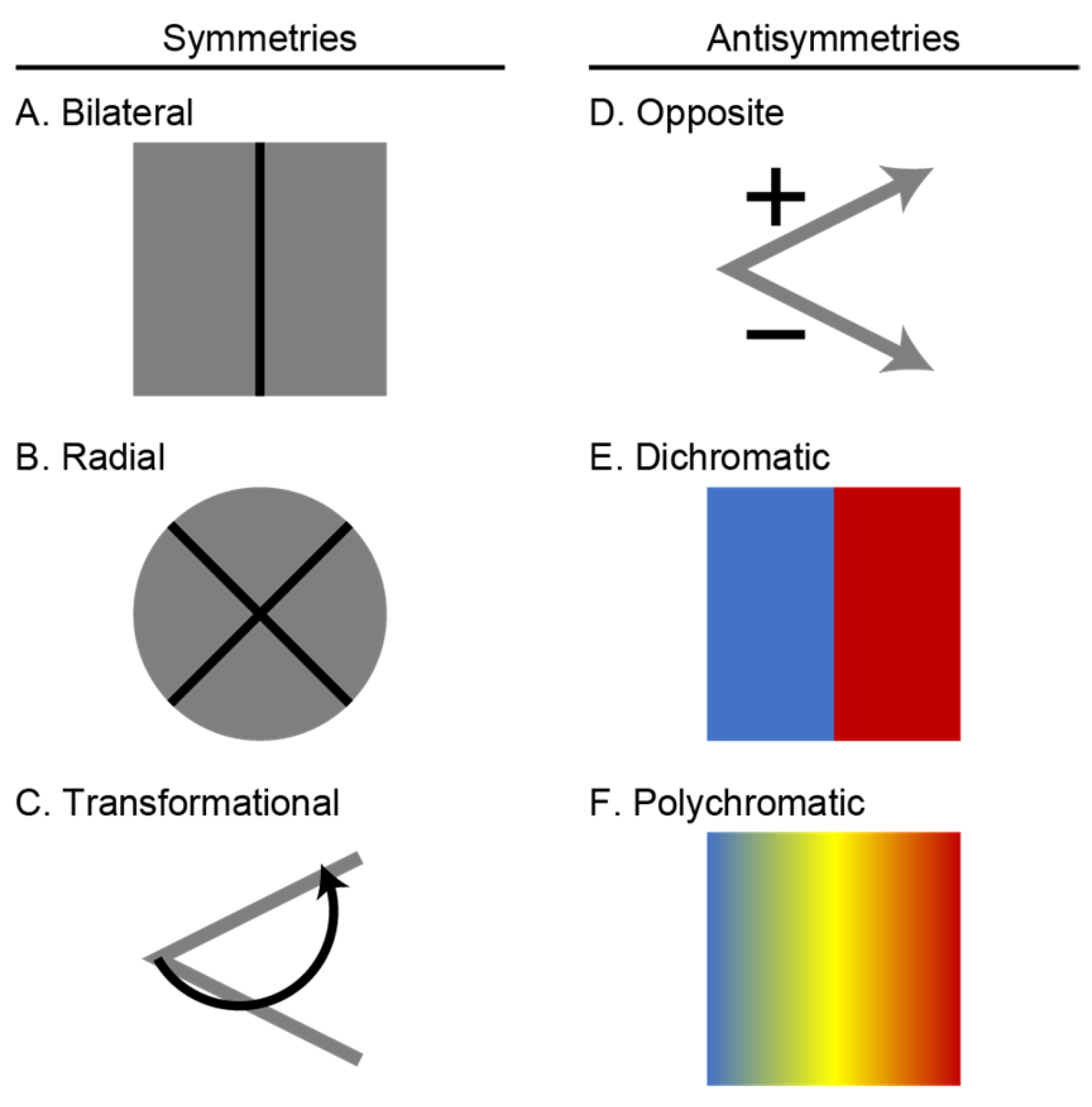
1. Introduction
Organic symmetry implies a wide range of evolutionary phenomena, including patterns of phylogenetic relationships [
1,
2], progressive change [
3,
4], co-adaptation of form and function [
5], developmental canalization [
6], esthetic preferences [
7,
8], and missing phenomena for apparent asymmetries [
9,
10]. Despite this rich potential, most evolutionary applications of symmetry consider only structural morphology in terms of body shapes, i.e., bilateral (vertebrates), radial (anthozoans), pentameral (echinoderms), or other geometric forms (
Figure 1A,B). However, symmetry concepts can be abstracted into various contexts. Thus, symmetry can also be applied to the physical geometry of any form that can be divided into a scheme of similar pieces. In this case, symmetry is still demonstrated if the form retains its overall shape despite transformations that move around the individual pieces. The specific symmetry then depends on piecemeal organization and transformation, which can include one (reflection, rotation, scaling, or translation) or more (glide reflection) operations [
11,
12]. For example, in simple line or reflection symmetries, features retain their inter-relationships when flipped across a dividing line or axis (
Figure 1C). This more formal type of symmetry can be further expanded to include operations that reverse the form or function, referred to as antisymmetry (
Figure 1D–F). Such considerations reveal that symmetry concepts can be applied to any situation wherein the underlying phenomena are preserved, despite arbitrary quantitative transformations. For this reason, symmetry has been generalized to mean
invariance under any transformation, which can apply just as well to a process as to a pattern [
13]. Moreover, disruptions to these relationships can be viewed as broken symmetries of the corresponding cases [
10]. Therefore, generalized symmetry concepts appear fundamental to the study of ecology and evolution, as they provide simplified and predictive frameworks that can help distinguish general phenomena amidst the diversity of nature.
In this regard, an expanded conception of symmetries may be an underappreciated aspect of the exceptional diversity in organismal colorations and their physical bases (wavelengths). In addition to more typical color symmetries associated with body form, color antisymmetries incorporate the reversal of wavelengths by which long (“red”) ones are interchanged with short (“blue”) ones [
14]. These reversals are dichromatic if only two colors or wavelengths are involved, or polychromatic if many are involved, as in a gradient (
Figure 1E,F). It is therefore noteworthy that animals that manipulate carotenoid pigments as plumage colorants appear to favor visible red shifts in response to an increased uptake of carotenoids in their diet, which is the ultimate source of these epidermal biochromes in virtually all animals [
15]. Thus, numerous asymmetric red shifts (to longer wavelengths), but no comparable blue shifts (to shorter wavelengths), have been documented within and among individuals or species that ingest greater quantities of these human-perceived yellow, orange, and red pigments in their diets [
16,
17,
18,
19,
20,
21,
22,
23]. Although perceptions of light are subjective, red shifts have an objective physical basis because of the association between perceived carotenoid yellowness and redness with distinct absorbance and reflectance properties [
24,
25,
26]. These dietary red shifts also transcend pigmentation details—being expressed by a yellower (shorter wavelength) pigment class at lower to a redder (longer wavelength) pigment class at higher dietary carotenoid levels [
16,
21]—by a single pigment class (e.g., concentrations of yellow, orange, or red) across dietary carotenoid levels [
16,
17,
20,
27,
28,
29], through various mechanisms influencing feather characteristics, such as thickness, nanostructures, and pigment × protein interactions alone or in combination [
20,
21,
22,
30,
31], and by patches or plumages [
20,
21]. Thus, plumage red shifts appear to be general physical phenomena not associated with any particular divergence level, chemistry, or anatomy, suggesting their pertinence to general patterns and processes. These shifts are of fundamental biological importance, as animals appear to have exploited connections between ecology and red shifts to provide visual information regarding the underlying qualities that affect individual survival and reproduction related to foraging, health, and fitness costs and benefits of carotenoid use [
18,
32,
33,
34,
35]. Despite intensive studies, current asymmetries in carotenoid-based plumage expression require further exploration to determine if they are part of even broader symmetries.
