Mobility Confers Resilience in Red Kangaroos (Osphranter rufus) to a Variable Climate and Coexisting Herbivores (Sheep, Goats, Rabbits and Three Sympatric Kangaroo Species) in an Arid Australian Rangeland
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Landclasses
2.3. Estimates of Mammalian Herbivore Densities
2.3.1. Kangaroos
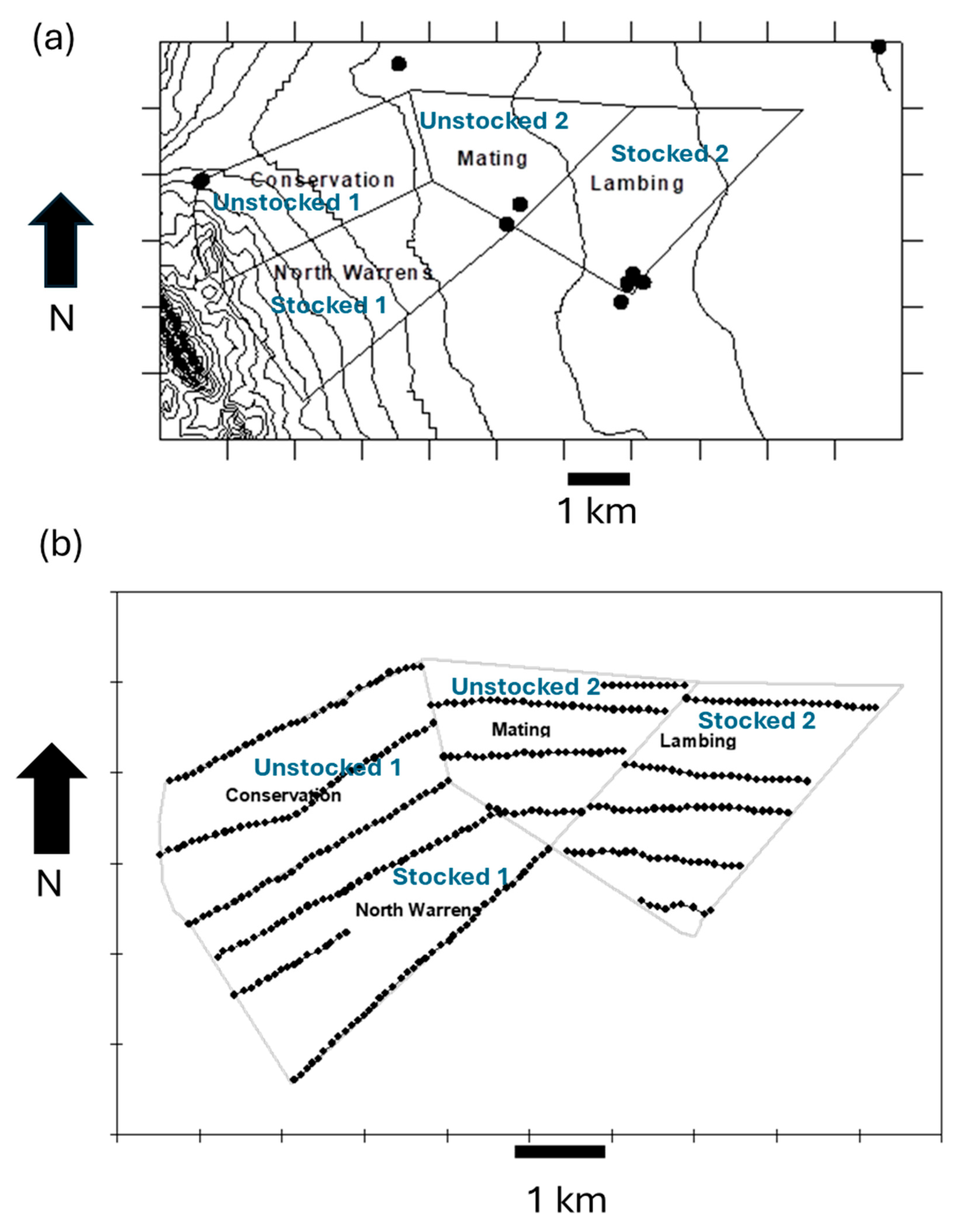
2.3.2. Sheep
2.3.3. Rabbits
2.3.4. Goats
2.4. Spatial Distributions of Herbivore Populations
2.5. Statistical Analysis
2.6. Analysis of Spatial Distributions
2.6.1. Landclass Selection
2.6.2. Other Habitat Features
2.7. Overlap of Species’ Population Home Ranges
2.8. Species Richness in Space and Time
2.9. Determinants of Densities and Distribution of Resident Herbivores Across Landclasses
2.10. Spatio-Temporal Behavior of Individuals
2.10.1. Capture and Radio-Collaring
2.10.2. Radio-Tracking
2.10.3. Data Handling
2.10.4. Other Mammalian Herbivores
2.11. Animal Ethics
3. Results
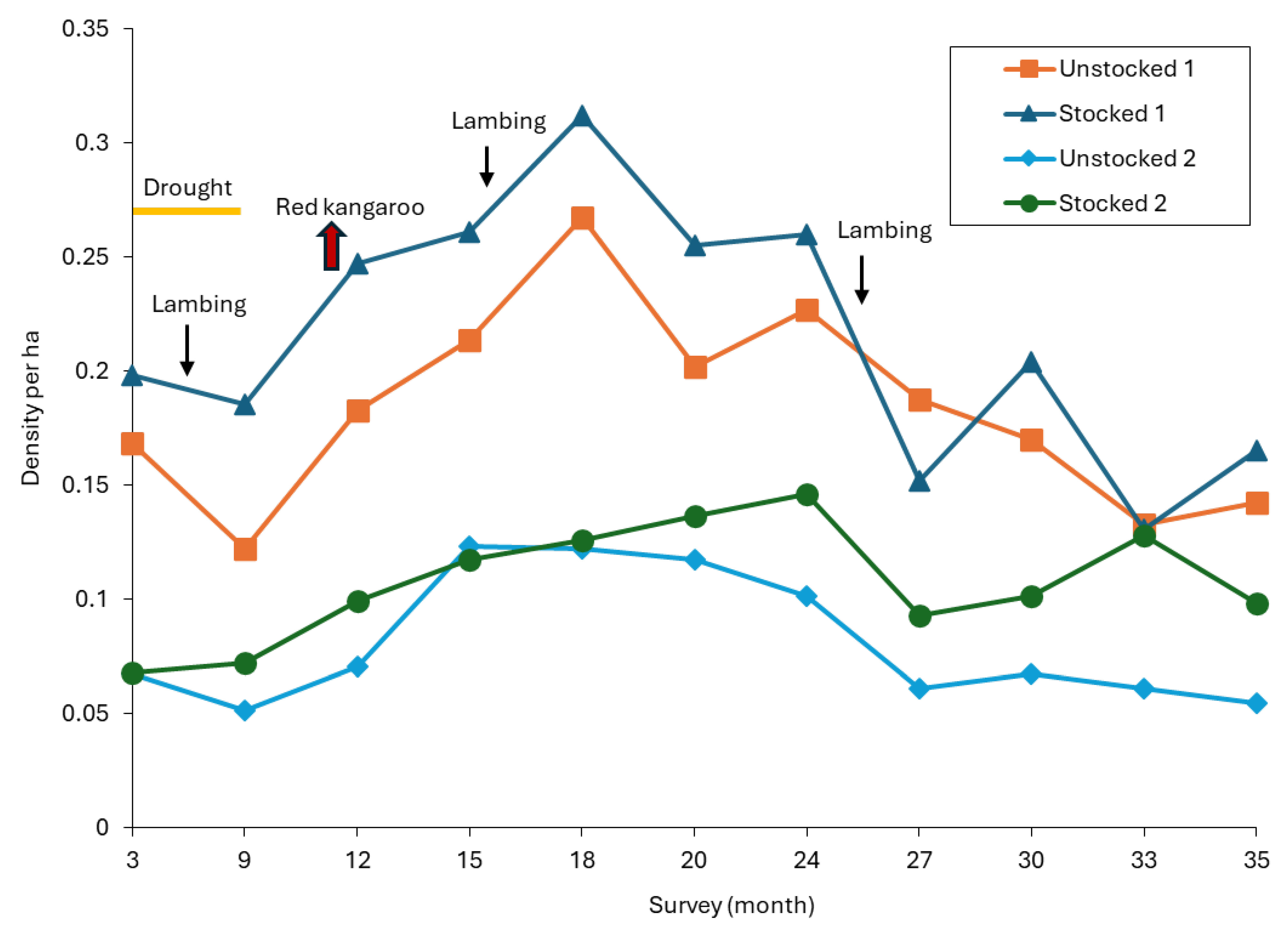
3.1. Variation in Density of Herbivores in the Study Site
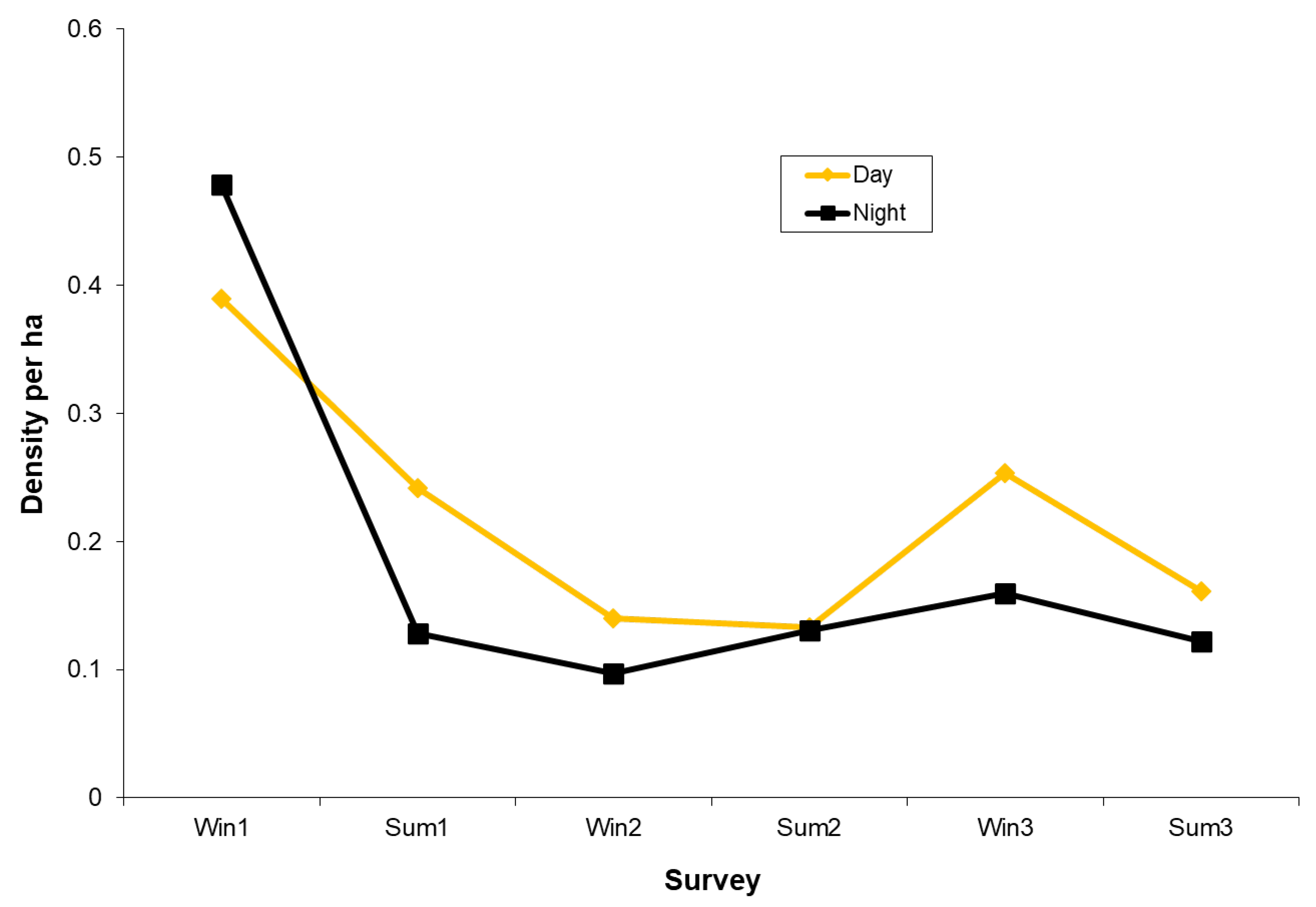
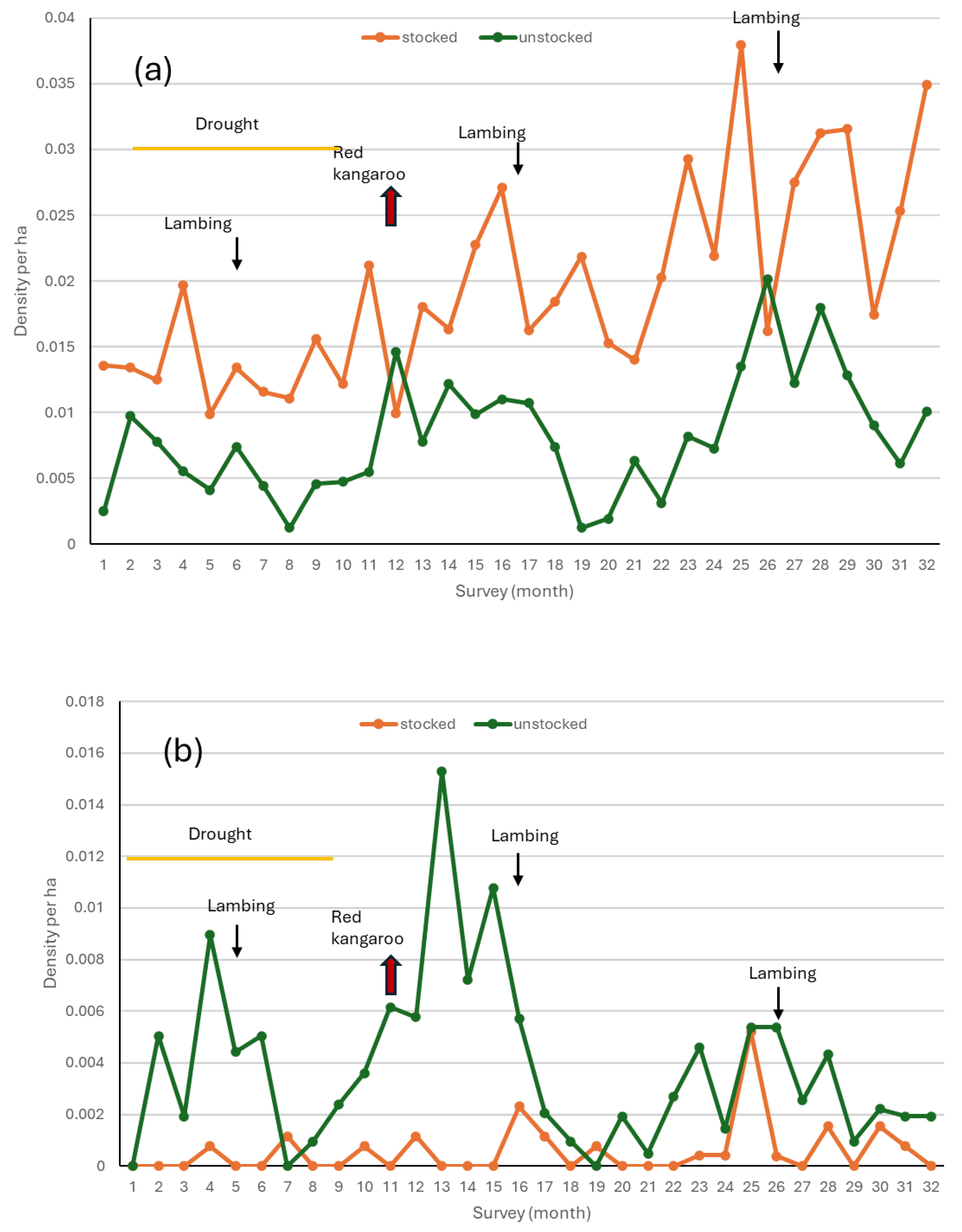
3.1.1. Density of Red Kangaroos
3.1.2. Density of Eastern and Western Grey Kangaroos and Common Wallaroos
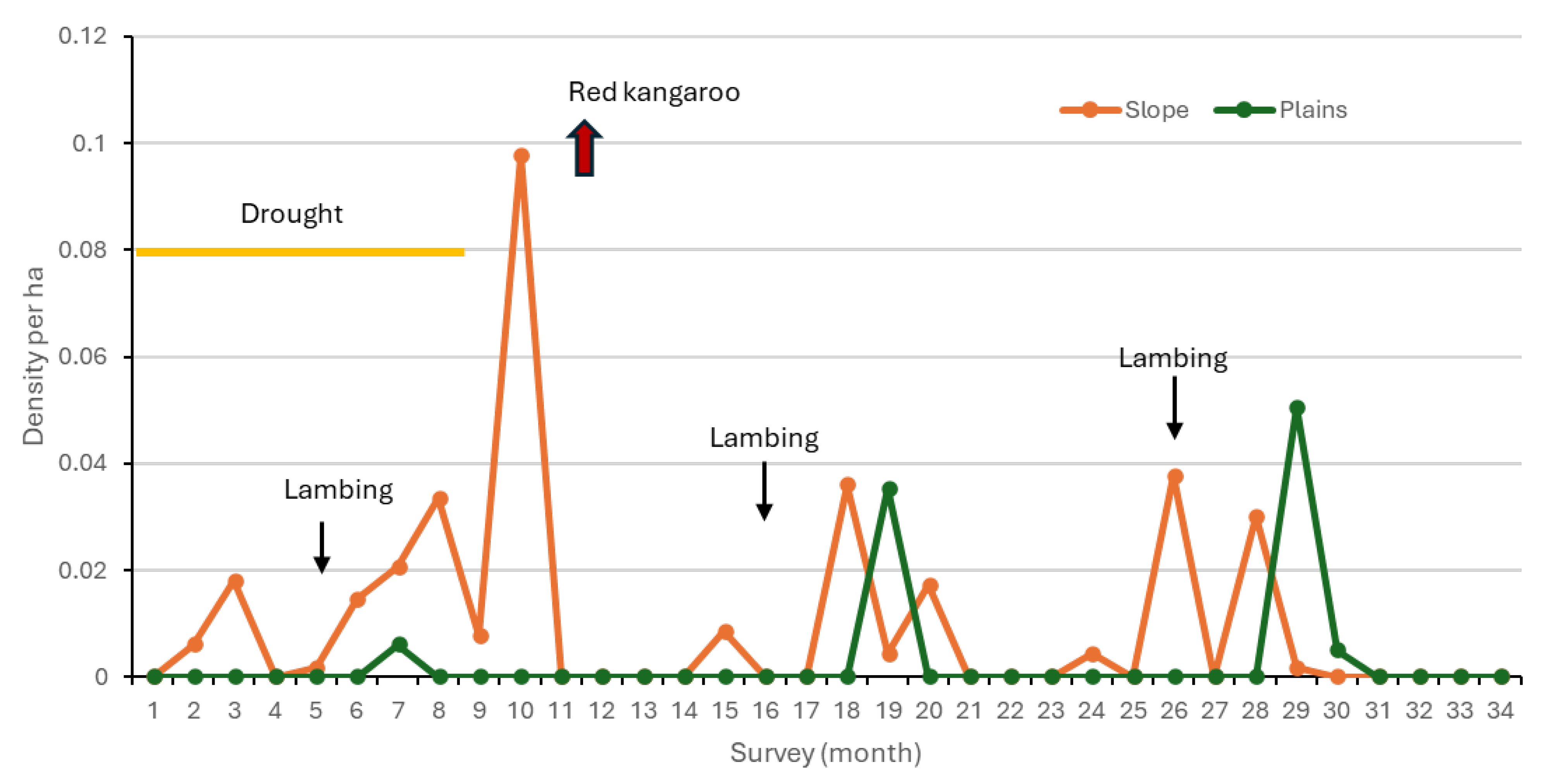
3.1.3. Density of Rabbits
3.1.4. Density of Goats
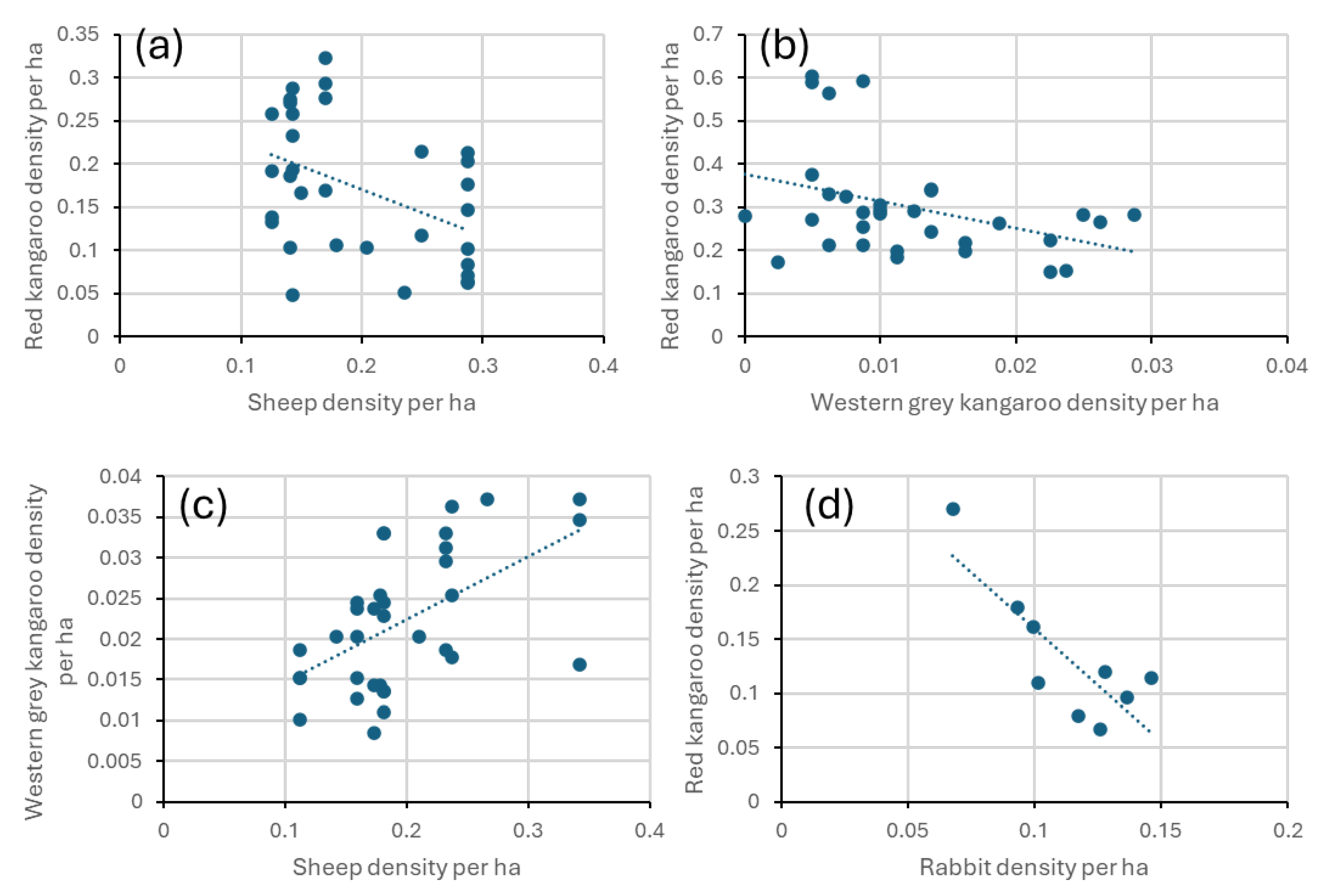
3.2. Correlations Between the Herbivore Populations
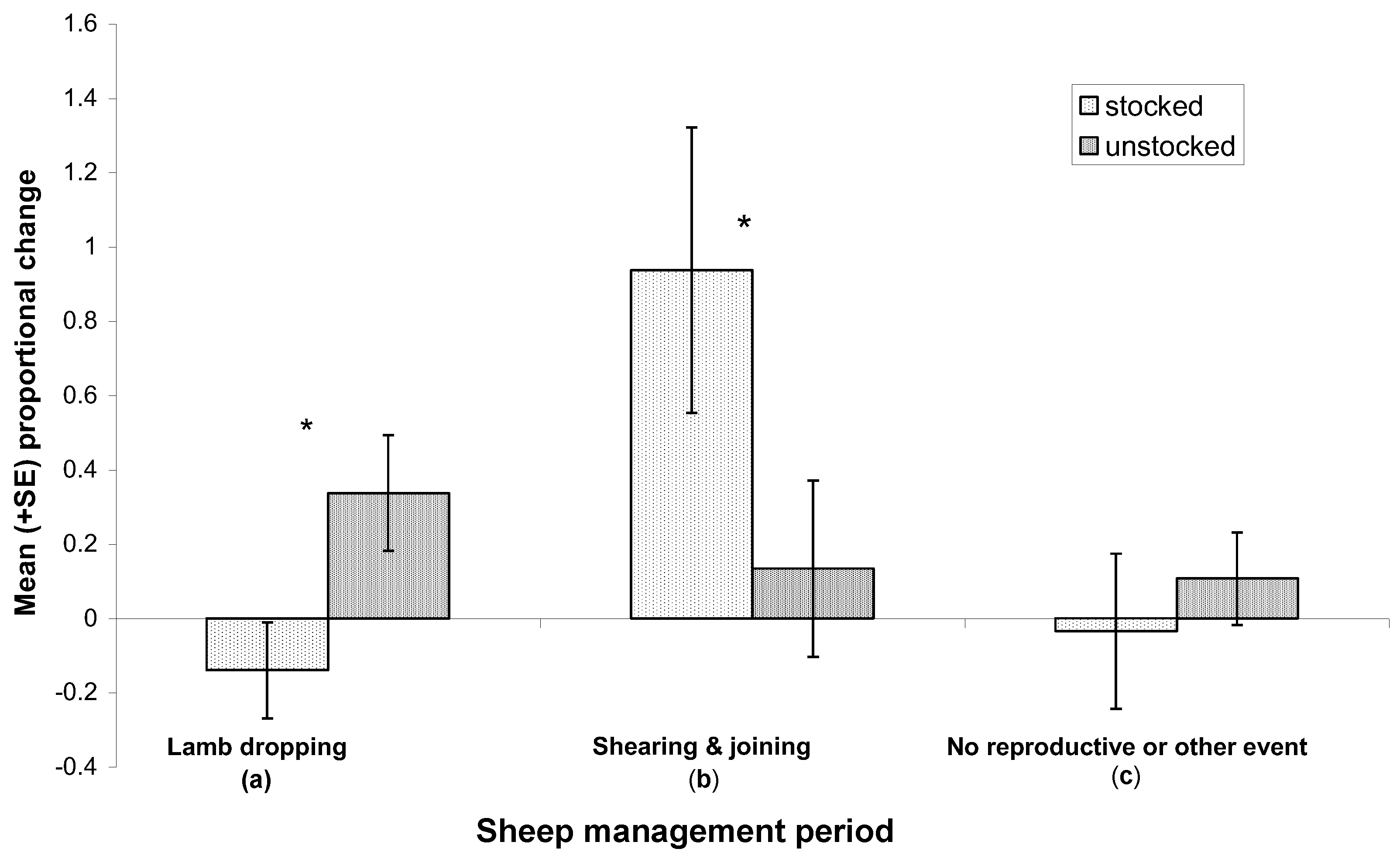
3.3. Reproductive Success and Mortality
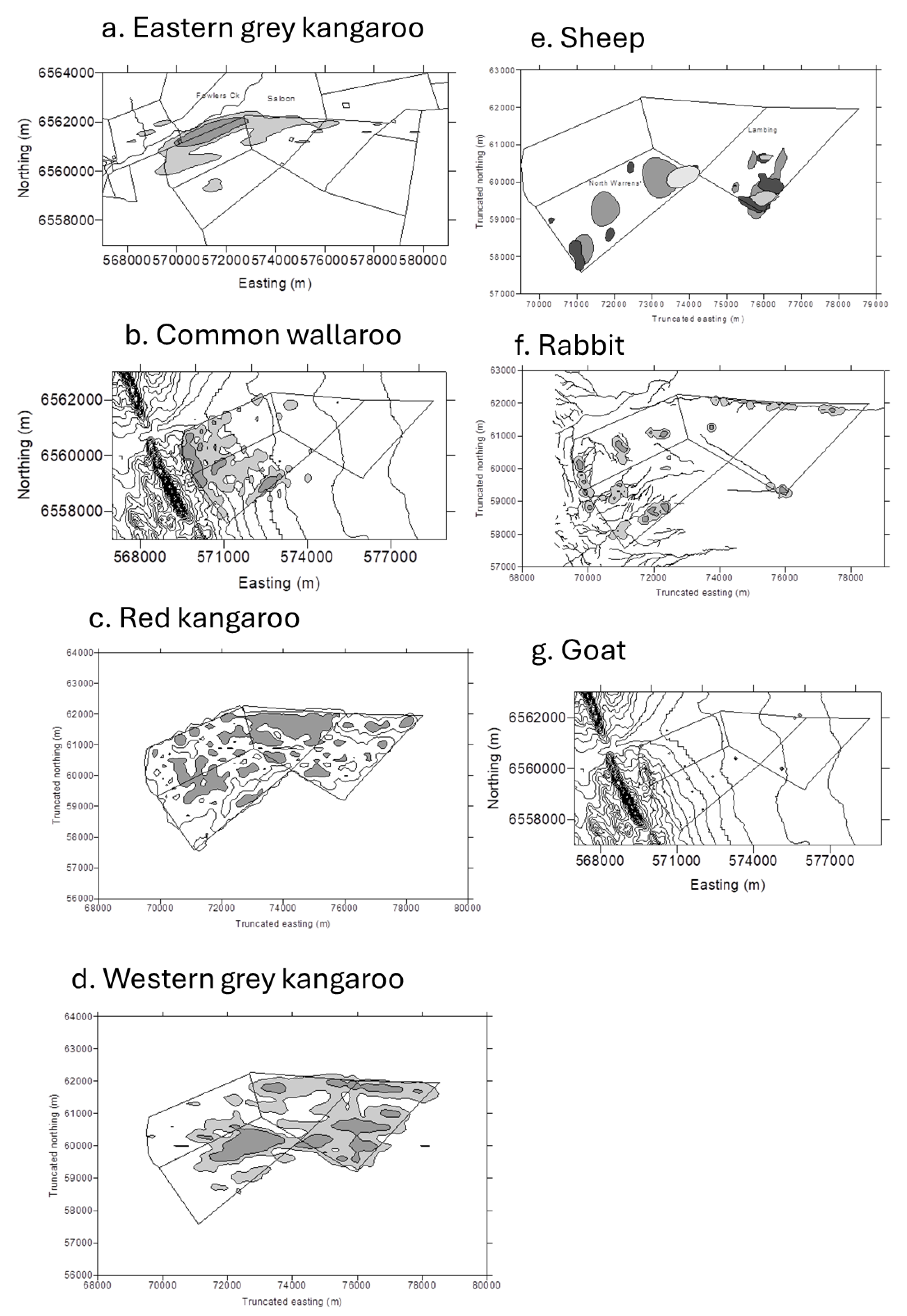
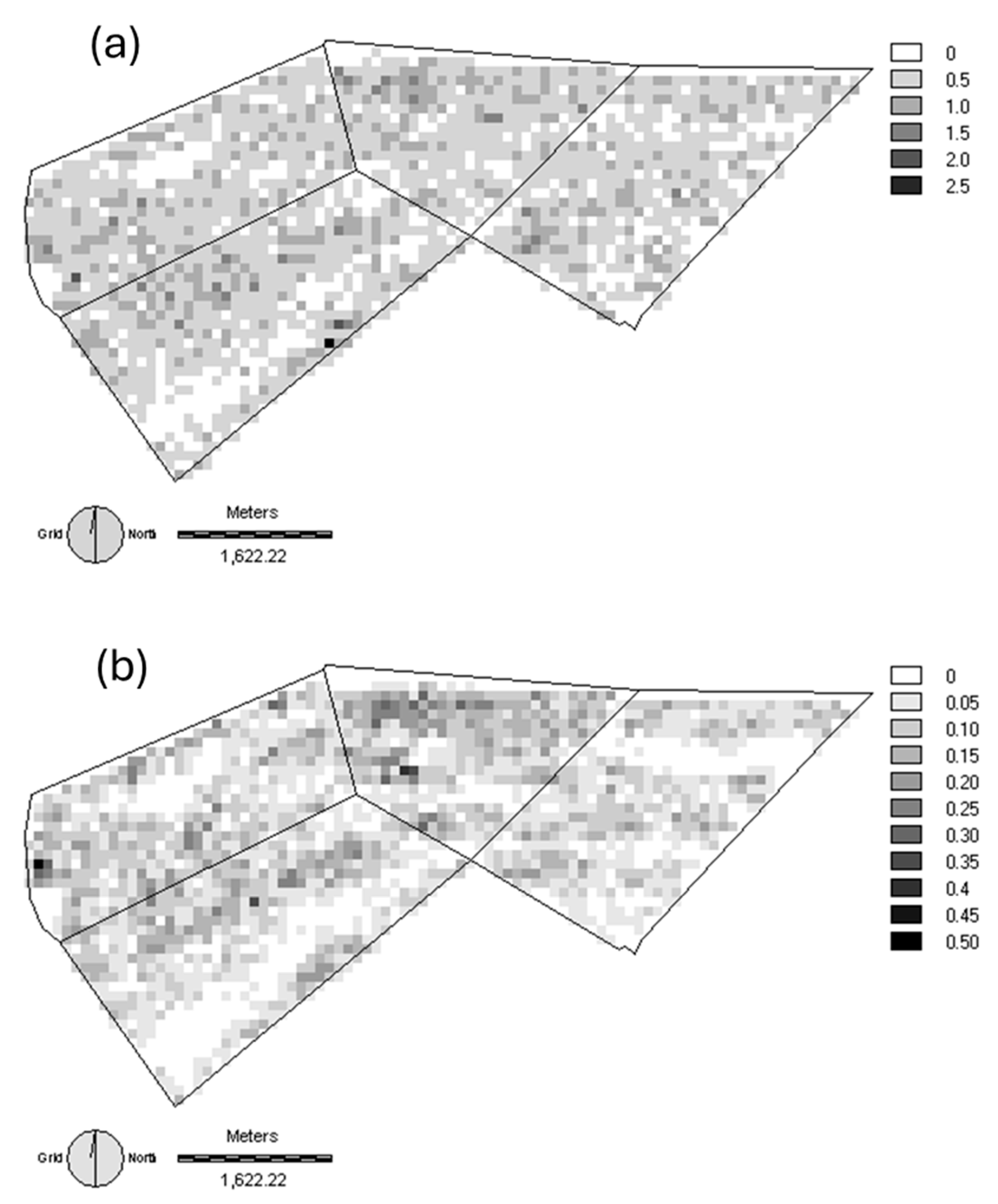
3.4. Population Utilization Distributions of Herbivores
3.5. Habitat Utilization of Herbivores
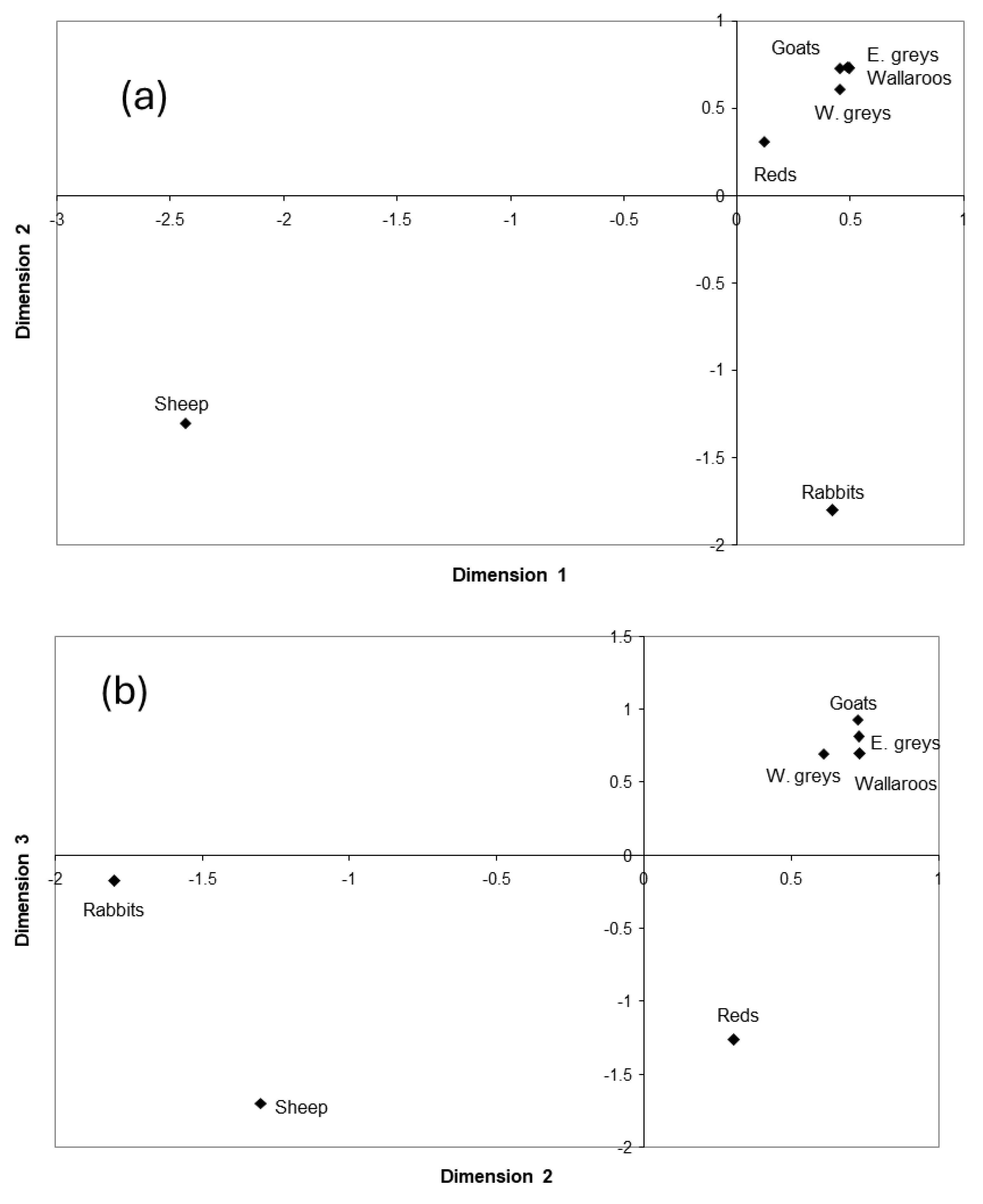
3.5.1. Multi-Dimensional Scaling
Unstocked Paddocks
Stocked Paddocks
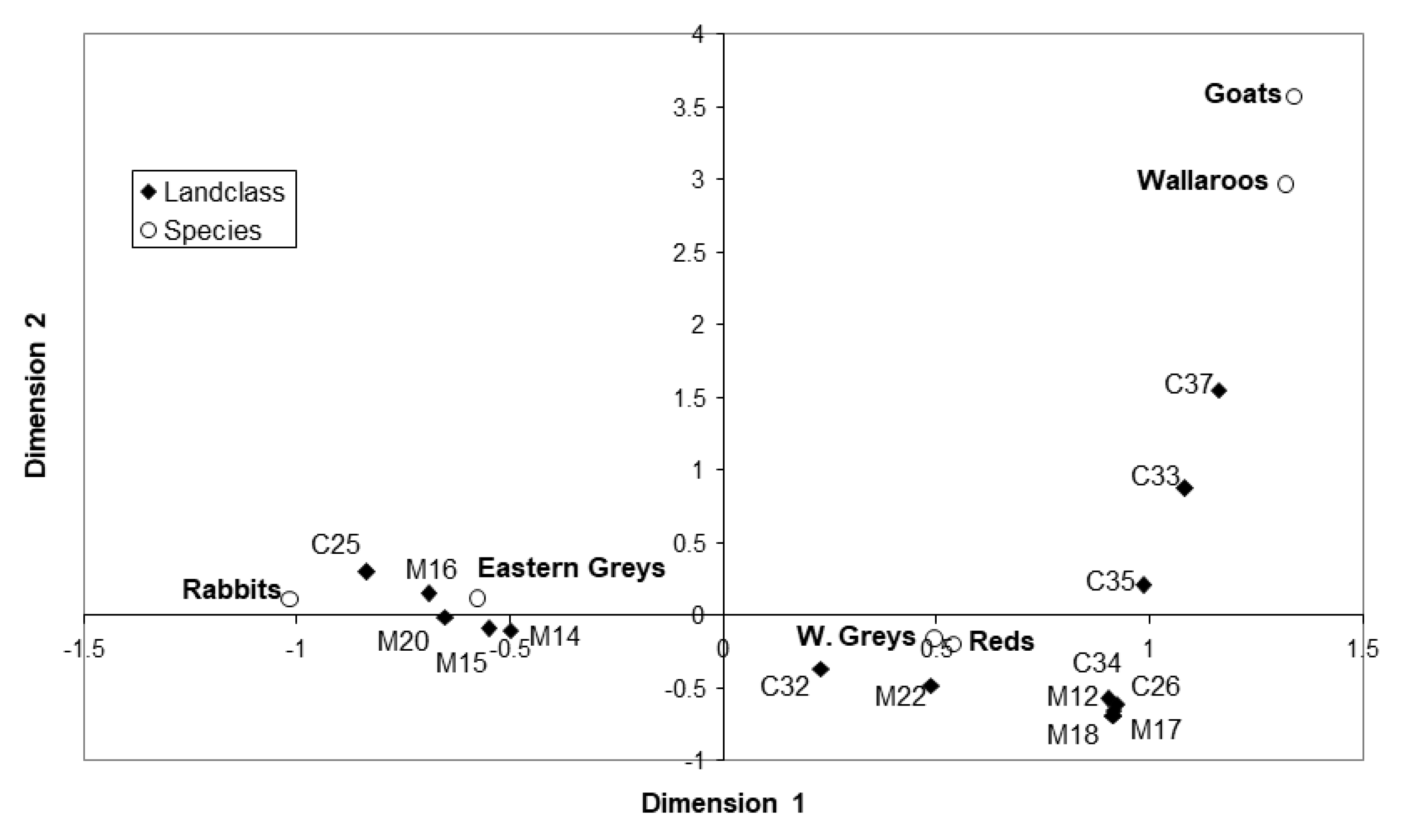
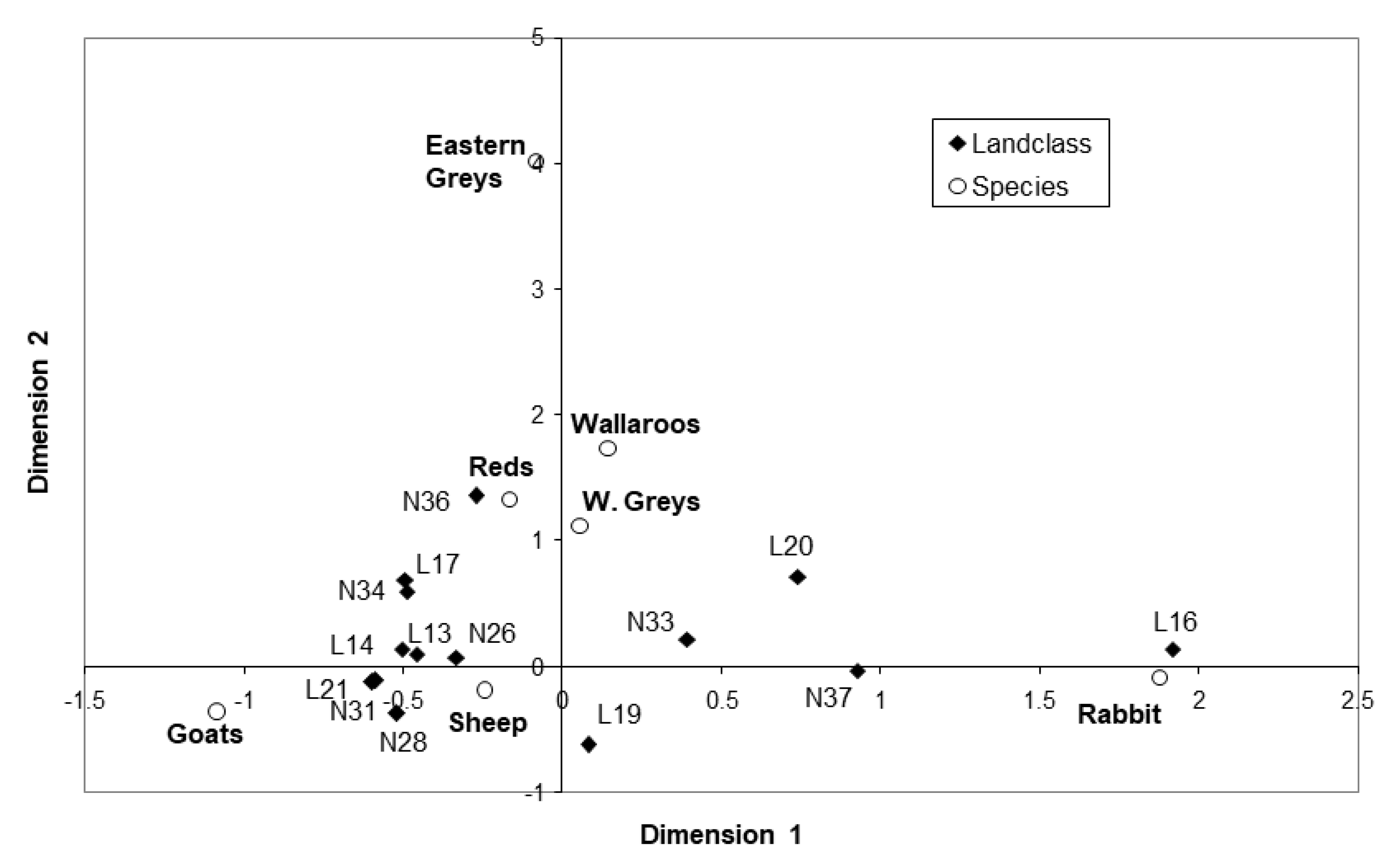
3.5.2. Correspondence Analysis
3.6. Overlap in Habitat Usage by Herbivores
3.6.1. Species Richness
3.6.2. Overlap of Core Ranges of Herbivore Species
3.7. Spatio-Temporal Habitat Use of Individual Radio-Tracked Herbivores
3.7.1. Home Range Estimates, Locations and Movement of Kangaroos
Eastern Grey Kangaroos
Red Kangaroos
Western Grey Kangaroo Females
3.7.2. Mobility of Red and Western Grey Kangaroos

3.7.3. Home Range Quality
3.7.4. Home Range Quality and Reproductive Success
4. Discussion
4.1. Population Dynamics
4.2. Habitat Selection
4.3. Spatio-Temporal Behaviour of Individuals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Landclass | Soil Type | Vegetation Community | Survey Year |
|---|---|---|---|
| 12 | 2—Plains with gilgais, channels and depressions: clay and texture contrast | Cottonbush | 1986 |
| 13 | 2—Plains with gilgais, channels and depressions: clay and some texture contrast | Saltbush and cottonbush | 1986 |
| 14 | 2—Broad low ridges with complex mosaic of hummocks, scalds and stony surfaces: heavy clay | Cottonbush | 1986 |
| 15 | 2—Plains with gilgais, channels and depressions: mixed clay and texture contrast | Copperburrs | 1986 |
| 16 | 2—Broad low ridges with complex mosaic of hummocks, scalds and stony surfaces: heavy clay | Cottonbush and copperburrs | 1986 |
| 17 | 1—Broad low ridges with complex mosaic of hummocks, scalds and stony surfaces: texture contrast | Copperburrs | 1986 |
| 18 | 2—Broad low ridges with complex mosaic of hummocks, scalds and stony surfaces: clay and texture contrast | Cottonbush | 1986 |
| 19 | 2—Plains with clay and texture contrast soils, scalded hummock surfaces, soft (reclaimed) scalds and depressions: heavy clay | Cottonbush | 1986 |
| 20 | 2—Plains with clay and texture contrast soils, scalded hummock surfaces, soft (reclaimed) scalds and depressions: heavy clay | Cottonbush and saltbush | 1986 |
| 21 | 2—Plains with clay and texture contrast soils, scalded hummock surfaces, soft (reclaimed) scalds and depressions: mixed | Cottonbush and copperburrs | 1986 |
| 22 | 2—Plains with clay and texture contrast soils, scalded hummock surfaces, soft (reclaimed) scalds and depressions: mixed | Cottonbush and copperburrs | 1986 |
| 25 | 1—Heavily scalded texture contrast | Cottonbush—saltbush plains | 1991 |
| 26 | 2—Texture contrast/heavy clay | Saltbush—cottonbush plains | 1991 |
| 28 | 2—Degraded texture contrast/heavy clay | Cottonbush | 1991 |
| 31 | 3—Heavy clay depressions with scalding | Mitchell grass | 1991 |
| 32 | 3—Heavy clay depressions | Saltbush plains | 1991 |
| 33 | 4—Foot slopes, desert loam soils | Grass | 1991 |
