Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here?
Abstract
1. Introduction
2. Habitat Spatial Structure and the Metapopulation Concept
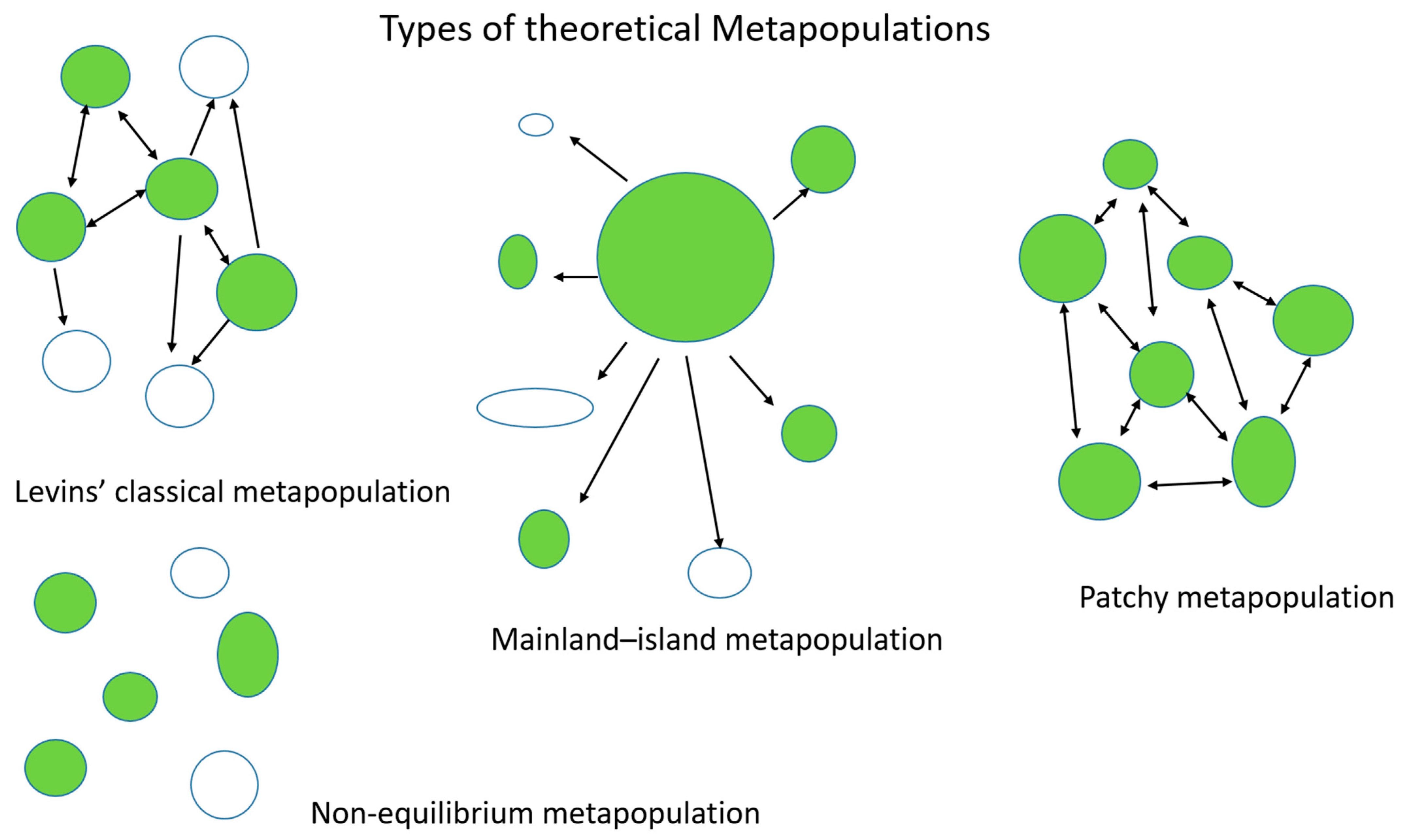
2.1. Metapopulation Theory
2.2. Tests of Metapopulation Theory
2.2.1. Classic Metapopulations
2.2.2. Mainland–Island Metapopulations
2.2.3. When Is Fragmented Habitat Fragmented? A Species Perspective
2.2.4. Metapopulation Theory and the Real World
2.3. Genetic Implications of Metapopulation Structure
2.4. Limitations of a Metapopulation Approac
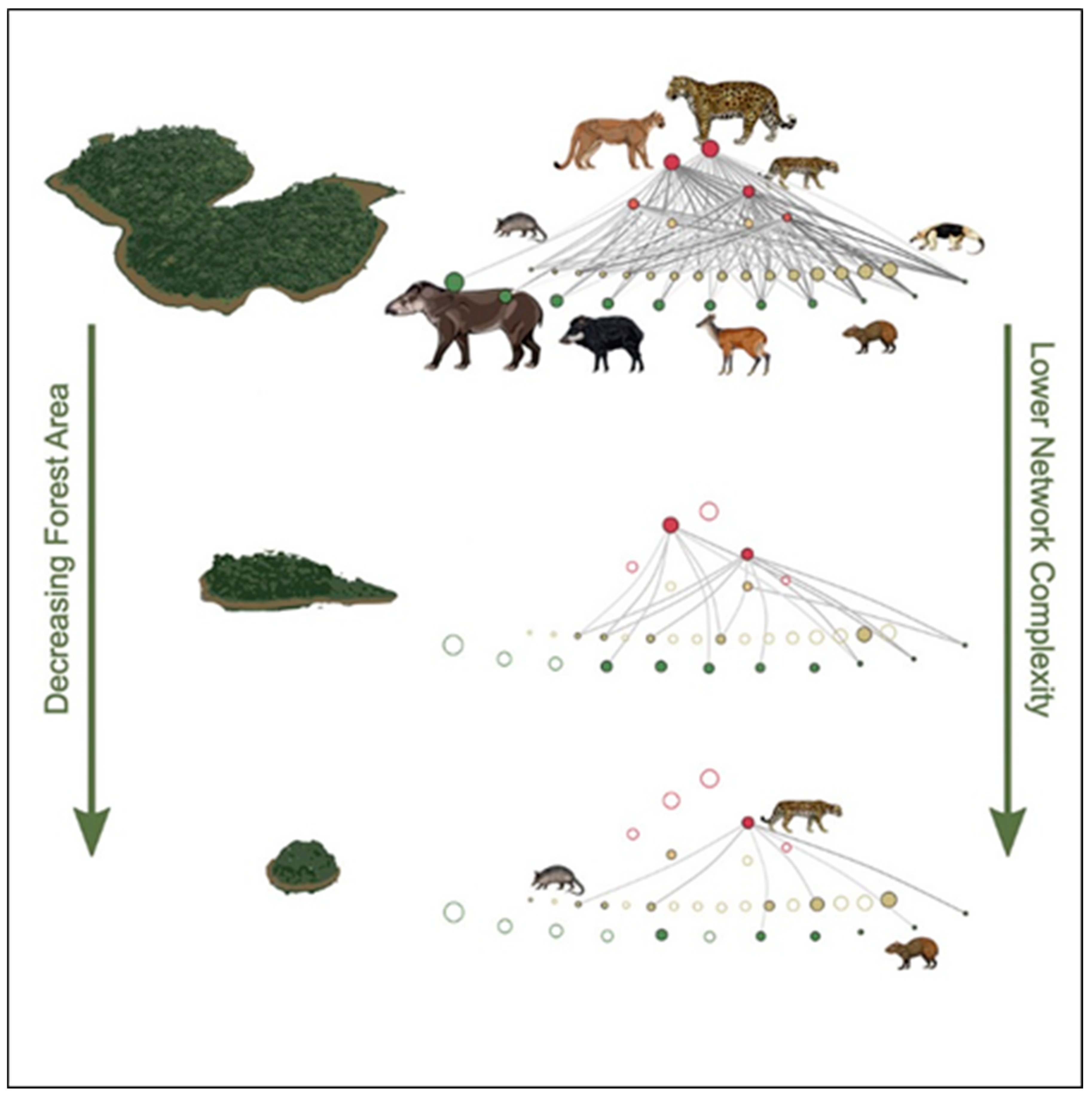
3. Food Webs, Biodiversity and Fragmented Habitats
3.1. Foundation Species
3.2. Keystone Species
3.3. Keystone Communities
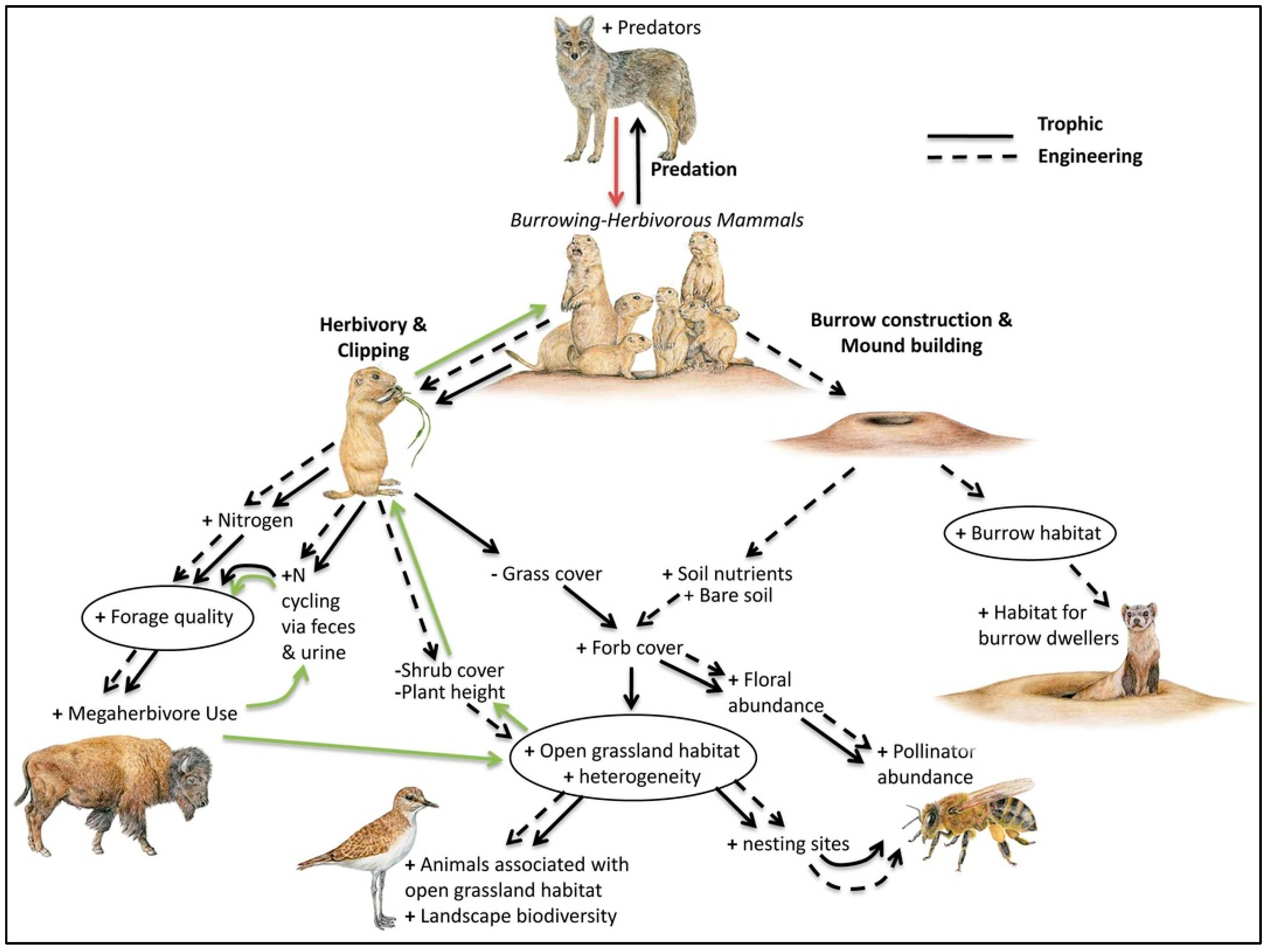
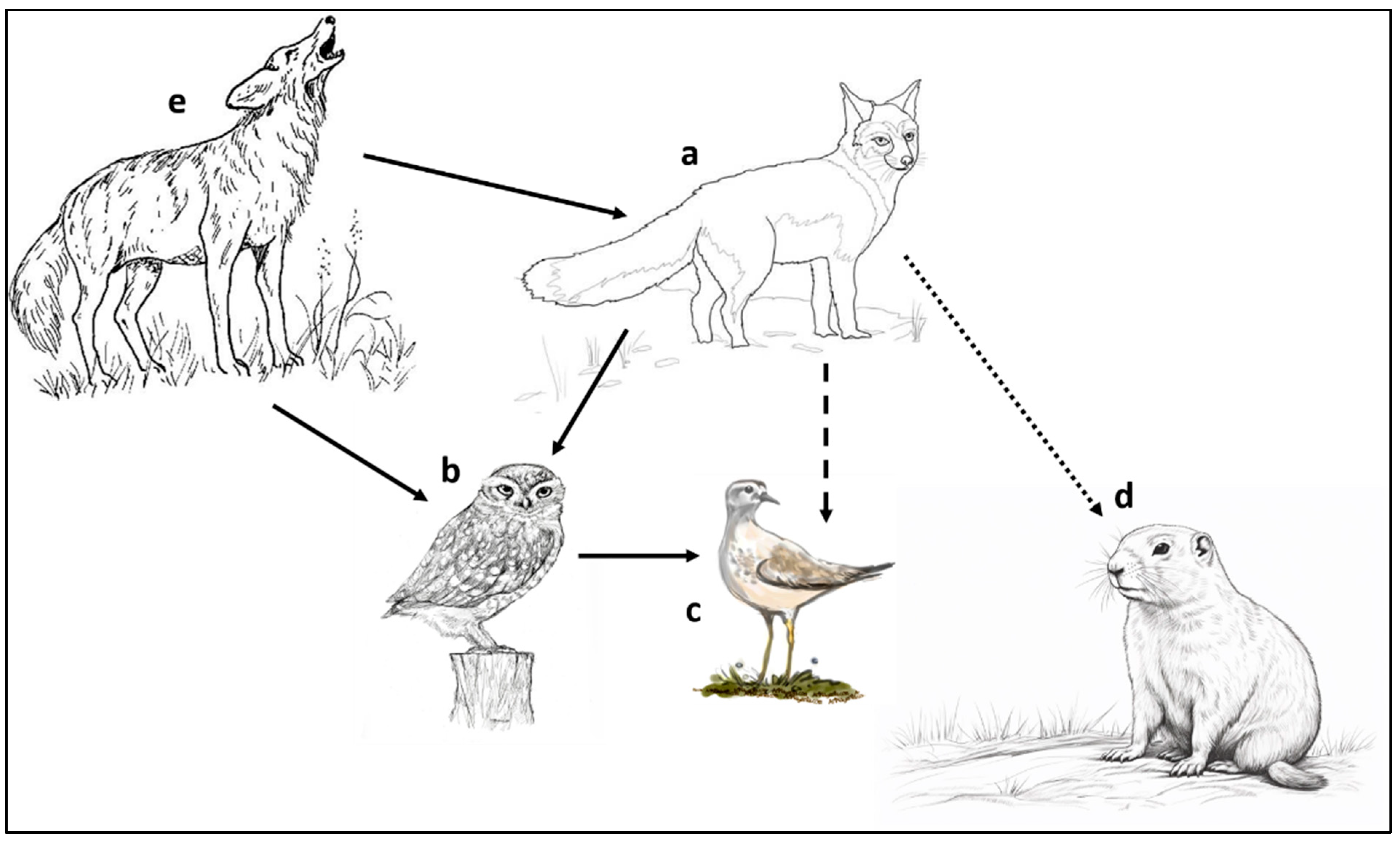
3.4. Habitat Fragmentation and Trophic Cascades—The Black-Tailed Prairie Dog
4. Ecosystem Function and Biodiversity in Fragmented Landscapes
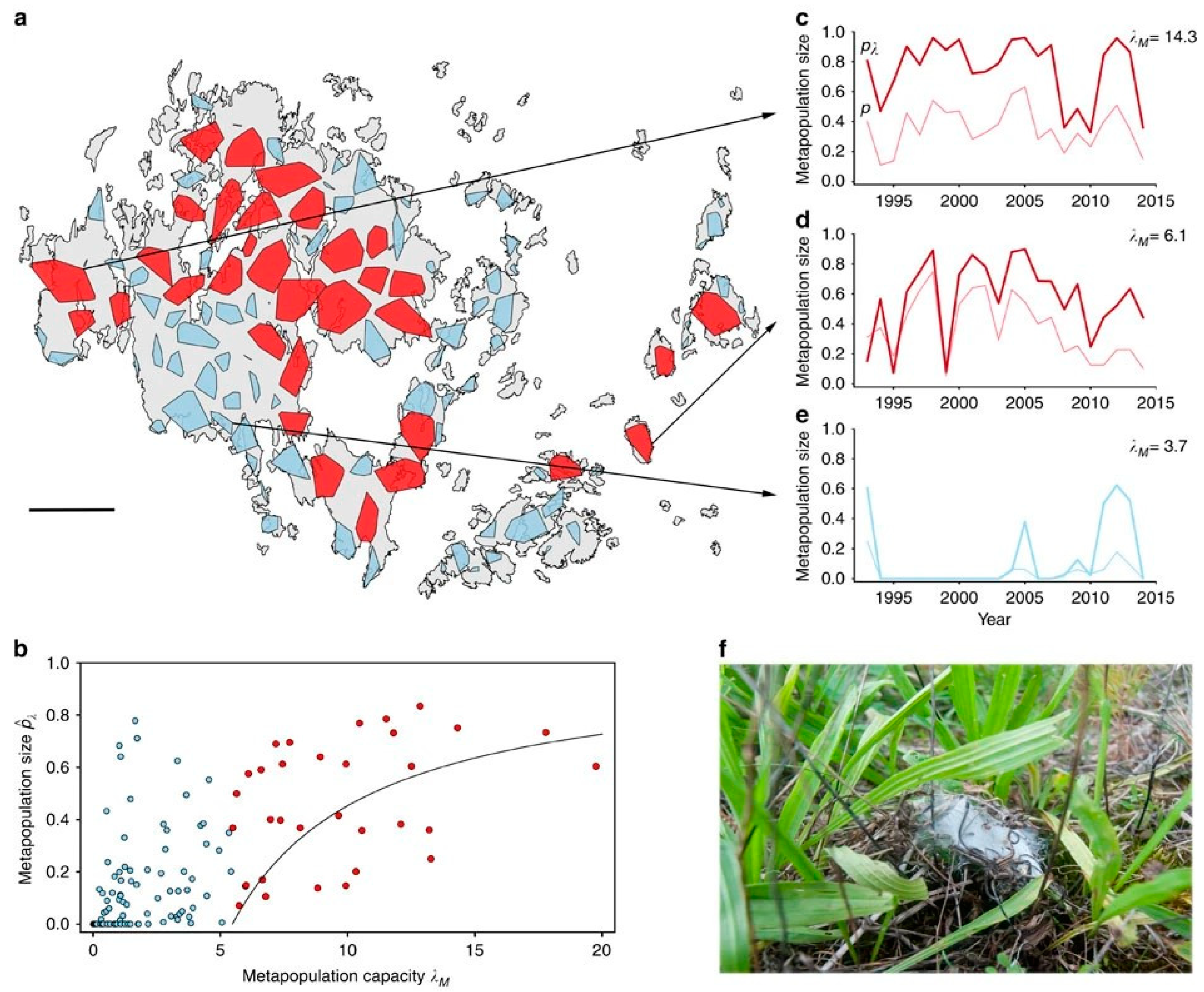
5. Biodiversity and Metapopulation Capacity
6. Landscape Genetics/Genomics and Biodiversity in a Fragmented World: Integration
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Avise, J.C.; Hamrick, J.L. (Eds.) Conservation Genetics: Case Histories from Nature; Chapman & Hall: New York, NY, USA, 1996. [Google Scholar]
- Hedrick, P.W. Recent developments in conservation genetics. Forest Ecol. Manag. 2004, 197, 3–19. [Google Scholar] [CrossRef]
- Frankham, R. Where are we in conservation genetics and where do we need to go? Conserv. Genet. 2010, 11, 661–663. [Google Scholar] [CrossRef]
- Kardos, M. Conservation genetics. Curr. Biol. 2021, 31, R1185–R1190. [Google Scholar] [CrossRef]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. Landscape modification and habitat fragmentation: A synthesis. Global. Ecol. Biogeogr. 2007, 16, 265–280. [Google Scholar] [CrossRef]
- Berry, P.; Ogawa-Onishi, Y.; McVey, A. The vulnerability of threatened species: Adaptive capability and adaptation opportunity. Biology 2013, 2, 872–893. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Fischer, J. Habitat Fragmentation and Landscape Change: An Ecological and Conservation Synthesis; Island Press: Washington, DC, USA, 2013. [Google Scholar]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef]
- Wilson, M.C.; Chen, X.Y.; Corlett, R.T.; Didham, R.K.; Ding, P.; Holt, R.D.; Holyoak, M.; Hu, G.; Hughes, A.C.; Jaing, L.; et al. Habitat fragmentation and biodiversity conservation: Key findings and future challenges. Landscape Ecol. 2016, 31, 219–227. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr.; Betts, M.G.; Damschen, E.I.; Hefley, T.J.; Hightower, J.; Smith, T.A.; Fortin, M.-J.; Haddad, N.M. Addressing the problem of scale that emerges with habitat fragmentation. Global. Ecol. Biogeogr. 2023, 32, 828–841. [Google Scholar] [CrossRef]
- Lienert, J. Habitat fragmentation effects on fitness of plant populations–a review. J. Nat. Conserv. 2004, 12, 53–72. [Google Scholar] [CrossRef]
- Stewart, J.R.; Lister, A.M.; Barnes, I.; Dalén, L. Refugia revisited: Individualistic responses of species in space and time. Proc. Roy. Soc. B-Biol. Sci. 2010, 277, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Hannah, L.; Flint, L.; Syphard, A.D.; Moritz, M.A.; Buckley, L.B.; McCullough, I.M. Fine-grain modeling of species’ response to climate change: Holdouts, stepping-stones, and microrefugia. Trends Ecol. Evol. 2014, 29, 390–397. [Google Scholar] [CrossRef]
- Klingler, K.B.; Jahner, J.P.; Parchman, T.L.; Ray, C.; Peacock, M.M. Genomic variation in the American pika: Signatures of geographic isolation and implications for conservation. BMC Ecol. Evol. 2021, 21, 2. [Google Scholar] [CrossRef] [PubMed]
- Cullingham, C.I.; Stephens, T.R.; Swan, K.D.; Wilson, S.C.; Janes, J.K.; Matchett, M.R.; Griebel, R.; Moehrenschlager, A. Genetic analysis reveals hidden threats and new motivation for conservation translocation of black-tailed prairie dogs at the northern limit of their range. Glob. Ecol. Conserv. 2023, 46, e02591. [Google Scholar] [CrossRef]
- Keppel, G.; Stralberg, D.; Morelli, T.L.; Bátori, Z. Managing climate-change refugia to prevent extinctions. Trends Ecol. Evol. 2024, 39, 800–808. [Google Scholar] [CrossRef]
- Johansson, M.; Primmer, C.R.; Merilä, J. Does habitat fragmentation reduce fitness and adaptability? A case study of the common frog (Rana temporaria). Mol. Ecol. 2007, 16, 2693–2700. [Google Scholar] [CrossRef]
- Leimu, R.; Vergeer, P.; Angeloni, F.; Ouborg, N.J. Habitat fragmentation, climate change, and inbreeding in plants. Ann. Ny. Acad. Sci. 2010, 1195, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.J.; Debes, P.V.; Bernatchez, L.; Hutchings, J.A. Population size, habitat fragmentation, and the nature of adaptive variation in a stream fish. Proc. Roy. Soc. B-Biol. Sci. 2014, 281, 20140370. [Google Scholar] [CrossRef] [PubMed]
- Cheptou, P.O.; Hargreaves, A.L.; Bonte, D.; Jacquemyn, H. Adaptation to fragmentation: Evolutionary dynamics driven by human influences. Philos. Trans. Roy. Soc. B 2017, 372, 20160037. [Google Scholar] [CrossRef]
- Mable, B.K. Conservation of adaptive potential and functional diversity: Integrating old and new approaches. Conserv. Genet. 2019, 20, 89–100. [Google Scholar] [CrossRef]
- Taylor Aiken, G.; Mabon, L. Where next for managed retreat: Bringing in history, community and under-researched places. Area 2024, 56, e12890. [Google Scholar] [CrossRef]
- Cincotta, R.P.; Wisnewski, J.; Engelman, R. Human population in the biodiversity hotspots. Nature 2000, 404, 990–992. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I. Habitat loss, the dynamics of biodiversity, and a perspective on conservation. Ambio 2011, 40, 248–255. [Google Scholar] [CrossRef]
- Crist, E.; Mora, C.; Engelman, R. The interaction of human population, food production, and biodiversity protection. Science 2017, 356, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Wilting, H.C.; Schipper, A.M.; Bakkenes, M.; Meijer, J.R.; Huijbregts, M.A. Quantifying biodiversity losses due to human consumption: A global-scale footprint analysis. Environ. Sci. Technol. 2017, 51, 3298–3306. [Google Scholar] [CrossRef]
- Elisha, O.D.; Felix, M.J. The loss of biodiversity and ecosystems: A threat to the functioning of our planet, economy and human society. IJEEDS 2020, 1, 30–44. [Google Scholar]
- Cafaro, P.; Hansson, P.; Götmark, F. Overpopulation is a major cause of biodiversity loss and smaller human populations are necessary to preserve what is left. Biol. Conserv. 2022, 272, 109646. [Google Scholar] [CrossRef]
- World Wildlife Fund. Living Planet Report 2024—A System in Peril; WWF: Gland, Switzerland, 2024. [Google Scholar]
- Yuan, R.; Zhang, N.; Zhang, Q. The impact of habitat loss and fragmentation on biodiversity in global protected areas. Sci. Total Environ. 2024, 931, 173004. [Google Scholar] [CrossRef]
- McCallum, H.; Dobson, A. Disease, habitat fragmentation and conservation. Proc. Roy. Soc. B-Biol. Sci. 2002, 269, 2041–2049. [Google Scholar] [CrossRef]
- Trudeau, K.M.; Britten, H.B.; Restani, M. Sylvatic plague reduces genetic variability in black-tailed prairie dogs. J. Wildlife Dis. 2004, 40, 205–211. [Google Scholar] [CrossRef][Green Version]
- Ewers, R.M.; Didham, R.K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. 2006, 81, 117–142. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Nascimento, H.E.; Laurance, S.G.; Andrade, A.; Ewers, R.M.; Harms, K.E.; Luizão, R.C.C.; Ribeiro, J.E. Habitat fragmentation, variable edge effects, and the landscape-divergence hypothesis. PLoS ONE 2007, 2, e1017. [Google Scholar] [CrossRef]
- Moilanen, A.; Wintle, B.A. The boundary-quality penalty: A quantitative method for approximating species responses to fragmentation in reserve selection. Conserv. Biol. 2007, 21, 355–364. [Google Scholar] [CrossRef]
- Gillespie, T.R.; Chapman, C.A. Forest fragmentation, the decline of an endangered primate, and changes in host–parasite interactions relative to an unfragmented forest. Am. J. Primatol. 2008, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Porensky, L.M.; Young, T.P. Edge-effect interactions in fragmented and patchy landscapes. Conserv. Biol. 2013, 27, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kiene, F.; Andriatsitohaina, B.; Ramsay, M.S.; Rakotondramanana, H.; Rakotondravony, R.; Radespiel, U.; Strube, C. Forest edges affect ectoparasite infestation patterns of small mammalian hosts in fragmented forests in Madagascar. Int. J. Parasitol. 2020, 50, 299–313. [Google Scholar] [CrossRef]
