An Elusive New Genus and Species of Subterranean Amphipod (Hadzioidea: Eriopisidae) from Barrow Island, Western Australia †
Abstract
1. Introduction
2. Materials and Methods
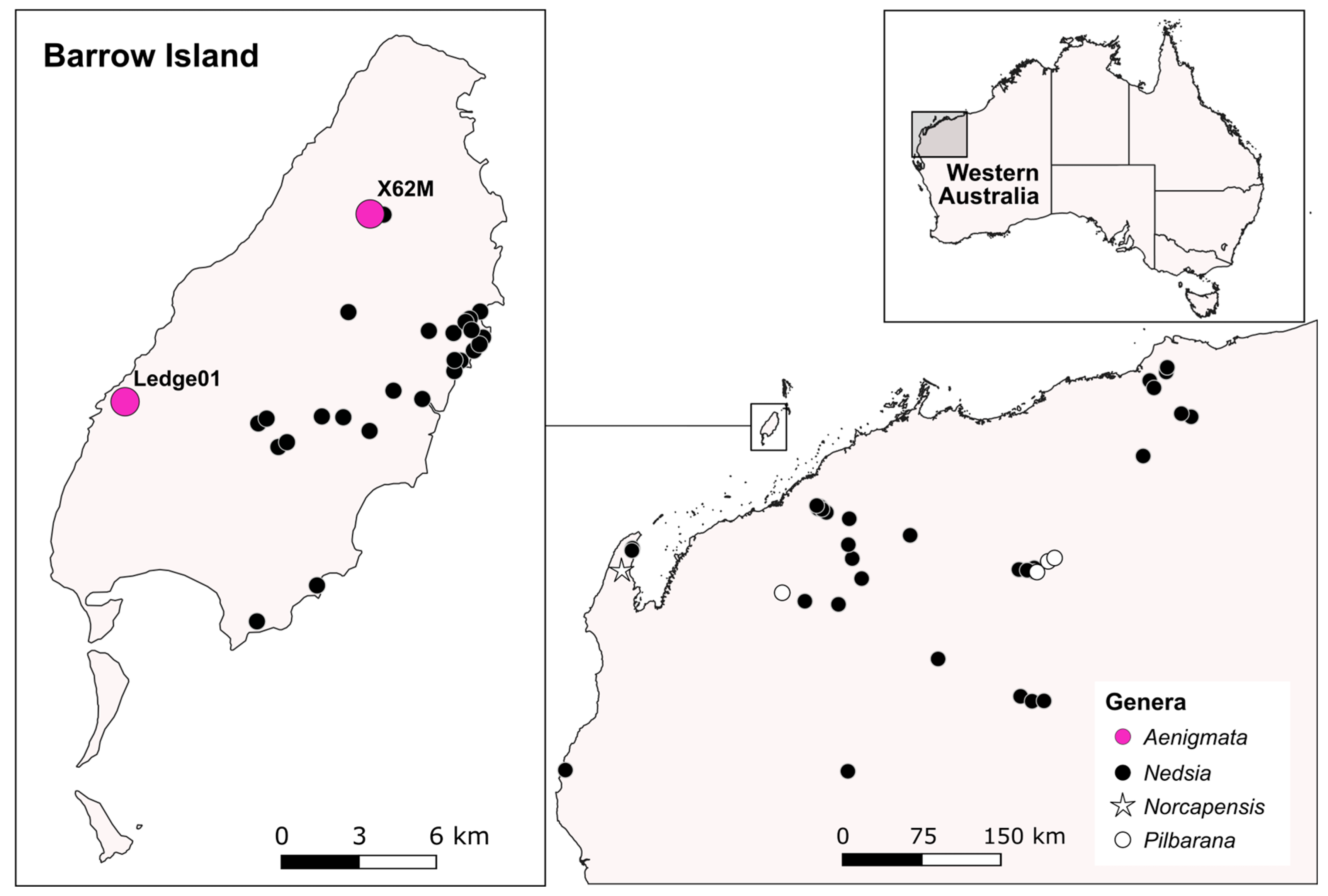
2.1. Study Site and Specimen Collection
