M.; Ernoult, A. Investigating the effect of habitat amount and landscape heterogeneity on the gamma functional diversity of grassland and hedgerow plants. J. Ecol. 2022, 110, 1871–1882. [Google Scholar] [CrossRef]
- Zimmerer, K.S.; de Haan, S.; Jones, A.D.; Creed-Kanashiro, H.; Tello, M.; Carrasco, M.; Meza, K.; Plasencia Amaya, F.; Cruz-Garcia, G.S.; Tubbeh, R.; et al. The biodiversity of food and agriculture (Agrobiodiversity) in the anthropocene: Research advances and conceptual framework. Anthropocene 2019, 25, 100192. [Google Scholar] [CrossRef]
- FAO. What is agrobiodiversity? In Building on Gender, Agrobiodiversity and Local Knowledge; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004. [Google Scholar]
- Donald, P.F.; Green, R.E.; Heath, M.F. Agricultural intensification and the collapse of Europe’s farmland bird populations. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 25–29. [Google Scholar] [CrossRef]
- Kleijn, D.; Kohler, F.; Báldi, A.; Batáry, P.; Concepción, E.D.; Clough, Y.; Díaz, M.; Gabriel, D.; Holzschuh, A.; Knop, E.; et al. On the relationship between farmland biodiversity and land-use intensity in Europe. Proc. R. Soc. B Biol. Sci. 2008, 276, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Reidsma, P.; Tekelenburg, T.; van den Berg, M.; Alkemade, R. Impacts of land-use change on biodiversity: An assessment of agricultural biodiversity in the European Union. Agric. Ecosyst. Environ. 2006, 114, 86–102. [Google Scholar] [CrossRef]
- Pellegrini, E.; Buccheri, M.; Martini, F.; Boscutti, F. Agricultural land use curbs exotic invasion but sustains native plant diversity at intermediate levels. Sci. Rep. 2021, 11, 8385. [Google Scholar] [CrossRef]
- Falcucci, A.; Maiorano, L.; Boitani, L. Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landsc. Ecol. 2007, 22, 617–631. [Google Scholar] [CrossRef]
- Clergue, B.; Amiaud, B.; Pervanchon, F.; Lasserre-Joulin, F.; Plantureux, S. Biodiversity: Function and Assessment in Agricultural Areas: A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 309–327. [Google Scholar]
- Duelli, P.; Obrist, M.K. Biodiversity indicators: The choice of values and measures. Agric. Ecosyst. Environ. 2003, 98, 87–98. [Google Scholar] [CrossRef]
- Gonthier, D.J.; Ennis, K.K.; Farinas, S.; Hsieh, H.-Y.; Iverson, A.L.; Batáry, P.; Rudolphi, J.; Tscharntke, T.; Cardinale, B.J.; Perfecto, I. Biodiversity conservation in agriculture requires a multi-scale approach. Proc. R. Soc. B Biol. Sci. 2014, 281, 20141358. [Google Scholar] [CrossRef]
- Dover, J.W.; Bunce, R.G.H. Key Concepts in Landscape Ecology; IALE UK, Coplin Cross Printers Ltd.: Garstang, UK, 1998. [Google Scholar]
- Forman, R.T.T. Land Mosaics: The Ecology of Landscapes and Regions, 1st ed.; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Forman, R.T.T.; Godron, M. Landscape Ecology; John Wiley and Sons: New York, NY, USA, 1986. [Google Scholar]
- Dramstad, W.E.; Olson, J.D.; Forman, R.T.T. Landscape Ecology Principles in Landscape Architecture and Land Use Planning; Island Press: Washington, DC, USA, 1996. [Google Scholar]
- Turner, M.G.; Gardner, R.H. Landscape Ecology in Theory and Practice, Pattern and Process; Springer: New York, NY, USA, 2015. [Google Scholar]
- Jonsen, I.D.; Fahrig, L. Response of generalist and specialist insect herbivores to landscape spatial structure. Landsc. Ecol. 1997, 12, 185–197. [Google Scholar] [CrossRef]
- Fahrig, L.; Baudry, J.; Brotons, L.; Burel, F.G.; Crist, T.O.; Fuller, R.J.; Sirami, C.; Siriwardena, G.M.; Martin, J.-L. Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol. Lett. 2011, 14, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- With, K.A. The Landscape Ecology of Invasive Spread. Conserv. Biol. 2002, 16, 1192–1203. [Google Scholar] [CrossRef]