- DeWoody, J.A.; Harder, A.M.; Mathur, S.; Willoughby, J.R. The long-standing significance of genetic diversity in conservation. Mol. Ecol. 2021, 30, 4147–4154. [Google Scholar] [CrossRef]
- Johnson, K.H. Trophic-dynamic considerations in relating species diversity to ecosystem resilience. Biol. Rev. 2000, 75, 347–376. [Google Scholar]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchy, O.L.; et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef]
- Truchy, A.; Angeler, D.G.; Sponseller, R.A.; Johnson, R.K.; McKie, B.G. Linking biodiversity, ecosystem functioning and services, and ecological resilience: Towards an integrative framework for improved management. Adv. Ecol. Res. 2015, 53, 55–96. [Google Scholar]
- Brussard, P.F. Update: Minimum viable populations: How many are too few? Restor. Manag. Notes 1985, 3, 21–25. [Google Scholar] [CrossRef]
- Soulé, M.E. (Ed.) Viable Populations for Conservation; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Nunney, L.; Campbell, K.A. Assessing minimum viable population size: Demography meets population genetics. Trends Ecol. Evol. 1993, 8, 234–239. [Google Scholar] [CrossRef]
- Reed, D.H.; O’Grady, J.J.; Brook, B.W.; Ballou, J.D.; Frankham, R. Estimates of minimum viable population sizes for vertebrates and factors influencing those estimates. Biol. Conserv. 2003, 113, 23–34. [Google Scholar] [CrossRef]
- Hanski, I.; Gilpin, M.E. Metapopulation dynamics: Brief history and conceptual domain. Biol. J. Linn. Soc. 1991, 42, 3–16. [Google Scholar] [CrossRef]
- Simberloff, D. Habitat fragmentation and population extinction of birds. IBIS 1995, 137, S105–S111. [Google Scholar] [CrossRef]
- Rieman, B.E.; Dunham, J.B. Metapopulations and salmonids: A synthesis of life history patterns and empirical observations. Ecol. Freshw. Fish 2000, 9, 51–64. [Google Scholar] [CrossRef]
- MacNally, R.; Bennett, A.F. Species-specific predictions of the impact of habitat fragmentation: Local extinction of birds in the box-ironbark forests of central Victoria, Australia. Biol. Conserv. 1997, 82, 147–155. [Google Scholar] [CrossRef]
- Hill, M.F.; Caswell, H. Habitat fragmentation and extinction thresholds on fractal landscapes. Ecol. Lett. 1999, 2, 121–127. [Google Scholar] [CrossRef]
- Fahrig, L. Effect of habitat fragmentation on the extinction threshold: A synthesis. Ecol. Appl. 2002, 12, 346–353. [Google Scholar]
- Gu, W.; Heikkilä, R.; Hanski, I. Estimating the consequences of habitat fragmentation on extinction risk in dynamic landscapes. Landsc. Ecol. 2002, 17, 699–710. [Google Scholar] [CrossRef]
- Reed, D.H. Extinction risk in fragmented habitats. In Animal Conservation Forum; Cambridge University Press: Cambridge, UK, 2004; Volume 7, pp. 181–191. [Google Scholar]
- Levins, R. Evolution in Changing Environments; Princeton University Press: Princeton, NJ, USA, 1969. [Google Scholar]
- Levins, R. Extinction. Lect. Notes Math. 1970, 2, 75–107. [Google Scholar]
- Hanski, I. Metapopulation ecology. In Population Dynamics in Ecological Space and Time; Rhodes, O.E., Jr., Chesser, R.K., Smith, M.H., Eds.; University of Chicago Press: Chicago, IL, USA, 1996; Volume 1, p. 13. [Google Scholar]
- Hanski, I. Metapopulation Ecology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hanski, I.; Simberloff, D. The metapopulation approach, its history, conceptual domain, and application to conservation. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 5–26. [Google Scholar]
- Harrison, S. Local extinction in a metapopulation context: An empirical evaluation. Biol. J. Linn. Soc. 1991, 42, 73–88. [Google Scholar] [CrossRef]
- Harrison, S.; Taylor, A.D. Empirical evidence for metapopulation dynamics. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 27–42. [Google Scholar]
- Lande, R. Risks of population extinction from demographic and environmental stochasticity and random catastrophes. Am. Nat. 1993, 142, 911–927. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.H.; Kepler, C.B.; Snyder, N.F.; Derrickson, S.R.; Dein, F.J.; Wiley, J.W.; Wunderle, J.M., Jr.; Lugo, A.E.; Graham, D.L.; Toone, W.D. Puerto Rican parrots and potential limitations of the metapopulation approach to species conservation. Conserv. Biol. 1994, 8, 114–123. [Google Scholar] [CrossRef]
- Elmhagen, B.; Angerbjörn, A. The applicability of metapopulation theory to large mammals. Oikos 2001, 94, 89–100. [Google Scholar] [CrossRef]
- Baguette, M. The classical metapopulation theory and the real, natural world: A critical appraisal. Basic Appl. Ecol. 2004, 5, 213–224. [Google Scholar] [CrossRef]
- Driscoll, D.A. How to find a metapopulation. Can. J. Zool. 2007, 85, 1031–1048. [Google Scholar] [CrossRef]
- Tao, Y.; Hastings, A.; Lafferty, K.D.; Hanski, I.; Ovaskainen, O. Landscape fragmentation overturns classical metapopulation thinking. Proc. Natl. Acad. Sci. USA 2024, 121, e2303846121. [Google Scholar] [CrossRef]
- Hanski, I. Effects of landscape pattern on competitive interactions. In Mosaic Landscapes and Ecological Processes; Forman, T.T., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 203–224. [Google Scholar]
- Moilanen, A.; Hanski, I. Metapopulation dynamics: Effects of habitat quality and landscape structure. Ecology 1998, 79, 2503–2515. [Google Scholar] [CrossRef]
- Fleishman, E.; Ray, C.; Sjögren-Gulve, P.; Boggs, C.L.; Murphy, D.D. Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv. Biol. 2002, 16, 706–716. [Google Scholar] [CrossRef]
- Bohonak, A.J.; Jenkins, D.G. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol. Lett. 2003, 6, 783–796. [Google Scholar] [CrossRef]
- Pellet, J.; Fleishman, E.; Dobkin, D.S.; Gander, A.; Murphy, D.D. An empirical evaluation of the area and isolation paradigm of metapopulation dynamics. Biol. Conserv. 2007, 136, 483–495. [Google Scholar] [CrossRef]
- Vandewoestijne, S.; Schtickzelle, N.; Baguette, M. Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol. 2008, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Guiney, M.S.; Andow, D.A.; Wilder, T.T. Metapopulation structure and dynamics of an endangered butterfly. Basic Appl. Ecol. 2010, 11, 354–362. [Google Scholar] [CrossRef]
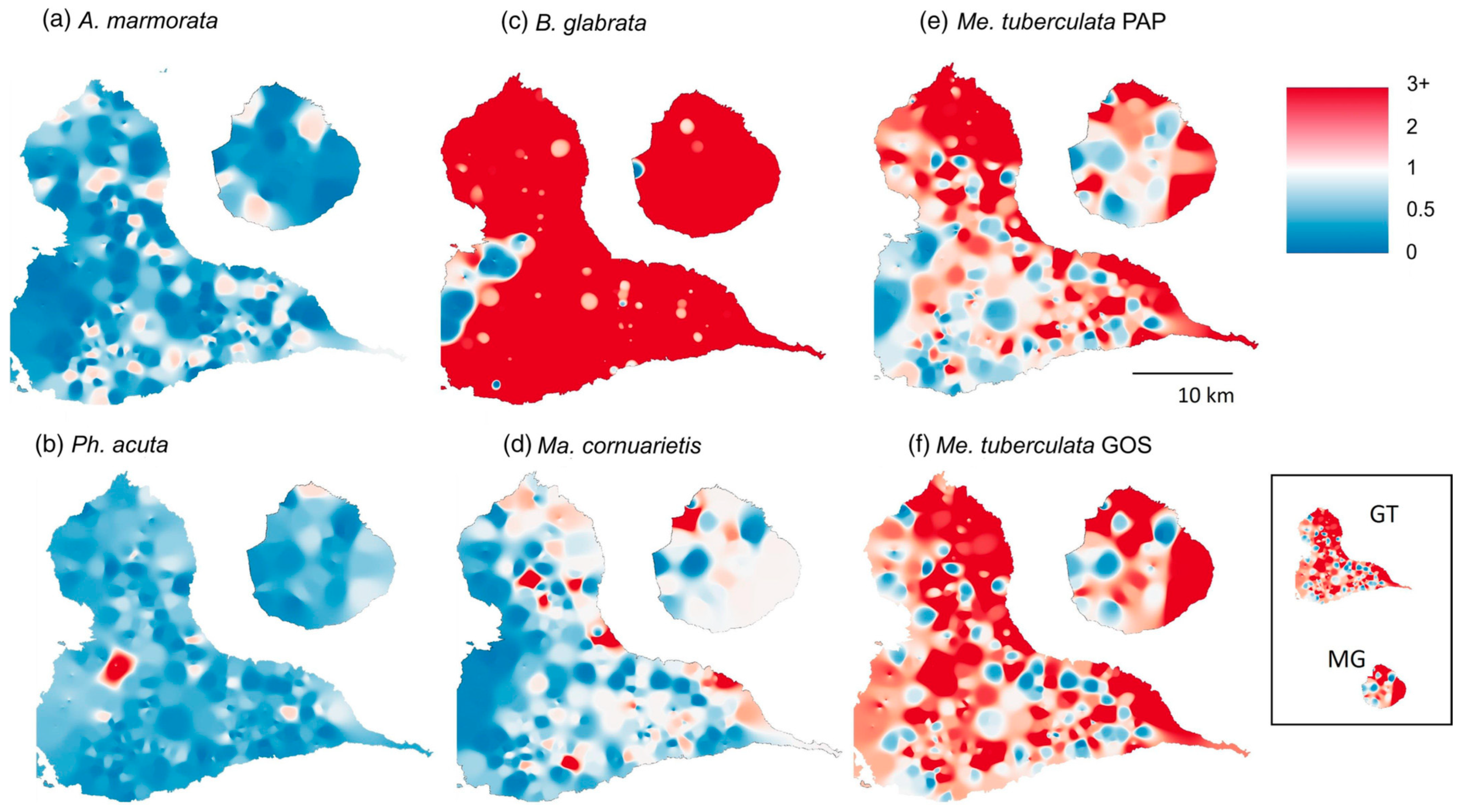
- Lamy, T.; Pointier, J.P.; Jarne, P.; David, P. Testing metapopulation dynamics using genetic, demographic and ecological data. Mol. Ecol. 2012, 21, 1394–1410. [Google Scholar] [CrossRef]
- Holyoak, M.; Lawler, S.P. Persistence of an extinction-prone predator-prey interaction through metapopulation dynamics. Ecology 1996, 77, 1867–1879. [Google Scholar] [CrossRef]
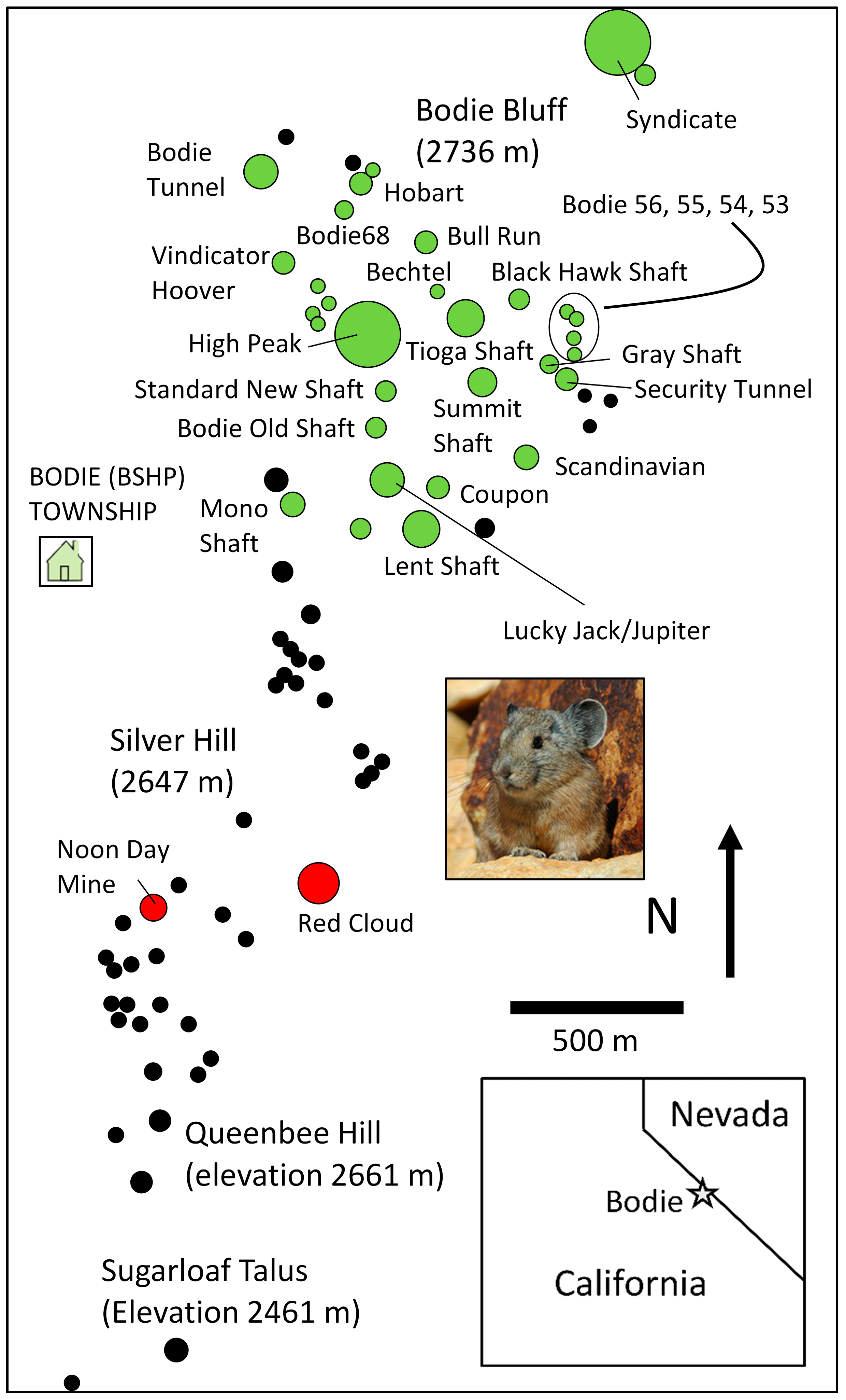
- Peacock, M.M.; Smith, A.T. The effect of habitat fragmentation on dispersal patterns, mating behavior, and genetic variation in a pika (Ochotona princeps) metapopulation. Oecologia 1997, 112, 524–533. [Google Scholar] [CrossRef]
- Moilanen, A.; Smith, A.T.; Hanski, I. Long-term dynamics in a metapopulation of the American pika. Am. Nat. 1998, 152, 530–542. [Google Scholar] [CrossRef]
- Sweanor, L.L.; Logan, K.A.; Hornocker, M.G. Cougar dispersal patterns, metapopulation dynamics, and conservation. Conserv. Biol. 2000, 14, 798–808. [Google Scholar] [CrossRef]
- Lambin, X.; Aars, J.; Piertney, S.B.; Telfer, S. Inferring pattern and process in small mammal metapopulations: Insights from ecological and genetic data. In Ecology, Genetics and Evolution of Metapopulations; Hanski, I., Gaggiotti, O.E., Eds.; Elsevier Academic Press: London, UK, 2004; pp. 515–540. [Google Scholar]
- Harrison, S.; Ray, C. Plant population viability and metapopulation-level processes. In Population Viability Analysis; Beissenger, S.R., McCullough, D.R., Eds.; University of Chicago Press: Chicago, IL, USA, 2002; pp. 109–122. [Google Scholar]
- Verheyen, K.; Vellend, M.; Van Calster, H.; Peterken, G.; Hermy, M. Metapopulation dynamics in changing landscapes: A new spatially realistic model for forest plants. Ecology 2004, 85, 3302–3312. [Google Scholar] [CrossRef]
