The Optimal Counting Number for Silicoflagellate Assemblages in the Western Arctic Ocean
Abstract
1. Introduction
2. Materials and Methods
2.1. Oceanographic Setting
2.2. Sinking Particles and Surface Sediments
2.3. Slide Preparation and Identification of Silicoflagellates
2.4. Counting Quadrat and Gradient Setting
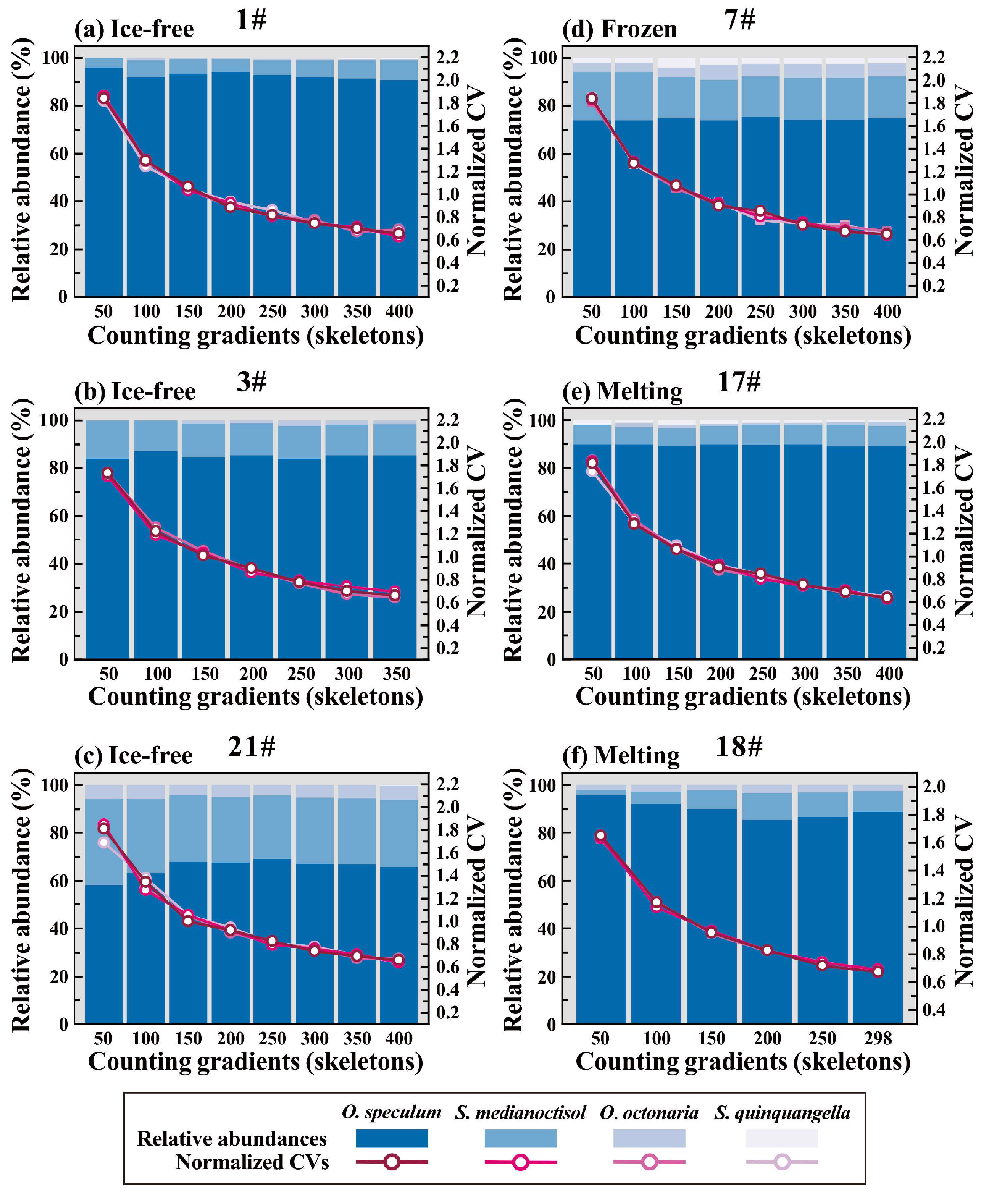
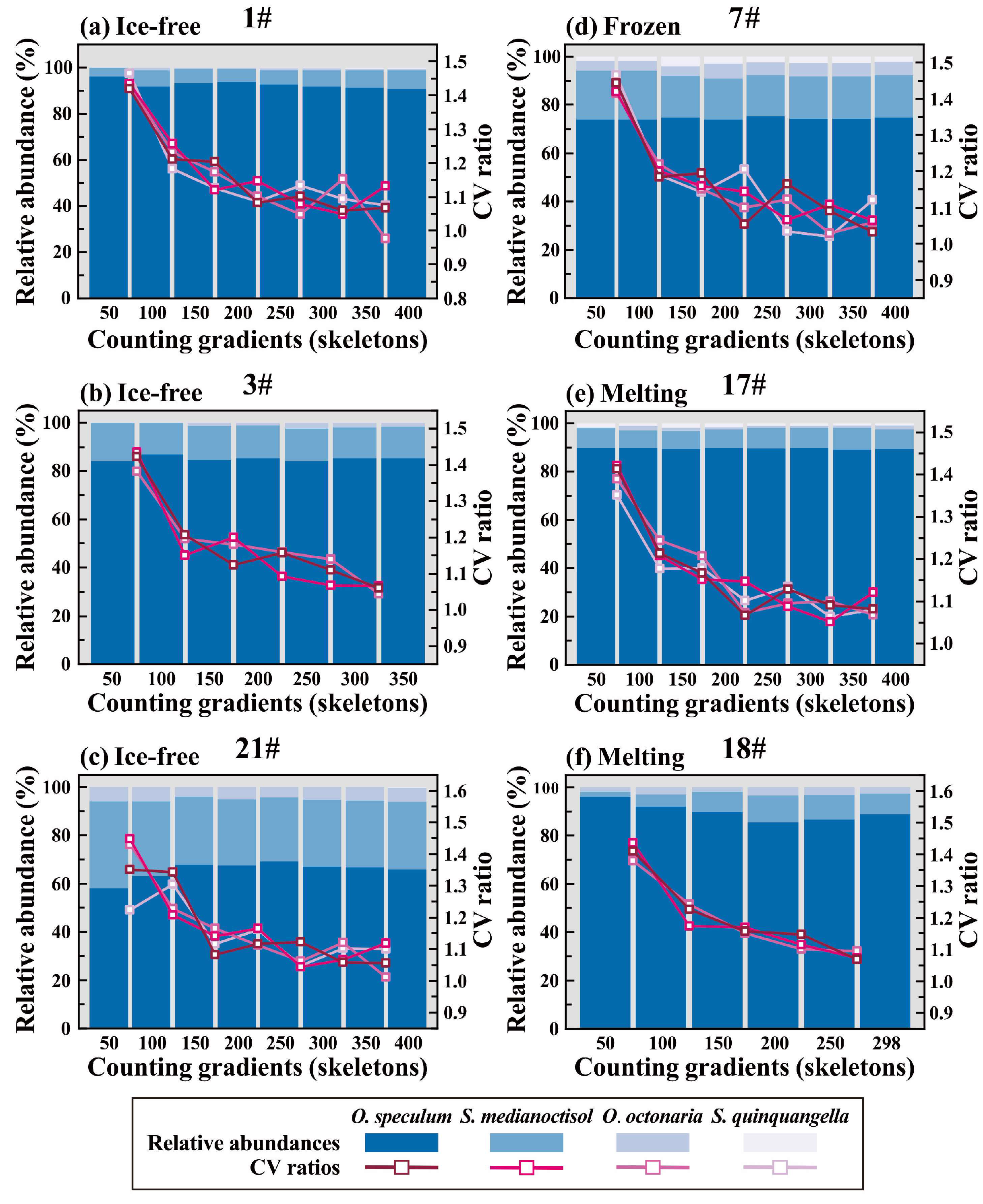
2.5. Optimal Counting Number Determination
3. Results and Discussion
3.1. Silicoflagellates in Insinking Particles
3.2. Silicoflagellates in the Surface Sediments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serreze, M.C.; Barrett, A.P.; Stroeve, J.C.; Kindig, D.N.; Holland, M.M. The emergence of surface-based Arctic amplification. Cryosphere 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Lenssen, N.J.L.; Schmidt, G.A.; Hansen, J.E.; Menne, M.J.; Persin, A.; Ruedy, R.; Zyss, D. Improvements in the GISTEMP Uncertainty Model. J. Geophys. Res. Atmos. 2019, 124, 6307–6326. [Google Scholar] [CrossRef]
- Jacobs, N.; Simpson, W.R.; Graham, K.A.; Holmes, C.; Hase, F.; Blumenstock, T.; Tu, Q.; Frey, M.; Dubey, M.K.; Parker, H.A.; et al. Spatial distribution of XCO2 seasonal cycle amplitude and phase over northern high-latitude regions. Atmos. Chem. Phys. 2021, 21, 16661–16687. [Google Scholar] [CrossRef]
- Rantanen, M.; Karpechko, A.Y.; Lipponen, A.; Nordling, K.; Hyvärinen, O.; Ruosteenoja, K.; Vihma, T.; Laaksonen, A. The Arctic has warmed nearly four times faster than the globe since 1979. Commun. Earth Environ. 2022, 3, 168. [Google Scholar] [CrossRef]
- Meier, W.N. Losing Arctic sea ice: Observations of the recent decline and the long-term context. In Sea Ice; Thomas, D.N., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2017; pp. 290–303. [Google Scholar]
