The Enigmatic Hadal Ophiuroid Has Found Its Place: A New Family Abyssuridae Links Ultra-Abyssal and Shallow-Water Fauna
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Nomenclatural Acts
2.3. Morphological Analysis
2.4. Molecular Analysis
3. Results
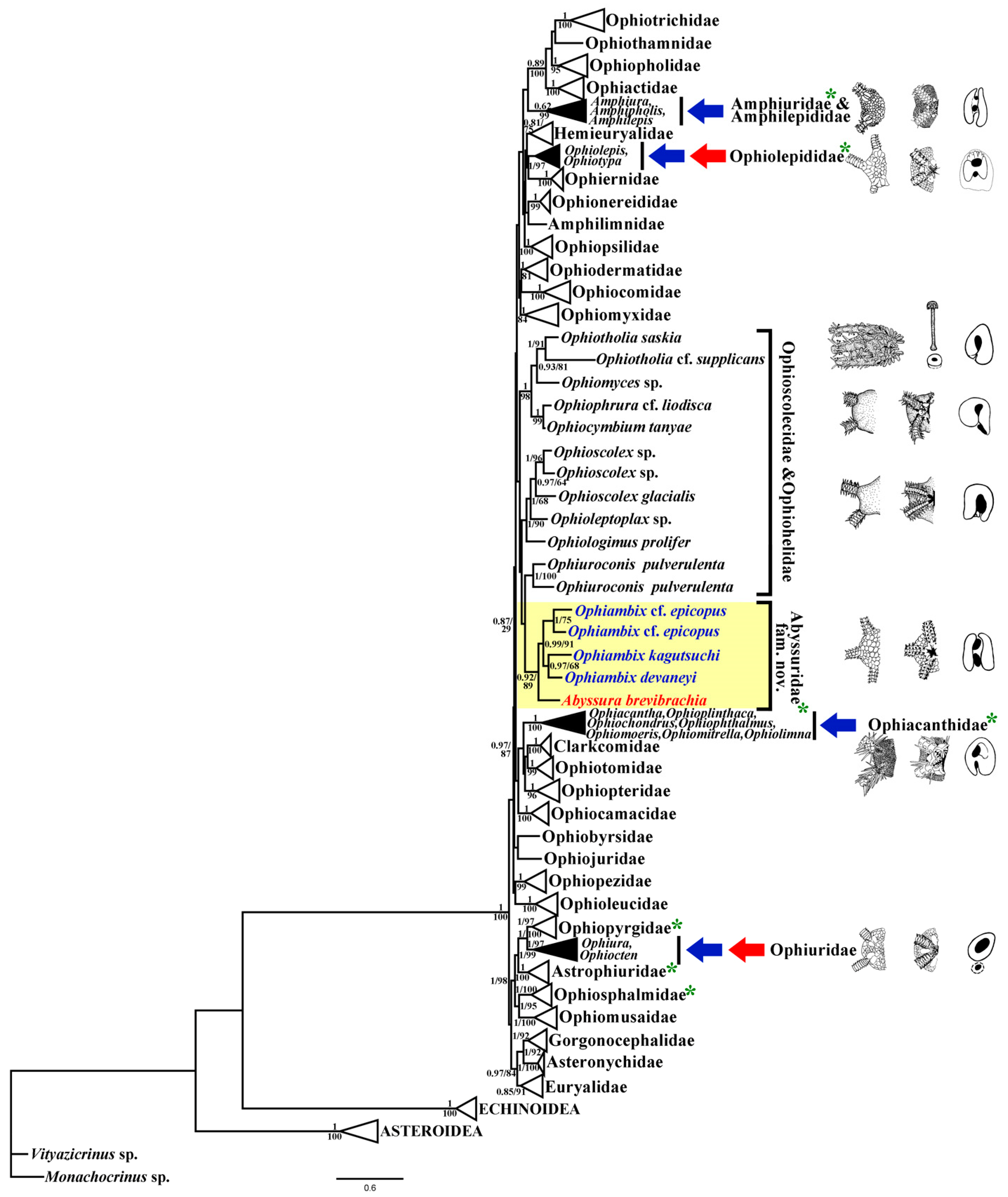
3.1. Molecular Phylogeny
3.2. Systematic Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martynov, A.V. Reassessment of the classification of the Ophiuroidea (Echinodermata), based on morphological characters. I. General character evaluation and delineation of the families Ophiomyxidae and Ophiacanthidae. Zootaxa 2010, 2697, 1–154. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Hugall, A.F.; Thuy, B.; Stöhr, S.; Martynov, A.V. Restructuring higher taxonomy using broad-scale phylogenomics: The living Ophiuroidea. Mol. Phyl. Evol. 2017, 107, 415–430. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Hugall, A.F.; Woolley, S.N.C.; Bribiesca-Contreras, G.; Bax, N.J. Contrasting processes drive ophiuroid phylodiversity across shallow and deep seafloors. Nature 2019, 565, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.; O’Hara, T.D.; Hugall, A.F.; Martinez Arbizu, P. Dark ophiuroid biodiversity in a prospective abyssal mine field. Curr. Biol. 2019, 29, 3909–3912.e3. [Google Scholar] [CrossRef]
- O’Hara, T.D.; Stohr, S.; Hugall, A.F.; Thuy, B.; Martynov, A.V. Morphological diagnoses of higher taxa in Ophiuroidea (Echinodermata) in support of a new classification. Europ. J. Taxon. 2018, 416, 1–35. [Google Scholar] [CrossRef]
- Wolf, T. The concept of the hadal or ultra-abyssal fauna. Deep-Sea Res. 1970, 17, 983–1003. [Google Scholar] [CrossRef]
- Mironov, A.N.; Kremenetskaia, A. Distribution of hadal genera depends on the lower limits of their bathymetrical ranges. Deep Sea Res. Part I Oceanogr. Res. Pap. 2022, 185, 103787. [Google Scholar] [CrossRef]
- Belyaev, G.M.; Litvinova, N.M. New and rare species of the deep water Ophiuroidea from Pacific and Indian oceans. Proc. Shirshov Instit. Oceanol. 1976, 99, 126–139. [Google Scholar]
- Paterson, G.L.J. The deep-sea Ophiuroidea of the North Atlantic Ocean. Bull. British Mus. Nat. Hist. 1985, 49, 1–162. [Google Scholar]
- Belyaev, G.M. Deep-Sea Oceanic Trenches and Their Fauna; Nauka: Moscow, Russia, 1989; 385p. [Google Scholar]
- Mironov, A.N.; Dilman, A.B.; Gebruk, A.V.; Kremenetskaia, A.V.; Minin, K.V.; Smirnov, I.S. Echinoderms of the Kuril-Kamchatka Trench. Prog. Ocean. 2019, 179, 102217. [Google Scholar] [CrossRef]
- Paterson, G.L.J.; Baker, A. A revision of the genus Ophiambix (Echinodermata: Ophiuroidea) including the description of a new species. J. Nat. Hist. 1988, 22, 1579–1590. [Google Scholar] [CrossRef]
- Okanishi, M.; Kato, M.; Watanabe, H.K.; Chen, C.; Fujita, T. Large populations of two new species of Ophiambix (Echinodermata, Ophiuroidea) discovered on Japanese hot vents and cold seeps. Raffl. Bull. Zool. 2020, 68, 196–213. [Google Scholar]
- Martynov, A.V. Structure of the arm spine articulation ridges as a basis for taxonomy of Ophiuroidea (a preliminary report). In Proceedings of the Twelfth International Echinoderm Conference, Durham, NH, USA, 6–12 August 2006; Harris, L., Ed.; Balkema: Rotterdam, The Netherlands, 2010; pp. 233–239. [Google Scholar]