- Uroy, L.; Ernoult, A.; Mony, C. Effect of landscape connectivity on plant communities: A review of response patterns. Landsc. Ecol. 2019, 34, 203–225. [Google Scholar] [CrossRef]
- Tscharntke, T.; Klein, A.; Kruess, A.; Steffan-Dewenter, I.; Thies, C. Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Lett. 2005, 8, 857–874. [Google Scholar] [CrossRef]
- O’Reilly-Nugent, A.; Palit, R.; Lopez-Aldana, A.; Medina-Romero, M.; Wandrag, E.; Duncan, R.P. Landscape Effects on the Spread of Invasive Species. Curr. Landsc. Ecol. Rep. 2016, 1, 107–114. [Google Scholar] [CrossRef]
- Chiaffarelli, G.; Sgalippa, N.; Vagge, I. The Landscape Ecological Quality of Two Different Farm Management Models: Polyculture Agroforestry vs. Conventional. Land 2024, 13, 1598. [Google Scholar] [CrossRef]
- Vagge, I.; Chiaffarelli, G. Validating the Contribution of Nature-Based Farming Solutions (NBFS) to Agrobiodiversity Values through a Multi-Scale Landscape Approach. Agronomy 2023, 13, 233. [Google Scholar] [CrossRef]
- Vagge, I.; Sgalippa, N.; Chiaffarelli, G. Agricultural Landscapes: A Pattern-Process-Design Approach to Enhance Their Ecological Quality and Ecosystem Services through Agroforestry. Diversity 2024, 16, 431. [Google Scholar] [CrossRef]
- Vagge, I.; Chiaffarelli, G.; Pirola, L.; Gibelli, M.G.; Sgalippa, N. Landscape Ecology and Ecosystem Services as Landscape Analysis and Assessment Tools for Ecological Landscape Planning. In Landscape Architecture and Design—Sustainability and Management; Sérgio, L., Ed.; IntechOpen: Rijeka, Croatia, 2024; pp. 1–29. [Google Scholar]
- Vagge, I.; Sgalippa, N.; Chiaffarelli, G. The role of agroforestry in solving the agricultural landscapes vulnerabilities in the Po Plain district. Community Ecol. 2024, 25, 361–387. [Google Scholar] [CrossRef]
- Urban, D.L.; O’Neill, R.V.; Shugart, H.H., Jr. Landscape Ecology, A Hierarquical Perspective Can Help Scientists Understand Spatial Patterns. BioScience 1987, 37, 119–127. [Google Scholar] [CrossRef]
- Burel, F.; Lavigne, C.; Marshall, E.J.P.; Moonen, A.C.; Ouin, A.; Poggio, S.L. Landscape ecology and biodiversity in agricultural landscapes. Agric. Ecosyst. Environ. 2013, 166, 1–2. [Google Scholar] [CrossRef]
- Burel, F. Effect of landscape structure and dynamics on species diversity in hedgerow networks. Landsc. Ecol. 1992, 6, 161–174. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Sáenz, S.; Penas, A. Worldwide bioclimatic classification system. Glob. Geobot. 2011, 1, 634. [Google Scholar]
- Rivas-Martínez, S. Global Bioclimatics, Clasificación Bioclimática de la Tierra; CIF: Madrid, Spain, 2004. [Google Scholar]
- Domina, G.; Galasso, G.; Bartolucci, F.; Guarino, R. Ellenberg Indicator Values for the vascular flora alien to Italy. Electronic Supplementary File 1. Flora Mediterr. 2018, 28, 53–61. [Google Scholar] [CrossRef]
- Guarino, R.; Domina, G.; Pignatti, S. Ellenberg’s Indicator values for the Flora of Italy—First update: Pteridophyta, Gymnospermae and Monocotyledoneae. Flora Mediterr. 2012, 22, 197–209. [Google Scholar] [CrossRef]
- Tichý, L.; Axmanová, I.; Dengler, J.; Guarino, R.; Jansen, F.; Midolo, G.; Nobis, M.P.; Van Meerbeek, K.; Aćić, S.; Attorre, F.; et al. Ellenberg-type indicator values for European vascular plant species. J. Veg. Sci. 2023, 34, e13168. [Google Scholar] [CrossRef]
- Pignatti, S.; Menegoni, P.; Pietrosanti, S. Valori di bioindicazione delle piante vascolari della flora d’Italia, Bioindicator values of vascular plants of the flora of Italy. Braun-Blanquetia Rev. Geobot. Monogr. 2005, 39, 1–97. [Google Scholar]
- Ellenberg, H.E. Aufgaben und methoden der vegetationskunde. In Einführung in die Phytologie; Walter, H., Ed.; Springer Nature: Stuttgart, Germany, 1956; Volume 8, pp. 1–136. [Google Scholar]