- Dahlgren, J.P.; Ehrlén, J. Distribution patterns of vascular plants in lakes–the role of metapopulation dynamics. Ecography 2005, 28, 49–58. [Google Scholar] [CrossRef]
- Meulebrouck, K.; Verheyen, K.; Brys, R.; Hermy, M. Metapopulation viability of an endangered holoparasitic plant in a dynamic landscape. Ecography 2009, 32, 1040–1050. [Google Scholar] [CrossRef]
- Vermaat, J.E.; Vigneau, N.; Omtzigt, N. Viability of metapopulations of wetland birds in a fragmented landscape: Testing the key-patch approach. Biodivers. Conserv. 2008, 17, 2263–2273. [Google Scholar] [CrossRef][Green Version]
- Huang, R.; Pimm, S.L.; Giri, C. Using metapopulation theory for practical conservation of mangrove endemic birds. Conserv. Biol. 2020, 34, 266–275. [Google Scholar] [CrossRef] [PubMed]
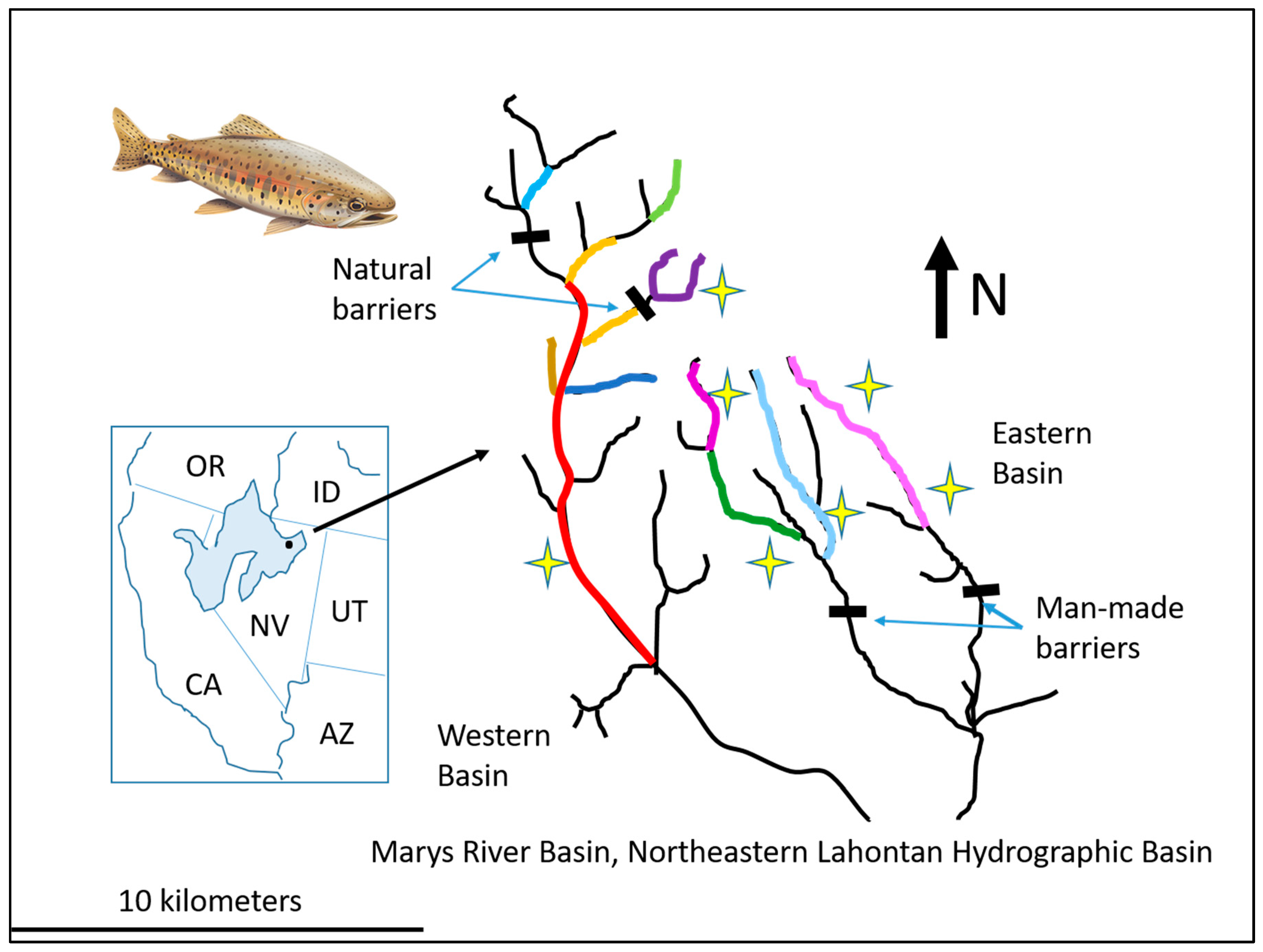
- Neville, H.M.; Dunham, J.B.; Peacock, M.M. Landscape attributes and life history variability shape genetic structure of trout populations in a stream network. Landsc. Ecol. 2006, 21, 901–916. [Google Scholar] [CrossRef]
- Neville, H.; Dauwalter, D.; Peacock, M. Monitoring demographic and genetic responses of a threatened inland trout to habitat reconnection. Trans. Am. Fish. Soc. 2016, 145, 610–626. [Google Scholar] [CrossRef]
- Vera-Escalona, I.; Senthivasan, S.; Habit, E.; Ruzzante, D.E. Past, present, and future of a freshwater fish metapopulation in a threatened landscape. Conserv. Biol. 2018, 32, 849–859. [Google Scholar] [CrossRef]
- Snead, A.A.; Tatarenkov, A.; Taylor, D.S.; Marson, K.; Earley, R.L. Centrality to the metapopulation is more important for population genetic diversity than habitat area or fragmentation. Biology Lett. 2024, 20, 20240158. [Google Scholar] [CrossRef]
- Hanski, I.; Pakkala, T.; Kuussaari, M.; Lei, G. Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 1995, 72, 21–28. [Google Scholar] [CrossRef]
- Hanski, I.; Moilanen, A.; Pakkala, T.; Kuussaari, M. The quantitative incidence function model and persistence of an endangered butterfly metapopulation. Conserv. Biol. 1996, 10, 578–590. [Google Scholar] [CrossRef]
- Hanski, I. Metapopulation dynamics: From concepts and observations to predictive models. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 69–91. [Google Scholar]
- Hanski, I.; Ovaskainen, O. The metapopulation capacity of a fragmented landscape. Nature 2000, 404, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, P.E.R. Extinction and isolation gradients in metapopulations: The case of the pool frog (Rana lessonae). Biol. J. Linn. Soc. 1991, 42, 135–147. [Google Scholar] [CrossRef]
- Gulve, P.S. Distribution and extinction patterns within a northern metapopulation of the pool frog, Rana lessonae. Ecology 1994, 75, 1357–1367. [Google Scholar] [CrossRef]
- Sjögren-Gulve, P. Spatial movement patterns in frogs: Target-oriented dispersal in the pool frog, Rana lessonae. Ecoscience 1998, 5, 31–38. [Google Scholar] [CrossRef]
- Kuussaari, M.; Nieminen, M.; Hanski, I. An experimental study of migration in the Glanville fritillary butterfly Melitaea cinxia. J. Anim. Ecol. 1996, 65, 791–801. [Google Scholar] [CrossRef]
- Hanski, I.; Singer, M.C. Extinction-colonization dynamics and host-plant choice in butterfly metapopulations. Am. Nat. 2001, 158, 341–353. [Google Scholar] [CrossRef]
- Hanski, I.; Saastamoinen, M.; Ovaskainen, O. Dispersal-related life-history trade-offs in a butterfly metapopulation. J. Anim. Ecol. 2006, 75, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Hanski, I.; Alho, J.; Moilanen, A. Estimating the parameters of survival and migration of individuals in metapopulations. Ecology 2000, 81, 239–251. [Google Scholar] [CrossRef]
- Hanski, I.; Thomas, C.D. Metapopulation dynamics and conservation: A spatially explicit model applied to butterflies. Biol. Conserv. 1994, 68, 167–180. [Google Scholar] [CrossRef]
- Lewis, O.; Thomas, C.; Hill, J.; Brookes, M.; Crane, T.P.; Graneau, Y.; Mallet, J.; Rose, O. Three ways of assessing metapopulation structure in the butterfly Plebejus argus. Ecol. Entomol. 1997, 22, 283–293. [Google Scholar] [CrossRef]
- Schtickzelle, N.; Choutt, J.; Goffart, P.; Fichefet, V.; Baguette, M. Metapopulation dynamics and conservation of the marsh fritillary butterfly: Population viability analysis and management options for a critically endangered species in Western Europe. Biol. Conserv. 2005, 126, 569–581. [Google Scholar] [CrossRef]
- Griffiths, R.A.; Sewell, D.; McCrea, R.S. Dynamics of a declining amphibian metapopulation: Survival, dispersal and the impact of climate. Biol. Conserv. 2010, 143, 485–491. [Google Scholar] [CrossRef]
- Fronhofer, E.A.; Kubisch, A.; Hilker, F.M.; Hovestadt, T.; Poethke, H.J. Why are metapopulations so rare? Ecology 2012, 93, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Murphy, D.D.; Ehrlich, P.R. Distribution of the bay checkerspot butterfly, Euphydryas editha bayensis: Evidence for a metapopulation model. Am. Nat. 1988, 132, 360–382. [Google Scholar] [CrossRef]
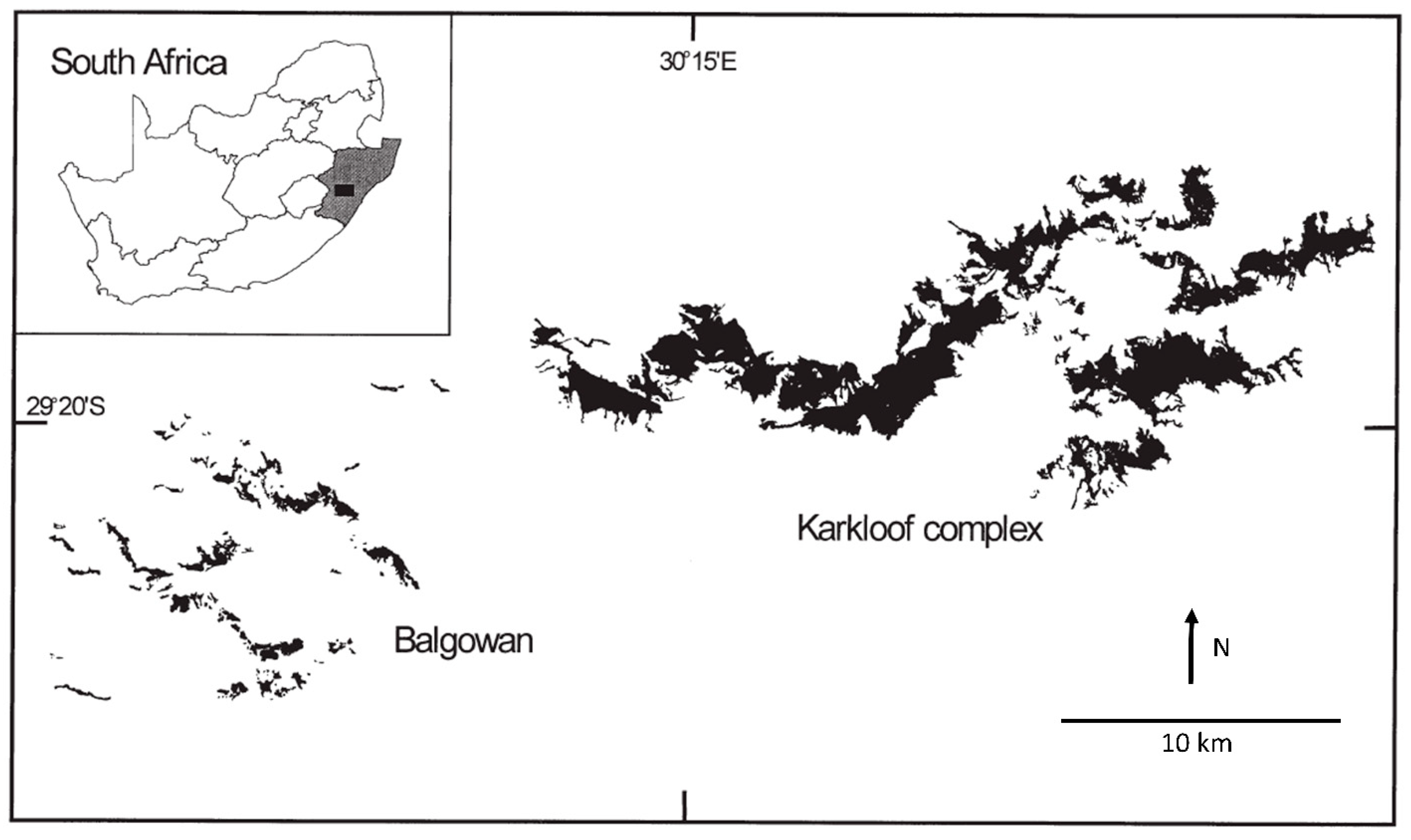
- Lawes, M.J.; Mealin, P.E.; Piper, S.E. Patch occupancy and potential metapopulation dynamics of three forest mammals in fragmented afromontane forest in South Africa. Conserv. Biol. 2000, 14, 1088–1098. [Google Scholar] [CrossRef]
- Onorato, D.P.; Hellgren, E.C.; Van Den Bussche, R.A.; Doan-Crider, D.L. Phylogeographic patterns within a metapopulation of black bears (Ursus americanus) in the American Southwest. J. Mammal. 2004, 85, 140–147. [Google Scholar] [CrossRef]
- Lawes, M.J.; Fly, S.; Piper, S.E. Gamebird vulnerability to forest fragmentation: Patch occupancy of the Crested guineafowl (Guttera edouardi) in Afromontane forests. Anim. Conserv. 2006, 9, 67–74. [Google Scholar] [CrossRef]
- Åström, J.; Bengtsson, J. Patch size matters more than dispersal distance in a mainland–island metacommunity. Oecologia 2011, 167, 747–757. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, J.L.; Bright, P.W. Metapopulation dynamics and a landscape approach to conservation of lowland water voles (Arvicola amphibius). Landsc. Ecol. 2011, 26, 1395–1404. [Google Scholar] [CrossRef]
- Glorvigen, P.; Andreassen, H.P.; Ims, R.A. Local and regional determinants of colonisation-extinction dynamics of a riparian mainland-island root vole metapopulation. PLoS ONE 2013, 8, e56462. [Google Scholar] [CrossRef]
- Murphy, D.D.; Weiss, S.B. Ecological studies and the conservation of the bay checkerspot butterfly, Euphydryas editha bayensis. Biol. Conserv. 1988, 46, 183–200. [Google Scholar] [CrossRef]
- Ross, J.V. Stochastic models for mainland-island metapopulations in static and dynamic landscapes. Bull. Math. Biol. 2006, 68, 417–449. [Google Scholar] [CrossRef] [PubMed]
- Klingler, K.B.; Nichols, L.B.; Hekkala, E.R.; Stewart, J.A.; Peacock, M.M. Life on the edge—A changing genetic landscape within an iconic American pika metapopulation over the last half century. PeerJ 2023, 11, e15962. [Google Scholar] [CrossRef] [PubMed]
- Matthysen, E.; Adriaensen, F.; Dhondt, A.A. Dispersal distances of nuthatches, Sitta europaea, in a highly fragmented forest habitat. Oikos 1995, 72, 375–381. [Google Scholar] [CrossRef]
- Stow, A.J.; Sunnucks, P.; Briscoe, D.A.; Gardner, M. The impact of habitat fragmentation on dispersal of Cunningham’s skink (Egernia cunninghami): Evidence from allelic and genotypic analyses of microsatellites. Mol. Ecol. 2001, 10, 867–878. [Google Scholar] [CrossRef]
- Parmesan, C.; Singer, M.C. Mosaics of climatic stress across species’ ranges: Tradeoffs cause adaptive evolution to limits of climatic tolerance. Philos. Trans. Roy. Soc. B 2022, 377, 20210003. [Google Scholar] [CrossRef]
- Rodhouse, T.J.; Jeffress, M.R.; Sherrill, K.R.; Mohren, S.R.; Nordensten, N.J.; Magnuson, M.L.; Schwalm, D.; Castillo, J.A.; Shinderman, M.; Epps, C.W. Geographical variation in the influence of habitat and climate on site occupancy turnover in American pika (Ochotona princeps). Divers. Distrib. 2018, 24, 1506–1520. [Google Scholar] [CrossRef]
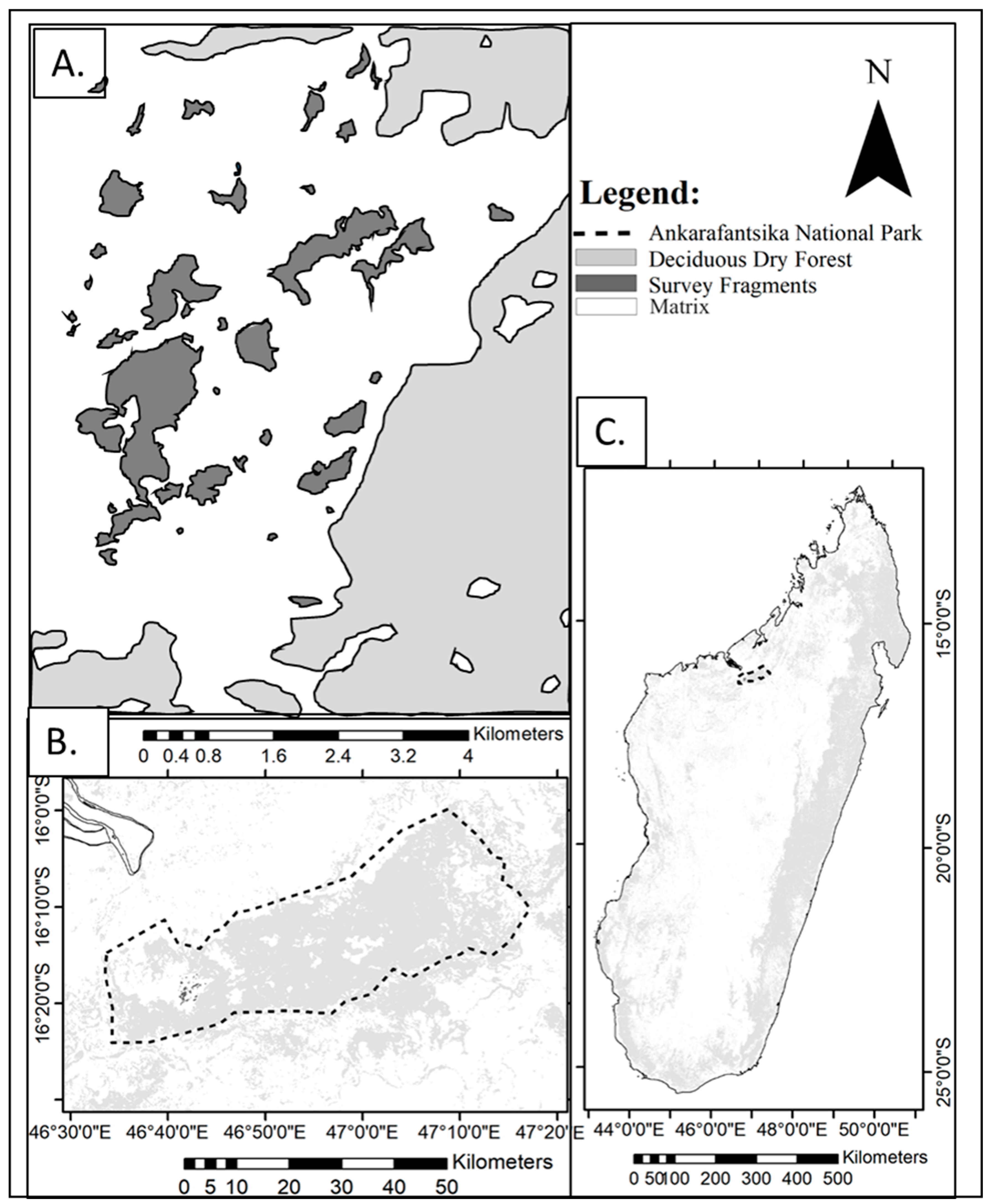
- Steffens, T.S.; Lehman, S.M. Lemur species-specific metapopulation responses to habitat loss and fragmentation. PLoS ONE 2018, 13, e0195791. [Google Scholar] [CrossRef]
