Elevation-Driven Variations in Species Composition and Biodiversity in a Protected Temperate Forest, Mount Gyebangsan, Korea
Abstract
1. Introduction
2. Materials and Methods
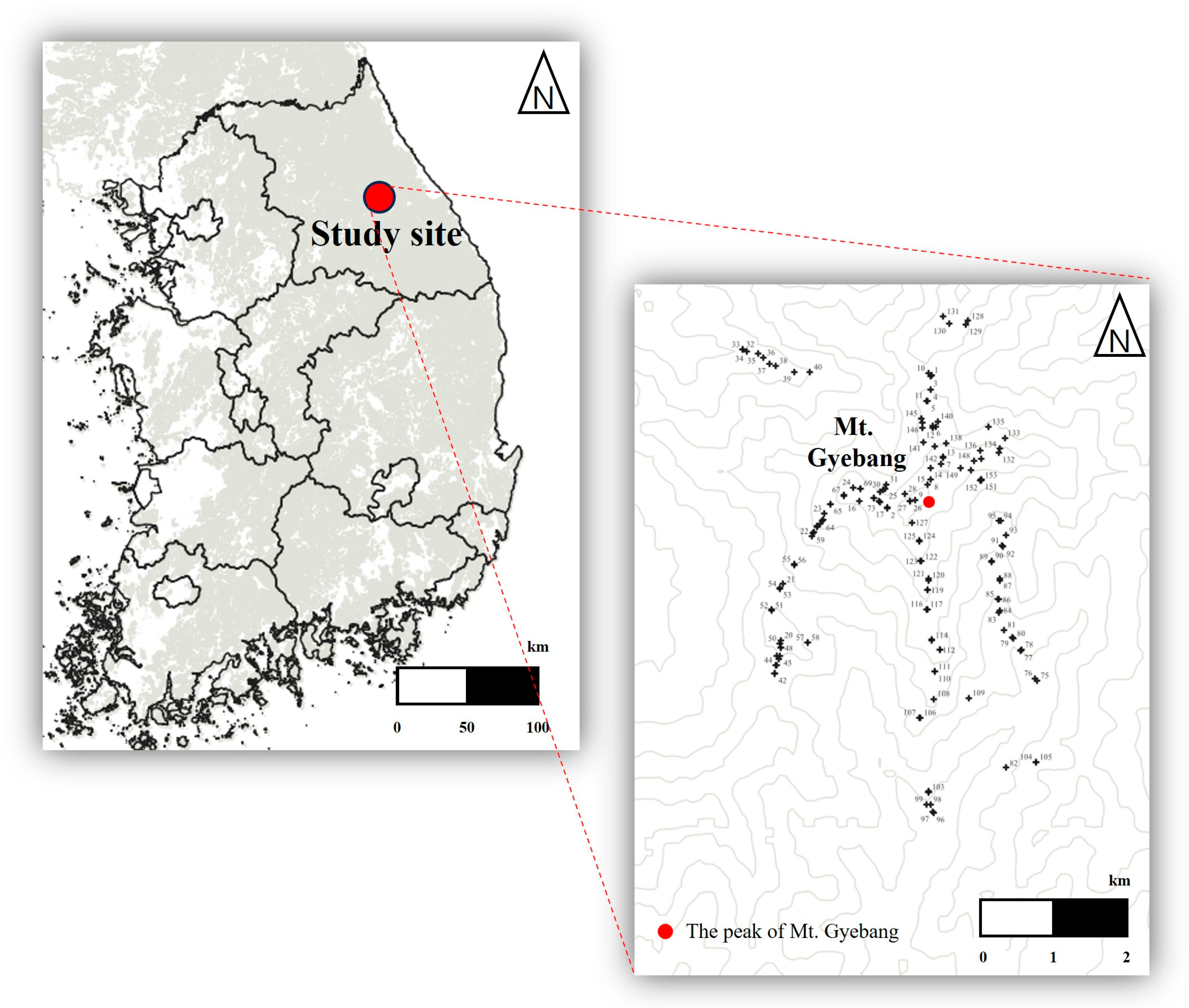
2.1. Study Area
2.2. Vegetation Sampling and Survey
2.3. Data Analysis
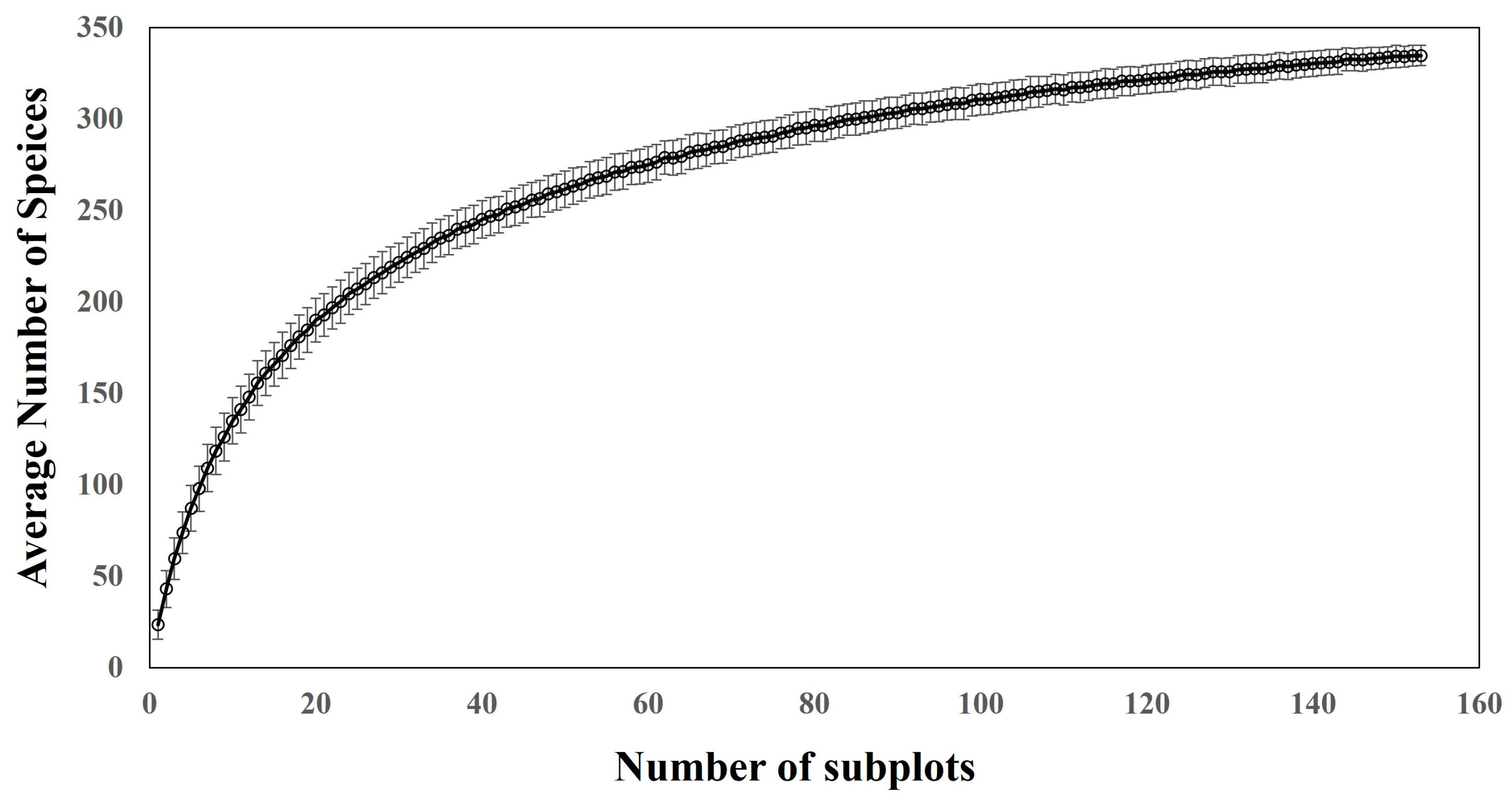
2.3.1. Plot Adequacy and Elevation Grouping
2.3.2. Environmental Variables
2.3.3. Diversity and Community Composition
2.3.4. Multivariate Analyses
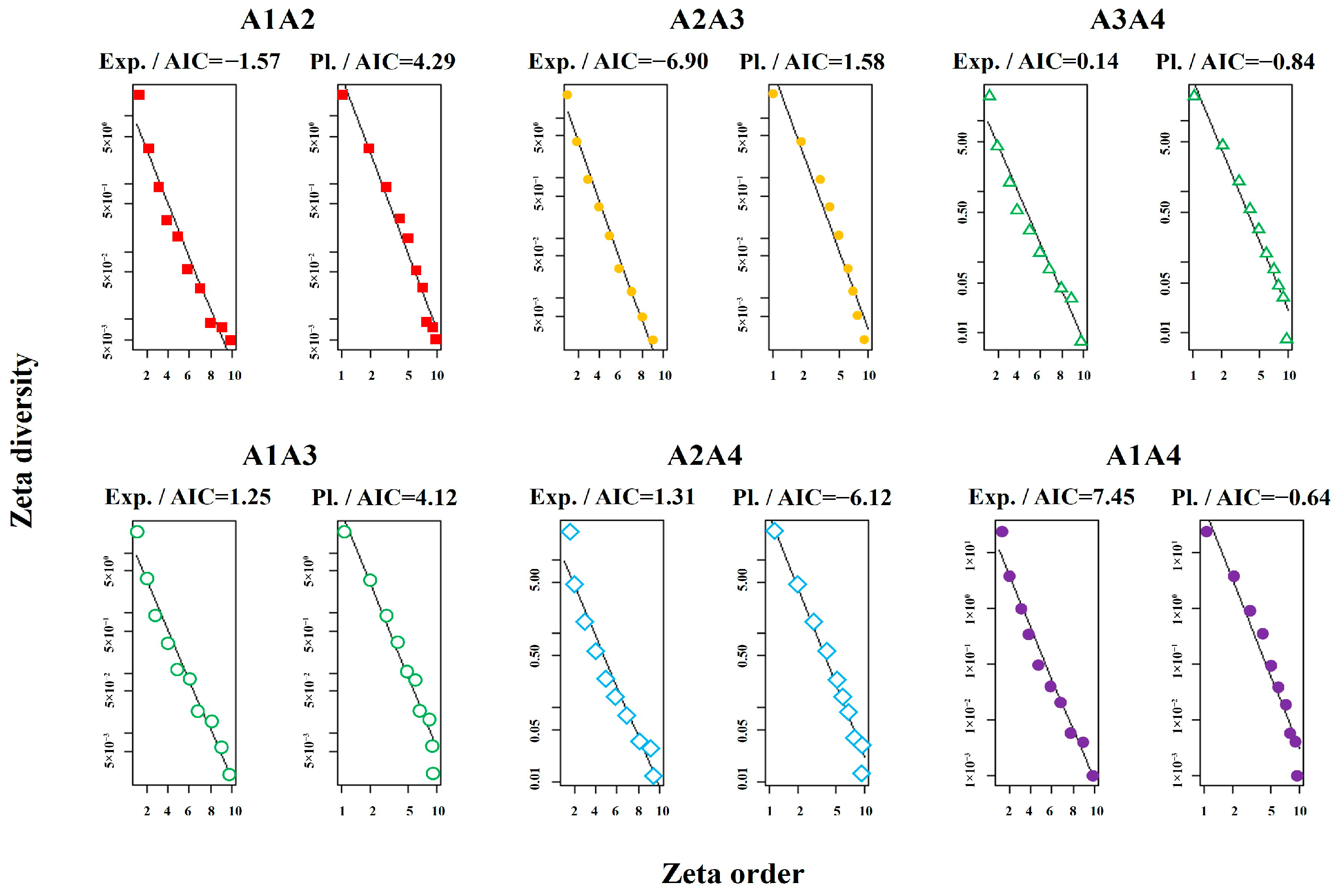
2.3.5. Zeta Diversity and Turnover Modeling
2.3.6. Statistical Testing
3. Results
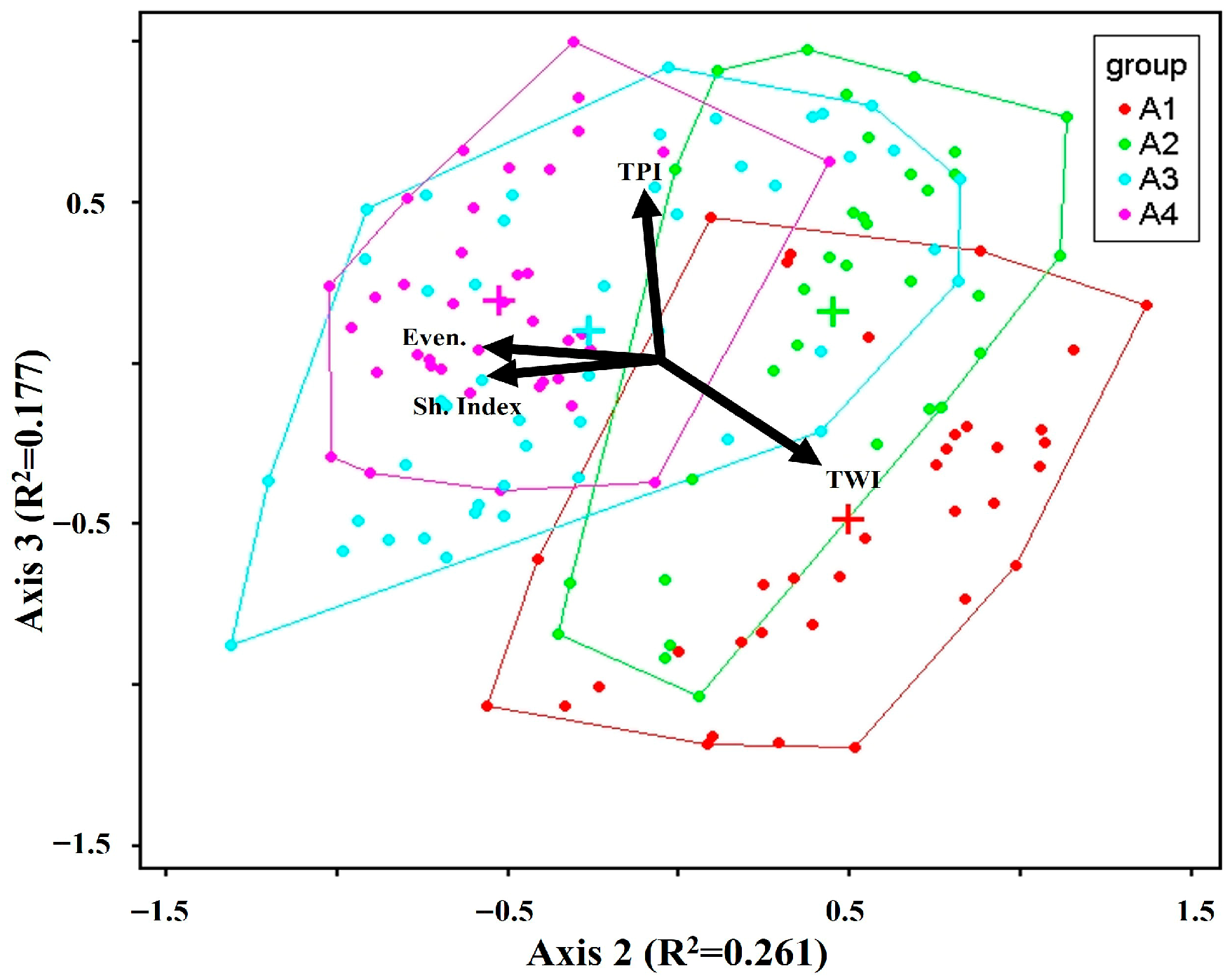
3.1. Species Composition and Environmental Factors
3.2. Biodiversity
4. Discussion
4.1. Species Composition and Environmental Factors
4.2. Biodiversity
4.3. Limitation and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMDS | non-metric multidimensional scaling |
| MRPP | multi-response permutation procedure |
| T | test statistic |
| A | within-group agreement |
| AIC | Akaike’s information criterion |
| TPI | topographic position index |
| TWI | topographic wetness index |
| RC | relative coverage |
| RF | relative frequency |
References
- Whittaker, R.H. Evolution and measurement of species diversity. Taxon 1972, 21, 213–251. [Google Scholar] [CrossRef]
