Environmental Drivers and Edge Effects on Anuran Diversity in Fragmented Forests of the Southwestern Brazilian Amazon
Abstract
1. Introduction
2. Materials and Methods
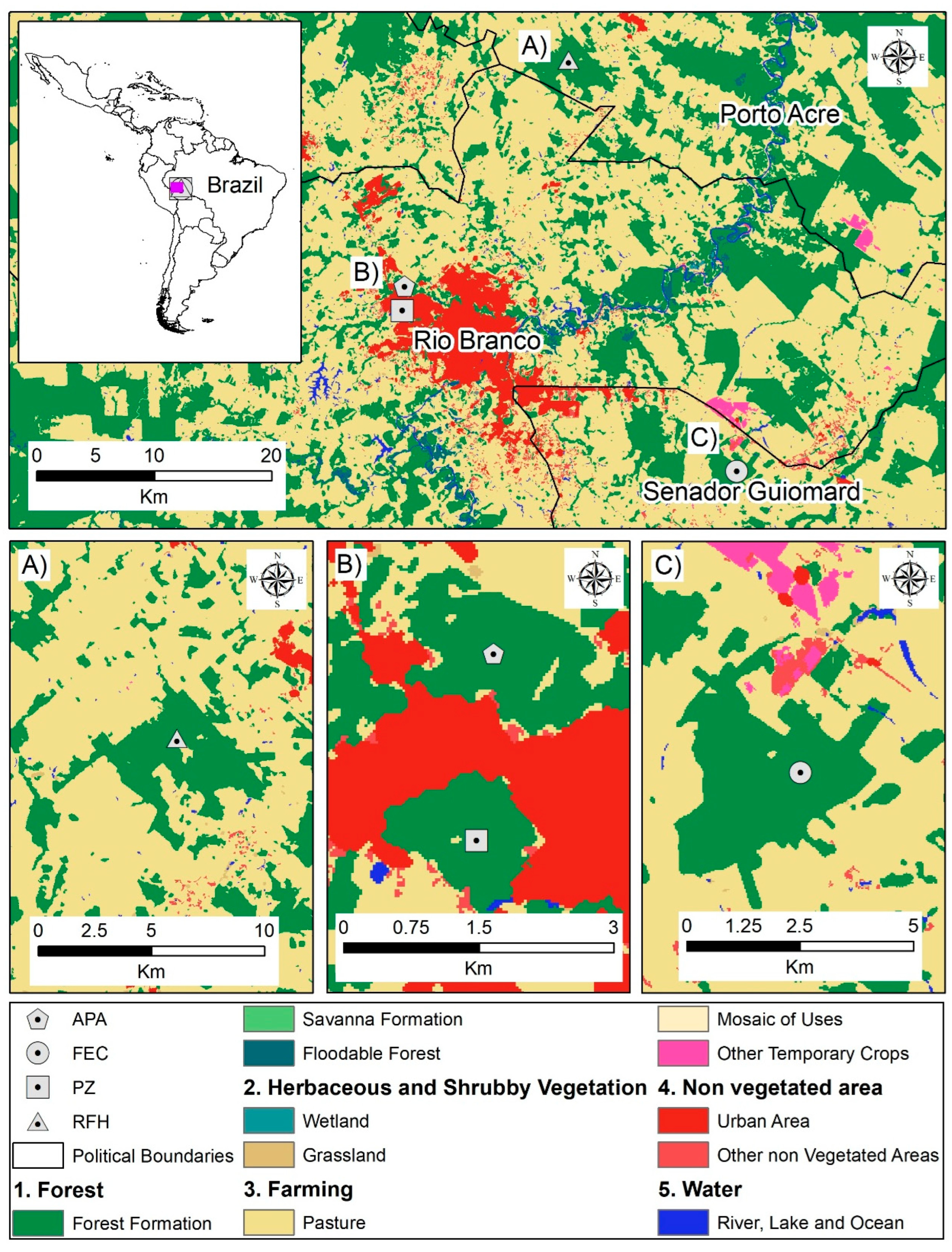
2.1. Study Area
- APA, located in a highly urbanized area, faces significant anthropogenic pressures from recent housing developments and constant human presence.
