Abstract
Tsuga forrestii Downie, a vulnerable species endemic to China, is confined to small, fragmented habitats in the northwestern Yunnan and southwestern Sichuan Provinces, China. Understanding the current status of its communities and populations is crucial for protecting existing natural forest resources. We surveyed 33 plots in Yunnan and Sichuan, where T. forrestii is the primary dominant species. We analyzed their community characteristics including vertical stratification, species diversity, and population structure, and classified them into four distinct forest types: (1) Tsuga forrestii evergreen coniferous forest; (2) Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest; (3) Tsuga forrestii-Quercus guyayifolia evergreen coniferous and broad-leaved mixed forest; (4) Tsuga forrestii-Abies forrestii evergreen coniferous forest. These forests exhibited a multilayered vertical structure, and T. forrestii as a dominant species appeared in the arborous and shrub layers, and the associated taxa were mainly species of Abies, Lithocarpus, Quercus, Castanopsis, and Rhododendron. No significant differences in species richness or diversity indices or phylogenetic relatedness metrics among the forest types were found. The maximum age of the remaining wild specimens was 344 years, with individuals under 20 years or over 170 years old being rare. The average growth rate of T. forrestii, based on ring width, fluctuated over time, and the range of mean values was 0.92 to 3.31 mm/year. Established seedlings/saplings were rare and mainly found in unstable microhabitats. These findings highlight the poor regeneration and a decline in its populations. Improving its regeneration status is crucial to maintaining its population status.
1. Introduction
Tsuga, commonly known as Hemlock, is a genus of the Pinaceae with a disjunct distribution across Asia and North America [1,2]. As relict plants, the fossil record of Tsuga includes reports of wood, leaves, seeds, pollen, and seed cones, indicating that the genus was once distributed throughout North America and Eurasia from the Late Cretaceous to the Plio–Pleistocene [3]. A total of 24 species have been described in the past, while only 10 species were once recognized [1,4,5,6], with three species occurring in China [1,5].
Although the morphological characteristics of seed cones have played important roles in distinguishing Tsuga at the species level [3], Tsuga species in East Asia are difficult to distinguish due to their minimal morphological differences [4], such as T. forrestii, and its characteristics place it between T. dumosa and T. chinensis [7]. In China, due to their morphological similarities and geographic overlap, T. forrestii was once regarded as T. chinensis or a variant of T. chinensis (as “T. chinensis var. forrestii (Downie) Silba”) [8,9]. With the popularization of molecular ecology, authors divided the 10 species of Tsuga into two clades based on the genetic information of Tsuga species, with the one clade grouped with T. mertensiana and T. heterophylla, which was from western North America; and the other clade grouped with T.canadensis and Tsuga species from East Asia (T. caroliniana was nested in as a sister species) [5,10].The six Tsuga species distributed in East Asia are sister species to each other [5,10,11]. As a vulnerable relict plant, T. forrestii is confined to a narrow area in northwestern Yunnan Province and the southwestern Sichuan Province of China [1,12] (Figure 1).
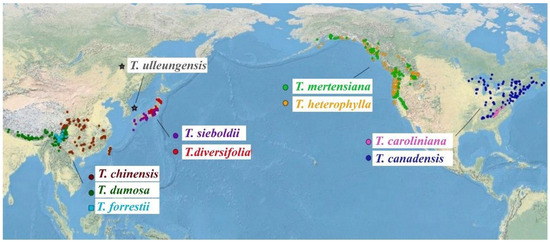
Figure 1.
Geographic distribution of each species of Tsuga (revised from [1]).
East Asia hosts an exceptional assemblage of relict genera of Paleogene–Neogene and even Cretaceous age [13]. Many relict tree species are threatened by climate restricted distribution, leading to a continuous decline in their populations. China is home to a large number of relict plants, exemplified by Ginkgo biloba, Metasequoia glyptostroboides, Glyptostrobus pensilis, Cathaya argyrophylla, Taiwania cryptomerioides, Davidia involucrata, etc. [13,14,15,16,17,18,19]. Most of the relict plant groups only survive in parts of southeastern, south-central, and southwestern China [13,14,15,16,17,20,21,22]. Relict flora includes some of the rarest and most endangered plant species in the world [23]. The distribution areas of relict plant groups have either been encroached upon by humans or are currently under human occupation, posing a constant threat of extinction. A comprehensive understanding of the population structure, statistics, and ecological niches of these species is crucial for ongoing conservation efforts and for revealing the mechanisms that shape their biogeography and ensure their persistence over millennia [17,24].
In China, T. forrestii is restricted to northwestern Yunnan and southwestern Sichuan [1,7,8]. While Ying et al. [9] included locations in northeastern Guizhou and western Hubei, these are likely incorrect identifications of Tsuga chinensis [1]. T. forrestii is a high-mountain species occurring between 2000 m and 3500 m. It is a key component of montane conifer forests, where it coexists with Abies spp., Picea spp., Larix potaninii, and various angiosperm trees, such as Betula albosinensis, Acer spp., Sorbus spp., Quercus spp., and Magnolia spp. [1]. Studying the communities of a threatened species can help identify its interactions with other species and its role in habitat provision. Studies on the current status of endangered and vulnerable relict plants of its forests and populations are an important task for the effective conservation of plant diversity [25]. However, no studies have focused on this species within its diverse topographic surroundings in southwestern China, including its community characteristics, population structure, regeneration status, and phylogenetic diversity.
The objective of this paper is to address the following questions: (1) What are the community characteristics of Tsuga forrestii? (2) What are growth trends and the age structure of T. forrestii? (3) What is the regeneration status of the species? (4) What is the phylogenetic diversity of the communities dominated by T. forrestii? Answers to these questions will enhance our understanding of the current status of this relict plant and provide insight into its population structure and survival prospects.
2. Materials and Methods
2.1. Study Area
We conducted extensive surveys to locate forest communities where Tsuga forrestii is the primary dominant species in its distribution range in northwestern Yunnan and southwestern Sichuan, southwestern China. A total of 33 plots were established in 11 locations across 5 counties, along the Yunnan–Sichuan border (27°37′21″–28°49′4″ N, 100°45′46″–102°24′37″ E) (Figure 2). The elevational of these sites ranges from 2330 to 3340 m above sea level, where T. forrestii forests predominantly occupy along steep slopes, gullies, riparian slopes, and stream sides in the mountainous areas.
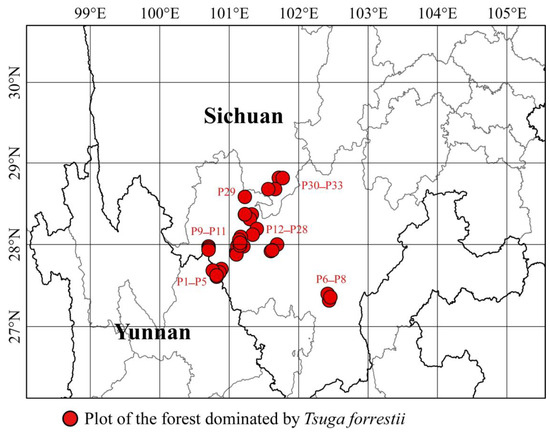
Figure 2.
The locations of 33 plots of forests dominated by Tsuga forrestii.