- Wassmann, P.; Duarte, C.M.; Agustí, S.; Sejr, M.K. Footprints of climate change in the Arctic marine ecosystem. Glob. Change Biol. 2011, 17, 1235–1249. [Google Scholar] [CrossRef]
- Coupel, P.; Jin, H.Y.; Joo, M.; Horner, R.; Bouvet, H.A.; Sicre, M.-A.; Gascard, J.-C.; Chen, J.F.; Garçon, V.; Ruiz-Pino, D. Phytoplankton distribution in unusually low sea ice cover over the Pacific Arctic. Biogeosciences 2012, 9, 4835–4850. [Google Scholar] [CrossRef]
- Nadaï, G.; Nöthig, E.-M.; Fortier, L.; Lalande, C. Early snowmelt and sea ice breakup enhance algal export in the Beaufort Sea. Prog. Oceanogr. 2021, 190, 102479. [Google Scholar] [CrossRef]
- Arrigo, K.R.; van Dijken, G.L. Continued increases in Arctic Ocean primary production. Prog. Oceanogr. 2015, 136, 60–70. [Google Scholar] [CrossRef]
- Mills, M.M.; Brown, Z.W.; Laney, S.R.; Ortega-Retuerta, E.; Lowry, K.E.; van Dijken, G.L.; Arrigo, K.R. Nitrogen limitation of the summer phytoplankton and heterotrophic prokaryote communities in the Chukchi Sea. Front. Mar. Sci. 2018, 5, 362. [Google Scholar] [CrossRef]
- Bukry, D. Silicoflagellates and their geologic applications. US Geol. Surv. 1995, 95-260, 1–27. [Google Scholar]
- Poelchau, H.S. Distribution of Holocene silicoflagellates in North Pacific sediments. Micropaleontology 1976, 22, 164. [Google Scholar] [CrossRef]
- Rühland, K.; Priesnitz, A.; Smol, J.P. Paleolimnological evidence from diatoms for recent environmental changes in 50 lakes across Canadian Arctic treeline. Arct. Antarct. Alp. Res. 2003, 35, 110–123. [Google Scholar] [CrossRef]
- Bjørklund, K.R.; Kruglikova, S.B. Polycystine radiolarians in surface sediments in the Arctic Ocean basins and marginal seas. Mar. Micropaleontol. 2003, 49, 231–273. [Google Scholar] [CrossRef]
