Unveiling Zooplankton Diversity Patterns: The Differential Influence of Macrophyte Belts on Species and Functional Metrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. Species Identification
2.3. Environmental Variables
2.4. Data Analysis
3. Results
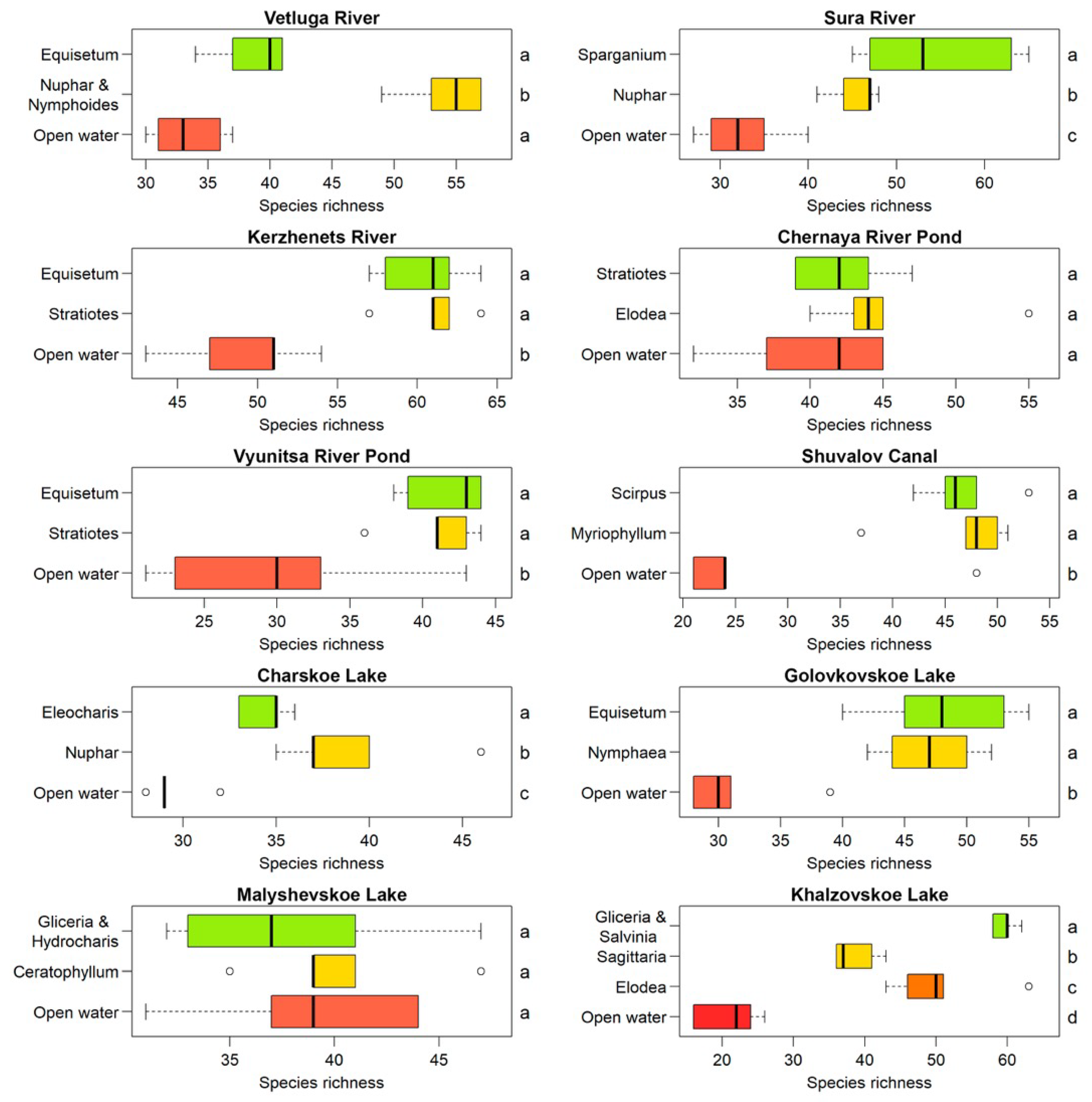
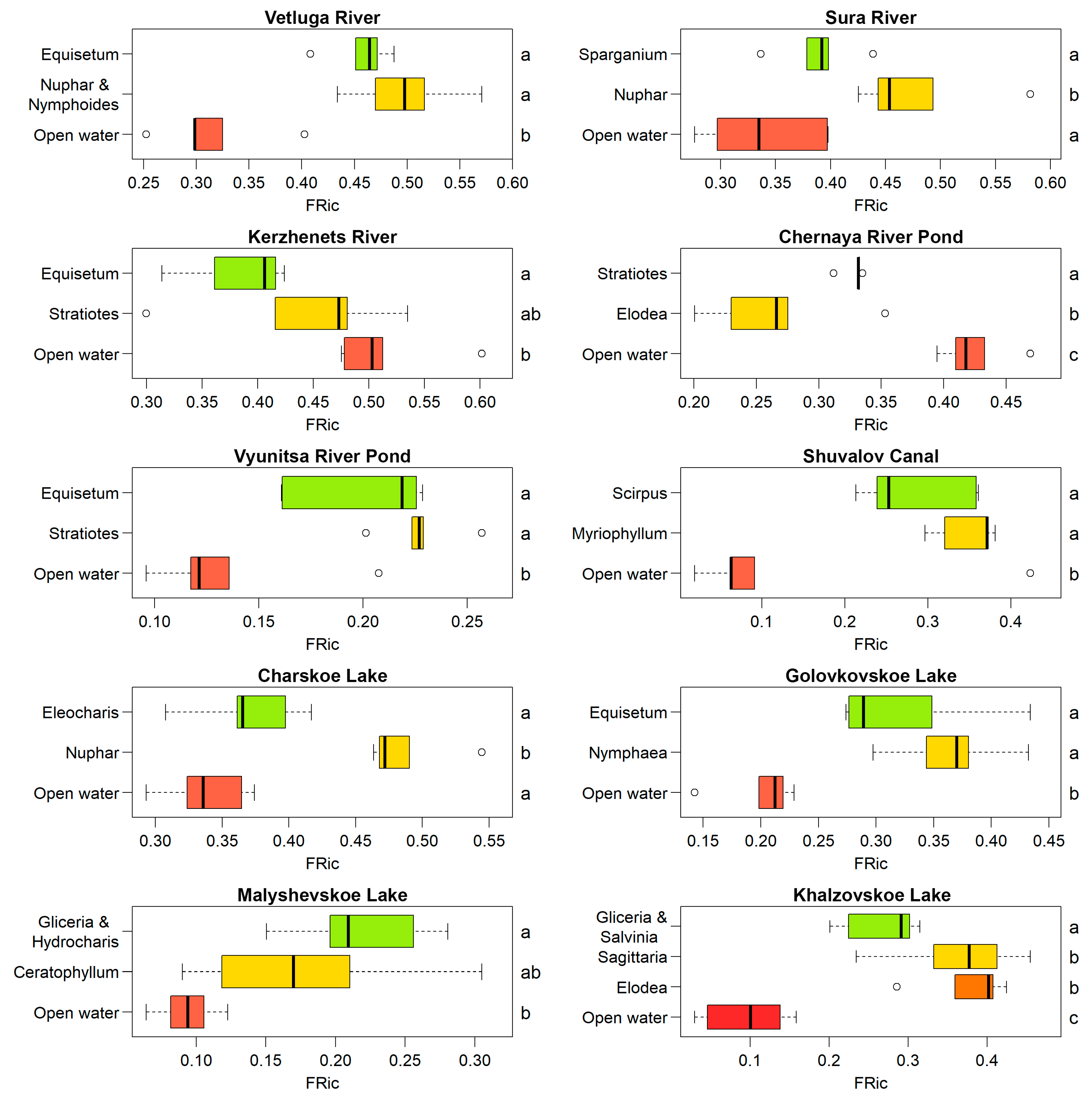
3.1. Species and Functional Diversity
3.2. Diversity and Environmental Variables
4. Discussion
4.1. Distribution of Species and Functional Diversity
4.2. The Influence of Environmental Variables on Species and Functional Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142620. [Google Scholar] [CrossRef]
- Lavorel, S.; Storkey, J.; Bardgett, R.D.; De Bello, F.; Berg, M.P.; Le Roux, X.; Moretti, M.; Mulder, C.; Pakeman, R.J.; Díaz, S.; et al. A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. J. Veg. Sci. 2013, 24, 942–948. [Google Scholar] [CrossRef]
- Mouillot, D.; Graham, N.A.J.; Villéger, S.; Mason, N.W.H.; Bellwood, D.R. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013, 28, 167–177. [Google Scholar] [CrossRef]
- Stephan, L.R.; Beisner, B.E.; Oliveira, S.G.M.; Castilho-Noll, M.S.M. Influence of Eichhornia crassipes (Mart) solms on a tropical microcrustacean community based on taxonomic and functional trait diversity. Water 2019, 11, 2423. [Google Scholar] [CrossRef]
- Braghin, L.d.S.M.; Dias, J.D.; Simões, N.R.; Bonecker, C.C. Food availability, depth, and turbidity drive zooplankton functional diversity over time in a Neotropical floodplain. Aquat. Sci. 2021, 83, 10. [Google Scholar] [CrossRef]
- Villéger, S.; Mason, N.W.H.; Mouillot, D. New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 2008, 89, 2290–2301. [Google Scholar] [CrossRef]
- Junka, W.J.; Piedade, M.T.F.; Lourival, R.; Wittmann, F.; Kandus, P.; Lacerda, L.D.; Bozelli, R.L.; Esteves, F.A.; Nunes da Cunha, C.; Maltchik, L.; et al. Brazilian wetlands: Their definition, delineation, and classification for research, sustainable management, and protection. Aquat. Conserv. 2013, 24, 5–22. [Google Scholar] [CrossRef]
