Abstract
In the Gulf of California, seabirds carry heavy loads to feed their chicks, making their takeoff capacity crucial for foraging. While some studies explore this, few consider the lift force, induced power, or aerobic vs. anaerobic performance. Moreover, the differences between individuals—such as size or sex—remain largely unexamined, leaving gaps in the understanding of seabird flight efficiency. In this work, the load capacity during takeoff of the Least Storm-Petrel (LSP) and the Black Storm-Petrel (BSP) in Isla Partida Norte, Gulf of California, was analyzed. Forty-nine individuals of the Least Storm-Petrel group and 23 of the Black Storm-Petrel group were evaluated. In both species, the carrying capacity was found to be independent of individual size, but the Least Storm-Petrel managed to take off with a higher proportion of its total mass than the Black Storm-Petrel. Although smaller, LSPs lift more than BSPs, and environmental factors like El Niño also influence seabird performance. This study found that both storm-petrel species were smaller and lighter than previously reported; yet, LSPs carried relatively heavier loads than BSPs. Although BSPs had higher absolute values for mass and lift, LSPs were more energy-efficient. The muscle mass proportions were similar and typical for takeoff. No significant sex-based differences were found. Both species used aerobic and anaerobic takeoff, with anaerobic flight likely being more efficient.
1. Introduction
The Gulf of California (hereafter, GoC) is recognized as a region of high species richness, many of which are endemic [1,2,3]. It also contains ecosystems with significant geographic isolation, such as the Midriff Islands [4]. Given the atmospheric and oceanic conditions, the islands within the GoC are strategic sites for reproduction, nesting, resting, and feeding for around 56 bird species [5]. Whether resident or migratory, foraging (i.e., the active search for resources) is an activity that constantly demands abundant amounts of energy, and the ability to carry a sizable weight determines how far these resources can be transported from one point to another.
In this context, the maximal load-lift during takeoff and flight efficiency are of the utmost importance because they directly affect the foraging capacity. This is an activity whose efficiency largely defines the possibility of survival, mating, and chick provisioning, and the viability of bird populations. Such an important aspect of life history has not been addressed in any bird species that inhabits the GoC. However, one of the few studies on the maximal load-lift during takeoff regarding seabirds has been performed on two species distributed very closely to the GoC, on the Pacific coast of the Baja California peninsula: the Leach’s Storm-Petrel (Oceanodroma leucorhoa (now Hydrobates leucorhous; https://checklist.americanornithology.org) URL accessed on 29 April 2025) and the Cassin’s Auklet (Ptychoramphus aleuticus) [6]. Although both species display very different ways of motion when interacting with the sea (the first one is a surface feeder with low wing loading, while the second one is a wing-propelled diver with high wing loading), they manage to keep safe margins to support extra load during takeoff. The study authors were able to estimate that both the Leach’s Storm-Petrel and Cassin’s Auklet supported a maximum extra load of 45% and 25% of their body mass, respectively. However, although their results were compared with the data on food intake and plumage wettability reported in the literature (see references in [6]), they did not evaluate the possible effect that intraspecies factors such as size or sex have on the maximal load-lift during takeoff.
Thus, Procellariiformes seabirds such as the Black Storm-Petrel (Hydrobates melania) and the Least Storm-Petrel (Hydrobates microsoma) represent a good opportunity for evaluating these aspects. The Black Storm-Petrel (hereafter, BSP) nests on seven islands in the North American Pacific and Gulf of California, while the Lesser Storm-Petrel (hereafter, LSP) is only present on four islands in the area [7]. Their most important colonies are found on Isla Partida Norte, San Benito and La Lobera islet, nearby Isla Espiritu Santo [7]. From all of them, Isla Partida Norte represents the most important nesting area, since it is in a good state of conservation, uninhabited, and free of introduced predators. The only known studies on these species so far are the identification of a divergent genetic population structure: while such structuring was proven for the LSP, this was not the case for the BSP [8].
The goal of this study is to estimate if the maximum carrying capacity at takeoff of the BSP and the LSP is significantly affected by size or sex. To this purpose, the model of Marden [9] was applied to determine their maximum load during takeoff. Moreover, we determined the aerial performance of each species as suggested in the model proposed by Marden [10]. The maximum load-lift at takeoff refers to the greatest amount of extra weight a bird can carry while still being able to successfully take off from the surface. It reflects the bird’s lift-generating ability relative to its body mass and is influenced by factors such as its wing size, muscle strength, and flight technique. It is a key indicator of flight performance and energy capacity. On the other hand, a takeoff model such as Marden’s model is a biomechanical framework used to estimate a bird’s maximum load-lifting capacity and related flight parameters. It typically incorporates variables like body mass, wing area, muscle mass, and induced power to predict how much weight a bird can lift during vertical or near-vertical takeoff, and to assess flight efficiency and energy use. Compared to historical accounts and previous measures [11], we found important differences between the size and weight of both species currently recognized and those found in our study. Moreover, we observed differences in the maximum extra load supported at takeoff between species, but not in terms of sex; however, although it was found that, in all cases, the females and males of each species achieved an efficient anaerobic takeoff, not all individuals achieved an aerobic takeoff.