A latent capacity for carotenoid-related blue shifts as opposed to red shifts and insights into how they may arise are suggested by subtle differences in plumage carotenoids between birds with ultraviolet-sensitive (UVS) or violet-sensitive (VS) tetrachromatic color vision systems [
36,
37]. Many diurnal birds with either of these main color vision systems develop various yellow-, orange-, and red-carotenoid-based plumages. However, a relation between plumage and visual pigment characteristics is suggested by an alignment between the maximal absorption wavebands of plumage carotenoids and those of the V and S single-cones distinguishing the color vision systems (350–500 nm) in the four-cone array (λ of maximum sensitivity ranked V
Violet < S
Short < M
Medium < L
Long). This connection appears to be borne out by evidence that the average spectral location of plumage carotenoid absorption maxima (i.e., of reflectance minima, λR
min) occurs at longer wavelengths for UVS Passeriformes [
21,
38] and Trogoniformes [
26] compared with VS Piciformes and Coraciiformes [
26]. Notably, the average spectral locations of λR
min are extremely similar between carotenoids in VS Piciformes–Coraciiformes and colorful plumage porphyrins unique to VS non-passerines (Galliformes, Musophagiformes, and Charadriiformes) [
39]. Thus, λR
min values for colored plumage pigments appear relatively “red-shifted” (to longer wavelengths) for UVS and relatively “blue-shifted” (to shorter wavelengths) for VS. The quantitative alignments of the λR
min of plumage carotenoids and porphyrins with diagnostic V and S single-cone maximum sensitivity values in each color vision system suggest that concerted UVS-red versus VS-blue shifts in λR
min have some relationship with the key differences between visual systems [
26,
38,
39]. These various shifts and alignments could relate to signaling as λR
min integrates many informational pigment properties, including their chemistry, optical density, and dietary or metabolic origin. The possible salience of wavelength-based shifts described for λR
min seems less intuitive than that of those described for reflectance maxima. Nevertheless, pigments create color via selective wavelength subtraction (absorption) from the white light spectrum [
24]. Hence, absorption and reflection are complementary phenomena in pigment-based color mechanisms.
Considering these patterns, sampling bias could explain the apparent asymmetry in shift patterns if the red-biased UVS system is the only one considered. This explanation is plausible, as relevant studies have focused mainly on the extraordinarily diverse Passerida passerine clade [
25,
40,
41], whose members appear exclusively UVS [
42,
43,
44]. To broaden the exploration of wavelength-based shifts in carotenoid-based plumage evolution, this study analyzes carotenoid-based plumage shift patterns in relation to dietary carotenoid composition in VS Piciformes–Coraciiformes with the physical blue shift in average λR
min described above. This species-rich assemblage, which includes woodpeckers, barbets, toucans, and bee-eaters, provides several advantages for examining plumage-shift dynamics. First, many members of the VS Piciformes–Coraciiformes orders have developed spectacular carotenoid-based plumages comparable to or rivaling those of many UVS groups [
45,
46,
47,
48]. Second, the VS piciform–coraciiform group is minimally paraphyletic in that only a single derived, depauperate, and carotenoid-lacking lineage (Momotidae) has evolved the UVS characteristic within this radiation [
37,
49,
50], providing a practical way to define the study system [