- PZ, though surrounded by residences, represents the best-preserved forest patch within Rio Branco’s urban perimeter.
- RFH, the largest and most remote fragment, maintains high conservation status.
- FEC, despite being partially protected, experiences illegal logging, hunting, and fishing pressures.
2.2. Sampling Methods
2.3. Local Environmental Variables
2.4. Data Analysis
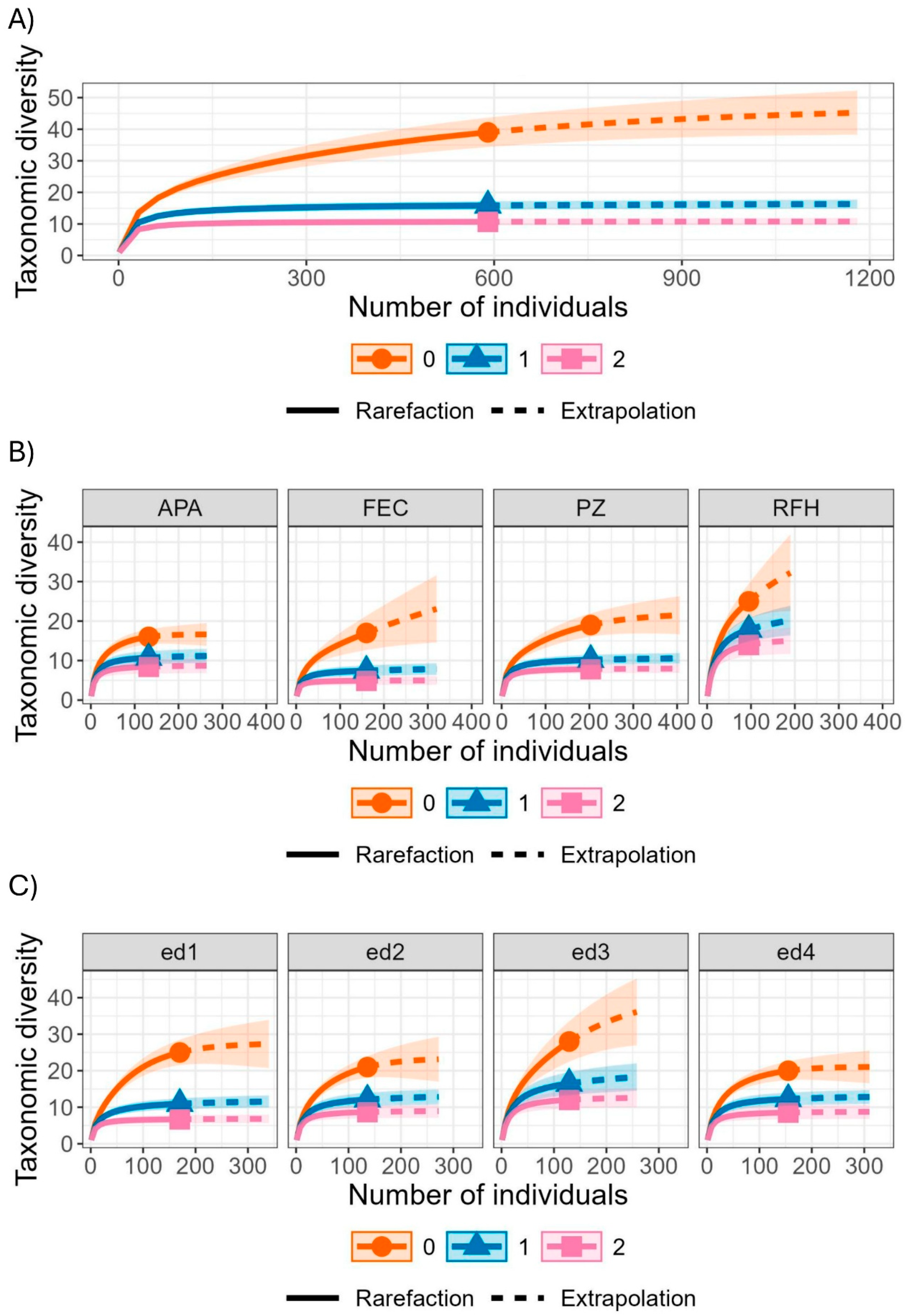
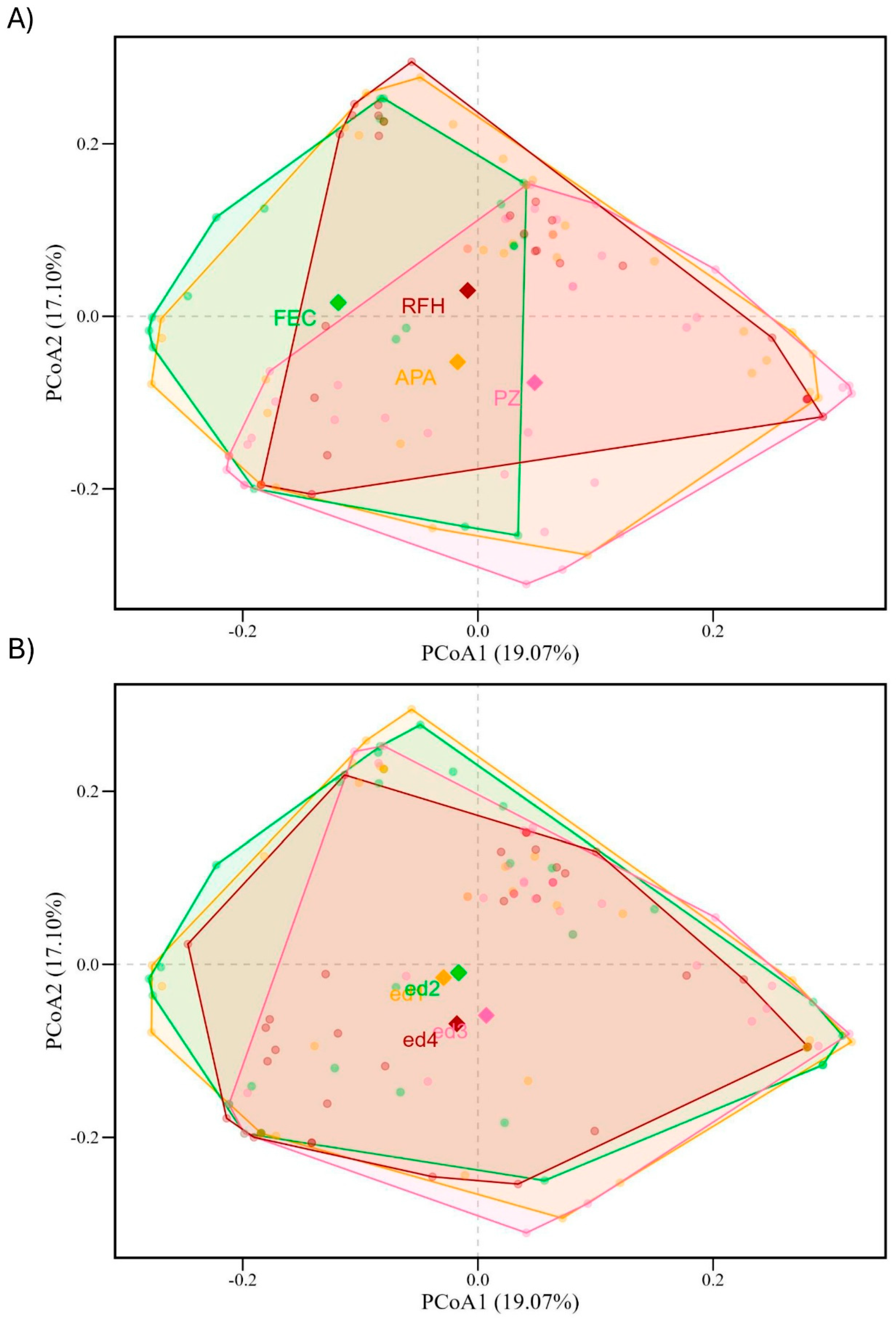
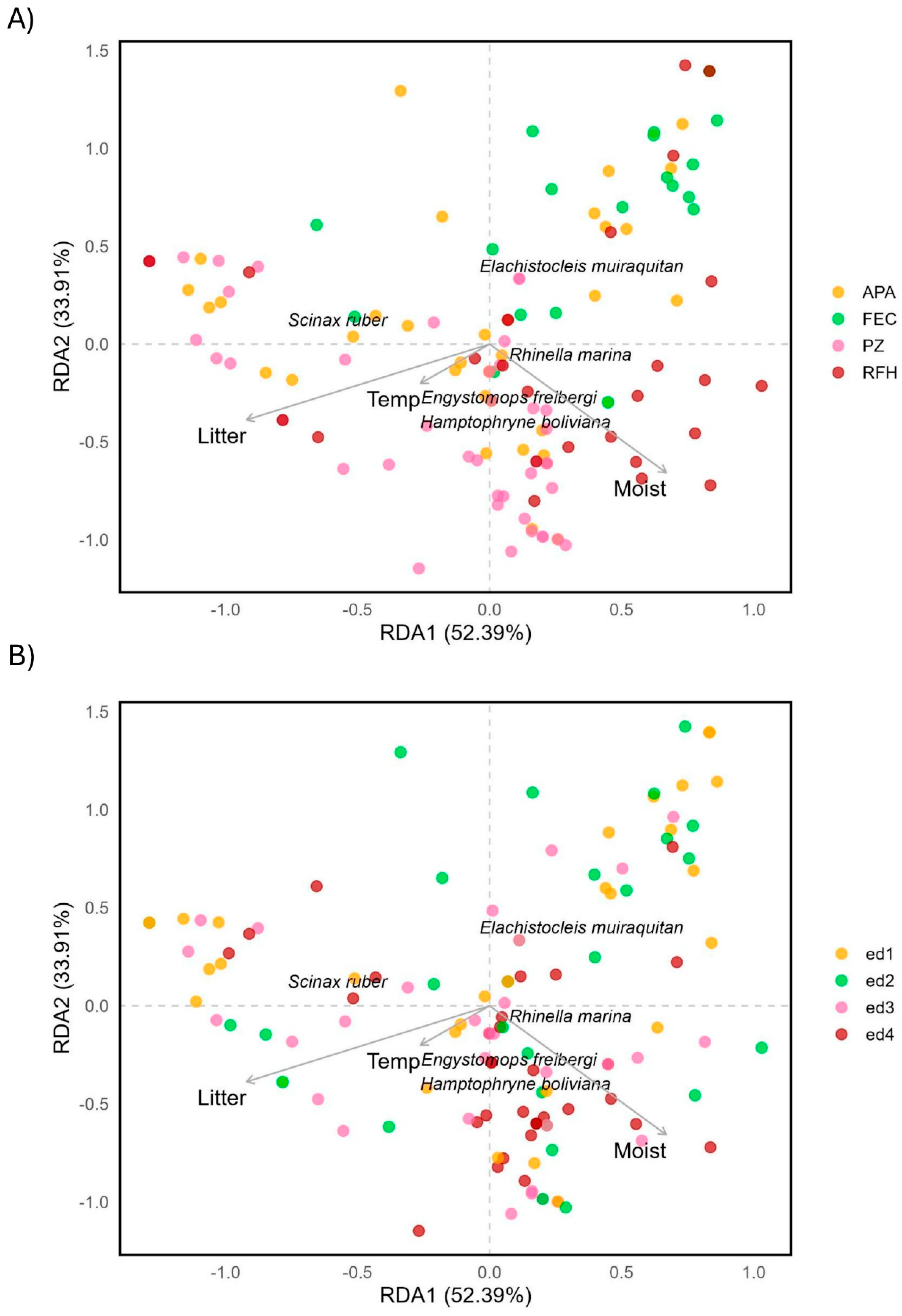
3. Results
4. Discussion
4.1. Species Diversity and Composition Among Fragments
4.2. Edge-Interior Gradient and Its Ecological Implications
4.3. Influence of Environmental Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beard, K.H.; Vogt, K.A.; Kulmatiski, A. Top-down Effects of a Terrestrial Frog on Forest Nutrient Dynamics. Oecologia 2002, 133, 583–593. [Google Scholar] [CrossRef]