While the previous work, such as the study on Leach’s Storm-Petrel and Cassin’s Auklet [6], focused on interspecific differences in the maximum load-carrying capacity and takeoff dynamics, the present study offers several novel contributions. First, it examines two closely related storm-petrel species (LSP and BSP) within the same ecological guild, allowing for finer-scale comparisons of their morphology, muscle mass proportion, and lift-to-power ratios under controlled conditions. Second, it uniquely highlights that, despite being smaller, LSPs exhibit a greater relative load-carrying efficiency and a higher lift-to-power ratio, suggesting a functional advantage in energy expenditure not previously reported. Third, this study incorporates sex-based comparisons and finds that, although females tend to have higher absolute performance metrics, sex has no significant effect on the relative carrying capacity—a dimension largely unaddressed in the earlier literature. Lastly, by comparing the current morphometric data with historical records, this work identifies potential temporal or environmental shifts in body size, prompting ecological questions about climate or habitat-driven adaptation. Together, these elements contribute novel insights into the biomechanics, energetics, and ecological implications of flight performance in small seabirds.
2. Materials and Methods
2.1. Study Area
This study was carried out on Isla Partida Norte, which is in the Midriff Island region of the GoC, about 60 km off the coast of Bahía de los Ángeles, Baja California (28°53′30″ N and 113°02′25″ W) (for the map, see Figure 1 in Mancilla-Morales et al. [8]). It is a small island that measures only 1.38 km2. The area has high primary productivity, since it is strongly influenced by upwellings of deep waters from the Canal de Ballenas, which is rich in nutrients that are used by both phytoplankton and macrophytes, and, later, by other organisms in the food chain [12]. The water temperature presents wide annual intervals (15–17 °C), with minimum winter temperatures of 14–15 °C and maximum temperatures of 30–31 °C in summer [12]. The dominant vegetation on the island is the desert scrub type [8,13]. The sampling was carried out from May 30 to June 6, 2016, a period within the breeding season of the two species of storm-petrels that runs from March to June (for exact location of sampled nests of each species within the island, see Mancilla-Morales et al. [8]).
2.2. Data Collection
All individuals sampled were either incubating their eggs or simply sitting at their roosts. None of them were foraging at the time of sampling. To prevent performing the experiments with individuals who recently fed or have a full stomach, regurgitating was stimulated (very few individuals did so), after which animals were allowed to rest (to complete digestion, if any) for two or three hours. The air temperature and atmospheric pressure recorded were 26 °C and 1008.8 mbar, respectively. The air density was estimated as 1.1591 kg·m−3. For the capture of birds, the work was carried out at night, once they returned from foraging to their nests. Night walks were carried out to locate the birds by means of their vocalizations, and, when the individuals were located, the rocks and small boulders were removed to capture them directly. Birds were placed in duly labeled blanket sacks with information on the individual’s number, species, nest number, and relevant data (e.g., presence of eggs). Once this has been performed, they were transported to the base camp where the work described below was carried out. The Secretaría de Medio Ambiente y Recursos Naturales, the Subsecretaría de Gestión para la Gestión Ambiental, and the Dirección Nacional de Vida Silvestre (Mexico) approved the fieldwork, animal experimentation, and sample collection (permit number: SGPA/DGVS/11605/16).
2.3. Sex Determination
There are no external elements in the morphology of either storm-petrel species as to clearly distinguish an individual’s sex. However, since birds have heterogametic chromosomes (ZW) in females and homogametic chromosomes (ZZ) in males, it is possible to determine the sex of birds. This is carried out by analyzing the DNA-binding Chromo-Helicase (CHD) gene, which is present on both chromosomes [14], and has been tested in other co-distributed species [15]. Although both sexes have the CHD gene, they show differences in length and size in the intergenic region. Thus, DNA Extraction, Polymerase Chain Reaction (PCR), and sex determination (using the CHD gene from samples of the collected individuals) was performed. First, blood samples (60 μL) were taken from the brachial vein with the help of sterile needles and capillaries. The blood was then placed in 1.5 mL tubes containing 300 µL of Longmire solution [16]. Subsequently, the DNeasy Blood and Tissue Extraction Kit (QIAGEN, Hilden, Germany) was used, following the manufacturer’s protocol. Once the DNA extraction was completed, the PCR technique was used to amplify a region of the CHD gene: ≈400 bp in the case of females (on the W chromosome) and ≈600 bp in the case of both males and females (on the Z chromosome). For each sample, the reagents were added as follows: each PCR reaction contained 20 ng of genomic DNA, 10 mg·mL−1 bovine serum albumin (BSA), dimethyl sulfoxide (DMSO) 0.2%, 0.25 mM of each dNTP, 2× Kapa Taq ReadyMix (Kapa Biosystems, Wilmington, MA, USA), and the primers reported by Fridolfsson and Ellegren [14]. Reactions were performed in an Arktik™ Thermal Cycler thermocycler (Thermo Fisher Scientific, Waltham, MA, USA), and cycling parameters were as follows: initial denaturalization, 120 s at 94 °C, 5 cycles of 40 s at 92 °C, 40 s at 50 °C, and 60 s at 72 °C; then, 35 cycles of 60 s at 94 °C, 60 s at 53 °C, and 60 s at 72 °C; and, lastly, a final extension of 5 min at 72 °C. The resulting PCR reaction products were visualized via electrophoresis on a 1% agarose gel, using a buffer based on Tris, acetate, and EDTA (Tris base, acetic acid, and EDTA, (TAE)). Subsequently, the resulting gels were stained with GelRedTM dye (Biotium, Fremont, CA, USA) and UV light was used to reveal the image of the resulting PCR products. Thus, two bands were observed for females (ZW) and a single band for males (ZZ).

Figure 1.
PCR amplification of the CHD gene for sex identification in storm-petrels. In Black Storm-Petrels, females (F) show two bands (~400 bp and ~600 bp) indicating ZW chromosomes, while males (M) show a single ~600 bp band corresponding to ZZ chromosomes. A 100–12,000 bp DNA ladder is included. The same banding pattern was observed in Lesser Storm-Petrels (not shown).