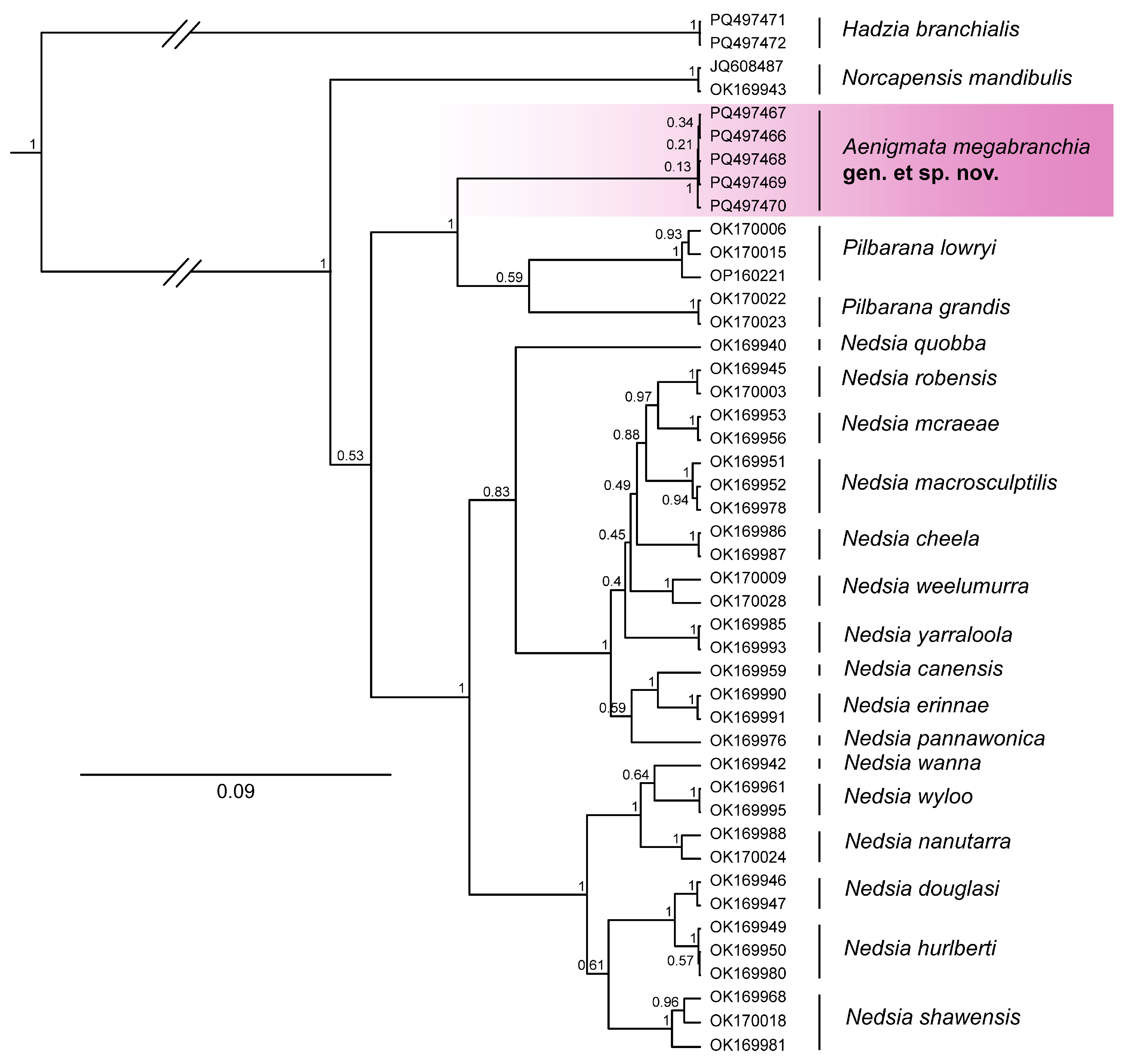
2.2. DNA Extraction, Sequencing, and Phylogenetics
2.3. Morphology
3. Results
Taxonomy
- Superfamily Hadzioidea S. Karaman, 1943 (Bousfield 1983)
- Family Eriopisidae Lowry & Myers, 2013
- Remarks
- 1.