- Driscoll, D. The frequency of metapopulations, metacommunities and nestedness in a fragmented landscape. Oikos 2008, 117, 297–309. [Google Scholar] [CrossRef]
- Pantel, J.H.; Lamy, T.; Dubart, M.; Pointier, J.P.; Jarne, P.; David, P. Metapopulation dynamics of multiple species in a heterogeneous landscape. Ecol. Monogr. 2022, 92, e1515. [Google Scholar] [CrossRef]
- Thomas, C.D. Dispersal and extinction in fragmented landscapes. Proc. Roy. Soc. B-Biol. Sci. 2000, 267, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dunham, J.B.; Vinyard, G.L.; Rieman, B.E. Habitat fragmentation and extinction risk of Lahontan cutthroat trout. N. Am. J. Fish. Manag. 1997, 17, 1126–1133. [Google Scholar] [CrossRef]
- Dunham, J.B.; Rieman, B.E. Metapopulation structure of bull trout: Influences of physical, biotic, and geometrical landscape characteristics. Ecol. Appl. 1999, 9, 642–655. [Google Scholar] [CrossRef]
- Loehle, C. Effect of ephemeral stepping stones on metapopulations on fragmented landscapes. Ecol. Complex. 2007, 4, 42–47. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Kaiser, T.S.; Frank, K.; Wiegand, T. Analyzing the effect of stepping stones on target patch colonisation in structured landscapes for Eurasian lynx. Landsc. Ecol. 2011, 26, 501–513. [Google Scholar] [CrossRef]
- Peacock, M.M.; Dochtermann, N.A. Evolutionary potential but not extinction risk of Lahontan cutthroat trout (Oncorhynchus clarkii henshawi) is associated with stream characteristics. Can. J. Fish. Aquat. Sci. 2012, 69, 615–626. [Google Scholar] [CrossRef]
- Edgardo, B.; Brandão, N.B.; Ribeiro, M.C.; Vieira, M.V. Dispersal movement through fragmented landscapes: The role of stepping stones and perceptual range. Landsc. Ecol. 2021, 36, 3249–3267. [Google Scholar]
- García-Antón, A.; Garza, V.; Traba, J. Connectivity in Spanish metapopulation of Dupont’s lark may be maintained by dispersal over medium-distance range and stepping stones. PeerJ 2021, 9, e11925. [Google Scholar] [CrossRef]
- Kindvall, O. Habitat heterogeneity and survival in a bush cricket metapopulation. Ecology 1996, 77, 207–214. [Google Scholar] [CrossRef]
- Graniero, P.A. The influence of landscape heterogeneity and local habitat effects on the response to competitive pressures in metapopulations. Ecol. Model. 2007, 203, 349–362. [Google Scholar] [CrossRef]
- Schooley, R.L.; Branch, L.C. Spatial heterogeneity in habitat quality and cross-scale interactions in metapopulations. Ecosystems 2007, 10, 846–853. [Google Scholar] [CrossRef]
- Shen, G.; He, F.; Waagepetersen, R.; Sun, I.F.; Hao, Z.; Chen, Z.S.; Yu, M. Quantifying effects of habitat heterogeneity and other clustering processes on spatial distributions of tree species. Ecology 2013, 94, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Szacki, J. Spatially structured populations: How much do they match the classic metapopulation concept? Landsc. Ecol. 1999, 14, 369–379. [Google Scholar] [CrossRef]
- Vandermeer, J.; Carvajal, R. Metapopulation dynamics and the quality of the matrix. Am. Nat. 2001, 158, 211–220. [Google Scholar] [CrossRef]
- Ricketts, T.H. The matrix matters: Effective isolation in fragmented landscapes. Am. Nat. 2001, 158, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jules, E.S.; Shahani, P. A broader ecological context to habitat fragmentation: Why matrix habitat is more important than we thought. J. Veg. Sci. 2003, 14, 459–464. [Google Scholar] [CrossRef]
- Prugh, L.R.; Hodges, K.E.; Sinclair, A.R.; Brashares, J.S. Effect of habitat area and isolation on fragmented animal populations. Proc. Natl. Acad. Sci. USA 2008, 105, 20770–20775. [Google Scholar] [CrossRef]
- Akçakaya, H.R.; Mills, G.; Doncaster, C.P. The role of metapopulations in conservation. Key Topics Cons. Biol. 2007, 1, 64–84. [Google Scholar]
- Hanski, I.; Schulz, T.; Wong, S.C.; Ahola, V.; Ruokolainen, A.; Ojanen, S.P. Ecological and genetic basis of metapopulation persistence of the Glanville fritillary butterfly in fragmented landscapes. Nat. Commun. 2017, 8, 14504. [Google Scholar] [CrossRef]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.M.; Van Groenendael, J.M.; Vosman, B. Genetic diversity and the survival of populations. Plant biol. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Battey, C.J.; Ralph, P.L.; Kern, A.D. Space is the place: Effects of continuous spatial structure on analysis of population genetic data. Genetics 2020, 215, 193–214. [Google Scholar] [CrossRef] [PubMed]
- Lande, R. Genetics and demography in biological conservation. Science 1988, 241, 1455–1460. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, M. The genetic effective size of a metapopulation. Biol. J. Linn. Soc. 1991, 42, 165–175. [Google Scholar] [CrossRef]
- Korn, H. Genetic, demographic, spatial, environmental and catastrophic effects on the survival probability of small populations of mammals. In Minimum Animal Populations; Remmert, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 33–49. [Google Scholar]
- Pannell, J.R.; Charlesworth, B. Neutral genetic diversity in a metapopulation with recurrent local extinction and recolonization. Evolution 1999, 53, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Pannell, J.R.; Charlesworth, B. Effects of metapopulation processes on measures of genetic diversity. Philos. Trans. Roy. Soc. B 2000, 355, 1851–1864. [Google Scholar] [CrossRef]
- Frankham, R. Effective population size/adult population size ratios in wildlife: A review. Genet Res. 1995, 66, 95–107. [Google Scholar] [CrossRef]
- Johnson, J.A.; Bellinger, M.R.; Toepfer, J.E.; Dunn, P. Temporal changes in allele frequencies and low effective population size in greater prairie-chickens. Mol. Ecol. 2004, 13, 2617–2630. [Google Scholar] [CrossRef]
- Johnson, W.E.; Onorato, D.P.; Roelke, M.E.; Land, E.D.; Cunningham, M.; Belden, R.C.; McBride, R.; Jansen, D.; Lotz, M.; Shindle, D.; et al. Genetic restoration of the Florida panther. Science 2010, 329, 1641–1645. [Google Scholar] [CrossRef]
- Waples, R.S.; Antao, T.; Luikart, G. Effects of overlapping generations on linkage disequilibrium estimates of effective population size. Genetics 2014, 197, 769–780. [Google Scholar] [CrossRef]
- Senneville, S.; Beaulieu, J.; Daoust, G.; Deslauriers, M.; Bousquet, J. Evidence for low genetic diversity and metapopulation structure in Canada yew (Taxus canadensis): Considerations for conservation. Can. J. Forest Res. 2001, 31, 110–116. [Google Scholar] [CrossRef]
- Gerlach, G.; Hoeck, H.N. Islands on the plains: Metapopulation dynamics and female biased dispersal in hyraxes (Hyracoidea) in the Serengeti National Park. Mol. Ecol. 2001, 10, 2307–2317. [Google Scholar] [CrossRef]
- Ortego, J.; Aparicio, J.M.; Cordero, P.J.; Calabuig, G. Individual genetic diversity correlates with the size and spatial isolation of natal colonies in a bird metapopulation. Proc. Roy. Soc. B-Biol. Sci. 2008, 275, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Kunz, F.; Klinga, P.; Sittenthaler, M.; Schebeck, M.; Stauffer, C.; Grünschachner-Berger, V.; Hackländer, K.; Nopp-Mayr, U. Assessment of drivers of spatial genetic variation of a ground-dwelling bird species and its implications for conservation. Ecol. Evol. 2022, 12, e8460. [Google Scholar] [CrossRef]
- Fountain, T.; Nieminen, M.; Sirén, J.; Wong, S.C.; Lehtonen, R.; Hanski, I. Predictable allele frequency changes due to habitat fragmentation in the Glanville fritillary butterfly. Proc. Natl. Acad. Sci. USA 2016, 113, 2678–2683. [Google Scholar] [CrossRef]
- Saccheri, I.; Kuussaari, M.; Kankare, M.; Vikman, P.; Fortelius, W.; Hanski, I. Inbreeding and extinction in a butterfly metapopulation. Nature 1998, 392, 491–494. [Google Scholar] [CrossRef]
- DiLeo, M.F.; Nair, A.; Kardos, M.; Husby, A.; Saastamoinen, M. Demography and environment modulate the effects of genetic diversity on extinction risk in a butterfly metapopulation. Proc. Natl. Acad. Sci. USA 2024, 121, e2309455121. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Spitze, K.; Kerfoot, W.C. Ephemeral metapopulations show high genetic diversity at regional scales. Ecology 2009, 90, 2670–2675. [Google Scholar] [CrossRef]
- Morrissey, M.B.; de Kerckhove, D.T. The maintenance of genetic variation due to asymmetric gene flow in dendritic metapopulations. Am. Nat. 2009, 174, 875–889. [Google Scholar] [CrossRef]