- Itaki, T.; Ito, M.; Narita, H.; Ahagon, N.; Sakai, H. Depth distribution of radiolarians from the Chukchi and Beaufort Seas, western Arctic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2003, 50, 1507–1522. [Google Scholar] [CrossRef]
- Brown, T.A.; Belt, S.T. Identification of the sea ice diatom biomarker IP25 in Arctic benthic macrofauna: Direct evidence for a sea ice diatom diet in Arctic heterotrophs. Polar Biol. 2012, 35, 131–137. [Google Scholar] [CrossRef]
- Ikenoue, T.; Bjørklund, K.R.; Kruglikova, S.B.; Onodera, J.; Kimoto, K.; Harada, N. Flux variations and vertical distributions of siliceous Rhizaria (Radiolaria and Phaeodaria) in the western Arctic Ocean: Indices of environmental changes. Biogeosciences 2015, 12, 2019–2046. [Google Scholar] [CrossRef]
- Onodera, J.; Watanabe, E.; Harada, N.; Honda, M.C. Diatom flux reflects water-mass conditions on the southern Northwind Abyssal Plain, Arctic Ocean. Biogeosciences 2015, 12, 1373–1385. [Google Scholar] [CrossRef]
- Ren, J.; Chen, J.; Bai, Y.; Sicre, M.-A.; Yao, Z.; Lin, L.; Zhang, J.; Li, H.; Wu, B.; Jin, H.; et al. Diatom composition and fluxes over the Northwind Ridge, western Arctic Ocean: Impacts of marine surface circulation and sea ice distribution. Prog. Oceanogr. 2020, 186, 102377. [Google Scholar] [CrossRef]
- Obrezkova, M.S.; Tsoy, I.B.; Kolyada, A.E.; Shi, X.; Liu, Y. Distribution of diatoms in seafloor surface sediments of the Laptev, East Siberian, and Chukchi Seas: Implication for environmental reconstructions. Polar Biol. 2023, 46, 21–34. [Google Scholar] [CrossRef]