The climate in the study area is largely influenced by the Indian Ocean monsoon during the summer. Across the plots, the mean annual temperature ranges from 7 °C to 15 °C, with average temperatures of 11 °C to 21 °C in the warmest month (July) and −1.5 °C to 7.9 °C in the coldest month (January). Annual precipitation ranges from 752 mm to 860 mm, with approximately 93% of rainfall occurring between March and October. Relative humidity remains high, exceeding 96% monthly, and the moisture index—defined as the ratio of mean annual actual evapotranspiration to mean annual potential evapotranspiration—ranges from 0.9 to 1.0. These data on the plot sites were extrapolated from the observed data over 50 years (1950–2000) at the local climatological stations.
2.2. Study Species
Tsuga forrestii, endemic to China, is an evergreen coniferous tree with a canopy that develops a more pronounced tower shape as the tree matures. It can reach heights of up to 30 m and 100 cm DBH (diameter at breast height) [8]. The bark is rough, scaly, and orange–brown, becoming brown–gray and furrowed with age. The crown is broadly conical in young trees, becoming flat-topped or irregular in older specimens. Leaves are flat and needle-like. The flowering period occurs from April to May, with cones maturing in October. The cones are larger, oval to oblong in shape, and are densely distributed on the outer branches of the crown. They are short pedunculate or sessile, ovoid–oblong when closed, and ripen from green to light brown. The seeds are oblong with wings, and the upper part of the seed wings tapers slightly [7,8].
The wood properties and uses of Tsuga forrestii are similar to those of T. Dumosa. T. forrestii wood is characterized by a straight grain, uniform texture, solid composition, and resistance to water and moisture, making it suitable for use in railway sleepers, construction, aircraft, furniture, appliances, boats, mine pillars, and as a raw material in the wood fiber industry. The bark can be used to extract roasted gum, and resin can be harvested from the trunk for the production of rosin and turpentine [7,8].
2.3. Data Collection and Analysis
2.3.1. Forest Structure and Species Diversity
In July 2021, September 2022, and December 2023, we surveyed Tsuga forrestii communities in the Yunnan and Sichuan Provinces. We established forest plots dominated primarily by Tsuga forrestii in Ninglang, Yanyuan, Dechang, Muli, and Jiulong Counties. Plot sizes ranged from 400 to 1000 m2, based on the smallest area for the maximum number of species and accessibility in a local topography. The plot area in total was 21,900 m2. Each plot was divided into 10 m × 10 m subplots. All woody individuals with a height of at least 1.3 m tall were identified to species level, numbered, and tagged. DBH and height were recorded, and additional site information (e.g., slope position, altitude, slope exposure, slope inclination, soil types, as well as human disturbance history) was noted.
Woody stems (≥1.3 m tall) were classified into two categories based on vertical position and height: (1) the arborous layer (height ≥ 5 m) and (2) the shrub layer (1.3 m ≤ height < 5 m). The arborous layer was further divided into 3 sublayers: emergent (height > 25 m), canopy (10 m ≤ height < 25 m), and subcanopy (5 m ≤ height < 10 m). For all woody species in the understory (height < 1.3 m), species were identified, counted, and measured for height and percent cover. We described forest stratification through the height classes of woody species (height ≥ 1.3 m).
In each subplot, five 1 m × 1 m squares were used to assess herbaceous taxa. These quadrats were placed at each corner and the center of the subplot. Herbaceous species were identified, and the coverage and abundance were recorded. Individuals of T. forrestii with heights between 90 and 130 cm were considered established seedlings/saplings.
For all woody individuals ≥ 1.3 m tall, we calculated basal area using DBH and determined species abundance via relative importance value (RIV), computed as follows: RIV = (Relative density + Relative basal area)/2 for overstory species, and RIV = (Relative density + Relative coverage)/2 for understory species [26]. Plant communities were classified using a floristic similarity dendrogram (Euclidean and Ward’s Method; [27]). The communities were named based on the dominant species of the overstory. Diversity indices for each community plot, including species richness (number of species), Simpson’s index D [28], and Shannon–Wiener’s diversity index H′ [29], were calculated.
For floristic characterization, we classified seed plant families and genera based on Wu’s system [30,31].
2.3.2. Tree Core Analysis of Tsuga forrestii
We obtained 65 increment cores from T. forrestii trees of varying DBHs in study plots. Each increment core was collected at 1.3 m above ground level. For saplings with heights ≤ 1.3 m, tree-ring data indicated an approximate growth time of eleven years to reach this height, which was added to the ages obtained from increment cores. Additionally, five T. forrestii saplings with DBH ≤ 5 cm were cut at 1.3 m to assess age through cross-sections, as increment borers were unsuitable for such small DBHs. It took approximately 11 years to reach 1.3 m in height. This eleven-year period was added to the ages obtained from the increment cores. Age was determined using the WinDENDRO tree-ring analysis system software (TSAPWin V4.81c) (Rinntech Instruments Inc., Heidelberg, Germany).
We used age and DBH data to derive an age–DBH correlation formula, applying it to DBH measurements to estimate tree ages. Tree-ring analysis also yielded ring widths for basal area increment (BAI), calculated using the formula: T–Y, where T is the basal area at year X (the most recent growth year), and Y is the basal area of the preceding year. BAI is a valuable metric for assessing forest growth, as it quantifies wood production, accounting for the increasing tree diameter over time [32].
2.3.3. Phylogenetic Analysis and Phylogenetic Relatedness
Phylogenetic diversity is crucial in understanding species coexistence mechanisms, such as competitive exclusion and habitat filtering [33]. We analyzed community phylogenetic diversity using three main indices: phylogenetic diversity (PD), mean phylogenetic distance (MPD), and mean nearest taxon distance (MNTD). Corresponding null models were used to compute their standardized values: net relatedness index (NRI) and nearest taxon index (NTI) [34,35,36].
To investigate the phylogenetic structure, we created a phylogenetic tree for 197 species from the Tsuga forrestii forest plots. Species names were verified with the “WFO Plant List” (https://wfoplantlist.org/plant-list (accessed on 19 November 2024)) and the time-calibrated phylogeny was generated using V.PhyloMaker (v.0.1.0; [37]) in R (v.4.1.0; [38]). We adopted Smith and Brown’s [39] dataset as the backbone tree and integrated 197 species from the T. forrestii forest plots. For species absent in the backbone tree, we applied the genus-level phylogenetic placement.
PD quantifies the total evolutionary history represented by all species in a community, calculated as the sum of the branch lengths on a phylogenetic tree [40]. MPD calculates the average phylogenetic distance among all possible pairs of species within a community, and MNTD measures the mean phylogenetic distance to the closest relative (nearest neighbor) for each species within a community [34]. NRI and NTI were calculated to assess phylogenetic relatedness: NRI (based on MPD) reflects deep evolutionary history, while NTI (based on MNTD) pertains to recent evolutionary divergence. Positive NRI and NTI values suggest phylogenetic clustering due to habitat filtering, while negative values indicate over-dispersion due to competitive exclusion [34,41]. We analyzed NRI and NTI values for each forest type and visualized the data using boxplots in R (v.4.1.0; [38]).
3. Results
3.1. Forest Types and Structure
Floristic similarity analysis, at a floristic similarity level of approximately 28%, classified 33 plots into four distinct forest communities (Figure 3A). The forest’s arborous and shrub layers were distinctly separated. The vertical stratification of woody species (height ≥ 1.3 m) is shown in Figure 3B.
