A New Species of Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Mesenteries of Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Upper Ouachita River Drainage, Arkansas, USA †
Abstract
1. Introduction
2. Methods
2.1. Collection and Necropsy
2.2. Morphological Characterization
2.3. Histology
2.4. Molecular Characterization
3. Results
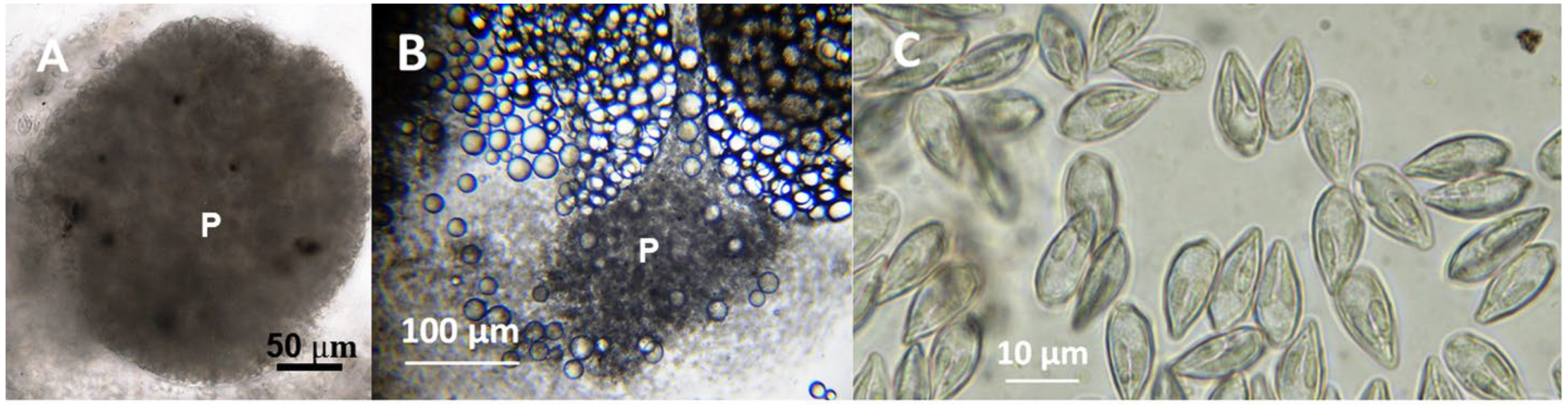
3.1. Description of the New Species
| Species | Fundulus spp. Host | PLS | PLL | PLW | MXS | MXL | MXW | MXT | PCS | LPCL | LPCW | SPCL | SPCW | PTC | ME | IV | SM | IP | Type/ Voucher Specimens | DNA | Site | Locality | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. bilineatum Bond, 1938 | F. heteroclitus | - | (250–1000) | - | Broadly obovoid | 10.5 (10–12) | (9–10) | (9–10) | Nearly globular | - | - | - | - | (7–9) | - | P | - | - | - | - | Brain, kidney, urinary, | MD, NY | [26] |
| gall bladder, gill arch | |||||||||||||||||||||||
| M. diaphanus (Fanthum, Porter, and Richardson, 1940), Landsberg and Lom, 1991 | F. diaphanus | - | - | Elong. ovoid-pyriform | (15.5–20) | (5.2–7.6) | - | Elong. pyriform | (7.4–9.6) | (1.5–2.2) | (7.4–9.6) | (1.5–2.2) | (11–15) | A | P | P | - | AY950664 | Testes | NS | [16,28,29,30] | ||
| M. diaphanus (Fanthum, Porter, and Richardson, 1940), Landsberg and Lom, 1991 | F. diaphanus | Round to elongate | <500 | - | Elong. pyriform | 19.1 (17.0–21.8) | 7.2 (6.8–7.7) | 5.8 | Elong. pyriform | 8.5 (7.4–9.8) | 2.3 (1.9–2.7) | - | - | (7–10) | A | A | P | - | USNM 95334 | AY950664 | Numerous organs | NS | [31] |
| M. diaphanus (Fanthum, Porter, and Richardson, 1940), Landsberg and Lom, 1991 | F. diaphanus | - | - | - | Elong. oval | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Numerous organs | MD, NS, ON | [32] |