26]. Third, the VS Piciformes–Coraciiformes have undergone dramatic diversification in their diets, covering the gamut of carotenoid contents in avian foods [
51,
52,
53,
54,
55]. Finally, a large body of evidence from wild [
56,
57] and captive [
58,
59,
60,
61] VS Piciformes–Coraciiformes strongly suggests that their carotenoid-based plumages are highly sensitive to dietary changes. Therefore, morphological, historical, ecological, and physiological factors appear to favor a response of carotenoid-based plumages to diet among VS Piciformes–Coraciiformes.
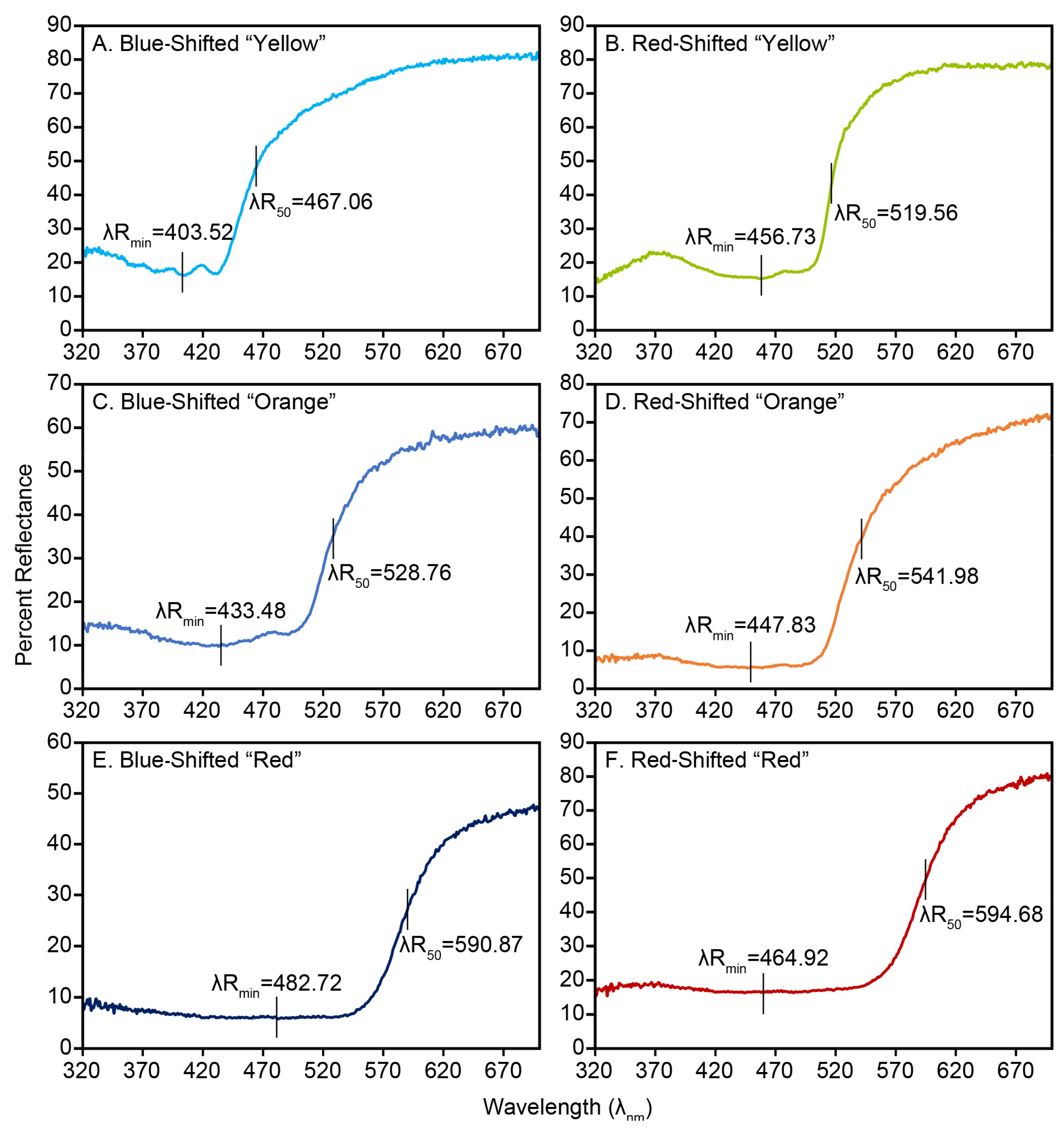
I evaluated carotenoid-based plumage responses to diet by VS Piciformes–Coraciiformes by analyzing carotenoid pigment classes and their key spectral features in the visible range of diurnal birds. These attributes were treated as standardized physical measures of fundamental traits potentially related to shift patterns that could be compared across taxa and used to diagnose physical and chemical processes that underlie plumage information content. Subjective visual models are not applied because of the paucity of realistic parameter values and categorical color spaces assigned for Piciformes–Coraciiformes [
62]. Relevant to the physical approach of evaluating dietary responses in relation to color vision, contour plumage carotenoids of UVS birds actually respond to higher dietary carotenoids with three forms of red shift. More intuitively, the characteristic main reflectance band shifts to longer wavelengths as dietary carotenoid amounts increase [
16,
17,
20,
28,
63,
64]. Less intuitively, the prominence of a characteristic secondary reflectance band at shorter wavelengths diminishes greatly as dietary carotenoid amounts increase [
20,
21,
22], reinforcing the parallel long-wavelength bias of the main reflectance band [
22]. Both of these responses are related to λR
min, which marks the boundary between the main (at its short-wavelength end) and secondary (at its long-wavelength end) reflectance bands. Consequently, λR
min can also be red-shifted at relatively high dietary carotenoid levels [
21]. Thus, wavelength-based reflectance enables shift patterns (wavelength compositions as longer = “redder” or shorter = “bluer”) and processes (chemistry, cost, and benefits) for these spectral components to be compared objectively on the same scale across studies, physical components, and color vision systems. Therefore, the aims of this study are twofold: (1) to assess shift
pattern symmetries in functional responses to dietary carotenoid contents across pigment classes and spectral features as related to the color vision system; (2) to explore internal evidence that shift
process symmetries in carotenoid limitations, honest signaling, and other factors may underlie these patterns. The results support the importance of the symmetry principles in plumage carotenoids, similar to other photonic systems.
4. Discussion
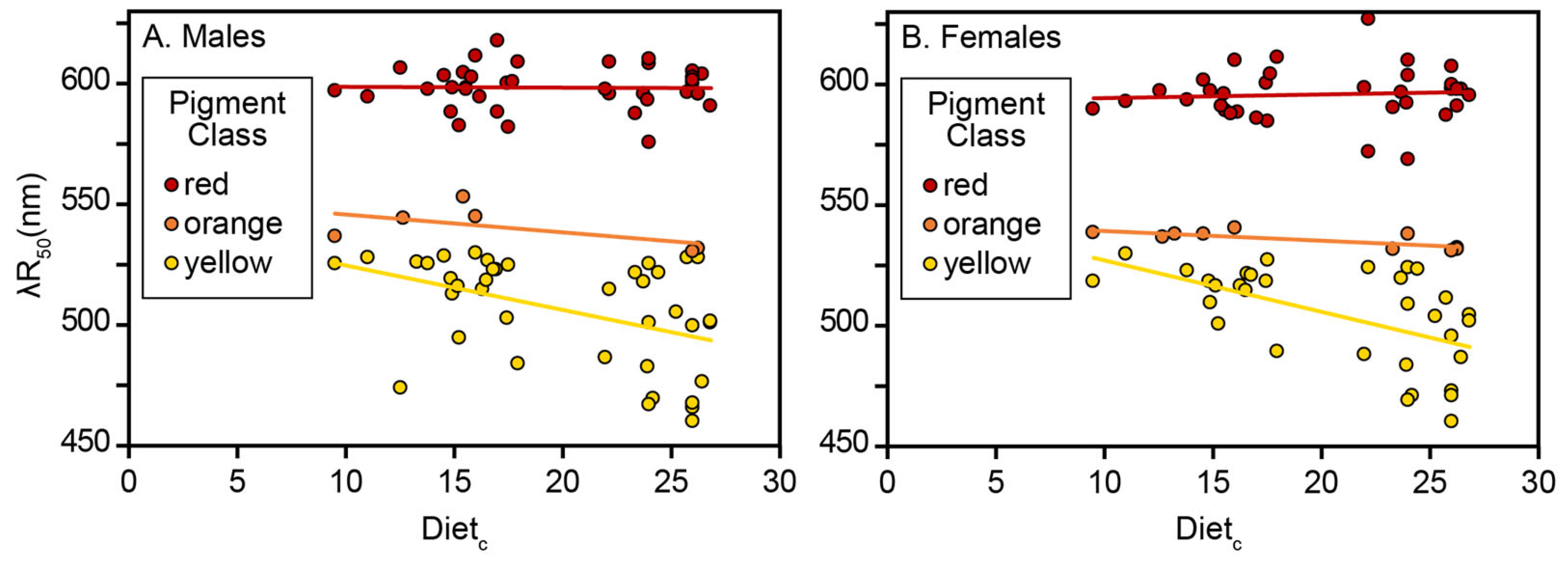
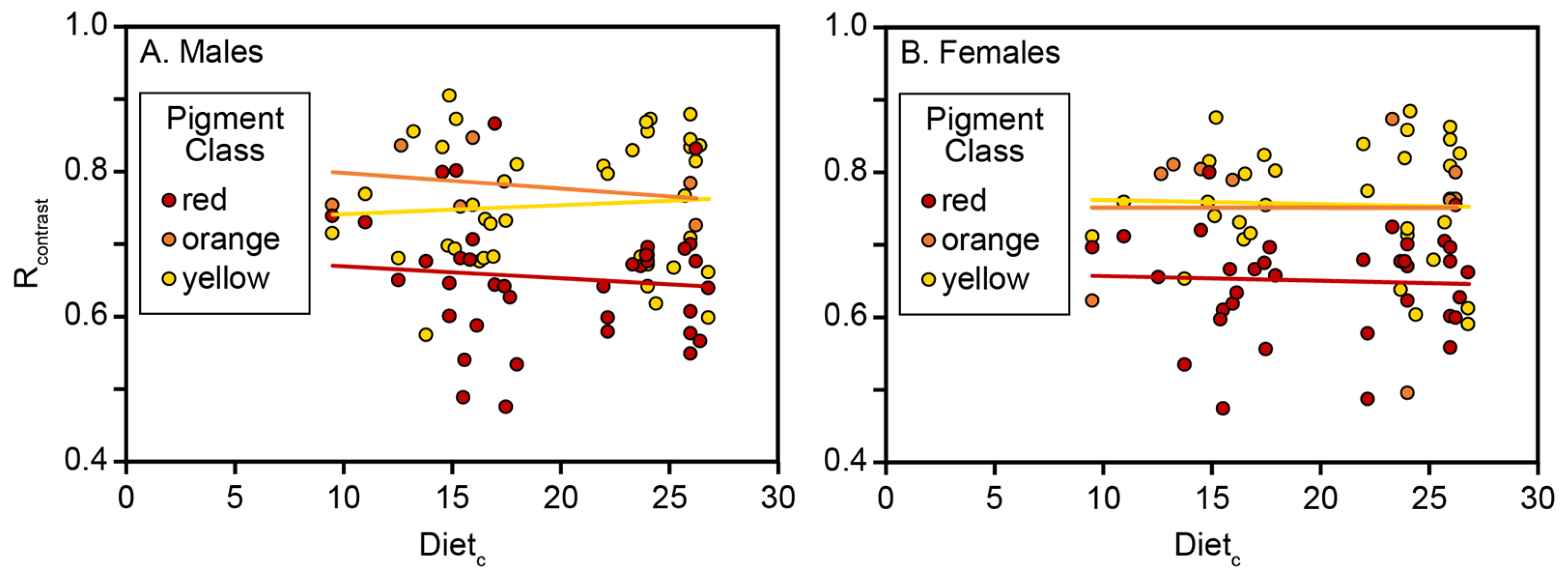
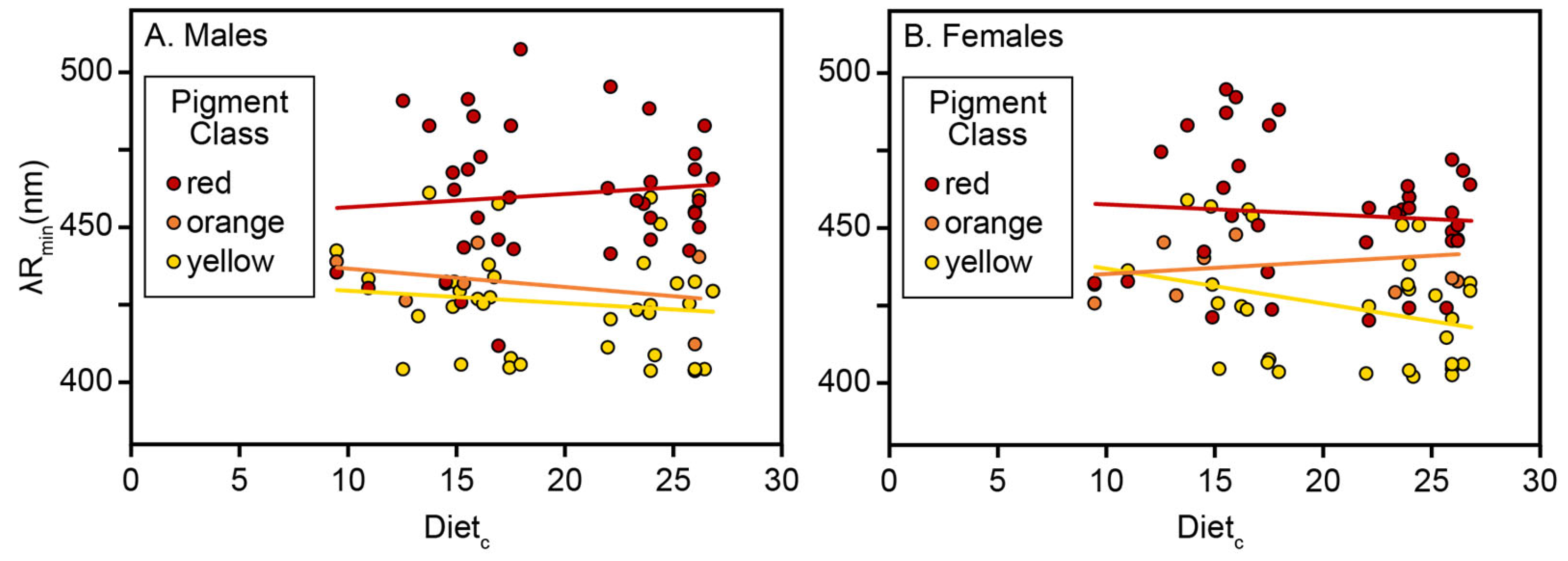
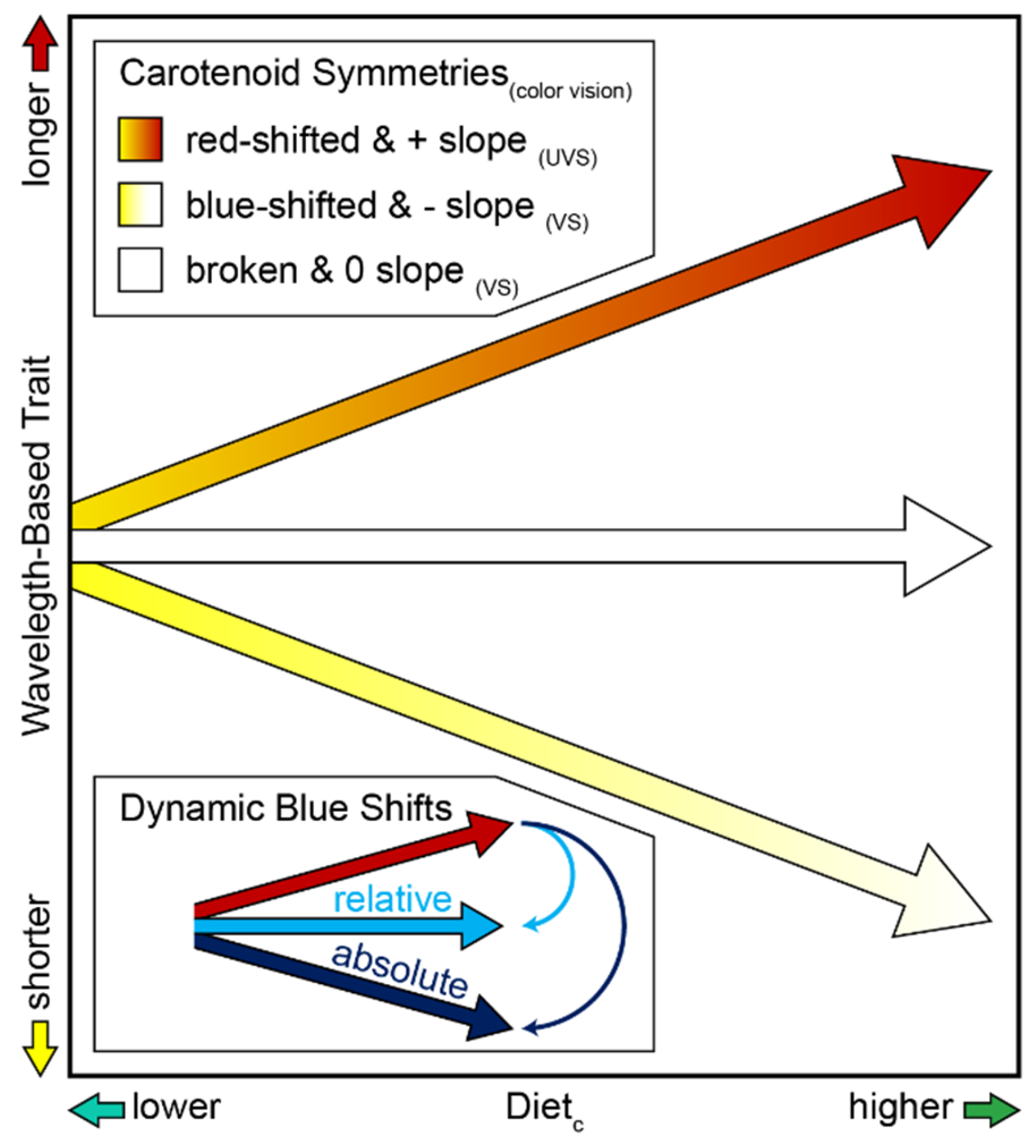
The analysis of carotenoid-based reflectance in an ecological context among VS Piciformes–Coraciiformes is consistent with earlier evidence based on mean λR
min alone for changes towards bluer (shorter) or redder (longer) wavelengths depending on color vision system (see
Section 1) [
26,
38,
39], extending this distinction to
dynamic responses at higher Diet
c. In the case of either sexual pattern in VS Piciformes–Coraciiformes, none of the three carotenoid-pigment classes were significantly red-shifted (i.e., no significant positive slopes) at higher Diet