- MacArthur, R.H. Patterns of species diversity. Biol. Rev. 1965, 40, 510–533. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; pp. 72–161. ISBN 9780632056330. [Google Scholar]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the alpine zone. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- McCune, B.; Grace, J.B. Analysis of Ecologcial Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; pp. 15–204. ISBN 9780972129008. [Google Scholar]
- Hui, C.; McGeoch, M.A. Zeta diversity as a concept and metric that unifies incidence-based biodiversity patterns. Am. Nat. 2014, 184, 684–694. [Google Scholar] [CrossRef]
- Latombe, G.; McGeoch, M.A.; Nipperess, D.A.; Hui, C. zetadiv: Functions to compute compositional turnover using zeta diversity. Methods Ecol. Evol. 2018, 9, 431–442. [Google Scholar]
- Koo, K.A.; Park, S.U. A review of ecological niche theory from the early 1000s to the present. Korean J. Environ. Ecol. 2021, 35, 316–335. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Available online: https://www.cbd.int/gbf (accessed on 29 July 2025).
- Lee, W.T. Coloured Standard Illustrations of Korean Plants; Academy Books: Seoul, Republic of Korea, 1996; pp. 1–1688. ISBN 9788976161598. [Google Scholar]
- Kim, C.H. A study on the plant community classification of Korean forests. Korean J. Ecol. 1992, 15, 125–140. [Google Scholar]
- Yun, S.H.; Kim, J.H.; Lee, J.H. Effects of habitat fragmentation on plant species diversity in Korean forests. J. Ecol. Environ. 2016, 39, 341–355. [Google Scholar]