2.4. Maximum Load-Lift at Takeoff
The methodology of Marden [9] was followed to determine the maximum carrying capacity in each storm-petrel species. The methodology established by Marden [6] was selected due to its widespread use and validation in avian flight performance studies, ensuring comparability and scientific rigor. The incremental loading technique (see below), using a known weight attached to the legs, allows for a precise and repeatable assessment of maximum carrying capacity while mimicking natural load-bearing scenarios like prey transport. A small bag with known weight (starting with 1.5 g, and, later, with successive increments using that same amount) was attached to each individual, before a flight attempt. This weight was placed on the legs and fastened with worsted thread. An acceptable flight was considered only when the movement was both forward and upward. To minimize stress and ensure ethical treatment, the protocol was adapted to include nighttime handling, red-light conditions, and sufficient rest between trials—measures supported by best practices in avian field research. The absence of observable exhaustion further supports the appropriateness of this approach. Birds were stimulated by clapping hands behind them, after which they were allowed to rest (until normal breathing) between each attempt. The experiments were performed until the animals were no longer capable of performing an acceptable flight (i.e., between 15–45 times in a row). As mentioned, no signs of exhaustion in any single individual were observed. At most, they were unable to fly due to excessive weight (see below). All the work was carried out inside a structural tent (3 × 3 m), avoiding any disturbance by surrounding wind gusts and/or breezes. Conducting the trials in a wind-sheltered tent ensured standardized environmental conditions, reducing confounding variables such as gusts and thermal drift. Additionally, the calculation of maximal lift force using the method of Ortega–Jiménez et al. [6] provided a robust and physiologically grounded measure of performance. Together, these elements offer a reliable, ethical, and scientifically defensible method for evaluating load-lifting capacity in storm-petrel species.
The maximal lift force (L) in Newtons (N) was calculated following Ortega–Jiménez et al. [6]:
where:
L = (mb + ML) (9.8 m·s−2)
- mb = body mass of the individual;
- ML = added mass.
Following the methodology of Marden [17], and outlined by Ortega–Jiménez et al. [6], the maximum induced power output (Pind) in Watts (W) was also calculated using the following formula:
where:
Pind = (L3/(2ρ·π·rb2)0.5
- L = maximal lifting force;
- ρ = air density;
- r = wing semi-span.
For r, the mean value of each species was considered (17.2 cm for the LSP and 25.7 cm for the BSP).
2.5. Takeoff Model
To test the model, five individuals of each bird species were sacrificed and kept refrigerated, to subsequently extract all the muscles (except those of the head) and weigh them. In addition, the flight muscles [6] were weighed separately to determine their proportion with respect to the total mass. With the data obtained, the mass specific lift (Lmb) was calculated, again following Marden [10] and Ortega-Jimenez et al. [6]. The resulting formula was as follows:
where:
Lmb = [Mtmus/(mb + ML)] [L/Pind] [Po,m]
- Mtmus = total muscle mass;
- Po,m = muscle-mass-specific power output (referring to the following constants: aerobic flight performance = 100 W·kg−1; and anaerobic flight performance = 225 W·kg−1).
2.6. Statistical Analysis
As the main purpose of this study was to measure if the maximum load at takeoff and the takeoff model estimates are influenced by the species, species size, or sex, only Student’s t-tests were performed to compare the arithmetic means and, thus, define if there are statistically significant differences. Therefore, Student’s t-test was employed to assess whether statistically significant differences exist between groups. This test is appropriate for comparing the means of two independent groups when the dependent variable (e.g., lift force or carrying capacity) is continuous and approximately normally distributed. Prior to analysis, data were checked for normality and homogeneity of variances, ensuring the assumptions of the test were met. Given the two-group comparison and the scale of measurement, the t-test provides a straightforward and powerful method to detect group differences. The value of the probability level at which the null hypothesis was rejected was p ≤ 0.05. All statistical comparisons (e.g., differences between species or sexes) were performed using Student’s t-tests in Microsoft Excel [18].
3. Results
3.1. Sex Determination of BSP and LSP Individuals
PCR products for the CHD gene yield two bands of 400 and 600 bp in females, while only one 600 bp band was observed in males (Figure 1). Sex determination by this molecular method was performed for 48 individuals of the LSP group, of which 26 were identified as males and 22 as females. For the BSP group, 23 samples were used, of which 15 were identified as males and 8 as females.
3.2. Carrying Capacity of BSP and LSP Individuals
3.2.1. Maximum Load, Takeoff Lift Force, and Maximum Induced Power
The average total mass per individual (mb) was 18.928 g (SD = 3.493) for the LSP and 54.804 g (SD = 6.612) for the BSP. The differences in weight between the species were statistically significant (t28 = 1.7; p << 0.001), since the weight of the former was almost a third of that of the latter. Regarding the maximum load (ML), this was 25.979 g (SD = 4.465) for the LSP, since it managed to lift 7.051 g extra of its own weight (37.25% more than its mb). For the BPS, a mean ML of 71.59 g (SD = 6.678) was obtained, indicating that it was able to lift an extra 16.783 g of its own weight (30.62% more than its mb). The difference in this carrying capacity proved to be statistically significant between the two species (t31 = 1.69; p << 0.001), indicating that, despite being smaller, the LSP is able to lift a greater proportion of its own weight than the BSP. Regarding the maximum lifting force (L), the value was 0.254 N (SD = 0.043) for the LSP and 0.702 N (SD = 0.065) for the BSP. In this case, the difference between the two species was also statistically significant (t31 = 1.69; p << 0.001); this capacity was 3.5 times greater in the BSP than in the LSP. For the maximum induced power (Pind), this was 0.278 W (SD = 0.072) for the LSP and 0.844 W (SD = 0.133) for the BSP. Here, the difference was also statistically significant (t28 = 1.7; p << 0.001), three times higher in the BSP than in the LSP. All the values described for the estimated variables, as well as the values of the Student’s t-tests, are shown in Table 1.