c, red-extended (i.e., no collinear yellow to red pigmentation) at higher Diet
c, or red-dimorphic (i.e., no red-male expression bias), contrary to the red-shifted, largely UVS groups [
16,
20,
21,
41]. Instead, the VS piciform–coraciiform response to higher Diet
c revealed both absolute (i.e., significant negative slopes) and relative (i.e., non-significant zero slopes) blue compared with absolute red shifts at higher Diet
c, covariant (equal) expression of all pigment classes at higher Diet
c, and roughly equal sexual pigment class expression (
Table 11 and
Table 12,
Figure 4,
Figure 5 and
Figure 6). Moreover, VS piciform–coraciiform tendencies were mutually reinforcing in that patterns at long (λR
50) wavelength were not contradicted by those at short (R
contrast non-shifts) or intermediate (λR
min blue shifts between those observed for λR
50 and R
contrast) wavelengths. These blue–red distinctions can be summarized as a series of physically objective opposite-pattern symmetries (
Figure 7). In the case of yellow and orange pigment classes in VS Piciformes–Coraciiformes, absolute blue shifts and their negative slopes in response to higher Diet
c by λR
50, and to a lesser extent by λR
min, are antisymmetries when compared with absolute red shifts and their positive slopes (
Figure 1D). The wavelength continuum within each shift pattern implied polychromatic symmetries between them, qualified by the fact that categorical perceptions could reduce these to dichromatic ones (
Figure 1E,F). The flat responses by λR
50 in the case of the red pigment class, and R
contrast for all pigment classes, are relatively blue-shifted but not strictly opposite (
Figure 7 bottom inset) to the positive slope (λR
50) or diminishing the short-wavelength reflectance (R
contrast) of their red-shifted counterparts at higher Diet
c. These responses can be considered broken symmetries [
10] rather than antisymmetries (
Figure 7). The nearly identical results of parallel OLS and favored PGLS models, and interpretation of their fitted parameter values (appropriate
R2adjusted; large α compared with small
t1/2 and
ρ), provided strong statistical support for VS blue biases and symmetries, with little evidence for historical, sexual, or preservation (Year) contributions but strong evidence for process models that incorporated trait adaptation or ecophenotypy (Diet
c distribution, OU model support). Therefore, the next step in developing a symmetry framework is to determine whether the underlying processes are similar (invariance symmetry) for the antisymmetries but not for the broken symmetries (
Figure 7). A certain level of process invariance is implied when concerted, antisymmetric shift responses to diet (higher Diet
c) are expressed between comparisons (e.g., color vision systems). However, no responses by VS Piciformes–Coraciiformes for other spectral features (e.g., R
contrast) or pigment classes (e.g., red) suggest altered processes. To further explore process symmetry and symmetry breaking, the well-studied red shifts of UVS Passerida passerines (UVS) best inform the VS piciform–coraciiform (VS) system.
Relation to Color Vision. Several observations suggest that the blue shifts in VS Piciformes–Coraciiformes are linked indirectly or directly to color vision. First, blue shifts in VS Piciformes–Coraciiformes are particularly striking, considering the many dietary red shifts associated with the UVS system (see
Section 1). This qualitative association is prima facie evidence that shifts and color vision patterns are conjoined. Second, VS Piciformes–Coraciiformes align the maximum sensitivity of their diagnostic V cone with the characteristic λR