| M. funduli (= M. musculi Hahn, 1915) Hahn, 1915, Kudo, 1920 | F. heteroclitus, F. majalis | - | - | - | Pyriform | 14.3 | 6.7 | 6.7 | Elong. Pyriform | 6.5 | 2 | - | - | (10–14) | - | P | - | - | - | - | Muscle, gills, connective tissue | MA | [29,30,33,34,35,36] |
| M. funduli (= Myxosoma funduli Kudo, 1918) (Kudo, 1918) | F. heteroclitus, F. majalis | Spherical | 150 (?–360) | - | Pyriform | 14 | 8 | 6 | Elong. Pyriform | 8 | 2 | - | - | - | - | A | P (7–10) | - | - | - | Gill lamellae | MA | [29,30,36,37] |
| M. funduli (= Myxosoma funduli Kudo, 1918) (Kudo, 1918) | F. heteroclitus, F. majalis, F. diaphanus | - | - | - | - | - | - | - | - | - | - | - | - | - | - | A | - | - | - | - | Gill lamellae | MD, NY | [26] |
| M. funduli (= Myxosoma funduli Kudo, 1918) (Kudo, 1918) | F. heteroclitus, F. majalis, F. diaphanus | - | 150 (?–360) | - | Pyriform | - | - | - | - | - | - | - | - | - | - | - | P (7–10) | - | - | - | Gills | MA, MD | [38] |
| M. funduli (= Myxosoma funduli Kudo, 1918) (Kudo, 1918) | F. kansae | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Gills | NE | [39,40,41] |
| M. funduli (= Myxosoma funduli Kudo, 1918) (Kudo, 1918) | F. Diaphanus | - | - | - | Pyriform | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Gill lamellae | MD, NB, NS | [32] |
| M. hudsonius (Bond, 1938), Landsberg and Lom, 1991, two morphs | F. heteroclitus | - | (230–305) | (170–260) | Scales at base of fins | NY | [26,29,30] | ||||||||||||||||
| Type A | Pyriform | - | - | - | Pyriform | (4–5) | (2–2.5) | (7–9) | - | A | A | - | - | - | |||||||||
| Type B | Obovoid | (11.5–12.5) | 7 | - | Pyriform | (4–5) | (2–2.5) | (7–9) | - | A | A | - | - | - | |||||||||