- Takahashi, K.; Onodera, J.; Katsuki, K. Significant Populations of seven-sided Distephanus (Silicoflagellata) in the sea-ice covered environment of the central Arctic Ocean, summer 2004. Micropaleontology 2009, 55, 313–325. [Google Scholar] [CrossRef]
- Onodera, J.; Watanabe, E.; Nishino, S.; Harada, N. Distribution and vertical fluxes of silicoflagellates, ebridians, and the endoskeletal dinoflagellate Actiniscus in the western Arctic Ocean. Polar Biol. 2016, 39, 327–341. [Google Scholar] [CrossRef]
- Ren, J.; Chen, J.; Li, H.; Wiesner, M.G.; Bai, Y.; Sicre, M.-A.; Yao, Z.; Jin, H.; Zhuang, Y.; Li, Y. Siliceous micro- and nanoplankton fluxes over the Northwind Ridge and their relationship to environmental conditions in the western Arctic Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2021, 174, 103568. [Google Scholar] [CrossRef]
- Zernova, V.V.; Nöthig, E.-M.; Shevchenko, V.P. Vertical microalga flux in the northern Laptev Sea (from the data collected by the yearlong sediment trap). Oceanology 2000, 40, 801–808. [Google Scholar]
- Koç Karpuz, N.; Schrader, H. Surface sediment diatom distribution and Holocene paleotemperature variations in the Greenland, Iceland and Norwegian Sea. Paleoceanography 1990, 5, 557–580. [Google Scholar] [CrossRef]
- Michelutti, N.; Douglas, M.S.V.; Smol, J.P. Diatom response to recent climatic change in a high arctic lake (Char Lake, Cornwallis Island, Nunavut). Glob. Planet. Change 2003, 38, 257–271. [Google Scholar] [CrossRef]
- Onodera, J.; Takahashi, K.; Jordan, R.W. Eocene silicoflagellate and ebridian paleoceanography in the central Arctic Ocean. Paleoceanography 2008, 23, PA1S15. [Google Scholar] [CrossRef]
- Barron, J.A.; Bukry, D.; Dean, W.E.; Addison, J.A.; Finney, B. Paleoceanography of the Gulf of Alaska during the past 15,000 years: Results from diatoms, silicoflagellates, and geochemistry. Mar. Micropaleontol. 2009, 72, 176–195. [Google Scholar] [CrossRef]