min of their strongly blue-shifted yellow pigments, suggesting a mutual relationship [
26]. Not only does alignment imply visual function, but λR
min embodies the chemical information content of any pigment [
24] used as a signal. Third, the failure to detect any response by short-wavelength reflectance (R
contrast) to Diet
c for any carotenoid pigment class in VS (
Table 5,
Table 6 and
Table 7) as compared with its strong response in UVS [
20,
21] is a pattern consistent with perceptual differences between the VS and UVS systems. Thus, the maximum sensitivities of the avian V and S cones mainly responsible for short-wavelength perception are 410 and 480 nm, respectively, in the VS system but 350 and 450 nm in the UVS system [
36], whereas the secondary short-wavelength reflectance band of carotenoids (
Figure 3) best covers the UVS sensitivity range [
22]. Fourth, the S single-cone maximum sensitivity in the VS system subtends their most blue-shifted (470–490 nm) λR
50 values (
Figure 4), whereas the maximum sensitivity of the S single-cone in the UVS system lies below these values [
26]. Consequently, the VS tuning patterns provide multiple reasons that favor λR
50 over R
contrast for signaling in the observed order yellow over orange over red. Parallel evidence linking red shifts to the UVS cone characteristics complements these arguments [
26,
38]. In contrast, the red shifts known for VS groups (particularly in waders) arise from cosmetic coloration, which is strongly impacted by the environment [
100,
101,
102].
Although most parameter values required to model the VS piciform–coraciiform system are lacking, the perceptual salience of blue-shift patterns is supported by experiments on VS visual behavior. The VS pigeon model (
Columba livia) can discriminate wavelength differences between 2 and 10 nm for narrow
-bandwidth visual stimuli (<10 nm) presented across a carotenoid-relevant spectral range of 450–625 nm [
103]. Even relatively more realistic behavioral choice experiments with broad-band stimuli similar to those of carotenoids suggest that VS jungle fowl (
Gallus gallus) are superior at discriminating wavelengths in the “yellow” and “orange” ranges, even when the physical differences are only a few nanometers and virtually indistinguishable to humans [
104,
105]. Remarkably, modeling studies of other pigeon data that account for subjective judgements indicate that maximal monochromatic discrimination also occurs in the physical yellow (500 nm) and orange (600 nm) ranges [
106]. Thus, VS discrimination or categorical perception abilities of VS models encompass much of the variation and spectral range within and among carotenoid pigment classes in VS Piciformes–Coraciiformes and particularly fit the stronger yellow and orange shift responses to the Diet
c of the latter group. Conversely, the implication that red wavelengths are relatively less discriminated matches their weak response to Diet
c. This consistency supports the putative signal value of the VS piciform–coraciiform blue shifts (
Figure 4). The opposite (absolute) or divergent (relative) signs of blue and red shifts between the color vision groups imply that these global patterns are even relatively more obvious for birds. Any dietary effects on oil droplet carotenoids [
107,
108,
109] that filter light before it reaches the retinal cones presumably would amplify these discriminations across species. Therefore, even without an explicit VS piciform–coraciiform visual model, participation in carotenoid-based pigment classes and blue shifts in signaling and communication is likely.