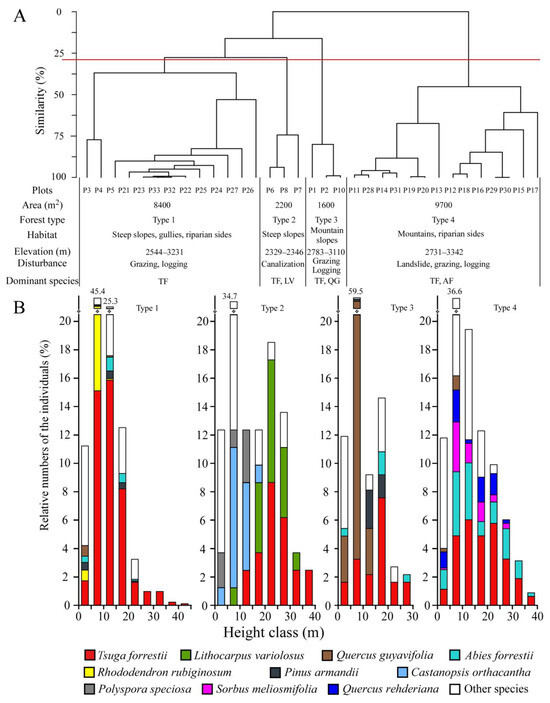
Figure 3.
Floristic dissimilarity dendrogram for the 33 plots (A) and frequency distribution of height classes of woody species (height ≥ 1.3 m) of each forest type (B). Type 1: Tsuga forrestii evergreen coniferous forest; Type 2: Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest; Type 3: Tsuga forrestii-Quercus guyayifolia evergreen coniferous and broad-leaved mixed forest; Type 4: Tsuga forrestii-Abies forrestii evergreen coniferous forest. TF = Tsuga forrestii, LV = Lithocarpus variolosus, QG = Quercus guyayifolia, and AF = Abies forrestii.
Type 1: Tsuga forrestii evergreen coniferous forest, occurring along steep slopes, gullies, and riversides, had an average inclination of 36° at elevations ranging from 2544 to 3231 m in Ninglang County of Yunnan and Yanyuan, Muli, and Jiulong Counties of Sichuan. T. forrestii dominates the canopy (10 m ≤ height < 25 m), subcanopy (5 m ≤ height < 10 m), and shrub layer (1.3 m ≤ height < 5 m). T. forrestii reached 41 m in the emergent sublayer (height > 25 m). Accompanying species were mainly coniferous Abies sq uamata, Pinus yunnanensis, P. armandii, evergreen broad-leaved Quercus rehderiana, and deciduous broad-leaved Populus davidiana occurring in canopy. The subcanopy mainly included evergreen broad-leaved Rhododendron rubiginosum, other conifers P. armandii, A. squamata, Taxus wallichiana, and broad-leaved trees, including Rhododendron traillianum, Q. rehderiana, and Acer davidii, were scattered in the subcanopy. In the shrub layer, along T. forrestii, the other species were Rhododendron decorum, Quercus guyavifolia, R. rubiginosum, Pinus armandii, and Abies forrestii. The understory included herbaceous species such as Ainsliaea yunnanensis, Ophiopogon intermedius, Fragaria moupinensis, etc.
Type 2: Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest, found along steep slopes, had an average inclination of 50°, at elevations of 2329 to 2346 m in Dechang County of Sichuan. In the canopy, T. forrestii and Lithocarpus variolosus were co-dominants, and they dominated the canopy and subcanopy. T. forrestii and L. variolosus reached 37 m and 32 m, respectively, in the emergent sublayer. The accompanying species mainly included evergreen broad-leaved tree, Castanopsis orthacantha, reaching 17 m in canopy. In the subcanopy, C. orthacantha was a dominant species. Accompanying species included Rhododendron simsii and Ternstroemia gymnanthera. Only Polyspora speciosa and Eurya loquaiana occasionally appeared in the shrub layer. In the understory, herbaceous species mainly included Blumea balsamifera, Rubus fockeanus, Pteris cretica, etc.
Type 3: Tsuga forrestii-Quercus guyavifolia evergreen coniferous and broad-leaved mixed forest, found along mountain slopes, had an average inclination of 34°, at elevations of 2783–3110 m in Ninglang County, Yunnan, and Yanyuan, Muli Counties, Sichuan. T. forrestii and Q. guyavifolia were co-dominants, with T. forrestii dominating the canopy, subcanopy, and shrub layer, and Q. guyavifolia mainly occurred in subcanopy. In the canopy, accompanying species included evergreen conifers Pinus armandii and P. yunnanensis. In the subcanopy, along the dominant Q. guyavifolia, only P. yunnanensis and Acer sinense were found. The shrub layer was mainly young trees of T. forrestii and Q. guyavifolia. Herbaceous species in the understory mainly included Fragaria moupinensis, Ainsliaea yunnanensis, Elsholtzia ciliata, etc.
Type 4: Tsuga forrestii-Abies forrestii evergreen coniferous forest, found along mountain slopes, riparian sides, had an average inclination of 40°, at elevations of 2731–3342 m in Yanyuan and Muli Counties, Sichuan. T. forrestii and A. forrestii were co-dominants in the canopy, subcanopy, and shrub layer. A. forrestii and T. forrestii reached 37 m and 36 m in the emergent sublayer, respectively, while Sorbus meliosmifolia reached 28 m. Accompanying species in the canopy mainly included evergreen broad-leaved Quercus rehderiana and deciduous broad-leaved S. meliosmifolia and Acer forrestii. In the subcanopy, besides the small trees of T. forrestii and A. forrestii, other species included Enkianthus deflexus, S. meliosmifolia, and Cornus macrophylla. The shrub layer featured young trees of dominant species, and accompanying Q. rehderiana, Enkianthus deflexus, and Rhododendron decorum. Herbaceous species in the understory mainly included Fragaria moupinensis, Carex breviculmis, and Ainsliaea yunnanensis.
Tsuga forrestii and its representative forest stands and habitats are shown in Figure 4A–G.

Figure 4.
Tsuga forrestii and its representative forest stands and habitats in Yunnan and Sichuan. (A): Seed cones of Tsuga forrestii. (B): A fragmented forest dominated by Tsuga forrestii by roads at ca. 2850 m a.s.l. in Xiqiuxiang, Muli County, Sichuan. (C): A Tsuga forrestii tree with 120 cm DBH and 26 m tall at ca. 2920 m a.s.l in Kalaxiang, Muli County, Sichuan. (D): A Tsuga forrestii forest on a steep slope above a stream at ca. 2700 m a.s.l in Wulaxizhen, Jiulong County, Sichuan. (E): A Tsuga forrestii forest on a steep slope prone to landslide at ca. 3230 m a.s.l in Gouzhuandong, Ninglang County, Yunnan. (F): A Tsuga forrestii forest on a steep slope above a stream at ca. 2730 m a.s.l in Kalaxiang, Muli County, Sichuan. (G): A Tsuga forrestii forest at ca. 2330 m a.s.l in Lediezhen, Dechang County, Sichuan.
