Abstract
This paper presents an analysis of helminth diversity in reptiles in eight provinces of the Middle Volga region (European Russia) based on the dataset recently published in the GBIF as the Darwin Core Archive. The dataset contains up-to-date information on the occurrence of parasitic worms in lizards and snakes and summarizes the records obtained during long-term helminthological studies conducted in 1996–2024. It includes 8576 helminth occurrence records in nine reptile species inhabiting the Middle Volga region. All helminth occurrence records are georeferenced. In total, we present data on 45 parasitic worm species, including 4 species of cestodes, 21 species of trematodes, 16 species of nematodes, and 4 species of acanthocephalans. The richest helminth fauna was found in Natrix natrix (26 species), Lacerta agilis (21), Natrix tessellata (16), and Vipera berus (15). Less diverse is the helminth fauna in Anguis colchica (8 species), Zootoca vivipara (7), Vipera renardi (6), Coronella austriaca (5), and Eremias arguta (3). The diversity of helminths in reptiles of the Middle Volga region does not reach its maximum compared to other European countries. Most helminth species found in lizards and snakes of the studied region belong to the Palearctic faunal complex (25 species). Eight species of parasites have a Holarctic distribution. Seven helminth species parasitize reptiles only in Europe. Five species of parasites are cosmopolitan. Of the 45 species of helminths found in reptiles, 3 species have medical and veterinary significance as causative agents of dangerous helminthiasis. Data on the diversity and distribution of parasitic worms in reptiles of the Middle Volga region remain incomplete, so further observations may provide new occurrence records of helminths and expand the knowledge about their hosts.
Keywords:
biodiversity; parasitic worms; Reptilia; occurrence records; Trematoda; Nematoda; Cestoda; Acanthocephala; Volga region 1. Introduction
Being a common component of natural biocenoses, reptiles are found almost everywhere and are hosts of a rich fauna of micro- and macroparasites, which varies significantly depending on the geographical location and the species of reptiles. Definitely, wild-living reptiles carry a wide range of viruses, bacteria, parasitic worms (leeches, monogeneans, trematodes, cestodes, acanthocephalans, and nematodes) and parasitic arthropods (pentastomes and mites). Helminths are parasitic worms that live inside their hosts, i.e., they are endoparasites. They have different life cycles, both with and without the involvement of intermediate hosts [1,2,3,4,5,6,7]. Lizards, snakes, and turtles serve as final, intermediate, and reservoir hosts for various helminths and are crucial for the implementation of their life cycles [1]. The role of reptiles as paratenic hosts for parasitic worms is especially significant [8,9,10,11]. A number of helminths that parasitize reptiles have epidemiological and epizootological significance as causative agents of parasitic zoonoses in domestic and wild animals, as well as in humans, creating global health and economic problems.
Parasites of reptiles in Russia have not been studied for a long time, as it was believed that they have no practical application and, therefore, do not play an important role in human life. The most extensive study of parasites in reptiles of the former USSR was conducted in the period from the 50s to the 80s of the 20th century. At that time, helminths of reptiles were actively studied in Ukraine and Central Asia [1].
In recent decades, interest in studying helminths of reptiles in Europe has been a paucity compared to parasites of other vertebrates. Helminthological data are usually devoted to one or a few species of reptiles [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52]. At the same time, helminthological studies covering the entire reptile fauna inhabiting a certain geographical area are still rare [1,53,54,55,56,57,58,59,60,61,62].
The herpetofauna of the Volga basin comprises 20 species; in the entire region, it accounts for 25% of the reptile fauna of Russia. Of these, 14 species of reptiles inhabit the Middle Volga region [63,64,65,66]. Despite the wide distribution of reptiles, their parasites in the European Russia and, in particular, the Volga basin have not been sufficiently studied. The first data on the helminth fauna of reptiles were obtained during the study of parasitic worms in the grass snake Natrix natrix (Linnaeus, 1758) from Tatarstan, the sand lizard Lacerta agilis Linnaeus, 1758, and the viviparous lizard Zootoca vivipara (Lichtenstein, 1823) from the Nizhny Novgorod Oblast [67,68,69,70].
Our study of helminths in reptiles of the Middle Volga region began in 1996. Helminths of reptiles have been studied unevenly in different provinces of the region. Research on helminths in reptiles was conducted mainly in the Republic of Mordovia, Orenburg Oblast, and the Samara Oblast. Over nearly 3 decades, we have studied the helminth fauna of lizards and snakes in 8 administrative provinces of the Middle Volga region out of 14 existing. Helminthological observations were conducted on 9 out of 14 species of reptiles inhabiting the territory of the studied region: grass snake N. natrix, dice snake Natrix tessellata (Laurenti, 1768), common European viper Vipera berus (Linnaeus, 1758), eastern steppe viper Vipera renardi (Christoph, 1861), smooth snake Coronella austriaca Laurenti, 1768, sand lizard L. agilis, viviparous lizard Z. vivipara, eastern slow worm Anguis colchica (Nordmann, 1840), and steppe-runner Eremias arguta (Pallas, 1773) [17,33,35,37,46,53,60,71,72,73,74]. The aim of our paper is to describe the diversity of helminths in lizards and snakes of the Middle Volga region based on our dataset recently published in the Global Biodiversity Information Facility (GBIF) as the Darwin Core Archive [75]. This paper is prepared in accordance with the “data paper” concept [76,77], which implies the presentation of usually hard-to-access primary data on biodiversity in the form of a scientific publication.
2. Materials and Methods
2.1. Study Area
The Middle Volga region is located in the southeastern part of the Russian Plain and occupies the territory within 52–57° northern latitude and 45–56° eastern longitude [78] (Figure 1).
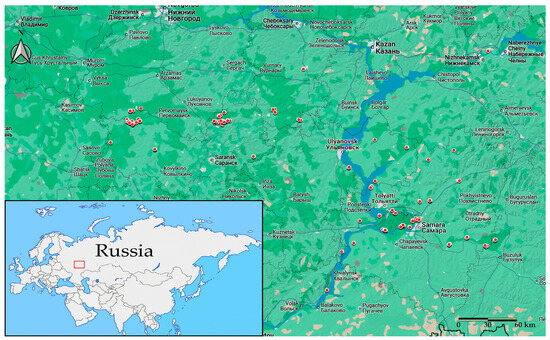
Figure 1.
The Middle Volga region and sampling sites for parasitic worms in reptiles in the study area. The locations of helminth occurrence are shown by red circles. The Middle Volga region (on the minor map, bottom left) is shown by a red frame.
The Middle Volga region has a diverse landscape: coniferous and mixed forests in the north and steppe in the south. The central and largest part of the territory is occupied by forest-steppe [79]. The location of the region within the forest, forest-steppe, and steppe zones determines the high diversity of plants and animals. Thus, the flora of the Middle Volga region has about 2050 species of plants [80,81], and the fauna includes 11 species of amphibians, 14 species of reptiles, 300 species of birds, and 85 species of mammals [63,64,65,66,82,83,84,85,86]. A more detailed description of the Middle Volga region is given in our earlier work [87].
2.2. Description of Parasitological Data
The new dataset we present summarizes our records obtained during long-term helminthological studies of lizards and snakes in the Middle Volga region over a 28-year period (1996–2024). The dataset is based on field studies by staff of the Institute of Ecology of the Volga River basin of the Russian Academy of Sciences (Togliatti) and the Joint Directorate of the Mordovia State Nature Reserve and National Park “Smolny”.
Data on parasites in reptiles from the studied region were published earlier, but without reference to geographic coordinates [17,33,35,37,46,53,60,71,72,73,74]. In our dataset, each occurrence of helminths in reptiles is referenced to geographic coordinates for the first time. All references were made by fixing the geographic coordinates using a GPS Navigator or Google Maps (https://www.google.ru/maps/, accessed on 14 March 2025) [88]. The accuracy of coordinate measurements was 10 m. The accuracy of defining geographic coordinates was up to the fourth digit. The WGS-84 coordinate system was used in all occurrence records.
Lizards and snakes were examined by the method of complete helminthological necropsy [89]. Helminths were collected from reptiles and fixed in 70% ethanol. Trematodes and cestodes were stained with aceto-carmine and dehydrated in a graded ethanol series (70–96%). Then, parasitic worms were cleared in clove oil and mounted in Canada balsam. Nematodes and acanthocephalans were translutened in lactic acid and mounted in Glycerin-Jelly [90,91].
Helminth species from reptiles were identified according to Sharpilo [1], Sharpilo and Iskova [92], and Dimitrova and Gibson [93]. In our study, most helminth species (96%) were identified to the species level. The taxonomy of helminths and their host reptiles is given according to the GBIF database (https://www.gbif.org/) [94].
Data visualization was performed using the “R” programming language (R Core Team, Vienna, Austria) [95] with the “treemapify” [96] and “ggplot2” [97] packages. To determine the species diversity of helminths, the Shannon’s diversity index (H’) was calculated. The significance of differences between the index values was estimated using Student’s t-test [98]. The similarity of helminth compositions was evaluated using Morisita’s overlap index (Cm). The degree of similarity is as follows: 0–0.33—low; 0.34–0.66—medium; 0.67–1—high. Statistical data processing was performed using the PAST 2.16 (University of Oslo, Norway) [99].
Most of the helminth specimens obtained from reptiles of the Middle Volga region are stored in the parasitological collection of the Institute of Ecology of Volga Basin of RAS (IEVB RAS), as well as in the personal collection of A.A. Kirillov and N.Yu. Kirillova. The reptile specimens examined are also deposited as a herpetological collection in the laboratory of zoology and parasitology of the IEVB RAS [100].
3. Results
3.1. Structure of Dataset
The dataset includes 8576 records of helminth occurrence in reptiles (Serpentes and Lacertilia) from the Republic of Mordovia (5379 records), the Samara Oblast (2254 records), the Orenburg Oblast (398 records), the Nizhny Novgorod Oblast (256 records), Chuvashia (188 records), the Ulyanovsk Oblast (75 records), the Saratov Oblast (20 records) and Tatarstan (6 records). In total, our records include 354,878 helminth specimens.