| M. neurophilus (Guilford, 1963) | F. heteroclitus, Perca flavescens | Diffuse | - | - | - | 11.8 | 4.5 | - | - | 5.6 | - | - | - | - | - | - | - | - | - | - | Optic tectum, spinal cord | NS | [42] |
| M. storeri sp. n. | F. olivaceus | Round to ovoid, or diffuse | 394 (135–699) | 270 (90–516) | Pyriform | 15.0 (13.1–16.9) | 7.1 (6.2–8.2) | 5.5 (4.5–5.8) | Narrowly pyriform | 8.6 (7.3–9.4) | 1.9 (1.6–2.2) | 8.2 (6.9–9.4) | 1.8 (1.3–2.2) | 9 (7–10) | A | - | P (2–6) | A | HWML 217936 | PQ776916 | Mesenteries | AR | Present study |
| M. subtecalis (Bond, 1938); Landsberg and Lom, 1991 | F. heteroclitus | Diffuse | (50–300) | Pyriform | (15–18) | (6.5–8) | 6 | Elong. pyriform | (7–8) | 2 | (11–12) | - | A | - | - | - | - | Viscera, brain, fins | MD, NY | [26,29,30,43] | |||
| Myxobolus sp. | F. chrysotus | - | - | - | Ovoidal | 16 (14–16) | 8 (7–9) | - | Deltoid-Narrowly pyriform | 9 (8–10) | 3 (2–3) | 8 (7–9) | 3 (2–3) | - | - | - | - | - | - | MH626584 | Peritoneum | AR | [44] |
| F. chrysotus | Spheroidal | (200–400) | Ovoidal | 17 (15–18) | 8 (7–9) | - | Deltoid-Narrowly pyriform | 9 (8–10) | 3 (2–3) | 8 (7–9) | 3 (2–3) | - | - | - | - | - | HWML139415 | - | Gill | AR | [44] | ||
| Myxobolus sp. | F. diaphanus | Diffuse mass | - | - | Round | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | Connective tissue of the brain, skull | NS | Cone et al. [32] |
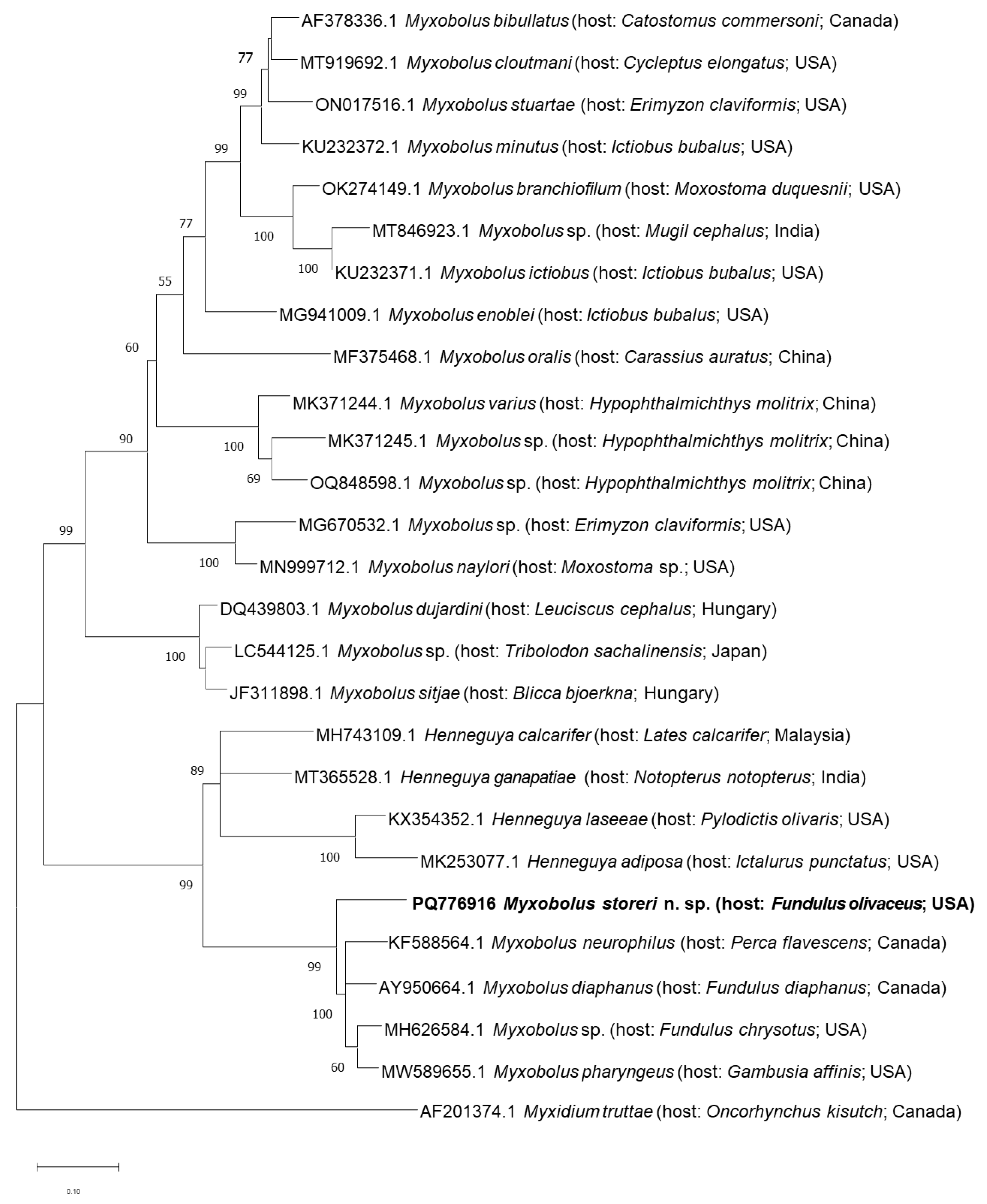
3.2. Molecular Characterization and Phylogenetic Analysis
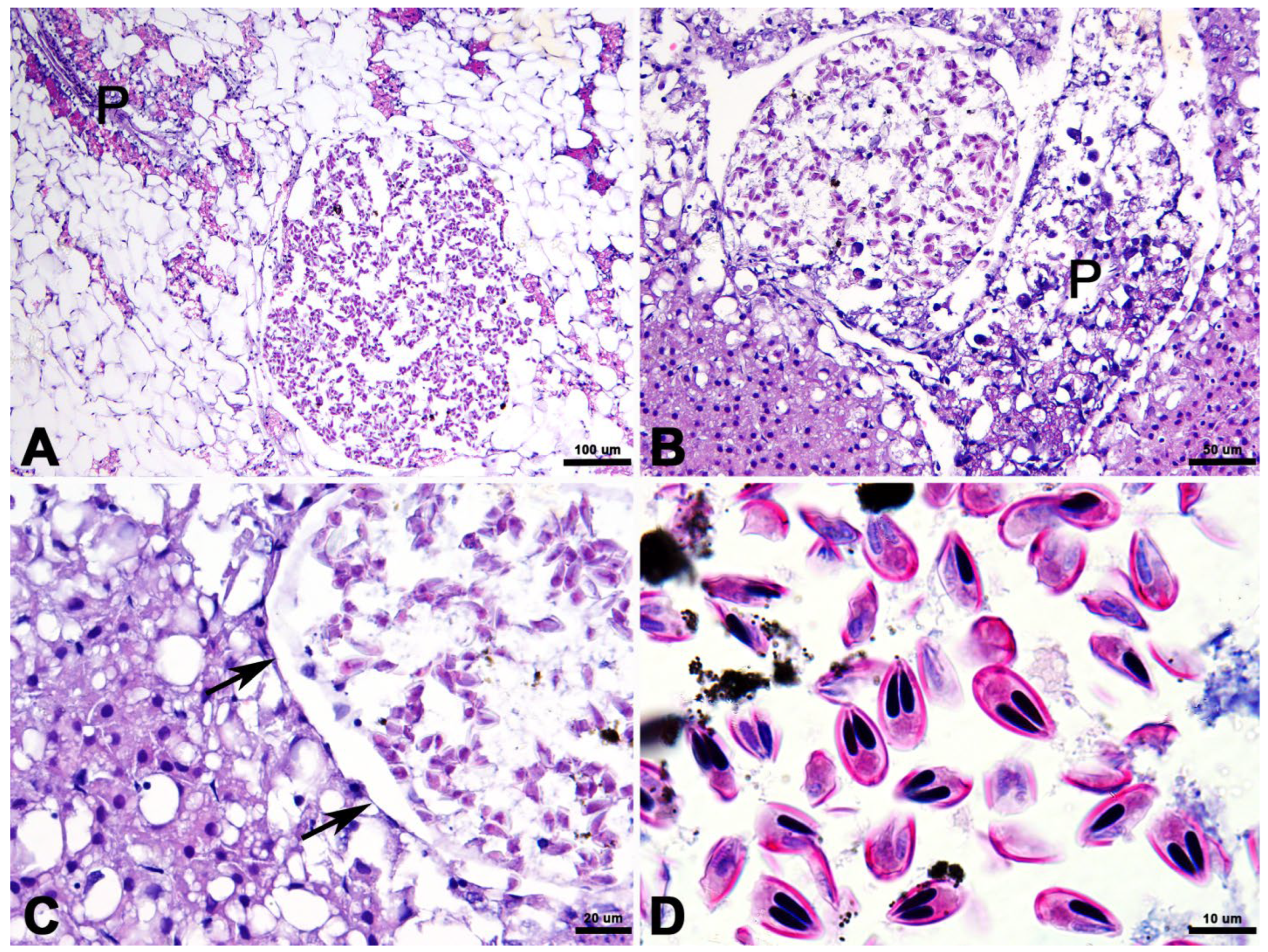
3.3. Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eiras, J.C. An overview on the myxosporean parasites in amphibians and reptiles. Acta Parasitol. 2005, 50, 267–275. [Google Scholar]