| 34 | 4—Desert loam soils | Bluebush plains | 1991 |
| 35 | 4—Desert loam soil | Bluebush plains | 1991 |
| 36 | 4—Desert loam soils | Saltbush plains | 1991 |
| 37 | 5—Foot slopes, brown gibber soils | Dense saltbush | 1991 |
Appendix B
| Reds | Western Greys | Eastern Greys | Common Wallaroos | Sheep | Goats | |
|---|---|---|---|---|---|---|
| Unstocked 1 | ||||||
| W grey | 0.308 | |||||
| E grey | 0.217 | 0.287 | ||||
| Wallaroo | 0.144 | −0.059 | 0.088 | |||
| Goats | 0.015 | −0.133 | −0.150 | 0.049 | ||
| Rabbits | 0.009 | 0.355 | 0.189 | 0.320 | −0.504 | |
| Stocked 1 | ||||||
| W greys | 0.178 | |||||
| Euros | 0.327 | 0.264 | ||||
| Sheep | −0.440 * | 0.090 | −0.106 | |||
| Rabbits | −0.161 | −0.234 | 0.145 | 0.215 | ||
| Unstocked 2 | ||||||
| W greys | −0.378 * | |||||
| E greys | 0.085 | 0.111 | ||||
| Rabbits | −0.240 | −0.114 | −0.193 | |||
| Stocked 2 | ||||||
| W greys | −0.270 | |||||
| Sheep | −0.370 | 0.553 * | ||||
| Rabbits | −0.815 * | 0.278 | 0.128 |
Appendix C
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| M12 | 1.9 ± 2.4 | 2.3 ± 7.9 | 1.7 ± 2.2 |
| M14 | 4.7 ± 14.2 | - | 6.2 ± 19.1 |
| M15 | 0.4 ± 1.4 | - | 0.6 ± 2.0 |
| M16 | 4.7 ± 6.3 | 11.3 ± 6.8 * | 2.5 ± 5.3 |
| M17 | - | - | - |
| M18 | - | - | - |
| M20 | 6.8 ± 14.5 | 5.5 ± 19.0 | 7.3 ± 18.7 |
| M22 | 0.7 ± 1.6 | 2.7 ± 4.9 | - |
| C25 | 5.1 ± 3.5 * | 3.2 ± 3.9 | 5.8 ± 4.2 * |
| C26 | - | - | - |
| C32 | 1.2 ± 2.8 | - | 0.6 ± 2.3 |
| C33 | 0.9 ± 3.0 | - | 1.2 ± 4.1 |
| C34 | - | - | - |
| C35 | 1.7 ± 4.6 | - | 2.3 ± 5.8 |
| C37 | - | - | - |
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| C25 | 1.3 ± 1.0 | 1.7 ± 1.0 | 1.1 ± 1.1 |
| C26 | 0.1 ± 0.2 * | - | 0.1 ± 0.3 * |
| N26 | 0.8 ± 0.8 | 0.5 ± 0.7 | 0.9 ± 1.2 |
| N28 | 0.6 ± 0.9 | 1.4 ± 1.0 | 0.2 ± 0.4 * |
| N31 | 0.1 ± 0.3 * | 0.3 ± 0.4 * | - |
| C32 | 0.1 ± 0.4 * | 0.4 ± 0.5 * | - |
| C33 | 3.5 ± 1.9 * | 2.8 ± 1.3 * | 3.9 ± 2.8 * |
| N33 | 3.1 ± 1.3 * | 2.8 ± 0.3 * | 3.3 ± 1.9 * |
| C34 | 0.3 ± 1.0 | - | 0.5 ± 1.5 |
| N34 | 3.4 ± 5.6 | - | 5.2 ± 7.7 |
| C35 | 1.6 ± 2.3 | 0.7 ± 0.9 | 2.1 ± 3.0 |
| N36 | 2.3 ± 3.3 | 2.7 ± 2.6 | 2.1 ± 4.9 |
| C37 | 5.5 ± 4.9 | 6.6 ± 3.2 * | 4.9 ± 7.4 |
| N37 | 2.6 ± 0.8 * | 2.3 ± 1.0 * | 2.8 ± 0.9 * |
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| M12 | 1.5 ± 0.4 * | 1.5 ± 1.1 | 1.5 ± 0.6 * |
| L13 | 0.9 ± 0.4 | 1.1 ± 0.7 | 0.7 ± 0.2 * |
| M14 | 1.9 ± 1.3 | 2.0 ± 2.5 | 1.8 ± 1.4 |
| L14 | 0.7 ± 0.5 | 1.0 ± 0.8 | 0.5 ± 0.3 * |
| M15 | 1.1 ± 0.4 | 1.0 ± 0.4 | 1.3 ± 0.7 |
| M16 | 0.9 ± 0.4 | 0.9 ± 0.6 | 0.9 ± 0.7 |
| L16 | 0.6 ± 1.6 | 1.1 ± 3.4 | 0.1 ± 0.4 * |
| M17 | 1.1 ± 0.8 | 0.9 ± 1.7 | 1.2 ± 0.5 |
| L17 | 0.8 ± 1.3 | 0.8 ± 1.9 | 0.8 ± 2.0 |
| M18 | 0.6 ± 0.4 | 0.4 ± 0.4 * | 0.7 ± 0.7 |
| L19 | 0.4 ± 0.3 * | 0.5 ± 0.4 * | 0.2 ± 0.4 |
| M20 | 1.9 ± 1.8 | 1.4 ± 2.3 | 2.4 ± 2.4 |
| L20 | 0.8 ± 0.6 | 1.1 ± 1.2 | 0.6 ± 0.2 * |
| L21 | 1.0 ± 0.4 | 1.2 ± 0.5 | 0.8 ± 0.5 |
| M22 | 1.4 ± 0.3 * | 1.5 ± 0.4 * | 1.4 ± 0.7 |
| C25 | 1.2 ± 0.3 | 1.1 ± 0.3 | 1.3 ± 0.5 |
| C26 | 1.2 ± 0.5 | 1.0 ± 0.9 | 1.3 ± 0.6 |
| N26 | 0.9 ± 0.5 | 0.8 ± 0.7 | 0.9 ± 0.8 |
| N28 | 0.5 ± 0.3 * | 0.5 ± 0.4 * | 0.5 ± 0.6 |
| N31 | 0.8 ± 0.5 | 0.7 ± 0.5 | 1.0 ± 0.9 |
| C32 | 1.1 ± 0.4 | 1.0 ± 0.7 | 1.3 ± 0.4 |
| C33 | 0.9 ± 0.5 | 0.9 ± 0.6 | 1.0 ± 0.7 |
| N33 | 0.9 ± 0.3 | 0.8 ± 0.5 | 0.9 ± 0.5 |
| C34 | 1.6 ± 1.2 | 1.8 ± 1.8 | 1.3 ± 1.5 |
| N34 | 1.2 ± 1.0 | 0.7 ± 0.6 | 1.7 ± 1.5 |
| C35 | 1.3 ± 0.6 | 1.2 ± 0.7 | 1.5 ± 1.0 |
| N36 | 1.4 ± 1.5 | 1.1 ± 1.1 | 1.8 ± 2.6 |
| C37 | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.6 ± 1.4 |
| N37 | 0.8 ± 0.3 | 0.8 ± 0.5 | 0.9 ± 0.6 |
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| M12 | 1.1 ± 0.7 | 0.6 ± 0.8 | 1.2 ± 0.8 |
| L13 | 1.1 ± 1.0 | 1.5 ± 1.5 | 1.0 ± 1.2 |
| M14 | 1.6 ± 2.6 | 1.1 ± 3.5 | 1.8 ± 3.3 |
| L14 | 1.7 ± 0.9 | 1.1 ± 1.9 | 1.9 ± 1.1 |
| M15 | 0.8 ± 1.1 | - | 1.0 ± 1.4 |
| M16 | 0.9 ± 1.3 | 2.2 ± 3.8 | 0.5 ± 1.2 |
| L16 | 3.0 ± 7.3 | 13.7 ± 24.0 | - |
| M17 | 0.3 ± 0.4 | 0.2 ± 0.6 | 0.3 ± 0.5 |
| L17 | 0.4 ± 1.4 | 2.0 ± 6.4 | - |
| M18 | - | - | - |
| L19 | 1.4 ± 1.2 | 1.5 ± 5.1 | 1.3 ± 0.8 |
| M20 | - | - | - |
| L20 | 1.5 ± 0.8 | 0.5 ± 0.3 * | 1.8 ± 0.7 * |
| L21 | 1.5 ± 0.8 | 2.3 ± 1.9 | 1.2 ± 0.9 |
| M22 | 0.5 ± 0.4 * | 0.3 ± 1.0 | 0.6 ± 0.5 |
| C25 | 0.3 ± 0.5 | 0.6 ± 1.0 | 0.2 ± 0.5 |
| C26 | 0.5 ± 0.8 | 0.9 ± 2.0 | 0.3 ± 0.8 |
| N26 | 3.4 ± 2.3 * | 2.4 ± 3.9 | 3.7 ± 2.7 * |
| N28 | 1.0 ± 1.2 | 0.9 ± 1.9 | 1.0 ± 1.5 |
| N31 | 2.8 ± 1.4 * | 1.2 ± 3.9 | 3.2 ± 1.1 * |
| C32 | 0.2 ± 0.3 * | 0.5 ± 1.1 | 0.1 ± 0.2 * |