Table 1.
Estimated parameters for both the BSP and LSP, along with the corresponding comparisons using the Student’s t-test. mb, body mass of the individual; ML, maximum load; L, maximum lifting force; Pind, maximum induced power; SD, standard deviation; n, number of individuals; *, p << 0.001.
3.2.2. Comparison of the Carrying Capacity Between Females and Males Within Each Species
For the LSP, the mb was 19.357 g (SD = 3.013) in females and 18.314 g (SD = 3.825) in males. For the BSP, the mb was 55.812 g (SD = 7.279) in females and 54.266 g (SD = 6.427) in males. Apparently, in both species, females have a higher mb than males; however, these differences are non-significant (t46 = 2.01; p = 0.29, and t22 = 2.16; p = 0.62 for the LSP and the BSP, respectively). The same occurs with the estimates of ML, L, and Pind; that is, although females also showed higher values compared to males (Table 2), the differences were non-significant (Table 3).

Table 2.
Effect of sex on the carrying capacity variables evaluated. mean; SD, standard deviation; n, number of individuals; mb, total mass of the individual; ML, maximum load; L, maximum lifting force; Pind, maximum induced power.

Table 3.
Student’s t-tests (exact values of p) performed to compare the means of variables evaluated between females and males of both the LSP and BSP. mb, total mass of the individual; ML, maximum load; L, maximum lifting force; Pind, maximum induced power.
3.3. Takeoff Model of the BSP and LSP Individuals
3.3.1. Determination of Muscle Mass Proportion in BSP and LSP Individuals
From the dissections and extraction of individual birds’ muscles, the percentage of the flight muscle mass (mf) with respect to the total muscle mass (Mtmus) was obtained. This was 75.87% (SD = 4.24) for the LSP and 72.21% (SD = 3.73) for the BSP. When comparing this same mf with the total mass of the individual (mb), it was obtained that mf represents 13.46% (SD = 3.62) of the mb for the LSP, and 11.81% (SD = 2.72) for the BSP (Table 4). Despite such differences between the mf values between the two species, they were not significant (t7 = 1.89; p = 0.22).

Table 4.
Muscle mass measurements for both BSP and LSP individuals. mb, total mass of birds; ML, maximum load; mf, flight muscle mass; Mtmus, total muscle mass; %mf/Mtmus, percentage of flight muscle mass with respect to total muscle mass; %mf/mb, percentage of flight muscle mass with respect to total mass; n, number of individuals; BSP mean; mean values for Black Storm-Petrel individuals; LSP mean; mean values for Least Storm-Petrel individuals; SD, standard deviation.
3.3.2. Mass-Specific Lift
Regarding the estimation of the proportion of flight muscles (Flight Muscle Ratio, FMR), this was 0.11 (SD = 0.021) for the LSP and 0.13 (SD = 0.024) for the BSP (Table 5). On the other hand, the ratio between the maximal lift force and the maximum induced power output (L/Pind) was 0.945 (SD = 0.102) for the LSP and 0.8 (SD = 0.08) for the BSP (Table 5). Thus, from these results, the calculation of the mass-specific lift (Lmb) was performed using the constants of 100 W·Kg−1 for the aerobic flight performance and 225 W·Kg−1 for the anaerobic performance (Table 5). The data indicates that BSP individuals have a larger mass-specific lift capacity, both aerobic and anaerobic, compared to LSP individuals.

Table 5.
Mass-specific lift and related measurements. ID, Individual ID; FMR, flight muscle ratio; L/Pind, lift-to-power ratio; m, mean; SD, standard deviation; Lmb, mass-specific lift.
The values obtained for the aerobic and anaerobic aerial performance in both species were higher than 9.8 N·Kg−1 (Figure 2), which is the minimum value necessary for a bird to take off. Therefore, both species can accomplish takeoff with a maximum load with either metabolism. The averages of the aerobic Lmb for both species were not significantly different from the critical value (9.8 N·Kg−1) (t48 = 2.13, p = 0.35 for the LSP; and t22 = 2.13, p = 0.24 for the BSP), unlike the anaerobic Lmb for both species, which was significantly different (t48 = 2.13, p = 0.006 for the LSP; and t22 = 2.13, p = 0.003 for the BSP).
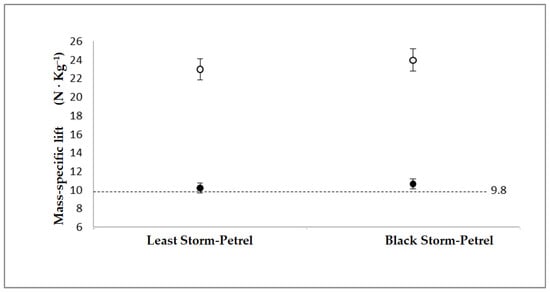
Figure 2.
Mass-specific lift with aerobic (black circles) and anaerobic (open circles) metabolism, as expected in the model by Marden [10], in 49 LSP and 23 BSP individuals, respectively. Error bars indicate standard deviation (SD). The dashed line indicates the minimum value necessary for a bird to take off (9.8 N·Kg−1).