We used standard terms from the Darwin Core (https://dwc.tdwg.org/list/#2-use-of-terms, accessed on 8 March 2025) to describe the dataset [101]. Each parasite occurrence record includes basic information about the helminth species, helminth taxonomy, host species, site of infection, location name with geographic coordinates (latitude/longitude), date of occurrence, the authors of the recording and identification of the species (Table S1). The taxa included in the dataset are presented in Table S2.
3.2. Data Description
The dataset contains data on 45 species of parasitic worms, including 21 species of trematodes, 4 species of cestodes, 16 species of nematodes, and 4 species of acanthocephalans (Table 1).

Table 1.
List of helminths in the dataset.
According to our database, trematodes are the predominant parasites of the studied reptiles and are represented by 21 species belonging to 10 families: Diplodiscidae, Encyclometridae, Leptophallidae, Macroderidae, Opisthorchiidae, Plagiorchiidae, Pleurogenidae, Telorchiidae, Diplostomidae, and Strigeidae (Figure 2). This set includes both adult (15 species) and larval (6) forms. Trematodes account for 66.7% (5719 records) of the total number of occurrence records (Figure 3).
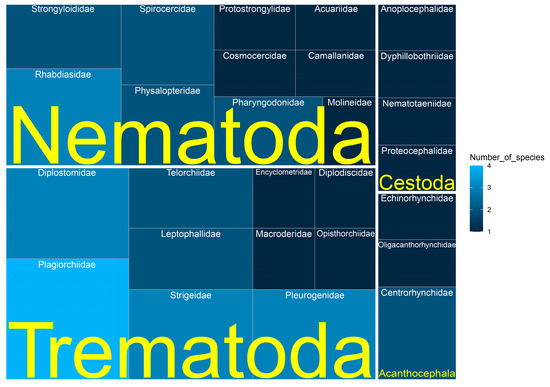
Figure 2.
Taxonomic distribution of helminths amongst families in the dataset.
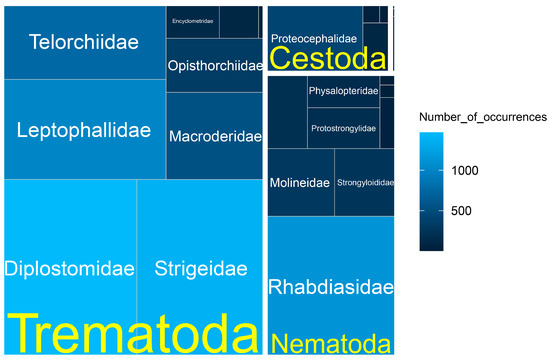
Figure 3.
Distribution of helminth occurrences amongst families in the dataset.
It should be noted that the number of records of adult and larval trematodes was almost the same (adults—2851, larvae—2868), but larval forms prevailed in abundance. Thus, the total number of trematode specimens in our dataset is 327,988, of which 274,459 specimens are at the larval stage.
Nematodes are represented by 16 species belonging to 10 families: Acuariidae, Camallanidae, Cosmocercidae, Molineidae, Pharyngodonidae, Physalopteridae, Protostrongylidae, Rhabdiasidae, Spirocercidae, and Strongyloididae (Figure 2). Among them there are larval (5) and adult (11) forms, together accounting for 26.7% (2291 records) of the total number of occurrence records (Figure 3). Both in occurrences and in abundance, adult forms predominate—1978 occurrence records (17997 specimens); larval forms account for 313 occurrence records (5103 specimens).
Cestodes are represented by four species from four families: Anoplocephalidae, Nematotaeniidae, Dyphillobothriidae, and Proteocephalidae (Figure 2). Of these, only one species (Spirometra erinaceieuropaei (Rudolphi, 1819)) was registered as a metacestode. The remaining three species are represented by mature forms. Cestodes account for 6.3% (539 records) of the dataset (Figure 3). Almost all of them are included in occurrence records of adult individuals, and only 20 records refer to plerocercoids of S. erinaceieuropaei. A total of 3750 specimens of cestodes were collected, of which, only 52 specimens were S. erinaceieuropaei, plc.
Four species of acanthocephalans were found, belonging to three families: Centrorhynchidae, Echinorhynchidae, and Oligacanthorhynchidae (Figure 2). Acanthocephalans account for only 0.3% (27 records) of the dataset (Figure 3). Of these, only two occurrences (one specimen each) of the adult acanthocephalan Acanthocephalus lucii were recorded. The remaining occurrence records refer to juveniles of 3 species (38 specimens).
Our database contains information on helminths that parasitize nine species of reptiles belonging to the suborders Serpentes and Lacertilia. The helminth fauna in snakes of the Middle Volga region includes 31 species; slightly fewer helminth species were found in lizards (24 species). Of these, 11 species of parasites are recorded in both snakes and lizards. Snakes also dominate in the occurrence of helminths—7325 (85.4%); in lizards, 1251 (14.6%) occurrences were recorded.
The greatest number of helminth species was found in the grass snake, N. natrix (26 species). The helminth fauna of the dice snake, N. tessellata, consists of 16 species of parasites, and the helminths of the common viper, V. berus, are represented by 15 species. The helminth fauna of the steppe viper, V. renardi, (6) and the common copperhead, C. austriaca, (5) is less diverse. The helminth fauna of N. natrix includes all taxonomic groups: trematodes, nematodes, cestodes, and acanthocephalans. Cestodes were not found in vipers of both species and C. austriaca.
Among lizards, the sand lizard, L. agilis, is the leader in the number of helminth species in the database, with 21 parasite species recorded. The helminth fauna in A. colchica (8 species) and Z. vivipara (7) is less diverse. Only three species of helminths were recorded in E. arguta. All four taxonomic groups of parasitic worms (trematodes, cestodes, nematodes, acanthocephalans) are represented in the helminth fauna in L. agilis. In contrast, the helminth fauna in Z. vivipara and E. arguta does not include cestodes and acanthocephalans, respectively. The helminth fauna in A. colchica includes only nematodes and trematodes.
Our database shows that the widest host ranges (six reptile species each) belong to the nematodes Oswaldocruzia filiformis (Goeze, 1782), Physaloptera clausa Rudolphi, 1819, juv., and Protostrongylus sp., juv. and the trematodes Alaria alata (Goeze, 1782), msc. and Strigea strigis (Schrank, 1788), mtc. The nematode Rhabdias fuscovenosa (Railliet, 1899) was found in five reptile species. The host range of the trematodes Plagiorchis elegans (Rudolphi, 1802) and Strigea sphaerula (Rudolphi, 1803), mtc., as well as the nematode Physocephalus sexalatus (Molin, 1860), juv. consists of four reptile species each. The cestode Oochoristica tuberculata (Rudolphi, 1819), the trematodes Leptophallus nigrovenosus (Bellingham, 1844), Paralepoderma cloacicola (Luhe, 1909), and Telorchis assula (Dujardin, 1845), the larvae of acanthocephalans Sphaerirostris picae (Rudolphi, 1819) and Macracanthorhynchus catulinus Kostylew, 1927, as well as the nematode Strongyloides sp., juv. have three host species. The remaining 29 species of parasitic worms were found in only one or two host species (Table 1).
According to the database, the richest fauna of helminths was found in reptiles of the Republic of Mordovia and Samara Oblast (37 species each). Smaller numbers of parasite species were found in lizards and snakes in the Orenburg Oblast (21), Chuvashia (17), Nizhny Novgorod Oblast (16), and Ulyanovsk Oblast (13). In the Saratov Oblast and Tatarstan, only seven and four species of helminths were found in reptiles, respectively.
The diversity of various taxonomic groups of helminths in reptiles in different provinces of the Middle Volga region is not the same. In the Republic of Mordovia, Samara Oblast, and Ulyanovsk Oblast, all four groups of parasitic worms are represented in the helminth fauna. On the contrary, in the Saratov, Nizhny Novgorod, and Orenburg Oblasts, acanthocephalans were not registered in reptiles, and in Tatarstan and Chuvashia, only trematodes and nematodes were found.
The trematodes A. alata, mtc., L. nigrovenosus, and T. assula are the most common helminth species in the Middle Volga region and were found in reptiles in seven of the eight provinces studied. Eight parasite species, namely, the cestode Ophiotaenia europaea Odening, 1963, trematodes S. sphaerula, mtc., S. strigis, mtc., P. elegans, and P. cloacicola, and nematodes Rh. fuscovenosa, Strongyloides mirzai Singh, 1954, and Ph. clausa, juv., were recorded in reptiles in six provinces of the Middle Volga region. Trematodes Macrodera longicollis (Abildgaard, 1788), Neodiplostomum spathoides Dubois, 1937, mtc., and Pharyngostomum cordatum (Diesing, 1850), mtc. and nematodes O. filiformis and Protostrongylus sp., juv. were registered in five provinces studied.
Less common are the trematode Strigea falconis Szidat, 1928 and nematode Oxysomatium brevicaudatum (Zeder, 1800), recorded in four provinces each. Trematodes Astiotrema monticellii Stossich, 1904, Encyclometra colubrimurorum (Rudolphi, 1819), and Opisthioglyphe ranae (Frölich, 1791), nematodes Entomelas dujardini (Maupas, 1916), Entomelas entomelas (Dujardin, 1845), and Ph. sexalatus, juv., and the acanthocephalan Centrorhynchus aluconis (Müller, 1780), juv., were recorded in three provinces. The remaining 20 species of helminths were found in reptiles in only one or two provinces studied (Table 1).
The analysis of helminth species diversity in different provinces of the Middle Volga region showed that greater diversity is characteristic of the Orenburg Oblast (H’ = 1.988), Chuvashia (1.986), and Samara Oblast (1.981). Less diverse is the helminth fauna in reptiles of the Republic of Mordovia (1.963), Nizhny Novgorod Oblast (1.890), and Saratov Oblast (1.406). Low helminth species diversity is typical for reptiles of Tatarstan (0.800) and the Ulyanovsk Oblast (0.414). Differences in the Shannon index values are statistically significant in most cases (p < 0.05). Insignificant differences in the Shannon index values were revealed between the Samara Oblast and the Orenburg Oblast, the Samara Oblast and Chuvashia, and the Orenburg Oblast and Chuvashia (p > 0.05).