| C33 | 0.3 ± 0.5 * | - | 0.3 ± 0.6 * |
| N33 | 0.8 ± 0.6 | 1.3 ± 1.1 | 0.6 ± 0.6 |
| C34 | 0.2 ± 0.5 * | - | 0.2 ± 0.7 |
| N34 | 0.4 ± 0.8 | 0.5 ± 1.5 | 0.4 ± 0.9 |
| C35 | 1.9 ± 3.9 | 6.9 ± 13.0 | 0.5 ± 1.5 |
| N36 | 3.6 ± 4.6 | 5.7 ± 16.1 | 2.9 ± 4.1 |
| C37 | 0.3 ± 0.7 | - | 0.4 ± 0.9 |
| N37 | 0.2 ± 0.3 * | 0.3 ± 0.1 * | 0.1 ± 0.2 * |
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| L13 | 0.7 0.3 | 0.7 ± 0.1 * | 0.9 ± 0.4 |
| L14 | 0.7 ± 0.5 | 0.9 ± 0.2 | 0.7 ± 0.6 |
| L16 | 0.4 ± 1.1 | - | 0.5 ± 1.5 |
| L17 | 0.4 ± 0.8 | - | 0.5 ± 1.1 |
| L19 | 3.2 ± 1.3 * | 3.8 ± 1.4 * | 3.1 ± 1.6 * |
| L20 | 0.3 ± 0.2 * | 0.4 ± 0.5 * | 0.3 ± 0.2 * |
| L21 | 1.2 ± 1.1 | 0.4 ± 0.9 | 1.5 ± 1.3 |
| N26 | 1.1 ± 0.8 | 1.9 ± 2.8 | 0.9 ± 0.6 |
| N28 | 1.4 ± 1.0 | 1.4 ± 1.9 | 1.4 ± 1.3 |
| N31 | 1.2 ± 0.8 | 0.8 ± 0.5 | 1.4 ± 1.0 |
| N33 | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.8 ± 0.5 |
| N34 | 0.8 ± 1.1 | 1.0 ± 2.2 | 0.8 ± 1.3 |
| N36 | 0.6 ± 1.0 | 0.6 ± 1.8 | 0.6 ± 1.3 |
| N37 | 1.0 ± 0.6 | 1.0 ± 1.0 | 1.0 ± 0.7 |
| Landclass | All Surveys | Drought | Non-Drought |
|---|---|---|---|
| M12 | - | - | - |
| L13 | 0.1 ± 0.04 * | 0.1 ± 0.1 * | 0.1 ± 0.05 * |
| M14 | 2.3 ± 1.6 | 2.7 ± 2.9 | 2.1 ± 2.0 |
| L14 | - | - | - |
| M15 | 1.5 ± 0.4 * | 1.5 ± 0.8 | 1.5 ± 0.4 * |
| M16 | 1.6 ± 0.3 * | 1.3 ± 0.2 * | 1.7 ± 0.4 * |
| L16 | 3.7 ± 3.5 | 3.8 ± 7.3 | 3.7 ± 4.1 |
| M17 | - | - | - |
| L17 | - | - | - |
| M18 | - | - | - |
| L19 | 3.4 ± 0.8 * | 3.1 ± 1.3 * | 3.6 ± 1.0 * |
| M20 | 2.9 ± 1.7 * | 3.9 ± 2.8 * | 2.4 ± 2.0 |
| L20 | 1.2 ± 0.2 | 1.2 ± 0.3 | 1.1 ± 0.3 |
| L21 | - | - | - |
| M22 | 0.3 ± 0.1 * | 0.3 ± 0.1 * | 0.3 ± 0.1 * |
| C25 | 2.8 ± 0.4 * | 2.7 ± 0.8 * | 2.8 ± 0.4 * |
| C26 | - | - | - |
| N26 | 0.3 ± 0.1 * | 0.3 ± 0.3 * | 0.3 ± 0.1 * |
| N28 | - | - | - |
| N31 | - | - | - |
| C32 | 0.4 ± 0.3 * | 0.5 ± 0.5 | 0.4 ± 0.3 * |
| C33 | - | - | - |
| N33 | 1.5 ± 0.2 * | 1.4 ± 0.3 * | 1.5 ± 0.3 * |
| C34 | - | - | - |
| N34 | - | - | - |
| C35 | - | - | - |
| N36 | 0.2 ± 0.4 * | 0.3 ± 0.8 | 0.2 ± 0.5 * |
| C37 | - | - | - |
| N37 | 3.2 ± 0.3 * | 3.4 ± 0.5 * | 3.1 ± 0.3 * |
| Soil Type | Proportion | Selection Ratio | 95% Confidence Interval |
|---|---|---|---|
| 1—Texture contrast | 0.11 | 0.36 | 0.01 |
| 2—Texture contrast/clay | 0.50 | 0.38 | 0.02 |
| 3—Heavy clay | 0.08 | 0.36 | 0.12 |
| 4—Desert loams | 0.20 | 1.54 * | 0.04 |
| 5—Brown gibber | 0.12 | 3.77 * | 0.08 |
Appendix D
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| Water distance | 0.921 | |||
| Slope | 0.877 | −0.436 | ||
| Wallaroo density | 0.803 | −0.152 | −0.433 | −0.128 |
| FLC biomass | 0.807 | 0.125 | −0.395 | −0.388 |
| Rabbit density | 0.781 | −0.107 | 0.331 | 0.309 |
| Green grass biomass | 0.725 | 0.290 | 0.167 | −0.267 |
| Sheep density | 0.917 | 0.177 | ||
| Red kangaroo density | −0.895 | 0.155 | ||
| Copperburr biomass | −0.629 | 0.136 | ||
| Western grey kangaroo density | 0.114 | 0.595 | 0.260 | −0.551 |
| Tree density | −0.103 | −0.105 | 0.814 | |
| Green forb biomass | 0.721 | |||
| RLC biomass | −0.0264 | 0.382 | 0.447 | 0.599 |
References
- Newsome, A.E. An ecological comparison of the two arid-zone kangaroos of Australia, and their anomalous prosperity since the introduction of ruminant stock to their environment. Q. Rev. Biol. 1975, 50, 389–428. [Google Scholar] [PubMed]
- Newsome, A.E. The abundance of red kangaroos, Megaleia rufa, (Desmarest), in central Australia. CSIRO Wildl. Res. 1965, 13, 269–287. [Google Scholar] [CrossRef]
- Neave, H.M. Assessment of the Conservation and Management of the Red Kangaroo Macropus rufus and Euro Macropus robustus in the Northern Territory; Biodiversity Conservation Division, Department of Natural Resouces Environment and the Arts, Northern Territory Government: Palmerston, NT, Australia, 2008; p. 71. [Google Scholar]
- Letnic, M.; Ritchie, E.G.; Dickman, C.R. Top predators as biodiversity regulators: The dingo Canis lupus dingo as a case study. Biol. Rev. 2012, 87, 390–413. [Google Scholar] [CrossRef] [PubMed]
- Bomford, M.; Caughley, J. Sustainable Use of Wildlife by Aboriginal Peeples and Torres Strait Islanders; Department of Primary Industries and Energy, Bureau of Resource Sciences, Australian Government Publishing Service: Canberra, ACT, Australia, 1996.
- Newsome, A.E. The influence of food on breeding in the red kangaroo in central Australia. CSIRO Wildl. Res. 1966, 11, 187–196. [Google Scholar] [CrossRef]
- Newsome, A.E. Anoestrus in the red kangaroo Megaleia rufa (Desmarest). CSIRO Wildl. Res. 1964, 12, 9–17. [Google Scholar] [CrossRef]