- Choi, H.S.; Park, S.J.; Lee, B.Y. Predicting future plant diversity changes in response to climate change in South Korea. Ecol. Model. 2020, 419, 108960. [Google Scholar]
- Lee, C.B.; Chun, J.H.; Kim, H.H. Elevational patterns and determinants of α and β plant diversity on the ridge of the Baekdudaegan Mountains, South Korea. J. Agric. Life Sci. 2014, 48, 93–104. [Google Scholar] [CrossRef]
- Park, B.J.; Byeon, J.G.; Heo, T.I.; Cheon, K.; Yang, J.C.; Oh, S.H. Comparison of species composition among Picea jezoensis (Siebold & Zucc.) Carrière forests in Northeast Asia (from China to South Korea). J. Asia-Pac. Biodivers. 2023, 16, 272–281. [Google Scholar]
- Park, B.J.; Kim, J.J.; Byeon, J.G.; Cheon, K.I.; Joo, S.H.; Lee, Y.G. Classification of forest community and characteristics of stand structure in Mt. Myeonbong—Focused on the research forest of Kyungpook National University, Cheongsong. J. Korean For. Soc. 2016, 105, 391–400. [Google Scholar] [CrossRef]
- Son, Y.H. Long-term ecological research: Importance and approaches. Nat. Resour. Res. 1997, 5, 29–42. [Google Scholar]
- Kim, H.G.; Oh, S.H.; Cho, Y.C. Structurization in community composition and diversity pattern of soil seed banks in Gwangneung Forest, South Korea. J. Korean For. Sci. 2021, 110, 577–589. [Google Scholar]
- Jung, S.; Sim, H.S.; Kim, J.S.; Bae, K.H.; Cho, Y.C. Processes driving understory community dynamics in Ulleungdo Island broadleaved forest, South Korea. Ecol. Res. 2021, 36, 686–700. [Google Scholar] [CrossRef]