- Gnathopod 2 propodus enlarged in both males and females, at least 3 times length of gnathopod 1 propodus...........................................................................................................2
- –
- Gnathopod 2 propodus no more than 2 times length of gnathopod 1 propodus..........3
- 2.
- Distinct antennal sinus; pereonite 1 with concave posterodistal corner, pereonites 2–7 laterally square-shaped, as long as broad, vermiform body shape; uropod 1 peduncle 2 times length of rami; uropod 3 outer ramus second article apically concave................................................................................................................................Pilbarana
- –
- No distinct antennal sinus; pereonite 1 lacking concave posterodistal corner, pereonites 2–7 around 2 times as long as broad, robust body shape; uropod 1 peduncle approximately equal in length to rami; uropod 3 outer ramus second article not apically concave .....................................................................................................................Norcapensis
- 3.
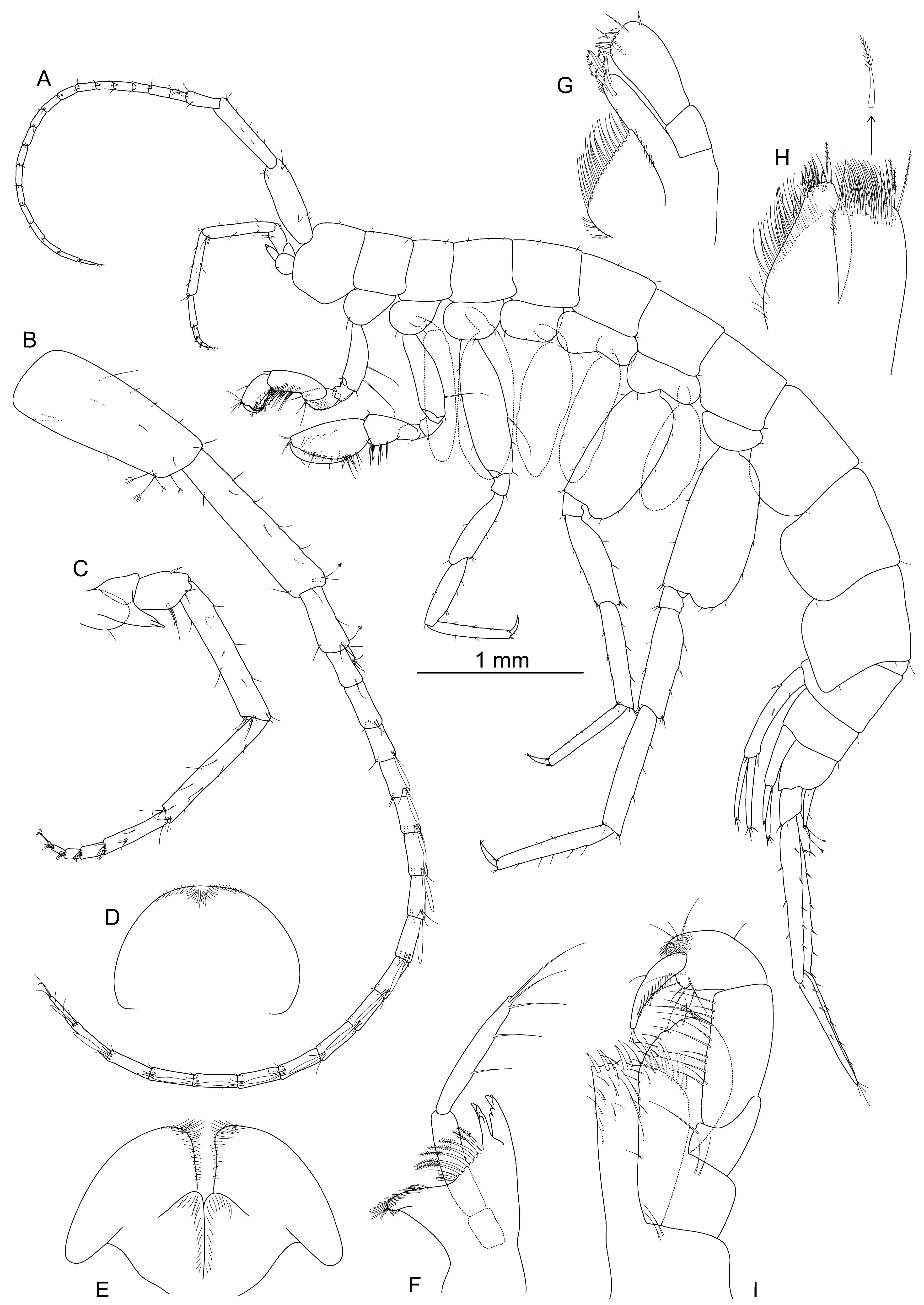
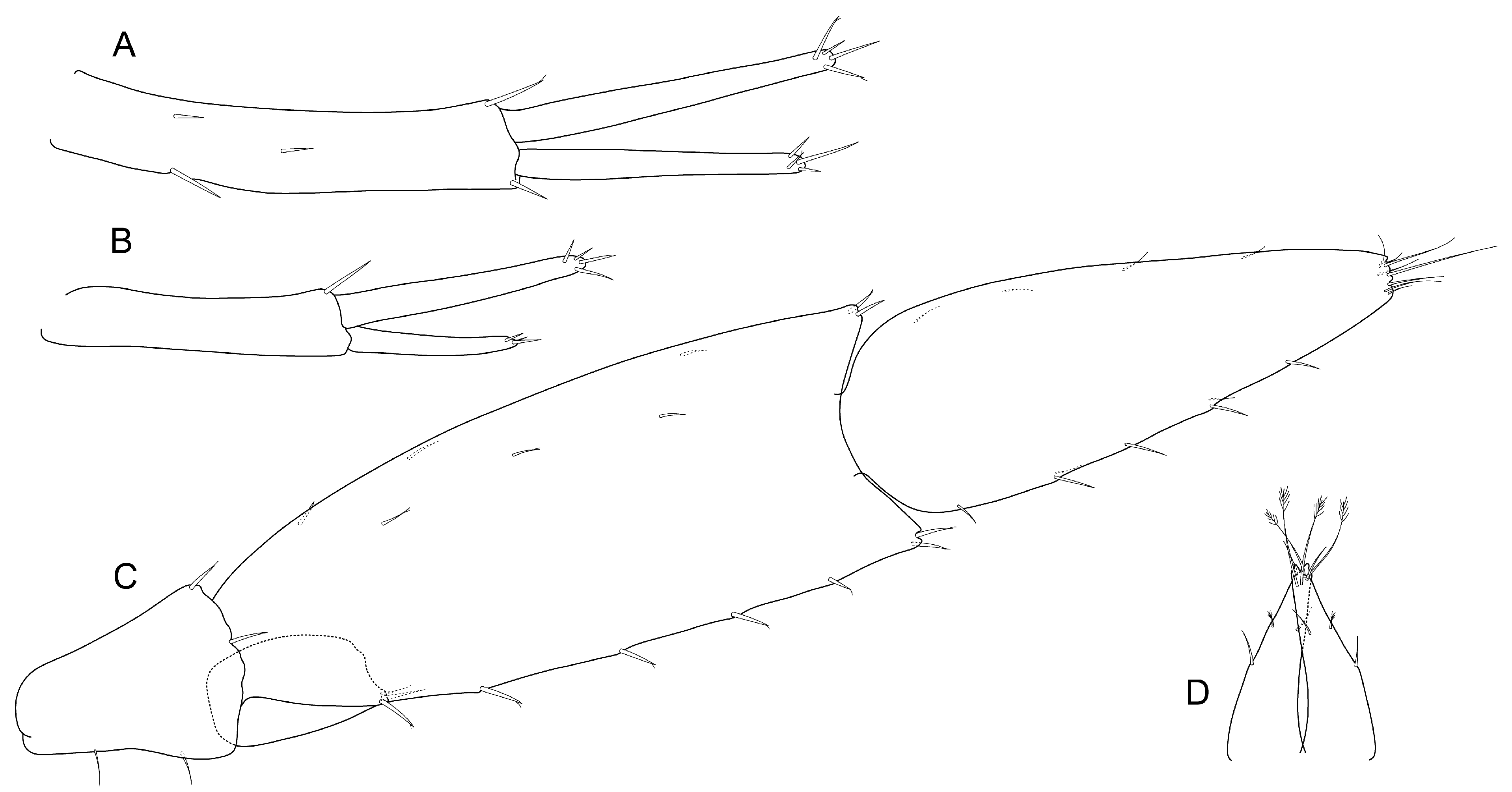
- Coxal gills elongated, around 2 or more times length of pereonites (Figure 3A); pereopods 5–7 bases with distinct lobe at posterodistal corner (Figure 4E–G)......................................................................................................................................Aenigmata gen. nov.
