Abstract
The high-elevation calcareous screes of the Southern Carpathians are ecologically important habitats characterised by extreme environmental conditions. These habitats support specialised plant communities, including endemic and relict species, shaped by climatic, edaphic and biogeographic factors. This study examines three scree vegetation communities in the Bucegi, Piatra Craiului and Făgăraș massifs to assess species composition, ecological strategies and environmental influences. Phytosociological surveys were carried out using the Braun-Blanquet method, diversity indices (species richness, Simpson indices and species evenness) and multivariate analyses, including ANOSIM (ANalysis Of SIMilarities), SIMPER (Similarity Percentage method) and PCA (Principal Component Analysis), and were applied to evaluate species–environment relationships. A total of 62 vascular plant species were recorded, with Caryophyllaceae and Asteraceae as the dominant families. Differences in lifeform composition and species distribution between the massifs were related to variations in soil moisture, nutrient availability and climatic conditions. The results highlight the role of calcareous substrates in supporting alpine endemism and underline the influence of abiotic stress on community structure. Conservation efforts should prioritise these fragile ecosystems, especially as climate change and human activities increase pressure on high-elevation habitats. The study contributes to a broader understanding of the Carpathian alpine flora and its biogeographic connections with other European mountain systems, and it highlights the need for targeted conservation strategies to preserve biodiversity in these vulnerable environments.
1. Introduction
High-elevation calcareous screes, typically found at altitudes above 1000 m, are ecologically important habitats distributed throughout the mountain ranges of the European Nemoral Zone []. These screes consist of rock debris, primarily base-rich rocks such as limestones, dolomites, calcareous shales and marls. The alkaline conditions created by these substrates, along with additional environmental factors such as instability, drought stress and temperature extremes, support highly specialised plant communities in major mountain ranges across Europe [,]. Stenotopic species contribute to the differentiation of vegetation in the different mountain ranges of Europe []. The species composition of these communities varies with elevation, exposition, inclination and geographical location of the rock habitat, reflecting a complex interplay of climatic, edaphic and biogeographic factors [,,].
In this context, the geographical isolation and limited extent of high-elevation cliffs have favoured a high degree of specialisation and endemism within their flora. These habitats often act as natural refuges, preserving many relict species that have survived past climatic changes, while local ecological pressures have driven the evolution of some endemic species [,,].
The traditional explanation for the clustering of Alpine endemism in the north-east, south-east and south-west Alps has been widely accepted for decades. Pils [] argued that current ecological conditions in the northeastern Alps play a more significant role in the restricted distribution of endemic plants, emphasising the prevalence of vicariance among endemic species. Vicariant alpine species are closely related species or subspecies that occupy similar ecological niches but are geographically separated, often due to historical processes such as geographic barriers [,]. These species arise through vicariance, when populations of a common ancestor become isolated and diverge over time. Vicariant species provide information about past geological and climatic events that have shaped current ecosystems. Because they are restricted to small areas, these species are vulnerable to habitat loss or climate change [,,].
Papaver corona-sancti-stephani Zapal. [] is a common species in the Carpathian region, particularly in Romania. It has been proposed as a diagnostic species for the Papavero-Thymion pulcherrimae alliance, which is endemic to Romania. The species grows on rocky, calcareous substrates and, as an alpine species, P. corona-sancti-stephani is adapted to extreme conditions, including temperature fluctuations and minimal soil nutrient levels [].
One of the Carpathian Cerastium species is Cerastium lerchenfeldianum Schur [], an endemic species mainly associated with calcareous cliffs and rocky slopes at high altitudes. Although it can occasionally be found in alpine meadows, its optimal habitat remains the rocky environment. C. lerchenfeldianum is part of the characteristic flora of the Carpathians and also contributes to the Papavero-Thymion pulcherrimae alliance []. It is a diagnostic species in these alpine communities, with its low growth form and ability to flower during short, harsh summers.
From a phytosociological point of view [,], the two species form an association that is endemic to the Southern Carpathians and belongs to the class Thlaspietea rotundifolii Br.-Bl. 1926. The phytocoenoses of this vegetation class develop on scree and rock outcrops, representing the initial stage of vegetation establishment on soils formed by the gradual accumulation of rock fragments []. The order Thlaspietalia rotundifolii Br.-Bl. 1926 comprises heliophilous plant associations inhabiting calcareous screes in alpine and montane zones. In this context, the Papavero-Thymion pulcherrimi Pop 1968 order represents the alpine calcareous scree vegetation of the Eastern and Southern Carpathians. It includes calcareous scree associations characteristic of the alpine steppe of the Carpathians [,].
The Cerastio lerchenfeldiani-Papaveretum Boșcaiu, Täuber et Coldea 1977 association, dominated by C. lerchenfeldianum and P. alpinum subsp. corona sancti-stephani, includes pioneer and stabilising phytocoenoses on crumbling calcareous rocks. These communities are well-adapted to the conditions of mobile scree [].
The most important species of this association are C. lerchenfeldianum, Arabis alpina, Thymus pulcherrimus, Papaver alpinum subsp. corona sancti-stephani, Ranunculus oreophilus and Myosotis alpestris [,].