We have conducted a comparative analysis of the species structure of helminths in reptiles from different provinces of the Middle Volga region. The dendrogram of similarity of the helminth fauna of different species of snakes and lizards is presented in Figure 4.
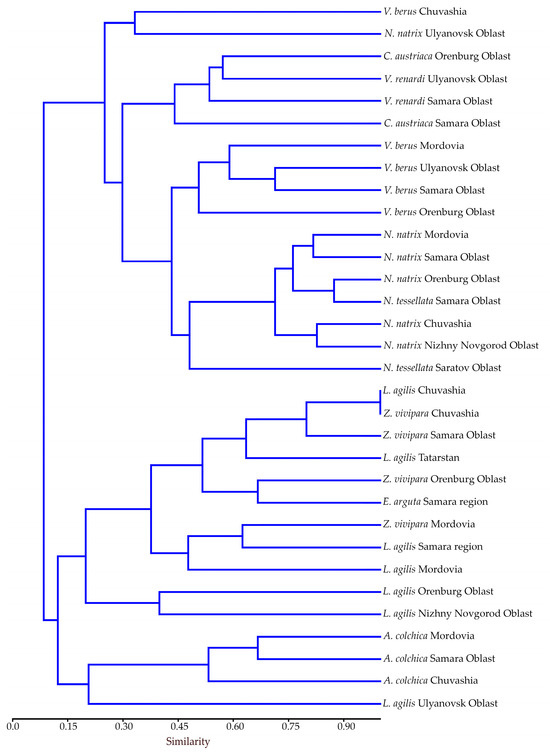
Figure 4.
Similarity dendrogram of helminth fauna of reptiles from different provinces of the Middle Volga Region obtained using the Morisita index (UPGMA). Correlation coefficient: r = 0.849.
As a result of clustering, all the studied species of reptiles were divided at the first level of branching into two clusters with the closest helminth fauna. The first cluster consists of the helminth fauna of snakes. It differs greatly from the second cluster formed by the helminth fauna of lizards. Each cluster in turn is divided into two groups (Figure 4).
In the first cluster, the highest degree of similarity in helminth fauna was found in the second group among Natrix spp. (Figure 4). Here, a high degree of similarity appears between N. natrix from the Orenburg Oblast and N. tessellata from the Samara Oblast (0.88), N. natrix from the Samara Oblast and Mordovia (0.82), and N. natrix from the Nizhny Novgorod Oblast and Chuvashia (0.83). A high degree of similarity was also revealed for vipers V. berus from the Samara and Ulyanovsk Oblasts (0.71).
In the second cluster, the greatest similarity was found in the first group (Figure 4). In this group, complete similarity of the helminth fauna was established for L. agilis and Z. vivipara from Chuvashia (1.0). A high degree of similarity was revealed for L. agilis from Chuvashia and Z. vivipara from the Samara Oblast, Z. vivipara from Chuvashia and the Samara Oblast (0.8 each), and Z. vivipara from the Orenburg Oblast and E. arguta from the Samara Oblast (0.67). In the second group, the helminth fauna of slow worms from the Samara Oblast and Mordovia is most similar (0.67).
Most of the helminth species found in reptiles of the Middle Volga region belong to the Palearctic faunal complex (25 species). Eight species of parasitic worms have a Holarctic distribution. Seven species of helminths parasitize only reptiles of Europe. Five species of parasites are cosmopolitan (Table 1).
Helminthological studies of various reptile species were conducted in many European countries and regions of Russia. Our analysis included only those countries where several reptile species were involved in the study (Figure 5). As a result, about 190 species of helminths were registered in reptiles, of which 170 were identified to the species level [1,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,67,68,69,70,71,72,73,74,75,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140].
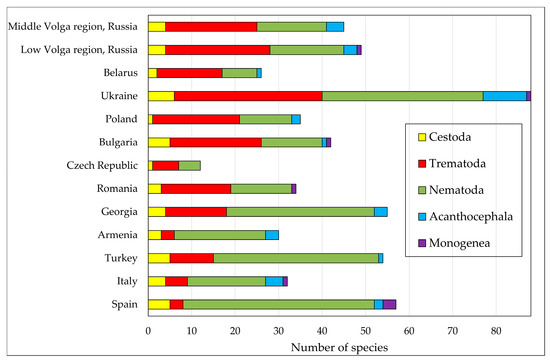
Figure 5.
The number of helminth species found in reptiles in different European countries.
The greatest number of helminth species was recorded in reptiles from Ukraine (88) [1,14,62], Spain (57) [13,18,19,20,21,24,27,28,32,36,40,41,42,43,48,120,126,127], Georgia (55) [56,57,140], and Turkey (54) [23,25,26,29,30,31,34,44,47,49,50,104]. Somewhat fewer parasitic worms were found in reptiles in the Middle Volga region and Lower Volga region—45 and 49 species, respectively [17,33,35,37,46,52,53,59,60,67,68,69,70,71,72,73,74,75,103,106,108,128,134,135,138]. The smallest number of parasite species was found in reptiles in the Czech Republic (12 species) [1,54] (Figure 5). Trematodes, nematodes, and cestodes were found in reptiles in all the countries studied. Acanthocephalans were not recorded in reptiles from the Czech Republic and Romania. Polystomes were recorded only in Ukraine, Romania, Bulgaria, Italy, Spain, and the Lower Volga region (Russia) [1,15,40,41,43,55,62] (Figure 5).
4. Discussion
This paper presents a description of our recently published database containing information on the fauna of parasitic worms in reptiles of the Middle Volga region (European Russia). As a result of our research, the list of these helminths was expanded and currently includes 45 species [75]. Despite the relatively high diversity of helminths in reptiles of the Middle Volga region, it still does not reach its maximum, since there are regions of Europe where reptiles serve as hosts for a larger number of helminth species (Figure 5).
During the period of our helminthological study of reptiles, 11 species of parasites were registered for the first time in the Russia, namely, the trematodes Plagiorchis maculosus (Rudolphi, 1802), Prosotocus confusus (Looss, 1896), Neoglyphe sobolevi (Shaldybin, 1953), and Pleurogenoides medians (Olsson, 1876), the acanthocephalan A. lucii, and the nematodes S. mirzai, Camallanus truncatus (Rudolphi, 1814), O. brevicaudatum, Streptocara crassicauda (Creplin, 1829), juv., Ph. clausa, juv., and Protostrongylus sp., juv.
For the first time in the Middle Volga region, 14 species of helminths were found in lizards and snakes: the trematode Pleurogenes claviger (Rudolphi, 1819), the cestodes Nematotaenia tarentolae Lopez-Neyra, 1944, and S. erinaceieuropaei, plc., the acanthocephalans C. aluconis, juv., M. catulinus, juv., and S. picae, juv., and the nematodes Abbreviata abbreviata (Rudolphi, 1819), E. entomelas, E. dujardini, Spauligodon lacertae Sharpilo, 1966, Spauligodon pseudoeremiasi Sharpilo, 1976, S. mirzai, Ascarops strongylina (Rudolphi, 1819), juv., and Ph. sexalatus, juv.
According to our dataset, the greatest species diversity of helminths in reptiles of the Middle Volga region is formed by trematodes. In addition, trematodes dominate in occurrence and abundance (Figure 2 and Figure 3). This is mainly due to the fact that reptiles feed as carnivores and the nature of their trophic relationships. Thus, snakes become infected with adults of most species when feeding on anurans, which often serve as second intermediate hosts for trematodes. Infection of reptiles with larval forms occurs mainly through topical routes [141]. Lizards become infected with all trematode species when eating larvae and adult near-aquatic insects, which are possible intermediate hosts of these parasites.
The high abundance of trematode larvae compared to adult forms is due to the different dynamics of infection and accumulation of these parasites in reptiles. Larval forms accumulate in the host’s body for several years, while the lifespan of adult parasites is usually limited to a period of several months to a year [142,143,144].
Like trematodes, nematodes parasitizing reptiles of the Middle Volga region are also characterized by high diversity. Among nematodes, species with both direct and indirect life cycles have been found. Lizards and snakes become infected with nematodes with an indirect life cycle when feeding on invertebrates. Reptiles become infected with geohelminths (nematodes with a direct life cycle) when they accidentally swallow parasite eggs or invasive larvae when feeding in a humid environment. Nematodes and trematodes are also the most diverse groups of parasites of reptiles from other European countries (Figure 5). Thus, the number of trematode species in different countries ranges from 2 to 33, and nematode species—from 5 to 38.
Among the helminths in reptiles of the Middle Volga region, cestodes are less common. Their occurrence and abundance are significantly lower than those of trematodes and nematodes. The most common cestode is O. europaea, a parasite of colubrid snakes. This is due, firstly, to the abundance and wide distribution of the main final hosts of this helminth—grass snakes and dice snakes; secondly, to the wide distribution of O. europaea in Europe. Grass snakes become infected with cestodes when feeding on fish, which is the main food item of N. tessellata and is included in the diet of N. natrix [145,146,147].
The cestodes N. tarentolae and O. tuberculata, which parasitize lizards, are rarely found in the Middle Volga region. This is explained, on the one hand, by the local distribution of the parasites, and on the other hand, by the fact that the northern boundary of the range of these cestode species passes through the Middle Volga region. Thus, according to Sharpilo [1], O. tuberculata is more often found in lizards in the southern regions of the former USSR.
The plerocercoid of S. erinaceieuropaei is a widespread but locally occurring parasite of reptiles [1]. In the Middle Volga region, it was recorded by us only in one region and only in one host (N. natrix). Infection occurs when snakes swallow intermediate hosts of the cestode, copepods (Cyclops spp.), together with food items, fish or amphibians [11,148,149]. The species richness of cestodes in reptiles in European countries is also low, and their greatest diversity was found in Ukraine (6 species) (Figure 5).
Acanthocephalans are rare parasites of reptiles in Europe [1]. In most European countries, their diversity does not exceed 4 species, with the exception of Ukraine, where 10 species have been found (Figure 5). In the Middle Volga region, reptiles serve as paratenic hosts for three species of acanthocephalans: Centrorhynchus aluconis (Müller, 1780), Sphaerirostris picae (Rudolphi, 1819), and Macracanthorhynchus catulinus Kostylew, 1927. Reptiles become infected with them when feeding on insects [1].