- –
- Coxal gills shorter than 2 times length of pereonites; pereopods 5–7 bases without lobe at posterodistal corner ...................................................................................................Nedsia

- Aenigmata Stringer & King gen. nov.
- urn:lsid:zoobank.org:act:0F7D34AB-0999-43CD-A2BC-F59BCB99F8CB
- Type species: Aenigmata megabranchia sp. nov.
- Diagnosis
- Description
- Etymology
- Remarks
- Aenigmata megabranchia Stringer & King sp. nov.
- urn:lsid:zoobank.org:act:70A76716-3FE2-44DE-AC13-71162E13BC9E
- Material examined
- Holotype: male, WAM C84784 (LN00302.1), bore X62M, Barrow Island, WA, 20°43′56″ S 115°25′34″ E, coll. N.B. Stevens, M. van der Heyde, and M. Jones, 1 December 2020.
- Description: Holotype, male, 5.5 mm
- Etymology
- Remarks
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lynch, A.J.J.; Beeton, R.J.S.; Greenslade, P. The conservation significance of the biota of Barrow Island, Western Australia. J. R. Soc. West. Aust. 2019, 102, 98–133. [Google Scholar]
- Chevron Australia. Final Environmental Impact Statement/Environmental Review and Management Programme for the Gorgon Gas Development; Chevron Australia: Perth, Australia, 2006; Volume 2, pp. 18–19. [Google Scholar]
- Moro, D.; Lagdon, R. History and environment of Barrow Island. Rec. West. Aust. Mus. Supp. 2013, 83, 1–8. [Google Scholar] [CrossRef]
- Humphreys, G.; Alexander, J.; Harvey, M.S.; Humphreys, W.F. The subterranean fauna of Barrow Island, north-western Australia: 10 years on. Rec. West. Aust. Mus. Supp. 2013, 83, 145–158. [Google Scholar] [CrossRef][Green Version]
- Majer, J.D.; Callan, S.K.; Edwards, K.; Gunawardene, N.R.; Taylor, C.K. Baseline survey of the terrestrial invertebrate fauna of Barrow Island. Rec. West. Aust. Mus. Supp. 2013, 83, 13–112. [Google Scholar] [CrossRef][Green Version]
- King, R.A.; Fagan-Jeffries, E.P.; Bradford, T.M.; Stringer, D.N.; Finston, T.L.; Halse, S.A.; Eberhard, S.M.; Humphreys, G.; Humphreys, B.F.; Austin, A.D.; et al. Cryptic diversity down under: Defining species in the subterranean amphipod genus Nedsia Barnard & Williams, 1995 (Hadzioidea: Eriopisidae) from the Pilbara, Western Australia. Invertebr. Syst. 2022, 36, 113–159. [Google Scholar] [CrossRef]
- Framenau, V.W. A new species in the ground spider genus Austrammo from Barrow Island, Western Australia (Araneae, Gnaphosidae). Aust. J. Taxon. 2023, 27, 1–6. [Google Scholar] [CrossRef]
- Humphreys, W.F. The subterranean fauna of Barrow Island, northwestern Australia, and its environment. Mem. Biospeol. 2001, 28, 107–127. [Google Scholar]
- Burbidge, A.A. Threatened Animals of Western Australia; Department of Conservation and Land Management: Perth, Australia, 2004; pp. 1–202. [Google Scholar]
- Hertzog, L. Crustacés de biotopes hypogées de la vallée du Rhin d’alsace. Bull. Soc. Zool. Fr. 1936, 61, 356–372. [Google Scholar]
- Bradbury, J.H.; Williams, W.D. Freshwater amphipods from Barrow Island, Western Australia. Rec. Aust. Mus. 1996, 48, 33–74. [Google Scholar] [CrossRef]