Previous studies have reported that both biotic and abiotic conditions influence alpine plant growth and possibly population dynamics [,]. By incorporating biogeographic and functional perspectives, Llambi et al. [] have argued for a more integrative and predictive understanding of vegetation dynamics in alpine environments, with implications for both biodiversity monitoring and climate change mitigation. This broader view is important to capture the complex relationships between species and their environment and to develop more effective conservation strategies in the face of environmental change []. The results of Aalto et al. [] emphasise the role of biological processes in influencing abiotic factors and show how vegetation can significantly alter local habitat conditions at fine spatial scales in alpine environments. In addition, Valachovič [] conducted a study comparing the rock vegetation of the Western Carpathians with that of neighbouring mountains in Central Europe.
This study focuses on three different calcareous scree vegetation communities in the Southern Carpathians: the Bucegi Massif community, the Fagaras Massif community and the Piatra Craiului community, with a particular emphasis on the dominant species P. corona-sancti-stephani and C. lerchenfeldianum. Our main aim is to improve our understanding of the role of these ecosystems by analysing species composition, adaptation strategies and environmental responses. Specifically, we address the following research questions: (1) How do species and environmental preferences vary among the three scree communities? (2) What are the dominant adaptive strategies that allow species to persist in these extreme habitats? (3) To what extent do abiotic factors such as light availability, temperature, nitrogen levels, soil response and moisture shape community structure? By answering these questions, our study contributes to the broader ecological knowledge needed for effective conservation strategies, particularly as climate change and anthropogenic pressures continue to affect these fragile habitats.
Both biotic and abiotic factors influence ecosystem structure and functioning. In this study, we assessed these influences by analysing species composition, environmental preferences and adaptation strategies. However, we did not perform ecological niche modeling or create predictive models for future scenarios. In habitats with low productivity, such as alpine regions with high abiotic stress, plant interactions can range between competition for limited resources and favoring. However, in scree habitats, biotic competition is generally low due to extreme environmental conditions, and favoring is more expected to have a more important role in increasing species survival. In such stressed environments, where plants are typically distributed throughout the habitat, species interactions are limited, and community composition is largely determined by abiotic factors.
2. Materials and Methods
2.1. Area of Study and Sampling Sites
The Southern Carpathians, part of the Carpathian Mountain range in Central and Eastern Europe, stretch across Romania. They are composed of rocks ranging from crystalline schists and granites in the central areas to limestones and sandstones in the peripheral zones []. This petrographic diversity has greatly influenced the relief. The Southern Carpathians show evidence of glaciation, with glacial valleys, cirques and moraines. The Southern Carpathians are characterised by a mountain climate with low temperatures, abundant rainfall and high climatic variability, characterised by significant fluctuations in temperature between seasons and even within a single day, as well as rainfall variations influenced by altitude, exposure and local topography. Compared to neighboring mountain ranges such as the Alps or the Balkans, the Carpathians face stronger continental influences, resulting in colder winters, frequent temperature inversions and extended snow cover at high altitudes. The high elevations are exposed to extreme weather phenomena such as blizzards, avalanches and freezing rain, especially because of the region’s exposure to cold air masses from Eastern Europe [,].
The study focused on the vegetation of strictly calcareous screes, including those formed on limestone and calcareous conglomerates in the Southern Carpathians, with particular emphasis on the Caraiman valley, Bucegi (B), Marele Grohotis, Piatra Craiului (PC) and Raiosu, Făgăraș (F), massifs (Figure 1). The map was created using Google Earth Pro 7.3.6. software, Google Ireland Limited, Dublin, Ireland [].
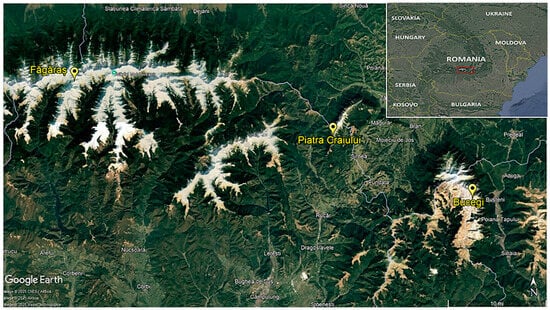
Figure 1.
Location of the study areas.
The floristic composition and vegetation community structure under extreme climatic conditions at altitudes above 1500 m were analysed. The exact elevation exposition, inclination and geographical location of each study site is given in the Supplementary Materials.
2.2. Field Methods
A robust methodological approach was employed to assess vegetation communities in diverse alpine areas across three Southern Carpathian massifs. Phytosociological surveys were conducted using the Braun-Blanquet method [], widely recognised for capturing plant community composition and abundance. We analysed 25 plots of 4 m2 of each massif, in the summer season. The collected data included detailed information such as locality, elevation, slope aspect, plot area, total vegetation cover and species abundance, assessed using the Braun-Blanquet scale. Species identification was based on World Flora Online standards [], supplemented by regional literature [] and expert knowledge. The study focused on the species C. lerchenfeldianum and P. corona-sancti-stephani.
We recorded life forms [] and specific ecological indicators (M-moisture, N-nitrogen, R = soil reaction, L-light, T-temperature) for each species []. Additionally, we analysed the floristic elements of the species in the study area [].
2.3. Data Analysis
We analysed the life forms and floristic elements across the three massifs to examine their distribution and variations among the identified plant communities [].
The statistical data analysis including ANOSIM, SIMPER, Kruskal–Wallis test and PCA were performed using the software package PAST 4.16c []. The Simpson index and Pielou evenness index were calculated using RStudio Desktop 2024.12.0-467, 2025 Posit Software PBC, Boston, MA, USA and Vegan packages, version 2.6-10, CRAN [,].