Columbidae (pigeons and doves) are especially informative for VS Piciformes–Coraciiformes, considering their similar ecological habits and responsiveness to diet by plumage carotenoids (extent in fruit doves) [
110]. The strong association of λR
50 with generic colorimetric perceptions such as hue and saturation in vertebrate visual systems [
35] is also useful for inferring avian from human perceptions of shift patterns. Thus, the ability of humans to distinguish blue-shift patterns based on generic color parameters (
Figure 2) implies salience to birds, given the latter’s ability to discriminate among yellows and oranges that are difficult for humans to distinguish [
104,
105]. For us, the physical blue shift in λR
50 (
Figure 3 and
Figure 4) by yellow plumage (pigment class) among species with higher Diet
c because of more plant-based diets (
Figure 2A,C,E) translates to a consistently less saturated (broader reflectance plateau), lemon-yellow (shorter λR
50) compared to a golden-yellow in species with more animal-based diets (
Figure 2B). Consistent with antisymmetry, the opposite saturation pattern applies to UVS red shifts. Compatible with its overall insignificant response to Diet
c, the red pigment class appears to lack any human-visual tendencies among species with more plant- (
Figure 2A,C,E) or animal-based (
Figure 2D,F) diets. Though slightly ambiguous, the intermediate response by orange λR
50 to Diet
c seems visually intermediate to humans in relation to these dietary distinctions between more plant- (
Figure 2C) versus more animal- (
Figure 2D) based diets. Moreover, the lack of any UV-based (<400 nm) trends (incorporated into R
contrast) that would be invisible to humans implies that our perceptions of plumage differences (compare
Figure 2 and
Figure 3) provide a conservative assessment of what avian systems can distinguish [
111]. Thus, quantitative and qualitative variations (perceivable to humans) are mutually consistent. Therefore, human-visible λR
50 variations should provide useful biomarkers of Diet
c for VS such that trophic habits can (yellow and orange) or cannot (red) be appreciated from plumage appearance, even to us.
General sexual similarities in color vision [
36,
37,
42,
112] are also consistent with the observed sexual similarities in carotenoid-based shift (
Figure 4,
Figure 5 and
Figure 6) and expression (
Table 12) patterns, or at least allow for them indirectly, including by genetic correlations between the sexes. Compatible with this interpretation, a few typical UVS Passerida passerines (Parulidae) exhibit sexual differences in their visual opsin sensitivities that correlate with plumage sexual dimorphisms [
43]. However, other considerations suggest that the inter-relationships between sexual distinctions in vision and plumage are more complicated. In particular, the primary diet data did not distinguish between the sexes or account for any diet-related sexual perceptions related to oil-droplet carotenoids [
107,
108,
109] or the foraging environment, which could have altered the interpretation of resemblance. The sexes of the UVS Passerida passerines and VS Piciformes-Coraciiformes may also differ in their visual environments. For example, most Passerida passerines build arboreal open-cup nests that expose the sexes to visual detection by predators, which can select for plumage divergence based on male versus female reproductive roles [
113]. In comparison, VS Piciformes–Coraciiformes are cavity nesters [
74], a habit that may better conceal both sexes from visually hunting predators, thereby reducing that source of divergent selection on plumage [
114]. Moreover, nest cavities are usually limited resources that lead to strong competition among pairs, which may select for mutual resemblance through social selection [
115,
116]. These general habits of VS Piciformes–Coraciiformes could, therefore, explain why their sexual carotenoid-based shifts and expression patterns are similar, even across a wide range of Diet
c values from associated foraging preferences. How these and other factors interact to produce the observed patterns requires further study.
Yellow and Orange Responses. Physically different plumage responses to diet by birds with different color vision systems could result from different adaptive processes. However, the VS absolute blue-shifted (yellow and orange pigment classes) and UVS absolute red-shifted responses to Diet
c share many attributes suggestive of process symmetries. The most descriptive of these is that in both systems, some plumage spectral features within one or more carotenoid-based pigment classes are reliable markers of diet. A relatively more mechanistic shared process is suggested by evidence for a shared role of limitations in dietary carotenoid content (Diet
c) on λR
50 and (less) λR
min plumage expression. This sensitivity has been supported in VS Piciformes–Coraciiformes by their captive counterparts [
58,
59,
60]. However, general fading of all carotenoid-based plumages for individuals on captive diets [
58] suggests that evolutionary in addition to ecophenotypic factors are responsible for the different sensitivities (yellow > orange > red) of pigment classes to Diet
c among wild taxa. Qualitatively, both color vision systems appear to lack any obvious bias in using carotenoids when they are scarce or abundant [
16,
32,
41,
115,
117]. In the VS system for example, carotenoid pigments were expressed in species over a wide span of trophic habits (
Table 11), whose Diet
c values range from 9.5 (emphasizing low-content arthropods) to 26.83 (emphasizing high-content fruits). Furthermore, the response by especially VS yellow λR
50 remained linear over the same Diet
c range (
Table 2,
Table 3 and
Table 4, and
Figure 4) whereas non-linear (saturation) effects occur in certain UVS birds [
21,
22]. Notably, many VS piciform–coraciiform frugivores favor so-called “superfruits” [
74], whose high nutritional along with carotenoid content could maintain informativeness regarding dietary limitations even at the highest Diet
c levels. In this regard, assessing dietary carotenoid content as the richness of food items (
Figure 4,
Figure 5 and
Figure 6, and
Table S2) rather than the amount of food consumed simply emphasizes signal design through dietary specialization rather than through food-gathering abilities per se. Conversely, the specific taxonomies for the carotenoid content of dietary items used to estimate Diet
c (see
Table S2) qualify other effects on bioavailability, including indirect climate–humidity [
34] or internal physiological [
118] regulations.
Whether the similar responsiveness of VS and UVS to dietary limitations reflects deeper shared processes depends on whether similar active mechanisms of carotenoid handling produce similar color vision-appropriate shift patterns. Further understanding can be gained by examining the underlying chemistries. In these terms, UVS that eat carotenoid-poor foods (typically grains or arthropods) often deposit dietary “native” yellow carotenoids (typically the xanthophylls lutein, zeaxanthin, β-cryptoxanthin, or rarely the carotene β-carotene) directly to produce yellow to orange plumage [
19,
25]. However, endogenous processes acting on higher levels of dietary natives in UVS produce mainly red shifts in individuals and species with higher Diet
c [
16,
20,
21], either by depositing carotenoids at higher concentration or greater thickness to increase optical density [
16,
17,
20,
21], by enhancing carotenoid X protein interactions [
30], or by converting yellower carotenoids to intrinsically more orange to red xanthophylls [
16,
17,
19,
25,
72,
119]. Many UVS also convert dietary natives to yellow metabolites, providing another way to encode Diet
c. However, these UVS yellow carotenoids (canary xanthophylls, 3′-dehydrolutein, 2′,3′-anhydrolutein) are not strongly blue-shifted (λR
min usually ≤ 3 nm, up to 9 nm shorter) compared with dietary natives [
17,
63,
120,
121], which they closely resemble. Birds may still be able to distinguish small blue shifts to a certain extent [
104]. However, the greatly restricted physical magnitude of these blue shifts leaves little room for encoding ecological radiation in Diet
c this way, thereby reinforcing a red bias in terms of interspecific wavelength-based shifts. Considering the basic yellow-to-orange carotenoid chemistry in UVS, the universal physical properties of carotenoids [
20,
21,
122] pertaining to increased optical density (by concentration, thickness, and carotenoid type) are more likely to produce a red shift in response to a higher Diet
c.
