Lots of Lancelets or Not? Diversity of Cephalochordates in the Tropical Eastern Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and Sequencing
2.3. Analysis of DNA Sequences
3. Results
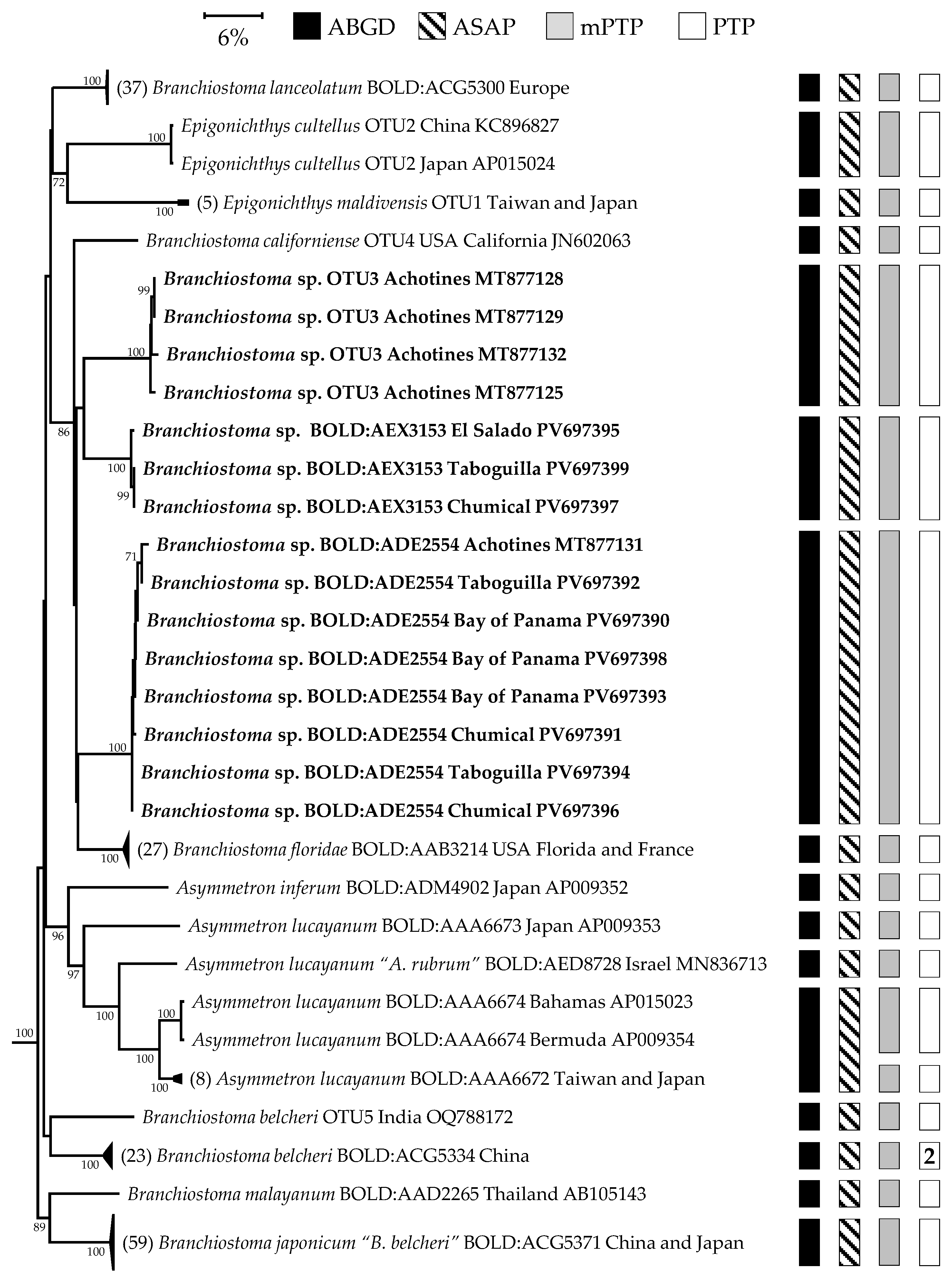
3.1. DNA Barcoding and Phylogeny of Branchiostoma Species from the Americas
3.2. Morphological Descriptions of Panamanian Branchiostoma Species
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, Z.; Xie, Y.; Fang, Q.; Li, J.; Liu, J.; Wang, J.; Huang, L.; Qu, T.; Xie, W. Current Population, Habitat Status and Species of Amphioxus in Dongshan Bay, Fujian Province. J. Ocean. Univ. China 2025, 24, 417–426. [Google Scholar] [CrossRef]
- Barboza, C.A.D.M.; Hadlich, H.L.; Sandrini-Neto, L.; Martins, C.D.C.; Lana, P.D.C. Is the Distribution of the Lancelet Branchiostoma caribaeum Affected by Sewage Discharges? An Analysis at Multiple Scales of Variability. Mar. Pollut. Bull. 2013, 69, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Campos-Dávila, L.; Pérez-Estrada, C.J.; Rodríguez-Estrella, R.; Morales-Bojórquez, E.; Brun-Murillo, F.G.; Balart, E.F. Seagrass Halodule wrightii as a New Habitat for the Amphioxus Branchiostoma californiense (Cephalochordata, Branchiostomidae) in the Southern Gulf of California, Mexico. ZooKeys 2019, 873, 113–131. [Google Scholar] [CrossRef]
- Kon, T.; Nohara, M.; Yamanoue, Y.; Fujiwara, Y.; Nishida, M.; Nishikawa, T. Phylogenetic Position of a Whale-Fall Lancelet (Cephalochordata) Inferred from Whole Mitochondrial Genome Sequences. BMC Evol. Biol. 2007, 7, 127. [Google Scholar] [CrossRef]
- Nishikawa, T. A New Deep-Water Lancelet (Cephalochordata) from off Cape Nomamisaki, SW Japan, with a Proposal of the Revised System Recovering the Genus Asymmetron. Zool. Sci. 2004, 21, 1131–1136. [Google Scholar] [CrossRef][Green Version]
- Rodríguez-Uribe, M.C.; Chávez-Dagostino, R.M.; Del Moral-Flores, L.F.; Bravo-Olivas, M.L. First Record of Amphioxus Branchiostoma californiense (Amphioxiformes: Branchiostomatidae) Adjacent to a Shallow Submarine Hydrothermal System at Banderas Bay (Mexico). Diversity 2019, 11, 227. [Google Scholar] [CrossRef]