- Hand, B.K.; Muhlfeld, C.C.; Wade, A.A.; Kovach, R.P.; Whited, D.C.; Narum, S.R.; Matala, A.P.; Ackerman, M.W.; Garner, B.A.; Kimball, J.S.; et al. Climate variables explain neutral and adaptive variation within salmonid metapopulations: The importance of replication in landscape genetics. Mol. Ecol. 2016, 25, 689–705. [Google Scholar] [CrossRef]
- Huntsman, B.M.; Petty, J.T.; Sharma, S.; Merriam, E.R. More than a corridor: Use of a main stem stream as supplemental foraging habitat by a brook trout metapopulation. Oecologia 2016, 182, 463–473. [Google Scholar] [CrossRef]
- Ardren, W.R.; Bernall, S.R. Dams impact westslope cutthroat trout metapopulation structure and hybridization dynamics. Conserv. Genet. 2017, 18, 297–312. [Google Scholar] [CrossRef]
- Peacock, M.M.; Hekkala, E.R.; Kirchoff, V.S.; Heki, L.G. Return of a giant: DNA from archival museum samples helps to identify a unique cutthroat trout lineage formerly thought to be extinct. Roy. Soc. Open Sci. 2017, 4, 171253. [Google Scholar] [CrossRef] [PubMed]
- Arntzen, J.W.; van Belkom, J. ‘Mainland-island’ population structure of a terrestrial salamander in a forest-bocage landscape with little evidence for in situ ecological speciation. Sci. Rep. 2020, 10, 1700. [Google Scholar]
- Moncrief, N.D.; Roberts, J.H.; Hallerman, E.M.; Van Den Bussche, R.A.; Porter, J.H.; Dueser, R.D. Landscape genetics of a raccoon (Procyon lotor) metapopulation in an undeveloped coastal island system. J. Mammal. 2017, 98, 1137–1155. [Google Scholar] [CrossRef]
- Stacey, P.B.; Taper, M.L.; Johnson, V.A. Migration within metapopulations: The impact upon local population dynamics. In Metapopulation Biology; Hanski, I., Gilpin, M.E., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 267–291. [Google Scholar]
- Sonsthagen, S.A.; McClaren, E.L.; Doyle, F.I.; Titus, K.; Sage, G.K.; Wilson, R.E.; Gust, J.R.; Talbot, S.L. Identification of metapopulation dynamics among Northern Goshawks of the Alexander Archipelago, Alaska, and coastal British Columbia. Conserv. Genet. 2012, 13, 1045–1057. [Google Scholar] [CrossRef]
- Severaid, J.H. The Natural History of the Pikas (Mammalian Genus Ochotona). Doctoral Dissertation, University of California, Berkeley, CA, USA, 1955. [Google Scholar]
- Smith, A.T. The distribution and dispersal of pikas: Consequences of insular population structure. Ecology 1974, 55, 1112–1119. [Google Scholar] [CrossRef]
- Peacock, M.M. Determining natal dispersal patterns in a population of North American pikas (Ochotona princeps) using direct mark-resight and indirect genetic methods. Behav. Ecol. 1997, 8, 340–350. [Google Scholar] [CrossRef]
- Nichols, L. Fecal pellets of American pikas (Ochotona princeps) provide a crude chronometer for dating patch occupancy. West. N. Am. Naturalist. 2011, 70, 500–507. [Google Scholar] [CrossRef]
- Nichols, L.B.; Klingler, K.B.; Peacock, M.M. American pikas (Ochotona princeps) extirpated from the historic Masonic Mining District of eastern California. West. N. Am. Naturalist. 2016, 76, 163–171. [Google Scholar] [CrossRef]
- Reiss, J.; Bridle, J.R.; Montoya, J.M.; Woodward, G. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 2009, 24, 505–514. [Google Scholar] [CrossRef]
- Villard, M.A.; Metzger, J.P. Beyond the fragmentation debate: A conceptual model to predict when habitat configuration really matters. J. Appl. Ecol. 2014, 51, 309–318. [Google Scholar] [CrossRef]
- Tilman, D.; Clark, M.; Williams, D.R.; Kimmel, K.; Polasky, S.; Packer, C. Future threats to biodiversity and pathways to their prevention. Nature 2017, 546, 73–81. [Google Scholar] [CrossRef]
- Brose, U.; Hillebrand, H. Biodiversity and ecosystem functioning in dynamic landscapes. Philos. Trans. Roy. Soc. B 2016, 371, 20150267. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M. The island dilemma: Lessons of modern biogeographic studies for the design of natural reserves. Biol. Conserv. 1975, 7, 129–146. [Google Scholar] [CrossRef]
- Quinn, J.F.; Harrison, S.P. Effects of habitat fragmentation and isolation on species richness: Evidence from biogeographic patterns. Oecologia 1988, 75, 132–140. [Google Scholar] [CrossRef]
- Baz, A.; Garcia-Boyero, A. The SLOSS dilemma: A butterfly case study. Biodivers. Conserv. 1996, 5, 493–502. [Google Scholar] [CrossRef]
- Tjørve, E. How to resolve the SLOSS debate: Lessons from species-diversity models. Theor. Popul. Biol. 2010, 264, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Ecological responses to habitat fragmentation per se. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 1–23. [Google Scholar] [CrossRef]
- Fahrig, L. Why do several small patches hold more species than few large patches? Global Ecol. Biogeogr. 2020, 29, 615–628. [Google Scholar] [CrossRef]
- Riva, F.; Fahrig, L. The disproportionately high value of small patches for biodiversity conservation. Conserv. Lett. 2022, 15, e12881. [Google Scholar] [CrossRef]
- Chase, J.M.; Liebergesell, M.; Sagouis, A.; May, F.; Blowes, S.A.; Berg, Å.; Bernard, E.; Brosi, B.J.; Cadotte, M.W.; Cayuela, L.; et al. FragSAD: A database of diversity and species abundance distributions from habitat fragments. Ecology 2019, 100, 2861. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wilson, M.; Hu, G.; Liu, J.; Wu, J.; Yu, M. How does habitat fragmentation affect the biodiversity and ecosystem functioning relationship? Landsc. Ecol. 2018, 33, 341–352. [Google Scholar] [CrossRef]
- Swihart, R.K.; Gehring, T.M.; Kolozsvary, M.B.; Nupp, T.E. Responses of ‘resistant’ vertebrates to habitat loss and fragmentation: The importance of niche breadth and range boundaries. Divers Distrib. 2003, 9, 1–18. [Google Scholar] [CrossRef]
- Layman, C.A.; Quattrochi, J.P.; Peyer, C.M.; Allgeier, J.E. Niche width collapse in a resilient top predator following ecosystem fragmentation. Ecol. Lett. 2007, 10, 937–944. [Google Scholar] [CrossRef]
- Birand, A.; Vose, A.; Gavrilets, S. Patterns of species ranges, speciation, and extinction. Am Nat. 2012, 179, 1–21. [Google Scholar] [CrossRef]
- Cagnolo, L.; Valladares, G.; Salvo, A.; Cabido, M.; Zak, M. Habitat fragmentation and species loss across three interacting trophic levels: Effects of life-history and food-web traits. Conserv. Biol. 2009, 23, 1167–1175. [Google Scholar] [CrossRef]
- Hagen, M.; Kissling, W.D.; Rasmussen, C.; De Aguiar, M.A.; Brown, L.E.; Carstensen, D.W.; Alves-Dos-Santos, I.; Dupont, Y.L.; Edwards, F.K.; Genini, J.; et al. Biodiversity, species interactions and ecological networks in a fragmented world. Adv. Ecol. Res. 2012, 46, 89–210. [Google Scholar]
- Valladares, G.; Cagnolo, L.; Salvo, A. Forest fragmentation leads to food web contraction. Oikos 2012, 121, 299–305. [Google Scholar] [CrossRef]
- Ellison, A.M.; Bank, M.S.; Clinton, B.D.; Colburn, E.A.; Elliott, K.; Ford, C.R.; Foster, D.R.; Kloeppel, B.D.; Knoepp, J.D.; Lovett, G.M.; et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front. Ecol. Environ. 2005, 3, 479–486. [Google Scholar] [CrossRef]
- Baiser, B.; Whitaker, N.; Ellison, A.M. Modeling foundation species in food webs. Ecosphere 2013, 4, 1–14. [Google Scholar] [CrossRef]
- Castorani, M.C.; Reed, D.C.; Miller, R.J. Loss of foundation species: Disturbance frequency outweighs severity in structuring kelp forest communities. Ecology 2018, 99, 2442–2454. [Google Scholar] [CrossRef]
- Ellison, A.M. Foundation species, non-trophic interactions, and the value of being common. Iscience 2019, 13, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Bangert, R.K.; Lonsdorf, E.V.; Wimp, G.M.; Shuster, S.M.; Fischer, D.; Schweitzer, J.A.; Allan, G.J.; Bailey, J.K.; Whitham, T.G. Genetic structure of a foundation species: Scaling community phenotypes from the individual to the region. Heredity 2008, 100, 121–131. [Google Scholar] [CrossRef]
- Yando, E.S.; Osland, M.J.; Jones, S.F.; Hester, M.W. Jump-starting coastal wetland restoration: A comparison of marsh and mangrove foundation species. Restor. Ecol. 2019, 27, 1145–1154. [Google Scholar] [CrossRef]