3.3.3. Takeoff Model and the Relationship with Bird Sex
The mass-specific lift was also compared between females and males within each species. In the case of the LSP, of the five individuals evaluated, only one corresponded to a female, for which the aerobic Lmb did not exceed the critical value (9.8 N·Kg−1), while the anaerobic Lmb was higher (17.834 N·Kg−1). For the four males, the aerobic Lmb value was lower than the critical value for two of them and higher for the remaining two. On the other hand, the anaerobic Lmb value was higher than the critical value in all four males (Table 5). For BSP individuals, three females and two males were recorded. For females, the aerobic and anaerobic Lmb values were higher than the critical value in all cases, while, for the two males, the aerobic Lmb was higher in one and lower in the other. Regarding the anaerobic Lmb value, it was higher in all individuals of both males and females (Table 5). In any case, no significant differences associated with sex were found.
4. Discussion
There are environmental factors that may be affecting the performance of birds (including the Procellariiformes reported in this study) in ways that are only recently being explored. For example, in terms of bird size, Samaniego et al. [11] reported an average weight of 21 g and a wingspan of 36 cm for the LSP and an average weight of 59 g and a wingspan of 53 cm for the BSP. However, in the present study, smaller sizes were found in both species. For the LSP, an average mass of 18.92 g (9.9% less) and a wingspan of 32 cm (5.5% shorter) were observed, while, for the BSP, an average weight of 54.8 g (7.11% less) and a wingspan of 50 cm (10.71% shorter) were obtained. Later, Sausner et al. [19] also report higher averages compared to the data in this work: for the LSP, they report an average weight of 21.9 g (13.6% more) and a wingspan of 34.6 cm (7.51% larger), while, for the BSP, they report a weight of 60.2 g (8.97% more) and a wingspan of 51 cm (1.96% larger). Thus, although it is not the object of this study to evaluate the reasons, it is possible that smaller, lighter individuals of both species are currently forced to cope with their normal activities and life histories at a disadvantage, as opposed to earlier generations. If so, the current individuals of both species are probably now struggling harder to obtain their resources, negatively affecting their performance in, for example, the maximum load at taking off, or other parameters such as FMR, L/Pind, or Lmb. Other, different possibilities are that the observed differences in both weight and size are the consequence of (1) the life histories of these organisms, or (2) the environmental circumstances that have been present in the Gulf of California in recent years. In the first case, the data reported in this study were obtained during the breeding season for both species, which runs from early spring to early summer. Although seabirds generally lose body mass during reproduction, this is considered an adaptation to reduce flight costs [20]. However, other authors propose it as an adaptation to allow the transportation of heavier load during foraging [6]. In the second case, the sampling year corresponded to an El Niño Southern Oscillation (ENSO) year, regarded as very severe [21]. This phenomenon has been shown to lead to negative consequences for marine species throughout the food chain, including the birds’ food supply [22]. Storm-petrel species are no exception, as they depend heavily on plankton for survival, which, in turn, depends heavily on the region’s strong currents. ENSO conditions weaken such currents, and favor the formation of thermoclines, which, in turn, impede the upwelling of nutrients from the seafloor.
The results of this study clearly show a positive relationship between the physical variables evaluated and the mass of birds. That is, these variables increase as mass increases. However, although it was expected that the BSP (which is larger) would show a higher ML, this was not the case. BSP individuals managed to lift an extra load of 30.62% of its own mass, while LSP individuals managed an extra load of 37.26%. Thus, the latter managed to lift more extra mass during takeoff. In the work by Ortega-Jiménez et al. [6], the authors observed the same trend, as the smallest bird (40.1 g), the Leach’s Storm-Petrel (Hydrobates leucorhous), managed to carry an extra load of 45% of its own mass, while the larger Cassin’s Auklet (Ptychoramphus aleuticus) (152.5 g) managed to carry only 25% more of its own mass. This apparent paradox is explained by the greater effort a larger bird must make to lift its own weight: In physical terms, the greater loading efficiency for the LSP than for BSP responds to a greater energy cost for the last species to lift its own weight, and, therefore, the extra weight it can lift could be limited.
Regarding the estimates of mb, ML, L, and Pind (Table 1), these were always higher in the BSP than in the LSP. When these values are compared between species in terms of proportions (Table 6), it is observed that, in almost all cases, the values for the larger species are about three times higher than those for the smaller one. Thus, the BSPs mb is 2.8× larger than the LSP, has a ML 2.75× larger, and has a L 2.7× higher. However, Pind does not increase in this same proportion. Rather, its increase is >3× higher. A similar trend was observed in the work of Ortega-Jiménez et al. [6], where they also compare birds with significant differences in size. In that case, the ratios of mb, ML, and L were ≈3.6× higher, but, for Pind, the increase was >6× higher for the Cassin’s Auklet than for the Leach’s Storm-Petrel. These results can be explained by the fact that, although flying species such as birds may be morphologically similar, the biomechanical power required for flight is relatively greater for larger species than for smaller ones, given that the required biomechanical power must scale with body mass [23,24]. Thus, the BSP must produce greater power to achieve takeoff. On the other hand, it was also observed that the wingspan of the BSP was 1.5× larger than that for the LSP, which was not proportional to the increase in mb. This is consistent with what was observed by Ortega-Jiménez et al. [6], who also reported that the increase in wingspan was not proportional to the increase in mb, as the Leach’s Storm-Petrel (≈44 cm wingspan) generated less Pind (0.7 W) compared to the Cassin’s Auklet (≈40 cm wingspan) which, despite having only 1.1 times the wingspan, generated 6.5× more Pind (4.58 W).

Table 6.