- Lowry, J.K.; Myers, A.A. A phylogeny and classification of the Senticaudata subord. nov. (Crustacea: Amphipoda). Zootaxa 2013, 3610, 1–80. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S. Die unterirdischen Amphipoden Südserbiens. Srp. Akad. Nauka Pos. Izd. 135 Prir. Matem. Spisi 1943, 34, 161–312. [Google Scholar]
- Bradbury, J.H.; Williams, W.D. Two new species of anchialine amphipod (Crustacea: Hadziidae: Liagoceradocus) from Western Australia. Rec. West. Aust. Mus. 1996, 17, 395–409. [Google Scholar]
- Bousfield, E.L. A new look at the systematics of gammaroidean amphipods of the world. Crustaceana Supp. 1977, 4, 282–316. [Google Scholar]
- Barnard, J.L.; Williams, W.D. The taxonomy of Amphipoda (Crustacea) from Australian fresh waters: Part 2. Rec. Aust. Mus. 1995, 47, 161–201. [Google Scholar] [CrossRef][Green Version]
- Bradbury, J.H.; Williams, W.D. The amphipod (Crustacea) stygofauna of Australia: Description of new taxa (Melitidae, Neoniphargidae, Paramelitidae), and a synopsis of known species. Rec. Aust. Mus. 1997, 49, 249–341. [Google Scholar] [CrossRef]
- Stringer, D.N.; King, R.A.; Austin, A.D.; Guzik, M.T. Pilbarana, a new subterranean amphipod genus (Hadzioidea: Eriopisidae) of environmental assessment importance from the Pilbara, Western Australia. Zootaxa 2022, 5188, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Chevreux, E. Sur quelques amphipodes nouveaux ou peu connus provenant des côtes de Bretagne. Bull. Soc. Zool. Fr. 1920, 45, 75–87. [Google Scholar]
- Barnard, J.L. Benthic marine Amphipoda of southern California: Families Tironidae to Gammaridae. Pac. Nat. 1962, 3, 73–115. [Google Scholar]
- Karaman, G.S.; Barnard, J.L. Classifactory revisions in gammaridean Amphipoda (Crustacea), part 1. Proc. Biol. Soc. Wash. 1979, 92, 106–165. [Google Scholar]
- Guzik, M.T.; Austin, A.D.; Cooper, S.J.B.; Harvey, M.S.; Humphreys, W.F.; Bradford, T.; Eberhard, S.M.; King, R.A.; Leys, R.; Muirhead, K.A.; et al. Is the Australian subterranean fauna uniquely diverse? Invertebr. Syst. 2011, 24, 407–418. [Google Scholar] [CrossRef]
- Saccò, M.; Guzik, M.T.; van der Heyde, M.; Nevill, P.; Cooper, S.J.B.; Austin, A.D.; Coates, P.J.; Allentoft, M.E.; White, N.E. eDNA in subterranean ecosystems: Applications, technical aspects, and future prospects. Sci. Total Environ. 2022, 820, 153223. [Google Scholar] [CrossRef] [PubMed]
- van der Heyde, M.; Alexander, J.; Nevill, P.; Austin, A.D.; Stevens, N.; Jones, M.; Guzik, M.T. Rapid detection of subterranean fauna from passive sampling of groundwater eDNA. Environ. DNA 2023, 5, 1706–1719. [Google Scholar] [CrossRef]
- van der Heyde, M.; White, N.E.; Nevill, P.; Austin, A.D.; Stevens, N.; Jones, M.; Guzik, M.T. Taking eDNA underground: Factors affecting eDNA detection of subterranean fauna in groundwater. Mol. Ecol. Resour. 2023, 23, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- QGIS Development Team. QGIS Geographic Information System Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 25 September 2024).