- Fiala, I.; Bartoŝová-Sojková, P.; Whipps, C.M. Classification and Phylogenetics of Myxozoa. In Myxozoan Evolution, Ecology and Development; Okamura, B., Gruhl, A., Bartholomew, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Bartholomew, J.L.; Atkinson, S.D.; Hallett, S.L.; Lowenstine, L.J.; Garner, M.M.; Gardiner, C.H.; Rideout, B.A.; Keel, M.K.; Brown, J.D. Myxozoan parasitism in waterfowl. Int. J. Parasitol. 2008, 38, 1199–1207. [Google Scholar] [CrossRef]
- Prunescu, C.-C.; Prunescu, P.; Lom, J. The first finding of myxosporean development from plasmodia to spores in terrestrial mammals: Soricimyxum fegati gen. et sp. n. (Myxozoa) from Sorex araneus (Soricomorpha). Folia Parasitol. 2007, 54, 159–164. [Google Scholar] [CrossRef]
- Székely, C.; Cech, G.; Atkinson, S.D.; Kálmán, M.; Egyed, L.; Gubányi, A. A novel myxozoan parasite of terrestrial mammals: Description of Soricimyxum minuti sp. n. (Myxosporea) in pygmy shrew Sorex minutus from Hungary. Folia Parasitol. 2015, 62, 45–49. [Google Scholar] [CrossRef][Green Version]
- Kent, M.L.; Margolis, L.; Corliss, J.O. The demise of a class of protists: Taxonomic and nomenclatural revisions proposed for the protist phylum Myxozoa Grasse, 1970. Can. J. Zool. 1994, 72, 932–937. [Google Scholar] [CrossRef]
- Eiras, J.C.; Molnár, K.; Lu, Y.S. Synopsis of the species of Myxobolus Bütschli, 1882. Parasitology 2005, 88, 11–36. [Google Scholar]
- Eiras, J.C.; Zhang, J.; Molnár, K. Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea: Myxobolidae) described between 2005 and 2013. Syst. Parasitol. 2014, 88, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C.; Cruz, C.F.; Saraiva, A.; Adriano, E.A. Synopsis of the species of Myxobolus (Cnidaria, Myxozoa, Myxosporea) described between 2014 and 2020. Folia Parasitol. 2021, 68, 012. [Google Scholar] [CrossRef]