Proportion of variables considered between the BSP and the LSP. The last two columns correspond to the number of times the values obtained for the Cassin’s Auklet increase with respect to the Leach’s Storm-Petrel [6].
Another aspect that could be related to the LSP’s reduced takeoff power could be the rounded shape of its tail. The tail acts as a rudder during flight; that is, it maintains aerodynamic stability. It is used to control the angle of attack of the wings and produces a substantial lift to augment the lift generated by the wings [25]. In Marden’s [10] model, the lift produced by the tail is not considered; however, its aerodynamic function has been verified in other birds. For example, Gatesy and Dial [26] analyzed how the tail muscles of the Harris’s Hawk (Parabuteo unicinctus) work and showed that the bird generates substantial forces with its tail during takeoff, and that, once the bird achieves stable flight, the tail extends and remains at a constant angle to the airflow. In contrast, Thomas [25] designed a model of tail aerodynamics, finding that the efficiency varies depending on the shape and size of the tail, and predicting that a longer, tightly feathered tail can generate greater power during takeoff. In this sense, both storm-petrels have relatively long tails; however, the LSP’s tail is spatulate or rounded, while the BSP’s is forked. Thus, if Thomas’ model is correct, then the LSP could generate greater power with its tail, contributing to the power generated by its wings.
Although the effects of bird sex on factors such as the carrying capacity or lift power have not been properly evaluated, their potential contribution has been minimized. For example, although Ortega-Jiménez et al. [6] argues that sex or age may not have an effect on the carrying capacity during takeoff, the statistical support for their results is low given the limited sample size. In contrast, the present study found that the evaluated females apparently did have higher values for mb, ML, L, and Pind, for both the LSP and the BSP. However, the Student’s t-tests showed no significant differences between total mass, carrying capacity, and flight performance between females and males, indicating that these differences were due to chance. Therefore, these results are consistent with the authors’ findings despite the differences in sample sizes.
Just as the effects of variables such as individual size or sex have been poorly studied in explaining the carrying capacity, the effect of the proportion of muscles used in flight is also unknown. To begin with, muscles generally perform approximately the same amount of work in proportion to their mass [27,28]. The pectoralis is a large muscle, approximately 8–11% of the body mass [29]. It produces mechanical work during descent, in addition to controlling wing pronation. In terms of mass, the supracoracoideus is only one-fifth of the pectoralis, that is, approximately 2% of body mass. It acts dorsally on the shoulder as a pulley, elevating and supinating the wing during the upstroke [30]. Thus, the combined mass of both muscles represents approximately 13% of the bird’s total mass. In comparison, the present study found that this proportion for both storm-petrels was very close to (but different from) 13% (13.6% and 11.81% for both the LSP and the BSP, respectively). Apparently, the LSP has a larger muscle mass for takeoff, which may partially explain its larger carrying capacity. The development of these muscles is important not only for flight, but also for their role in heat production, especially during the early stages of the life history of these species. This is because the growth rate of these muscles can limit the overall growth rate of the hatchlings [31]. On the other hand, Marden’s [10] model suggests that the lift-to-power ratio (L/Pind) increases as the wingspan increases in flying birds, since the induced power requirements decrease with the wingspan size [32]. This is consistent with the data obtained in this study, since the L/Pind ratio was 0.94 for the LSP and 0.79 for the BSP. As mentioned, the wingspan of the latter does not increase proportionally to the body mass.
The mass-specific lift estimates from Marden’s model [10] suggest that, if the aerobic or anaerobic Lmb value obtained for a flying animal exceeds the critical value (9.8 W·Kg−1), the animal is considered capable of achieving a takeoff (aerobic or anaerobic, as it corresponds) with maximum load and minimum energy cost. For example, in the work of Ortega-Jiménez et al. [6], the Leach’s Storm-Petrel achieved a takeoff with ML both aerobically and anaerobically, since both values were higher than the critical value. In contrast, the Cassin’s Auklet only achieved an anaerobic takeoff, since the aerobic Lmb value did not exceed the critical value. In this study, the same trend observed for the Leach’s Storm-Petrel was found, as both the LSP and the BSP were able to achieve takeoff with both aerobic and anaerobic ML, with the latter being more efficient. Thus, in all three storm-petrels, it was observed that they could barely achieve aerobic takeoffs, while an anaerobic takeoff was well above the critical value, probably because of the muscle composition.
Future research should investigate the physiological and biomechanical mechanisms behind the LSP’s greater relative load-carrying capacity and energy efficiency, including its muscle composition and wing dynamics. Comparative studies across different populations may clarify whether the observed reductions in body mass and wingspan reflect geographic, ecological, or climatic influences. Given the potential impact of environmental stressors like El Niño, longitudinal studies examining how such factors affect the flight performance and body condition are recommended. Additionally, tracking individuals across life stages and seasons could reveal ontogenetic or temporal changes in the carrying capacity. While no significant sex-based differences were found, further research with larger sample sizes may uncover subtle variations. Finally, integrating aerodynamic modeling with field tracking would also enhance the understanding of flight performance under natural conditions, and examining the long-term fitness consequences of lift efficiency may provide insight into the ecological significance of these traits.