- Cheon, K.I.; Joo, S.H.; Sung, J.H.; Chun, J.H.; Lee, Y.G. Understory vegetation structure by altitude and azimuth slope and indicator species analysis in Mt. Gyebang. J. Korean For. Sci. 2014, 103, 165–174. [Google Scholar] [CrossRef]
- Leith, H.; Aston, D.H. The light compensation point of some herbaceous plants inside and outside deciduous woods in Germany. Can. J. Bot. 1961, 39, 1255–1259. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- Mabry, C.M.; Gerken, M.E.; Thompson, J.R. Seasonal storage of nutrients by perennial herbaceous species in undisturbed and disturbed deciduous hardwood forests. Appl. Veg. Sci. 2008, 11, 37–44. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 2009, 18, 346–360. [Google Scholar] [CrossRef]
- Davidar, P.; Rajagopal, B.; Mohandass, D.; Puyravaud, J.P.; Condit, R.; Wright, S.J.; Leigh, E.G., Jr. The effect of climatic gradients, topographic variation and species traits on the beta diversity of rainforest trees. Glob. Ecol. Biogeogr. 2007, 16, 510–518. [Google Scholar] [CrossRef]
- Korea National Arboretum. Forest of Korea(IV) Biogeography of Korea: Flora and Vegetation; Sumeungil: Seoul, Republic of Korea, 2020; pp. 42–79. ISBN 9791190509497. [Google Scholar]
- The Services of Climatic Data Portal of Korea Meteorological Administration. Available online: https://data.kma.go.kr/cmmn/main.do/ (accessed on 1 August 2025).