- Onodera, J.; Takahashi, K. Oceanographic conditions influencing silicoflagellate flux assemblages in the Bering Sea and subarctic Pacific Ocean during 1990–1994. Deep Sea Res. Part II Top. Stud. Oceanogr. 2012, 61–64, 4–16. [Google Scholar] [CrossRef]
- Onodera, J.; Takahashi, K.; Nagatomo, R. Diatoms, silicoflagellates, and ebridians at Site U1341 on the western slope of Bowers Ridge, IODP Expedition 323. Deep Sea Res. Part II Top. Stud. Oceanogr. 2016, 125–126, 8–17. [Google Scholar] [CrossRef]
- Okazaki, Y.; Onodera, J.; Tanizaki, K.; Nishizono, F.; Egashira, K.; Tomokawa, A.; Sagawa, T.; Horikawa, K.; Ikehara, K. Silicoflagellate assemblages in the North Pacific surface sediments: An application of the modern analog method to reconstruct the glacial sea surface temperature in the Japan Sea. Prog. Earth Planet. Sci. 2024, 11, 62. [Google Scholar] [CrossRef]
- Jakobsson, M. Hypsometry and volume of the Arctic Ocean and its constituent seas. Geochem. Geophys. Geosyst. 2002, 3, 1–18. [Google Scholar] [CrossRef]
- Woodgate, R.A.; Aagaard, K.; Weingartner, T.J. A year in the physical oceanography of the Chukchi Sea: Moored measurements from autumn 1990–1991. Deep Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 3116–3149. [Google Scholar] [CrossRef]
- Coachman, L.K.; Aagaard, K. On the water exchange through Bering Strait. Limnol. Oceanogr. 1966, 11, 44–59. [Google Scholar] [CrossRef]
- Grebmeier, J.M.; Cooper, L.W.; Feder, H.M.; Sirenko, B.I. Ecosystem dynamics of the Pacific-influenced Northern Bering and Chukchi Seas in the Amerasian Arctic. Prog. Oceanogr. 2006, 71, 331–361. [Google Scholar] [CrossRef]