- Moss, B.; Kornijow, R.; Measey, G.J. The effects of nymphaeid (Nuphar lutea) density and predation by perch (Perca fluviatilis) on the zooplankton communities in a shallow lake. Freshw. Biol. 1998, 39, 689–697. [Google Scholar] [CrossRef]
- Scheffer, M. Ecology of Shallow Lakes; Springer Science & Business Media: New York, NY, USA, 2004; 457p. [Google Scholar]
- Litchman, E.; Ohman, M.D.; Kiorboe, T. Trait-based approaches to zooplankton communities. J. Plankton Res. 2013, 35, 473–484. [Google Scholar] [CrossRef]
- Deosti, S.; Bomfim, F.d.F.; Lansac-Tôha, F.M.; Quirino, B.A.; Bonecker, C.C.; Lansac-Tôha, F.A. Zooplankton taxonomic and functional structure is determined by macrophytes and fish predation in a Neotropical River. Hydrobiologia 2021, 848, 1475–1490. [Google Scholar] [CrossRef]
- Lauridsen, T.L.; Jeppesen, E.; Søndergaard, M.; Lodge, D. Horizontal migration of zooplankton: Predator-mediated use of macrophyte habitat. In The Structuring Role of Submerged Macrophytes in Lakes; Springer: New York, NY, USA, 1998; pp. 233–239. [Google Scholar]
- Lucena-Moya, P.; Duggan, I.C. Macrophyte architecture affects the abundance and diversity of littoral microfauna. Aquat. Ecol. 2011, 45, 279–287. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Chang, K.H.; Joo, G.J. The influence of aquatic macrophytes on the distribution and feeding habits of two Asplanchna species (A. priodonta and A. herrickii) in shallow wetlands in South Korea. J. Limnol. 2015, 74, 1–11. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N. Zooplankton structure in architecturally differentiated macrophyte habitats of shallow lakes in the Wielkopolska Region, Poland. Int. J. Ocean. Hyd. 2006, 35, 179–191. [Google Scholar]
- Kurbatova, S.A.; Lapteva, N.A.; Bykova, S.N.; Yershov, I.Y. Aquatic plants as a factor that changes trophic relations and the structure of zooplankton and microperiphytone communities. Biol. Bull. 2019, 46, 284–293. [Google Scholar] [CrossRef]
