New Species and Old Semaphoronts: Updating Taxonomic Knowledge of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) with an Integrative Approach
Abstract
1. Introduction
2. Materials and Methods
3. Results
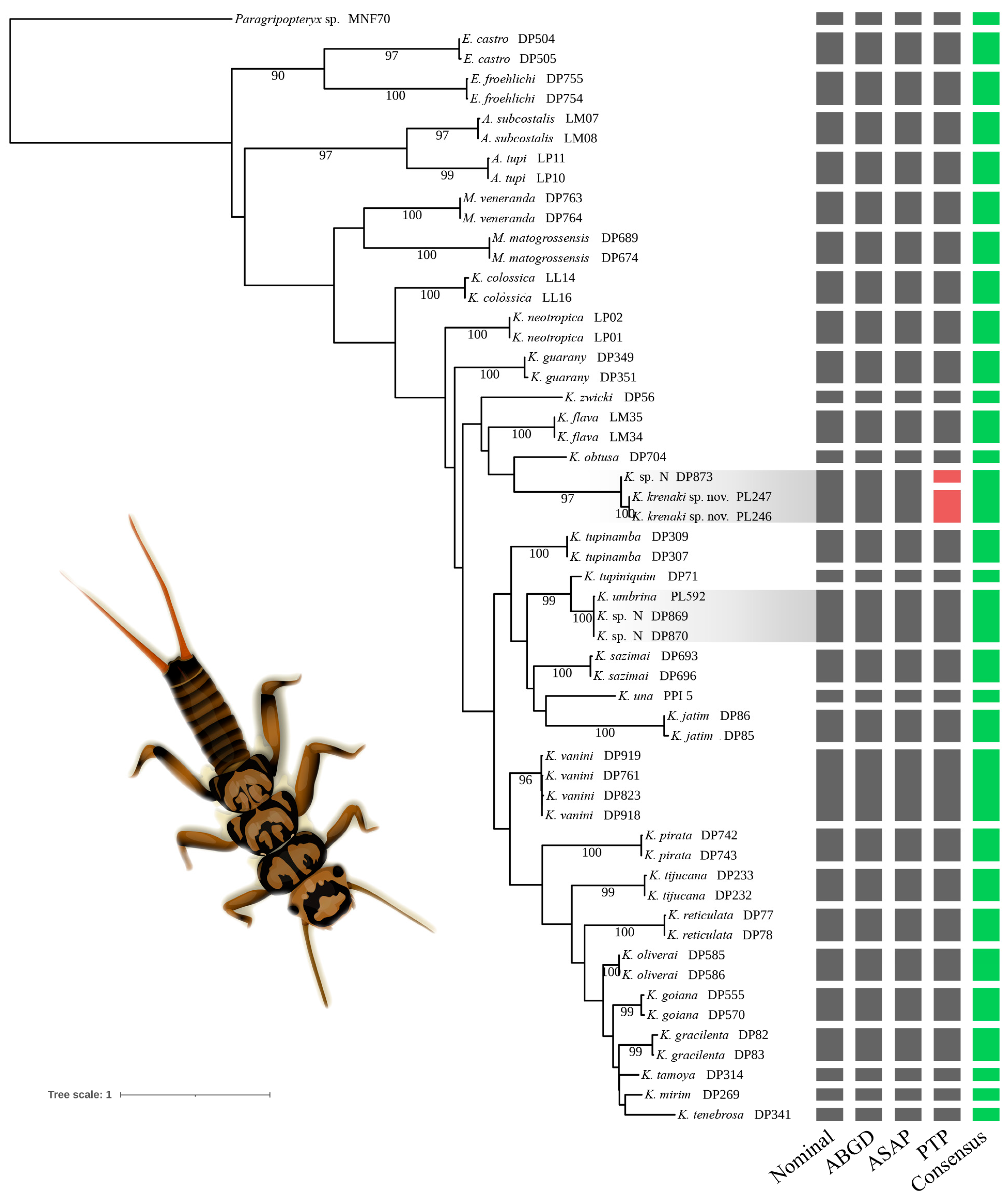
3.1. Molecular
3.2. Morphological Taxonomy
4. Discussion
| Species | COI | Life Stages | Distribution | ||
|---|---|---|---|---|---|
| M | F | N | |||
| K. alterosarum Froehlich, 1988 [21] | ● | ● | ● | ● | BA; MG; RJ. |
| K. auberti Froehlich, 1996 [42] | ● | PR; SP. | |||
| *K. brasilica (Navás, 1932) [44] | ● | RJ. | |||
| K. brasiliensis (Pictet, Kollar. Mss., 1841) [45] | ● | Brazil. | |||
| K. colossica (Navás, 1934) [7] | ● | ● | ● | ● | MG; PR; RJ; SC; SP. |
| K. couriae Avelino-Capistrano, Barbosa and Takiya, 2016 [8] | ● | ● | ● | ● | RJ. |
| K. flava Klapálek, 1916 [12] | ● | ● | ● | ES; MG; RJ; SP. | |
| K. goiana Bispo and Froehlich, 2004 [9] | ● | ● | GO; TO. | ||
| K. gracilenta (Enderlein, 1909) [10] | ● | ● | ● | ● | BA; ES; MG; RJ; SP. |
| K. guarany Almeida, Gonçalves and Bispo, 2024 [4] | ● | ● | SC. | ||
| K. guassu Froehlich, 1988 [21] | ● | ● | ● | RJ. | |
| K. jatim Froehlich, 1988 [21] | ● | ● | ● | BA; ES; MG; RJ; SP. | |
| *K. kaingang Froehlich, 2011 [35] | ● | ● | SC. | ||
| *K. klugii (Pictet, 1841) [45] | ● | Brazil. | |||
| K. krenaki sp. nov. Gastaldo, Almeida, Salles and Bispo | ● | ● | ● | MG. | |
| K. mirim Froehlich, 1984 [46] | ● | ● | ● | MG; SC; SP. | |
| K. neotropica (Jacobson and Bianchi, 1905) [11] | ● | ● | ● | ● | BA; ES; GO; MG; PR; RJ; RS; SC; SP. |
| K. obtusa Klapálek, 1916 [12] | ● | ● | ● | ● | ES; MG; RJ; SP. |
| *K. ocellata Froehlich, 2011 [35] | ● | RJ. | |||
| K. oliveirai Bispo and Froehlich, 2004 [9] | ● | ● | ● | GO. | |
| *K. petropolitana (Navás, 1929) [47] | ● | RJ. | |||
| K. pinhoi Froehlich, 2011b [43] | ● | ● | SC. | ||
| K. pirata Froehlich, 2011 [35] | ● | ● | ● | ES; RJ; SP. | |
| K. puri Avelino-Capistrano, Souza and Nessimian, 2013 [48] | ● | RJ. | |||
| K. reichardti Froehlich, 1984 [46] | ● | ● | MG; SP. | ||
| *K. remota (Banks, 1920) [49] | ● | RJ. | |||
| K. reticulata (Klapálek, 1916) [12] | ● | ● | ● | ● | ES. |
| K. sazimai Froehlich, 1988 [21] | ● | ● | MG. | ||
| K. serrana Navás, 1936 [50] | ● | ES; RJ; SP. | |||
| *K. sordida Klapálek, 1916 [12] | ● | Brazil. | |||
| K. tamoya Froehlich, 1984 [46] | ● | ● | ● | SP. | |
| *K. taunayi Navás, 1936 [50] | ● | RJ. | |||
| K. tenebrosa Klapálek, 1916 [12] | ● | ● | ● | RJ; SC. | |
| K. tijucana Dorvillé and Froehlich, 1997 [13] | ● | ● | ● | ● | RJ. |
| K. tupinamba Froehlich, 2011 [35] | ● | ● | ● | SP. | |
| K. tupiniquim Almeida, Gonçalves and Bispo, 2024 [4] | ● | ● | ES. | ||