- Cornwell, W.K.; Grubb, P.J. Regional and local patterns in plant species richness with respect to resource availability. Oikos 2003, 100, 417–428. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. Distribution pattern of the flora in a peri-urban forest: An effect of the city–forest ecotone. Landsc. Urban Plan. 2003, 65, 169–185. [Google Scholar] [CrossRef]
- Fanelli, G.; Tescarollo, P.; Testi, A. Ecological indicators applied to urban and suburban floras. Ecol. Indic. 2006, 6, 444–457. [Google Scholar] [CrossRef]
- Midolo, G.; Herben, T.; Axmanová, I.; Marcenò, C.; Pätsch, R.; Bruelheide, H.; Karger, D.N.; Aćić, S.; Bergamini, A.; Bergmeier, E.; et al. Disturbance indicator values for European plants. Glob. Ecol. Biogeogr. 2023, 32, 24–34. [Google Scholar] [CrossRef]
- Herben, T.; Klimešová, J.; Chytrý, M. Effects of disturbance frequency and severity on plant traits: An assessment across a temperate flora. Funct. Ecol. 2018, 32, 799–808. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Wien, Austria; New York, NY, USA, 1964. [Google Scholar]
- Biondi, E. Phytosociology today: Methodological and conceptual evolution. Plant Biosyst. 2011, 145, 19–29. [Google Scholar] [CrossRef]
- Géhu, J.M. L’analyse symphytosociologique et géosymphytosociologique de l’espace, Théorie et métodologie. Coll. Phytosoc. 1988, 17, 11–46. [Google Scholar]
- Géhu, J.M. Pour un approche nouvelle des paysages végétaux: La symphytosociologie. Bull. Soc. Bot. Fr. 1979, 126, 213–223. [Google Scholar]
- Rivas-Martìnez, S. Notions on dynamic-catenal phytosociology as a basis of landscape science. Plant Biosyst. 2005, 139, 135–144. [Google Scholar] [CrossRef]
- Tüxen, R. Sigmeten und Geosigmeten, ihre Ordnung und ihre Bedeutug für, wissenschaft, naturschutz und planung. Biogeographie 1979, 16, 79–92. [Google Scholar]
- Taffetani, F.; Rismondo, M. Bioindicators system for the evaluation of the environment quality of agro-ecosystems. Fitosociologia 2009, 46, 3–22. [Google Scholar]
- Taffetani, F.; Rismondo, M.; Lancioni, A. Integrated tools and methods for the analysis of agro-ecosystem’s functionality through vegetational investigations. Fitosociologia 2011, 48, 41–52. [Google Scholar]
- Grime, J.P. Plant Strategies, Vegetation Processes, and Ecosystem Properties, 2nd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Martin, C.A. An early synthesis of the habitat amount hypothesis. Landsc. Ecol. 2018, 33, 1831–1835. [Google Scholar] [CrossRef]
- Fahrig, L. How Much Habitat Is Enough? Biol. Conserv. 2001, 100, 65–74. [Google Scholar] [CrossRef]
- Fahrig, L.; Girard, J.; Duro, D.; Pasher, J.; Smith, A.; Javorek, S.; King, D.; Lindsay, K.F.; Mitchell, S.; Tischendorf, L. Farmlands with smaller crop fields have higher within-field biodiversity. Agric. Ecosyst. Environ. 2015, 200, 219–234. [Google Scholar] [CrossRef]
- Godefroid, S.; Koedam, N. How important are large vs. small forest remnants for the conservation of the woodland flora in an urban context? Glob. Ecol. Biogeogr. 2003, 12, 287–298. [Google Scholar] [CrossRef]
- Duflot, R.; Georges, R.; Ernoult, A.; Aviron, S.; Burel, F. Landscape heterogeneity as an ecological filter of species traits. Acta Oecologica 2014, 56, 19–26. [Google Scholar] [CrossRef]
- Maskell, L.C.; Botham, M.; Henrys, P.; Jarvis, S.; Maxwell, D.; Robinson, D.A.; Rowland, C.S.; Siriwardena, G.; Smart, S.; Skates, J.; et al. Exploring relationships between land use intensity, habitat heterogeneity and biodiversity to identify and monitor areas of High Nature Value farming. Biol. Conserv. 2019, 231, 30–38. [Google Scholar] [CrossRef]
- Poggio, S.L.; Chaneton, E.J.; Ghersa, C.M. Landscape complexity differentially affects alpha, beta, and gamma diversities of plants occurring in fencerows and crop fields. Biol. Conserv. 2010, 143, 2477–2486. [Google Scholar] [CrossRef]
- Liccari, F.; Boscutti, F.; Bacaro, G.; Sigura, M. Connectivity, landscape structure, and plant diversity across agricultural landscapes: Novel insight into effective ecological network planning. J. Environ. Manag. 2022, 317, 115358. [Google Scholar] [CrossRef]