- Weingartner, T.J.; Danielson, S.; Sasaki, Y.; Pavlov, V.; Kulakov, M. The Siberian Coastal Current: A wind- and buoyancy-forced Arctic coastal current. J. Geophys. Res. Ocean. 1999, 104, 29697–29713. [Google Scholar] [CrossRef]
- Timmermans, M.-L.; Toole, J.M. The Arctic Ocean’s Beaufort Gyre. Annu. Rev. Mar. Sci. 2023, 15, 223–248. [Google Scholar] [CrossRef] [PubMed]
- Comiso, J.C. Large decadal decline of the arctic multiyear ice cover. J. Clim. 2012, 25, 1176–1193. [Google Scholar] [CrossRef]
- Stroeve, J.C.; Markus, T.; Boisvert, L.; Miller, J.; Barrett, A. Changes in Arctic melt season and implications for sea ice loss. Geophys. Res. Lett. 2014, 41, 1216–1225. [Google Scholar] [CrossRef]
- Serreze, M.C.; Crawford, A.D.; Stroeve, J.C.; Barrett, A.P.; Woodgate, R.A. Variability, trends, and predictability of seasonal sea ice retreat and advance in the Chukchi Sea. J. Geophys. Res. Ocean. 2016, 121, 7308–7325. [Google Scholar] [CrossRef]
- Kato, Y.; Morono, Y.; Ijiri, A.; Terada, T.; Ikehara, M. A simple method for taxon-specific purification of diatom frustules from ocean sediments using a cell sorter. Prog. Earth Planet. Sci. 2023, 10, 13. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1996, 77, 350–363.43. [Google Scholar] [CrossRef]
- Tilman, D.; Lehman, C.; Bristow, C. Diversity-stability relationships: Statistical inevitability or ecological consequence? Am. Nat. 1998, 151, 277. [Google Scholar] [CrossRef] [PubMed]
- Resampling Stats. Resampling Stats Add-in for Excel User’s Guide (Version 4); Statistics.com, LLC: Arlington, VA, USA, 2009. [Google Scholar]
- Wang, B.; Zhu, Q.; Zhuang, Y.; Jin, H.; Li, H.; Zhang, Y.; Chen, J. Biogenic silica distribution in Chukchi Sea and its implications for silicate pump process. Bull. Mineral. Petrol. Geochem. 2015, 34, 1131–1141, (In Chinese with English Abstract). [Google Scholar]