- Bertrand, S.; Escriva, H. Evolutionary Crossroads in Developmental Biology: Amphioxus. Development 2011, 138, 4819–4830. [Google Scholar] [CrossRef]
- Holland, L.Z.; Albalat, R.; Azumi, K.; Benito-Gutiérrez, È.; Blow, M.J.; Bronner-Fraser, M.; Brunet, F.; Butts, T.; Candiani, S.; Dishaw, L.J.; et al. The Amphioxus Genome Illuminates Vertebrate Origins and Cephalochordate Biology. Genome Res. 2008, 18, 1100–1111. [Google Scholar] [CrossRef]
- Holland, L.Z.; Holland, N.D. Cephalochordates: A Window into Vertebrate Origins. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2021; Volume 141, pp. 119–147. ISBN 978-0-12-814968-3. [Google Scholar]
- Marlétaz, F.; Firbas, P.N.; Maeso, I.; Tena, J.J.; Bogdanovic, O.; Perry, M.; Wyatt, C.D.R.; De La Calle-Mustienes, E.; Bertrand, S.; Burguera, D.; et al. Amphioxus Functional Genomics and the Origins of Vertebrate Gene Regulation. Nature 2018, 564, 64–70. [Google Scholar] [CrossRef]
- Bi, C.; Lu, N.; Huang, Z.; Chen, J.; He, C.; Lu, Z. Whole-genome Resequencing Reveals the Pleistocene Temporal Dynamics of Branchiostoma belcheri and Branchiostoma floridae Populations. Ecol. Evol. 2020, 10, 8210–8224. [Google Scholar] [CrossRef]
- Igawa, T.; Nozawa, M.; Suzuki, D.G.; Reimer, J.D.; Morov, A.R.; Wang, Y.; Henmi, Y.; Yasui, K. Evolutionary History of the Extant Amphioxus Lineage with Shallow-Branching Diversification. Sci. Rep. 2017, 7, 1157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-L.; Zhang, G.-L.; Yuan, M.-L.; Dong, Z.-X.; Li, H.-W.; Guo, J.; Wang, F.; Deng, X.-Y.; Chen, J.-Y.; Lin, L.-B. A Phylogenomic Framework and Divergence History of Cephalochordata Amphioxus. Front. Physiol. 2018, 9, 1833. [Google Scholar] [CrossRef] [PubMed]
- Caccavale, F.; Osca, D.; D’Aniello, S.; Crocetta, F. Molecular Taxonomy Confirms That the Northeastern Atlantic and Mediterranean Sea Harbor a Single Lancelet, Branchiostoma lanceolatum (Pallas, 1774) (Cephalochordata: Leptocardii: Branchiostomatidae). PLoS ONE 2021, 16, e0251358. [Google Scholar] [CrossRef]