- Walsh, E.J. Habitat-specific predation susceptibilities of a littoral rotifer to two invertebrate predators. Hydrobiologia 1995, 313, 205–211. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N.; Nagengast, B. The impact of the architecture of macrophytes on the spatial structure of zooplankton of the Wielkowiejskie lake. Rocz. AR Pozn. CCCLIV 2003, 6, 121–129. [Google Scholar]
- Choi, J.Y.; Jeong, K.S.; La, G.H.; Joo, G.J. Effect of removal of free-floating macrophytes on zooplankton habitat in shallow wetland. Knowl. Manag. Aquat. Ecos. 2014, 414, 11. [Google Scholar] [CrossRef]
- Jeong, K.S.; Choi, J.Y.; Jeong, K.S. Influence of aquatic macrophytes on the interactions among aquatic organisms in shallow wetlands (Upo Wetland, South Korea). J. Ecol. Environ. 2014, 37, 185–194. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jeong, K.S.; Kim, S.K.; La, G.H.; Chang, K.H.; Joo, G.J. Role of macrophytes as microhabitats for zooplankton community in lentic freshwater ecosystems of South Korea. Ecol. Inform. 2014, 24, 177–185. [Google Scholar] [CrossRef]
- Bolduc, P.; Bertolo, A.; Pinel-Alloul, B. Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system. Hydrobiologia 2016, 778, 151–165. [Google Scholar] [CrossRef]
- Grzybkowska, M.; Dukowska, M.; Leszczyn’ska, J.; Lik, J.; Szczerkowska-Majchrzak, E.; Przybylski, M. The food resources exploitation by small-sized fish in a riverine macrophyte habitat. Ecol. Indic. 2018, 90, 206–214. [Google Scholar] [CrossRef]
- Manatunge, J.; Asaeda, T.; Priyadarshana, T. The influence of structural complexity on fish-zooplankton interactions: A study using artificial submerged macrophytes. Environ. Biol. Fishes 2000, 58, 425–438. [Google Scholar] [CrossRef]
- Celewicz-Goldyn, S.; Kuczynska-Kippen, N. Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 2017, 12, e0177317. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, B.; Dai, Z.; Zhou, Q.; Kong, L.; Zhang, Y.; He, F.; Wu, Z. Analyzing the effects of four submerged macrophytes with two contrasting architectures on zooplankton: A mesocosm experiment. J. Limnol. 2017, 76, 581–590. [Google Scholar] [CrossRef]