The overall significance of the difference between the three sites was assessed with multivariate test one-way ANOSIM based on the Bray–Curtis similarity index. Differences between groups are indicated by a large positive R (up to 1) []. The Bray–Curtis similarity index was used because it accounts for species cover []. Also, this index does not treat species’ joint absence in both communities as similarity []. This is particularly important given the low species numbers in our dataset. Moreover, Bray–Curtis is widely used in ecological studies for evaluation of community composition differences.
The ANOSIM test and diversity indices were performed after the transformation of the Braun-Blanquet scale values [] to percentage cover: + = 1.0%; 1 = 5%; 2 = 17%; 3 = 37%; 4 = 62%; 5 = 87%.
We use the SIMPER method to determine which taxa have principally contributed to observed variation across sample groups [].
The Kruskal–Wallis test was performed to analyse the difference between the plant life forms in the three massifs.
The study estimated mean species richness, the Simpsons index and the Pielou index.
The Simpson index measures the probability that two randomly selected individuals in a group belong to the same species []. The index was calculated as 1 − D [].
where = and N is the total number of counts.
The value of Simpson’s index falls between 0 and 1. In this index, limitless diversity is represented by 1 [,].
The Pielou evenness index (J) measures the proportion of observed diversity to maximum diversity [,],
where H′ is the Shannon index and S is the number of species in the community.
J′ = H′/log(S),
The PCA was conducted to examine the relationship between plant species and their preference for five variables—soil moisture (M), soil nitrogen (N), soil reaction (R), light (L) and temperature (T).
3. Results
A total of 62 vascular plant species were recorded: 32 B, 13 F, 42 PC (Table S1). Asteraceae and Caryophyllaceae have the highest number of species, with an important contribution from PC and B, whereas Poaceae and Saxifragaceae also show high diversity, with all three categories represented. Asteraceae has a strong presence of B, while Caryophyllaceae has a higher number of PC. Saxifragaceae and Rubiaceae show a more balanced contribution from all three groups (Figure 2).
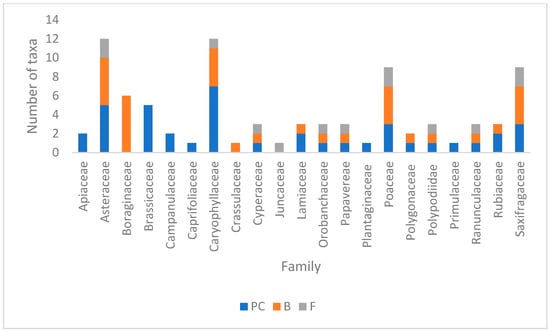
Figure 2.
Species families by massifs (PC-Piatra Craiului, B-Bucegi, F-Fagaras).
In this study, 75 relevés were conducted to analyse the scree vegetation communities. The number of taxa per plot ranged from 5 to 19, with an average of 11.93 taxa per plot. The surveyed plots were distributed across an elevational range of 1600 to 2200 m, covering various slope inclinations (10–65°) and expositions (V, VE, S, E, NE). The vegetation cover varied between 15% and 75%. Each plot had a uniform surface area of 4 m2, ensuring consistency in sampling.
Our analysis revealed that hemicryptophytes were the most abundant, comprising 75% of the total. Chamaephytes made up 16%, with the remaining 7% attributed to therophytes and 2% to geophytes (Table 1).

Table 1.
Life form percentage in studied massifs.
- H (Hemicryptophytes): perennial plants with regenerating shoots at ground level, protected by leaves or snow, adapted to cold or extreme climates.
- Ch (Chamaephytes): low, woody or semi-woody plants, with shoots close to the ground, adapted to dry and exposed environments. These plants, being low, are characteristic of extreme environments.
- Th (Therophytes): annual plants that survive the unfavorable season as seeds, characteristic of disturbed areas.
- G (Geophytes): Perennial plants that keep their regenerating organs underground (bulbs, tubers, rhizomes), adapted to variable conditions.The Kruskal–Wallis test indicated that the number of species belonging to each bioform (Ch, Th, H, and G) did not differ significantly among the three massifs.
The analysis of floristic elements is presented in Figure 3.
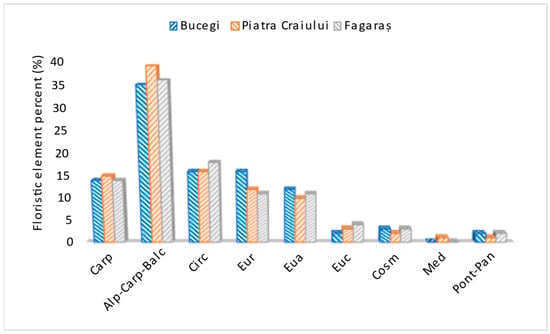
Figure 3.
Floristic elements in the three massifs.
- Carp (Carpathian): Endemic species, with restricted distribution or specific to the Carpathian Mountain range.
- Alp-Carp-Balc (Alpine-Carpathian-Balkan): Species with a common distribution in the Alps, Carpathians and Balkans.
- Circ (Circumpolar): Species distributed in cold or temperate.
- Eur (European): Species with a distribution restricted to Europe.
- Eua (Eurasian): Species distributed in both Europe and Asia.
- Euc (Europeo-Centrale): Species characteristic of Central Europe.
- Cosm (Cosmopolitan): Globally distributed species, adapted to different ecological conditions and found on most continents.