- Kon, T.; Nohara, M.; Nishida, M.; Sterrer, W.; Nishikawa, T. Hidden Ancient Diversification in the Circumtropical Lancelet Asymmetron lucayanum Complex. Mar. Biol. 2006, 149, 875–883. [Google Scholar] [CrossRef]
- Subirana, L.; Farstey, V.; Bertrand, S.; Escriva, H. Asymmetron Lucayanum: How Many Species Are Valid? PLoS ONE 2020, 15, e0229119. [Google Scholar] [CrossRef]
- WoRMS Editorial Board World Register of Marine Species. VLIZ. Available online: https://www.marinespecies.org (accessed on 13 March 2025).
- Lewin, H.A.; Richards, S.; Lieberman Aiden, E.; Allende, M.L.; Archibald, J.M.; Bálint, M.; Barker, K.B.; Baumgartner, B.; Belov, K.; Bertorelle, G.; et al. The Earth BioGenome Project 2020: Starting the Clock. Proc. Natl. Acad. Sci. USA 2022, 119, e2115635118. [Google Scholar] [CrossRef]
- Poss, S.G.; Boschung, H.T. Lancelets (Cephalochordata: Branchiostomatidae): How Many Species Are Valid? Isr. J. Zool. 1996, 42, S13–S66. [Google Scholar]
- Del Moral-Flores, L.F.; Guadarrama-Martínez, M.Á.; Flores-Coto, C. Taxonomic Composition and Distribution of Cephalochordates (Cephalochordata: Amphioxiformes) from Mexico. Lat. Am. J. Aquat. Res. 2017, 44, 497–503. [Google Scholar] [CrossRef]
- Galván-Villa, C.M.; Ríos-Jara, E.; Ayón-Parente, M. New Records of the Californian Lancelet Branchiostoma Californiense (Cephalochordata: Branchiostomidae) from the Pacific Coast of Mexico. Rev. Mex. Biodivers. 2017, 88, 995–998. [Google Scholar] [CrossRef]
- Hubbs, C.L. A List of the Lancelets of the World with Diagnosis of Five New Species of Branchiostoma. In Occasional Papers of the Museum of Zoology; University of Michigan: Ann Arbor, MI, USA, 1922; Volume 105, pp. 1–16. [Google Scholar]
- Kirkaldy, T.W. A Revision of the Genera and Species of the Branchiostomidae. J. Cell Sci. 1895, 2, 303–324. [Google Scholar] [CrossRef]
- Global Biodiversity Information Facility GBIF. Occurrence Download 2025. The Dataset Includes 7838 Records from 269 Constituent Datasets. Available online: https://www.gbif.org/occurrence/download/0007176-250310093411724 (accessed on 13 March 2025).