- Litvinova, N.M. The brittle-stars of the genus Amphiophiura of the Pacific and Indian Oceans collected by the soviet expeditions on the RV ‘Vityaz’ and ‘Academik Kurchatov’. Proc. Shirshov Instit. Oceanol. 1971, 92, 298–316. [Google Scholar]
- Stöhr, S.; O’Hara, T.D. Deep-sea Ophiuroidea (Echinodermata) from the Danish Galathea II Expedition, 1950–1952, with taxonomic revisions. Zootaxa 2021, 4963, 505–529. [Google Scholar] [CrossRef]
- Belyaev, G.M.; Litvinova, N.M. New genera and species of deep sea Ophiuroidea. Bull. Mos. Soc. Nat. Investig. 1972, 3, 5–20. [Google Scholar]
- Bribiesca-Contreras, G.; Dahlgren, T.G.; Amon, D.J.; Cairns, S.; Drennan, R.; Durden, J.M.; Eléaume, M.P.; Hosie, A.M.; Kremenetskaia, A.; McQuaid, K.; et al. Benthic megafauna of the western Clarion-Clipperton Zone, Pacific Ocean. ZooKeys 2022, 1113, 1–110. [Google Scholar] [CrossRef]
- Christodoulou, M.; O’Hara, T.; Hugall, A.F.; Khodami, S.; Rodrigues, C.F.; Hilario, A.; Vink, A.; Martinez Arbizu, P. Unexpected high abyssal ophiuroid diversity in polymetallic nodule fields of the northeast Pacific Ocean and implications for conservation. Biogeosciences 2020, 17, 1845–1876. [Google Scholar] [CrossRef]
- Nethupul, H.; Stöhr, S.; Zhang, H. Deepest known novel species of the genus Ophiuroglypha Hertz, 1927 (Echinodermata: Ophiuroidea) from the central rift zone, Philippine Sea. Europ. J. Tax. 2023, 891, 167–185. [Google Scholar] [CrossRef]
- O’Hara, T.D.; England, P.R.; Gunasekera, R.M.; Naughton, K.M. Limited phylogeographic structure for five bathyal ophiuroids at continental scales. Deep-Sea Res. Part I 2014, 84, 18–28. [Google Scholar] [CrossRef]
- Dayrat, B.; Tillier, A.; Lecointre, G.; Tillier, S. New clades of euthyneuran gastropods (Mollusca) from 28S rRNA sequences. Mol. Phyl. Evol. 2001, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Le, H.L.V.; Lecointre, G.; Perasso, R. A 28S rRNA based phylogeny of the Gnathostomes: First steps in the analysis of conflict and congruence with morphologically based cladograms. Mol. Phyl. Evol. 1993, 2, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucl. Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis Version 11. Mol. Biol. Evol. 2012, 38, 3022–3027. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J.A. Rapid bootstrap algorithm for the RAxML web-servers. Syst. Biol. 2008, 75, 758–771. [Google Scholar] [CrossRef]
- Lyman, T. Ophiuridae and Astrophytidae of the “Challenger” expedition. Part I. Bull. Mus. Comp. Zool. Harvard Coll. 1878, 5, 65–168. [Google Scholar]
- Clark, H.L. North Pacific Ophiurans in the Collection of the United States National Museum; US Government Printing Office: Washington, DC, USA, 1911; Volume 75, pp. 1–302.
- Matsumoto, H. A monograph of Japanese Ophiuroidea, arranged according to a new classification. J. Coll. Sci. Imp. Univ. Tokyo 1917, 38, 1–408. [Google Scholar]
- O’Hara, T.D.; Thuy, B.; Hugall, A.F. Relict from the Jurassic: New family of brittle-stars from a New Caledonian seamount. Proc. R. Soc. B 2021, 288, 20210684. [Google Scholar] [CrossRef]