- Honnay, O.; Verheyen, K.; Butaye, J.; Jacquemyn, H.; Bossuyt, B.; Hermy, M. Possible effects of habitat fragmentation and climate change on the range of forest plant species. Ecol. Lett. 2002, 5, 525–530. [Google Scholar] [CrossRef]
- Billeter, R.; Liira, J.; Bailey, D.; Bugter, R.; Arens, P.; Augenstein, I.; Aviron, S.; Baudry, J.; Bukacek, R.; Burel, F.; et al. Indicators for biodiversity in agricultural landscapes: A pan-European study. J. Appl. Ecol. 2008, 45, 141–150. [Google Scholar] [CrossRef]
- Sowińska-Świerkosz, B. Critical review of landscape-based surrogate measures of plant diversity. Landsc. Res. 2020, 45, 819–840. [Google Scholar] [CrossRef]
- Duelli, P. Biodiversity Evaluation in Agricultural Landscapes: An Approach at Two Different Scales. Agric. Ecosyst. Environ. 1997, 62, 81–91. [Google Scholar] [CrossRef]
- Burkhard, B.; Kroll, F.; Müller, F.; Windhorst, W. Landscapes‘ Capacities to Provide Ecosystem Services—A Concept for Land-Cover Based Assessments. Landsc. Online 2009, 15, 1–12. [Google Scholar] [CrossRef]
- Celesti-Grapow, L.; Alessandrini, A.; Arrigoni, P.V.; Assini, S.; Banfi, E.; Barni, E.; Bovio, M.; Brundu, G.; Cagiotti, M.R.; Camarda, I.; et al. Non-native flora of Italy: Species distribution and threats. Plant Biosyst. 2010, 144, 12–28. [Google Scholar] [CrossRef]
- Domina, G. Invasive Aliens in Italy. In Invasive Alien Species; Wiley: Hoboken, NJ, USA, 2021; pp. 190–214. [Google Scholar]
- Chiaffarelli, G.; Tambone, F.; Vagge, I. The Contribution of the Management of Landscape Features to Soil Organic Carbon Turnover among Farmlands. Soil Syst. 2024, 8, 95. [Google Scholar] [CrossRef]
- Ingegnoli, V. Landscape Ecology: A Widening Foundation; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Ingegnoli, V.; Giglio, E. Ecologia del Paesaggio: Manuale per Conservare, Gestire e Pianificare L’ambiente; Sistemi Editoriali: Napoli, Italy, 2005. [Google Scholar]
- Ingegnoli, V. Landscape Bionomics: Biological-Integrated Lanscape Ecology; Springer: Milan, Italy, 2015. [Google Scholar]
- Geoportale Regione Lombardia. Available online: https://www.geoportale.regione.lombardia.it (accessed on 10 October 2022).
- Geoportale Piemonte. Available online: https://www.geoportale.piemonte.it/cms/ (accessed on 10 October 2022).
- ARPA Lombardia Archivio Agrometeo. Available online: https://www.arpalombardia.it/temi-ambientali/meteo-e-clima/form-richiesta-dati/ (accessed on 6 June 2025).
- Arpa Piemonte. Available online: https://www.arpa.piemonte.it/ (accessed on 27 May 2024).
- Globalbioclimatics. Available online: https://www.globalbioclimatics.org (accessed on 21 October 2022).
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Prodromo Della Vegetazione Italiana. Available online: https://www.prodromo-vegetazione-italia.org (accessed on 21 October 2022).
- Blasi, C. La Vegetazione d’italia con Carta Delle Serie di Vegetazione Scala 1:500,000; Palombi Editori: Rome, Italy, 2010; 539p. [Google Scholar]
- Vagge, I. Le foreste di farnia e carpino bianco della pianura lombarda. In Bosco: Biodiversità, Diritti e Culture dal Medioevo al Nostro Tempo; I libri di Viella 411; Viella: Roma, Italy, 2022. [Google Scholar]
- Vagge, I.; Chiaffarelli, G. The Alien Plant Species Impact in Rice Crops in Northwestern Italy. Plants 2023, 12, 12. [Google Scholar] [CrossRef]
- Flora Italiae ActaPlantarum. Available online: https://www.actaplantarum.org/forum/link (accessed on 11 October 2022).
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole: Bologna, Italy, 2019. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982. [Google Scholar]
- Dryades. Available online: http://dryades.units.it/cercapiante/index.php (accessed on 11 October 2022).