- Braun–Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde; Springer: Wien, Austria, 1964; pp. 17–205. ISBN 9783709181119. [Google Scholar]
- Lee, T.B. Coloured Flora of Korea; Hyangmoonsa: Seoul, Republic of Korea, 2003; Volume 1, pp. 1–916. ISBN 9788971871959. [Google Scholar]
- Lee, T.B. Coloured Flora of Korea; Hyangmoonsa: Seoul, Republic of Korea, 2003; Volume 2, pp. 1–910. ISBN 9788971871966. [Google Scholar]
- Korea Fern Society. Ferns and Fern Allies of Korea; Geobook: Seoul, Republic of Korea, 2005; pp. 1–399. ISBN 9788995504925. [Google Scholar]
- Korea National Arboretum. Rare Plants Data Book in Korea; Geobook: Seoul, Republic of Korea, 2008; pp. 1–332. ISBN 9788991458352. [Google Scholar]
- Korea National Arboretum. Invasive Alien Plant Impact on Forest; Sumeungil: Seoul, Republic of Korea, 2015; pp. 1–280. ISBN 9791187031185. [Google Scholar]
- Korea National Arboretum. Checklist of Vascular Plants in Korea; Samsung edCOM: Seoul, Republic of Korea, 2017; pp. 1–1000. ISBN 9791188720125. [Google Scholar]
- Cho, Y.H.; Kim, J.H.; Park, S.H. Grasses and Sedges in South Korea; Geobook: Seoul, Republic of Korea, 2016; pp. 1–528. ISBN 9788994242422. [Google Scholar]
- Knowledge System of National Species in Korea. Available online: http://www.nature.go.kr/kpni/index.do (accessed on 3 August 2025).
- Korea National Arboretum: The List of National Species: Vascular Plants. Available online: https://www.nature.go.kr/kpni/stndasrch/dtl/selectNtnStndaPlantList1.do (accessed on 3 August 2025).
- Park, I.H.; Seo, Y.K. Plot size for investigating forest community structure (V): Adequate number of plots for tree and shrub strata in a mixed forest community of broad-leaved trees at Guryongsan area. Korean J. Environ. Ecol. 2002, 15, 394–400. [Google Scholar]
- Chao, A.; Chazdon, R.L.; Colwell, R.K.; Shen, T.J. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 2005, 8, 148–159. [Google Scholar] [CrossRef]
- Mielke, P.W., Jr. Meteorological applications of permutation techniques based on distance functions. In Handbook of Statistics; Krishnaiah, P.R., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; Volume 4, pp. 813–830. [Google Scholar]
- Cheon, K.; Byeon, J.G.; Kim, J.I.; Park, B.J. Community structure of Abies nephrolepis habitats and characteristics of understory vegetation in Mt. Gyebang and Mt. Odae. J. Korean Environ. Restor. Technol. 2022, 25, 59–76. [Google Scholar]
- Petroselli, A.; Vessella, F.; Lucia, C.; Piovesan, G.; Schirone, B. Ecological behavior of Quercus suber and Quercus ilex inferred by topographic wetness index (TWI). Trees 2013, 27, 1201–1215. [Google Scholar] [CrossRef]
- Zwolinski, Z.; Stefańska, E. Relevance of moving window size in landform classification by TPI. In Geomorphometry for Geosciences; Bogucki Wydawnictwo Naukowe: Poznań, Poland, 2015; pp. 273–277. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. An upland forest continuum in the prairie–forest border region of Wisconsin. Ecology 1951, 32, 476–496. [Google Scholar] [CrossRef]
- Park, B.J.; Heo, T.I.; Cheon, K.I. Analyzing generalist plant species using topographic characteristics of Picea jezoensis (Siebold & Zucc.) Carrière forests in East Asia: From China (Mt. Changbai) to South Korea. Int. J. Plant Biol. 2024, 15, 320–339. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Gaston, K.J.; He, F. The distribution of species range size: A stochastic process. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1079–1086. [Google Scholar] [CrossRef]
- Zuppinger-Dingley, D.; Schmid, B.; Petermann, J.S.; Yadav, V.; De Deyn, G.B.; Flynn, D.F. Selection for niche differentiation in plant communities increases biodiversity effects. Nature 2014, 515, 108–111. [Google Scholar] [CrossRef]
- Newton, A.C. Forest Ecology and Conservation; Oxford University Press Inc.: New York, NY, USA, 2007; pp. 85–146. ISBN 9780198567455. [Google Scholar]