- Eichsteller, A.; Martynov, A.V.; O’Hara, T.D.; Christodoulou, M.; Korshunova, T.A.; Bribiesca-Contreras, G.; Martinez Arbizu, P. Ophiotholia (Echinodermata: Ophiuroidea): A little known deep-sea genus present in polymetallic nodule fields with the description of a new species. Front. Mar. Sci. 2023, 10, 1056282. [Google Scholar] [PubMed]
- Bartsch, I. Ophiambix meteoris n. sp., ein neuer Schlangenstern aus der Iberische Tiefsee. Spixiana 1983, 6, 97–100. [Google Scholar]
- Martynov, A.V.; Lundin, K.; Korshunova, T. Ontogeny, phylotypic periods, paedomorphosis, and ontogenetic systematics. Front. Ecol. Evol. 2022, 10, 806414. [Google Scholar] [CrossRef]
- Worsaae, K.; Rouan, A.; Seaver, E.; Miyamoto, N.; Tilic, E. Postembryonic development and male paedomorphosis in Osedax (Siboglinidae, Annelida). Front. Neurosci. 2024, 18, 1369274. [Google Scholar] [CrossRef] [PubMed]


| Species (Alphabetic Order) | Previous Family Placement | Current Family Placement | Depth Range, Meters | Distribution | Molecular Data | Reference |
|---|---|---|---|---|---|---|
| Abyssura brevibrachia Belyaev & Litvinova, 1976 | Ophiuridae, Ophiolepididae s.str., “Ophiurida incertae sedis” | Abyssuridae fam. nov. | 6156–7295 | Japan, Kuril-Kamchatic, Aleut Trenches | Present study | [1,8,14]; present study |
| Amphiophiura bullata (Thomson, 1877) | Ophiuridae, Ophiolepididae s.l. | Ophiopyrgidae | 2268–6035 | Atlantic, Indian Oceans, Antarctic waters | [4] | [10,15,16] Taxonomy of “A. bullata” complex is unresolved |
| Amphiophiura bullata vitjazi Litvinova, 1971 | Ophiuridae, Ophiolepididae s.l. | Ophiopyrgidae | 6810 | Ryukyu Trench | Absent | [15], it was recorded from North Atlantic (5100 m) [9] without confirmation; assignment of data for this taxon in [4] is uncertain |
| Amphiophiura convexa (Lyman, 1878) | Ophiuridae, Ophiolepididae s.l. | Ophiopyrgidae | 1950–6810 | North Pacific | [4] | [10,15,16] |
| Bathylepta pacifica Belyaev & Litvinova, 1972 | Ophioleucidae | “Ophiurida incertae sedis” | 5740–8006 | Bougainville, New Hebrides Trenches | Absent | [1,5,10,17] |
| Ophiacantha cosmica Lyman, 1878, s.l. | Ophiacanthidae | Ophiacanthidae | 3566–6235 | Pacific Ocean, Antarctic waters | [4] | [16,17]; present study |
| Ophiocymbium rarispinum Martynov, 2010 | Ophiacanthidae, Ophiomyxidae s.l. | “Ophioscolecidae” s.l. | 6740–6850 | Izu-Bonin Trench | Absent | [1] O. cf. rarispinum was reported in [18] from Clarion-Clipperton Zone (5206 m) |
| Ophiocymbium tanyae Martynov, 2010 | Ophiacanthidae, Ophiomyxidae s.l. | “Ophioscolecidae” s.l. | 5204–6850 | Izu-Bonin Trench, Clarion-Clipperton Zone | [18,19] | [1,18,19]; present study |
| Ophioplinthus madseni (Belyaev & Litvinova, 1972) | Ophiuridae, Ophiolepididae s.l. | Ophiopyrgidae | 6675–7230 | Kuril-Kamchatic, Japan Trenches | Absent | [10,17] |
| Ophiotypa simplex Koehler, 1897 | Ophiuridae | Ophiolepididae | 3595–6487 | Atlantic, Indian, Pacific Oceans, Javanese Trench | [4] | [4,9,10]; present study |
| Ophiura bathybia H.L. Clark, 1911 | Ophiuridae, Ophiolepididae s.l. | Ophiuridae | 2870–6328 | North Pacific, including Aleutic and Kuril-Kamchatic Trenches | Absent | [10] |
| Ophiuroglypha fendouzhe Nethupul, Stöhr, Zhang, 2023 | Ophiopyrgidae | Ophiopyrgidae | 7729 | Philippine sea, central rift zone | [20] | [20] |