- Newsome, A.E. Reproductive anomalies in the red kangaroo in central Australia explained by aboriginal traditional knowledge and ecology. In Marsupial Biology: Recent Research, New Perspectives; Saunders, N.R., Hinds, L.A., Eds.; University of New South Wales Press: Sydney, NSW, Australia, 1997; pp. 227–236. [Google Scholar]
- Croft, D.B.; Witte, I. The Perils of Being Populous: Control and Conservation of Abundant Kangaroo Species. Animals 2021, 11, 1753. [Google Scholar] [CrossRef]
- Calaby, J.H.; Grigg, G.C. Changes in macropodid communities and populations in the past 200 years, and the future. In Kangaroos, Wallabies and Rat-Kangaroos; Grigg, G.C., Jarman, P.J., Hume, I.D., Eds.; Surry Beatty & Sons: Sydney, NSW, Australia, 1989; Volume 2, pp. 813–820. [Google Scholar]
- Caughley, G. Introduction to the sheep rangelands. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 1–13. [Google Scholar]
- Freudenberger, D.; Hodgkinson, K.; Noble, J. Causes and consequences of landscape dysfunction in rangelands. In Landscape Ecology—Function and Management; Ludwig, J., Tongway, D., Freudenberger, D., Noble, J., Hodgkinson, K., Eds.; CSIRO Publishing: Melbourne, VIC, Australia, 1997; pp. 63–77. [Google Scholar]
- Witte, I.; Croft, D.B. Who eats the grass? Grazing pressure and interactions between wild kangaroos, feral goats and rabbits and domestic sheep on an arid Australian rangeland. Wild 2025, 2, 5. [Google Scholar] [CrossRef]
- Dawson, S.J.; Kreplins, T.L.; Kennedy, M.S.; Renwick, J.; Cowan, M.A.; Fleming, P.A. Land use and dingo baiting are correlated with the density of kangaroos in rangeland systems. Integr. Zool. 2023, 18, 299–315. [Google Scholar] [CrossRef]
- Wilson, G.R.; Edwards, M. Options and rationale for regional property-based kangaroo production. Ecol. Manag. Restor. 2021, 22, 176–185. [Google Scholar] [CrossRef]
- Lee, E.; Kloecker, U.; Croft, D.B.; Ramp, D. Kangaroo-vehicle collisions in Australia’s sheep rangelands, during and following drought periods. Aust. Mammal. 2004, 26, 215–226. [Google Scholar] [CrossRef]
- Smith, D.; King, R.; Allen, B.L. Impacts of exclusion fencing on target and non-target fauna: A global review. Biol. Rev. 2020, 95, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
- Letnic, M.; Laffan, S.W.; Greenville, A.C.; Russell, B.G.; Mitchell, B.; Fleming, P.J.S. Artificial watering points are focal points for activity by an invasive herbivore but not native herbivores in conservation reserves in arid Australia. Biodivers. Conserv. 2015, 24, 1–16. [Google Scholar] [CrossRef]
- McCarthy, M.A. Red kangaroo (Macropus rufus) dynamics: Effects of rainfall, density dependence, harvesting and environmental stochasticity. J. Appl. Ecol. 1996, 33, 45–53. [Google Scholar] [CrossRef]
- Jonzen, N.; Pople, A.; Knape, J.; Skold, M. Stochastic demography and population dynamics in the red kangaroo Macropus rufus. J. Anim. Ecol. 2010, 79, 109–116. [Google Scholar] [CrossRef]
- McLeod, S.R.; Finch, N.; Wallace, G.; Pople, A.R. Assessing the spatial and temporal organization of Red Kangaroo, Western Grey Kangaroo and Eastern Grey Kangaroo populations in eastern Australia using multivariate autoregressive state-space models. Ecol. Manag. Restor. 2021, 22, 106–123. [Google Scholar] [CrossRef]
- Dawson, T.J.; Ellis, B.A. Diets of mammalian herbivores in Australian arid shrublands: Seasonal effects on overlap between red kangaroos, sheep and rabbits and on dietary niche breadths and electivities. J. Arid Environ. 1994, 26, 257–271. [Google Scholar] [CrossRef]
- Edwards, G.P.; Croft, D.B.; Dawson, T.J. Competition between red kangaroos (Macropus rufus) and sheep (Ovis aries) in the arid rangelands of Australia. Aust. J. Ecol. 1996, 21, 165–172. [Google Scholar] [CrossRef]
- Edwards, G.P.; Dawson, T.J.; Croft, D.B. The dietary overlap between red kangaroos (Macropus rufus) and sheep (Ovis aries) in the arid rangelands of Australia. Aust. J. Ecol. 1995, 20, 324–334. [Google Scholar] [CrossRef]
- Croft, D.B. Locomotion, foraging competition and group size. In Comparison of Marsupial and Placental Behaviour; Croft, D.B., Ganslosser, U., Eds.; Filander Verlag GmbH: Fuerth, Germany, 1996; pp. 134–157. [Google Scholar]
- Tyndale-Biscoe, H. Kangaroos and sheep: The unequal contest. Australas. Sci. 2005, 26, 29–32. [Google Scholar]
- Bayliss, P. Kangaroo dynamics. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 119–134. [Google Scholar]