Findings of the host-specific fish parasite, A. lucii, in the grass snake, as well as the findings of the nematode C. truncatus in the dice snake, are cases of postcyclic parasitism. The phenomenon of postcyclic parasitism probably also includes the findings of specific amphibian parasites in N. natrix—the trematodes D. subclavatus, P. claviger and O. ranae [150]. In the Middle Volga region, reptiles serve as definitive hosts for 25 species of helminths. For 15 species of parasitic worms, reptiles serve as paratenic and/or intermediate hosts. Findings of larval stages of parasitic worms in reptiles indicate their involvement in the life cycles of helminths of birds and mammals of a higher trophic levels. It is no coincidence that reptiles are prey for many species of vertebrates [63,151,152].
Most of the helminth species found in snakes and lizards of the Middle Volga region are host-specific parasites of reptiles. They are found in reptiles practically throughout their geographical ranges. Only 10 species (the trematodes D. subclavatus, O. ranae, P. confusus, N. sobolevi, P. claviger, P. medians, P. elegans, and P. maculosus, the nematode C. truncatus, and the acanthocephalan A. lucii) are facultative parasites of reptiles and parasitize lizards and snakes accidentally.
Helminths of reptiles characterize the ecology of their hosts. Our study of the species diversity of helminths in reptiles of the Middle Volga region showed that the most abundant and widespread host species, N. natrix, has the richest helminth fauna. In addition, this is due to the fact that N. natrix feeds on anurans (intermediate hosts of specific trematodes of the grass snake) and lives in semi-aquatic habitats, where favorable conditions for infection with nematodes and digenean larvae are formed. Compared to N. natrix, the dice snake N. tessellata has a narrower ecological niche (feeding mainly on fish and having a semi-aquatic lifestyle) and has a depleted composition of helminths.
The species composition, prevalence, and abundance of helminths in V. berus and V. renardi are closely related to the diet of vipers. In the Middle Volga region, the basis of the diet of both species is myomorph rodents, which are not a source of helminth infection for vipers. The helminth fauna of vipers is more diverse if their diet includes amphibians and fish. Among all snakes, the smallest number of helminth species is characteristic of C. austriaca, which is due to dry habitats and a diet consisting only of lizards.
Like the grass snake, the sand lizard L. agilis is a common and widespread species in the Middle Volga region. Compared with other lizards, L. agilis has the largest number of helminth species. This is primarily due to the diversity of the diet in different habitats. In contrast, the smaller size of the viviparous lizard Z. vivipara limits its diet by reducing the size of food items. In addition, the distribution of Z. vivipara is limited to humid forest habitats. All this leads to the depletion of its helminth fauna. The helminth fauna in the slow worm A. colchica is unique, with nematodes predominating among the helminths. Metacercariae of S. strigis were also accidentally found in slow worms of the Middle Volga region. Two registered nematode species, E. entomelas and E. dujardini, parasitize only slow worms, which is an extremely rare phenomenon among parasites of reptiles [1]. The infection of this legless lizard mainly with nematodes is explained by the fact that the reptile lives on a humid forest litter, i.e., in a favorable environment for the development of nematode eggs and larvae. Anguis colchica also feeds on invertebrates, which serve as intermediate hosts or reservoirs for the invasive nematode larvae [73,111].
The smallest number of helminth species was found in E. arguta, which is due to the preference of this lizard for drier sandy habitats. In addition, the level of helminth infection and the species richness of parasites decrease at the boundaries of the host’s range [153]. Thus, the territory of the Middle Volga region is the northern boundary of the range of E. arguta, as well as N. tessellata, which causes the depletion of the helminth fauna in these reptile species.
Comparative analysis of the species structure of helminths in reptiles from different provinces of the Middle Volga region showed the uniqueness of the helminth fauna of snakes and lizards. On the other hand, a great similarity was found between the helminth fauna of snakes within the genera Natrix and Vipera, as well as lizards within the families Lacertidae and Anguidae (Figure 4). The uniqueness of the helminth fauna of snakes is due to the presence of 21 species of helminths that parasitize exclusively in them. At the same time, 10 species of helminths parasitize exclusively in lizards (Table 1). The similarity of the helminth fauna of various reptile species from different provinces of the Middle Volga region is mainly due to the host–parasite specificity, as well as the wide distribution of host-specific helminths of reptiles and the geographical proximity of the studied territories.
The helminth fauna of reptiles varies in different provinces of the Middle Volga region. These differences are due to a number of factors, such as the diversity of biocenoses, the richness of the vertebrate and invertebrate fauna, including the final and intermediate hosts of helminths, and the number of reptile species studied. Over 28 years of helminthological studies of reptiles in the Middle Volga region, nine species of lizards and snakes have been investigated to varying degrees. The most complete data on parasitic worms were obtained in the Samara Oblast (for nine host species) and the Republic of Mordovia (for five host species). Here, the studies covered a larger number of habitats, as well as a larger number of reptile specimens. Therefore, the largest number of parasitic worms was recorded in these provinces—37 species in each (Table 1). In the Saratov Oblast and Tatarstan, less data on parasites have been obtained, since only two species of reptiles have been studied—N. tessellata (seven parasite species) and L. agilis (four parasite species), respectively. Helminthological studies of reptiles in Mari El and Penza Oblast have not yet been conducted.
The helminth fauna of reptiles varies significantly in different European countries depending on the host species examined. Involvement of new reptile species in research expands knowledge about the helminth fauna of a specific geographical area. For example, a helminthological study of land and freshwater turtles in southern European countries supplemented the list of helminths registered in reptiles with turtle-specific parasites [22,23,27,36,40,41,43].
Of the 45 species of parasitic worms found in reptiles of the Middle Volga region, 3 species have medical and veterinary significance as causative agents of dangerous helminthiasis. These include the trematode A. alata (causes alariosis in humans and domestic animals), the cestode S. erinaceieuropaei (spirometrosis and sparganosis in humans and wild and domestic animals), and the nematode Ph. sexalatus (physocephalosis in humans, domestic pigs, and wild boars) [9,154,155,156].
5. Conclusions
According to our database, the helminth fauna of reptiles in the Middle Volga region to date includes 45 species of parasitic worms. The most diverse helminth fauna is found in the most common and abundant reptile species—the grass snake N. natrix (26 helminth species) and the sand lizard L. agilis (21). The helminth fauna of N. tessellata (16) and V. berus (15) is less diverse. Several species of parasitic worms were found in A. colchica (8), Z. vivipara (7), V. renardi (6), C. austriaca (5), and E. arguta (3).
Among the helminths found in reptiles of the Middle Volga region, trematodes have the greatest species diversity, also prevailing in occurrence and abundance. Nematodes are also characterized by great diversity, but their occurrence and abundance are significantly lower. Cestodes are even less common than nematodes and especially trematodes. Acanthocephalans are rare parasites of lizards and snakes of the Middle Volga region.
Most species of helminths recorded in lizards and snakes have a Palearctic distribution (25 species). Eight species of parasites are distributed in the Holarctic. The distribution of seven species of helminths is limited to Europe. Five species of reptile parasites are cosmopolitan. Of the 45 species of helminths found in reptiles, 3 species (A. alata, S. erinaceieuropaei, Ph. sexalatus) are of medical and veterinary importance as causative agents of parasitic zoonoses.
Data on the diversity and distribution of helminths parasitizing reptiles in the Middle Volga region remain incomplete, since there are still unexplored (Mari El, Penza Oblast) and poorly studied (Republic of Chuvashia, Nizhny Novgorod, Ulyanovsk and Saratov Oblasts) territories. Our database, published in GBIF, can be supplemented with new data on the occurrence of parasitic worms in reptiles and modified in accordance with new concepts of the taxonomy of helminths and their hosts.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d17060380/s1, Table S1: Description of the data in the dataset; Table S2: Taxa included in the dataset.
Author Contributions
Conceptualization, A.A.K., A.B.R. and N.Y.K.; methodology, A.A.K., N.Y.K. and S.V.S.; software, A.A.K., S.V.S. and A.I.F.; validation, A.A.K., N.Y.K., S.V.S. and A.B.R.; formal analysis, A.A.K., N.Y.K. and S.V.S.; investigation, A.A.K., N.Y.K. and A.B.R.; resources, A.A.K., A.B.R., N.Y.K. and A.I.F.; data curation, A.A.K., N.Y.K. and A.B.R.; writing—original draft preparation, A.A.K., N.Y.K. and S.V.S.; writing—review and editing, A.A.K. and N.Y.K.; visualization, A.A.K., N.Y.K. and S.V.S.; supervision, A.A.K., N.Y.K. and A.B.R.; project administration, N.Y.K., A.I.F. and A.B.R.; funding acquisition, A.I.F. and A.B.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Bioethics Committee of the Institute of Ecology of Volga River Basin of RAS (Registration number: 1/24; 26 March 2025). All appropriate international, national, and institutional guidelines for the use and care of wild animals were followed. Our research was conducted in compliance with the ethical standards of humane treatment of animals in accordance with the recommended standards described in the Directive of the European Parliament and of the Council of the European Union (22 September 2010) “On the protection of animals used for scientific purposes” (EU Directive 2010/63/EU). Trapping and research of reptiles was carried out in accordance with agreements on scientific cooperation with the National Park “Samarskaya Luka”, Zhiguli Nature Reserve in 1999–2022 and the Federal State Budgetary Institution “Reserved Mordovia” (“Zapovednaya Mordovia”) in 2018–2024.
Data Availability Statement
The data are presented in our database at https://doi.org/10.15468/yntugs.