5. Conclusions
This study concludes that both Black Storm-Petrels (BSPs) and Lesser Storm-Petrels (LSPs) exhibited significantly smaller body sizes and masses compared to historical records, with LSPs being notably lighter and shorter. Despite their smaller size, LSPs demonstrated a higher relative load-carrying capacity and greater energy efficiency, as indicated by the superior lift-to-power ratio (0.94 vs. 0.79 for BSPs). Although BSPs had higher absolute values for their body mass, maximum load, and lift, their induced power requirements were disproportionately larger, reducing their overall efficiency. Their muscle mass proportions were consistent with the established norms, suggesting other factors like wing dynamics or muscle composition contribute to performance differences. No statistically significant differences were found between sexes in carrying capacity or flight metrics, though females tended to show higher raw values. All individuals achieved an efficient anaerobic takeoff, but not all succeeded in aerobic takeoff, highlighting the potential physiological limits. The use of Marden’s model effectively quantified these traits, underscoring its relevance in avian flight studies. These findings suggest that relative performance rather than absolute size may be more critical for load-lifting efficiency, particularly in smaller seabird species like the LSP.
Author Contributions
Conceptualization, E.A.R.; methodology, E.A.R., Z.G.-L., M.D.M.-M. and A.C.-F.; software, E.A.R. and A.C.-F.; validation, E.A.R., Z.G.-L., A.C.-R., J.J.F.-M. and A.C.-F.; formal analysis, E.A.R., Z.G.-L., A.C.-R. and M.D.M.-M.; investigation, E.A.R., Z.G.-L., A.C.-R., A.C.-F., J.J.F.-M. and M.D.M.-M.; resources, E.A.R. and Z.G.-L.; data curation, E.A.R., Z.G.-L. and A.C.-F.; writing—original draft preparation, E.A.R., Z.G.-L. and A.C.-F.; writing—review and editing, E.A.R., Z.G.-L., A.C.-R., A.C.-F., J.J.F.-M. and M.D.M.-M.; visualization, E.A.R., Z.G.-L. and A.C.-F.; supervision, E.A.R. and Z.G.-L.; project administration, E.A.R. and Z.G.-L.; funding acquisition, E.A.R. and Z.G.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politécnico Nacional (IPN), with projects SIP-20161532, SIP-20171835, and SIP-20181044 granted to E.A.R., and projects SIP-20221553, SIP-20231001, and SIP-20240526 to Z.G.-L.
Institutional Review Board Statement
This study complies with Mexican regulations regarding the ethical treatment of research subjects. The Dirección General de Vida Silvestre, Subsecretaría de Gestión para la Protección Ambiental, Secretaría de Medio Ambiente y Recursos Naturales, and Secretaría de Gobernación granted the corresponding research and sample collection permit (SGPA/DGVS/11605/16).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The study was conducted with permits from The Dirección General de Vida Silvestre, Subsecretaría de Gestión para la Protección Ambiental, Secretaría de Medio Ambiente y Recursos Naturales. We thank the APFF Islas del Golfo de California (Rosalía Avalos), the Prescott College Kino Bay Center (Lorayne Meltzer), the Secretaría de Gobernación, and the Secretaría de Marina-Armada de México for their logistic support to visit Isla Rasa.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brusca, R.C.; Findley, L.T.; Hastings, P.A.; Hendrickx, M.E.; Cosio, J.T.; Heiden, A.M.V.D. Macrofaunal Diversity in the Gulf of California. In Biodiversity, Ecosystems, and Conservation in Northern Mexico; Cartron, J.-L.E., Ceballos, G., Felger, R.S., Eds.; Oxford University Press: Oxford, UK, 2005. [Google Scholar] [CrossRef]
- The Gulf of California: Biodiversity and Conservation; Brusca, R.C., Ed.; University of Arizona Press: Tucson, AZ, USA, 2010. [Google Scholar] [CrossRef]
- Enríquez-Andrade, R.; Anaya-Reyna, G.; Barrera-Guevara, J.C.; Carvajal-Moreno, M.d.l.Á.; Martínez-Delgado, M.E.; Vaca-Rodríguez, J.; Valdés-Casillas, C. An analysis of critical areas for biodiversity conservation in the Gulf of California Region. Ocean. Coast. Manag. 2005, 48, 31–50. [Google Scholar] [CrossRef]
- Alvarez-Borrego, S. Physical Oceanography. In A new Island Biography of the Sea of Cortes; Chase, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 41–59. [Google Scholar]
- Arizmendi, M.d.C.; Marquez Valdemar, L. Áreas de Importancia para la Conservación de las aves en México; CONABIO: Mexico City, Mexico, 2000; p. 440. [Google Scholar]
- Ortega-Jimenez, V.M.; Alvarez-Borrego, S.; Arriaga-Ramirez, S.; Bridge, E.S.; Renner, M. Maximum Load-carrying During Takeoff of Leach’s Storm-Petrel Oceanodroma leucorhoa and Cassin’s Auklet Ptychoramphus aleuticus. Waterbirds 2011, 34, 102–106. [Google Scholar] [CrossRef]
- Carmona, R.; Guzmán, J.; Ramírez, S.; Fernández, G. Breeding waterbirds of La Paz Bay, Baja California Sur, Mexico. West. Birds 1994, 25, 151–157. [Google Scholar]
- Mancilla-Morales, M.D.; Romero-Fernández, S.; Contreras-Rodríguez, A.; Flores-Martínez, J.J.; Sánchez-Cordero, V.; Herrera M, L.G.; López, M.F.; Ruiz, E.A. Diverging Genetic Structure of Coexisting Populations of the Black Storm-Petrel and the Least Storm-Petrel in the Gulf of California. Trop. Conserv. Sci. 2020, 13, 1940082920949177. [Google Scholar] [CrossRef]