- Bidle, K.D.; Azam, F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999, 397, 508–512. [Google Scholar] [CrossRef]
- Pichon, J.-J.; Bareille, G.; Labracherie, M.; Labeyrie, L.D.; Baudrimont, A.; Turon, J.-L. Quantification of the biogenic silica dissolution in Southern Ocean sediments. Quat. Res. 1992, 37, 361–378. [Google Scholar] [CrossRef]
- Onodera, J.; Takahashi, K. Silicoflagellate fluxes and environmental variations in the northwestern North Pacific during December 1997–May 2000. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 371–388. [Google Scholar] [CrossRef]
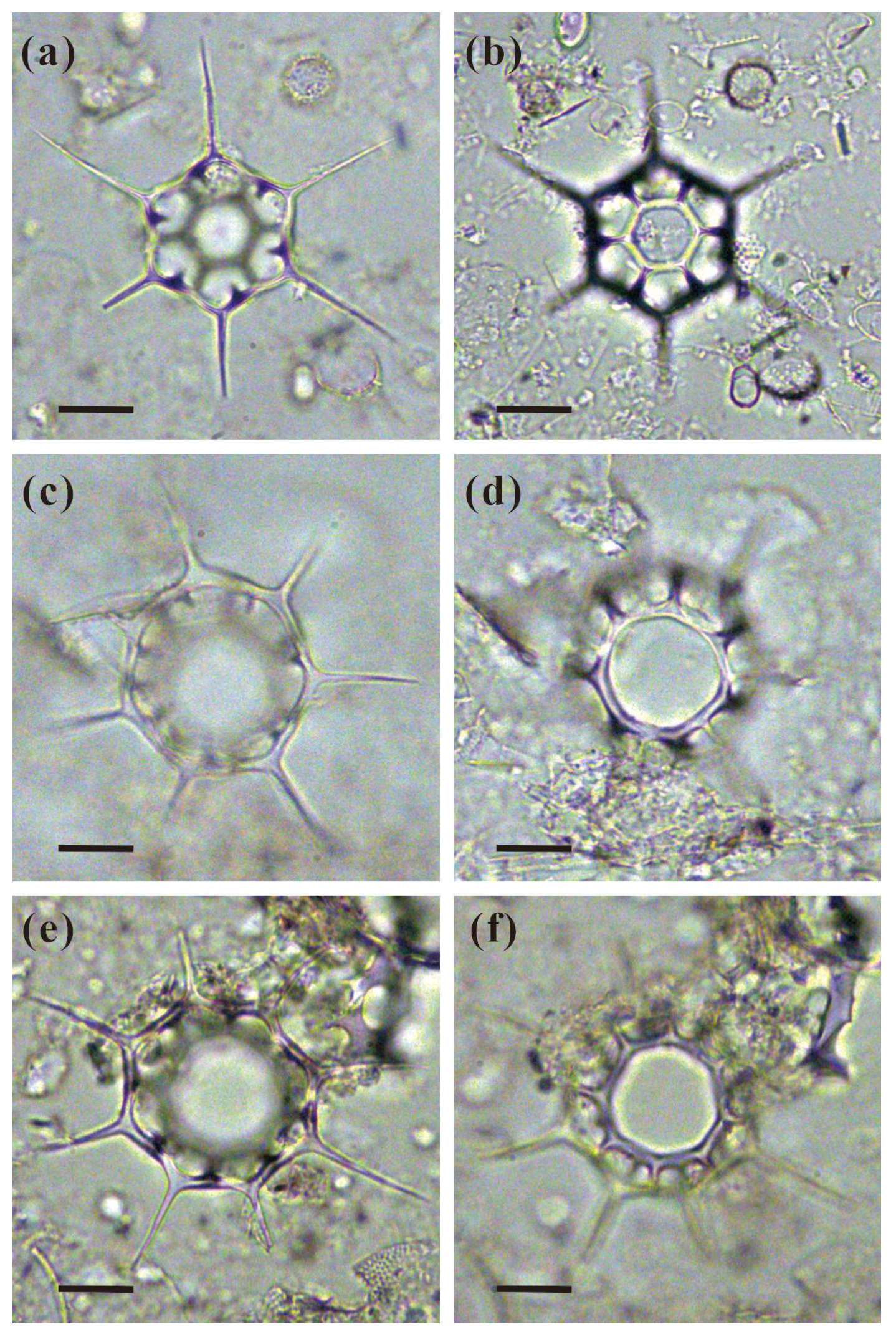
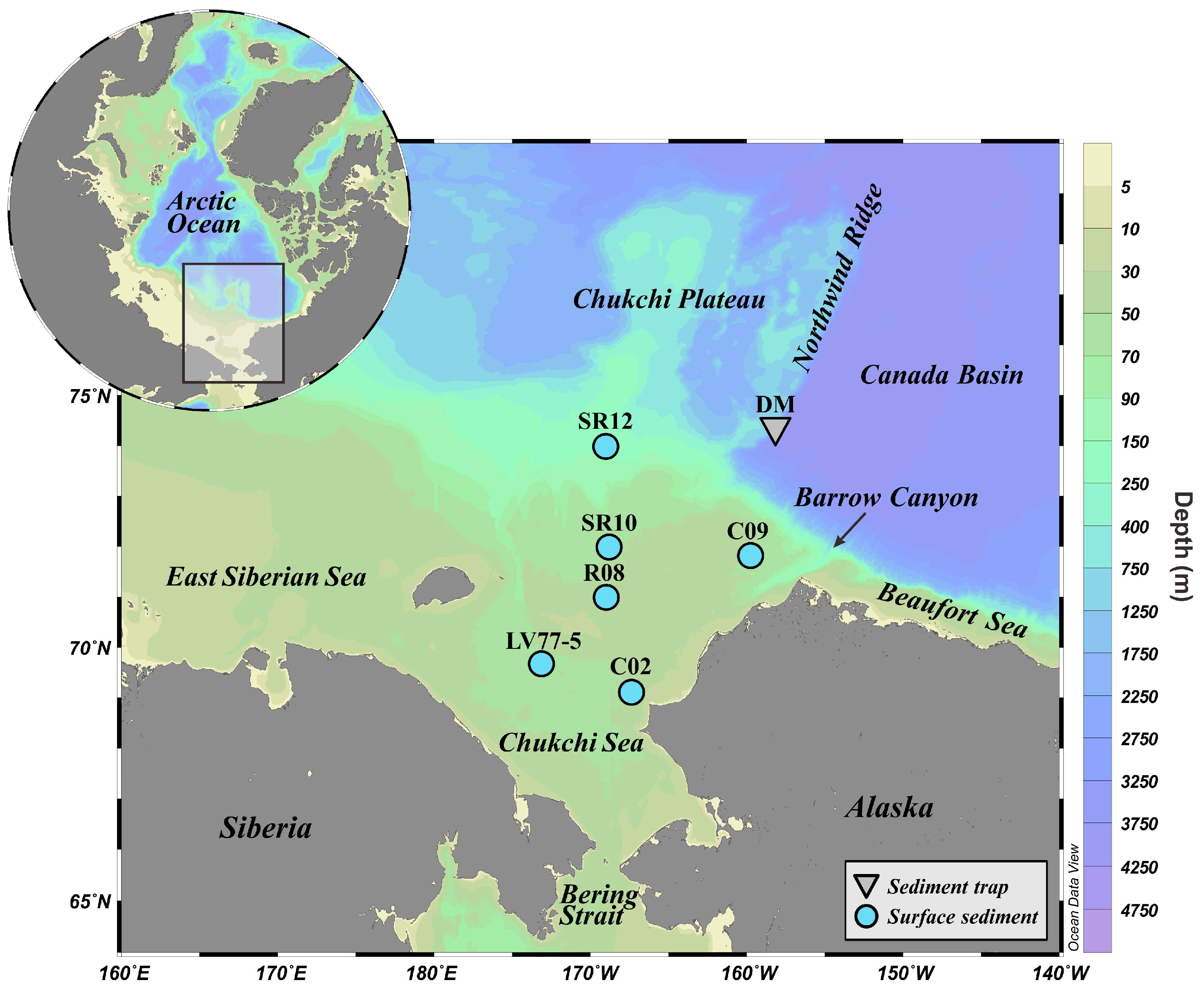

| No. of Cup | Periods of Collection | Environment |
|---|---|---|
| 1# | 7 August 2008–15 August 2008 | Ice-free |
| 3# | 1 September 2008–15 September 2008 | Ice-free |
| 21# | 16 September 2009–30 September 2009 | Ice-free |
| 7# | 1 November 2008–15 November 2008 | Frozen |
| 17# | 16 July 2009–31 July 2009 | Ice melting |
| 18# | 1 August 2009–15 August 2009 | Ice melting |
| Stations | Silicoflagellate Species | ||||||
|---|---|---|---|---|---|---|---|
| O. speculum | S. medianoctisol | O. octonaria | S. quinquangella | D. octangulata | Others | ||
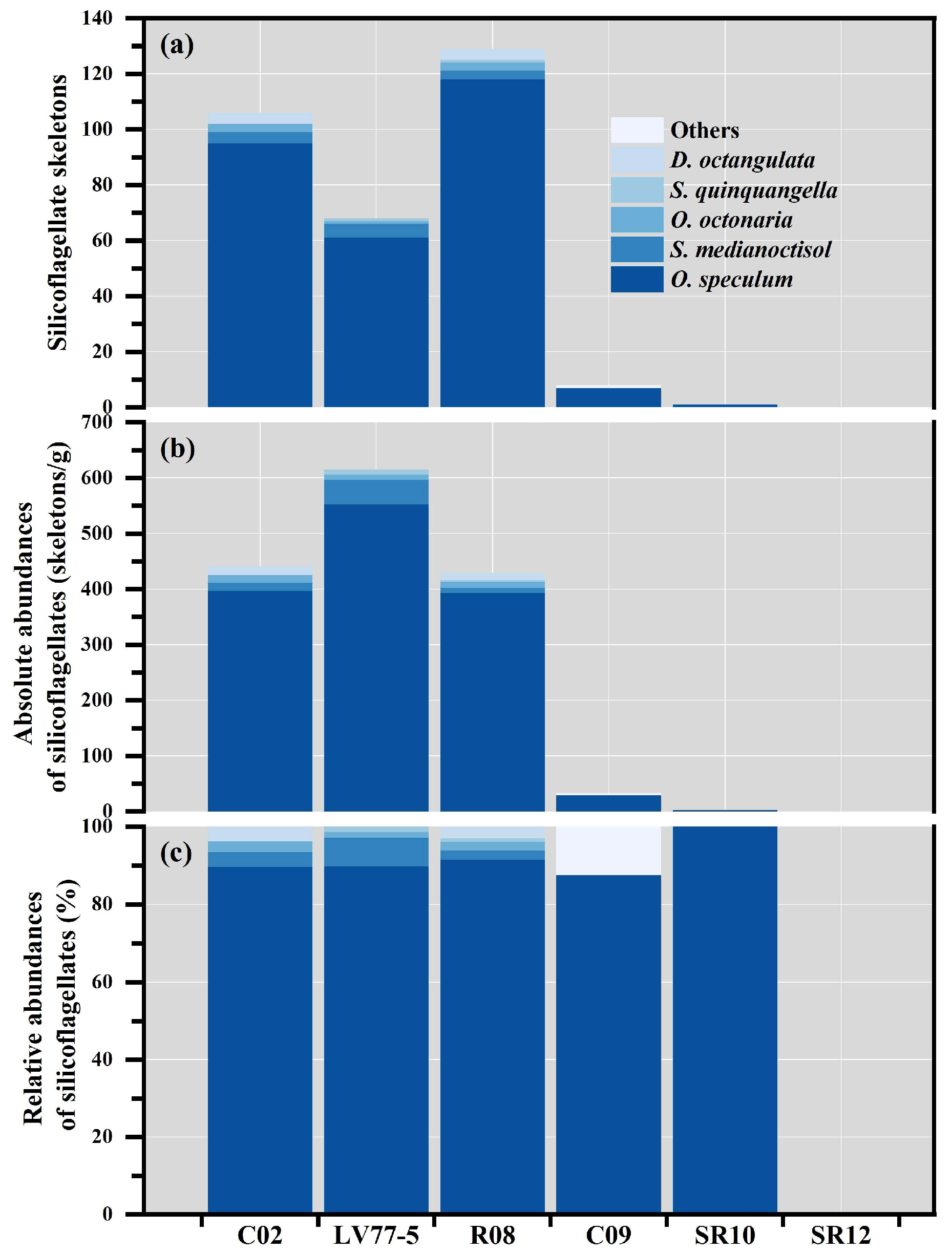
| C02 | Total skeletons | 95 | 4 | 3 | n.o | 4 | n.o |
| Absolute abundance (skeletons/g) | 396 | 16 | 13 | n.o | 16 | n.o | |
| Relative abundance (%) | 89.60 | 3.80 | 2.80 | n.o | 3.80 | n.o | |
| LV77-5 | Total skeletons | 61 | 5 | 1 | 1 | n.o | n.o |
| Absolute abundance (skeletons/g) | 553 | 44 | 9 | 9 | n.o | n.o | |