- Park, B.J.; Cheon, K. Herbaceous layer response to Overstory vegetation change in Quercus mongolica Fisch. ex Ledeb. Forests in Korea. Forests 2025, 16, 1344. [Google Scholar] [CrossRef]
- Germino, M.J.; Smith, W.K.; Resor, A.C. Conifer seedling distribution and survival in an alpine-treeline ecotone. Plant Ecol. 2002, 162, 157–168. [Google Scholar] [CrossRef]
- Kong, W.S. Species composition and distribution of Korean Alpine Plants. J. Korean Geogr. Soc. 2002, 37, 357–370. [Google Scholar]
- Mori, A.; Mizumachi, E.; Osono, T.; Doi, Y. Substrate-associated seedling recruitment and establishment of major conifer species in an old-growth subalpine forest in central Japan. For. Ecol. Manag. 2004, 196, 287–297. [Google Scholar] [CrossRef]
- Han, A.R.; Lee, S.K.; Suh, G.U.; Park, Y.; Park, P.S. Wind and topography influence the crown growth of Picea jezoensis in a subalpine forest on Mt. Deogyu, Korea. Agric. For. Meteorol. 2012, 166–167, 207–214. [Google Scholar] [CrossRef]
- Cha, Y.J.; Lee, K.J. Nutrient contents and biomass production of dwarf bamboo (Sasa borealis) along the developmental stages of deciduous forests after clearcutting in Mt. Baekwoon, Chonnam Province, Korea. J. Korean For. Soc. 2002, 91, 396–404. [Google Scholar]
- Park, S.G.; Yi, M.H.; Yoon, J.W.; Shin, H.T. Environmental factors and growth properties of Sasa borealis (Hack.) Makino community and its effects on the development of lower vegetation in Jirisan National Park. Korean J. Environ. Ecol. 2012, 26, 82–90. [Google Scholar]
- Kim, J.D.; Lim, J.H.; Yun, C.W. Dynamics of Abies nephrolepis seedlings in relation to environmental factors in Seorak Mountain, South Korea. Forests 2019, 10, 702. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.G. Classification and Assessment of Plant Communities; World Science: Seoul, Republic of Korea, 2006; pp. 14–72. ISBN 9788958810605. [Google Scholar]
- Lee, A.R.; Shin, D.B.; Lee, S.J.; Byeon, J.G.; Kim, J.S.; Oh, S.H. Vegetation structure and characteristic analysis of the Abies nephrolepis forest according to the appearance of Tripterygium regelii. J. Korean For. Soc. 2023, 112, 405–416. [Google Scholar]
- Park, B.J.; Heo, T.I. Vegetation structure of Mt. Bangtae among protected areas for forest genetic resource conservation. Korean J. Plant Resour. 2024, 37, 461–474. [Google Scholar]
- Pickett, S.T.A. Population patterns through twenty years of old-field changes. Plant Ecol. 1982, 49, 45–59. [Google Scholar] [CrossRef]
- Li, G.; Gong, Z.; Li, W. Niches and Interspecifc associations of dominant populations in three changed stages of natural secondary forests on Loess Plateau, P.R. China. Sci. Rep. 2017, 7, 6604. [Google Scholar]
- Jeong, B.K.; Oh, C.H. Analysis on the community structure of Quercus mongolica in the Baekdudaegan Mountains by elevation. Korean J. Environ. Ecol. 2013, 27, 449–461. [Google Scholar]
- Ko, D.W.; Lee, D.W. Dendroecological reconstruction of disturbance dynamics and human legacy in an old-growth hardwood forest in Korea. For. Ecol. Manag. 2013, 302, 43–53. [Google Scholar] [CrossRef]
- Kim, C.H.; Oh, J.G.; Kang, E.O.; Yun, C.S.; Lim, J.K. Community distribution on mountain forest vegetation of the Gyebangsan area in the Odaesan National Park, Korea. Korean J. Ecol. Environ. 2014, 47, 135–145. [Google Scholar] [CrossRef]
- Cheon, K.I.; Chun, J.H.; Yang, H.M.; Lim, J.H.; Shin, J.H. Changes of understory vegetation structure for 10 years in long-term ecological research site at Mt. Gyebang. J. Korean Soc. For. Sci. 2014, 103, 1–11. [Google Scholar] [CrossRef]
- Hawkins, B.A.; Field, R.; Cornell, H.V.; Currie, D.J.; Guégan, J.F.; Kaufman, D.M.; Kerr, J.T.; Mittelbach, G.G.; Oberdorff, T.; O’Brien, E.M.; et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology 2003, 84, 3105–3117. [Google Scholar] [CrossRef]