Acknowledgments
This research was performed within the framework of the state assignment 1-22-31-1 from Ministry of Natural Resources and Ecology of the Russian Federation and the research topic of the Institute of Ecology of the Volga River Basin, a branch of the Samara Federal Research Center of the Russian Academy of Sciences No. 1023062000002-6-1.6.20;1.6.19 “Land vertebrates of the Middle Volga region and adjacent territories and their parasitic worms: ecological, faunal and biological aspects of the organization and functioning of communities against the background of natural and anthropogenic changes”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GBIF | Global Biodiversity Information Facility |
| IEVB RAS | Institute of Ecology of the Volga River basin of the Russian Academy of Sciences |
| msc. | Mesocercaria |
| mtc. | Metacercaria |
| plc. | Plerocercoid |
| juv. | Juvenile |
| UPGMA | Unweighted pair group method with arithmetic mean |
References
- Sharpilo, V.P. Parasitic Worms of Reptiles of Fauna of USSR; Naukova Dumka: Kiev, Ukraine, 1976; pp. 3–286. [Google Scholar]
- Pare, J.A. An overview of pentastomiasis in reptiles and other vertebrates. J. Exot. Pet Med. 2008, 17, 285–294. [Google Scholar] [CrossRef]
- Corn, J.L.; Mertins, J.W.; Hanson, B.; Snow, S. First reports of ectoparasites collected from wild-caught exotic reptiles in Florida. J. Med. Entomol. 2011, 48, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Fajfer, M. Acari (Chelicerata)—Parasites of reptiles. Acarina 2012, 20, 108–129. [Google Scholar]
- Schmidt, V.; Mock, R.; Burgkhardt, E.; Junghanns, A.; Ortlieb, F.; Szabo, I.; Marschang, R.; Blindow, I.; Krautwald-Junghanns, M.E. Cloacal aerobic bacterial flora and absence of viruses in free-living slow worms (Anguis fragilis), grass snakes (Natrix natrix) and European adders (Vipera berus) from Germany. Ecohealth 2014, 11, 571–580. [Google Scholar] [CrossRef]
- Zajac, M.; Wasyl, D.; Rozycki, M.; Bilska-Zajac, E.; Fafinski, Z.; Iwaniak, W.; Krajewska, M.; Hoszowski, A.; Konieczna, O.; Fafinska, P.; et al. Free-living snakes as a source and possible vector of Salmonella spp. and parasites. Eur. J. Wildl. Res. 2016, 62, 161–166. [Google Scholar] [CrossRef]
- Pham, V.T.; Le Duc, O.; Leprince, B.; Bordes, C.; Ducotterd, C.; Luu, V.Q.; An, L.T.; Nguyen, M.H.; Lo, V.O.; Ha, V.N.; et al. An assessment of turtle communities in Bach Ma National Park, Vietnam. Nat. Conserv. Res. 2023, 8, 72–80. [Google Scholar] [CrossRef]
- Sharpilo, V.P.; Salamatin, R.V. Paratenic Parasitism: Fonnation and Development of the Conception; Historical Sketch and Bibliography: Kiev, Ukraine, 2005; pp. 3–240. [Google Scholar]
- Mohl, K.; Grosse, K.; Hamedy, A.; Wuste, T.; Kabelitz, P.; Lucker, E. Biology of Alaria spp. and human exposition risk to Alaria mesocercariae—A review. Parasitol. Res. 2009, 105, 1–15. [Google Scholar] [CrossRef]
- Duscher, G.G.; Leschnik, M.; Fuehrer, H.-P.; Joachim, A. Wildlife reservoirs for vector-borne canine, feline and zoonotic infections in Austria. Int. J. Parasitol. Parasites Wildl. 2014, 4, 88–96. [Google Scholar] [CrossRef]
- Kondzior, E.; Tokarska, M.; Kowalczyk, R.; Ruczynska, I.; Sobocinski, W.; Kolodziej-Sobocinska, M. The first case of genetically confirmed sparganosis (Spirometra erinaceieuropaei) in European reptiles. Parasitol. Res. 2018, 117, 3659–3662. [Google Scholar] [CrossRef]
- Buchvarov, G.; Kirin, D.; Kostadinova, A. Platyhelminth parasite assemblages in two species of snakes Natrix natrix and Natrix tessellata (Reptilia, Colubridae) from Bulgaria: Seasonal variation. JEPE 2000, 1, 124–131. [Google Scholar]
- Sanchis, V.; Roig, J.M.; Carretero, M.A.; Roca, V.; Llorente, G.A. Host-parasite relationships of Zootoca vivipara (Sauria: Lacertidae) in the Pyrenees (North Spain). Fol. Parasitol. 2000, 47, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Sharpilo, V.P.; Biserkov, V.A.; Kostadinova, J.; Behnke, M.; Kuzmin, Y.I. Helminths of the sand lizard, Lacerta agilis (Reptilia, Lacertidae), in the Palaearctic: Faunal diversity and spatial patterns of variation in the composition and structure of component communities. Parasitology 2001, 123, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kirin, D.A. New data on the helminth fauna of Emys orbicularis L. (1758) Reptilia, (Emydidae) in South Bulgaria. Comp. Rend. l’Acad. Bulg. Sci. 2001, 54, 95–98. [Google Scholar]
- Kirin, D. New records of the Helminth fauna from grass snake, Natrix natrix L., 1758 and dice snake, Natrix tessellata Laurenti, 1768 (Colubridae: Reptilia) in South Bulgaria. Acta Zool. Bulg. 2002, 54, 49–53. [Google Scholar]
- Kirillov, A.A.; Bakiev, A.G. Study of helminthes of vipers (Viperidae) in Middle Volga region. Sam. Luka Probl. Reg. Glob. Ecol. 2003, 13, 331–336. [Google Scholar]
- Martin, J.E.; Roca, V.; Galdon, M.A.; Sanchez-Mut, J.; Muniesa, J. Helminth fauna of Gallotia caesaris caesaris (Lehrs, 1914) from El Hierro Island and Gallotia caesaris gomerae (Boettger et Muller, 1914) from La Gomera Island (Sauria: Lacertidae). Rev. Iber. Parasitol. 2003, 63, 30–35. [Google Scholar]
- Martin, J.E.; Roca, V. Helminth infracommunities of Gallotia caesaris caesaris and Gallotia caesaris gomerae (Sauria: Lacertidae) from the Canary Islands (Eastern Atlantic). J. Parasitol. 2004, 90, 266–270. [Google Scholar] [CrossRef]
- Martin, J.E.; Llorente, G.A.; Roca, V.; Carretero, M.A.; Montori, A.; Santos, X.; Romeu, R. Relationship between diet and helminths in Gallotia caesaris (Sauria: Lacertidae). Zoology 2005, 108, 121–130. [Google Scholar] [CrossRef]
- Roca, V.; Sanchez-Torres, N.; Martin, J.E. Intestinal helminths parasitizing Mauremys leprosa (Chelonia: Bataguridae) from Extremadura (western Spain). Rev. Esp. Herpetol. 2005, 19, 47–55. [Google Scholar]
- Traversa, D.; Capelli, G.; Iorio, R.; Bouamer, S.; Cameli, A.; Giangaspero, A. Epidemiology and biology of nematodofauna affecting Testudo hermanni, Testudo graeca and Testudo marginata in Italy. Parasitol. Res. 2005, 98, 14–20. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Sahin, R. The helminth fauna of Emys orbicularis (European pond turtle) (Linnaeus, 1758) living in freshwater. Türk. Parazitol. Derg. 2005, 29, 56–62. [Google Scholar]
- Santos, X.; Martinez-Freiria, F.; Pleguezuelos, J.M.; Roca, V. First helminthological data on Iberian vipers: Helminth communities and host-parasite relationships. Act. Parasitol. 2006, 51, 130–135. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Goldberg, S.R.; Bursey, C.R. Helminth parasites of the Caucasian agama, Laudakia caucasia, and the roughtail rock agama, Laudakia stellio (Squamata: Agamidae), from Turkey. Comp. Parasitol. 2006, 2, 257–262. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Bursey, C.R.; Goldberg, S.R. Helminth parasites of the Grass snake, Natrix natrix, and the Dice snake, Natrix tessellata (Serpentes: Colubridae), from Turkey. Comp. Parasitol. 2007, 74, 343–354. [Google Scholar] [CrossRef]
- Hidalgo-Vila, J.; Diaz-Paniagua, C.; Ribas, A.; Florencio, M.; Perez-Santigosa, N.; Casanova, J.C. Helminth communities of the exotic introduced turtle, Trachemys scripta elegans in southwestern Spain: Transmission from native turtles. Res. Vet. Sci. 2009, 86, 463–465. [Google Scholar] [CrossRef]
- Foronda, P.; Abreu-Acosta, N.; Casanova, J.C.; Ribas, A.; Valladares, B. A new Anoplocephalid (Cestoda: Cyclophyllidea) from Gallotia atlantica (Reptilia, Lacertidae) in the Canary Islands, Spain. J. Parasitol. 2009, 95, 678–680. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Yilmaz, N.; Incedogan, S. Helminth fauna of the Anatolian worm lizard, Blanus strauchi (Bedriaga, 1884) from Hatay. Türk. Parazitol. Derg. 2009, 33, 327–329. [Google Scholar]
- Dusen, S.; Ugurtas, I.H.; Altunel, F.N. Nematode parasites of the smooth snake, Coronella austriaca Laurenti, 1768 and the Aesculapian snake, Zamenis longissimus (Laurenti, 1768) (Ophidia: Colubridae), collected from North-Western Turkey. North-West. J. Zool. 2010, 6, 86–89. [Google Scholar]