3.2. Forest Floristic Features and Species Diversity
A total of 195 plant species from 70 families and 138 genera were recorded across all the Tsuga forrestii forest communities. This included 12 species from three families and seven genera of gymnosperms, 163 species from sixty families and one hundred and twenty genera of angiosperms, and 20 species from seven families and eleven genera of ferns. Notably, approximate 51.2% of the families and 35.7% of the genera represented tropical elements, while 48.8% of the families and 64.3% of the genera consisted of temperate elements. The high proportion of temperate elements in genus level indicated that the species composition in the T. forrestii forests is primarily temperate.
The species composition of the arborous, shrub, and understory layers for each forest type is detailed in Tables S1–S3. Species richness and diversity indices for each forest type are shown in Figure 5.
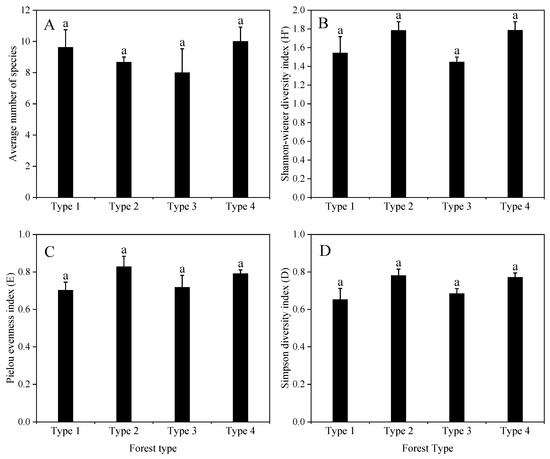
Figure 5.
Diversity of woody species (height ≥ 1.3 m) in each forest type. (A) Average number of species; (B) Shannon–Wiener diversity index; (C) Pielou evenness index; (D) Simpson diversity index. Forests sharing the same letters do not differ significantly by the non-parametric Kruskal–Wallis all-pairwise comparisons test (p < 0.05). Bars represent standard deviation. Type 1 = Tsuga forrestii evergreen coniferous forest; Type 2 = Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest; Type 3 = Tsuga forrestii-Quercus guyayifolia evergreen coniferous and broad-leaved mixed forest; Type 4 = Tsuga forrestii-Abies forrestii evergreen coniferous forest. Forests sharing the same letter do not differ significantly according to the non-parametric Kruskal–Wallis all-pairwise comparisons test (p < 0.05). Bars represent standard deviation.
Among the four forest types, the average number of woody species, ranging from 8 to 10 (Figure 5A), as well as the Shannon–Wiener diversity index ranging from 1.4 to 1.8 (Figure 5B), Pielou evenness index (E) ranging from 0.7 to 0.8 (Figure 5C), Simpson diversity index ranging from 0.7 to 0.8 (Figure 5D), showed no statistically significant differences (p < 0.05).
3.3. Phylogenetic Relatedness Among the Forest Types
Phylogenetic relatedness among the four forest types indicated clustered over-dispersion for the Tsuga forrestii evergreen coniferous forest (Type 1) (NRI = −0.63), Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest (Type 2) (NRI = −0.17), Tsuga forrestii-Quercus guyayifolia evergreen coniferous and broad-leaved mixed forest (Type 3) (NRI = −0.19), and Tsuga forrestii-Abies forrestii evergreen coniferous forest (Type 4) (NRI = −0.68); the values of NRI meant closely related species were less likely to coexist, which was due to competitive exclusion. Among all the forest types, there was no significant difference (Figure 6A) (p < 0.05, Table S4). However, NTI values indicated that all four forest types were phylogenetically clustered (mean values from 0.66 to 1.19), which meant closely related species were more similar in their ecological requirements and, thus, are more likely to coexist (Figure 6B), and forests were not significantly different (p < 0.05, Table S4).
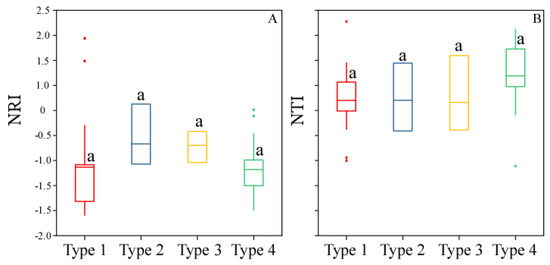
Figure 6.
Phylogenetic metrics (NRI, NTI) of each forest type. (A) NRI (Net relatedness index) of each forest type; (B) NTI (Nearest taxon index) of each forest type. Type 1 = Tsuga forrestii evergreen coniferous forest; Type 2 = Tsuga forrestii-Lithocarpus variolosus evergreen coniferous and broad-leaved mixed forest; Type 3 = Tsuga forrestii-Quercus guyayifolia evergreen coniferous and broad-leaved mixed forest; Type 4 = Tsuga forrestii-Abies forrestii evergreen coniferous forest. Forests sharing the same letter do not differ significantly according to the non-parametric Kruskal–Wallis all-pairwise comparisons test (p < 0.05).
3.4. Population Characteristics
3.4.1. Growth Trends
The relationship between DBH and age in Tsuga forrestii shows a strong positive correlation (y = 0.0003x3 − 0.0636x2 +5.1127x + 7.7886, R2 = 0.7715, n = 65, p < 0.05) (Figure 7A), allowing DBH to be used as an effective predictor of age for each Tsuga forrestii individual.
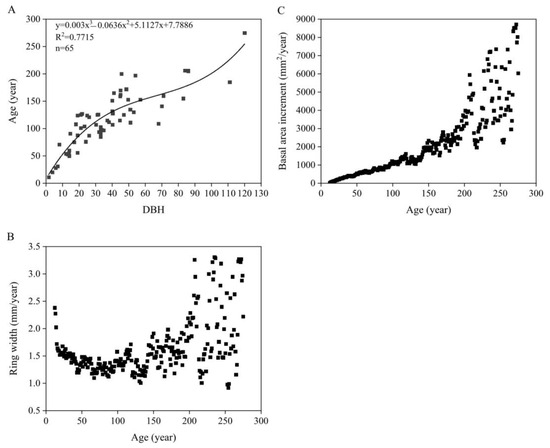
Figure 7.
Growth trends of Tsuga forrestii (height ≥ 1.3 m). (A) Relationships between DBH and age; (B) Changes in ring width over the years; (C) changes in basal area increment over the years.
The average annual ring-width growth of T. forrestii was 1.6 mm/year. The ring-width growth rate decreased gradually from 2.4 mm/year to 1.4 mm/year, averaging 1.5 mm/year, during the first 49 years. Between 50 and 98 years, trees maintained a stable growth rate, averaging 1.3 mm/year. From 99 to 118 years, the ring-width growth rate increased to an average of 1.5 mm/year. Between 119 and 142 years, the ring-width growth rate decreased to an average of 1.2 mm/year. Between 143 and 275 years, growth fluctuated greatly but generally trended upward, averaging 1.9 mm/year (Figure 7B).
The basal area increment (BAI) remained steady up to 213 years, with an average annual increase of 1459 mm2/year. From 214 to 275 years, despite fluctuations, BAI showed an overall increase, with an average annual increment of 4793.1 mm2 (Figure 7C).