- Hunziker, U.; Brang, P. Microsite patterns of conifer seedling establishment and growth in a mixed stand in the southern Alps. For. Ecol. Manag. 2005, 210, 67–79. [Google Scholar] [CrossRef]
- Kong, W.S.; Kim, K.; Lee, S.; Park, H.; Cho, S.H. Distribution of high mountain plants and species vulnerability against climate change. J. Environ. Impact Assess. 2014, 23, 119–136. [Google Scholar] [CrossRef]
- Körner, C. Concepts in alpine plant ecology. Plants 2023, 12, 2666. [Google Scholar] [CrossRef]
- Park, B.J.; Heo, T.I.; Cheon, K. Ecological niches of generalist and specialist plants in the subalpine conifer habitats (Abies sp.) of Northeast Asia: From South Korea to the Manchurian region of China. Forests 2024, 15, 2119. [Google Scholar] [CrossRef]
- Grime, J.P. Competitive exclusion in herbaceous vegetation. Nature 1973, 242, 344–347. [Google Scholar] [CrossRef]
- Veblen, T.T. Regeneration dynamics. In Plant Succession: Theory and Prediction; Glenn-Lewin, D.C., Peet, R.K., Veblen, T.T., Eds.; Chapman & Hall: London, UK, 1992; pp. 152–187. [Google Scholar]
- McCain, C.M. Area and mammalian elevational diversity. Ecology 2007, 88, 76–86. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995; pp. 1–436. ISBN 9780521493463. [Google Scholar]
- Kim, J.; Lee, M.; Kong, W.; Kim, T.; Kang, C.; Park, K.; Park, B.; Park, H.; Sung, H.; Son, M.; et al. The Physical Geography of Korea; Seoul National University Press: Seoul, Republic of Korea, 2022; pp. 1–496. ISBN 9788952121929. [Google Scholar]
- Kim, J.U.; Park, S.Y.; Lee, C.H. Geomorphology; Seoul National University Press: Seoul, Republic of Korea, 2003; pp. 1–420. ISBN 9788970965513. [Google Scholar]
- National Institute of Forest Science. Forest Ecosystems of Korea; Korea Forest Service: Daejeon, Republic of Korea, 2015; pp. 1–310. ISBN 9788989863709. [Google Scholar]
- McGeoch, M.A.; Latombe, M.; Andrew, N.R.; Nakagawa, S.; Nipperess, D.A.; Roigé, M.; Marzinelli, E.M.; Campbell, A.H.; Vergés, A.; Thomas, T.; et al. Measuring continuous compositional change using decline and decay in zeta diversity. Ecology 2019, 100, e02832. [Google Scholar] [CrossRef]
- Habel, J.C.; Assmann, T.; Schmitt, T.; Avise, J.C. Relict Species: From Past to Future; Springer: Berlin, Germany, 2010; pp. 1–5. ISBN 9783540921608. [Google Scholar]
- Grandcolas, P.; Nattier, R.; Trewick, S. Relict species: A relict concept? Trends Ecol. Evol. 2014, 29, 655–663. [Google Scholar] [CrossRef]
- Legg, S. IPCC, 2021: Climate Change 2021-The Physical Science Basis. Interaction 2021, 49, 44–45. [Google Scholar]
- An, J.H.; Park, H.J.; Nam, G.H.; Lee, B.Y.; Park, C.H.; Kim, J.H. Vertical distribution of vascular plant species along an elevational gradient in the Gyebangsan area of Odaesan National Park. Korean J. Ecol. Environ. 2017, 50, 381–402. [Google Scholar] [CrossRef]
- Yang, J.C.; Hwang, H.S.; Lee, H.J.; Jung, S.Y.; Ji, S.J.; Oh, S.H.; Lee, Y.M. Distribution of vascular plants along the altitudinal gradient of Gyebangsan (Mt.) in Korea. J. Asia-Pac. Biodivers. 2014, 7, e40–e71. [Google Scholar] [CrossRef]

| Contents | Family | Genus | Species | Sub- Species | Variant | Form | Total | Ratio (%) |
|---|---|---|---|---|---|---|---|---|
| Pteridophyta | 8 | 16 | 27 | - | 2 | - | 29 | 7.9 |
| Gymnospermae | 3 | 5 | 7 | - | - | - | 7 | 1.9 |
| Angiospermae | 70 | 181 | 286 | 3 | 38 | 4 | 331 | 90.2 |
| Dicotyledoneae | 62 | 149 | 232 | 3 | 32 | 3 | 270 | 73.6 |
| Monocotyledoneae | 8 | 32 | 54 | - | 6 | 1 | 61 | 16.6 |
| Total | 81 | 202 | 320 | 3 | 40 | 4 | 367 | 100.0 |
| Contents | A1 | A2 | A3 | A4 | F | p | |
|---|---|---|---|---|---|---|---|
| Total plots | 35 | 33 | 47 | 38 | - | - | |
| Aspect (No. of plots) | N | 7 | 7 | 11 | 14 | - | - |
| E | 7 | 8 | 13 | 7 | - | - | |
| S | 12 | 8 | 11 | 7 | - | - | |
| W | 9 | 10 | 12 | 10 | - | - | |