- Croft, D.B. Socio-ecology of the antilopine wallaroo, Macropus antilopinus, in the Northern Teritory, with observations on sympatric M. robustus woodwardii and M. agilis. Aust. Wildl. Res. 1987, 14, 243–255. [Google Scholar] [CrossRef]
- Garnick, S.; Di Stefano, J.; Elgar, M.A.; Coulson, G. Ecological specialisation in habitat selection within a macropodid herbivore guild. Oecologia 2016, 180, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.B.; Gregory, S.D.; Brook, B.W.; Ritchie, E.G.; Croft, D.B.; Coulson, G.; Fordham, D.A. The influence of non-climate predictors at local and landscape resolutions depends on the autecology of the species. Austral Ecol. 2014, 39, 710–721. [Google Scholar] [CrossRef]
- Norbury, G.L.; Norbury, D.C.; Oliver, A.J. Facultative behaviour in unpredictable environments: Mobility of red kangaroos in arid Western Australia. J. Anim. Ecol. 1994, 63, 410–418. [Google Scholar] [CrossRef]
- Croft, D.B. Home range of the red kangaroo Macropus rufus. J. Arid Environ. 1991, 20, 83–98. [Google Scholar] [CrossRef]
- Priddel, D.; Shepherd, N.; Ellis, M. Homing by the red kangaroo, Macropus rufus (Marsupialia: Macropodidae). Aust. Mammal. 1988, 11, 171–172. [Google Scholar] [CrossRef]
- Priddel, D.; Wellard, G.; Shepherd, N. Movements of sympatric red kangaroos, Macropus rufus, and western grey kangaroos, M. fuliginosus, in western New South Wales. Aust. Wildl. Res. 1988, 15, 339–346. [Google Scholar] [CrossRef]
- Priddel, D.; Shepherd, N.; Wellard, G. Home ranges of sympatric red kangaroos, Macropus rufus, and western grey kangaroos, M. fuliginosus, in western New South Wales. Aust. Wildl. Res. 1988, 15, 405–411. [Google Scholar] [CrossRef]
- McCarron, H.C.K. Environmental physiology of the eastern grey kangaroo (Macropus giganteus Shaw). Ph.D. Thesis, University of New South Wales, Sydney, NSW, Australia, 1990. [Google Scholar]
- Croft, D.B. Home range of the euro, Macropus robustus erubescens. J. Arid Environ. 1991, 20, 99–111. [Google Scholar] [CrossRef]
- Caughley, G.; Brown, B.; Dostine, P.; Grice, D. The grey kangaroo overlap zone. Aust. Wildl. Res. 1984, 11, 1–10. [Google Scholar] [CrossRef]
- Cairns, S.C.; Grigg, G.C.; Beard, L.A.; Pople, A.R.; Alexander, P. Western grey kangaroos (Macropus fuliginosus) in the South Australian pastoral zone: Populations at the edge of their range. Wildl. Res. 2000, 27, 309–318. [Google Scholar] [CrossRef]
- Munn, A.J.; Kalkman, L.; Skeers, P.; Roberts, J.A.; Bailey, J.; Dawson, T.J. Field metabolic rate, movement distance, and grazing pressures by western grey kangaroos (Macropus fuliginosus melanops) and Merino sheep (Ovis aries) in semi-arid Australia. Mamm. Biol. 2016, 81, 423–430. [Google Scholar] [CrossRef]
- Munn, A.J.; Skeers, P.; Kalkman, L.; McLeod, S.R.; Dawson, T.J. Water use and feeding patterns of the marsupial western grey kangaroo (Macropus fuliginosus melanops) grazing at the edge of its range in arid Australia, as compared with the dominant local livestock, the Merino sheep (Ovis aries). Mamm. Biol. 2014, 79, 1–8. [Google Scholar] [CrossRef]
- Dawson, T.J.; Maloney, S.K. Functional interactions between coat structure and colour in the determination of solar heat load on arid living kangaroos in summer: Balancing crypsis and thermoregulation. J. Comp. Physiol. B 2024, 194, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.J. Kangaroos—Biology of the Largest Marsupials, 2nd ed.; CSIRO Publishing: Melbourne, VIC, Australia, 2012. [Google Scholar]
- Andrew, M.H.; Lange, R.T. The spatial distributions of sympatric populations of kangaroos and sheep: Examples of dissociation between the species. Aust. Wildl. Res. 1986, 13, 367–373. [Google Scholar] [CrossRef]
- Dudzinski, M.L.; Lowe, W.A.; Müller, W.J.; Lowe, B.S. Joint use of habitat by red kangaroos and shorthorn cattle in arid central Australia. Aust. J. Ecol. 1982, 7, 69–74. [Google Scholar] [CrossRef]
- Frank, A.S.K.; Wardle, G.M.; Greenville, A.C.; Dickman, C.R. Cattle removal in arid Australia benefits kangaroos in high quality habitat but does not affect camels. Rangel. J. 2016, 38, 73–84. [Google Scholar] [CrossRef]
- Bell, F.C. Climate of Fowlers Gap Station. In Lands of Fowlers Gap Station, New South Wales; Research Series No. 3; Mabbutt, J.A., Ed.; The University of New South Wales: Sydney, NSW, Australia, 1973; pp. 45–64. [Google Scholar]
- Pickard, J. Illustrated Glossary of Australian Rural Fence Terms; Heritage Branch Report HB 09/01; Heritage Branch, News South Wales Department of Planning: Sydney, NSW, Australia, 2009.