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon press: Oxford, UK, 1934. [Google Scholar]
- Taffetani, F.; Rismondo, M.; Lancioni, A. Environmental Evaluation and Monitoring of Agro-Ecosystems Biodiversity. In Ecosystems Biodiversity; Venora, G., Grillo, O., Eds.; InTech: London, UK, 2011; pp. 333–370. [Google Scholar] [CrossRef]
- Géhu, J.M.; Rivas-Martínez, S. Notions fondamentales de phytosociologie. Ber. Int. Simp. Int. Ver. Veg. 1981, 980, 5–33. [Google Scholar]
- Pirola, A. Elementi di Fitosociologia; CLUEB: Bologna, Italy, 1970. [Google Scholar]
- Rivas-Martínez, S. Nociones sobre Fitosociología, Biogeografía e Bioclimatología. In La Vegetation de España; Universidad de Alcalá de Henares: Alcalá de Henares, Spain, 1987; pp. 19–45. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde; Springer: Berlin/Heidelberg, Germany, 1928. [Google Scholar]
- van der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Vagge, I.; Blasi, C. New and validated syntaxa for the checklist of Italian vegetation. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 318–332. [Google Scholar] [CrossRef]
- Biondi, E.; Blasi, C.; Allegrezza, M.; Anzellotti, I.; Azzella, M.M.; Carli, E.; Casavecchia, S.; Copiz, R.; Del Vico, E.; Facioni, L.; et al. Plant communities of Italy: The Vegetation Prodrome. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2014, 148, 728–814. [Google Scholar] [CrossRef]
- Biondi, E.; Allegrezza, M.; Casavecchia, S.; Galdenzi, D.; Gasparri, R.; Pesaresi, S.; Soriano, P.; Tesei, G.; Blasi, C. New insight on Mediterranean and sub-Mediterranean syntaxa included in the Vegetation Prodrome of Italy. Flora Mediterr. 2015, 25, 77–102. [Google Scholar] [CrossRef]
- Verde, S.; Assini, S.; Andreis, C. Le Serie di Vegetazione Della Regione Lombardia. In La Vegetazione d’italia con Carta Delle Serie di Vegetazione Scala 1:500,000; Blasi, C., Ed.; Palombi Editori: Rome, Italy, 2010; pp. 53–82. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois: Urbana, IL, USA; Chicago, IL, USA; London, UK, 1949; pp. 3–24. [Google Scholar]
- Morabito, A.; Musarella, C.M.; Caruso, G.; Spampinato, G. Biodiversity as a Tool in the Assessment of the Conservation Status of Coastal Habitats: A Case Study from Calabria (Southern Italy). Diversity 2024, 16, 535. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Google. Immagini (c) 2023 TerraMetrics, Dati cartografici (c) 2023. Available online: https://terrametrics.com/v2/ (accessed on 6 June 2025).
- European Environment Agency. Updated CLC Illustrated Nomenclature Guidelines; European Environment Agency: Wien, Austria, 2019. [Google Scholar]
- Fabbri, P. Ecologia del Paesaggio per la Pianificazione/Pompeo Fabbri; Aracne: Roma, Italy, 2005. [Google Scholar]
- Ingegnoli, V.; Giglio, E. Proposal of a synthetic indicator to control ecological dynamics at an ecological mosaic scale. Ann. Di Bot. 1999, 57. [Google Scholar] [CrossRef]
- Ingegnoli, V. The study of vegetation for a diagnostical evaluation of agricultural landscapes, some examples fom Lombardy. Ann. Di Bot. Nuova Ser. 2006, 6, 111–120. [Google Scholar]
- Burkhard, B.; Kandziora, M.; Hou, Y.; Müller, F. Ecosystem Service Potentials, Flows and Demands—Concepts for Spatial Localisation, Indication and Quantification. Landsc. Online 2014, 34, 1–32. [Google Scholar] [CrossRef]
- Berghöfer, A.; Mader, A.; Patrickson, S.; Calcaterra, E.; Smit, J.; Blignaut, J.; de Wit, M.; Van Zyl, H. TEEB Manual for Cities: Ecosystem Services in Urban Management; TEEB: Dallas, TX, USA, 2011. [Google Scholar]
- Dal Borgo, A.G.; Chiaffarelli, G.; Capocefalo, V.; Schievano, A.; Bocchi, S.; Vagge, I. Agroforestry as a Driver for the Provisioning of Peri-Urban Socio-Ecological Functions: A Trans-Disciplinary Approach. Sustainability 2023, 15, 1020. [Google Scholar] [CrossRef]
- Roschewitz, I.; Gabriel, D.; Tscharntke, T.; Thies, C. The effects of landscape complexity on arable weed species diversity in organic and conventional farming. J. Appl. Ecol. 2005, 42, 873–882. [Google Scholar] [CrossRef]
- Janišová, M.; Michalcová, D.; Bacaro, G.; Ghisla, A. Landscape effects on diversity of semi-natural grasslands. Agric. Ecosyst. Environ. 2014, 182, 47–58. [Google Scholar] [CrossRef]
- Ernoult, A.; Alard, D. Species richness of hedgerow habitats in changing agricultural landscapes: Are α and γ diversity shaped by the same factors? Landsc. Ecol. 2011, 26, 683–696. [Google Scholar] [CrossRef]
- Dogra, K.; Sood, S.; Dobhal, P.; Sharma, S. Alien plant invasion and their impact on indigenous species diversity at global scale: A review. J. Ecol. Nat. Environ. 2010, 2, 175–186. Available online: https://academicjournals.org/article/article1379601216_Kuldip%2520Dogra.pdf (accessed on 6 June 2025).
- Clout, M.; De Poorter, M. IUCN Guidelines for the Prevention of Biodiversity Loss Caused By Alien Invasive Species. Aliens 2000, 11, 25. Available online: https://portals.iucn.org/library/efiles/documents/Rep-2000-052.pdf (accessed on 6 June 2025).