- Barraza Sandoval, J.E.; Melara Pérez, V.E. New Records of Branchiostoma californiense (Amphioxiformes: Branchiostomatidae) in El Salvador. Cuad. Investig. UNED 2022, 14, e4302. [Google Scholar] [CrossRef]
- Mujica, A.; Nava, M.L.; Leiva-Dietz, F. On the Presence of Branchiostoma elongatum Juveniles (Cephalochordata: Branchiostomatidae) on the North-Central Coast of Chile. Lat. Am. J. Aquat. Res. 2022, 50, 622–627. [Google Scholar] [CrossRef]
- Velázquez-Velázquez, E. First Record of Branchiostoma californiense (Cephalochordata: Branchiostomatidae) in a Lagoon-Estuarine System from Chiapas, Mexico. Hidrobiológica 2021, 30, 107–110. [Google Scholar] [CrossRef]
- Vergara, M.; Oliva, M.E.; Riascos, J.M. Population Dynamics of the Amphioxus Branchiostoma elongatum from Northern Chile. J. Mar. Biol. Ass. 2012, 92, 591–599. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Macdonald, K.; Driskell, A.C.; Boyle, M.J. Knots, Spoons, and Cloches: DNA Barcoding Unusual Larval Forms Helps Document the Diversity of Neotropical Marine Annelids. Invertebr. Biol. 2021, 140, e12311. [Google Scholar] [CrossRef]
- Collin, R.; Madrid, M.; Venera-Pontón, D.E.; Macdonald, K.S.; De León, A.; Vrdoljak, D.; Boyle, M.J.; Bryant, P.; Arehart, T.; Driskell, A.C. Diversity and Genetic Connectivity of Heteropod (Pterotracheoidea) Gastropods in the Tropical Eastern Pacific. Invertebr. Biol. 2023, 142, e12395. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Paulay, G.; Boyle, M.J. World Travelers: DNA Barcoding Unmasks the Origin of Cloning Asteroid Larvae from the Caribbean. Biol. Bull. 2020, 239, 73–79. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Driskell, A.C.; Macdonald, K.S.; Boyle, M.J. Unexpected Molecular and Morphological Diversity of Hemichordate Larvae from the Neotropics. Invertebr. Biol. 2019, 138, e12273. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Driskell, A.C.; Macdonald, K.S.; Boyle, M.J. How I Wonder What You Are: Can DNA Barcoding Identify the Larval Asteroids of Panama? Invertebr. Biol. 2020, 139, e12303. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Driskell, A.C.; Macdonald, K.S.; Chan, K.K.; Boyle, M.J. Documenting Neotropical Diversity of Phoronids with DNA Barcoding of Planktonic Larvae. Invertebr. Biol. 2019, 138, e12242. [Google Scholar] [CrossRef]
- Collin, R.; Venera-Pontón, D.E.; Driskell, A.C.; Macdonald, K.S.; Geyer, L.B.; Lessios, H.A.; Boyle, M.J. DNA Barcoding of Echinopluteus Larvae Uncovers Cryptic Diversity in Neotropical Echinoids. Invertebr. Biol. 2020, 139, e12292. [Google Scholar] [CrossRef]