- Marden, J.H. Maximum Lift Production during Takeoff in Flying Animals. J. Exp. Biol. 1987, 130, 235–258. [Google Scholar] [CrossRef]
- Marden, J.H. From damselflies to pterosaurs: How burst and sustainable flight performance scale with size. Am. J. Physiol. 1994, 266, R1077–R1084. [Google Scholar] [CrossRef]
- Samaniego-Herrera, A.; Peralta-García, A.; Valdez Villavicencio, J.H.; Luna Mendóza, L.; Aguirre Muñoz, A. Vertebrados de las Islas del Pacífico de Baja California. Guia de Campo; Samaniego-Herrera, A., Peralta-García, A., Aguirre Muñoz, A., Eds.; Grupo de Ecología y Conservación de las Islas, A.C.: Mexico City, Mexico, 2007; p. 178. [Google Scholar]
- Pacheco-Ruiz, I.; Zertuche-González, J.; Espinosa-Ávalo, J.; Riosmena-Rodríguez, R.; Galindo-Bect, J.; Gálves-Télles, A.; Meling-López, A.; Orduña-Rojas, J. Macroalgas. In Bahía de los Ángeles: Recursos Naturales y Comunidad Línea Base 2007; Danemann, G.D., Ezcurra, E., Eds.; Secretaría de Medio Ambiente y Recursos Naturales, Tlalpan, México D.F.: Mexico City, Mexico, 2008; pp. 181–213. [Google Scholar]
- Cody, M.; Moran, R.; Rebman, J.; Thompson, H. Plants. In A New Island Biogeography of the Sea of Cortés; Case, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: Oxford, UK; New York, NY, USA, 2002; pp. 63–110. [Google Scholar]
- Fridolfsson, A.K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratit birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Ruiz, E.A.; Contreras-Rodríguez, A.; Araiza, O.; Aguilera-Arreola, M.G.; Hernández-García, J.A.; Flores-Martínez, J.J.; Sánchez-Cordero, V.; Gomez-Lunar, Z. Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California. Diversity 2024, 16, 617. [Google Scholar] [CrossRef]
- Longmire, L.J.; Maltbie, M.; Baker, J.R. Use of “Lysis Buffer” in DNA Isolation and Its Implication for Museum Collections; Museum of Texas Tech University: Lubbock, TX, USA, 1997; pp. 1–3. [Google Scholar]
- Marden, J.H. Maximum Load-Lifting and Induced Power Output of Harris Hawks Are General Functions of Flight-Muscle Mass. J. Exp. Biol. 1990, 149, 511–514. [Google Scholar] [CrossRef]
- Corporation, M. Microsoft Excel, Version 16.0; Microsoft Corporation: Redmond, WA, USA, 2022. [Google Scholar]
- Sausner, J.; Torres-Mura, J.C.; Robertson, J.; Hertel, F. Ecomorphological differences in foraging and pattering behavior among storm-petrels in the eastern Pacific Ocean. Auk 2016, 133, 397–414+318. [Google Scholar] [CrossRef]
- Elliott, K.H.; Jacobs, S.R.; Ringrose, J.; Gaston, A.J.; Davoren, G.K. Is mass loss in Brünnich’s guillemots Uria lomvia an adaptation for improved flight performance or improved dive performance? J. Avian Biol. 2008, 39, 619–628. [Google Scholar] [CrossRef]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The Defining Characteristics of ENSO Extremes and the Strong 2015/2016 El Niño. Rev. Geophys. 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Páez-Osuna, F.; Sanchez-Cabeza, J.A.; Ruiz-Fernández, A.C.; Alonso-Rodríguez, R.; Piñón-Gimate, A.; Cardoso-Mohedano, J.G.; Flores-Verdugo, F.J.; Carballo, J.L.; Cisneros-Mata, M.A.; Álvarez-Borrego, S. Environmental status of the Gulf of California: A review of responses to climate change and climate variability. Earth-Sci. Rev. 2016, 162, 253–268. [Google Scholar] [CrossRef]
- Pennycuick, C.J. The Flight of Petrels and Albatrosses (Procellariiformes), Observed in South Georgia and its Vicinity. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1982, 300, 75–106. [Google Scholar]
- Rayner, J.M.V. A New Approach to Animal Flight Mechanics. J. Exp. Biol. 1979, 80, 17–54. [Google Scholar] [CrossRef]
- Thomas, A.L.R. On the Tails of Birds. BioScience 1997, 47, 215–225. [Google Scholar] [CrossRef]
- Gatesy, S.M.; Dial, K.P. Tail Muscle Activity Patterns in Walking and Flying Pigeons (Columba livia). J. Exp. Biol. 1993, 176, 55–76. [Google Scholar] [CrossRef]
- Alexander, R.M. The work that muscles can do. Nature 1992, 357, 360–361. [Google Scholar] [CrossRef]
- Marsh, R.L. How muscles deal with real-world loads: The influence of length trajectory on muscle performance. J. Exp. Biol. 1999, 202, 3377–3385. [Google Scholar] [CrossRef]
- Roberts, T.J.; Marsh, R.L.; Weyand, P.G.; Taylor, C.R. Muscular force in running turkeys: The economy of minimizing work. Science 1997, 275, 1113–1115. [Google Scholar] [CrossRef]
- Dial, K.P.; Biewener, A.A. Pectoralis Muscle Force and Power Output During Different Modes of Flight in Pigeons (Columba livia). J. Exp. Biol. 1993, 176, 31–54. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Susan, C.W.; Cullen, J. Energetics of Postnatal Growth in Leach’s Storm-Petrel. Auk 1980, 97, 566–575. [Google Scholar]
- Pennycuick, C.J. Mechanics of flight. In Avian Biology; Farner, D.S., King, J.R., Eds.; Academic Press: Cambridge, MA, USA, 1975; Volume 5, pp. 1–75. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).