- Narwani, A.; Reyes, M.; Pereira, A.L.; Penson, H.; Dennis, S.R.; Derrer, S.; Spaak, P.; Matthews, B. Interactive effects of foundation species on ecosystem functioning and stability in response to disturbance. Proc. R. Soc. Ser. B Biol. Sci. 2019, 286, 20191857. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Altieri, A.H.; Angelini, C.; Bishop, M.J.; Bulleri, F.; Farhan, R.; Frühling, V.M.M.; Gribben, P.E.; Harrison, S.B.; He, Q.; et al. Heterogeneity within and among co-occurring foundation species increases biodiversity. Nat. Commun. 2022, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, Q.; Shen, Y.; Wang, H.; Yao, X. Using landscape genomics to assess local adaptation of fruit trees to current and future climatic conditions. Fruit Res. 2024, 4, e003. [Google Scholar] [CrossRef]
- Sorte, C.J.; Davidson, V.E.; Franklin, M.C.; Benes, K.M.; Doellman, M.M.; Etter, R.J.; Hannigan, R.E.; Lubchenco, J.; Menge, B.A. Long-term declines in an intertidal foundation species parallel shifts in community composition. Global Change Biol. 2017, 23, 341–352. [Google Scholar] [CrossRef]
- Sarà, G.; Giommi, C.; Giacoletti, A.; Conti, E.; Mulder, C.; Mangano, M.C. Multiple climate-driven cascading ecosystem effects after the loss of a foundation species. Sci. Total Environ. 2021, 770, 144749. [Google Scholar] [CrossRef]
- Paine, R.T. Food web complexity and species diversity. Am. Nat. 1966, 100, 65–75. [Google Scholar] [CrossRef]
- Paine, R.T. A note on trophic complexity and community stability. Am. Nat. 1969, 103, 91–93. [Google Scholar] [CrossRef]
- Powers, M.E.; Tilman, D.; Estes, J.A.; Menge, B.A.; Bond, W.J.; Mills, L.S.; Daily, G.; Castilla, J.C.; Lubchenco, J.; Paine, R.T. Challenges in the quest for keystones: Identifying keystone species is difficult—But essential to understanding how loss of species will affect ecosystems. BioScience 1996, 46, 609–620. [Google Scholar] [CrossRef]
- Davic, R.D. Linking keystone species and functional groups: A new operational definition of the keystone species concept. Conserv Ecol. 2003, 7, r11. Available online: http://www.consecol.org/vol7/iss1/resp11/ (accessed on 8 March 2025). [CrossRef]
- Paine, R.T. A conversation on refining the concept of keystone species. Conserv. Biol. 1995, 9, 962–964. [Google Scholar] [CrossRef]
- Mills, L.S.; Soulé, M.E.; Doak, D.F. The keystone-species concept in ecology and conservation. BioScience 1993, 43, 219–224. [Google Scholar] [CrossRef]
- Bond, W.J. Keystone species. In Biodiversity and Ecosystem Function; Schulze, E., Mooney, H.A., Eds.; Springer: Berlin, Germany, 1994; pp. 237–253. [Google Scholar]
- Jordán, F. Keystone species and food webs. Philos. Trans. Roy. Soc. B 2009, 364, 1733–1741. [Google Scholar] [CrossRef]
- Valls, A.; Coll, M.; Christensen, V. Keystone species: Toward an operational concept for marine biodiversity conservation. Ecol. Monogr. 2015, 85, 29–47. [Google Scholar] [CrossRef]
- Luther, D.A.; Cooper, W.J.; Wolfe, J.D.; Bierregaard, R.O.; Gonzalez, A.; Lovejoy, T.E. Tropical forest fragmentation and isolation: Is community decay a random process? Glob. Ecol. Conserv. 2020, 23, e01168. [Google Scholar] [CrossRef]
- Navarrete, S.A.; Menge, B.A. Keystone predation and interaction strength: Interactive effects of predators on their main prey. Ecol. Monogr. 1996, 66, 409–429. [Google Scholar] [CrossRef]
- Suzuki, S.S.; Baba, Y.G.; Toju, H. Dynamics of species-rich predator-prey networks and seasonal alternations of core species. Nat. Ecol. Evol. 2023, 7, 1432–1443. [Google Scholar] [CrossRef]
- Roffler, G.H.; Eriksson, C.E.; Allen, J.M.; Levi, T. Recovery of a marine keystone predator transforms terrestrial predator-prey dynamics. Proc. Natl. Acad. Sci. USA 2023, 120, e2209037120. [Google Scholar] [CrossRef] [PubMed]
- Vandermeer, J.; Maruca, S. Indirect effects with a keystone predator: Coexistence and chaos. Theor. Popul. Biol. 1998, 54, 38–43. [Google Scholar] [CrossRef]
- Barrios-O’Neill, D.; Bertolini, C.; Collins, P.C. Trophic cascades and the transient keystone concept. Biol. Conserv. 2017, 212, 191–195. [Google Scholar] [CrossRef]
- Wallach, A.D.; Dekker, A.H.; Lurgi, M.; Montoya, J.M.; Fordham, D.A.; Ritchie, E.G. Trophic cascades in 3D: Network analysis reveals how apex predators structure ecosystems. Methods Ecol. Evol. 2017, 8, 135–142. [Google Scholar] [CrossRef]
- Traveset, A.; Tur, C.; Eguíluz, V.M. Plant survival and keystone pollinator species in stochastic coextinction models: Role of intrinsic dependence on animal-pollination. Sci. Rep. 2017, 7, 6915. [Google Scholar] [CrossRef]
- Easton-Calabria, A.; Demary, K.C.; Oner, N.J. Beyond pollination: Honey bees (Apis mellifera) as zootherapy keystone species. Front. Ecol. Evol. 2019, 6, 161. [Google Scholar] [CrossRef]
- Simpson, D.T.; Weinman, L.R.; Genung, M.A.; Roswell, M.; MacLeod, M.; Winfree, R. Many bee species, including rare species, are important for function of entire plant–pollinator networks. Proc. Roy. Soc. B-Biol. Sci. 2022, 289, 20212689. [Google Scholar] [CrossRef]
- Gove, A.D.; Majer, J.D.; Dunn, R.R. A keystone ant species promotes seed dispersal in a “diffuse” mutualism. Oecologia 2007, 153, 687–697. [Google Scholar] [CrossRef]
- Longland, W.S.; Ostoja, S.M. Ecosystem services from keystone species: Diversionary seeding and seed-caching desert rodents can enhance Indian rice grass seedling establishment. Restor. Ecol. 2013, 21, 285–291. [Google Scholar] [CrossRef]
- Mello, M.A.R.; Rodrigues, F.A.; Costa, L.D.F.; Kissling, W.D.; Şekercioğlu, Ç.H.; Marquitti, F.M.D.; Kalko, E.K.V. Keystone species in seed dispersal networks are mainly determined by dietary specialization. Oikos 2015, 124, 1031–1039. [Google Scholar] [CrossRef]
- Vitali, A.; Sasal, Y.; Vázquez, D.P.; Miguel, M.F.; Rodríguez-Cabal, M.A. The disruption of a keystone interaction erodes pollination and seed dispersal networks. Ecology 2022, 103, e03547. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.E.; Cuddington, K.; Jones, C.G.; Talley, T.S.; Hastings, A.; Lambrinos, J.G.; Crooks, J.A.; Wilson, W.G. Using ecosystem engineers to restore ecological systems. Trends Ecol. Evol. 2006, 21, 493–500. [Google Scholar] [CrossRef]
- de Visser, S.; Thébault, E.; de Ruiter, P.C. Ecosystem engineers, keystone species. In Ecological Systems: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Leemans, R., Ed.; Springer: New York, NY, USA, 2012; pp. 59–68. [Google Scholar]
- Johnson, S.A.; Ober, H.K.; Adams, D.C. Are keystone species effective umbrellas for habitat conservation? A spatially explicit approach. J. Nat. Conserv. 2017, 37, 47–55. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Smith, G.A. Terrestrial vertebrate communities at black-tailed prairie dog (Cynomys ludovicianus) towns. Biol. Conserv. 2004, 115, 89–100. [Google Scholar] [CrossRef]
- Ishida, Y.; Gugala, N.A.; Georgiadis, N.J.; Roca, A.L. Evolutionary and demographic processes shaping geographic patterns of genetic diversity in a keystone species, the African forest elephant (Loxodonta cyclotis). Ecol. Evol. 2018, 8, 4919–4931. [Google Scholar] [CrossRef] [PubMed]
- Boswell, G.P.; Britton, N.F.; Franks, N.R. Habitat fragmentation, percolation theory and the conservation of a keystone species. Proc. Roy. Soc. B-Biol. Sci. 1998, 265, 1921–1925. [Google Scholar] [CrossRef]
- Williams, D.A.; Wang, Y.; Borchetta, M.; Gaines, M.S. Genetic diversity and spatial structure of a keystone species in fragmented pine rockland habitat. Biol. Conserv. 2007, 138, 256–268. [Google Scholar] [CrossRef]
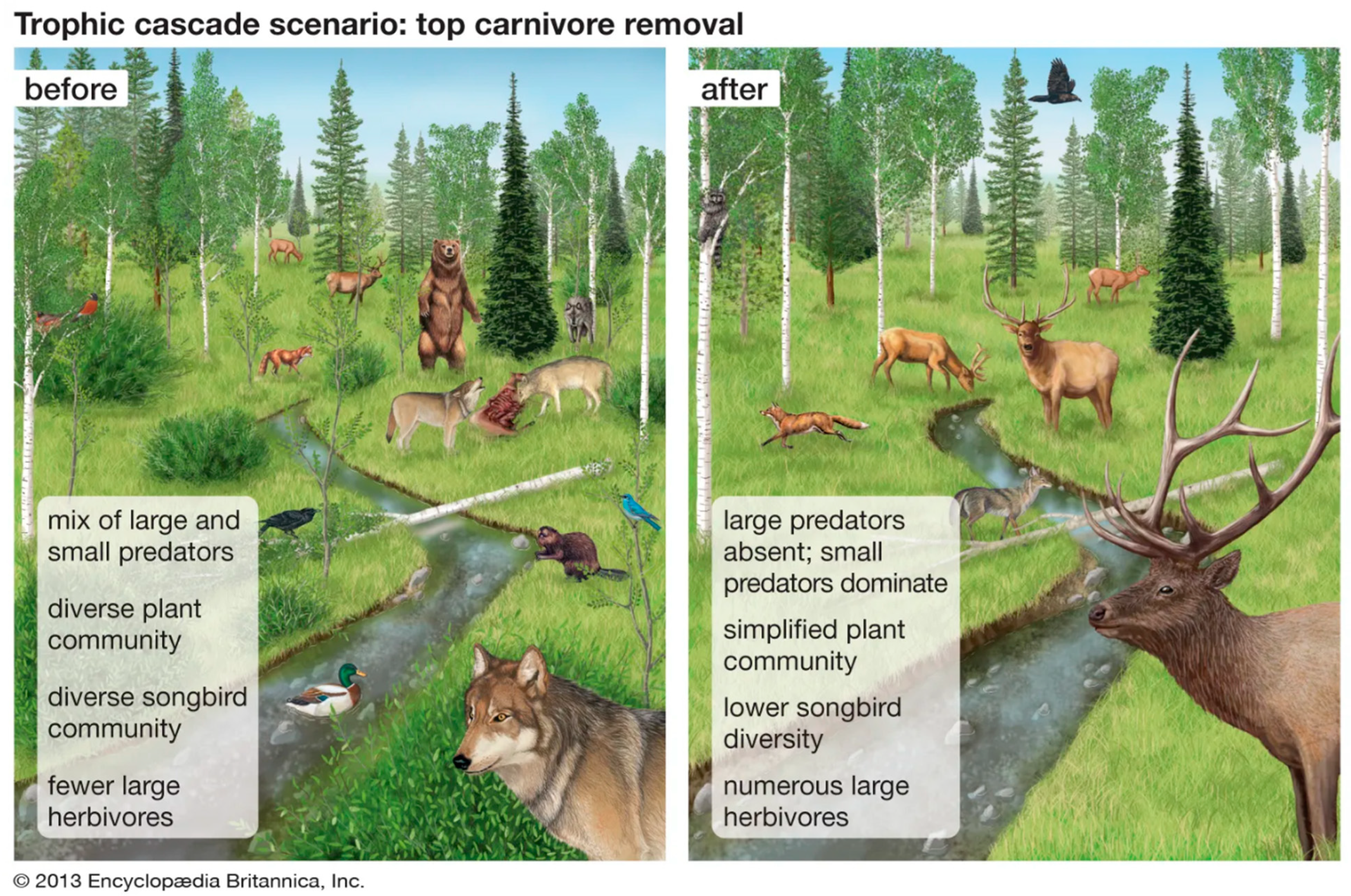
- Ripple, W.J.; Beschta, R.L. Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol. Conserv. 2012, 145, 205–213. [Google Scholar] [CrossRef]
- Kareiva, P.; Estes, J.A.; Marvier, M. Restore protected status for gray wolves. Science 2021, 373, 632. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L.; Wolf, C.; Painter, L.E.; Wirsing, A.J. The strength of the Yellowstone trophic cascade after wolf reintroduction. Glob. Ecol. Conserv. 2025, 58, e03428. [Google Scholar] [CrossRef]
- Kitchell, J.F.; Boggs, C.H.; He, X.; Walters, C.J. Keystone predators in the central Pacific. In Ecosystem Approaches for Fisheries Management; Proceedings of the Symposium on Ecosystem Considerations in Fisheries Management; 1999; pp. 665–683. Available online: https://repository.library.noaa.gov/ (accessed on 8 March 2025).