- Med (Mediterraneans): Species characteristic of the Mediterranean region, adapted to the warm and dry climates.
- Pont-Pan (Pontic-Pannonian): Species in the Pontic (around the Black Sea) and Pannonian (Pannonian Plain) regions.
The results highlight differences in ecological stability and community structure between the three massifs, with the PC massif representing the most diverse and resilient ecosystem.
The multivariate one-way test ANOSIM, based on the Bray–Curtis similarity index, highlighted the dissimilarities between sites (Table 2). The ANOSIM R-value was 0.593, and the mean rank between sites (184.9) was higher than the mean rank within sites (95.95).

Table 2.
ANOSIM dissimilarity between plants from the three sites. The values in the lower left corner are R-values, and in the upper right corner are Bonferroni-corrected p-values.
The SIMPER test points out an overall average dissimilarity of 63.13. Two species (C. lerchenfeldianum and P. corona-sancti-stephani) contributed to this dissimilarity (Table 3).

Table 3.
Responsible species for the observed difference between plants from the three sites.
The Pielou index has the highest value in Piatra Craiului, suggesting a more even distribution of species. Făgăraș has the lowest Pielou index value, indicating the dominance of some species over others.
The Simpson indices Piatra Craiului with the highest value (0.883), indicating high diversity and low species dominance. Făgăraș has the lowest value, again confirming a higher dominance of certain species (Figure 4).
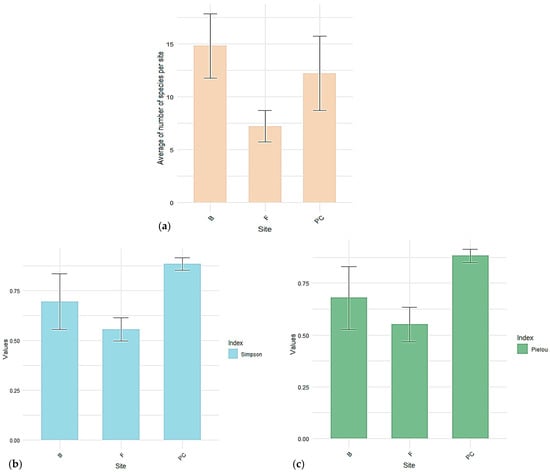
Figure 4.
The indices in the three massifs: (a) mean species richness, (b) the Simpsons’s index, (c) Pielou index.
Plant species were grouped by PCA analysis according to light (L), temperature (T), soil response (R), nitrogen (N) and moisture preferences (M) (Figure 5).
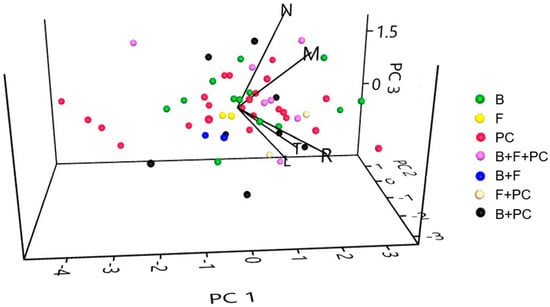
Figure 5.
The 3D scatter plot of PCA analysis of plant species in relationship to five indices—soil moisture (M), soil nitrogen (N), soil reaction (R), light (L) and temperature (T).
The first three Principal Components explain 81.985 percent of the total variance. Principal Component 1 (PC 1) is positively shaped by all variables, but the Principal Component 2 (PC 2) was strongly negatively influenced by soil reaction and positively by soil nitrogen. Principal component 3 (PC 3) was shaped positively especially by soil nitrogen and moisture and negatively by light and temperature (Table 4).

Table 4.
Eigenvalue and variance of principal factors and PCA loadings.
4. Discussion
The study identified 62 species of vascular plants. Asteraceae and Caryophyllaceae are the most diverse, with PC and B contributing most. Poaceae and Saxifragaceae also show high diversity across all massifs. The results of this study showed a high similarity with the general trends observed in the flora of the Alps and Corsica, in particular the dominance of Caryophyllaceae and Asteraceae. This suggests that the Alpine study area shares ecological characteristics with these alpine belts, favouring these plant families. Brassicaceae and Poaceae are present both in the alpine flora [] and in Corsica. However, their representation in our study (11% each) is more pronounced than in the Alpine flora, where they are less dominant. This could be explained by specific environmental conditions or different historical factors. However, for a more precise assessment of floristic similarity, further analysis at genus or species level would be needed.
The data showed that the PC massif is dominated by hemicryptophytes, while the B massif has a more balanced representation of chamaephytes. In contrast, F showed a higher proportion of therophytes and the presence of geophytes, indicating a different ecological dynamic, probably influenced by major disturbances or environmental pressures. Hemicryptophytes also dominate the mid-elevation areas of the Romanian Carpathians [] due to their ability to sprout from protected shoots, which provides resistance to seasonal changes and moderate disturbances. Other studies, such as Di Biase et al. [], have highlighted the adaptability of hemicryptophytes and chamaephytes to extreme conditions such as low temperatures and drought. Similarly, chamaephytes are predominantly found at high elevations, where their ground-level buds provide resilience to extreme cold and wind [,,,].