| K. umbrina Froehlich, 1988 [21] | ● | ● | ● | ● | MG; RJ; SP. |
| K. una Almeida and Bispo, 2024 [4] | ● | ● | SP. | ||
| K. vanini Froehlich, 1988 [21] | ● | ● | ● | GO; MG; SP. | |
| K. varipes Klapálek, 1916 [12] | ● | ● | ● | RJ. | |
| K. zwicki Almeida, Gonçalves and Bispo, 2024 [4] | ● | ● | ES. | ||
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DeWalt, R.E.; Ower, G.D. Ecosystem Services, Global Diversity, and Rate of Stonefly Species Descriptions (Insecta: Plecoptera). Insects 2019, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Pessacq, P.; Zuñiga, M.D.C.; Duarte, T. An Updated Checklist of Neotropical Plecoptera. Zoosymposia 2019, 16, 182–209. [Google Scholar] [CrossRef]
- Klapálek, F. Analytická tabulka fam. Perlidae a její dvou subfam., Perlinae a Acroneuriinae (Plecoptera). Cas. Ceskoslovenské Spol. Entomol. 1914, 11, 53–69. [Google Scholar]
- de Almeida, L.H.; Gonçalves, M.C.; Bispo, P.d.C. An Integrative Approach to the Study of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) from Brazil: Support for the Description of Four New Species and a Basis for Future Studies. PLoS ONE 2024, 19, e0305824. [Google Scholar] [CrossRef]
- Stark, B.P.; Gaufin, A.R. The Neartic Genera of Perlidae (Plecoptera). Misc. Publ. Entomol. Soc. Am. 1976, 10, 1–80. [Google Scholar]
- Stark, B.P.; Froehlich, C.G.; Zúñiga, M.C. South American Stoneflies (Plecoptera). In Aquatic Biodiversity in Latin America; Adis, J., Arias, J.R., Rueda-Delgado, G., Wattzen, K.M., Eds.; Pensoft: Sofia, Bulgaria; Moscow, Russia, 2009; p. 154. [Google Scholar]
- Navás, L. Família Pérlidos. Insectos Suramericanos. Rev. Real Acad. Cienc. Madr. 1934, 31, 22–23. [Google Scholar]
- Avelino-Capistrano, F.; Barbosa, L.S.; Takiya, D.M. Description of a New Kempnyia Klapálek from Brazil (Plecoptera: Perlidae) with Life Stages Associated Using DNA Barcodes. Zootaxa 2016, 4079, 372–380. [Google Scholar] [CrossRef]
- Bispo, P.C.; Froehlich, C.G. The First Records of Kempnyia (Plecoptera: Perlidae) from Central Brazil, with Descriptions of New Species. Aquat. Insects 2004, 530, 1–7. [Google Scholar] [CrossRef]
- Enderlein, G. Klassifikation Der Plecopteren Sowie Diagnosen Neuer Gattungen Und Arten. Zool. Anz. 1909, 34, 385–419. [Google Scholar]
- Jacobson, G.G.; Bianchi, V.L. Orthoptera and Pseudoneuroptera from the Russian Empire and Neighboring Lands; Izdanie A. F. Devriena: Leningrad, Russia, 1905; 952p. [Google Scholar]
- Klapálek, F. Subfamilia Acroneuriinae Klp. Časopis České Společnosti Entomol. 1916, 13, 45–84. [Google Scholar]
- Dorvillé, L.F.M.; Froehlich, C.G. Kempnyia Tijucana Sp.n. from Southeastern Brazil (Plecoptera, Perlidae). Aquat. Insects 1997, 19, 177–181. [Google Scholar] [CrossRef]