3.4.2. Age Structure and Regeneration
The estimated age class frequency distribution of individuals of Tsuga forrestii as a whole was sporadic (Figure 8). The observed maximum age of T. forrestii was 344 years in forest Type 1, followed by 305, 224, and 164 in Types 4, 3, and 2, respectively. In Type 1, it is a multimodal distribution pattern, with sparse representation below 30 years and beyond 170 years, indicating a moderately aged population with limited recruitment of young individuals. Type 2 is characterized by a dominance of older individuals, most exceeding 125 years, with no individuals below 90 years, reflecting a senescent population structure with no regeneration in recent 90 years. In Type 3, individuals are primarily concentrated around 130–135 years, with sparse, discontinuous representation in other age classes and no individuals under 20 years, suggesting limited recruitment and age cohort clustering. Lastly, Type 4 shows a concentration of individuals between 130 and 145 years, accompanied by a few individuals over 185 years, while only a few younger trees (ages: 20–60 years) were found, and individuals with ages under 20 years are entirely absent. In general, T. forrestii as a whole had a notable absence in ages younger than 20 years and had only a few trees older than 170 years.
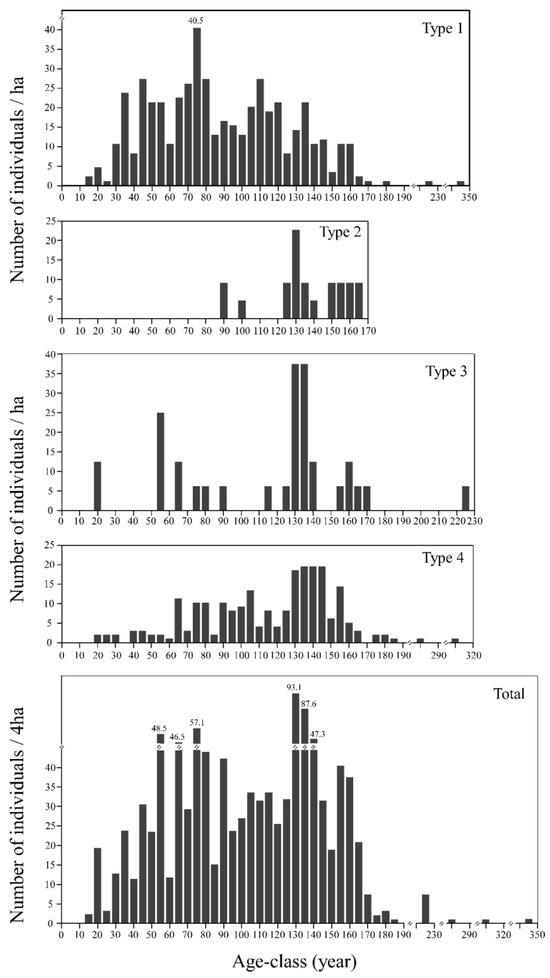
Figure 8.
The estimated age structure of Tsuga forrestii (height ≥ 1.3 m) of each forest type.
Tsuga forrestii seedlings and saplings had a lower survival rate, because the number of seedling and saplings in each microhabitat (forest edges, canopy gaps, and roadsides) decreased significantly with age. At the initial stage of seedling growth (0–30 cm tall), T. forrestii individuals were observed at high density beneath the forest canopy gaps. The number of seedlings under the canopy was rather limited, especially when the height of the seedling reached 60 cm. Established seedlings/saplings (with a height of 90–130 cm) ultimately remained in quite open microhabitats, including canopy gaps, roadsides, and forest edges where moderate disturbances occurred (Figure S1).
4. Discussion
4.1. Distribution, Forest Characteristics, and Habitats
Table 1 compares the habitats and forest characteristics of Tsuga forrestii with other Tsuga species worldwide.
Compared to Tsuga dumosa, which is primarily distributed in the southeastern Himalayas at altitudes ranging from 2600 to 3200 m [1,42], and T. chinensis, found across the Guizhou, Shanxi, Hunan, Jiangxi, Fujian, and Zhejiang Provinces in China with altitudes between 550 and 2500 m, T. forrestii occupies a higher altitudinal range (2320–3350 m) in northwestern Yunnan and southeastern Sichuan. However, its distribution is narrower and more localized. The species composition of T. forrestii forests differs from that of T. dumosa and T. chinensis. T. dumosa is typically associated with species such as Abies, Picea, Larix, and Pinus in high-altitude conifer belts [1]. In contrast, forests of T. chinensis include companion species like Taxus wallichiana var. mairei, Quercus phillyraeoides, Photinia bodinieri, Engelhardia roxburghiana, and Pinus armandii, and a diverse mix of broad-leaved trees and shrubs (e.g., Acer davidii, Schima superba, Rhododendron latoucheae, and Castanopsis eyrei) [43,44,45,46].
The other three Asian Tsuga species are distributed in eastern Asia. Tsuga sieboldii is found in central Honshu, Shikoku, and Kyushu (Japan), thriving on hills and mountains (steep slopes) at altitudes of 400–1500 m [47]. It grows alongside conifers such as Abies firma, Pseudotsuga japonica, Chamaecyparis obtusa, Cryptomeria japonica, Pinus densiflora, P. parviflora, and Sciadopitys verticillata, as well as various angiosperms in mixed forests [1]. T. diversifolia is mainly distributed in central Honshu, particularly in Nagano, Tochigi, Gunma, and Yamanashi prefectures, at altitudes between 700 and 2000 m. It is often the dominant species but coexists with several other conifers. T. ulleungensis is endemic to Ulleungdo Island, South Korea, where it grows on northern slopes at 310–500 m in well-drained ridge communities dominated by Pinus parviflora, alongside Acer, Camellia, and Fagus species [4].
In North America, four Tsuga species are distributed across eastern and western regions:
T. mertensiana, found in Alaska and Canada, grows in coastal and mountainous areas at altitudes of 1200–3350 m a.s.l., and it is commonly associated with Abies lasiocarpa, A. amabilis, Picea glauca, P. sitchensis, P. engelmannii, Pinus spp., T. heterophylla, Xanthocyparis nootkatensis, Juniperus occidentalis, and Betula papyrifera [1,7]. T. heterophylla occurs in the northwestern United States, thriving at altitudes between 600 and 1800 m. T. caroliniana, found in Georgia, North Carolina, South Carolina, Tennessee, and Virginia, grows in mountainous and valley habitats at altitudes of 600–1500 m, often alongside broad-leaved trees and shrubs. T. canadensis inhabits the eastern United States, growing at 600–1500 m, and commonly associating with Pinus strobus, Picea rubens, Abies balsamea, and species of Betula and Populus [1].
Although the distribution of the Tsuga species varies widely in altitude and regions across the world, the major species composition in communities dominated by Tsuga—component genera such as Abies, Pinus, Quercus, Acer, and Betula—exhibits certain similarities.

Table 1.
General characteristics of Tsuga forrestii compared with other species of Tsuga worldwide. LC: Least Concern; NT: Near Threatened; VU: Vulnerable; CR: Critically Endangered.
Table 1.