In contrast to UVS, however, many VS Piciformes-Coraciiformes can convert dietary native yellow to metabolized yellow “picofulvin” (dihydro- or tetrahydro- derivatives of dietary natives) [
25,
123,
124] carotenoids characterized by strong blue shifts of up to approximately 70 nm compared with dietary natives. At least five empirical observations support the hypothesis that picofulvins cause absolute blue shifts in yellow carotenoids at high Diet
c in VS Piciformes–Coraciiformes. First, picofulvins are common in this group (various woodpeckers) [
123]. Second, λR
min of picofulvins in vitro [
123,
124] match those observed for the most blue-shifted yellow plumages (
Figure 3A and
Figure 6) [
26]. Third, the maximum blue shifts of λR
50 (
Figure 4) and λR
min (
Figure 6) are similar. Fourth, the distinctive undulating series of R
mins across the overall low reflectance at short visible (400–500 nm) wavelengths in spectra from species with the highest Diet
c (
Figure 3A) resemble those obtained from yellow plumages known to be picofulvin-rich [
26,
119,
124]. Fifth, endogenous metabolite use is consistent with evolutionary influences. Conversely, picofulvins are rare or absent in UVS [
25], whereas native-like yellow carotenoid metabolites produced by UVS are rare (small amounts in the red plumage of one toucan) [
46] or absent [
72,
119,
123] in VS Piciformes–Coraciiformes. These patterns also imply that more red-shifted (λR
50 = 510–530 nm) yellows produced by many VS Piciformes-Coraciiformes (
Figure 3B and
Figure 4) at low Diet
c are dietary natives, consistent with chemical analyses of a few woodpeckers (
Colaptes,
Picoides) [
123]. Therefore, for VS Piciformes–Coraciiformes, absolute or relative amounts of picofulvins in reciprocal combination with their relatively red-shifted (
Figure 3A,B) dietary native precursors provide a consistent basis for the variation in blue shifts with Diet
c. Intermediate blue shifts (
Figure 4) by oranges most parsimoniously arise from mixes of yellow picofulvins with orange or red xanthophylls, as suggested by orange’s intermediate spectra (
Figure 3C,D) and occasional formation within yellow patches in species that also produce red (
Ramphastos dicolorus;
Figure 2C). Mixes containing metabolized picofulvins also seem to be the only way to achieve a blue shift with a higher Diet
c, as greater amounts of dietary or metabolized orange or red xanthophylls would create a red shift. Therefore, species in both color vision systems appear to use native yellows at lower Diet
c but diverge in the carotenoid metabolites they produce at higher Diet
c.
Although these chemical hypotheses require confirmation, they are consistent with the active control of plumage carotenoids, as suggested by the greater specificity of responses among species compared to those by individuals in feeding trials (see above). Consequently, the yellow carotenoids available to VS Piciformes–Coraciiformes can produce dramatic (λR
50) or modest (λR
min) absolute blue shifts at higher Diet
c through the increased deposition of their metabolized picofulvins over dietary natives, whereas comparable shifts are constrained for the combinations of metabolized and dietary yellows available to UVS (see above). In turn, these properties represent divergent processes between VS and UVS yellows, but convergent ones between VS yellows and UVS reds. In the convergent cases, a metabolized carotenoid that is strongly λR
50-shifted compared to dietary native carotenoids strengthens the shift response to higher Diet
c [
19]; however, this produces absolute blue shifts in VS and absolute red shifts in UVS. Higher concentrations of yellows at higher Diet
c apparently also contribute to corresponding shifts, but the VS blue shift may require metabolized picofulvins to avoid the red shift created by higher concentrations of dietary native [
17] or UVS-type metabolized [
30] yellows. The production and deposition of more or metabolized pigmentation at higher Diet
c in both VS (blue) and UVS (red) shift systems suggests invariant processes consistent with several models of honest signaling based on costs and benefits [
16,
17,
34,
64]. This mutually reinforcing evidence for shared honesty could extend to more detailed invariances related to diet, including ecological signaling [
20,
21,
22], imposition of signal costs (higher concentrations and metabolites) when carotenoids are abundant (higher Diet
c) to avoid cheating [
34], possible costs or benefits of native or metabolite carotenoids in (different) physiological processes [
19,
34,
125], trade-offs between these or other influences [
118], or some combinations thereof. Notably, λR
min values typical of picofulvins also occur at low Diet
c (
Figure 6). In the one species (Pileated Woodpecker,
Dryocopus pileatus; Picidae) analyzed both ecologically (Diet
c = 12.543, λR
50 = 474.155 nm) and chemically [
123], the blue shift is associated with mostly picofulvins but at very low concentrations, consistent with a lower cost. Accordingly, using picofulvins alone at various concentrations may augment the changing ratios of picofulvins and dietary natives (see above) to produce an honest-signaling system that achieves even greater flexibility across Diet
c. The spread-out distribution of picofulvins may also explain why λR
min has an intermediate association with Diet
c, even though this feature should correlate strongly with λR
50 [
21,
22]. With regard to λR
50, however, a strong functional blue shift by yellow and orange in VS Piciformes–Coraciiformes up to nearly the maximal possible terrestrial Diet
c (30 for a pure fruit diet; see
Table S2) suggests little limitation on their signal informativeness, which is consistent with predictions that honest signals must span the range of costs and benefits to be fully reliable [
7]. Nevertheless, ambiguities for orange through its physical proximity to yellow and possible mixed signal content (informative yellow + uninformative red), combined with avoidance of common dietary orange carotenes as pure plumage colorants [
25], may help explain the surprising scarcity of orange plumages in honesty systems, both in VS (herein) and UVS (“orange gap” in UVS Thraupidae) [
22] birds.
Red Response. Considering that red plumage in VS Piciformes–Coraciiformes responds to individual diets in captivity [
58], the absence of a response by any of the measured spectral features for red pigments to Diet
c across wild VS piciform–coraciiform taxa suggests a certain evolutionary control in the latter context. This inference is consistent with the evidence that most terrestrial birds produce red plumage by metabolizing red keto-carotenoids from yellow dietary precursors [
25,
123] rather than by ingesting red pigments [
41]. However, a countergradient selection-type mechanism [
126] appears unlikely because red plumage in VS Piciformes–Coraciiformes otherwise contains little, if any, of the diet-dependent yellow natives or their picofulvin derivatives (woodpeckers) [
123], whose relative blue shifts could otherwise cancel a genetic red shift expressed in birds with a higher Diet
c. The reduced response of red to Diet
c is consistent with claims for the same pattern (in plumage coverage) in UVS [
41], but the complete absence of a shift response in VS Piciformes–Coraciiformes challenges expectations for carotenoids, considering the past emphasis on metabolized red carotenoids as central to honest plumage colors in UVS [
16,
32]. Instead, different (yellow and orange versus red within VS) or the same (red between VS and UVS) carotenoid pigment classes may evolve under different constraints or functional specializations. As with yellow and orange, the different physical mechanisms for producing red [
66] presumably vary in costs and benefits, which could undermine honesty if their deployment strategy varies with respect to Diet
c across species. However, both the chemical data [
123] and the distinctness of VS piciform–coraciiform pigment class spectra (
Figure 3) in λR
50 phenotype space (
Figure 4) suggest that reds here are distinctive keto-carotenoid metabolites, inconsistent with the main alternative route to increasing carotenoid-based redness through greater optical densities of yellow to orange pigments [
17,
20,
31]. Any inherent limitations on red-pigment variation (e.g., saturation) also seem unrelated to the non-response of red carotenoids to Diet
c in VS Piciformes–Coraciiformes, in that the same red keto-carotenoids [
123] participate in plumage red shifts in UVS [
16]. How much the different functional response by reds in VS and UVS systems indicate divergent processes depends on whether mechanisms differ only in degree (remain honesty-enforcing) or also in kind (related to dishonesty, or to influences outside the honesty rubric).