- Dusen, S.; Ugurtas, I.H.; Aydogdu, A. Nematode parasites of the two limbless lizards: Turkish worm lizard, Blanus strauchi (Bedriaga, 1884) (Squamata: Amphisbaenidae), and slow worm, Anguis fragilis Linnaeus 1758 (Squamata: Anguidae), from Turkey. Helmintologia 2010, 47, 158–163. [Google Scholar] [CrossRef]
- Ribas, A.; Lopez, S.; Roca, V. Helminths from snakes in Northeast Spain. Bol. Asoc. Herpetol. Esp. 2010, 21, 44–46. [Google Scholar]
- Kirillov, A.A. Helminth communities of the grass snake, Natrix natrix L. (Reptilia: Colubridae) from south of Middle Volga area. Proc. Sam. Sci. Cent. RAS 2011, 13, 127–134. [Google Scholar]
- Yildirimhan, H.S.; Bursey, C.R.; Altunel, F.N. Helminth parasites of the Balkan green lizard, Lacerta trilineata Bedriaga 1886, from Bursa, Turkey. Turk. J. Zool. 2011, 35, 1–17. [Google Scholar] [CrossRef]
- Bakiev, A.G.; Kirillov, A.A.; Mebert, K. Diet and parasitic helminths of dice snakes from the Volga basin, Russia. Mertensiella 2011, 18, 325–329. [Google Scholar]
- Chavarri, M.; Berriatua, E.; Gimenez, A.; Gracia, E.; Martinez-Carrasco, C.; Ortiz, J.M.; de Ybanez, R.R. Differences in helminth infections between captive and wild spur-thighed tortoises Testudo graeca in southern Spain: A potential risk of reintroductions of this species. Vet. Parasitol. 2012, 187, 491–497. [Google Scholar] [CrossRef]
- Ruchin, A.B.; Kirillov, A.A. Helminths of Natrix natrix L. in Mordovia. Biol. Sci. Kazakh. 2012, 4, 344–348. [Google Scholar]
- Santoro, M.; Aznar, F.J.; Mattiucci, S.; Kinsella, J.M.; Pellegrino, F.; Cipriani, P.; Nascetti, G. Parasite assemblages in the Western whip snake Hierophis viridiflavus carbonarius (Colubridae) from southern Italy. J. Helminthol. 2013, 87, 277–285. [Google Scholar] [CrossRef]
- Sargsyan, N.; Arakelyan, M.; Danielyan, F.; Vartanyan, L. Helminthes of some species of reptiles from Republic of Armenia. El. J. Nat. Sci. 2014, 1, 50–54. [Google Scholar]
- Iglesias, R.; Garcia-Estevez, J.M.; Ayres, C.; Acuna, A.; Cordero-Rivera, A. First reported outbreak of severe spirorchiidiasis in Emys orbicularis, probably resulting from a parasite spillover event. Dis. Aquat. Organ. 2015, 113, 75–80. [Google Scholar] [CrossRef]
- Meyer, L.; Du Preez, L.; Bonneau, E.; Heritier, L.; Quintana, M.F.; Valdeon, A.; Sadaoui, A.; Kechemir-Issad, N.; Palacios, C.; Verneau, O. Parasite host-switching from the invasive American red-eared slider Trachemys scripta elegans, to the native Mediterranean pond turtle, Mauremys leprosa, in natural environments. Aq. Inv. 2015, 10, 79–91. [Google Scholar] [CrossRef]
- Roca, V.; Jorge, F.; Ilgaz, C.; Kumlutas, Y.; Durmus, S.H.; Carretero, M.A. The intestinal helminth community of the spiny-tailed lizard Darevskia rudis (Squamata, Lacertidae) from northern Turkey. J. Helminthol. 2015, 90, 144–151. [Google Scholar] [CrossRef]
- Heritier, L.; Valdeon, A.; Sadaoui, A.; Gendre, T.; Ficheux, S.; Bouamer, S.; Kechemir-Issad, N.; Du Preez, L.; Palacios, C.; Verneau, O. Introduction and invasion of the red-eared slider and its parasites in freshwater ecosystems of Southern Europe: Risk assessment for the European pond turtle in wild environments. Biodivers. Conserv. 2017, 26, 1817–1843. [Google Scholar] [CrossRef]
- Birlic, S.; Yildirimhan, H.S.; Ilgas, C.; Kumlutas, Y. Helminth fauna of spiny tailed lizard, Darevskia rudis (Bedriaga, 1886) (Sauria: Lacertidae) from Turkey. Helminthologia 2018, 55, 45–51. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Guven, A.; Sumer, N. The helminth parasites of the Mediterranean spur-thighed tortoise, Testudo graeca (L., 1758) from Bursa, Turkey. Biharean Biol. 2018, 12, 10–12. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y. Comparative analysis of the helminth fauna of Natrix natrix and Natrix tessellata (Reptilia, Colubridae) in the Samarskaya Luka National Park (Russia). Nat. Conserv. Res. 2019, 4, 12–25. [Google Scholar] [CrossRef]
- Sumer, N.; Birlik, S.; Yildirimhan, H.S. Morphological and molecular taxonomy of helminths of the slow worm, Anguis fragilis (Linnaeus) (Squamata: Anguidae) from Turkey. Biharean Biol. 2019, 13, 36–38. [Google Scholar]
- Roca, V.; Belliure, J.; Santos, X.; Pausas, J.G. New reptile hosts for helminth parasites in a mediterranean region. J. Herpetol. 2020, 54, 268–271. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Karaman, D.; Bursey, C.R. Helminth fauna of the European green lizard, Lacerta viridis (Laurenti, 1768), from Bursa, Turkey. Comp. Parasitol. 2020, 87, 56–67. [Google Scholar] [CrossRef]
- Yildirimhan, H.S.; Sumer, N.; Bursey, C.R. Helminh parasites of two lacertid species, Anatololacerta anatolica (Wermer, 1902) and Darevskia rudis (Bedriaga, 1886) (Sauria: Lacertidae) from Bursa province, North-Western Turkey. Acta Zool. Bulg. 2020, 72, 315–320. [Google Scholar]
- Belcik, A.; Rozycki, M.; Korpysa-Dzirba, W.; Marucci, G.; Fafinski, Z.; Fafinska, P.; Karamon, J.; Kochanowski, M.; Cencek, T.; Bilska-Zajac, E. Grass snakes (Natrix natrix) as a reservoir of Alaria alata and other parasites. Pathogens 2022, 11, 156. [Google Scholar] [CrossRef]
- Kirillova, A.A.; Kirillova, N.Y.; Bakiev, A.G.; Gorelov, R.A. Ecological analysis of the helminth fauna in Natrix tessellata (Reptilia, Colubridae) from the Low Volga region (Russia). Inl. Wat. Biol. 2023, 16, 357–368. [Google Scholar] [CrossRef]
- Kirillov, A.A. Helminths fauna of reptiles of Samara region. Proc. Sam. Sci. Cent. RAS 2000, 2, 324–329. [Google Scholar]
- Borkovcova, M.; Kopriva, J. Parasitic helminths of reptiles (Reptilia) in South Moravia (Czech Republic). Parasitol. Res. 2005, 95, 77–78. [Google Scholar] [CrossRef]
- Mihalca, A.D.; Gherman, C.; Ghira, I.; Cozma, V. Helminth parasites of reptiles (Reptilia) in Romania. Parasitol. Res. 2007, 101, 491–492. [Google Scholar] [CrossRef]
- Murvanidze, L.; Lomidze, T.; Nikolaishvili, K.; Jankarashvili, E. The annotated list of reptile helminthes of Georgia. Proc. Inst. Zool. 2008, 23, 54–61. [Google Scholar]
- Murvanidze, L.P.; Gogebashvili, I.Y.; Nikolaishvili, K.G.; Lomidze, T.V.; Kakalova, E.S.; Arabuli, L.S. To the study of parasitofauna of amphibians and reptiles of coastal line of Tbilisi Reservoir. Vest. Zool. 2009, 23, 149–152. [Google Scholar]
- Shimalov, V.V. Helminthofauna of reptiles in the Republic of Belarus. Parazitologiya 2010, 44, 22–29. [Google Scholar]
- Kalmykov, A.P.; Tulendeev, R.N. The helminth fauna of reptiles of the Astrakhan region. In Proceedings of the All-Russian Scientific Conference “Natural Ecosystems of the Caspian Region: Past, Present, Future”, Astrakhan Oblast, Russia, 3–5 September 2019; Litvinov, K.V., Kalmykov, A.P., Meshcheryakova, N.O., Eds.; Mir: Astrakhan, Russia, 2019; pp. 205–208. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y. Overview of helminths in reptiles of the National Park “Samarskaya Luka” (Russia). Nat. Conserv. Res. 2018, 3 (Suppl. S1), 73–82. [Google Scholar] [CrossRef]
- Kusmierek, N.; Pyrka, E.; Popiolek, M. Diversity of helminths in polish reptiles: A review. Biologia 2020, 75, 733–739. [Google Scholar] [CrossRef]
- Marushchak, O.; Syrota, Y.; Dmytrieva, I.; Kuzmin, Y.; Nechai, A.; Lisitsyna, O.; Svitin, R. Helminths found in common species of the herpetofauna in Ukraine. Biodivers. Data J. 2024, 12, e113770. [Google Scholar] [CrossRef]
- Bakiev, A.G.; Garanin, V.I.; Litvinov, N.A.; Pavlov, A.V.; Ratnikov, V.Y. Snakes of Volzhsko-Kamskij Region; Publish of Samara Scientific Center RAS: Samara, Russia, 2004; pp. 3–192. [Google Scholar]
- Dunaev, E.A.; Orlova, V.F. Amphibians and Reptiles of Russia: Determinant Atlas; Fiton+: Moscow, Russia, 2017; pp. 3–328. [Google Scholar]
- Bakiev, A.; Kirillov, A.; Kirillova, N.; Ruchin, A.; Klenina, A.; Gorelov, R.; Kostina, N. Reptile occurrences data in the Volga River basin (Russia). Biodivers. Data J. 2020, 8, e58033. [Google Scholar] [CrossRef]
- Kirillov, A.; Kirillova, N.; Bakiev, A.; Ruchin, A.; Klenina, A.; Gorelov, R. Occurrence of the Reptiles in the Volga River Basin (Russia). Occurrence Dataset. Version 1.9. 2020. GBIF. Available online: https://www.gbif.org/dataset/2fdc8f80-3f12-482e-ac28-8683d448e881 (accessed on 15 March 2025).