- Dorvillé, L.F.M.; Froehlich, C.G. Description of the Nymph of Kempnyia tijucana Dorvillé and Froehlich (Plecoptera, Perlidae), with Notes on Its Development and Biology. In Trends in Research in Ephemeroptera and Plecoptera; Domínguez, E., Ed.; Kluwer Academic/Plenum: New York, NY, USA, 2001; pp. 385–392. [Google Scholar]
- Bispo, P.C.; Froehlich, C.G. Description of the Larva and Redescription of the Adult of Kempnyia neotropica (Jacobsen and Bianchi) (Plecoptera: Perlidae) with Biological Notes. Aquat. Insects 2008, 30, 61–67. [Google Scholar] [CrossRef]
- Avelino-Capistrano, F.; Barbosa, L.S.; Almeida, G.L. Complementary Descriptions of Kempnyia gracilenta (Enderlein 1909) and Kempnyia Reticulata (Klapálek 1916) (Plecoptera: Perlidae). Illiesia 2011, 7, 142–147. [Google Scholar]
- Bispo, P.C.; Cardoso-Leite, R.; Lecci, L.S. Description of the Larva of Kempnyia colossica (Navás) (Plecoptera: Perlidae) with Biological Notes. Aquat. Insects 2013, 34, 217–221. [Google Scholar] [CrossRef]
- Rippel, M.L.S.; Novaes, M.C.; Krolow, T.K. First Records of Kempnyia and Macrogynoplax (Plecoptera: Perlidae) from Tocantins State, Brazil with Description of the Immatures and the Adult Female. Zootaxa 2019, 4700, 471–478. [Google Scholar] [CrossRef]
- Avelino-Capistrano, F.; Nessimian, J.L.; Santos-Mallet, J.R.; Takiya, D.M. DNA Based Identification and Descriptions of Immatures of Kempnyia Klapálek (Insecta: Plecoptera) from Macaé River Basin, Rio de Janeiro State, Brazil. Freshw. Sci. 2014, 33, 325–337. [Google Scholar] [CrossRef]
- de Almeida, L.H.; Bispo, P.d.C. Perlidae (Plecoptera) from the Paranapiacaba Mountains, Atlantic Forest, Brazil: Diversity and Implications of the Integrative Approach and Teneral Specimens on Taxonomy. PLoS ONE 2020, 15, e0243393. [Google Scholar] [CrossRef]
- Froehlich, C.G. Brazilian Plecoptera 5. Old and New Species of Kempnyia (Perlidae). Aquat. Insects 1988, 10, 153–170. [Google Scholar] [CrossRef]
- Shepard, W.D.; Stewart, K.W. A Comparative Study of Nymphal Gills in North American Stonefly (Plecoptera) Genera and a New, Proposed Paradigm of Plecoptera Gill Evolution. Entomol. Soc. Am. 1983, 13, 1–58. [Google Scholar]
- Gastaldo, R.B.; de Almeida, L.H.; Salles, F.F. On Anacroneuriini (Plecoptera: Perlidae) Morphology: Standardizing Vocabulary and a Morphological Atlas. Arthropod Struct. Dev. 2025, 86, 101427. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S.; DeWaard, J.R. Barcoding Animal Life: Cytochrome c Oxidase Subunit 1 Divergences among Closely Related Species. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological Identifications through DNA Barcodes. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the Sensitivity of Progressive Multiple Sequence Alignment through Sequence Weighting, Position-Specific Gap Penalties and Weight Matrix Choice. Nucleid Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble Species by Automatic Partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A General Species Delimitation Method with Applications to Phylogenetic Placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Stark, B.P. A Synopsis of Neotropical Perlidae (Plecoptera). In Trends in Research in Ephemeroptera and Plecoptera; Dominguez, E., Ed.; Kluwer Academic Plenum Publisher: New York, NY, USA, 2001; pp. 405–422. [Google Scholar]
- Froehlich, C.G. Catalogue of Neotropical Plecoptera. Illiesia 2010, 6, 118–205. [Google Scholar]
- Froehlich, C.G. Checklist Dos Plecoptera (Insecta) Do Estado de São Paulo, Brasil. Biota Neotropica 2011, 11, 601–606. [Google Scholar] [CrossRef]
- Taniguti, P.N.; Sarmento, F.R.P.; Oliveira, R.; Cardoso, A.; Almeida, L.H. De Perlidae (Plecoptera) Do Estado de São Paulo, Brasil: Lista de Espécies, Problemas Taxonômicos e Amostragem. Acta Biol. Bras. 2023, 6, 5–14. [Google Scholar] [CrossRef]
- Almeida, L.H.; Gonçalves, M.C.; Novaes, M.C.; Paresqui, R.C.; Bispo, P.C. Anacroneuria flintorum Froehlich 2002 (Plecoptera: Perlidae): Notes, Distribution, and Life Stages Association Using Molecular Tools. Zootaxa 2018, 4370, 409–420. [Google Scholar] [CrossRef]
- de Almeida, L.H.; Duarte, T.; Bispo, P.d.C. Complementary Studies of the Perlidae (Insecta: Plecoptera) Fauna from the Paranapiacaba Mountains Using DNA Barcode Data. Zootaxa 2024, 5496, 500–508. [Google Scholar] [CrossRef]
- Faria, L.R.R.; Pie, M.R.; Salles, F.F.; Soares, E.D.G. The Haeckelian Shortfall or the Tale of the Missing Semaphoronts. J. Zool. Syst. Evol. Res. 2021, 59, 359–369. [Google Scholar] [CrossRef]
- Molineri, C.; Romero, F.; González, J.C.; Zúñiga, M.d.C. New Species and New Stages of Anacroneuria (Plecoptera: Perlidae) from Southern Yungas (Argentina and Bolivia). Rev. Soc. Entomol. Argentina 2023, 82, 1–23. [Google Scholar] [CrossRef]
- de Almeida, L.H.; Taniguti, P.N.; Lopez, V.M.; Bispo, P.d.C. Taxonomic Contributions on Macrogynoplax Enderlein and Enderleina Jewett (Plecoptera: Perlidae) from Brazil, Including a New Species Supported by Molecular and Morphological Data. Neotrop. Entomol. 2023, 52, 1119–1128. [Google Scholar] [CrossRef]
- Froehlich, C.G. Two New Species of Kempnyia from Southern Brazil (Plecoptera: Perlidae). Mitteilungen Schweizerische Entomol. Gesellschaft 1996, 69, 117–120. [Google Scholar]
- Froehlich, C.G. Kempnyia (Plecoptera) from the Mantiqueira Mountains of Brazil. Zootaxa 2011, 2999, 20–32. [Google Scholar] [CrossRef]