- Burnham, K.P.; Anderson, D.R. The need for distance data in transect counts. J. Wildl. Manag. 1984, 48, 1248–1254. [Google Scholar] [CrossRef]
- Wolf, I.D.; Croft, D.B. Minimizing disturbance to wildlife by tourists approaching on foot or in a car: A study of kangaroos in the Australian rangelands. Appl. Anim. Behav. Sci. 2010, 126, 75–84. [Google Scholar] [CrossRef]
- Watson, D.M.; Dawson, T.J. The effects of age, sex, reproductive status, and temporal factors on the time-use of free-ranging red kangaroos (Macropus rufus) in western new South Wales. Wildl. Res. 1993, 20, 785–801. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R.; Laake, J.L. Estimating density from line transect sampling of biological populations. Wildl. Monogr. 1980, 72, 5–202. [Google Scholar]
- Dawson, T.J. Kangaroos—Biology of the Largest Marsupials; University of New South Wales Press: Sydney, NSW, Australia, 1995. [Google Scholar]
- Parer, I.; Wood, D.H. Further observations of the use of warren entrances as an index of the number of rabbits, Oryctolagus cuniculus. Aust. Wildl. Res. 1986, 13, 331–332. [Google Scholar] [CrossRef]
- Anderson, D.J. The home range: A new nonparametric estimation technique. Ecology 1982, 63, 103–112. [Google Scholar] [CrossRef]
- Van Winkle, W. Comparison of several probabilistic home-range models. J. Wildl. Manag. 1975, 39, 118–123. [Google Scholar] [CrossRef]
- Harris, S.; Cresswell, W.J.; Forde, P.G.; Trewhella, W.J.; Woollard, T.; Wray, S. Home range analysis using radio-tracking data: A review of problems and techniques particularly as applied to the study of mammals. Mammal Rev. 1990, 20, 97–123. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L. Resource Selection by Animals; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Manly, B.F.J. Multivariate Statistical Methods: A Primer; Chapman and Hall: New York, NY, USA, 1986. [Google Scholar]
- Day, G.I.; Schemnitz, S.D.; Taber, R.D. Capturing and marking wild animals. In Wildlife Management Techniques Manual, 4th ed.; Schemitz, S.D., Ed.; The Wildlife Society: Washington, DC, USA, 1980; pp. 61–88. [Google Scholar]
- Robertson, G.G.; Gepp, B. Capture of kangaroos by ‘stunning’. Aust. Wildl. Res. 1982, 9, 393–396. [Google Scholar] [CrossRef]
- Shepherd, N.C.; Hopwood, P.R.; Dostine, P.L. Capture myopathy: Two techniques for estimating its prevalence and severity in red kangaroos, Macropus rufus. Aust. Wildl. Res. 1988, 15, 83–90. [Google Scholar] [CrossRef]
- Croft, D.B. Behaviour of red kangaroos, Macropus rufus (Desmarest, 1822) in northwestern New South Wales, Australia. Aust. Mammal. 1981, 4, 5–58. [Google Scholar] [CrossRef]
- Clancy, T.F.; Croft, D.B. Home range of the common wallaroo, Macropus robustus erubescens, in far western New South Wales. Aust. Wildl. Res. 1990, 17, 659–673. [Google Scholar] [CrossRef]
- McRae, I.R. A Study of the Feral Goat in Far Western New South Wales. Master’s Thesis, University of New South Wales, Sydney, NSW, Australia, 1984. [Google Scholar]
- King, D. Home ranges of feral goats in a pastoral area in Western Australia. Wildl. Res. 1992, 19, 643–649. [Google Scholar] [CrossRef]
- Williams, K.; Parer, I.; Coman, B.; Burley, J.; Braysher, M. Managing Vertebrate Pests: Rabbits—Bureau of Resource Sciences, CSIRO Division of Wildlife and Ecology; Australian Government Publishing Service: Canberra, ACT, Australia, 1995.
- Schiffman, S.S.; Reynolds, M.L.; Young, F.W. Introduction to Multi-Dimensional Scaling; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Norusis, M.J. SPSS for Windows Professional Statistics Release 6.0; SPSS Inc.: Chicago, IL, USA, 1993. [Google Scholar]
- Scott, J.M.; Davis, F.W.; Csuti, B.; Noss, R.; Butterfield, B.; Groves, C.; Anderson, H.; Caicco, S.; D’Erchia, F.; Edwards, T.C.J.; et al. Gap Analysis: A geographic approach to protection of biological diversity. Wildl. Monogr. 1993, 123, 3–41. [Google Scholar]
- Conroy, M.J.; Noon, B.R. Mapping of species richness for conservation of biological diversity: Conceptual and methodological issues. Ecol. Appl. 1996, 6, 763–773. [Google Scholar] [CrossRef]
- Hooper, P.; Lunt, R.; Gould, A.; Hyatt, A.; Russell, G.; Kattenbelt, J.; Blacksell, S.; Reddacliff, L.; Kirkland, P.; Davis, R.; et al. Epidemic of blindness in kangaroos—Evidence of a viral aetiology. Aust. Vet. J. 1999, 77, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Edwards, G.P.; Croft, D.B.; Dawson, T.J. Observations of differential sex/age class mobility in red kangaroos (Macropus rufus). J. Arid Environ. 1994, 27, 169–177. [Google Scholar] [CrossRef]
- Witte, I. Kangaroos—Misunderstood and maligned reproductive miracle workers. In Kangaroos: Myths and Realities; Wilson, M., Croft, D.B., Eds.; Australian Wildlife Protection Council: Melbourne, VIC, Australia, 2005; pp. 118–207. [Google Scholar]
- Moss, G.L. Home Range, Grouping Patterns and the Mating System of the Red Kangaroo (Macropus rufus) in the Arid Zone. Ph.D. Thesis, University of New South Wales, Sydney, NSW, Australia, 1995. [Google Scholar]
- Bayliss, P.G. The population dynamics of red and western grey kangaroos in arid New South Wales, Australia. I. Population trends and rainfall. J. Anim. Ecol. 1985, 54, 111–125. [Google Scholar] [CrossRef]
- Bayliss, P.G. The population dynamics of red and western grey kangaroos in arid New South Wales, Australia. II. The numerical response function. J. Anim. Ecol. 1985, 54, 127–135. [Google Scholar] [CrossRef]
- Newsome, A.E. The distribution of red kangaroos, Megaleia rufa (Desmarest), about sources of persistent food and water in central Australia. Aust. J. Zool. 1965, 13, 289–299. [Google Scholar] [CrossRef]
- Newsome, A.E. Reproduction in natural populations of the red kangaroo, Megaleia rufa (Desmarest), in central Australia. Aust. J. Zool. 1965, 13, 735–759. [Google Scholar] [CrossRef]
- Bailey, P.T. The red kangaroo, Megaleia rufa (Desmarest), in north-western New South Wales—I. Movements. CSIRO Wildl. Res. 1971, 16, 11–28. [Google Scholar] [CrossRef]
- Priddel, D. The mobility and habitat utilization of kangaroos. In Kangaroos: Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Ealey, E.H.M.; Main, A.R. Ecology of the euro, Macropus robustus (Gould), in north-western Australia—III. Seasonal changes in nutrition. CSIRO Wildl. Res. 1967, 12, 53–65. [Google Scholar] [CrossRef]
- Caughley, G. Ecological relationships. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 159–187. [Google Scholar]
- Shepherd, N. Condition and recruitment of kangaroos. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 135–158. [Google Scholar]
- Short, J. Factors affecting food intake of rangeland herbivores. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 84–99. [Google Scholar]
- Moss, G.L.; Croft, D.B. Body condition of the red kangaroo (Macropus rufus) in arid Australia: The effect of environmental condition, sex and reproduction. Aust. J. Ecol. 1999, 24, 97–109. [Google Scholar] [CrossRef]
- Edwards, G.P. Competition between red kangaroos and sheep in arid New South Wales. Ph.D. Thesis, University of New South Wales, Sydney, NSW, Australia, 1990. [Google Scholar]
- Cairns, S.C.; Grigg, G.C. Population dynamics of red kangaroos (Macropus rufus) in relation to rainfall in the South Australian pastoral zone. J. Appl. Ecol. 1993, 30, 444–458. [Google Scholar] [CrossRef]
- Gilroy, J. Kangaroo monitoring in relation to kangaroo management in New South Wales. Aust. Zool. 1999, 31, 306–308. [Google Scholar] [CrossRef]
- Zanker, L. Kangaroos, drought and a frustrated landowner. Ecol. Manag. Restor. 2021, 22, 66. [Google Scholar] [CrossRef]
- Clancy, T.F.; Croft, D.B. Differences in habitat use and grouping behavior between macropods and eutherian herbivores. J. Mammal. 1991, 72, 441–449. [Google Scholar] [CrossRef]