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.-H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST2: A software platform for Bayesian evolutionary analysis. PLoS Comp. Biol. 2014, 10, e1003537. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.M.; Lo, N.; Ho, S.Y.W. The impact of the tree prior on molecular dating of data sets containing a mixture of inter- and intraspecies sampling. Syst. Biol. 2017, 66, 413–425. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, K. The general lineage concept of species, species criteria, and the process of speciation. In Endless Forms: Species and Speciation; Howard, D.J., Berlocher, S.H., Eds.; Oxford University Press: New York, NY, USA, 1998; pp. 57–75. [Google Scholar]
- de Queiroz, K. Species concepts and species delimitation. Syst. Biol. 2007, 56, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Havermans, C.; Sonet, G.; d’Udekem d’Acoz, C.; Nagy, Z.T.; Martin, P.; Brix, S.; Riehl, T.; Agrawal, S.; Held, C. Genetic and morphological divergences in the cosmopolitan deep-sea amphipod Eurythenes gryllus reveal a diverse abyss and a bipolar species. PLoS ONE 2013, 8, e74218. [Google Scholar] [CrossRef] [PubMed]
- Tempestini, A.; Rysgaard, S.; Dufresne, F. Species identification and connectivity of marine amphipods in Canada’s three oceans. PLoS ONE 2018, 13, e0197174. [Google Scholar] [CrossRef] [PubMed]
- Krapp-Schickel, T. What has happened with the Maera-clade (Crustacea, Amphipoda) during the last decades? Boll. Mus. Civ. Stor. Nat. Verona 2008, 32, 3–32. [Google Scholar]
- Bousfield, E.L. Shallow-Water Gammaridean Amphipoda of New England; Cornell University Press: Ithaca, NY, USA, 1973; 312p. [Google Scholar]
- Harvey, M.S. Short-range endemism amongst the Australian fauna: Some examples from non-marine environments. Invertebr. Syst. 2002, 16, 555–570. [Google Scholar] [CrossRef]
- Guzik, M.T.; Stringer, D.N.; Murphy, N.P.; Cooper, S.J.B.; Taiti, S.; King, R.A.; Humphreys, W.F.; Austin, A.D. Molecular phylogenetic analysis of Australian arid-zone oniscidean isopods (Crustacea: Haloniscus) reveals strong regional endemicity and new putative species. Invertebr. Syst. 2019, 33, 556–574. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stringer, D.N.; King, R.A.; Austin, A.D.; Guzik, M.T. An Elusive New Genus and Species of Subterranean Amphipod (Hadzioidea: Eriopisidae) from Barrow Island, Western Australia. Diversity 2025, 17, 84. https://doi.org/10.3390/d17020084
Stringer DN, King RA, Austin AD, Guzik MT. An Elusive New Genus and Species of Subterranean Amphipod (Hadzioidea: Eriopisidae) from Barrow Island, Western Australia. Diversity. 2025; 17(2):84. https://doi.org/10.3390/d17020084
Chicago/Turabian StyleStringer, Danielle N., Rachael A. King, Andrew D. Austin, and Michelle T. Guzik. 2025. "An Elusive New Genus and Species of Subterranean Amphipod (Hadzioidea: Eriopisidae) from Barrow Island, Western Australia" Diversity 17, no. 2: 84. https://doi.org/10.3390/d17020084
APA StyleStringer, D. N., King, R. A., Austin, A. D., & Guzik, M. T. (2025). An Elusive New Genus and Species of Subterranean Amphipod (Hadzioidea: Eriopisidae) from Barrow Island, Western Australia. Diversity, 17(2), 84. https://doi.org/10.3390/d17020084