- Page, L.M.; Burr, B.M. Peterson Field Guide to Freshwater Fishes of North America North of Mexico, 2nd ed.; Houghton Mifflin Harcourt: New York, NY, USA, 2011. [Google Scholar]
- Shute, J.R. Fundulus olivaceus. In Atlas of North American Freshwater Fishes; Lee, D.S., Gilbert, C.R., Hocutt, C.H., Jenkins, R.E., McAllister, D.E., Stauffer, J.R., Jr., Eds.; North Carolina State Museum of Natural History: Raleigh, NC, USA, 1980. [Google Scholar]
- Robison, H.W.; Buchanan, T.M. Fishes of Arkansas, 2nd ed.; University of Arkansas Press: Fayetteville, AR, USA, 2020. [Google Scholar]
- Rice, L.A. The food of seventeen Reelfoot Lake fishes in 1941. J. Tenn. Acad. Sci. 1942, 17, 4–13. [Google Scholar]
- Ross, S.T. The Inland Fishes of Mississippi; University Press of Mississippi: Jackson, MS, USA, 2001. [Google Scholar]
- Wellborn, T.L., Jr.; Rogers, W.A. Five new species of Gyrodactylus (Trematoda: Monogenea) from the southeastern U.S. J. Parasitol. 1967, 53, 10–14. [Google Scholar] [CrossRef]
- Hoffman, G.L. Parasites of North American Freshwater Fishes, 2nd ed.; Comstock Publishing Associates: Ithaca, NY, USA, 1999. [Google Scholar]
- McAllister, C.T.; Bursey, C.R.; Fayton, T.J.; Font, W.F.; Robison, H.W.; Connior, M.B.; Cloutman, D.G. Helminth parasites of the blackspotted topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Interior Highlands of Arkansas. J Ark. Acad. Sci. 2015, 69, 135–138. [Google Scholar] [CrossRef]
- Use of Fishes in Research Committee (Joint Committee of the American Fisheries Society, the American Institute of Fishery Research Biologists, and the American Society of Ichthyologists and Herpetologists). Guidelines for the Use of Fishes in Research. 2014. Available online: https://fisheries.org/docs/wp/Guidelines-for-Use-of-Fishes.pdf (accessed on 1 July 2024).