| Ophiuroglypha irrorata (Lyman, 1878) s.l. | Ophiuridae, Ophiolepididae s.l. | Ophiopyrgidae | 2510–7340 (lowest depth ranges for O. irrorata s.l. are given as 91 m [10] or 403 m [9], which definitely belong to a different species) | Atlantic, Indian, and Pacific Oceans | [4] | [10] |
| Perlophiura profundissima Belyaev & Litvinova, 1972 | Ophiuridae | Ophiosphalmidae | 2265–8135 | Atlantic, Indian and Pacific Oceans | [4] | [4,9,10,17]; present study |
| Abyssuridae fam. nov. | Ophiuridae | Ophiopyrgidae | Ophiacanthidae | “Ophioscolecidae” s.l. | Ophiohelidae | Amphiuridae | Ophiolepididae | |
|---|---|---|---|---|---|---|---|---|
| Disk | Fully scaled, naked or with granules, without evident, thick skin | Fully scaled, commonly naked, without evident, thick skin | Fully scaled, commonly naked, without evident skin, thick skin in a few currently included taxa | Fully scaled, commonly with some evident amount of skin, spines and granules, or naked (without evident skin) in some taxa | In type genus of family (Ophioscolex) thick skin on disk and arms, and no disk scales, in several other currently included taxa (e.g., Ophiophrura) some disk scales are present | Fully scaled, sac-shaped | Fully scaled, commonly naked, without evident or with thick skin | Fully scaled, commonly naked, without evident, thick skin |
| Arms position in relation to disk | Adjacent to disk only at basal segments | Adjacent to disk only at basal segments | Adjacent to disk only at basal segments | Adjacent to disk only at basal segments | Adjacent to disk only at basal segments | Commonly adjacent to sac-shaped disk along its whole length | Adjacent to disk only at basal segments | Adjacent to disk only at basal segments |
| Arm spine articulation on lateral arm plates | Subparallel ridges open at both ends, framing two openings nearly equal in size | Large irregularly rounded muscle opening on elevation and nerve opening at basis of this knob, dorsal-most articulation usually placed at an angle in relation to nerve opening. In juvenile specimens, nerve opening is reduced | Large irregularly- rounded muscle opening on elevation and nerve opening at basis of this knob, dorsal-most articulation is usually placed at angle in relation to nerve opening. In some small-sized taxa and in juvenile specimens, nerve opening is reduced | Volute-shaped articulations with large muscle opening and small slit-shaped nerve opening, and sigmoidal fold | In type genus of family, Ophioscolex, single-opening pattern, sometimes horseshoe-shaped with broad low border. In other currently included representatives can be double-opening articulations, somewhat in part subparallel with low dorsal lobe (e.g., Ophiophrura, Ophiologimus) | Articulation with nearly vertical dorsal and ventral lobes, connected at their proximal ends, opening into wide y- or horseshoe-shaped, bordering single muscle/nerve opening, dorsal lobe larger and bent | Nearly parallel, quite often slightly curved high ridges, elongated in proximal- distal direction, framing two openings of nearly equal | Parallel or subparallel ridges open at both ends, framing two openings nearly equal in size. The ridges are considerably depressed on the lateral plate |