The alpine flora was dominated by endemic Carpathian (as Achillea schurii, Campanula kladniana, Dianthus callizonus) and montane species and circumpolar elements typical of alpine habitats []. European and Eurasian species added diversity, while rare elements reflected less significant influences. This biogeographical spectrum highlights the floristic links between the Carpathians, the Alps and the Balkans, while emphasising local specificities. Aeschimann et al. [] demonstrated the importance of calcareous substrates for alpine endemic species, with 83.2% of these species occurring on calcareous substrates. Similar patterns have been observed in southeastern France [], Corsica and Sardinia [], further emphasising the role of calcareous habitats in speciation. Anyway, speciation in calcareous habitats is influenced not only by substrate conditions but also by multiple factors, including the ‘island effect’, biogeographic junctions and ecosystem diversity, which together drive species diversification and adaptation.
The formation and persistence of these communities depend on several factors, including boulder size, exposure, slope and altitude. These communities grow as long as the slopes are continuously replenished with freshly disaggregated material, maintaining an active state of mobility. Such environments are typical of the alpine zone, where intense processes of rock disaggregation and erosion dominate [].
The results showed a moderate separation between the three massifs and a significant dissimilarity between PC and both B and F. Interspecific differences in water uptake [] and the effects of climate change [] could explain the differences in species composition. Preferences for uniform conditions were found, with some species negatively influenced by soil reaction but positively influenced by soil nitrogen and moisture, and others negatively influenced by light and temperature.
The Bucegi (B) species grow in warmer, sunnier habitats with minimal dependence on soil fertility or moisture. In contrast, the Făgăraș (F) species prefer humid habitats and are less dependent on temperature or light. Piatra Craiului (PC) species prefer cooler, wetter habitats with higher soil fertility. Common species from Făgăraș and Piatra Craiului grow in nutrient-rich, moist soils under varying light and temperature conditions. The species common to all three massifs have broad ecological niches, tolerating intermediate environmental gradients. These results are consistent with previous studies in the Swiss Alps [], where soil variables, particularly geochemical and drainage factors, significantly improved models of species distribution in montane regions []. Increased nutrient availability stimulates growth and flowering of generalist alpine species, potentially increasing their abundance and altering snowbed community structures [,,].
Conservation
The PC massif therefore emerges as the most stable and diverse habitat, characterised by high species richness and high species evenness, with no dominant species. This represents a mature, well-balanced ecosystem in which species are evenly distributed. Massif B presents an intermediate structure with moderate dominance. Although some species are more abundant, their presence is not extreme, and the relatively high equitability indicates that species distribution remains fairly even. Finally, massif F has the lowest species richness but also the lowest dominance. Thus, although few species are present, their abundance is relatively uniform, with no one species dominating.
Plant diversity and evenness were strongly correlated with corresponding community stability, suggesting that high levels of diversity lead to higher levels of complementary resource use via niche complementation [,,]. Alpine ecosystems currently support a limited number of non-native plant species, mainly due to the limited availability of propagules and the resilience of undisturbed native vegetation, which acts as a natural barrier to invasion. However, these constraints may diminish in the future as climate change and increasing anthropogenic pressures facilitate the dispersal of species to higher elevations [,]. While non-native species are already widespread at lower and middle elevations, ongoing environmental changes and human activities could allow their expansion into alpine zones, potentially altering these fragile ecosystems. Despite the nearly 200 non-native species recorded in alpine areas worldwide, research suggests that plant invasions have so far had relatively little impact on these environments [,].
In the Carpathians, the prevalence of alpine-Carpathian-Balkan floristic elements in the studied massifs highlights a common regional biodiversity, while the high proportion of endemic Carpathian species underlines the conservation importance of these areas. The increased presence of Pontic-Pannonian elements in the Făgăraș Mountains suggests a transitional or unique ecological character. The preservation of these floristic elements, especially the endemic ones and those shaped by the local microclimate, is crucial for the conservation of biodiversity [,].
Alpine plant communities on calcareous scree show remarkable diversity and resilience, shaped by both biotic and abiotic factors. However, they remain vulnerable to climate change, human disturbance and the potential spread of alien species. Biodiversity and species evenness are strongly linked to ecosystem stability, as greater diversity promotes resource partitioning and reduces competitive exclusion [,]. In calcareous soils, species adapted to harsh and unstable substrates show high ecological specialisation, which increases their resilience to environmental fluctuations []. By optimising resource use and reducing competition, these specialised plants contribute to the long-term stability of alpine ecosystems.
However, alpine meadow ecosystems face significant threats, which vary by region. While mining activities contribute to soil and vegetation disturbance in some areas [], tourism-related pressures, such as hiking and ski resort development, represent the primary threat in the European Alps []. To address these threats, researchers have proposed targeted conservation strategies to protect alpine meadows on the Qinghai–Tibetan Plateau []. In addition, the effectiveness of protected areas (PAs) in safeguarding biodiversity hotspots within alpine grasslands remains inadequate [,]. Reassessing and redefining PA boundaries based on biodiversity distribution patterns and buffer zones could improve conservation efforts and mitigate grassland degradation [].
5. Conclusions
This study highlights the distinct floristic composition and ecological dynamics of alpine habitats in the Piatra Craiului, Bucegi and Făgăraș massifs. The dominance of hemicryptophytes and chamaephytes reflects the adaptation of these species to extreme alpine conditions, characterised by low temperatures, strong winds and a short growing season. At the same time, variability in therophyte representation suggests different disturbance regimes influenced by factors such as geomorphological activity, grazing or local climatic variations.
Soil moisture appears as an important ecological factor in the distribution of species, leading to differences between massifs. This influence emphasises the role of microhabitat diversity in shaping alpine plant communities, highlighting how local environmental variations generate distinct patterns of floristic composition and ecological structure.