- Urata, M.; Yamaguchi, N.; Henmi, Y.; Yasui, K. Larval Development of the Oriental Lancelet, Branchiostoma belcheri, in Laboratory Mass Culture. Zool. Sci. 2007, 24, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Geller, J.; Meyer, C.; Parker, M.; Hawk, H. Redesign of PCR Primers for Mitochondrial Cytochrome c Oxidase Subunit I for Marine Invertebrates and Application in All-taxa Biotic Surveys. Mol. Ecol. Resour. 2013, 13, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.P. Molecular Systematics of Cowries (Gastropoda: Cypraeidae) and Diversification Patterns in the Tropics. Biol. J. Linn. Soc. 2003, 79, 401–459. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Benzie, J. Large Mitochondrial DNA Differences between Morphologically Similar Penaeid Shrimp. Mol. Mar. Biol. Biotechnol. 1991, 1, 27–34. [Google Scholar]
- Arnedo, M.A.; Oromí, P.; Ribera, C. Radiation of the Spider Genus Dysdera (Araneae, Dysderidae) in the Canary Islands: Cladistic Assessment Based on Multiple Data Sets. Cladistics 2001, 17, 313–353. [Google Scholar] [CrossRef]
- Schubart, C.D.; Cuesta, J.A.; Felder, D.L. Glyptograpsidae, a New Brachyuran Family from Central America: Larval and Adult Morphology, and a Molecular Phylogeny of the Grapsoidea. J. Crustac. Biol. 2002, 22, 28–44. [Google Scholar] [CrossRef]
- Sevigny, J.; Leasi, F.; Simpson, S.; Di Domenico, M.; Jörger, K.M.; Norenburg, J.L.; Thomas, W.K. Target Enrichment of Metazoan Mitochondrial DNA with Hybridization Capture Probes. Ecol. Indic. 2021, 121, 106973. [Google Scholar] [CrossRef]
- Katoh, K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis Version 12 for Adaptive and Green Computing. Mol. Biol. Evol. 2024, 41, msae263. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.L.; Valdivia, P.; Rosas-Puchuri, U.; Valdivia, N.L. SPdel: A Pipeline to Compare and Visualize Species Delimitation Methods for Single-locus Datasets. Mol. Ecol. Resour. 2023, 23, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for Primary Species Delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble Species by Automatic Partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A General Species Delimitation Method with Applications to Phylogenetic Placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-Rate Poisson Tree Processes for Single-Locus Species Delimitation under Maximum Likelihood and Markov Chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2016, 34, 772–773. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Carvalho, J.E.; Lahaye, F.; Yong, L.W.; Croce, J.C.; Escrivá, H.; Yu, J.-K.; Schubert, M. An Updated Staging System for Cephalochordate Development: One Table Suits Them All. Front. Cell Dev. Biol. 2021, 9, 668006. [Google Scholar] [CrossRef]