- Newsome, A.E. The ages of non-breeding red kangaroos. Aust. Wildl. Res. 1977, 4, 7–11. [Google Scholar] [CrossRef]
- Johnson, C.N.; Bayliss, P.G. Habitat selection by sex, age and reproductive class in the red kangaroo, Macropus rufus, in western New South Wales. Aust. Wildl. Res. 1981, 8, 465–474. [Google Scholar] [CrossRef]
- Munn, A.; Dawson, T.J. Energy requirements of the red kangaroo (Macropus rufus): Impacts of age, growth and body size in a large desert-dwelling herbivore. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2003, 173, 575–582. [Google Scholar] [CrossRef]
- Frith, H.J.; Sharman, G.B. Breeding in wild populations of the red kangaroo, Megaleia rufa. CSIRO Wildl. Res. 1964, 9, 86–114. [Google Scholar] [CrossRef]
- McLeod, S. The Foraging Behaviour of the Arid Zone Herbivores, the Red Kangaroo (Macropus rufus) and the Sheep (Ovis aries) and Their Role in Its Competitive Interactions, Population Dynamics and Life-History Strategies. Ph.D. Thesis, University of New South Wales, Sydney, NSW, Australia, 1996. [Google Scholar]
- Robertson, G. The mortality of kangaroos in drought. Aust. Wildl. Res. 1986, 13, 349–354. [Google Scholar] [CrossRef]
- Reddacliff, L.; Kirkland, P.; Philbey, A.; Davis, R.; Vogelnest, L.; Hulst, F.; Blyde, D.; Deykin, A.; Smith, J.; Hooper, P.; et al. Experimental reproduction of viral chorioretinitis in kangaroos. Aust. Vet. J. 1999, 77, 522–528. [Google Scholar] [CrossRef]
- Tyndale-Biscoe, C.H.; Renfree, M.B. Reproductive Physiology of Marsupials; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Barker, R.D. The diet of herbivores in the sheep rangelands. In Kangaroos Their Ecology and Management in the Sheep Rangelands of Australia; Caughley, G., Shepherd, N., Short, J., Eds.; Cambridge University Press: Cambridge, UK, 1987; pp. 69–83. [Google Scholar]
- McCarron, H.C.; Buffenstein, R.; Fanning, F.D.; Dawson, T.J. Free-ranging heart rate, body temperature and energy metabolism in eastern grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus) in the arid regions of South East Australia. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2001, 171, 401–411. [Google Scholar]
- McCarron, H.C.K.; Dawson, T.J. Thermal relations of Macropodoidea in hot environments. In Kangaroos, Wallabies and Rat-kangaroos; Grigg, G.C., Jarman, P.J., Hume, I.D., Eds.; Surrey Beatty & Sons: Sydney, NSW, Australia, 1989; Volume 1, pp. 255–263. [Google Scholar]
- Dawson, T.J.; Ellis, B.A. Diets of mammalian herbivores in Australian arid, hilly shrublands: Seasonal effects on overlap between euros (hill kangaroos), sheep and feral goats, and on dietary niche breadths and electivities. J. Arid Environ. 1996, 34, 491–506. [Google Scholar] [CrossRef]
- Clancy, T.F.; Croft, D.B. Space use patterns of the Common Wallaroo, Macropus robustus erubescens, in the arid zone. In Kangaroos, Wallabies and Rat-Kangaroos; Grigg, G.C., Jarman, P.J., Hume, I.D., Eds.; Surrey Beatty: Sydney, NSW, Australia, 1989; Volume 2, pp. 603–609. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant; O. and B. Books: Corvallis, OR, USA, 1982. [Google Scholar]
- Dawson, T.J.; Denny, M.J.S.; Russell, E.M.; Ellis, B.A. Water usage and diet preferences of free ranging kangaroos, sheep and feral goats in the Australian arid zone during summer. J. Zool. 1975, 177, 1–23. [Google Scholar] [CrossRef]
- Myers, K.; Parker, B.S. A study of the biology of the wild rabbit in climatically different regions in eastern Australia. VI. Changes in numbers and distribution related to climate and land systems in semi-arid north-western New South Wales. Aust. Wildl. Res. 1975, 2, 11–32. [Google Scholar] [CrossRef]
- Cooke, B.D. Factors limiting the distribution of the wild rabbit in Australia. Proc. Ecol. Soc. Aust. 1977, 10, 113–120. [Google Scholar]
- Belovsky, G.E.; Schmitz, O.J.; Slade, J.B.; Dawson, T.J. Effects of spines and thorns on Australian arid zone herbivores of different body masses. Oecologia 1991, 88, 521–528. [Google Scholar] [CrossRef]
- Parer, I. The population ecology of the wild rabbit, Oryctolagus cuniculus (L.), in a mediteranean-type climate in New South Wales. Aust. Wildl. Res. 1977, 4, 171–205. [Google Scholar] [CrossRef]
- Newsome, A.E. Biological interactions. In Proceedings of the Australian Rabbit Control Conference, Adelaide, SA, Australia, 2–3 April 1993; Cooke, B.D., Ed.; Anti-Rabbit Research Foundation of Australia: Adelaide, SA, Australia, 1993; pp. 23–25. [Google Scholar]
- Silva, L.M.; Croft, D.B. Nest-site selection, diet and parental care of the Wedge-tailed Eagle Aquila audax in Western New South Wales. Corella 2007, 31, 23–31. [Google Scholar]
- Parkes, J.; Henzell, R.; Pickles, G. Managing Vertebrate Pest: Feral Goats; Government Publishing Service: Canberra, ACT, Australia, 1996. [Google Scholar]
- Coulson, G. Forage biomass and kangaroo populations (Marsupialia: Macropodidae) in summer and autumn at Hattah-Kulkyne National Park, Victoria. Aust. Mammal. 1990, 13, 215–218. [Google Scholar] [CrossRef]
- Coulson, G. Habitat separation in the grey kangaroos, Macropus giganteus Shaw and M. fuliginosus (Desmarest) (Marsupialia: Macropodidae), in Grampians National Park, western Victoria. Aust. Mammal. 1990, 13, 33–40. [Google Scholar]
- Terpstra, J.W.; Wilson, A.D. Grazing distribution of sheep and kangaroos in a semi-arid woodland. Appl. Anim. Behav. Sci. 1989, 24, 343–352. [Google Scholar] [CrossRef]
- Newsome, A.E. Competition between wildlife and domestic stock. Aust. Vet. J. 1971, 47, 577–586. [Google Scholar] [CrossRef]
- Norbury, G.L.; Norbury, D.C.; Hacker, R.B. Impact of red kangaroos on the pasture layer in the Western Australian arid zone. Rangel. J. 1993, 15, 12–23. [Google Scholar] [CrossRef]
- Coulson, G. Use of heterogenous habitat by the Western Grey Kangaroo, Macropus fuliginosus. Wildl. Res. 1993, 20, 137–149. [Google Scholar] [CrossRef]
- Cairns, S.C.; Pople, A.R.; Grigg, G.C. Density distributions and habitat associations of red kangaroos, Macropus rufus, and western grey kangaroos, M. fuliginosus, in the South Australian Pastoral Zone. Wildl. Res. 1991, 18, 377–402. [Google Scholar] [CrossRef]
- Lynch, J.J.; Hinch, G.N.; Adams, D.B. The Behaviour of Sheep: Biological Principles and Implications for Production; CSIRO Publications: East Melbourne, VIC, Australia, 1992. [Google Scholar]
- Anderson, V.J.; Hodgkinson, K.N. Grass-mediated capture of resource flows and the maintenance of banded mulga in a semi-arid woodland. Aust. J. Bot. 1997, 45, 331–342. [Google Scholar] [CrossRef]
- Squires, V.R. Walking, watering and grazing behaviour of merino sheep on two semi-arid rangelands in south-western New South Wales. Aust. Rangel. J. 1976, 1, 13–23. [Google Scholar] [CrossRef]
- Leigh, J.H.; Wood, D.H.; Holgate, M.D.; Slee, A.; Stanger, M.G. Effects of rabbit and kangaroo grazing on two semi-arid grassland communities in central-western New South Wales. Aust. J. Bot. 1989, 37, 375–396. [Google Scholar] [CrossRef]
- Caughley, G. What is this thing called carrying capacity? In North American Elk: Ecology, Behaviour and Management; Boyce, M.S., Hayden-Wing, L.D., Eds.; University of Wyoming Press: Laramie, WY, USA, 1979. [Google Scholar]
- Caughley, G.; Short, J.; Grigg, G.C.; Nix, H. Kangaroos and climate: An analysis of distribution. J. Anim. Ecol. 1987, 56, 751–761. [Google Scholar] [CrossRef]
- McLeod, S.R. Is the concept of carrying capacity useful in variable environments? Oikos 1997, 79, 529–542. [Google Scholar] [CrossRef]
- Hobbs, N.T.; Hanley, T.A. Habitat evaluation: Do use/availability data reflect carrying capacity? J. Wildl. Manag. 1990, 54, 515–522. [Google Scholar] [CrossRef]
- Dawson, T.J.; Ellis, B.A. Competition between native and introduced herbivores in the Australian arid zone. In Arid Australia: Proceedings of a Symposium on the Origins, Biota and Ecology of Australia’s Arid Regions; Cogger, H.G., Cameron, E.E., Eds.; Australian Museum: Sydney, NSW, Australia, 1984. [Google Scholar]