In the context of the increasing impacts of climate change on alpine ecosystems—through changing precipitation patterns, rising temperatures and retreating glaciers—understanding these ecological relationships becomes significant for predicting future changes in biodiversity and ecosystem functioning. In this context, conservation strategies should prioritise the conservation of habitat heterogeneity, as microenvironmental diversity provides an essential basis for the resilience and adaptability of alpine flora in the face of environmental change.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17040283/s1, Table S1: The synoptic table.
Author Contributions
Conceptualization, C.B.-N.; methodology, C.B.-N.; software, D.M.; validation, C.B.-N. and D.M.; investigation, C.B.-N. and O.G.P.; writing—original draft preparation, C.B.-N.; writing—review and editing, C.B.-N. and D.M.; visualization, C.B.-N.; supervision, C.B.-N., D.M. and O.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by projects numbers RO1567-IBB01/2024 and RO1567-IBB04/2024 from the Institute of Biology Bucharest of Romanian Academy.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| B | Bucegi Massif |
| PC | Piatra Craiului Massif |
| F | Făgăraș Massif |
References
- Körner, C. Mountain biodiversity, its causes and function: An overview. Mt. Biodivers. 2024, 33, 11–17. [Google Scholar]
- Leuschner, C.; Ellenberg, H. Vegetation of the alpine and nival belts. In Ecology of Central European Non-Forest Vegetation: Coastal to Alpine, Natural to Man-Made Habitats: Vegetation Ecology of Central Europe, Volume II; Springer: Cham, Switzerland, 2017; pp. 271–431. [Google Scholar]
- Varga, Z. Extra-Mediterranean refugia, post-glacial vegetation history and area dynamics in Eastern Central Europe. In Relict Species: Phylogeography and Conservation Biology; Habel, J.C., Assmann, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–87. [Google Scholar]
- Zheng, G.; Gu, J.; Zhao, W.; Zhang, Y.; Guan, Z.; Lei, M.; He, C. Spatial, Geographical, Climatic, and Edaphic Influences on Moss Community Structure: A Case Study from Qinhuangdao, China. Forests 2024, 15, 424. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. R. Soc. B Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef]
- Pils, G. Systematics, distribution, and karyology of the Festuca violacea Group (Poaceae) in the Eastern Alps. Plant Syst. Evol. 1980, 136, 73–124. [Google Scholar] [CrossRef]
- Kropf, M.; Comes, H.P.; Kadereit, J.W. Long-distance dispersal vs. vicariance: The origin and genetic diversity of alpine plants in the Spanish Sierra Nevada. New Phytol. 2006, 172, 169–184. [Google Scholar] [CrossRef]
- Parisod, C. Plant speciation in the face of recurrent climate changes in the Alps. Alp. Bot. 2022, 132, 21–28. [Google Scholar] [CrossRef]
- Vintsek, L.; Klichowska, E.; Nowak, A.; Nobis, M. Genetic differentiation, demographic history and distribution models of high alpine endemic vicariants outline the response of species to predicted climate changes in a Central Asian biodiversity hotspot. Ecol. Indic. 2022, 144, 109419. [Google Scholar] [CrossRef]
- The World Checklist of Vascular Plants (WCVP). Available online: www.gbif.org (accessed on 20 November 2024).
- Coldea, G.; Cristea, V. Description of the plant associations distinguished in the Retezat National Park. In The Vascular Plant Communities of the Retezat National Park (Southern Carpathians); Springer: Cham, Switzerland, 2022; pp. 85–245. [Google Scholar]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G. Vegetation of Europe: Hierarchical floristic classification system of vascular plant, bryophyte, lichen, and algal communities. Appl. Veg. Sci. 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Biţă-Nicolae, C.D. The screes vegetation of the higher basin of the Prahova river. Rom. J. Biol.-Plant Biol. 2009, 54, 157–172. [Google Scholar]
- Rodwell, J.S.; Schaminée, J.H.J.; Mucina, L.; Pignatti, S.; Dring, J.; Moss, D. The diversity of European vegetation. An overview of phytosociological alliances and their relationships to EUNIS habitats. Rep. EC-LNV 2002, 54, 168. [Google Scholar]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate change impacts in alpine environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Llambi, L.D.; Gámez, L.E.; Pelayo, R.; Azócar, C.J.; Torres, J.E.; Márquez, N.J.; Berdugo, M.B.; Cuesta, F.; Ramirez, L.A. Species, Growth Form, and Biogeographic Diversity of Summit Vegetation along an Elevation Gradient in the Tropical Andes: A Baseline for Climate Change Monitoring. J. Mt. Sci. 2022, 19, 3441–3457. [Google Scholar] [CrossRef]
- Squires, V.R.; Dengler, J.; Hua, L.; Feng, H. Grasslands of the Mediterranean Basin and the Middle East and their Management. In Grasslands of the World; CRC Press: Boca Raton, FL, USA, 2018; pp. 103–126. [Google Scholar]
- Aalto, J.; le Roux, P.C.; Luoto, M. Vegetation Mediates Soil Temperature and Moisture in Arctic-Alpine Environments. Arct. Antarct. Alp. Res. 2013, 45, 429–439. [Google Scholar] [CrossRef]
- Valachovic, M. Papaverion tatrici, a vicarious alliance of alpine limestone-scree communities in the Western Carpathians. Biol.-Bratisl. 1995, 50, 377–390. [Google Scholar]
- Onaca, A.; Ardelean, F.; Urdea, P.; Magori, B. Southern Carpathian rock glaciers: Inventory, distribution and environmental controlling factors. Geomorphology 2017, 293, 391–404. [Google Scholar] [CrossRef]
- Shijin, W.; Yuande, Y.; Yanjun, C. Global snow-and ice-related disaster risk: A review. Nat. Hazards Rev. 2022, 23, 03122002. [Google Scholar] [CrossRef]
- Google Earth Pro. 7.3.6. Available online: https://www.google.com/earth/about/versions/#earth-pro (accessed on 15 December 2024).