- Vargas, J.A.; Dean, H.K. On Branchiostoma californiense (Cephalochordata) from the Gulf of Nicoya Estuary, Costa Rica. Rev. Biol. Tropical 2010, 58, 1143–1148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sibaja-Cordero, J.A.; Troncoso, J.S.; Cortés, J. The Lancelet Asymmetron lucayanum Complex in Cocos Island National Park, Pacific Costa Rica. Pac. Sci. 2012, 66, 523–528. [Google Scholar] [CrossRef]
- Meerhoff, E.; Veliz, D.; Vega-Retter, C.; Yannicelli, B. The Amphioxus Epigonichthys maldivensis (Forster Cooper, 1903) (Cephalochordata Branchiostomatidae) Larvae in the Plankton from Rapa Nui (Chile) and Ecological Implications. Biodivers. J. 2016, 7, 7–11. [Google Scholar]
- Ellison, C.I.; Frey, M.R.; Sanford, E.; Maslakova, S. Ribbon Worms (Phylum Nemertea) from Bodega Bay, California: A Largely Undescribed Diversity. ZooKeys 2024, 1204, 15–64. [Google Scholar] [CrossRef]
- Chen, Y. The Ecology and Biology of Amphioxus in Hong Kong. Ph.D. Thesis, Department of Biology and Chemistry, City University of Hong Kong, Hong Kong, China, 2007. [Google Scholar]
- Au, M.F.F.; Au, H.M.; Chu, W.K.V.; Kwok, C.K.; Cheung, S.G.; Leung, K.M.Y.; Qiu, J.-W. Spatial Distribution, Abundance, Seasonality and Environmental Relationship of Amphioxus in Subtropical Hong Kong Waters. Reg. Stud. Mar. Sci. 2023, 57, 102726. [Google Scholar] [CrossRef]
- Holland, N.D.; Holland, L.Z.; Heimberg, A. Hybrids Between the Florida Amphioxus (Branchiostoma floridae) and the Bahamas Lancelet (Asymmetron lucayanum): Developmental Morphology and Chromosome Counts. Biol. Bull. 2015, 228, 13–24. [Google Scholar] [CrossRef]
- Holland, N.D.; Holland, L.Z. The Ups and Downs of Amphioxus Biology: A History. Int. J. Dev. Biol. 2017, 61, 575–583. [Google Scholar] [CrossRef]
- Krug, P.J.; Vendetti, J.E.; Rodriguez, A.K.; Retana, J.N.; Hirano, Y.M.; Trowbridge, C.D. Integrative Species Delimitation in Photosynthetic Sea Slugs Reveals Twenty Candidate Species in Three Nominal Taxa Studied for Drug Discovery, Plastid Symbiosis or Biological Control. Mol. Phylogenet. Evol. 2013, 69, 1101–1119. [Google Scholar] [CrossRef]