- Gallardo, B.; Bacher, S.; Bradley, B.; Comín, F.; Gallien, L.; Jeschke, J.; Sorte, C.; Vilà, M. InvasiBES: Understanding and managing the impacts of Invasive alien species on Biodiversity and Ecosystem Services. NeoBiota 2019, 50, 109–122. [Google Scholar] [CrossRef]
- Baker, R.; Cannon, R.; Bartlett, P.; Barker, I. Novel strategies for assessing and managing the risks posed by alien species to global crop production and biodiversity. Ann. Appl. Biol. 2005, 146, 177–191. [Google Scholar] [CrossRef]
- Chiaffarelli, G.; Vagge, I. Cities vs countryside: An example of a science-based Peri-urban Landscape Features rehabilitation in Milan (Italy). Urban For. Urban Green. 2023, 86, 128002. [Google Scholar] [CrossRef]
- Stapanian, M.A.; Sundberg, S.D.; Baumgardner, G.A.; Liston, A. Alien plant species composition and associations with anthropogenic disturbance in North American forests. Plant Ecol. 1998, 139, 49–62. [Google Scholar] [CrossRef]
- Kowarik, I. Some responses of flora and vegetation to urbanization in Central Europe. In Plants and Plant Communities in the Urban Environment; Sukopp, H., Hejny, S., Kowarik, I., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1990; pp. 45–75. [Google Scholar]
- Chytry, M.; Jarosik, V.; Pyšek, P.; Hájek, O.; Knollova, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.M.G.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S.; et al. An updated checklist of the vascular flora alien to Italy. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Pierce, S.; Brusa, G.; Vagge, I.; Cerabolini, B.E.L. Allocating CSR plant functional types: The use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 2013, 27, 1002–1010. [Google Scholar] [CrossRef]
- Pausas, J.G.; Keeley, J.E. Evolutionary ecology of resprouting and seeding in fire-prone ecosystems. New Phytol. 2014, 204, 55–65. [Google Scholar] [CrossRef]
- McIntyre, S.; Lavorel, S.; Tremont, R.M. Plant Life-History Attributes: Their Relationship to Disturbance Response in Herbaceous Vegetation. J. Ecol. 1995, 83, 31–44. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Angiolini, C.; Bacaro, G.; Bacchetta, G.; Bagella, S.; Barni, E.; Bonari, G.; Buffa, G.; Caldarella, O.; Calderisi, G.; et al. Uniqueness matters: Patterns of α and β-diversity highlight conservation priorities for plant communities in Italian agricultural landscapes. In Proceedings of the Forum Nazionale Della Biodiversità, Università degli Studi di Palermo, Palermo, Italy, 20–21 May 2024. [Google Scholar]
- Baum, K.A.; Haynes, K.J.; Dillemuth, F.P.; Cronin, J.T. The matrix enhances the effectiveness of corridors and stepping stones. Ecology 2004, 85, 2671–2676. [Google Scholar] [CrossRef]
- Donald, P.F.; Evans, A.D. Habitat connectivity and matrix restoration: The wider implications of agri-environment schemes. J. Appl. Ecol. 2006, 43, 209–218. [Google Scholar] [CrossRef]
- Jules, E.S.; Shahani, P. A broader ecological context to habitat fragmentation: Why matrix habitat is more important than we thought. J. Veg. Sci. 2003, 14, 459–464. [Google Scholar] [CrossRef]