- Griffith, G.P.; Strutton, P.G.; Semmens, J.M.; Fulton, E.A. Identifying important species that amplify or mitigate the interactive effects of human impacts on marine food webs. Conserv. Biol. 2019, 33, 403–412. [Google Scholar] [CrossRef]
- Motivarash Yagnesh, B.; Fofandi Durga, C.; Dabhi Raj, M.; Makrani Rehanavaz, A.; Tanna Poojaben, D. Importance of sharks in ocean ecosystem. J. Entomol. Zool Stud. 2020, 8, 611–613. [Google Scholar]
- Banks-Leite, C.; Ewers, R.M.; Folkard-Tapp, H.; Fraser, A. Countering the effects of habitat loss, fragmentation, and degradation through habitat restoration. One Earth 2020, 3, 672–676. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Westgate, M.J. Are flagship, umbrella and keystone species useful surrogates to understand the consequences of landscape change? Curr. Landsc. Ecol. Rep. 2020, 5, 76–84. [Google Scholar] [CrossRef]
- Christianou, M.; Ebenman, B. Keystone species and vulnerable species in ecological communities: Strong or weak interactors? J. Theor. Biol. 2005, 235, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ebenman, B.; Jonsson, T. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol. Evol. 2005, 20, 568–575. [Google Scholar] [CrossRef]
- Steneck, R.S.; Graham, M.H.; Bourque, B.J.; Corbett, D.; Erlandson, J.M.; Estes, J.A.; Tegner, M.J. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ. Conserv. J. 2002, 29, 436–459. [Google Scholar] [CrossRef]
- Foster, M.S.; Schiel, D.R. Loss of predators and the collapse of southern California kelp forests: Alternatives, explanations and generalizations. J. Exp. Mar. Biol. Ecol. 2010, 393, 59–70. [Google Scholar] [CrossRef]
- Beschta, R.L.; Ripple, W.J. Riparian vegetation recovery in Yellowstone: The first two decades after wolf reintroduction. Biol. Conserv. 2016, 198, 93–103. [Google Scholar] [CrossRef]
- Atkins, J.L.; Long, R.A.; Pansu, J.; Daskin, J.H.; Potter, A.B.; Stalmans, M.E.; Tarnita, C.E.; Pringle, R.M. Cascading impacts of large-carnivore extirpation in an African ecosystem. Science 2019, 364, 173–177. [Google Scholar] [CrossRef]
- Polis, G.A.; Strong, D.R. Food web complexity and community dynamics. Am. Nat. 1996, 147, 813–846. [Google Scholar] [CrossRef]
- Petchey, O.L.; Downing, A.L.; Mittelbach, G.G.; Persson, L.; Steiner, C.F.; Warren, P.H.; Woodward, G. Species loss and the structure and functioning of multitrophic aquatic systems. Oikos 2004, 10, 467–478. [Google Scholar] [CrossRef]
- Oro, D.; Martínez-Abraín, A. Ecological non-equilibrium and biological conservation. Biol. Conserv. 2023, 286, 110258. [Google Scholar] [CrossRef]
- Mouquet, N.; Gravel, D.; Massol, F.; Calcagno, V. Extending the concept of keystone species to communities and ecosystems. Ecol. Lett. 2013, 16, 1–8. [Google Scholar] [CrossRef]
- Loreau, M.; Mouquet, N.; Gonzalez, A. Biodiversity as spatial insurance in heterogeneous landscapes. Proc. Natl. Acad. Sci. USA 2003, 100, 12765–12770. [Google Scholar] [CrossRef]
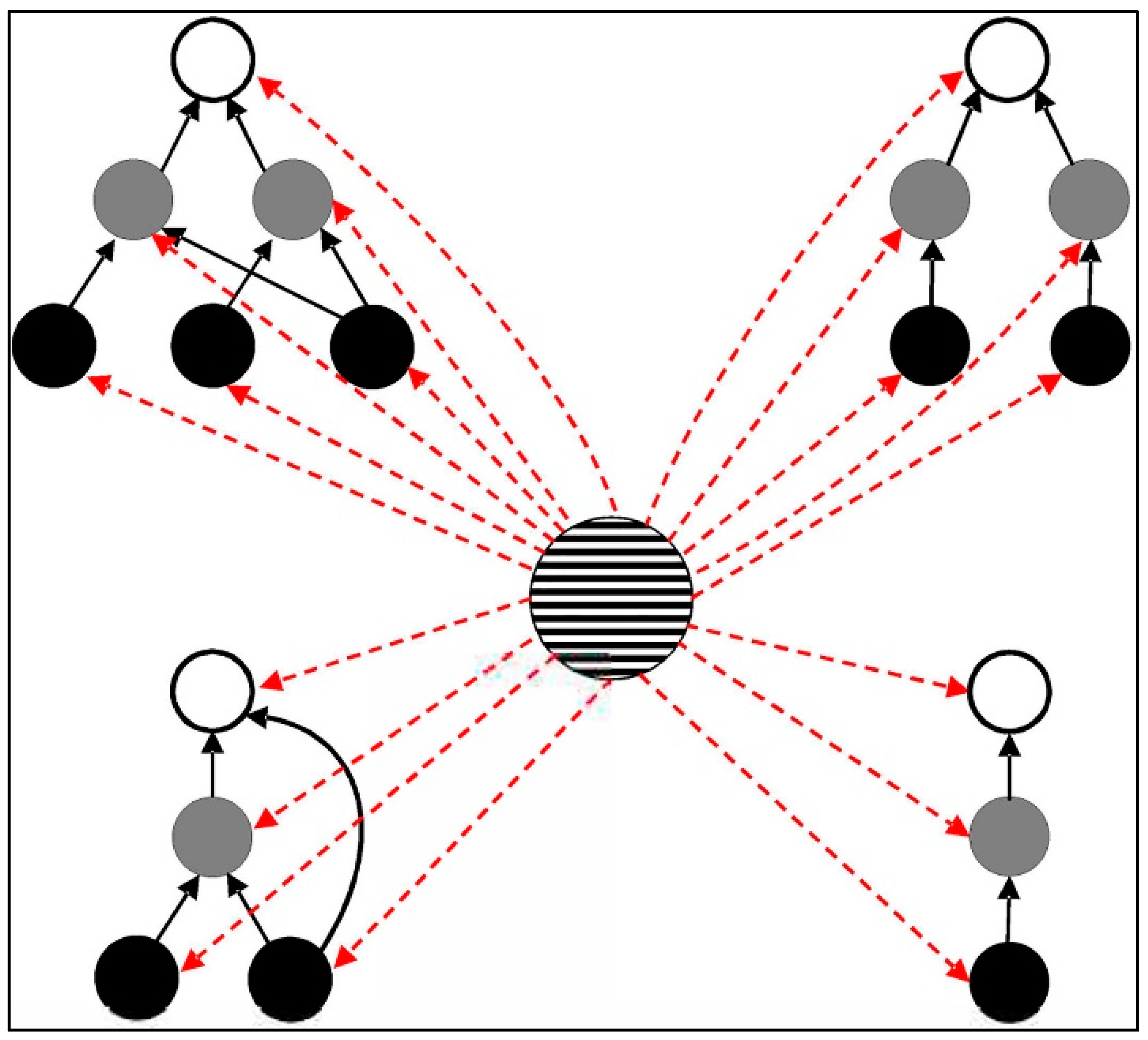
- Leibold, M.A.; Holyoak, M.; Mouquet, N.; Amarasekare, P.; Chase, J.M.; Hoopes, M.F.; Holt, R.D.; Shurin, J.B.; Law, R.; Tilman, D.; et al. The metacommunity concept: A framework for multi-scale community ecology. Ecol. Lett. 2004, 7, 601–613. [Google Scholar] [CrossRef]
- Leibold, M.A.; Norberg, J. Biodiversity in metacommunities: Plankton as complex adaptive systems? Limnol. Oceanogr. 2004, 49, 1278–1289. [Google Scholar] [CrossRef]
- Jamoneau, A.; Chabrerie, O.; Closset-Kopp, D.; Decocq, G. Fragmentation alters beta-diversity patterns of habitat specialists within forest metacommunities. Ecography 2012, 35, 124–133. [Google Scholar] [CrossRef]
- Willig, M.R.; Presley, S.J.; Cullerton, E.I.A. Canonical metacommunity structure over 3 decades: Ecologically consistent but spatially dynamic patterns in a hurricane-prone montane forest. Oecologia 2021, 196, 919–933. [Google Scholar] [CrossRef]
- Resetarits, E.J.; Cathey, S.E.; Leibold, M.A. Testing the keystone community concept: Effects of landscape, patch removal, and environment on metacommunity structure. Ecology 2018, 99, 57–67. [Google Scholar] [CrossRef]
- Yang, X.; Tan, J.; Sun, K.H.; Jiang, L. Experimental demonstration of the importance of keystone communities for maintaining metacommunity biodiversity and ecosystem functioning. Oecologia 2020, 193, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.D.; Thiesen Brum, F.; Houts, M.; Menefee, M.; Williamson, M.; Sterling Krank, L.; Van Pelt, B.; Augustine, D.J. Potential landscapes for conservation of the black-tailed prairie dog ecosystem. Divers. Distrib. 2025, 31, e13945. [Google Scholar] [CrossRef]
- Sierra–Corona, R.; Davidson, A.; Fredrickson, E.L.; Luna-Soria, H.; Suzan-Azpiri, H.; Ponce-Guevara, E.; Ceballos, G. Black-tailed prairie dogs, cattle, and the conservation of North America’s arid grasslands. PLoS ONE 2015, 10, e0118602. [Google Scholar] [CrossRef]
- Magle, S.B.; Ruell, E.W.; Antolin, M.F.; Crooks, K.R. Population genetic structure of black-tailed prairie dogs, a highly interactive species, in fragmented urban habitat. J. Mammal. 2010, 91, 326–335. [Google Scholar] [CrossRef]
- Parker, R.A.; Duchardt, C.J.; Dwyer, A.M.; Painter, C.; Pierce, A.K.; Michels, T.J.; Wunder, M.B. Trophic ecology warrants multispecies management in a grassland setting: Proposed species interactions on black-tailed prairie dog colonies. Rangelands 2019, 41, 135–144. [Google Scholar] [CrossRef]
- Roach, J.L.; Stapp, P.; Van Horne, B.; Antolin, M.F. Genetic structure of a metapopulation of black-tailed prairie dogs. J. Mammal. 2001, 82, 946–959. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Smith, G.A.; Vidal, V. Long-term persistence of prairie dog towns: Insights for designing networks of prairie reserves. Biol. Conserv. 2004, 115, 111–120. [Google Scholar] [CrossRef]
- Jones, P.H.; Britten, H.B. The absence of concordant population genetic structure in the black-tailed prairie dog and the flea, Oropsylla hirsuta, with implications for the spread of Yersinia pestis. Mol. Ecol. 2010, 19, 2038–2049. [Google Scholar] [CrossRef]
- Collinge, S.K.; Johnson, W.C.; Ray, C.; Matchett, R.; Grensten, J.; Cully, J.F., Jr.; Gage, K.L.; Kosoy, M.Y.; Loye, J.E.; Martin, A.P. Landscape structure and plague occurrence in black-tailed prairie dogs on grasslands of the western USA. Landsc. Ecol. 2005, 20, 941–955. [Google Scholar] [CrossRef]
- George, D.B.; Webb, C.T.; Pepin, K.M.; Savage, L.T.; Antolin, M.F. Persistence of black-tailed prairie-dog populations affected by plague in northern Colorado, USA. Ecology 2013, 94, 1572–1583. [Google Scholar] [CrossRef]
- Hoogland, J.L. The Black-Tailed Prairie Dog: Social Life of a Burrowing Mammal; Chicago University Press: Chicago, IL, USA, 1995. [Google Scholar]
- Goguen, C.B. Habitat use by mountain plovers in prairie dog colonies in northeastern New Mexico. J. Field Ornithol. 2012, 83, 154–165. [Google Scholar] [CrossRef]
- Baker, B.W.; Augustine, D.J.; Sedgwick, J.A.; Lubow, B.C. Ecosystem engineering varies spatially: A test of the vegetation modification paradigm for prairie dogs. Ecography 2013, 36, 230–239. [Google Scholar] [CrossRef]
- Duchardt, C.J.; Beck, J.L.; Augustine, D.J. Mountain plover habitat selection and nest survival in relation to weather variability and spatial attributes of black-tailed prairie dog disturbance. Condor 2020, 122, duz059. [Google Scholar] [CrossRef]
- Haun, A.J.; Dreelin, R.A.; Boyce, A.J. Prairie dog towns increase grassland bird diversity at the landscape scale. Wilson J. Ornithol. 2024, 136, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Shaughnessy, M.J., Jr.; Cifelli, R.L. Influence of black-tailed prairie dog towns (Cynomys ludovicianus) on carnivore distributions in the Oklahoma panhandle. West. N. Am. Naturalist. 2004, 64, 184–192. [Google Scholar]
- Shipley, B.K.; Reading, R.P. A comparison of herpetofauna and small mammal diversity on black-tailed prairie dog (Cynomys ludovicianus) colonies and non-colonized grasslands in Colorado. J. Arid Environ. 2006, 66, 27–41. [Google Scholar] [CrossRef]
- Bangert, R.K.; Slobodchikoff, C.N. Conservation of prairie dog ecosystem engineering may support arthropod beta and gamma diversity. J. Arid Environ. 2006, 67, 100–115. [Google Scholar] [CrossRef]
- Goodrich, J.M.; Buskirk, S.W. Spacing and ecology of North American badgers (Taxidea taxus) in a prairie-dog (Cynomys leucurus) complex. J. Mammal. 1998, 79, 171–179. [Google Scholar] [CrossRef]
- Eads, D.A.; Biggins, D.E. Aboveground predation by an American badger (Taxidea taxus) on black-tailed prairie dogs (Cynomys ludovicianus). West. N. Am. Naturalist. 2008, 68, 396–401. [Google Scholar] [CrossRef]
- Grassel, S.M.; Rachlow, J.L. When generalists behave as specialists: Local specialization by American badgers (Taxidea taxus). Can. J. Zool. 2018, 96, 592–599. [Google Scholar] [CrossRef]
- Livieri, T.M.; Forrest, S.C.; Matchett, M.R.; Breck, S. Conserving endangered black-footed ferrets: Biological threats, political challenges, and lessons learned. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: New York, NY, USA, 2021. [Google Scholar]
- Rocke, T.E. 13 Plague and distemper threats to black-footed ferret conservation. In Wildlife Disease and Health in Conservation; Jessup, D.A., Radcliff, R.W., Eds.; John Hopkins University Press: Baltimore, MD, USA, 2023; pp. 217–236. [Google Scholar]
- Davidson, A.D.; Detling, J.K.; Brown, J.H. Ecological roles and conservation challenges of social, burrowing, herbivorous mammals in the world’s grasslands. Front. Ecol. Environ. 2012, 10, 477–486. [Google Scholar] [CrossRef]
- Klute, D.S.; Ayers, L.W.; Green, M.T.; Howe, W.H.; Jones, S.L.; Sohaffer, J.A.; Sheffield, S.R.; Zimmerman, T.S. Status Assessment and Conservation Plan for the Western Burrowing Owl in the United States; Biological Technical Publication FWS/BTP-R6001-2003; U.S. Department of Interior, Fish and Wildlife Service: Washington, DC, USA, 2003.