- Lom, J.; Arthur, J.R. A guideline for the preparation of species description in Myxosporea. J. Fish Dis. 1989, 12, 151–156. [Google Scholar] [CrossRef]
- Clopton, R.E. Standard nomenclature and metrics of plane shapes for use in gregarine taxonomy. Comp. Parasitol. 2004, 71, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Page, L.M.; Bemis, K.E.; Dowling, T.E.; Espinosa-Pérez, H.S.; Findley, L.T.; Gilbert, C.R.; Hartel, K.E.; Lea, R.N.; Mandrak, N.E.; Neighbors, M.A.; et al. Common and Scientific Names of Fishes from the United States, Canada, and Mexico, 8th ed.; Special Publication 37; American Fisheries Society: Bethesda, MD, USA, 2023. [Google Scholar]
- Leis, E.M.; Rosser, T.G.; Baumgartner, W.A.; Griffin, M.J. Two novel myxozoans from pirate perch Aphredoderus sayanus (Gilliams, 1824) in the Upper Mississippi River, including the first North American species of Hennegoides Lom, Tonguthai, & Dykova, 1991. J. Parasitol. 2019, 105, 918–927. [Google Scholar] [PubMed]
- ICZN (International Commission on Zoological Nomenclature). Declaration 45—Addition of recommendations to Article 73 and of the term “specimen, preserved” to the glossary. Bull. Zool. Nomen. 2017, 73, 96–97. [Google Scholar] [CrossRef]
- Lom, J.; Dyková, I. Myxozoan genera: Definition and notes on taxonomy, life-cycle, terminology and pathogenic species. Folia Parasitol. 2006, 53, 1–36. [Google Scholar] [CrossRef]
- Bond, F.F. Cnidosporidia from Fundulus heteroclitus Lin. Trans. Amer. Micros. Soc. 1938, 57, 107–122. [Google Scholar] [CrossRef]
- Scudder, S.H. David Humphreys Storer. Proc. Amer. Acad. Arts Sci. 1891, 27, 388–391. [Google Scholar]
- Fantham, H.B.; Porter, A.; Richardson, L.R. Some more Myxosporidia observed in Canadian fishes. Parasitology 1940, 32, 333–353. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Lom, J. Taxonomy of the genera of the Myxobolus/Myxosoma group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst. Parasitol. 1991, 18, 165–186. [Google Scholar] [CrossRef]
- Cone, D.K.; Raesly, R.L. Redescription of Myxobolus rhinichthidis (Myxosporea) parasitizing Rhinichthys cataractae, with a revised taxonomic list of species of Myxobolus known from North American freshwater fishes. Can. J. Fish. Aquat. Sci. 1995, 52, 7–12. [Google Scholar] [CrossRef]
- Cone, D.; Easy, R. Supplemental diagnosis and molecular taxonomy of Myxobolus diaphanus (Fantham, Porter et Richardson, 1940) (Myxozoa) parasitizing Fundulus diaphanus (Cyprinodontiformes) in Nova Scotia, Canada. Folia Parasitol. 2005, 52, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Cone, D.K.; Marcogliese, D.J.; Barse, A.M.; Burt, M.D.B. The myxozoan fauna of Fundulus diaphanus (Cyprinodontidae) from freshwater localities in eastern North America: Prevalence, community structure, and geographic distribution. J. Parasitol. 2006, 92, 52–57. [Google Scholar] [CrossRef]
- Hahn, C.W. Sporozoon parasites of certain fishes in the vicinity of Woods Hole, Massachusetts. Bull. U. S. Bureau Fish. 1915, 33, 191–214. [Google Scholar]
- Hahn, C.W. On the sporozoon parasites of the fishes of Woods Hole and Vicinity. I. Further observations on Myxobolus musculi from Fundulus. J. Parasitol. 1917, 3, 91–104. [Google Scholar] [CrossRef]
- Hahn, C.W. On the sporozoon parasites of the fishes of Woods Hole and Vicinity. II. Additional observations on Myxobolus musculi. J. Parasitol. 1917, 3, 150–162. [Google Scholar] [CrossRef]
- Kudo, R. Contributions to the study of parasitic Protozoa. IV. Note on some Myxosporidia from certain fish in the vicinity of Woods Hole. J. Parasitol. 1918, 25, 11–16. [Google Scholar]
- Kudo, R. Studies on Myxosporidia. A synopsis of genera and species of myxosporidia. Ill. Biol. Monogr. 1920, 5, 1–265. [Google Scholar]
- Hoffman, G.L.; Putz, R.E.; Dunbar, C.E. Studies on Myxosoma cartilaginis n. sp. (Protozoa: Myxosporidia) of centrarchid fish and a synopsis of the Myxosoma of North American freshwater fishes. J. Protozool. 1965, 12, 319–332. [Google Scholar] [CrossRef]