- Machado, A. An index of naturalness. J. Nat. Conserv. 2004, 12, 95–110. [Google Scholar] [CrossRef]
- Morelli, F. High nature value farmland increases taxonomic diversity, functional richness and evolutionary uniqueness of bird communities. Ecol. Indic. 2018, 90, 540–546. [Google Scholar] [CrossRef]
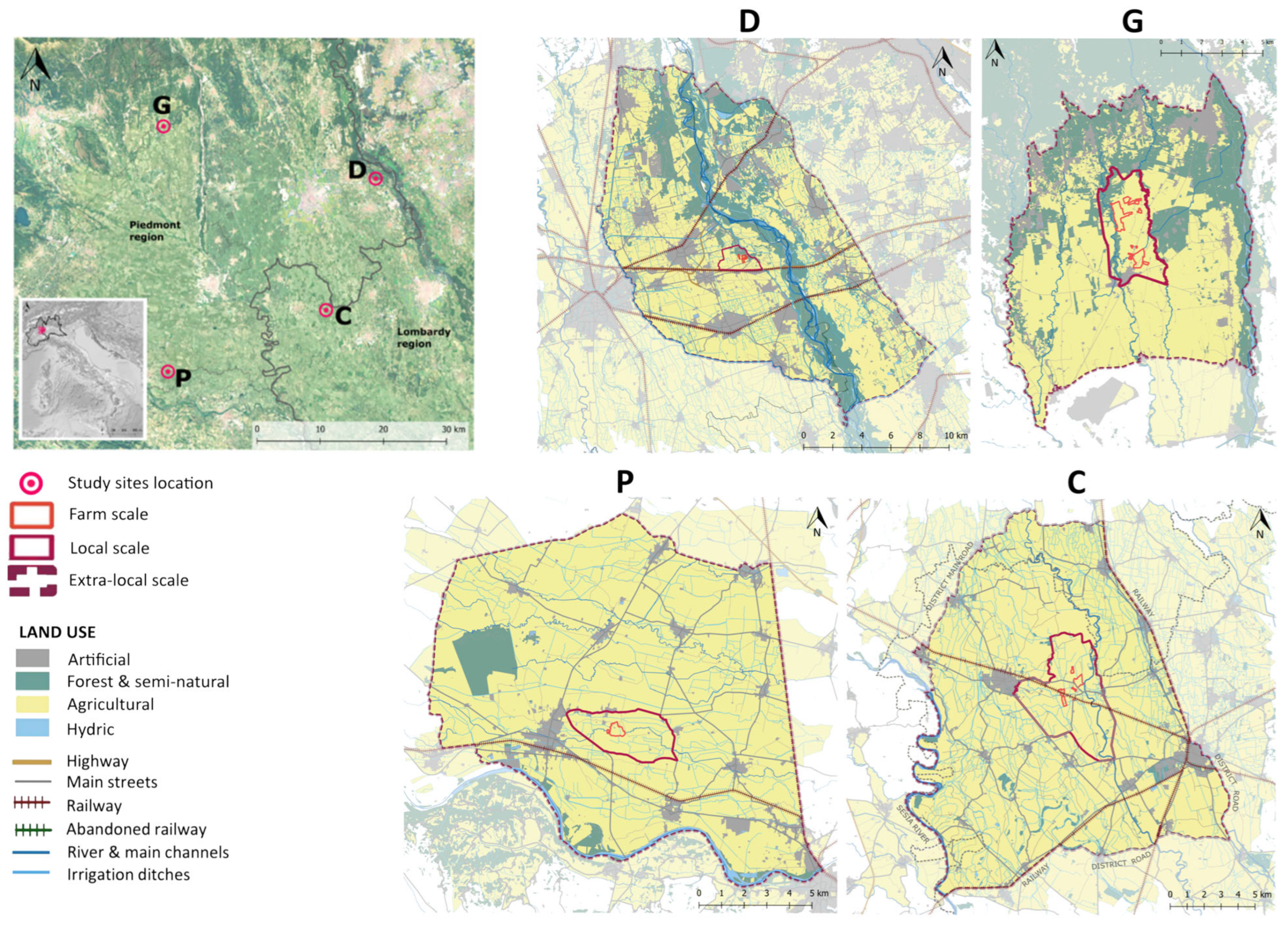
| D | G | P | C | ||
|---|---|---|---|---|---|
| PEDOLOGY | ST/WRB CLASSES | Inceptisols | Alfisols (ancient terraces), inceptisols | Inceptisols, entisols | Luvisols, arenosols |
| Geomorphology | Fluvial terrace | Riss alluvial terrace | Fluvial deposits | Fluvial terrace | |
| Main soil texture | Loamy skeletal | Fine silty | Loamy coarse, loamy sand | Loamy sand, sandy loam | |
| Development | Low pedogenesis | Intense pedogenesis | Low pedogenesis | Medium pedogenesis | |
| Permeability | High permeability | Surface hydromorphy | Medium permeability | Medium–low permeability | |
| pH | Acid to sub-acid | Acid | Sub-alkaline to alkaline | Sub-acid | |
| Land use capacity | III (stoniness) | III (oxygen availability) | II (oxygen availability) | IIw (waterlog) | |
| Specific traits | Dark epipedon | ||||
| CLIMATE [1990–2022 data] | Annual rainfall [mm] | 973 | 872 | 737 | 668 |
| Annual mean temperature [°C] | 11.8 | 12.3 | 13.2 | 13.1 | |
| Average maximum temperature [°C] | 17.9 | 18.9 | 18.8 | 18.6 | |
| Average minimum temperature [°C] | 6.4 | 7.0 | 8.5 | 8.19 | |
| BIOCLIMATE [1990–2022 data] | Bioclimate (variant) | Temperate continental | Temperate continental (steppic) | Temperate continental (steppic) | Temperate oceanic (sub-Mediterranean) |
| Bioclimatic belt | Upper mesotemperate Low humid | Upper mesotemperate Upper subhumid | Upper mesotemperate Low subhumid | Upper mesotemperate Low humid | |
| VEGETATION | Climactic series | Western neutral-acidophilous Po Plain series of lower plain oak–hornbeam forests (Carpinion betuli Isler 1931 alliance) | Mosaic between western neutral-acidophilous Po Plain series of upper plain oak–hornbeam forests (Carpinion betuli Isler 1931 alliance), Central–Western pre-alpine acidophilous series of sessile oak forests (Quercion roboris Malcuit 1929 alliance, Phyteumato betonicifolium—Querco petraea sigmetum) | Western neutral-acidophilous Po Plain series of lower plain oak–hornbeam forests (Carpinion betuli Isler 1931 alliance) | Western neutral-acidophilous Po Plain series of lower plain oak–hornbeam forests (Carpinion betuli Isler 1931 alliance) |
| Category | Indicator | Abbreviation | Equation | Reference | |
|---|---|---|---|---|---|
| FLORA | Richness | Species richness | SP_RICH | ||
| Biological forms | He/I | He/I | [103] | ||
| T | T | [103] | |||
| G | G | [103] | |||
| H | H | [103] | |||
| C | C | [103] | |||
| P | P | [103] | |||
| Chorotypes | Adventitious | ADV | |||
| Eurasiatic | EUR | ||||
| Wide distr. | WIDE | ||||
| Allochthony | Alien/total species | ALIEN | |||
| Invasive alien/alien | INVAS | ||||