| Umbrella (parasol)-shaped spines | Absent in all genera | Absent in all genera | Absent in all genera | Absent in all genera | Absent in all genera | Present in two from three currently included genera | Absent in all genera | Absent in all genera |
| Vertebrae | Not keeled, streptospondylous articulation | Keeled, zygospondylous articulation | Keeled, zygospondylous articulation | Keeled, zygospondylous, or rarely reduced towards streptospondy-lous articulation | Not keeled zygospondylous in type genus Ophioscolex, to partly keeled and zygospondylous (e.g., in Ophiophrura), or streptospondylous | Partly keeled with specific articulation | Keeled, zygospondylous articulation | Keeled, zygospondylous articulation |
| Tentacle pores | Large | Moderate | Moderate | Moderate | Large | Moderate | Moderate | Moderate |
| Tentacle scales | Reduced or absent | Commonly well-defined | Commonly well-defined | Commonly well-defined | Absent or reduced | Well-defined | Well-defined | Well-defined |
| Radial shield | Present | Present | Present | Present | Present | Absent | Present | Present |
| Oral and tooth papillae | Weakly differentiated, short | Well-differentiated, commonly spiniform or elongate | Well-differentiated, commonly spiniform or elongate, in small taxa may become short | Well-differentiated, commonly oval to elongate | Well-differentiated, commonly spiniform | Well-differentiated, long, flattened, densely placed on jaws | Well-differentiated, elongate or spiniform, tooth papillae commonly double | Well-differentiated, elongate to square |
| Teeth | Weakly differentiated, few in number, spiniform | Well-differentiated, spiniform, numerous | Well-differentiated, spiniform, numerous, in small taxa may become fewer | Well-differentiated, commonly broadly flattened and numerous | Well-differentiated, spiniform, numerous | Well-differentiated, spiniform, numerous | Well-differentiated commonly broadly flattened and numerous | Well-differentiated commonly broadly flattened and numerous |
| Oral shield | Weakly differentiated | Well-differentiated | Well-differentiated | Well-differentiated | Weakly differentiated in genus Ophioscolex, in other taxa well-differentiated | Well-differentiated | Well-differentiated | Well- differentiated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martynov, A.; Korshunova, T. The Enigmatic Hadal Ophiuroid Has Found Its Place: A New Family Abyssuridae Links Ultra-Abyssal and Shallow-Water Fauna. Diversity 2025, 17, 827. https://doi.org/10.3390/d17120827
Martynov A, Korshunova T. The Enigmatic Hadal Ophiuroid Has Found Its Place: A New Family Abyssuridae Links Ultra-Abyssal and Shallow-Water Fauna. Diversity. 2025; 17(12):827. https://doi.org/10.3390/d17120827
Chicago/Turabian StyleMartynov, Alexander, and Tatiana Korshunova. 2025. "The Enigmatic Hadal Ophiuroid Has Found Its Place: A New Family Abyssuridae Links Ultra-Abyssal and Shallow-Water Fauna" Diversity 17, no. 12: 827. https://doi.org/10.3390/d17120827
APA StyleMartynov, A., & Korshunova, T. (2025). The Enigmatic Hadal Ophiuroid Has Found Its Place: A New Family Abyssuridae Links Ultra-Abyssal and Shallow-Water Fauna. Diversity, 17(12), 827. https://doi.org/10.3390/d17120827