General characteristics of Tsuga forrestii compared with other species of Tsuga worldwide. LC: Least Concern; NT: Near Threatened; VU: Vulnerable; CR: Critically Endangered.
| Species | Distribution Region | Major Habitats and Elevations | DBH (cm) | Height (m) | Age (year) | Major Associated Species | IUCN Threat Categories | Source |
|---|---|---|---|---|---|---|---|---|
| Tsuga forrestii | NW Yunnan and SW Sixhuan | Crest ridge, mountain slopes, steep inclines, and riversides at ca. 2330–3340 m a.s.l | 0.5–148 | 1.3–41 | 12–344 | Abies forrestii, Picea likiangensis, A. squamata, Lithocarpus variolosus, Castanopsis orthacantha, Quercus guyavifolia, Q. rehderiana, Polyspora speciosa, Sorbus meliosmifolia, Betula utilis, and Rhododendron rubiginosum | VU | This study |
| Tsuga dumosa | Sichuan, S Xizang, N and W Yunnan, Bhutan, N India, N Myanmar, Nepal, Sikkim, and N Vietnam | Mountain slopes and river basins at ca. 2300–3500 m a.s.l | 150–250 (–270) | 40–50 | (max age) 1007 | Abies, Picea, Larix, and Pinus | LC | [7,8,42] |
| Tsuga chinensis | Anhui, Fujian, S Gansu, Guangdong, Guangxi, N Guizhou, W Henan, W Hubei, Hunan, Jiangxi, S Shaanxi, Sichuan, Taiwan, Yunnan, and Zhejiang | Mountains, valleys, and river basins at ca. 550–(–1000)–2500(–3500) m a.s.l | 50–60 (–160) | 20–25 (–50) | 140 | Taxus wallichiana var. mairei, Quercus phillyraeoides, Photinia bodinieri, Engelhardia roxburghiana, Pinus armandi, Toxicodendron vernicifluum, Acer davidii, Rhododendron ovatum, Schima argentea, Camellia cuspidate, R. latoucheae, Q. multinervis, Betula luminifera, Prunus dielsiana, Schima superba, R. simiarum, and Castanopsis eyrei | LC | [7,8,43,44,45,46,48] |
| Tsuga sieboldii | Central Honshu, Shikoku, and Kyushu, Japan | Mountains (steep ridges) and hills at ca. 400–1500 m a.s.l | (150)–250 | 25–30 | 500–800 | Abies firma, Pseudotsuga japonica, Chamaecyparis obtusa, Cryptomeria japonica, Pinus densiflora, P. parviflora, and Sciadopitys verticillata | NT | [1,7,47] |
| Tsuga diversifolia | Central Honshu, particularly in Nagano, Tochigi, Gunma, and Yamanashi prefectures, Japan | Mountains, 700–2000 m | 50–60 | 20–25 | Picea jezoensis, Abies homolepis, A. veitchii, A. mariesii, Larix kaempferi, Pinus parviflora, Thuja standishii, Thujopis dola brata var. hondae, Betula ermanii, B. corylifolia, Sorbus japonica, Alnus hirsuta var. sibirica, Quercus mongolica var. grosseserrata, and Rhododendron spp. | LC | [1,7] | |
| Tsuga ulleungensis | Ulleungdo in South Korea | Mountain slopes, 310–500 m | Acer, Camelia, and Fagus | CR | [4,12] | |||
| Tsuga mertensiana | Alaska and Canada | Coast and mountains, 1200–3350 m | 100–150 | 30–40 (–45) | Abies lasiocarpa, A. amabilis, Picea glauca, P. sitchensis, P. engelmannii, Pinus spp., Tsuga heterophylla, Xanthocyparis nootkatensis, Juniperus occidentalis, and Betula papyrifera | LC | [1,7] | |
| Tsuga heteropylla | NW of North America | Coast and mountains, 600–1800 | 150–250 | 60–70 | LC | [1,7] | ||
| Tsuga caroliniana | States of Georgia, North Carolina, South Carolina, Tennessee, and Virginia | Mountains and valleys, 600–1500 m | 50–60 | 20–25 | Broad-leaved trees and shrubs | NT | [1,7] | |
| Tsuga canadensis | Eastern half of North America | Coast and mountains, 600–1500 | 150–200 | 30–40 (–48) | Pinus strobus, Picea rubens, Abies balsamea, and Betula or Populus | NT | [1,7] |
4.2. Growth Trends
For Tsuga forrestii in our study, the average DBH growth rate during the first 30 years was 3.3 mm/year, which is higher than that (2.1 mm/year for the same period reported, recalculated from [48]) for its congener T. chinensis var. tchekiangensis in Guangze, Fujian Province, southeastern China. However, from 31 to 45 years of age, the average DBH growth rate of T. forrestii drops to 2.7 mm/year, which is lower than the 4.3 mm/year (recalculated from [48]) for T. chinensis var. tchekiangensis during the same age range. This may be caused by the two species’ different biological traits. Moreover, T. chinensis var. tchekiangensis at lower elevations (1445–1625 m) in Fujian experiences warmer conditions (16.2 °C annual average) and significantly higher rainfall (2000–2200 mm/year) compared to the study site for T. forrestii [48]. These climatic gradients likely contribute to the observed growth differences, as different temperature and water availability are critical regulators of cambial activity in conifers [49].
Beyond abiotic drivers, intrinsic factors such as genetic diversity and resource allocation strategies may further explain the reduced growth rates in T. forrestii. For instance, slow-growing high-elevation species often prioritize stress tolerance (e.g., dense wood, resin production) over rapid biomass accumulation, a trade-off linked to survival in harsh environments [50]. Comparative studies of Tsuga species’ physiological traits, such as leaf area-to-sapwood ratios or hydraulic conductivity, could clarify these mechanisms. Additionally, anthropogenic pressures (e.g., historical logging in T. forrestii forests) may have been selected for faster-growing individuals, introducing confounding effects when comparing natural vs. disturbed populations [51].
4.3. Regeneration Challenges and Conservation Recommendations
The regeneration of Tsuga forrestii mainly depends on the opening of the forest canopy. Seedlings are scattered sparsely on the forest floor under the mature canopy. They rarely reach the shrub layer, however, unless there is an opening of the canopy; therefore, we can infer that the regeneration period of T. forrestii will take a long time. Suzuki and Tsukahara [47] inferred that, in a mature stand of the forest, the length of one regeneration period of T. sieboldii was 70 years.
Seed dispersal distance, seed characteristics, and in situ habitats influenced the recruitment of species [52,53]. Our investigation found that the regeneration of Tsuga forrestii forests mainly occurs in canopy gaps, followed by forest edges and roadside areas, with almost no seedlings present under the forest canopy. This indicates that the early growth of T. forrestii seedlings requires a certain amount of light.
The absence of Tsuga forrestii saplings suggests that its population is in decline, and this outcome mirrors the development trend of declining T. chinensis populations found in Guizhou, Hunan, and Shaanxi [44,45].
Based on our exclusive survey, Tsuga forrestii populations in some areas, including Ninglang, Yanyuan, Dechang, and Muli Counties, are threatened by human disturbances such as grazing, logging, canalization, and herb medicine collection. In the forest communities of the T. forrestii distributed across Ninglang County and Yanyuan County, human activities such as grazing and firewood collection are prevalent. To mitigate these impacts, we recommend regulating grazing areas and specifying tree species such as Rhododendron rubiginosum that can be sustainably harvested for firewood, ensuring that the forest ecosystem remains healthy. In Dechang County, the T. forrestii community is facing diminished water resources due to a water diversion project. We suggest implementing measures to protect the water source, while also prioritizing the conservation of the surrounding vegetation’s original ecological integrity. This dual approach will help maintain both the hydrological and biodiversity functions of the ecosystem. At No. 911 and No. 913 Linchang in Muli County, the T. forrestii regeneration was adversely affected by mountain landslides, a consequence of intensive logging activities that occurred prior to 1998. We suggest cultivating T. forrestii seedlings in No. 911 and No. 913 Linchang to enhance its regeneration.