- Krämer, D.; Schmidt, C.; Podsiadlowski, L.; Beckers, P.; Horn, L.; Von Döhren, J. Unravelling the Lineus ruber/Viridis Species Complex (Nemertea, Heteronemertea). Zool. Scr. 2017, 46, 111–126. [Google Scholar] [CrossRef]

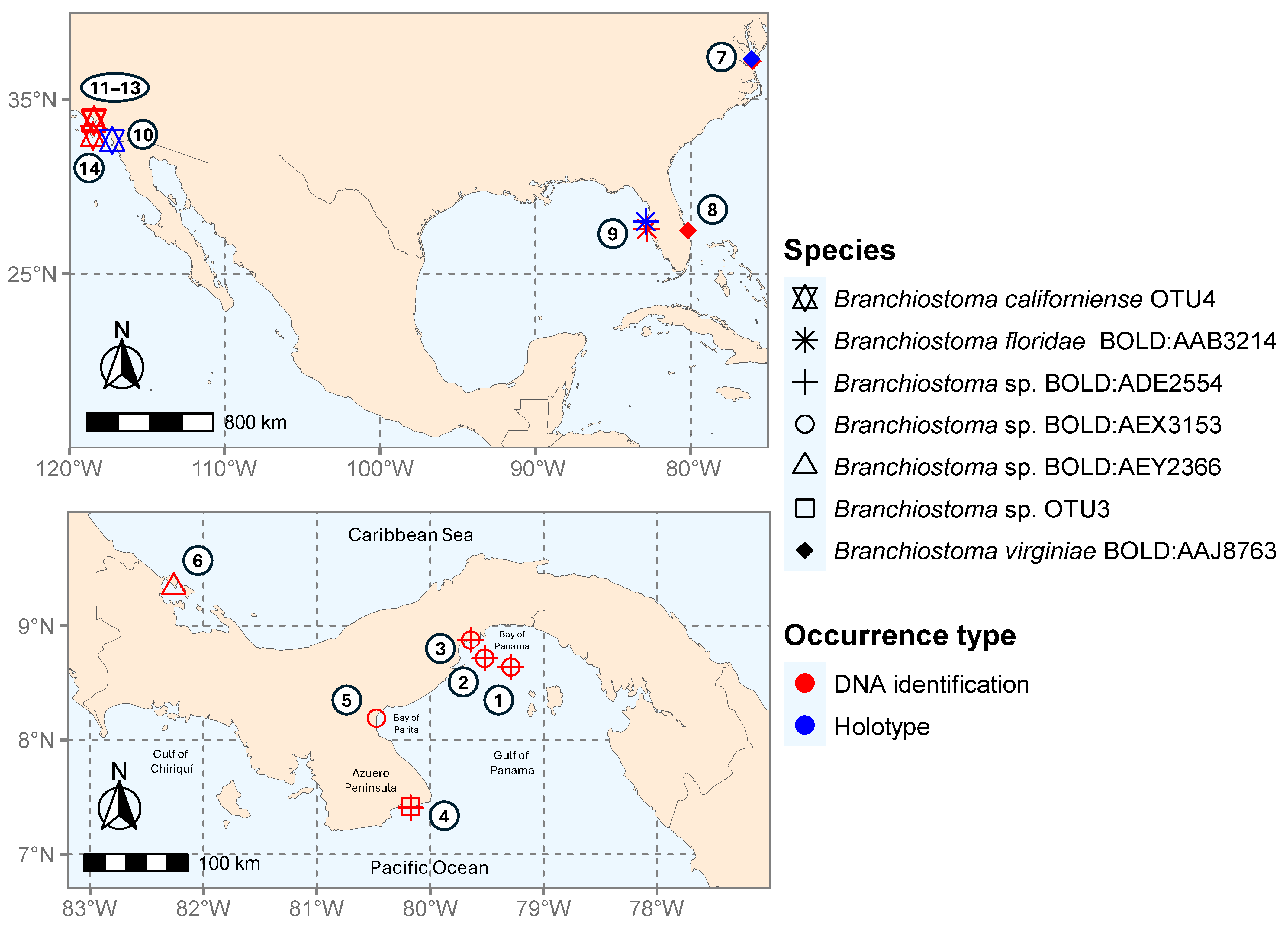





| BOLD BIN# | BOLD Process ID | Sample ID | COI | 16S | Life Stage | Locality Number in Figure 2 | Collection Date | Depth |
|---|---|---|---|---|---|---|---|---|
| PACIFIC | ||||||||
| ADE2554 | ABBAK057-17 | RCMB0657 | PV711381 | PV697394 | Larva (w/photo) | (2) SE side of Taboguilla Island PA | 18 November 2014 | Plankton tow |
| ABBAK059-17 | RCMB0659 | PV711378 | PV697392 | Larva (w/photo) | (2) SE side of Taboguilla Island PA | 18 November 2014 | Plankton tow | |
| ABBAI1544-16 | RCMB084 | PV711379 | PV697393 | Larva (w/photo) | (1) Bay of Panama PA | 13 March 2014 | Plankton tow | |
| ABBAI1545-16 | RCMB085 | PV711376 | PV697390 | Larva | (1) Bay of Panama PA | 13 March 2014 | Plankton tow | |
| ABBAI1546-16 | RCMB086 | PV711383 | PV697398 | Larva | (1) Bay of Panama PA | 13 March 2014 | Plankton tow | |
| ABBAL1292-21 | RCMicro 580 | PV711377 | PV697391 | Adult (w/photo) | (3) Chumical PA | 10 March 2020 | Intertidal | |
| ABBAL1297-21 | RCMicro 585 | PV711382 | PV697396 | Adult | (3) Chumical PA | 10 March 2020 | Intertidal | |
| LANGB001-22 | Achotines-F1 | MT877131 | MT877131 | Adult | (4) Achotines PA [42] | 8 March 2016 | 1 m | |
| AEX3153 | ABBAK058-17 | RCMB0658 | PV711384 | PV697399 | Larva (w/photo) | (2) SE side of Taboguilla Island PA | 18 November 2014 | Plankton tow |
| ABBAL1298-21 | RCMicro 586 | - | PV697397 | Adult | (3) Chumical PA | 10 March 2024 | Intertidal | |
| ABBAL968-21 | RCMicro 256 | - | PV697395 | Adult (w/photo) | (5) El Salado PA | 14 January 2020 | Intertidal | |
| OTU3 | LANGB005-22 | Achotines-E1 | MT877132 | MT877132 | Adult | (4) Achotines PA [42] | 8 March 2016 | 1 m |
| LANGB004-22 | Achotines-H1 | MT877125 | MT877125 | Adult | (4) Achotines PA [42] | 8 March 2016 | 1 m | |
| LANGB003-22 | Achotines-D1 | MT877128 | MT877128 | Adult (w/photo) | (4) Achotines PA [42] | 8 March 2016 | 1 m | |
| LANGB002-22 | Achotines-G1 | MT877129 | MT877129 | Adult | (4) Achotines PA [42] | 8 March 2016 | 1 m | |
| OTU4 | - | 15904 | PV366828 | - | Adult | (11) Redondo Beach CA | 21 August 2019 | 9.75 m |
| - | 16987 | PV366829 | - | Adult | (12) Christmas Tree Cove CA | 26 August 2019 | 20 m | |
| - | 17911 | PV366830 | - | Adult | (13) Point Vicente CA | 26 August 2019 | 30 m | |
| - | SIO 10-94 | - | JN602063 | Adult | (14) San Clement Island CA, unpublished | 1 July 2010 | 22.86 m | |