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde, 3rd ed.; Springer: Vienna, Austria; New York, NY, USA, 1964; 631p. [Google Scholar]
- World Flora Online. Available online: http://wfoplantlist.org/ (accessed on 20 November 2024).
- Biță-Nicolae, C.; Sanda, V. Cormophlora of Romania: Spontaneous and Cultivated Cormophytes in Romania; Lambert Academic Publishing: Saarbrucken, Germany, 2011. [Google Scholar]
- Ellenberg, H.H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 20 December 2024).
- RStudio Team. RStudio Desktop 2024.12.0-467: Integrated Development for; RStudio, PBC: Boston, MA, USA, 2020; Available online: https://posit.co/ (accessed on 12 February 2025).
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. R package version 2.6-10, 2025. Available online: https://CRAN.R-project.org/package=vegan (accessed on 12 February 2025).
- Bacaro, G.; Gioria, M.; Ricotta, C. Testing for differences in beta diversity from plot-to-plot dissimilarities. Ecol. Res. 2012, 27, 285–292. [Google Scholar] [CrossRef]
- Clarke, K.; Somerfield, P.; Chapman, M.G. On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray-Curtis coefficient for denuded assemblages. J. Exp. Mar. Biol. Ecol. 2006, 330, 55–80. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; 132p. [Google Scholar]
- Somerfield, P.; Clarke, K.; Warwick, R. Simpson Index. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3252–3255. [Google Scholar]
- Lande, R. Statistics and Partitioning of Species Diversity and Similarity among Multiple Communities. Oikos 1996, 76, 5–13. [Google Scholar] [CrossRef]
- Aeschimann, D.; Rasolofo, N.; Theurillat, J.P. Analyse de la flore des Alpes. 2: Biodiversité et chorologie. Candollea 2011, 66, 225–253. [Google Scholar] [CrossRef]
- Biță-Nicolae, C.; Florescu, L.I.; Purice, D.; Kaya, O. Riparian woody plant communities in the Romanian Carpathians: Species diversity and community structure of Salix and Hippophaë communities. Ecol. Evol. 2024, 14, e11361. [Google Scholar] [CrossRef] [PubMed]
- Di Biase, L.; Pace, L.; Mantoni, C.; Fattorini, S. Variations in Plant Richness, Biogeographical Composition, and Life Forms along an Elevational Gradient in a Mediterranean Mountain. Plants 2021, 10, 2090. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Vetaas, O.R. Variation in plant species richness of different life forms along a subtropical elevation gradient in the Himalayas, east Nepal. Glob. Ecol. Biogeogr. 2003, 12, 327–340. [Google Scholar] [CrossRef]
- Giménez, E.; Melendo, M.; Valle, F.; Gómez-Mercado, F.; Cano, E. Endemic flora biodiversity in the south of the Iberian Peninsula: Altitudinal distribution, life forms and dispersal modes. Biodivers. Conserv. 2004, 13, 2641–2660. [Google Scholar] [CrossRef]
- Mota, G.S.; Luz, G.R.; Mota, N.M.; Coutinho, E.S.; Veloso, M.D.D.M.; Fernandes, G.W.; Nunes, Y.R.F. Changes in species composition, vegetation structure, and life forms along an altitudinal gradient of rupestrian grasslands in south-eastern Brazil. Flora 2018, 238, 32–42. [Google Scholar] [CrossRef]
- Bita-Nicolae, C.; Yildiz, F.; Kaya, O. Exploring the biodiversity and conservation value of alpine grasslands in the Bucegi Massif, Romanian Carpathians. Sustainability 2023, 15, 12643. [Google Scholar] [CrossRef]
- Médail, F.; Verlaque, R. Ecological characteristics and rarity of endemic plants from southeast France and Corsica: Implications for biodiversity conservation. Biol. Conserv. 1997, 80, 269–281. [Google Scholar] [CrossRef]
- Fenu, G.; Fois, M.; Cañadas, E.M.; Bacchetta, G. Using endemic-plant distribution, geology and geomorphology in biogeography: The case of Sardinia (Mediterranean Basin). Syst. Biodivers. 2014, 12, 181–193. [Google Scholar] [CrossRef]
- Kullman, L. A richer, greener and smaller alpine world: Review and projection of warming-induced plant cover change in the Swedish Scandes. AMBIO 2010, 39, 159–169. [Google Scholar] [CrossRef]
- Escolar, C.; Martínez, I.; Bowker, M.A.; Maestre, F.T. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: Implications for ecosystem structure and functioning. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3087–3099. [Google Scholar] [CrossRef]
- Buri, A.; Grand, S.; Yashiro, E.; Adatte, T.; Spangenberg, J.E.; Pinto-Figueroa, E.; Verrecchia, E.; Guisan, A. What are the most crucial soil variables for predicting the distribution of mountain plant species? A comprehensive study in the Swiss Alps. J. Biogeogr. 2020, 47, 1143–1153. [Google Scholar] [CrossRef]