| Altitude (m) ** | 871.3 ± 12.3 a | 1112.5 ± 6.5 b | 1325.6 ± 6.8 c | 1462.7 ± 6.2 d | 951.920 | <0.001 | |
| Slope (°) ns | 22.5 ± 1.3 | 18.0 ± 1.1 | 24.9 ± 1.3 | 23.5 ± 1.5 | 4.371 | 0.068 | |
| Rock exposure (%) ns | 26.3 ± 3.2 | 11.0 ± 2.8 | 23.2 ± 3.5 | 18.4 ± 3.5 | 3.512 | 0.074 | |
| Basal area (m2/ha) ** | 35.25 ± 2.10 ab | 40.63 ± 2.39 b | 38.24 ± 3.75 ab | 27.77 ± 2.82 a | 3.913 | 0.009 | |
| Stand density (stem/ha) ** | 556 ± 28 a | 577 ± 33 a | 970 ± 101 b | 814 ± 52 b | 8.269 | <0.001 | |
| Mean temperature (°C) | 6.88 ± 0.07 d | 6.24 ± 0.05 c | 5.31 ± 0.04 b | 5.06 ± 0.03 a | 304.246 | <0.001 | |
| Mean wind speed (m/s) | 2.79 ± 0.02 a | 2.94 ± 0.02 b | 3.31 ± 0.02 c | 3.39 ± 0.06 d | 283.269 | <0.001 | |
| TPI ** | −5.81 ± 2.38 a | 4.58 ± 2.45 b | 5.65 ± 1.78 b | 10.39 ± 1.22 b | 11.238 | <0.001 | |
| TWI ** | 8.73 ± 0.46 c | 7.33 ± 0.33 b | 6.36 ± 0.11 a | 6.23 ± 0.07 a | 17.874 | <0.001 | |
| Scientific Name | Group | |||
|---|---|---|---|---|
| A1 | A2 | A3 | A4 | |
| Sasa borealis (Hack.) Makino | 3.74 | 14.80 | 6.79 | 0.07 |
| Tripterygium regelii Sprague & Takeda | 3.00 | 5.72 | 6.23 | 6.79 |
| Rhododendron schlippenbachii Maxim. | 6.81 | 1.24 | 2.45 | 1.51 |
| Isodon excisus (Maxim.) Kudo | 0.89 | 4.61 | 1.68 | 2.05 |
| Calamagrostis arundinacea (L.) Roth | 0.61 | 0.37 | 5.09 | 2.27 |
| Quercus mongolica Fisch. ex Ledeb. | 5.34 | 2.05 | 0.62 | 0.32 |
| Carex siderosticta Hance | 1.73 | 2.04 | 1.43 | 2.58 |
| Schisandra chinensis (Turcz.) Baill. | 2.94 | 3.60 | 0.80 | 0.32 |
| Acer pseudosieboldianum (Pax) Kom. | 4.16 | 3.76 | 2.57 | 1.53 |
| Acer pictum subsp. mono (Maxim.) Ohashi | 1.53 | 1.66 | 0.75 | 0.11 |
| Acer komarovii Pojark. | 0.08 | 0.51 | 4.50 | 4.59 |
| Acer barbinerve Maxim. | 0.82 | 0.07 | 4.43 | 5.16 |
| Acer ukurunduense Trautv. & C.A.Mey. | - | 0.19 | 1.12 | 3.14 |
| Weigela florida (Bunge) A.DC. | 0.14 | 0.81 | 1.16 | 1.28 |
| Picea jezoensis (Siebold & Zucc.) Carriere | - | - | 0.61 | 0.69 |
| Abies nephrolepis (Trautv.) Maxim. | - | - | 1.16 | 0.63 |
| Symplocos chinensis f. pilosa (Nakai) Ohwi | 0.76 | 2.88 | 2.75 | 1.26 |
| Omitted species | 67.44 (163 taxa) | 55.69 (171 taxa) | 55.85 (154 taxa) | 65.68 (163 taxa) |
| Contents | A1 | A2 | A3 | A4 | F | p | |
|---|---|---|---|---|---|---|---|
| Alpha diversity | Species richness ns | 23.26 ± 1.70 | 23.24 ± 1.31 | 22.60 ± 0.91 | 25.79 ± 1.14 | 1.312 | 0.273 |
| Shannon index ** | 2.59 ± 0.08 a | 2.60 ± 0.08 a | 2.65 ± 0.05 a | 2.89 ± 0.03 b | 3.904 | 0.010 | |
| Evenness ** | 0.840 ± 0.012 a | 0.838 ± 0.015 a | 0.859 ± 0.011 a | 0.899 ± 0.007 b | 5.373 | 0.002 | |
| Compared Groups | T | A | p |
|---|---|---|---|
| A3 vs. A4 | −22.9027 | 0.2305 | <0.001 |
| A3 vs. A2 | −15.8899 | 0.1434 | |
| A3 vs. A1 | −10.0133 | 0.1099 | |
| A4 vs. A2 | −12.7717 | 0.1146 | |
| A4 vs. A1 | −3.68345 | 0.0315 | |
| A2 vs. A1 | −22.1104 | 0.2304 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheon, K.; Lee, E.-S.; Park, B.-J. Elevation-Driven Variations in Species Composition and Biodiversity in a Protected Temperate Forest, Mount Gyebangsan, Korea. Diversity 2025, 17, 828. https://doi.org/10.3390/d17120828
Cheon K, Lee E-S, Park B-J. Elevation-Driven Variations in Species Composition and Biodiversity in a Protected Temperate Forest, Mount Gyebangsan, Korea. Diversity. 2025; 17(12):828. https://doi.org/10.3390/d17120828
Chicago/Turabian StyleCheon, Kwangil, Eun-Seo Lee, and Byeong-Joo Park. 2025. "Elevation-Driven Variations in Species Composition and Biodiversity in a Protected Temperate Forest, Mount Gyebangsan, Korea" Diversity 17, no. 12: 828. https://doi.org/10.3390/d17120828
APA StyleCheon, K., Lee, E.-S., & Park, B.-J. (2025). Elevation-Driven Variations in Species Composition and Biodiversity in a Protected Temperate Forest, Mount Gyebangsan, Korea. Diversity, 17(12), 828. https://doi.org/10.3390/d17120828