- Jaremovic, R.V.; Croft, D.B. Social organisation of the eastern grey kangaroo (Macropodidae, Marsupialia) in southeastern New South Wales. I. Groups and group home ranges. Mammalia 1991, 55, 169–185. [Google Scholar]
- Caughley, G.; Grice, D.; Barker, R.; Brown, B. The edge of the range. J. Anim. Ecol. 1988, 57, 771–785. [Google Scholar] [CrossRef]
- Blaney, C.E.; Dawson, T.J.; McCarron, H.C.K.; Buffenstein, R.; Krockenberger, A.K. Water metabolism and renal function and structure in eastern grey kangaroos (Macropus giganteus): Response to water deprivation. Aust. J. Zool. 2000, 48, 335–345. [Google Scholar] [CrossRef]
- Dawson, T.J.; Blaney, C.E.; McCarron, H.C.K.; Maloney, S.K. Dehydration, with and without heat, in kangaroos from mesic and arid habitats: Different thermal responses including varying patterns in heterothermy in the field and laboratory. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2007, 177, 797–807. [Google Scholar] [CrossRef]
- Dawson, T.J.; Blaney, C.E.; Munn, A.J.; Krockenberger, A.K.; Maloney, S.K. Thermoregulation by kangaroos from mesic and arid habitats: Influence of temperature on routes of heat loss in grey kangaroos (Macropus giganteus) and red kangaroos (Macropus rufus). Physiol. Biochem. Zool. 2000, 73, 374–381. [Google Scholar] [CrossRef]
- Frith, H.J. Mobility of the red kangaroo, Megaleia rufa. CSIRO Wildl. Res. 1964, 9, 1–19. [Google Scholar] [CrossRef]
- Denny, M.J.S. Red Kangaroo Arid Zone Studies; Final Report to the National Parks and Wildlife Service; National Parks and Wildlife Service: Canberra, ACT, Australia, 1980. [Google Scholar]
- Norbury, G.L.; Norbury, D.C. The distribution of red kangaros in relation to range regeneration. Rangel. J. 1993, 15, 3–11. [Google Scholar] [CrossRef]
- Priddel, D. Habitat utilisation by sympatric red kangaroos Macropus rufus, and western grey kangaroos M. fuliginosus, in western New South Wales. Aust. Wildl. Res. 1988, 15, 413–421. [Google Scholar] [CrossRef]
- Herbert, C.A. Long-acting contraceptives: A new tool to manage overabundant kangaroo populations in nature reserves and urban areas. Aust. Mammal. 2004, 26, 67–74. [Google Scholar]
- Hunt, M.; Slotnick, B.; Croft, D. Olfactory function in the red kangaroos (Macropus rufus) assessed using odor-cued taste avoidance. Physiol. Behav. 1999, 67, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Pepler, A.; Osman, M. Recent trends in extratropical lows and their rainfall over Australia. J. South. Hemisph. Earth Syst. Sci. 2024, 74, ES24002. [Google Scholar] [CrossRef]
- Read, J.L.; Wilson, G.R.; Coulson, G.; Cooney, R.; Paton, D.C.; Moseby, K.E.; Snape, M.A.; Edwards, M.J. Improving Kangaroo Management: A Joint Statement. Ecol. Manag. Restor. 2021, 22, 186–192. [Google Scholar] [CrossRef]







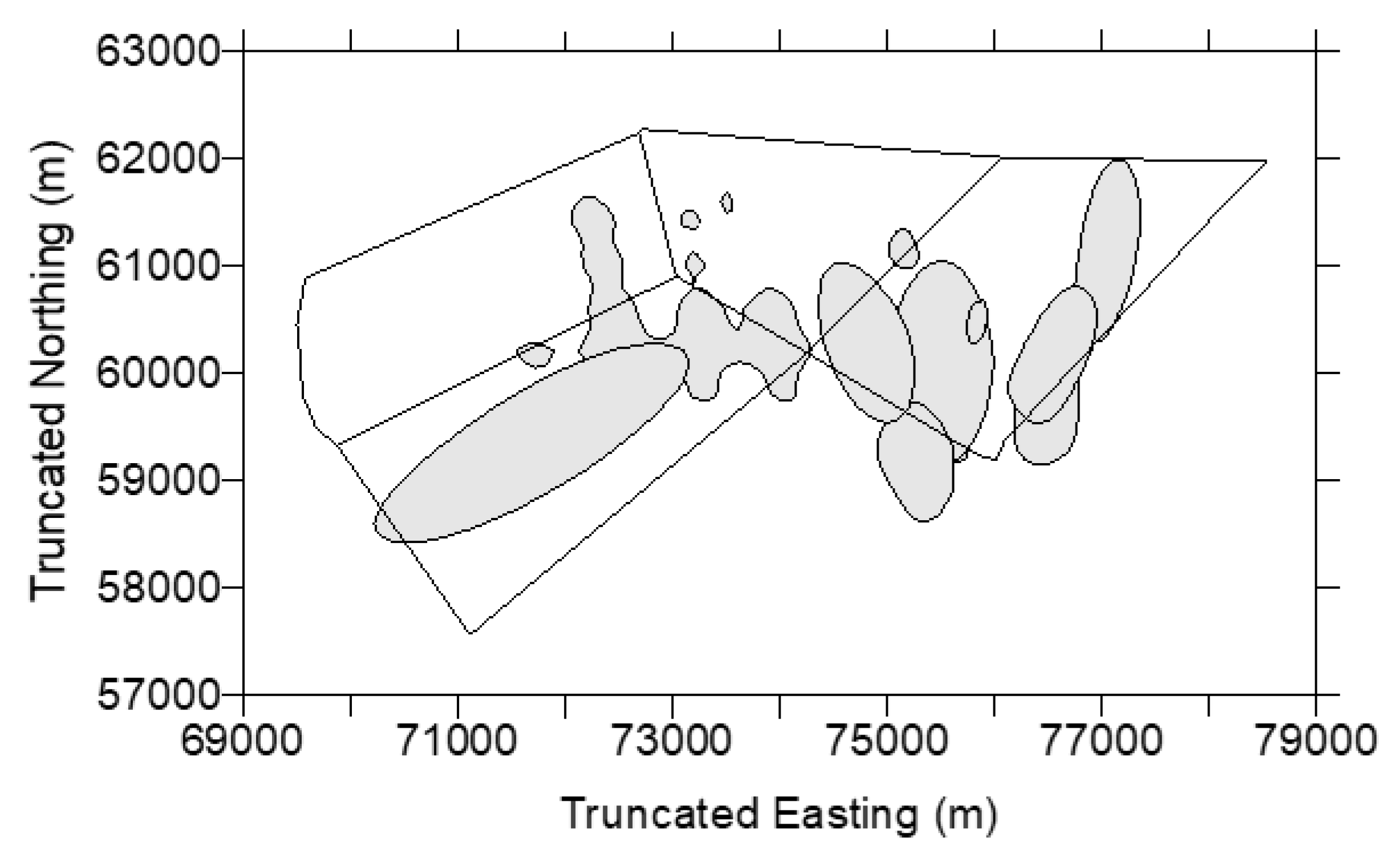
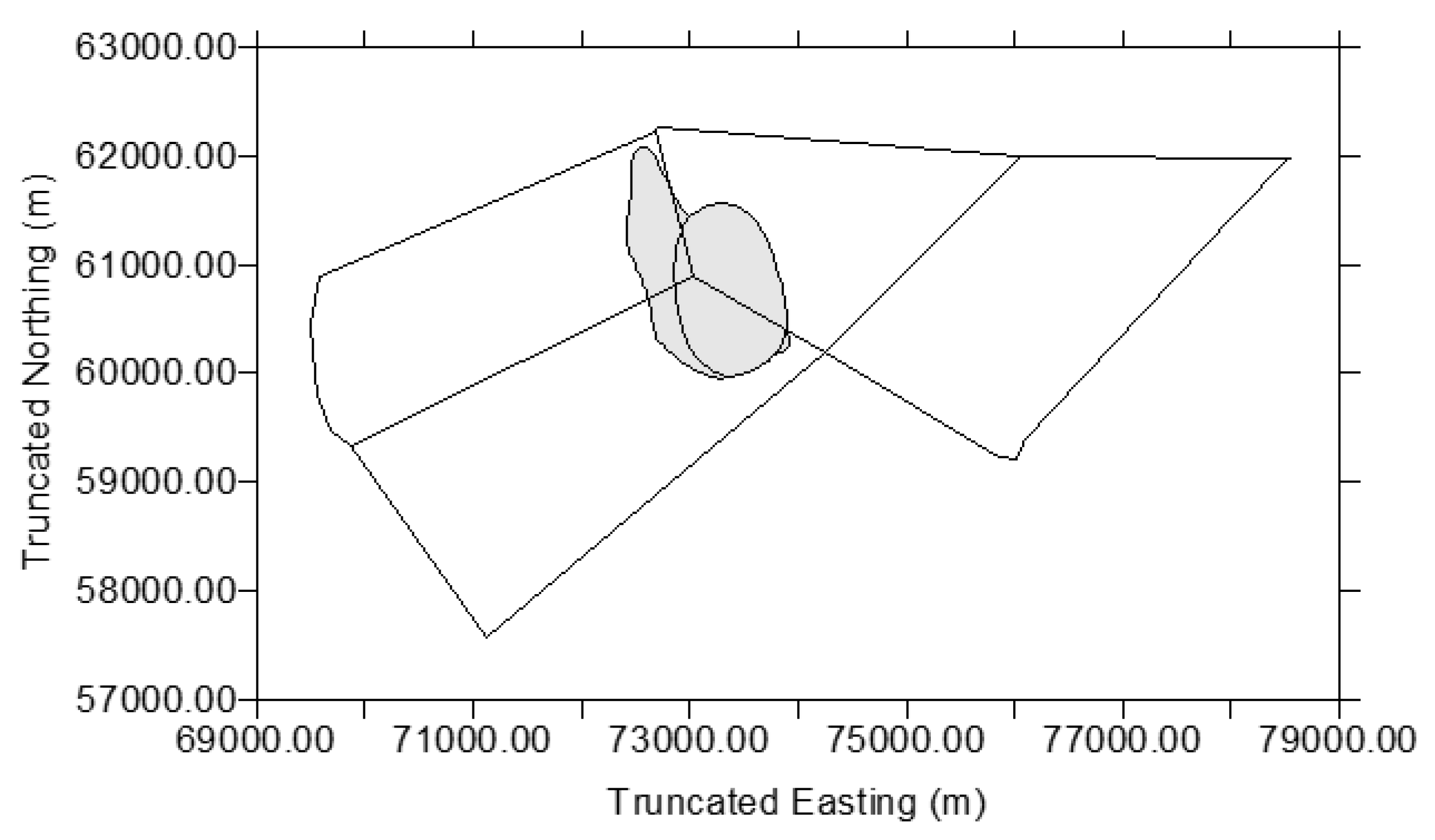
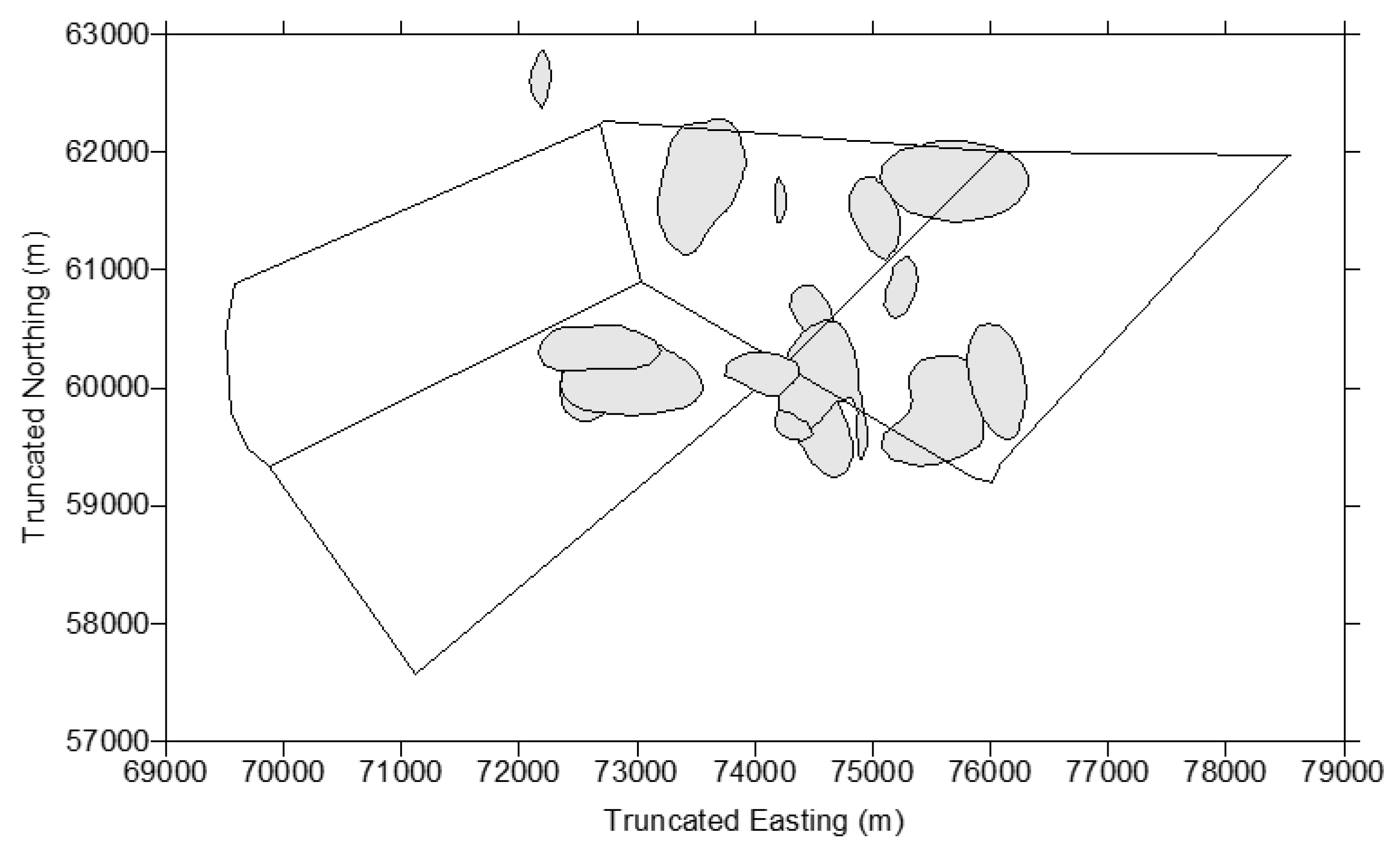
| Red Kangaroos (n = 34) | Western Grey Kangaroos (n = 15) | Eastern Grey Kangaroos (n = 5) | |
|---|---|---|---|
| Mean weight ± SE Range | 27.1 ± 0.6 21–33 | 26.5 ± 0.7 23.5–34 | 24.7 ± 0.9 23–28 |
| Mean locations ± SE Range | 69 ± 6 9–133 | 81 ± 12 10–140 | 72 ± 25 10–140 |
| Mean days tracked ± SE Range | 562 ± 51 90–1023 | 612 ± 86 63–1023 | 537 ± 191 68–1005 |
| Habitat Characteristic | Variable 1 |
|---|---|
| Geographic | Slope (contour at 5 m intervals ASL) |
| Distance from water (m) | |
| Vegetation 2 | Tree density (number ha−1) |
| Proportion of green grass biomass | |
| Proportion of green forb biomass | |
| Copperburr biomass (kg ha−1) | |
| Flat-leafed chenopod biomass (kg ha−1) | |
| Round-leafed chenopod biomass (kg ha−1) | |
| Herbivore density | Red kangaroo (abundance ha−1) |
| Western grey kangaroo (abundance ha−1) | |
| Common wallaroo (abundance ha−1) | |
| Sheep (abundance ha−1) | |
| Rabbit (abundance ha−1) |
| Size/Sex Class | Unstocked | Stocked | p |
|---|---|---|---|
| Adult female | 0.14 ± 0.01 | 0.08 ± 0.01 | *** |
| Adult male | 0.06 ± 0.01 | 0.04 ± 0.01 | * |
| Juvenile/YAF | 0.05 ± 0.01 | 0.03 ± 0.01 | *** |
| Year | Red Kangaroo 1 | Western Grey Kangaroo 2 | Sheep 3 |
|---|---|---|---|
| 1 | 43 | - | 80 |
| 2 | 16 | 49 | 84 |
| 3 | 38 | 98 | 96 |
| Age Class | ≤3 y | 4–12 y | >12 y |
|---|---|---|---|
| Female | 8 | 44 | 48 |
| Male | 4 | 28 | 68 |
| Landclass | E. Grey | Wallaroo | Red | W. Grey | Sheep | Rabbit |
|---|---|---|---|---|---|---|
| M12 | ✓ | - | ||||
| M15 | - | - | ✓ | |||
| M16 | - | - | ✓ | |||
| L19 | - | - | ✕ | ✓ | ✓ | |
| M20 | - | - | - | ✓ | ||
| L20 | - | - | ✕ | ✓ | ||
| M22 | - | ✓ | ✕ | - | ||
| C25 | ✓ | - | ✓ | |||
| N26 | - | ✓ | ||||
| N31 | - | ✓ | ||||
| C33 | ✓ | ✕ | - | |||
| N33 | - | ✓ | ✓ | |||
| N37 | - | ✓ | ✕ | ✓ |
| P | Red | W Grey | Wallaroo | Rabbit | Sheep | |
|---|---|---|---|---|---|---|
| S2 | Red | 30.1, 36.8, 28.7 | 0, 1.1, 0 | 0.7, 12.6, 19.0 | ||
| W grey | 31.1, 23.2, 45.5 | 0, 2.9, 0 | 1.5, 21.0, 27.3 | |||
| Wallaroo | ||||||
| Rabbit | 0, 16.7, 0 | 0, 6.7, 0 | 37.5, 33.3, 62.5 | |||
| Sheep | 2.9, 10.9, 29.2 | 5.9, 26.6, 26.5 | 8.8, 1.8, 4.4 | |||
| S1 | Red | 28.2, 14.3, 22.0 | 28.9, 7.7, 8.5 | 1.4, 2.4, 4.3 | 0.7, 11.3, 12.1 | |
| W grey | 49.4, 49.0, 56.4 | 14.8, 0, 3.6 | 0, 0, 0 | 12.3, 36.7, 41.8 | ||
| Wallaroo | 36.3, 14.3, 38.7 | 10.6, 0, 6.5 | 0, 12.1, 16.1 | |||
| Rabbit | 6.1, 16.7, 20.0 | 0, 0, 0 | 15.2, 20.8, 13.3 | 4.4, 5.5, 12.9 | 0, 12.5, 36.7 | |
| Sheep | 1.6, 17.8, 10.5 | 15.6, 16.8, 14.2 | 0, 10.3, 3.1 | 0, 2.8, 6.8 | ||
| U2 | Red | 35.9, 13.2, 33.0 | 6.8, 8.8, 10.3 | 1.0, 0, 0 | ||
| W grey | 48.7, 21.7, 49.2 | 17.1, 39.1, 13.8 | 3.9, 0, 1.5 | |||
| Wallaroo | 53.8, 22.7, 50.0 | 100, 61.4, 45.0 | 0, 0, 0 | |||
| Rabbit | 12.5, 0, 0 | 37.5, 0, 14.3 | 0, 0, 0 | |||
| U1 | Red | 3.5, 18.9, 13.9 | 4.9, 0, 16.8 | 1.4, 0.8, 0.7 | ||
| Wallaroo | 45.5, 38.5, 35.2 | 0, 0, 0 | 0, 1.5, 0 | |||
| Euro | 15.9, 0, 31.9 | 0, 0, 0 | 15.9, 12.1, 6.9 | |||
| Rabbit | 9.1, 6.3, 6.3 | 0, 6.3, 0 | 31.8, 25.0, 31.3 |
| Variable | Red Kangaroo | Western Grey Kangaroo |
|---|---|---|
| Red density | 0.39 | 0.12 |
| Western grey density * | 0.24 | 2.02 |
| Sheep density * | −0.22 | 0.82 |
| Wallaroo density | −0.70 | −0.83 |
| Rabbit density | −0.45 | −0.53 |
| Copperburr biomass | 0.06 | −0.04 |
| a Green forb biomass | −0.02 | 0.00 |
| b Green grass biomass | −0.04 | 0.02 |
| FLC biomass | −0.54 | −0.49 |
| RLC biomass | −0.04 | 0.11 |
| Tree density | 0.47 | 1.87 |
| Slope | −0.49 | −0.52 |
| Water distance | −0.32 | −0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croft, D.B.; Witte, I. Mobility Confers Resilience in Red Kangaroos (Osphranter rufus) to a Variable Climate and Coexisting Herbivores (Sheep, Goats, Rabbits and Three Sympatric Kangaroo Species) in an Arid Australian Rangeland. Diversity 2025, 17, 389. https://doi.org/10.3390/d17060389
Croft DB, Witte I. Mobility Confers Resilience in Red Kangaroos (Osphranter rufus) to a Variable Climate and Coexisting Herbivores (Sheep, Goats, Rabbits and Three Sympatric Kangaroo Species) in an Arid Australian Rangeland. Diversity. 2025; 17(6):389. https://doi.org/10.3390/d17060389
Chicago/Turabian StyleCroft, David B., and Ingrid Witte. 2025. "Mobility Confers Resilience in Red Kangaroos (Osphranter rufus) to a Variable Climate and Coexisting Herbivores (Sheep, Goats, Rabbits and Three Sympatric Kangaroo Species) in an Arid Australian Rangeland" Diversity 17, no. 6: 389. https://doi.org/10.3390/d17060389
APA StyleCroft, D. B., & Witte, I. (2025). Mobility Confers Resilience in Red Kangaroos (Osphranter rufus) to a Variable Climate and Coexisting Herbivores (Sheep, Goats, Rabbits and Three Sympatric Kangaroo Species) in an Arid Australian Rangeland. Diversity, 17(6), 389. https://doi.org/10.3390/d17060389