| Relative abundance (%) | 89.71 | 7.35 | 1.47 | 1.47 | n.o | n.o | |
| R08 | Total skeletons | 118 | 3 | 3 | 1 | 4 | n.o |
| Absolute abundance (skeletons/g) | 393 | 10 | 10 | 3 | 13 | n.o | |
| Relative abundance (%) | 91.50 | 2.30 | 2.30 | 0.80 | 3.10 | n.o | |
| C09 | Total skeletons | 7 | n.o | n.o | n.o | n.o | 1 |
| Absolute abundance (skeletons/g) | 29 | n.o | n.o | n.o | n.o | 4 | |
| Relative abundance (%) | 87.50 | n.o | n.o | n.o | n.o | 12.50 | |
| SR10 | Total skeletons | 1 | n.o | n.o | n.o | n.o | n.o |
| Absolute abundance (skeletons/g) | 4 | n.o | n.o | n.o | n.o | n.o | |
| Relative abundance (%) | 100 | n.o | n.o | n.o | n.o | n.o | |
| SR12 | Total skeletons | n.o | n.o | n.o | n.o | n.o | n.o |
| Absolute abundance (skeletons/g) | n.o | n.o | n.o | n.o | n.o | n.o | |
| Relative abundance (%) | n.o | n.o | n.o | n.o | n.o | n.o | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Ren, J.; Xu, R.; Jin, H.; Chen, J. The Optimal Counting Number for Silicoflagellate Assemblages in the Western Arctic Ocean. Diversity 2025, 17, 201. https://doi.org/10.3390/d17030201
Feng X, Ren J, Xu R, Jin H, Chen J. The Optimal Counting Number for Silicoflagellate Assemblages in the Western Arctic Ocean. Diversity. 2025; 17(3):201. https://doi.org/10.3390/d17030201
Chicago/Turabian StyleFeng, Xiaohang, Jian Ren, Ruowen Xu, Haiyan Jin, and Jianfang Chen. 2025. "The Optimal Counting Number for Silicoflagellate Assemblages in the Western Arctic Ocean" Diversity 17, no. 3: 201. https://doi.org/10.3390/d17030201
APA StyleFeng, X., Ren, J., Xu, R., Jin, H., & Chen, J. (2025). The Optimal Counting Number for Silicoflagellate Assemblages in the Western Arctic Ocean. Diversity, 17(3), 201. https://doi.org/10.3390/d17030201