- Adams, A.M. Parasites on the gills of the plains killifish, Fundulus kansae, in the South Platte River, Nebraska. Trans. Am. Microscop. Soc. 1985, 104, 278–284. [Google Scholar] [CrossRef]
- Janovy, J., Jr.; Hardin, E.L. Population dynamics of the parasites in Fundulus zebrinus in the Platte River of Nebraska. J. Parasitol. 1987, 73, 689–696. [Google Scholar] [CrossRef]
- Janovy, J., Jr.; Hardin, E.L. Diversity of the parasite assemblage of Fundulus zebrinus in the Platte River of Nebraska. J. Parasitol. 1988, 74, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Guilford, H.G. New species of Myxosporidia found in percid fishes from Green Bay (Lake Michigan). J. Parasitol. 1963, 49, 474–478.43. [Google Scholar] [CrossRef]
- Bond, F.F. Experimental studies on myxosporidiosis of Fundulus heteroclitus (Linn.) and F. diaphanus (Le Sueur). Trans. Am. Microsc. Soc. 1939, 58, 164–169. [Google Scholar] [CrossRef]
- McAllister, C.T.; Fayton, T.J.; Cloutman, D.G.; Bursey, C.R.; Robison, H.W.; Trauth, S.E.; Whipps, C.W. Parasites of the golden topminnow, Fundulus chrysotus (Cyprinodontiformes: Fundulidae), from Arkansas, U.S.A. Comp. Parasitol. 2019, 87, 19–32. [Google Scholar] [CrossRef]
- Parker, J.D.; Spall, R.D.; Warner, M.C. Two new species of myxosporida, Henneguya gambusi sp. n. and Myxospora pharyngeus sp. n., in the mosquitofish, Gambusia affinis (Baird and Girard). J. Parasitol. 1971, 57, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Khoo, L.; Rommel, F.A.; Smith, S.A.; Griffin, M.J.; Pote, L.M. Myxobolus neurophilus: Morphologic, histopathologic and molecular characterization. Dis. Aquat. Organ. 2010, 89, 51–61. [Google Scholar] [CrossRef]
- Stilwell, J.M.; Rosser, T.G.; Leary, J.H.; Woodyard, E.T.; Richardson, B.; Lopez-Porras, A.; Mischke, C.C.; Camus, A.C.; Griffin, M.J. Characterisation of myxozoan fauna of western mosquitofish, Gambusia affinis (Baird and Gerard 1853) (Cyprinodontiformes: Poeciliidae), inhabiting experimental catfish ponds in Mississippi, USA. Syst. Parasitol. 2021, 98, 423–441. [Google Scholar] [CrossRef]
- Holzer, A.S.; Piazzon, M.C.; Barrett, D.; Bartholomew, J.L.; Sitjà-Bobadilla, A. To react or not to react: The dilemma of fish immune systems facing myxozoan infections. Front. Immunol. 2021, 12, 734238. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McAllister, C.T.; Leis, E.M.; Cloutman, D.G.; Camus, A.C.; Fayton, T.J.; Robison, L.R.S.; Burrows, G.; Rodriguez, M.R.; Robison, H.W. A New Species of Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Mesenteries of Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Upper Ouachita River Drainage, Arkansas, USA. Diversity 2025, 17, 192. https://doi.org/10.3390/d17030192
McAllister CT, Leis EM, Cloutman DG, Camus AC, Fayton TJ, Robison LRS, Burrows G, Rodriguez MR, Robison HW. A New Species of Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Mesenteries of Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Upper Ouachita River Drainage, Arkansas, USA. Diversity. 2025; 17(3):192. https://doi.org/10.3390/d17030192
Chicago/Turabian StyleMcAllister, Chris T., Eric M. Leis, Donald G. Cloutman, Alvin C. Camus, Thomas J. Fayton, Logan R. S. Robison, George Burrows, Michael R. Rodriguez, and Henry W. Robison. 2025. "A New Species of Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Mesenteries of Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Upper Ouachita River Drainage, Arkansas, USA" Diversity 17, no. 3: 192. https://doi.org/10.3390/d17030192
APA StyleMcAllister, C. T., Leis, E. M., Cloutman, D. G., Camus, A. C., Fayton, T. J., Robison, L. R. S., Burrows, G., Rodriguez, M. R., & Robison, H. W. (2025). A New Species of Myxobolus (Cnidaria: Myxosporea: Myxobolidae) from the Mesenteries of Blackspotted Topminnow, Fundulus olivaceus (Cyprinodontiformes: Fundulidae), from the Upper Ouachita River Drainage, Arkansas, USA. Diversity, 17(3), 192. https://doi.org/10.3390/d17030192