- Meerhoff, M.; Iglesias, C.; De Mello, F.T.; Clemente, J.M.; Jensen, E.; Lauridsen, T.L.; Jeppesen, E. Effects of habitat complexity on community structure and predator avoidance behaviour of littoral zooplankton in temperate versus subtropical shallow lakes. Freshw. Biol. 2007, 52, 1009–1021. [Google Scholar] [CrossRef]
- Carniatto, N.; Cunha, E.R.; Thomaz, S.M.; Quirino, B.A.; Fugi, R. Feeding of fish inhabiting native and non-native macrophyte stands in a Neotropical reservoir. Hydrobiologia 2020, 847, 1553–1563. [Google Scholar] [CrossRef]
- Gavrilko, D.E.; Bubnov, V.A.; Sarapkin, A.Y.; Zhikharev, V.S. Spatial distribution of zooplankton communities in the littoral zone in small lakes of Nizhny Novgorod Region (European Russia). Limnol. Freshw. Biol. 2025, 4, 659–680. [Google Scholar] [CrossRef]
- Błędzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe: Cladocera & Copepoda (Calanoida, Cyclopoida) Key to Species Identification, with Notes on Ecology, Distribution, Methods and Introduction to Data Analysis; Springer: Cham, Switzerland, 2016; p. 918. [Google Scholar]
- Rogers, D.C.; Thorp, J.H. Keys to Palaearctic Fauna. Thorp and Covichʹs Freshwater. Invertebrates; Academic Press: Oxford, UK, 2019; Volume IV, p. 920. [Google Scholar]
- Korovchinsky, N.M.; Kotov, A.A.; Sinev, A.Y.; Neretina, A.N.; Garibyan, P.G. Cladocera (Crustacea: Cladocera) of Northern Eurasia; KMK Scientific Press Ltd.: Moscow, Russia, 2021; Volume II, p. 544. [Google Scholar]
- Papchenkov, V.G. Vegetation Cover of Reservoirs and Watercourses of the Middle Volga Region; SMR MUBiNT: Yaroslavl, Russia, 2001. (In Russian) [Google Scholar]
- Carlson, R.E. A trophic state index for lakes. Limnol. Oceanogr. 1977, 22, 361–369. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Oxford, UK, 2012; p. 990. [Google Scholar]