5. Conclusions
Tsuga forrestii is solely dominant or co-dominant with coniferous species of Abies, or broad-leaved species of Lithocarpus and Quercus, confined to steep slopes, gullies, or along riversides within an elevational range of 2329–3342 m in the Yunnan and Sichuan Provinces. T. forrestii exhibits slow growth in annual ring width. It has a sporadic distribution in age structure. The seedling/sapling bank is poor, and regeneration faces great challenges. The results underscore the urgent need for conservation efforts. We recommend implementing an effective policy to control the grazing and logging and to cultivate T. forrestii seedlings in their natural habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17030148/s1, Table S1: Species composition in the arborous layer of each forest type. Species with IV ≥ 0.01% are shown; Table S2: Species composition in the Shrub layer of each forest type. Species with IV ≥ 0.01% are shown; Table S3 Species composition in the understory of each forest type. Species with IV ≥ 0.01% are shown; Table S4: The significant difference among forest types in phylogenetic relatedness metrics (NRI, NTI); Figure S1 Density of seedlings/saplings of Tsuga forrestii in various micro-habitats.
Author Contributions
Conceptualization, C.Q.T.; Formal Analysis, P.-B.H.; Investigation, P.-B.H.; Methodology, C.Q.T.; Project Administration, S.-G.L. and C.Q.T.; Supervision, C.Q.T.; Validation, C.Q.T.; Visualization, C.Q.T.; Writing—Original Draft, P.-B.H.; Writing—Review and Editing C.Q.T., S.-G.L. and P.-B.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Major Program for Basic Research Project of Yunnan Province, China (grant number 202101BC070002), and Ministry of Science and Technology of China, the Special Foundation for National Science and Technology Basic Resources Investigation of China [2019FY202300], and the APC was funded by [2019FY202300].
Institutional Review Board Statement
Not applicable.
Data Availability Statement
All data are available from the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Brill: Leiden, The Netherlands, 2013. [Google Scholar]
- Wu, Z.-Y.; Raven, P.H.; Hong, D.-Y. (Eds.) Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1998. [Google Scholar]
- Lepage, B.A. A new species of Tsuga (Pinaceae) from the middle Eocene of Axel Heiberg Island, Canada, and an assessment of the evolution and biogeographical history of the genus. Bot. J. Linn. Soc. 2003, 141, 257–296. [Google Scholar] [CrossRef]
- Holman, G.; Del Tredici, P.; Havill, N.; Lee, N.S.; Cronn, R.; Cushman, K.; Campbell, C.S. A new species and introgression in eastern Asian hemlocks (Pinaceae: Tsuga). Syst. Bot. 2017, 42, 733–746. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Shen, T.T.; Shao, C.C.; Du, H.; Ran, J.H.; Wang, X.Q. Phylotranscriptomics reveals the complex evolutionary and biogeographic history of the genus Tsuga with an East Asian-North American disjunct distribution. Mol. Phylogenet. Evol. 2021, 157, 107066. [Google Scholar] [CrossRef]
- Chen, Z.; Shang, X.; Xie, Z.; Zhang, X. Phylogenetic relationship and evolutionary patterns of Tsuga pollen morphology: A cluster analysis-based study. J. Nanjing For. Univ. 2024, 48, 37. [Google Scholar]
- Farjon, A. A Handbook of the World’s Conifers: Revised and Updated Edition; Brill: Leiden, The Netherlands, 2010; pp. 1007–1019. [Google Scholar]
- Fu, L.; Li, N.; Mill, R.R. Tsuga. Flora of China; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MO, USA, 1999; Volume 4, pp. 39–41. [Google Scholar]
- Ying, T.S.; Chew, M.L.; Chang, H.C. Atlas of the Gymnosperms of China; China Science and Technology Press: Beijing, China, 2003. [Google Scholar]
- Havill, N.P.; Campbell, C.S.; Vining, T.F.; LePage, B.; Bayer, R.J.; Donoghue, M.J. Phylogeny and biogeography of Tsuga (Pinaceae) inferred from nuclear ribosomal ITS and chloroplast DNA sequence data. Syst. Bot. 2008, 33, 478–489. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Wang, Z.; Wu, X.; Hong, K.; Xie, C. Comparative Chloroplast Genome Analyses of Six Hemlock Trees in East Asia: Insights into Their Genomic Characterization and Phylogenetic Relationship. Forests 2023, 14, 2136. [Google Scholar] [CrossRef]
- IUCN (The IUCN Red List of Threatened Species), 2010. Available online: https://www.iucnredlist.org/ (accessed on 19 November 2024).
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Dong, Y.-F.; Momohara, A.; Herrando-Moraira, S.; Qian, S.; Yang, Y.; Ohsawa, M.; Luu, H.T.; et al. Identifying long-term stable refugia for relict plant species in East Asia. Nat. Commun. 2018, 9, 4488. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Ohsawa, M. Tertiary relic deciduous forests on a humid subtropical mountain, Mt. Emei, Sichuan, China. Folia Geobot. 2002, 37, 93–106. [Google Scholar] [CrossRef]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Momohara, A.; Hara, M.; Cheng, S.; Fan, S. Population structure of relict Metasequoia glyptostroboides and its habitat fragmentation and degradation in south-central China. Biol. Conserv. 2011, 144, 279–289. [Google Scholar] [CrossRef]
- Tang, C.Q.; Yang, Y.; Ohsawa, M.; Yi, S.R.; Momohara, A.; Su, W.H.; Wu, Z.L.; Zhang, Z.Y.; Peng, M.C.; Wu, Z.L. Evidence for the persistence of wild Ginkgo biloba (Ginkgoaceae) populations in the Dalou Mountains, southwestern China. Am. J. Bot. 2012, 99, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q. The Subtropical Vegetation of Southwestern China: Plant Distribution, Diversity and Ecology; Springer: Berlin/Heidelberg, Germany, 2015; Volume 11. [Google Scholar]
- He, L.Y.; Tang, C.Q.; Wu, Z.L.; Wang, H.C.; Ohsawa, M.; Yan, K. Forest structure and regeneration of the Tertiary relict Taiwania cryptomerioides in the Gaoligong Mountains, Yunnan, southwestern China. Phytocoenologia 2015, 45, 135–156. [Google Scholar] [CrossRef]
- Qian, S.; Yang, Y.; Tang, C.Q.; Momohara, A.; Yi, S.; Ohsawa, M. Effective conservation measures are needed for wild Cathaya argyrophylla populations in China: Insights from the population structure and regeneration characteristics. For. Ecol. Manag. 2016, 361, 358–367. [Google Scholar] [CrossRef]
- Tang, C.Q.; He, L.Y. Population status of the Tertiary relict Tetracentron sinense in the subtropical Ailao Mountains, Yunnan, SW China, and proposed conservation efforts. Annu. Rep. Pro Nat. Fund 2013, 21, 141–150. [Google Scholar]