| VEGETATION | Diversity | Shannon equitability index | H’/H’max | with | [114] |
| Naturalness index | N | with | [115] | ||
| Gini–Simpson diversity index | 1-lambda | [116] | |||
| Maturity | Index of maturity | IM | [66,67,104] |
| Indicator | Scale | Equation | References | |
|---|---|---|---|---|
| Basic structural traits | Matrix (MTX) x = [NAT; AGR] | E_La La | Ai = total area of each land use categories patch Atot = total area | [32] |
| Diversity indices | Diversity (DIV) X = [DIV_TOT; DIV_NAT; DIV_AGR] | E_La La | [32] | |
| Connectivity indices | Connectivity (CON) | La | L = no. of links N = no. of nodes | [119] |
| Weighted connectivity (WCON) | La | Li = no. of links for each Ecological Quality Class (EQCi = [1,2,3,4,5]) Wi = EQCi weight: | [41] | |
| Circuitry (CIR) | La | [119] | ||
| Weighted circuitry (WCIR) | La | Li = no. of links for each Ecological Quality Class (EQCi = [1,2,3,4,5]) Wi = EQCi weight (as above) | [41] | |
| Indices on ecological functionality | Mean biological territorial capacity (MBTC) X = [MBTC_TOT; MBTC _NAT; MBTC_AGR] | E_La La (only MBTC_TOT) | [88,120,121] |
| (A) | FLORISTIC–VEGETATIONAL INDICES | |||||||||||||||||
| Farm scale | ||||||||||||||||||
| SP_RICH | H’/H’max | N | 1-lambda | He/I. | T. | G. | H. | C. | P. | ADV | EUR | WIDE | ALIEN | INVAS | IM | |||
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| Shapiro–Wilk W | 0.99 | 0.84 | 0.98 | 0.77 | 0.80 | 0.73 | 0.92 | 0.98 | 0.91 | 0.90 | 0.97 | 0.93 | 0.96 | 0.87 | 0.95 | 0.77 | ||
| p(normal) | 0.98 | 0.21 | 0.89 | 0.06 | 0.10 | 0.03 | 0.52 | 0.88 | 0.47 | 0.42 | 0.83 | 0.59 | 0.80 | 0.30 | 0.73 | 0.06 | ||
| LANDSCAPE ECOLOGY INDICES | ||||||||||||||||||
| (B) | Extra-local scale | Local scale | ||||||||||||||||
| AGR | NAT | DIV_TOT | DIV_AGR | DIV_NAT | MBTC_TOT | MBTC_AGR | MBTC_NAT | AGR | NAT | DIV_TOT | DIV_NAT | DIV_AGR | MBTC_TOT | CON | WCON | CIR | WCIR | |
| N | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Shapiro–Wilk W | 0.82 | 0.82 | 0.87 | 0.91 | 0.85 | 0.90 | 0.65 | 0.99 | 0.75 | 0.79 | 0.85 | 0.86 | 0.87 | 0.84 | 0.92 | 0.96 | 0.94 | 0.95 |
| p(normal) | 0.15 | 0.13 | 0.31 | 0.49 | 0.22 | 0.45 | 0.002 | 0.93 | 0.04 | 0.08 | 0.23 | 0.25 | 0.29 | 0.19 | 0.55 | 0.78 | 0.64 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiaffarelli, G.; Vagge, I. Biodiversity in Agricultural Landscapes: Inter-Scale Patterns in the Po Plain (Italy). Diversity 2025, 17, 418. https://doi.org/10.3390/d17060418
Chiaffarelli G, Vagge I. Biodiversity in Agricultural Landscapes: Inter-Scale Patterns in the Po Plain (Italy). Diversity. 2025; 17(6):418. https://doi.org/10.3390/d17060418
Chicago/Turabian StyleChiaffarelli, Gemma, and Ilda Vagge. 2025. "Biodiversity in Agricultural Landscapes: Inter-Scale Patterns in the Po Plain (Italy)" Diversity 17, no. 6: 418. https://doi.org/10.3390/d17060418
APA StyleChiaffarelli, G., & Vagge, I. (2025). Biodiversity in Agricultural Landscapes: Inter-Scale Patterns in the Po Plain (Italy). Diversity, 17(6), 418. https://doi.org/10.3390/d17060418