- Conway, C.J. Spatial and temporal patterns in population trends and burrow usage of burrowing owls in North America. J. Raptor. Res. 2018, 52, 129–142. [Google Scholar] [CrossRef]
- Johnson, W.C.; Collinge, S.K. Landscape effects on black-tailed prairie dog colonies. Biol. Conserv. 2004, 115, 487–497. [Google Scholar] [CrossRef]
- Windell, R.M.; Bailey, L.L.; Livieri, T.M.; Eads, D.A.; Biggins, D.E.; Breck, S.W. Coyote use of prairie dog colonies is most frequent in areas used by American badgers. J. Mammal. 2024, 105, 1309–1321. [Google Scholar] [CrossRef]
- Butler, A.R.; Bly, K.L.S.; Harris, H.; Inman, R.M.; Moehrenschlager, A.; Schwalm, D.; Jachowski, D.S. Life on the edge: Habitat fragmentation limits expansion of a restored carnivore. Anim. Conserv. 2021, 24, 108–119. [Google Scholar] [CrossRef]
- Sackett, L.C.; Cross, T.B.; Jones, R.T.; Johnson, W.C.; Ballare, K.; Ray, C.; Collinge, S.K.; Martin, A.P. Connectivity of prairie dog colonies in an altered landscape: Inferences from analysis of microsatellite DNA variation. Conserv. Genet. 2012, 13, 407–418. [Google Scholar] [CrossRef]
- Castellanos-Morales, G.; Gasca-Pineda, J.; Ceballos, G.; Ortega, J. Genetic variation in a peripheral and declining population of black-tailed prairie dogs (Cynomys ludovicianus) from Mexico. J. Mammal. 2014, 95, 467–479. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity losses and ecosystem function in freshwaters: Emerging conclusions and research directions. BioScience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Pires, M.M.; Benchimol, M.; Cruz, L.R.; Peres, C.A. Terrestrial food web complexity in Amazonian forests decays with habitat loss. Curr. Biol. 2023, 33, 389–396. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Hitchman, S.M.; Mather, M.E.; Smith, J.M.; Fencl, J.S. Identifying keystone habitats with a mosaic approach can improve biodiversity conservation in disturbed ecosystems. Glob. Change Biol. 2018, 24, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Regolin, A.L.; Ribeiro, M.C.; Martello, F.; Melo, G.L.; Sponchiado, J.; Campanha, L.F.D.C.; Sugae, L.S.M.; Silva, T.S.F.; Cáceres, N.C. Spatial heterogeneity and habitat configuration overcome habitat composition influences on alpha and beta mammal diversity. Biotropica 2020, 52, 969–980. [Google Scholar] [CrossRef]
- Dufour, A.; Gadallah, F.; Wagner, H.H.; Guisan, A.; Buttler, A. Plant species richness and environmental heterogeneity in a mountain landscape: Effects of variability and spatial configuration. Ecography 2006, 29, 573–584. [Google Scholar] [CrossRef]
- Costanza, J.K.; Moody, A.; Peet, R.K. Multi-scale environmental heterogeneity as a predictor of plant species richness. Landsc. Ecol. 2011, 26, 851–864. [Google Scholar] [CrossRef]
- Udy, K.; Fritsch, M.; Meyer, K.M.; Grass, I.; Hanß, S.; Hartig, F.; Kneib, T.; Kreft, H.; Kukunda, C.B.; Pe’er, G.; et al. Environmental heterogeneity predicts global species richness patterns better than area. Glob. Ecol. Biogeogr. 2021, 30, 842–851. [Google Scholar] [CrossRef]
- Agra, J.; Cornelissen, T.; Viana-Junior, A.B.; Callisto, M. A global synthesis and meta-analysis of the environmental heterogeneity effects on the freshwater biodiversity. Oikos 2024, e10186. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Stein, A. Environmental heterogeneity–species richness relationships from a global perspective. Front. Biogeogr. 2015, 7, 4. [Google Scholar]
- Seiferling, I.; Proulx, R.; Wirth, C. Disentangling the environmental-heterogeneity–species-diversity relationship along a gradient of human footprint. Ecology 2014, 95, 2084–2095. [Google Scholar] [CrossRef]
- de Lima Filho, J.A.; Vieira, R.J.; de Souza, C.A.; Ferreira, F.F.; de Oliveira, V.M. Effects of habitat fragmentation on biodiversity patterns of ecosystems with resource competition. Phys. A Stat. Mech. Its Appl. 2021, 564, 125497. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.-J.; Marquet, P.A. Environmental heterogeneity as a driver of terrestrial biodiversity on a global scale. Prog. Phys. Geog. 2023, 47, 912–930. [Google Scholar] [CrossRef]
- Albrecht, J.; Peters, M.K.; Becker, J.N.; Behler, C.; Classen, A.; Ensslin, A.; Ferger, S.W.; Gebert, F.; Gerschlauer, F.; Helbig-Bonitz, M.; et al. Species richness is more important for ecosystem functioning than species turnover along an elevational gradient. Nat. Ecol. Evol. 2021, 5, 1582–1593. [Google Scholar] [CrossRef]
- Feld, C.K.; Martins da Silva, P.; Paulo Sousa, J.; De Bello, F.; Bugter, R.; Grandin, U.; Hering, D.; Lavorel, S.; Mountford, O.; Pardo, I.; et al. Indicators of biodiversity and ecosystem services: A synthesis across ecosystems and spatial scales. Oikos 2009, 118, 1862–1871. [Google Scholar] [CrossRef]
- Gonzalez, A.; Germain, R.M.; Srivastava, D.S.; Filotas, E.; Dee, L.E.; Gravel, D.; Thompson, P.L.; Isbell, F.; Wang, S.; Kéfi, S.; et al. Scaling-up biodiversity-ecosystem functioning research. Ecol. Lett. 2020, 23, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.L.; Kéfi, S.; Zelnik, Y.R.; Dee, L.E.; Wang, S.; de Mazancourt, C.; Loreau, M.; Gonzalez, A. Scaling up biodiversity–ecosystem functioning relationships: The role of environmental heterogeneity in space and time. Proc. Roy. Soc. B-Biol. Sci. 2021, 288, 20202779. [Google Scholar] [CrossRef]
- Fahrig, L.; Nuttle, W.K. Population ecology in spatially heterogeneous environments. In Ecosystem Function in Heterogeneous Landscapes; Lovett, G.M., Turner, M.G., Jones, C.G., Weathers, K.C., Eds.; Springer: New York, NY, USA, 2005; pp. 95–118. [Google Scholar]
- Laanisto, L.; Tamme, R.; Hiiesalu, I.; Szava-Kovats, R.; Gazol, A.; Pärtel, M. Microfragmentation concept explains non-positive environmental heterogeneity–diversity relationships. Oecologia 2013, 171, 217–226. [Google Scholar] [CrossRef]
- Foster, E.; Love, J.; Rader, R.; Reid, N.; Drielsma, M.J. Integrating a generic focal species, metapopulation capacity, and connectivity to identify opportunities to link fragmented habitat. Landsc. Ecol. 2017, 32, 1837–1847. [Google Scholar] [CrossRef]
- Wang, S.; Brose, U.; van Nouhuys, S.; Holt, R.D.; Loreau, M. Metapopulation capacity determines food chain length in fragmented landscapes. Proc. Natl. Acad. Sci. USA 2021, 118, e2102733118. [Google Scholar] [CrossRef]
- Brodie, J.F.; Mohd-Azlan, J.; Schnell, J.K. How individual links affect network stability in a large-scale, heterogeneous metacommunity. Ecology 2016, 97, 1658–1667. [Google Scholar] [CrossRef]
- Loreau, M.; Mouquet, N.; Holt, R.D. From metacommunities to metaecosystems. In Metacommunities: Spatial Dynamics and Ecological Communities; Holyoak, M., Leibold, M.A., Holt, R.D., Eds.; University of Chicago Press: Chicago, IL, USA, 2005; pp. 418–438. [Google Scholar]
- Clark, W. Principles of Landscape Ecology. In Nature Education Knowledge; Island Press: Washington, DC, USA, 2010; Volume 3, p. 34. Available online: https://www.nature.com/scitable/knowledge/ (accessed on 8 March 2025).
- Holderegger, R.; Wagner, H.H. Landscape genetics. Bioscience 2008, 58, 199–207. [Google Scholar] [CrossRef]
- Richardson, J.L.; Brady, S.P.; Wang, I.J.; Spear, S.F. Navigating the pitfalls and promise of landscape genetics. Mol. Ecol. 2016, 25, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Borokini, I.T.; Klingler, K.B.; Peacock, M.M. Life in the desert: The impact of geographic and environmental gradients on genetic diversity and population structure of Ivesia webberi. Ecol. Evol. 2021, 11, 17537–17556. [Google Scholar] [CrossRef]
- Manel, S.; Joost, S.; Epperson, B.K.; Holderegger, R.; Storfer, A.; Rosenberg, M.S.; Scribner, K.T.; Bonin, A.; Fortin, M.J. Perspectives on the use of landscape genetics to detect genetic adaptive variation in the field. Mol. Ecol. 2010, 19, 3760–3772. [Google Scholar] [CrossRef]
- Manel, S.; Holderegger, R. Ten years of landscape genetics. Trends Ecol. Evol. 2013, 28, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Vellend, M.; Geber, M.A. Connections between species diversity and genetic diversity. Ecol. Lett. 2005, 8, 767–781. [Google Scholar] [CrossRef]
- Struebig, M.J.; Kingston, T.; Petit, E.J.; Le Comber, S.C.; Zubaid, A.; Mohd-Adnan, A.; Rossiter, S.J. Parallel declines in species and genetic diversity in tropical forest fragments. Ecol. Lett. 2011, 14, 582–590. [Google Scholar] [CrossRef]
- Bragg, J.G.; Supple, M.A.; Andrew, R.L.; Borevitz, J.O. Genomic variation across landscapes: Insights and applications. New Phytol. 2015, 207, 953–967. [Google Scholar] [CrossRef]
- Balkenhol, N.; Dudaniec, R.Y.; Krutovsky, K.V.; Johnson, J.S.; Cairns, D.M.; Segelbacher, G.; Selkoe, K.A.; von der Hayden, S.; Wang, I.J.; Selmoni, O.; et al. Landscape genomics: Understanding relationships between environmental heterogeneity and genomic characteristics of populations. In Population Genomics: Concepts, Approaches and Applications; Rajora, O.P., Ed.; Springer: New York, NY, USA, 2019; pp. 261–322. [Google Scholar]
- Hand, B.K.; Lowe, W.H.; Kovach, R.P.; Muhlfeld, C.C.; Luikart, G. Landscape community genomics: Understanding eco-evolutionary processes in complex environments. Trends Ecol. Evol. 2015, 30, 161–168. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Mao, R.L.; Yang, J.; Miao, C.Y.; Li, Z.; Qiu, Y.X. Ten years of landscape genomics: Challenges and opportunities. Front. Plant Sci. 2017, 8, 2136. [Google Scholar] [CrossRef]
- Segelbacher, G.; Cushman, S.A.; Epperson, B.K.; Fortin, M.J.; Francois, O.; Hardy, O.J.; Holderegger, R.; Taberlet, P.; Waits, L.P.; Manel, S. Applications of landscape genetics in conservation biology: Concepts and challenges. Conserv. Genet. 2010, 11, 375–385. [Google Scholar] [CrossRef]
- Chambers, E.A.; Bishop, A.P.; Wang, I.J. Individual-based landscape genomics for conservation: An analysis pipeline. Mol. Ecol. Resour. 2023. Available online: https://doi-org.unr.idm.oclc.org/10.1111/1755-0998.13884 (accessed on 8 March 2025). [CrossRef] [PubMed]
- Alahuhta, J.; Tukiainen, H.; Toivanen, M.; Ala-Hulkko, T.; Farrahi, V.; Hjort, J.; Ikäheimo, T.M.; Lankila, T.; Maliniemi, T.; Puhakka, S.; et al. Acknowledging geodiversity in safeguarding biodiversity and human health. Lancet. Planet Health 2022, 6, e987–e992. [Google Scholar] [CrossRef]
- Crisp, J.R.; Ellison, J.C.; Fischer, A.; Tan, J.S. Geodiversity inclusiveness in biodiversity assessment. Prog. Phys. Geog. 2023, 47, 414–437. [Google Scholar] [CrossRef]
- Hjort, J.; Gordon, J.E.; Gray, M.; Hunter, M.L., Jr. Why geodiversity matters in valuing nature’s stage. Conserv. Biol. 2015, 29, 630–639. [Google Scholar] [CrossRef]
- Sutton, P.C.; Anderson, S.J.; Costanza, R.; Kubiszewski, I. The ecological economics of land degradation: Impacts on ecosystem service values. Ecol. Econ. 2016, 129, 182–192. [Google Scholar] [CrossRef]
- Haines-Young, R.; Potschin, M. The links between biodiversity, ecosystem services and human well-being. In Ecosystem Ecology: A New Synthesis; Raffaelli, D.G., Frid, C.L.J., Eds.; Cambridge University Press: Cambridge, UK, 2010; pp. 110–139. [Google Scholar]
- Mestre, F. Synergistic effects of climate change and habitat fragmentation on species range shifts and metapopulation persistence. Front. Biogeogr. 2018, 9. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Change Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Gahlawat, I.N.; Lakra, P. Global Climate change and its effects. JISS 2020, 7, 14–23. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peacock, M.M. Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity 2025, 17, 200. https://doi.org/10.3390/d17030200
Peacock MM. Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity. 2025; 17(3):200. https://doi.org/10.3390/d17030200
Chicago/Turabian StylePeacock, Mary M. 2025. "Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here?" Diversity 17, no. 3: 200. https://doi.org/10.3390/d17030200
APA StylePeacock, M. M. (2025). Negotiating a Fragmented World: What Do We Know, How Do We Know It, and Where Do We Go from Here? Diversity, 17(3), 200. https://doi.org/10.3390/d17030200