- Gavrilko, D.E.; Shurganova, G.V.; Kudrin, I.A.; Yakimov, B.N. Identification of Freshwater Zooplankton Functional Groups Based on the Functional Traits of Species. Biol. Bull. 2021, 48, 1849–1856. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2020. Available online: http://www.R--project.org/ (accessed on 26 October 2025).
- Lauridsen, T.L.; Lodge, D. Avoidance by Daphnia magna of fish and macrophytes: Chemical cues and predator-mediated use of macrophytes habitat. Limnol. Oceanogr. 1996, 41, 794–798. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Sondergaard, M.; Lauridsen, T.; Pedersen, L.J.; Jensen, L. Top-down control in freshwater lakes: The role of nutrient status, submerged macrophytes and water depth. Hydrobiologia 1997, 342/343, 151–164. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J.; Strzalek, M. Biodiversity of zooplankton (Rotifera and Crustacea) in water soldier (Stratiotes aloides) habitats. Biologia 2016, 71, 563–569. [Google Scholar] [CrossRef]
- Kuczynska-Kippen, N. The Impact of the macrophyte substratum and season on crustacean zooplankton communities of three shallow and macrophyte-dominated lakes. J. Freshw. Ecol. 2009, 24, 375–382. [Google Scholar] [CrossRef]
- Semenchenko, V.P.; Razlutsky, V.I. Factors determining the daily distribution and movements of zooplankton in the littoral zone of freshwater lakes. J. Sib. Fed. Univ. Ser. Biol. 2009, 2, 191–225. (In Russian) [Google Scholar]
- Barnett, A.J.; Beisner, B.E. Zooplankton biodiversity and lake trophic state: Explanations invoking resource abundance and distribution. Ecology 2007, 88, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, P.; Frenette, J.-J.; Proulx, R.; Pinel-Alloul, B.; Bertolo, A. Riverscape heterogeneity explains spatial variation in zooplankton functional evenness and biomass in a large river ecosystem. Landsc. Ecol. 2014, 29, 67–79. [Google Scholar] [CrossRef]