- Smirnova, M.I. Study of biocenotic relationships of helminths of some vertebrates in the coast of the Kuibyshev Reservoir. In Formation of Coastal Biogeocenoses of Reservoirs; Popov, V.A., Ed.; Nauka: Moscow, Russia, 1969; pp. 153–164. [Google Scholar]
- Smirnova, M.I. Helminth fauna of Natrix natrix in the Saralov area of the Volzhsko-Kamsky Nature Reserve. In Natural Resources of the Volzhsko-Kamsky Region: Animals; Popov, V.A., Ed.; Nauka: Moscow, Russia, 1971; Issue 3, pp. 164–167. [Google Scholar]
- Borisova, V.I. Basic patterns of distribution of parasites in lizards of the genus Lacerta L. Land Aquat. Ecosyst. 1981, 4, 115–120. [Google Scholar]
- Al-Zavahra, H.A. Snakes of Tatarstan. Ph.D. Thesis, Kazan State University, Kazan, Russia, 1992. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y. Trematodes (Trematoda) of reptiles of Middle Volga region. Proc. Sam. Sci. Cent. RAS 2011, 13, 139–147. [Google Scholar]
- Kirillov, A.A.; Ruchin, A.B.; Fayzulin, A.I.; Chikhlyaev, I.V. Helminths of reptiles in Mordovia: Preliminary data. Proc. Mord. Nat. Reserv. 2015, 14, 243–255. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y.; Ruchin, A.B. Overwiew of the helminth fauna of slow worm of genus Anguis (Reptilia, Anguidae) in the Western Palaearctic. Entomol. Appl. Sci. Lett. 2019, 6, 51–65. [Google Scholar]
- Kirillov, A.A.; Kirillova, N.Y. First finding of spirurid larva (Chromadorea, Spirurida) in the common European viper Vipera berus (Linnaeus, 1758) of the Russian fauna. IOP Conf. Ser. Earth Environ. Sci. 2021, 818, 012017. [Google Scholar] [CrossRef]
- Kirillov, A.A.; Kirillova, N.Y.; Ruchin, A.B.; Vekhnik, V.A. Helminths of Reptiles (Reptilia) in the Middle Volga Region (European Russia). Occurrence Dataset. 2025. Available online: https://www.gbif.org/dataset/db5c4d4f-b3ec-419a-9535-4c3e7c71fbd8 (accessed on 15 March 2025).
- Chavan, V.; Penev, L. The data paper: A mechanism to incentivize data publishing in biodiversity science. BMC Bioinformatics 2011, 12 (Suppl. S15), S2. [Google Scholar] [CrossRef]
- Penev, L.; Mietchen, D.; Chavan, V.; Hagedorn, G.; Smith, V.; Shotton, D.; Tuama, E.O.; Senderov, V.; Georgiev, T.; Stoev, P.; et al. Strategies and guidelines for scholarly publishing of biodiversity data. RIO 2017, 3, e12431. [Google Scholar] [CrossRef]
- Mil’kov, F.N. Middle Volga Region. Physical and Geographical Description; Academy of Sciences of the USSR: Moscow, Russia, 1953; pp. 3–262. [Google Scholar]
- Gorelov, M.S.; Matveev, V.I.; Ustinova, A.A. (Eds.) Nature of the Kuibyshev Region; Book Publishing House: Kuibyshev, Russia, 1990; pp. 3–464. [Google Scholar]
- Saksonov, S.V.; Lysenko, T.M.; Il’ina, V.N.; Koneva, N.V.; Lobanova, A.V.; Matveev, V.M.; Mitroshenkova, A.E.; Simonova, N.I.; Solovyeva, V.V.; Uzhametskaya, E.A. Green Book of the Samara Region: Rare and Protected Plant Communities; Samara Scientific Center of RAS: Samara, Russia, 2006; pp. 3–201. [Google Scholar]
- Senator, S.A.; Saxonov, S.V.; Rozenberg, G.S. Red Book of the Volga basin: Tactics for preserving the floristic diversity of a large ecoregion. In Rarities of the Volga Basin Flora; Saxonov, S.V., Senator, S.A., Eds.; Kassandra: Togliatti, Russia, 2012; pp. 218–230. [Google Scholar]
- Karyakin, I.V. Summary of the Bird Fauna of the Republic of Bashkortostan; Center Field Research Union for the Protection of Animals of the Urals: Perm, Russia, 1998; pp. 3–253. [Google Scholar]
- Stepanyan, L.S. Summary of the Ornithological Fauna of Russia and Adjacent Territories; ICC Akademkniga: Moscow, Russia, 2003; pp. 3–808. [Google Scholar]
- Kuzmin, S.L. Amphibians of the Former USSR, 2nd ed.; KMK: Moscow, Russia, 2012; pp. 3–369. [Google Scholar]
- Artaev, O.N.; Smirnov, D.G. Bats (Chiroptera; Mammalia) of Mordovia: Specific structure and features of distribution. Nat. Conserv. Res. 2016, 1, 38–51. [Google Scholar] [CrossRef]
- Mammals of Russia. Available online: https://rusmam.ru/info/index?sort=sort (accessed on 20 March 2025).
- Kirillova, N.Y.; Kirillov, A.A.; Vekhnik, V.A.; Shchenkov, S.V.; Fayzulin, A.I.; Ruchin, A.B. Trematodes of small mammals (Erinaceomorpha, Soricomorpha, Rodentia and Chiroptera) in the Middle Volga Region (Russia). Diversity 2023, 15, 796. [Google Scholar] [CrossRef]
- Google Maps. Available online: https://www.google.ru/maps/ (accessed on 14 March 2025).
- Skryabin, K.I. Method of Complete Helminthological Dissections of Vertebrates, Including Humans; Moscow State University: Moscow, Russia, 1928; pp. 3–45. [Google Scholar]
- Anikanova, V.S.; Bugmyrin, S.V.; Ieshko, E.P. Methods of the Collection and Studies of Helminths of Small Mammals; Karelian Scientific Center of RAS: Petrozavodsk, Russia, 2007; pp. 3–145. [Google Scholar]
- Zander, R.H. Four water-soluble mounting media for microslides. Phytoneuron 2014, 32, 1–4. [Google Scholar]
- Sharpilo, V.P.; Iskova, N.I. Trematodes. Plagiorchiata. Fauna of Ukraine; Naukova Dumka: Kiev, Ukraine, 1989; Volume 34, pp. 3–280. [Google Scholar]
- Dimitrova, Z.M.; Gibson, D.I. Some species of Centrorhynchus Luhe, 1911 (Acanthocephala: Centrorhynchidae) from the collection of the Natural History Museum, London. Syst. Parasitol. 2005, 62, 117–134. [Google Scholar] [CrossRef] [PubMed]
- GBIF. Available online: https://www.gbif.org/ (accessed on 12 March 2025).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: http://www.r-project.org/index.html (accessed on 23 March 2025).
- Wilkins, D. Treemapify: Draw Treemaps in ‘ggplot2’. R Package Version 2.5.5. 2021. Available online: https://CRAN.R-project.org/package=treemapify (accessed on 20 March 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 3–256. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 24 March 2025).
- Atyasheva, T.N.; Bakiev, A.G.; Gorelov, R.A.; Klenina, A.A. Reptiles of the Volga Basin in the Funds of Zoological Collections; Anna: Togliatti, Russia, 2021; pp. 3–76. [Google Scholar]
- Darwin Core. Available online: https://dwc.tdwg.org/ (accessed on 8 March 2025).
- Rizzo, A. La fauna helmintologica dei Rettili nell provincia di Catania. Arch. Parasitol. 1902, 6, 26–41. [Google Scholar]
- Dubinina, M.N. Dynamics of the parasite fauna of snakes in the coastal part of the Volga delta. Proc. Zool. Inst. Akad. Sci. USSR 1953, 13, 171–190. [Google Scholar]
- Schad, G.A.; Kuntz, R.E.; Wells, W.H. Nematode parasites from Turkish vertebrates: An annotated list. Can. J. Zool. 1960, 38, 949–963. [Google Scholar] [CrossRef]
- Gradba-Kazubska, B. Parasites of the grass snake Natrix natrix (L.) in Poland. Wiad. Parazytol. 1961, 7, 199–201. [Google Scholar]
- Markov, G.S.; Ivanov, V.P.; Nikulin, V.P.; Chernobai, V.F. Helminth fauna of reptiles in the Volga delta and the Caspian steppes. Proc. Astrakhan Nat. Res. 1962, 6, 145–170. [Google Scholar]
- Sharpilo, V.P. Towards the study of the helminth fauna of reptiles in Transcaucasia. Proc. Zool. Mus. Acad. Sci. Ukr. SSR 1962, 31, 63–69. [Google Scholar]
- Markov, G.S.; Kosareva, N.A.; Kubantsev, B.S. Materials on ecology and parasitology of lizards and snakes in the Volgograd region. In Parasitic Animals; Markov, G.S., Ed.; Volgograd Pedagogical Institute: Volgograd, Russia, 1969; pp. 198–220. [Google Scholar]
- Capuse, I. Contributions a l’etude des trematodes parasites chez les reptiles du Roumanie. Trav. Mus. d’Hist. Nat. Grigore Antipa 1971, 11, 33–40. [Google Scholar]
- Bertman, M.; Okulewicz, A. Lizards (Anguis fragilis L.) and snakes (Natrix natrix (L.)) as new hosts of Oswaldocruzia filiformis (Goeze, 1782) Travassos 1917 (Nematoda). Wiad. Parazytol. 1987, 33, 209–212. [Google Scholar]
- Lewin, J. Parasitic worms in a slow worm (Anguis fragilis L.) population from the Bieszczady Mountains (Poland). Acta Parasitol. Polon. 1990, 35, 207–215. [Google Scholar]
- Hornero, M.J.; Roca, V. Helmintofauna de Podarcis lilfordi (Günther, 1874) (Sauria, Lacertidae) de los islotes de Menorca (islas Baleares, Mediterraneo occidental). Misc. Zool. 1992, 16, 1–6. [Google Scholar]
- Lewin, J. Parasites of the sand lizard (Lacerta agilis L.) in Poland. Acta Parasitol. 1992, 37, 19–24. [Google Scholar]