| CARIBBEAN | ||||||||
| AEY2366 | ABBAI111-15 | CCLV062 | PV711380 | - | Larva (w/photo) | (4) Bahía Almirante PA | 1 July 2013 | Plankton tow |
| Loci | Species and BIN | Mean Intra. (%) | Maximum Intra. (%) | Nearest Neighbor | Min. Dist. to NN (%) |
|---|---|---|---|---|---|
| COI | B. belcheri BOLD:ACG5334 | 0.85 | 3.65 | Branchiostoma sp. BOLD:AEX3153 | 13.08 |
| B. californiense OTU4 | 0.51 | 0.76 | Branchiostoma sp. BOLD:AEX3153 | 14.5 | |
| B. floridae BOLD:AAB3214 | 1.51 | 3.17 | B. virginiae BOLD:AAJ8763 | 6.11 | |
| Branchiostoma sp. BOLD:AEX3153 | NA | NA | Branchiostoma sp. OTU3 | 10.99 | |
| Branchiostoma sp. BOLD:ADE2554 | 0.3 | 0.77 | Branchiostoma sp. BOLD:AEX3153 | 12.31 | |
| Branchiostoma sp. BOLD:AEY2366 | NA | NA | Branchiostoma sp. OTU3 | 12.06 | |
| Branchiostoma sp. OTU3 | 1.13 | 1.61 | Branchiostoma sp. BOLD:AEX3153 | 10.99 | |
| B. virginiae BOLD:AAJ8763 | 0.4 | 0.76 | B. floridae BOLD:AAB3214 | 6.11 | |
| 16S | B. floridae BOLD:AAB3214 | 0.94 | 2.03 | Branchiostoma sp. BOLD:ADE2554 | 10.99 |
| Branchiostoma sp. BOLD:ADE2554 | 0.23 | 0.4 | B. floridae BOLD:AAB3214 | 10.99 | |
| Branchiostoma sp. BOLD:AEX3153 | 0.37 | 0.55 | B. floridae BOLD:AAB3214 | 11.44 | |
| Branchiostoma sp. OTU3 | 0.75 | 1.02 | Branchiostoma sp. BOLD:AEX3153 | 12.15 | |
| B. californiense OTU4 | NA | NA | B. floridae BOLD:AAB3214 | 11.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madrid Concepcion, M.E.; Macdonald, K.S., III; Driskell, A.C.; Wetzer, R.; Di Domenico, M.; Collin, R. Lots of Lancelets or Not? Diversity of Cephalochordates in the Tropical Eastern Pacific. Diversity 2025, 17, 411. https://doi.org/10.3390/d17060411
Madrid Concepcion ME, Macdonald KS III, Driskell AC, Wetzer R, Di Domenico M, Collin R. Lots of Lancelets or Not? Diversity of Cephalochordates in the Tropical Eastern Pacific. Diversity. 2025; 17(6):411. https://doi.org/10.3390/d17060411
Chicago/Turabian StyleMadrid Concepcion, Maycol Ezequiel, Kenneth S. Macdonald, III, Amy C. Driskell, Regina Wetzer, Maikon Di Domenico, and Rachel Collin. 2025. "Lots of Lancelets or Not? Diversity of Cephalochordates in the Tropical Eastern Pacific" Diversity 17, no. 6: 411. https://doi.org/10.3390/d17060411
APA StyleMadrid Concepcion, M. E., Macdonald, K. S., III, Driskell, A. C., Wetzer, R., Di Domenico, M., & Collin, R. (2025). Lots of Lancelets or Not? Diversity of Cephalochordates in the Tropical Eastern Pacific. Diversity, 17(6), 411. https://doi.org/10.3390/d17060411