- Gliwicz, Z.M. Between Hazards of Starvation and Risk of Predation: The Ecology of Offshore Animals; International ecology institute: Oldendorf/Luhe, Germany, 2003; 377p. [Google Scholar]
- Vogt, R.J.; Peres-Neto, P.R.; Beisner, B.E. Using functional traits to investigate the determinants of crustacean zooplankton community structure. Oikos 2013, 122, 1700–1709. [Google Scholar] [CrossRef]
- Webster, K.E.; Peters, R.H. Some size dependent inhibition of larger cladocerans filter in filamentous suspensions. Limnol. Oceanogr. 1978, 23, 1238–1245. [Google Scholar] [CrossRef]
- Gliwicz, M.Z.; Lampert, W. Food thresholds in Daphnia in the absence and presence of blue-green filaments. Ecology 1990, 71, 691–702. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J. The usefulness of zooplankton as lake ecosystem indicators: Rotifer trophic state index. Pol. J. Ecol. 2012, 60, 339–350. [Google Scholar]
- Karpowicz, M.; Ejsmont-Karabin, J. Diversity and structure of pelagic zooplankton (Crustacea, Rotifera) in NE Poland. Water 2021, 13, 456. [Google Scholar] [CrossRef]
- Ochocka, A. Zooplankton index for shallow lakes assessment: Elaboration of a new classification method for Polish lakes. Water 2024, 16, 2730. [Google Scholar] [CrossRef]
- Afonina, E.Y.; Tashlykova, N.A. Structural and functional diversity of plankton communities along lake salinity gradients. Aquat. Ecol. 2024, 58, 717–740. [Google Scholar] [CrossRef]
- Afonina, E.; Tashlykova, N.; Borzenko, S. Saline lakes of Transbaikalia (Russia): Limnology and diversity of plankton communities. Limnology 2025, 26, 333–348. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Tavşanoğlu, Ű.N.; Vidal, N.; Yu, J.; Teixeira de Mello, F.; Cakiroglu, A.; He, H.; Liu, Z.; Jeppesen, E. Salinity shapes zooplankton communities and functional diversity and has complex effects on size structure in lakes. Hydrobiologia 2018, 813, 237–255. [Google Scholar] [CrossRef]