- Lewin, J. Parasites of the water snake, Natrix natrix L., in Poland. Acta Parasitol. 1992, 37, 195–199. [Google Scholar]
- Lewin, J. Parasites of Lacerta vivipara Jacquin, 1787 in Poland. Acta Parasitol. 1992, 37, 79–82. [Google Scholar]
- Lewin, J. A contribution to the knowledge of parasites of Elaphe longissima Laurenti, 1768 in Poland. Acta Parasitol. 1993, 38, 55–57. [Google Scholar]
- Bertman, M. Diplodiscus subclavatus (Pallas, 1760) (Trematoda) and Acanthocephalus ranae (Schrank, 1788) (Acanthocephala) in grass snake—Natrix natrix (L.). Wiad. Parazytol. 1993, 39, 405–406. [Google Scholar]
- Biserkov, V.Y. New records of nematodes and acanthocephalans from snakes in Bulgaria. Comp. Rend. l’Acad. Bulg. Sci. 1995, 48, 87–89. [Google Scholar]
- Kirin, D. A contribution to the cestodofauna of reptiles (Reptilia) in Bulgaria. Univ. Plovdiv “P. Hilendarski” Trav. Sci. 1995, 31, 77–80. [Google Scholar]
- Roca, V. An approach to the knowledge of the helminth infracommunities of Mediterranean insular lizards (Podarcis spp.). In Scientia Herpetologica; Llorente, G.A., Montori, A., Santos, X., Carretero, M.A., Eds.; AHE: Barcelona, Spain, 1995; pp. 285–292. [Google Scholar]
- Biserkov, V.Y. New records of platyhelminth parasites from snakes in Bulgaria. Comp. Rend. l’Acad. Bulg. Sci. 1996, 49, 73–76. [Google Scholar]
- Kirin, D. Helminths (Plathelminthes) of reptiles (Reptilia) in southern Bulgaria (morphology, fauna, ecology and distribution). Ph.D. Thesis, Faculty of Biology, Plovdiv University “P. Hilendarski”, Plovdiv, Bulgaria, 1996. [Google Scholar]
- Kirin, D. Helminths (class Trematoda, class Monogenea) of reptiles (Reptilia) from some regions of south Bulgaria. Sci. Works PU “P Hilendarski”–Biol. Anim. 1996, 32, 5–11. [Google Scholar]
- Lewin, J.; Grabda-Kazubska, B. Parasites of Vipera berus L. in Poland. Acta Parasitol. 1997, 42, 92–96. [Google Scholar]
- Biserkov, V.; Kostadinova, A. Intestinal helminth communities in Lacerta viridis Laurenti, 1768 (Reptilia, Lacertidae) from Bulgaria. J. Helminthol. 1998, 3, 267–271. [Google Scholar] [CrossRef]
- Roca, V. Relacion entre las faunas endoparasitas de reptiles y su tipo de alimentacion. Rev. Esp. Herpetol. 1999, 13, 101–121. [Google Scholar]
- Roca, V.; Martin, J.E.; Carbonell, E. Helminths parasitising endemic geckoes from Canary lslands. Misc. Zool. 1999, 22, 101–108. [Google Scholar]
- Ivanov, V.M.; Semenova, N.N. Species composition and ecological peculiarities of trematodes from reptiles in the Volga delta. Parazitologiya 2000, 34, 228–233. [Google Scholar]
- Shimalov, V.; Shimalov, V. Helminth fauna of snakes (Reptilia, Serpentes) in Belorussian Polesye. Parasitol. Res. 2000, 86, 340–341. [Google Scholar] [CrossRef]
- Shimalov, V.V.; Shimalov, V.T.; Shimalov, A.V. Helminth fauna of lizards (Reptilia, Sauria) in the Southern part of Belarus. Parasitol. Res. 2000, 86, 343. [Google Scholar] [CrossRef]
- Movsessian, S.O.; Chubarian, F.A.; Nikoghosian, M.A. Trematodes. In Fauna of the South of the Low Caucasus; Nauka: Moscow, Russia, 2004; pp. 3–279. [Google Scholar]
- Movsessian, S.O.; Chubarian, F.A.; Nikoghosian, M.A. Cestodes. In Fauna of the South of the Low Caucasus; Nauka: Moscow, Russia, 2006; pp. 3–331. [Google Scholar]
- Lhermitte, N.; Bain, O.; Virga, A. Skrjabinelazia rizzoi n. sp. (Nematoda: Seuratoidea) from a sicilian lacertid, with comments on specific and biological diversity in the genus. Parasite 2008, 15, 45–52. [Google Scholar] [CrossRef][Green Version]
- Ivanov, V.M.; Semenova, N.N. Reptiles as intermediate hosts of trematodes in the Volga delta. Proc. Astrakhan Nat. Res. 2009, 14, 317–319. [Google Scholar]
- Ivanov, V.M.; Semenova, N.N.; Kalmykov, A.P. Helminths in the Ecosystem of the Volga Delta; Trematodes; Volga: Astrakhan, Russia, 2012; Volume 1, pp. 3–254. [Google Scholar]
- Sargsyan, N.; Danielyan, F.; Arakelyan, M. Seven new species of helminths for reptiles from Armenia. Acta Parasitol. 2014, 59, 442–447. [Google Scholar]
- Bychkova, E.I.; Akimova, L.N.; Degtyarik, S.M.; Yakovich, M.M. Helminths of Vertebrates and Man in Belarus; Belarusskaya Nauka: Minsk, Belarus, 2017; pp. 3–316. [Google Scholar]
- Kalmykov, A.P.; Semenova, N.N.; Ivanov, V.M. Helminths in the Ecosystem of the Volga Delta; Nematodes; Print: Izhevsk, Russia, 2017; Volume 2, pp. 3–348. [Google Scholar]
- Movsessian, S.O.; Nikoghosian, M.A.; Petrosian, R.A.; Kuznetsov, D.N. Nematodes and Acanthocephales. In Fauna of the South of the Low Caucasus; KMK: Moscow, Russia, 2017; pp. 3–445. [Google Scholar]
- Arabuli, L.; Murvanidze, L.; Faltynkova, A.; Mumladze, L. Checklist of digeneans (Platyhelminthes, Trematoda, Digenea) of Georgia. Biodivers. Data J. 2024, 12, e110201. [Google Scholar] [CrossRef] [PubMed]
- Sudarikov, V.E.; Shigin, A.A.; Kurochkin, Y.V.; Lomakin, V.V.; Sten’ko, R.P.; Yurlova, N.I. Metacercariae of Trematodes—Parasites of Freshwater Aquatic Organisms in Central Russia; Nauka: Moscow, Russia, 2002; Volume 1, pp. 3–298. [Google Scholar]
- Kennedy, C.R. Ecological Animal Parasitology; Blackwell Scientific Publications: Oxford, UK, 1975; pp. 3–163. [Google Scholar]
- Zhokhov, A.E. Distribution of helminthes in the digestive tract of the ide Leuciscus idus: Analysis of interspecific relation. Zool. Zhurn. 2004, 83, 515–525. [Google Scholar]
- Kirillova, N.Y.; Kirillov, A.A. Life cycle of Cosmocerca ornata (Nematoda: Cosmocercidae), a parasite of amphibians. Inl. Wat. Biol. 2021, 14, 316–330. [Google Scholar] [CrossRef]
- Biserkov, V.; Genov, T. On the life cycle of Ophiotaenia europaea Odening, 1963 (Cestoda: Ophiotaeniidae). Helmintologia 1988, 25, 7–14. [Google Scholar]
- Sokolov, S.G.; Protasova, E.N.; Kholin, S.K. Parasites of the introduced Amur sleeper, Perccottus glenii (Osteichthyes): Alpha-diversity of parasites and age of the host. Biol. Bull. 2011, 38, 500–508. [Google Scholar] [CrossRef]
- Reshetnikov, A.N.; Sokolov, S.G.; Chikhlyaev, I.V.; Fayzulin, A.I.; Kirillov, A.A.; Kuzovenko, A.E.; Protasova, E.N.; Skomorokhov, A.O. Direct and indirect interactions between an invasive alien fish (Perccottus glenii) and two native semi-aquatic snakes. Copeia 2013, 1, 103–110. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, L.; Gong, S.; Deng, Y.; Zou, J.; Wu, J.; Liu, W.; Hou, F. Severe infection of wild-caught snakes with Spirometra erinaceieuropaei from food markets in Guangzhou, China involves a risk for zoonotic sparganosis. J. Parasitol. 2011, 97, 170–171. [Google Scholar] [CrossRef]
- Oda, F.H.; Borteiro, C.; da Graca, R.J.; Tavares, L.E.R.; Crampet, A.; Guerra, V.; de Lima, F.S.; Bellay, S.; Karling, L.C.; Castro, O.; et al. Parasitism by larval tapeworms genus Spirometra in South American amphibians and reptiles: New records from Brazil and Uruguay, and a review of current knowledge in the region. Acta Tropica 2016, 164 (Suppl. S1), 150–164. [Google Scholar] [CrossRef]
- Chikhlyaev, I.V.; Kirillov, A.A.; Kirillova, N.Y. Trematodes (Trematoda) of amphibians (Amphibia) of the Middle Volga region. 1. Order Plagiorchiida. Parazitologiya 2012, 46, 290–313. [Google Scholar]
- Filippi, E.; Luiselli, L. Negative effect of the wild boar (Sus scrofa) on the populations of snakes at a protected mountains forest in central Italy. Ecol. Mediterr. 2002, 28, 93–98. [Google Scholar]
- Bakiev, A.G. Snakes of the Volga River basin as nutrition objects for vertebrates. Curr. Stud. Herpetol. 2007, 7, 124–132. [Google Scholar]
- Dogel, V.A. General Parasitology; Leningrad State University: Leningrad, Russia, 1962; pp. 3–461. [Google Scholar]
- Akbaev, M.S.; Vodyanov, A.A.; Kosminkov, N.E.; Yatusevich, A.I.; Pashkin, P.I.; Vasilevich, F.I. Parasitology and Invasive Animal Diseases; Kolos: Moscow, Russia, 1998; pp. 3–743. [Google Scholar]
- Roepstorff, A.; Nansen, P. Epidemiology, Diagnosis, and Control of Helminth Parasites of Swine; Food and Agriculture Organization of the UN: Rome, Italy, 1998; pp. 3–161. [Google Scholar]
- Kuchta, R.; Kolodziej-Sobocinska, M.; Brabec, J.; Mlocicki, D.; Salamatin, R.; Scholz, T. Sparganosis (Spirometra) in Europe in the molecular era. Clin. Infect. Dis. 2021, 72, 882–890. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).