- Navás, L. Insectos suramericanos. Serie 06. Plecópteros. Rev. la Real Acad. Ciencias Madrid 1932, 29, 52–66. [Google Scholar]
- Pictet, F.J. Histoire Naturelle Générale et Particulière des Insectes Névroptères; Familie des Perlides, Ed.; J. Kessmann: Genève, Switzerland, 1841; Volume 1, Issue 1; p. 423. [Google Scholar]
- Froehlich, C.G. Brazilian Plecoptera 2. Species of the serrana-group of Kempnyia (Plecoptera). Aquat. Insects 1984, 6, 137–147. [Google Scholar] [CrossRef]
- Navás, L. Insectos del Museo de Hamburgo. 2.a serie. Bol. Soc. Ent. Esp. 1929, 12, 73–99. [Google Scholar]
- Avelino-Capistrano, F.; Souza, M.R.D.; Nessiman, J.L. Kempnyia puri, a new species of Perlidae (Plecoptera) from Rio de Janeiro, Brazil. Zootaxa 2013, 3619, 554–556. [Google Scholar]
- Banks, N. New Neuropteroid Insects. Bull. Mus. Comp. Zool. 1920, 64, 299–362. [Google Scholar]
- Navás, L. Insectos del Brasil. 5.a serie. Rev. Mus. Paul. 1936, 20, 721–734. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]




| Species (Life Stage) | Voucher Code | Collection | COI GenBank Accession |
|---|---|---|---|
| Kempnyia krenaki sp. nov. (M) | PL00246 | UFVB | PV553778 |
| Kempnyia krenaki sp. nov. (M) | PL00247 | UFVB | PV553777 |
| Kempnyia krenaki sp. nov. (N) | DP873 | CIACGF | PV553779 |
| Kempnyia umbrina (M) | PL00592 | UFVB | PV553774 |
| Kempnyia umbrina (N) | DP869 | CIACGF | PV553776 |
| Kempnyia umbrina (N) | DP870 | CIACGF | PV553775 |
| Kempnyia vanini (M) | DP918 | CIACGF | PV553772 |
| Kempnyia vanini (M) | DP919 | CIACGF | PV553773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, L.H.d.; Gastaldo, R.B.; Salles, F.F.; Bispo, P.d.C. New Species and Old Semaphoronts: Updating Taxonomic Knowledge of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) with an Integrative Approach. Diversity 2025, 17, 416. https://doi.org/10.3390/d17060416
Almeida LHd, Gastaldo RB, Salles FF, Bispo PdC. New Species and Old Semaphoronts: Updating Taxonomic Knowledge of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) with an Integrative Approach. Diversity. 2025; 17(6):416. https://doi.org/10.3390/d17060416
Chicago/Turabian StyleAlmeida, Lucas Henrique de, Rodrigo Braga Gastaldo, Frederico Falcão Salles, and Pitágoras da Conceição Bispo. 2025. "New Species and Old Semaphoronts: Updating Taxonomic Knowledge of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) with an Integrative Approach" Diversity 17, no. 6: 416. https://doi.org/10.3390/d17060416
APA StyleAlmeida, L. H. d., Gastaldo, R. B., Salles, F. F., & Bispo, P. d. C. (2025). New Species and Old Semaphoronts: Updating Taxonomic Knowledge of Kempnyia Klapálek, 1914 (Plecoptera: Perlidae) with an Integrative Approach. Diversity, 17(6), 416. https://doi.org/10.3390/d17060416