- Simões, N.R.; Dias, J.D.; Leal, C.M.; Braghin, L.S.M.; Lansac-Tôha, F.A.; Bonecker, C.C. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a Neotropical floodplain. Aquat. Sci. 2013, 75, 607–617. [Google Scholar] [CrossRef]
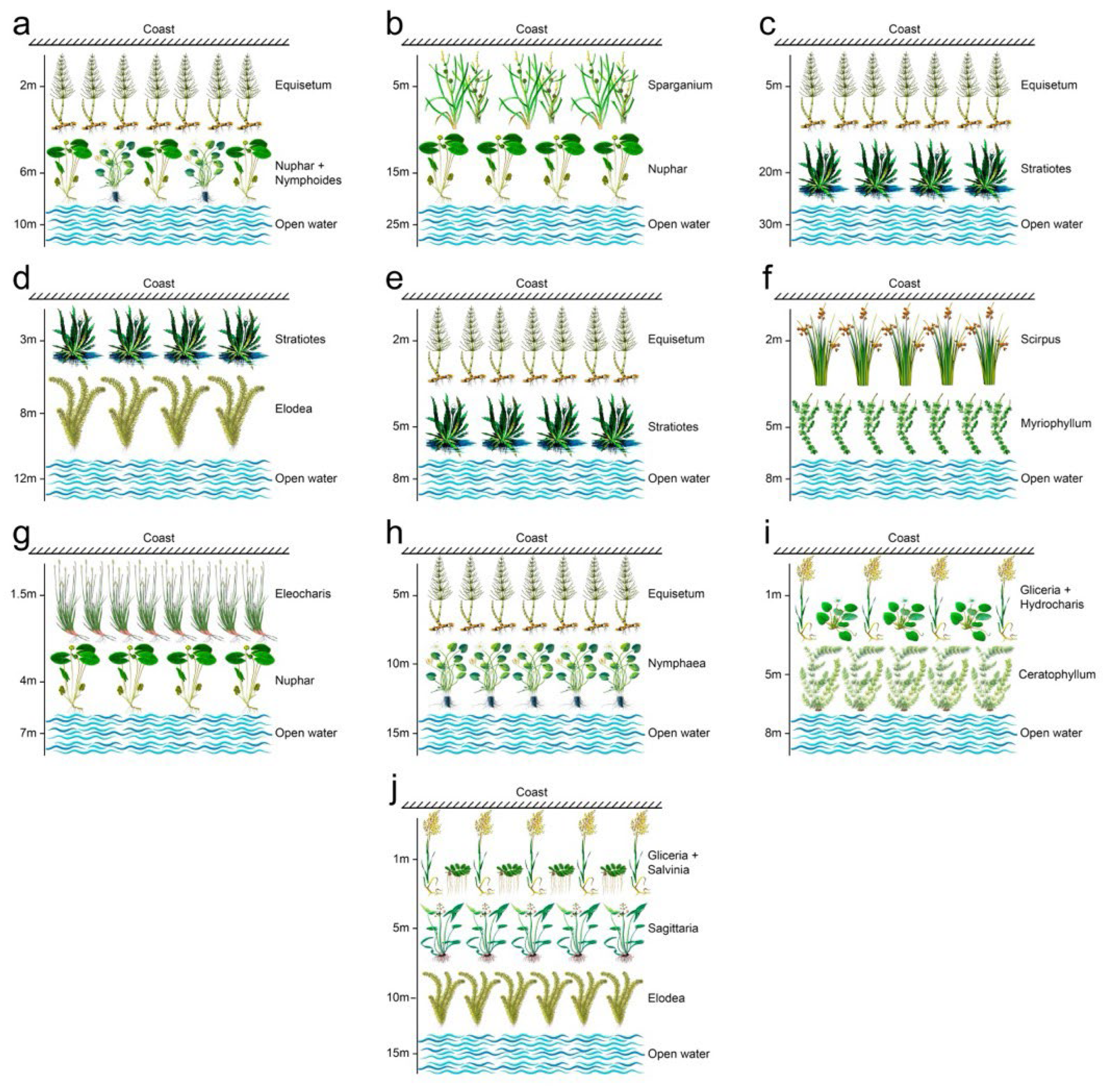
| Water Body | Year | Coordinates (N, E) |
|---|---|---|
| Vetluga River | 2022 | 56°42′32″, 46°27′31″ |
| Sura River | 56°11′43″, 46°00′62″ | |
| Kerzhenets River | 2023 | 56°08′95″, 44°96′86″ |
| Charskoe Lake | 2024 | 55°51′84″, 43°18′69″ |
| Golovkovskoe Lake | 56°33′28″, 43°70′54″ | |
| Malyshevskoe Lake | 56°20′27″, 43°82′81″ | |
| Khaltzovskoe Lake | 56°38′43″, 43°86′02″ | |
| Pond of the Vyunitsa River | 56°24′97″, 43°74′21″ | |
| Pond of the Chernaya River | 56°39′56″, 43°77′25″ | |
| Shuvalov Canal | 56°28′16″, 43°86′76″ |
| Water Body | Total | Rotifera | Cladocera | Copepoda |
|---|---|---|---|---|
| Vetluga River | 105 | 50 | 39 | 16 |
| Sura River | 100 | 49 | 37 | 14 |
| Kerzhenets River | 156 | 84 | 51 | 21 |
| Charskoe Lake | 74 | 37 | 26 | 11 |
| Golovkovskoe Lake | 85 | 55 | 21 | 9 |
| Malyshevskoe Lake | 102 | 63 | 26 | 13 |
| Khaltzovskoe Lake | 114 | 61 | 40 | 13 |
| Pond the Vyunitsa River | 89 | 43 | 32 | 14 |
| Pond of the Chernaya River | 107 | 52 | 38 | 17 |
| Shuvalov Canal | 94 | 52 | 28 | 14 |
| Predictor | Statistics | Species Richness | FRic | FEve | FDiv | Shannon Index | Pielou Index | Simpson Index |
|---|---|---|---|---|---|---|---|---|
| Water temperature | Est.Coef | −1.720 | −0.001 | 0.0005 | −0.009 | −0.135 | −0.020 | 0.021 |
| Std.Error | 0.489 | 0.006 | 0.004 | 0.005 | 0.033 | 0.006 | 0.007 | |
| p-value | <0.001 | 0.802 | 0.896 | 0.057 | <0.001 | 0.002 | 0.002 | |
| pH | Est.Coef | −5.453 | −0.110 | −0.012 | −0.009 | 0.173 | 0.055 | −0.040 |
| Std.Error | 1.522 | 0.017 | 0.011 | 0.014 | 0.102 | 0.019 | 0.020 | |
| p-value | <0.001 | <0.001 | 0.283 | 0.533 | 0.091 | 0.005 | 0.051 | |
| Electrical conductivity | Est.Coef | 0.008 | −0.00008 | −0.000005 | −0.00003 | 0.001 | 0.00009 | −0.00001 |
| Std.Error | 0.005 | 0.00005 | 0.00004 | 0.00005 | 0.0003 | 0.00006 | 0.00006 | |