- Tang, C.Q.; Yang, Y.; Momohara, A.; Wang, H.-C.; Luu, H.T.; Li, S.; Song, K.; Qian, S.; LePage, B.; Dong, Y.-F.; et al. Forest characteristics and population structure of Glyptostrobus pensilis, a globally endangered relict species of southeastern China. Plant Divers. 2019, 41, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Ai, X.; Yao, L.; Huang, J.; Xu, Y.; Lu, X.; Zang, R. Do climate and human disturbance determine the sizes of endangered Metasequoia glyptostroboides trees in their native range? Glob. Ecol. Conserv. 2020, 21, e00850. [Google Scholar] [CrossRef]
- Milne, R.I.; Abbott, R.J. The origin and evolution of Tertiary relict floras. Adv. Bot. Res. 2002, 38, 281–314. [Google Scholar]
- Tang, C.Q.; Yao, S.-Q.; Han, P.-B.; Wen, J.-R.; Li, S.; Peng, M.-C.; Wang, C.-Y.; Matsui, T.; Li, Y.-P.; Lu, S.; et al. Forest characteristics, population structure and growth trends of threatened relict Pseudotsuga forrestii in China. Plant Divers. 2023, 45, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.Q.; Matsui, T.; Ohashi, H.; Nualart, N.; Herrando-Moraira, S.; Dong, Y.-F.; Grote, P.J.; Van Ngoc, N.; Van Sam, H.; Li, S.; et al. Identifying long-term stable refugia for dominant Castanopsis species of evergreen broad-leaved forests in East Asia: A tool for ensuring their conservation. Biol. Conserv. 2022, 273, 109663. [Google Scholar] [CrossRef]
- Tang, C.Q.; Lu, X.; Du, M.-R.; Xiao, S.-L.; Li, S.; Han, P.-B.; Zeng, J.-L.; Wen, J.-R.; Yao, S.-Q.; Shi, Y.-C.; et al. Forest characteristics and population structure of a threatened palm tree Caryota obtusa in the karst forest ecosystem of Yunnan, China. J. Plant Ecol. 2022, 15, 829–843. [Google Scholar] [CrossRef]
- McCune, B.; Mefford, M.J. PC-ORD: Multivariate Analysis of Ecological Data, Version 698 7.0 for Windows; Wild Blueberry Media: Corvallis, OR, USA, 2016. [Google Scholar]
- Lande, R. Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 1996, 76, 5–13. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Wu, Z.Y. The areal-types of Chinese genera of seed plants. Plant Divers. 1991, 13, 1–139. (In Chinese) [Google Scholar]
- Wu, Z.Y. The areal-types of the world families of seed plants. Revised version. Acta Bot. Yunnanica 2003, 5, 535–538. (In Chinese) [Google Scholar]
- Rubino, D.L.; McCarthy, B.C. Dendroclimatological analysis of white oak (Quercus alba L., Fagaceae) from an old-growth forest of southeastern Ohio, USA. J. Torrey Bot. Soc. 2000, 127, 240–250. [Google Scholar] [CrossRef]
- Miller, E.T.; Farine, D.R.; Trisos, C.H. Phylogenetic community structure metrics and null models: A review with new methods and software. Ecography 2017, 40, 461–477. [Google Scholar] [CrossRef]
- Webb, C.O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145–155. [Google Scholar] [CrossRef]
- Helmus, M.R.; Bland, T.J.; Williams, C.K.; Ives, A.R. Phylogenetic measures of biodiversity. Am. Nat. 2007, 169, E68–E83. [Google Scholar] [CrossRef]
- Du, W.B. Patterns of Plant Diversity and Formation Mechanism in the Kunlun Mountains; Lanzhou University: Lanzhou, China, 2021. [Google Scholar]
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. Available online: https://www.R-project.org/ (accessed on 23 September 2024).
- Smith, S.A.; Brown, J.W. Constructing a broadly inclusive seed plant phylogeny. Am. J. Bot. 2018, 105, 302–314. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Swenson, N.G. Phylogenetic resolution and quantifying the phylogenetic diversity and dispersion of communities. PLoS ONE 2009, 4, e4390. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Yang, Y.M.; Du, F. A Study on Seed Plant Floristic Characteristics of Tsuga dumosa Community in Lancangjiang Nature Reserve. J. West China For. Sci. 2007, 2, 80–85. (In Chinese) [Google Scholar]
- Wang, D.X. Growth law of Tsuga chinensis in Huoditang Forest Region. J. Northwest A F Univ. (Nat. Sci. Ed.) 2008, 4, 83–88. (In Chinese) [Google Scholar]
- Feng, X.L.; Hu, G.; Liu, Z.H. Research on the Community Characteristics and Population Dynamics of Tsuga chinesis var. tchekiangensis on Gaopo in Guiyang City. Guizhou For. Sci. Technol. 2011, 39, 26–29. (In Chinese) [Google Scholar]
- Li, J.; Liu, P.; Dong, G.; He, X.; Kang, B. Community Structure and Species Composition of Tsuga chinensis Natural Forests in Micangshan Nature Reserve of Shaanxi. Shaanxi For. Sci. Technol. 2014, 3, 35–38. (In Chinese) [Google Scholar]
- Chen, B.; Zhao, J.T.; Guan, Q.W. Community composition and structure of a mid-subtropical coniferous (Tsuga chinensis var. tchekiangensis) and broadleaf mixed forest in Jiangxi Wuyishan, China. Acta Ecol. Sin. 2018, 38, 7359–7372. (In Chinese) [Google Scholar]
- Suzuki, E.; Tsukahara, J. Age structure and regeneration of old growth Cryptomeria japonica forests on Yakushima Island. Bot. Mag. 1987, 100, 223–241. [Google Scholar] [CrossRef]
- Luo, J.W. Growth and biomass of Tsuga chinensis var. tchekiangensis in a natural forest in Guangze, Fujian, China. J. Fujian Coll. For. 2011, 31, 156–160. (In Chinese) [Google Scholar]
- Rossi, S.; DesLauriers, A.; Griçar, J.; Seo, J.-W.; Rathgeber, C.B.K.; Anfodillo, T.; Morin, H.; Levanic, T.; Oven, P.; Jalkanen, R. Critical temperatures for xylogenesis in conifers of cold climates. Glob. Ecol. Biogeogr. 2008, 17, 696–707. [Google Scholar] [CrossRef]
- Hood, S.; Sala, A. Ponderosa pine resin defenses and growth: Metrics matter. Tree Physiol. 2015, 35, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Puettmann, K.J.; Wilson, S.M.; Baker, S.C.; Donoso, P.J.; Drössler, L.; Amente, G.; Harvey, B.D.; Knoke, T.; Lu, Y.; Nocentini, S.; et al. Silvicultural alternatives to conventional even-aged forest management-what limits global adoption? For. Ecosyst. 2015, 2, 8. [Google Scholar] [CrossRef]
- Gautier-Hion, A.; Duplantier, J.M.; Quris, R.; Feer, F.; Sourd, C.; Decoux, J.P.; Dubost, G.; Emmons, L.; Erard, C.; Hecketsweiler, P.; et al. Fruit characters as a basis of fruit choice and seed dispersal in a tropical forest vertebrate community. Oecologia 1985, 65, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Peres, C.A. Synergistic effects of subsistence hunting and habitat fragmentation on Amazonian forest vertebrates. Conserv. Biol. 2001, 15, 1490–1505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).