- Huston, M.A.; McBride, A.C. Evaluating the relative strengths of biotic versus abiotic controls on ecosystem processes. In Biodiversity and Ecosystem Functioning: Synthesis and Perspectives; Loreau, M., Naeem, S., Inchausti, P., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 47–60. [Google Scholar]
- Callaway, R.M.; Walker, L.R. Competition and facilitation: A synthetic approach to interactions in plant communities. Ecology 1997, 78, 1958–1965. [Google Scholar] [CrossRef]
- Tarnocai, C.; Canadell, J.G.; Schuur, E.A.; Kuhry, P.; Mazhitova, G.; Zimov, S. Soil organic carbon pools in the northern circumpolar permafrost region. Glob. Biogeochem. Cycles 2009, 23, GB2023. [Google Scholar] [CrossRef]
- Larcher, W.; Kainmüller, C.; Wagner, J. Survival types of high mountain plants under extreme temperatures. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 3–18. [Google Scholar] [CrossRef]
- Sturm, M.; Racine, C.; Tape, K. Increasing shrub abundance in the Arctic. Nature 2001, 411, 546–547. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Zavaleta, E.; Eviner, V.; Naylor, R.; Vitousek, P.; Reynolds, H.; Hooper, D.; Lavorel, S.; Sala, O.; Hobbie, S.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Anisimov, O.; Vaughan, D.G.; Callaghan, T.V.; Furgal, C.; Marchant, H.J.; Prowse, T.D.; Vilhjalmsson, H.; Walsh, J.E. Polar Regions (Arctic and Antarctic). In Climate Change 2007—Impacts, Adaptations and Vulnerability; Parry, M., Canziani, O., Palutikof, J., van der Linden, P., Hanson, C., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 653–685. [Google Scholar]
- Epstein, H.E.; Raynolds, M.K.; Walker, D.A.; Bhatt, U.S.; Tucker, C.J.; Pinzon, J.E. Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the past three decades. Environ. Res. Lett. 2012, 7, 015506. [Google Scholar] [CrossRef]
- Körner, C.; Hiltbrunner, E. Why is the alpine flora comparatively robust against climatic warming? Diversity 2021, 13, 383. [Google Scholar] [CrossRef]
- Sumner, E.E.; Venn, S.E. Thermal tolerance and growth responses to in situ soil water reductions among alpine plants. Plant Ecol. Divers. 2022, 15, 297–308. [Google Scholar] [CrossRef]
- Alexander, J.M.; Lembrechts, J.J.; Cavieres, L.A.; Daehler, C.C.; Haider, S.; Kueffer, C.; Liu, G.; McDougall, K.L.; Milbau, A.; Pauchard, A.; et al. Plant Invasions into Mountains and Alpine Ecosystems: Current Status and Future Challenges. Alp. Bot. 2016, 126, 89–103. [Google Scholar] [CrossRef]
- Hou, X.; Liu, S.; Zhao, S.; Dong, S.; Sun, Y.; Beazley, R. The alpine meadow around the mining areas on the Qinghai-Tibetan Plateau will degenerate as a result of the change of dominant species under the disturbance of open-pit mining. Environ. Pollut. 2019, 254, 113111. [Google Scholar] [CrossRef] [PubMed]
- Steiger, R.; Knowles, N.; Pöll, K.; Rutty, M. Impacts of climate change on mountain tourism: A review. J. Sustain. Tour. 2024, 32, 1984–2017. [Google Scholar] [CrossRef]
- Chapin III, F.S.; Sturm, M.; Serreze, M.C.; McFadden, J.P.; Key, J.R.; Lloyd, A.H.; Welker, J.M. Role of land-surface changes in Arctic summer warming. Science 2005, 310, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Tape, K.; Sturm, M.; Racine, C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Glob. Change Biol. 2006, 12, 686–702. [Google Scholar] [CrossRef]
- Callaghan, T.V.; Björn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Biodiversity, Distributions and Adaptations of Arctic Species in the Context of Environmental Change. AMBIO 2004, 33, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Haskell, D.E.; Flaspohler, D.J.; Webster, C.R.; Meyer, M.W. Variation in soil temperature, moisture, and plant growth with the addition of downed woody material on lakeshore restoration sites. Restor. Ecol. 2012, 20, 113–121. [Google Scholar] [CrossRef]
- Leng, X.; Cui, J.; Zhang, S.; Zhang, W.; Liu, Y.; Liu, S.; An, S. Differential water uptake among plant species in humid alpine meadows. J. Veg. Sci. 2013, 24, 138–147. [Google Scholar] [CrossRef]
- Li, C.; Peng, F.; Xue, X.; You, Q.; Lai, C.; Zhang, W.; Cheng, Y. Productivity and quality of alpine grassland vary with soil water availability under experimental warming. Front. Plant Sci. 2018, 9, 1790. [Google Scholar] [CrossRef]
- Nagy, L.; Grabherr, G. The Biology of Alpine Habitats; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Su, X.; Han, W.; Liu, G.; Zhang, Y.; Lu, H. Substantial gaps between the protection of biodiversity hotspots in alpine grasslands and the effectiveness of protected areas on the Qinghai-Tibetan Plateau, China. Agric. Ecosyst. Environ. 2019, 278, 15–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).