In principle, chemical constraints on red plumage could also prevent its use as an accurate indicator of dietary carotenoid content. Thus, if red was too cheap (cheatable) to be honest, then we would expect Diet
c values for red to move lower relative to yellow. Conversely, if red was ineffective because it was too expensive (limiting) to be universally honest, then we would expect Diet
c values for red to move higher relative to yellow. Instead, the extensive overlap among pigment classes across Diet
c (
Table 11,
Figure 4,
Figure 5 and
Figure 6) suggests that red is not different from other carotenoids in terms of more traditional honesty potential in relation to Diet
c. With respect to alternative honesty-enforcing processes, the shared pathway hypothesis [
18,
19,
33,
125] suggests that carotenoids are more sensitive to the internal (cellular) environment than to the external (dietary) environment because of links between carotenoid metabolism and vital cellular processes. Accordingly, carotenoid-based signal honesty may still vary, but its expression may arise from the disruption of these internal cellular processes rather than from a response to external factors, such as Diet
c. This mechanism is, therefore, consistent with the metabolic origin [
123] and dietary independence (herein) demonstrated by red carotenoids in VS Piciformes–Coraciiformes. Moreover, vital effects favoring plumage redness have been linked specifically to physiology, which may depend on many traits not examined here [
125]. However, in light of traditional links between diet, carotenoids, and liver function, for example [
127], variation in reds that is strongly independent of Diet
c (
Table 13,
Figure 4,
Figure 5 and
Figure 6) implies additional or other complex processes. Additionally, the blue shift of red pigments in VS relative to their red shifts in UVS suggests an active mechanism against greater redness with a higher Diet
c among VS Piciformes–Coraciiformes. Notably, studies on a few woodpeckers suggest that their red plumage functions differently based on species-specific morphological or ecological contexts [
128,
129]. Any widespread selection influenced by this plasticity could eliminate or obscure dietary trends in red plumages with Diet
c. Therefore, other natural history features of red plumage in VS Piciformes–Coraciiformes may also incorporate additional or alternative signal properties and functions that account for the unresponsiveness of red pigment reflectance to Diet
c.
Based on these considerations, variation by red in all three spectral features at each level of Diet
c (
Table 13,
Figure 4,
Figure 5 and
Figure 6) suggests that the red pigment class has some potential to respond to Diet
c but that selection operates differently. Adaptive explanations based on processes actively favoring resemblance among reds plausibly explain no significant functional response to Diet
c. An intrinsic sensory preference [
126,
130,
131,
132,
133] shared among the VS Piciformes–Coraciiformes would potentially be strong enough to override any response to Diet
c. In this regard, a striking feature of VS Piciformes–Coraciiformes is that red carotenoids are surprisingly ubiquitous among the plumages of both sexes (
Table 11 and
Table 12,
Figure 4,
Figure 5 and
Figure 6), compared with the general avian patterns for yellow plumage carotenoids to predominate [
116] and for a red bias in males [
32]. Moreover, many of these red plumage patches occur around the head, face, and rump and are most likely to be important in visual communication. These patterns suggest a deep-seated preference for red pigmentation by various means. For example, if the red carotenoid content of neither plumage nor the oil droplets of longer wavelength-sensitive M and L cones respond to Diet
c, then a bias for certain reds may emerge or be reinforced. Sensory preference also could compromise honest Diet
c signaling through related foraging biases that allow “expensive” red pigments to be obtained more cheaply as a component of sought-after foods [
134]. Thus, certain
Colaptes woodpeckers appear to obtain their red pigments through narrow myrmecophilous diets [
56], which otherwise have a very low overall carotenoid content (
Table S2). Foraging specializations associated with terrestrial habits in
Colaptes [
55] could further reduce the cost of seeking these red pigment sources, thereby circumventing the usual intrinsic physiological costs and benefits relating red pigmentation to Diet
c and honesty. However, broad variation in reds at each level of Diet
c challenges sensory preference explanations whereas the relative constancy of variation in reds across Diet
c values supports them (see
Figure 4,
Figure 5 and
Figure 6). Other sensory factors such as habitat, light environment, and biotic interactions may contribute to these complexities.
For example, honest or dishonest resemblances driven by social or aposematic mimicry towards competitors or predators are also particularly relevant to Piciformes-Coraciiformes [
45,
135,
136,
137]. Typical VS piciform–coraciiform traits favoring such resemblances include bold markings, large size, physical prowess, sexual resemblance, intense competition (e.g., for nest cavities), and interspecific communication [
137,
138,
139,
140,
141]. Moreover, the strong OLS and OU model support for the non-response of red to Diet
c is consistent with evolutionary convergence and not relatedness as the cause of resemblance. Evolved resemblance does not need to be independent of any tendency for red to saturate (physically similar) or create sensory biases (perceptually similar), as both phenomena enhance functional resemblance. Furthermore, the many physical mechanisms by which a red appearance can be achieved with carotenoids [
17,
22] and other pigments [
66] could produce corresponding variations in production costs and benefits that would further undermine honest signaling, potentially favoring numerous social and aposematic mechanisms based on deception/dishonesty. Reduced variation of red compared with yellow λR
50 (
Table 13), the large gap between yellow + orange versus red λR
50 values (
Figure 4), and overall strong support for OU models of λR
50 covariation (
Table 2,
Table 3 and
Table 4) suggest a favored zone of long-wavelength (red) plumage phenotypes consistent with resemblance hypotheses. Conversely, the scatter of λR
50 at a given Diet
c is not disqualifying in that resemblance need not be exact to be effective, as different species may be perceived as members of the same category, be at different stages of perfecting resemblance, or belong to different resemblance rings [
137]. The only requirement in the present case is that the importance of resemblance overrides the response to carotenoid limitation, resulting in no functional response to Diet
c (cf. yellow and orange). The slight and non-significant but more positive functional response to higher Diet
c by red λR
50 in females (
B~0.15) than in males (
B~0.02) may, therefore, indicate marginally stronger selection for more honest resemblances in females, consistent with sexual differences in certain behaviors (see above references). Physical and perceived similarities could favor red over yellow and orange under any resemblance scenario for the red response, although all pigments, particularly their Diet
c-independent wavebands, could participate. Thus, if VS incorporates yellow and orange as diet-limited to honesty-based signals, then the different characteristics of reds may lead to alternative functions, including ones related to dishonesty.