| p-value | 0.107 | 0.159 | 0.883 | 0.449 | 0.060 | 0.129 | 0.875 | |
| Oxygen content | Est.Coef | 0.979 | 0.031 | 0.001 | −0.0004 | −0.063 | −0.017 | 0.010 |
| Std.Error | 0.415 | 0.005 | 0.003 | 0.004 | 0.028 | 0.005 | 0.006 | |
| p-value | 0.020 | <0.001 | 0.687 | 0.927 | 0.024 | 0.002 | 0.065 | |
| TSI | Est.Coef | 0.188 | −0.003 | −0.002 | 0.003 | 0.009 | 0.001 | −0.003 |
| Std.Error | 0.136 | 0.002 | 0.001 | 0.001 | 0.009 | 0.002 | 0.002 | |
| p-value | 0.168 | 0.047 | 0.139 | 0.054 | 0.333 | 0.691 | 0.130 | |
| Projective covering of macrophytes | Est.Coef | 14.370 | 0.103 | −0.031 | 0.070 | 0.214 | −0.023 | −0.021 |
| Std.Error | 1.892 | 0.021 | 0.014 | 0.018 | 0.126 | 0.024 | 0.025 | |
| p-value | <0.001 | <0.001 | 0.029 | <0.001 | 0.092 | 0.348 | 0.403 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavrilko, D.; Bubnov, V.; Sarapkin, A.; Zhikharev, V.; Zolotareva, T.; Yakimov, B. Unveiling Zooplankton Diversity Patterns: The Differential Influence of Macrophyte Belts on Species and Functional Metrics. Diversity 2025, 17, 812. https://doi.org/10.3390/d17120812
Gavrilko D, Bubnov V, Sarapkin A, Zhikharev V, Zolotareva T, Yakimov B. Unveiling Zooplankton Diversity Patterns: The Differential Influence of Macrophyte Belts on Species and Functional Metrics. Diversity. 2025; 17(12):812. https://doi.org/10.3390/d17120812
Chicago/Turabian StyleGavrilko, Dmitry, Viktor Bubnov, Alexandr Sarapkin, Vyacheslav Zhikharev, Tatyana Zolotareva, and Basil Yakimov. 2025. "Unveiling Zooplankton Diversity Patterns: The Differential Influence of Macrophyte Belts on Species and Functional Metrics" Diversity 17, no. 12: 812. https://doi.org/10.3390/d17120812
APA StyleGavrilko, D., Bubnov, V., Sarapkin, A., Zhikharev, V., Zolotareva, T., & Yakimov, B. (2025). Unveiling Zooplankton Diversity Patterns: The Differential Influence of Macrophyte Belts on Species and Functional Metrics. Diversity, 17(12), 812. https://doi.org/10.3390/d17120812