- Best, M.L.; Welsh, H.H., Jr. The Trophic Role of a Forest Salamander: Impacts on Invertebrates, Leaf Litter Retention, and the Humification Process. Ecosphere 2014, 5, 1–19. [Google Scholar] [CrossRef]
- Toledo, L.F.; Ribeiro, R.S.; Haddad, C.F.B. Anurans as Prey: An Exploratory Analysis and Size Relationships between Predators and Their Prey. J. Zool. 2007, 271, 170–177. [Google Scholar] [CrossRef]
- Haddad, C.F.B.; Prado, C.P.A. Reproductive Modes in Frogs and Their Unexpected Diversity in the Atlantic Forest of Brazil. Bioscience 2005, 55, 207–217. [Google Scholar] [CrossRef]
- Gottsberger, B.; Gruber, E. Temporal Partitioning of Reproductive Activity in a Neotropical Anuran Community. J. Trop. Ecol. 2004, 20, 271–280. [Google Scholar] [CrossRef]
- Menin, M.; Waldez, F.; Lima, A.P. In Central Amazonia. Herpetolofical J. 2011, 21, 255–261. [Google Scholar]
- Giaretta, A.A.; Facure, K.G.; Sawaya, R.J.; Meyer, J.H.D.M.; Chemin, N. Diversity and Abundance of Litter Frogs in a Montane Forest of Southeastern Brazil: Seasonal and Altitudinal Changes 1. Biotropica 1999, 31, 669–674. [Google Scholar] [CrossRef]
- Oliveira, J.C.F.; Pralon, E.; Coco, L.; Pagotto, R.V.; Rocha, C.F.D. Environmental Humidity and Leaf-Litter Depth Affecting Ecological Parameters of a Leaf-Litter Frog Community in an Atlantic Rainforest Area. J. Nat. Hist. 2013, 47, 2115–2124. [Google Scholar] [CrossRef]
- Santos-Pereira, M.; Candaten, A.; Milani, D.; Oliveira, F.B.; Gardelin, J.; da Rocha, C.F.D. Seasonal Variation in the Leaf-Litter Frog Community (Amphibia: Anura) from an Atlantic Forest Area in the Salto Morato Natural Reserve, Southern Brazil. Zoologia 2011, 28, 755–761. [Google Scholar] [CrossRef]
- Menin, M.; Lima, A.P.; Magnusson, W.E.; Waldez, F. Topographic and Edaphic Effects on the Distribution of Terrestrially Reproducing Anurans in Central Amazonia: Mesoscale Spatial Patterns. J. Trop. Ecol. 2007, 23, 539–547. [Google Scholar] [CrossRef]
- Deichmann, J.L.; Lima, A.P.; Williamson, G.B. Effects of Geomorphology and Primary Productivity on Amazonian Leaf Litter Herpetofauna. Biotropica 2011, 43, 149–156. [Google Scholar] [CrossRef]
- Ramalho, W.P.; Andrade, M.S.; de Matos, L.R.A.; Vieira, L.J.S. Amphibians of Varzea Environments and Floating Meadows of the Oxbow Lakes of the Middle Purus River, Amazonas, Brazil. Biota Neotrop. 2016, 16, 1–15. [Google Scholar] [CrossRef]
- Venâncio, N.M.; de Souza, M.B. Anfíbios Do Parque Ambiental Chico Mendes, Rio Branco—Acre, Brasil. Biotemas 2016, 29, 85. [Google Scholar] [CrossRef]
- França, D.P.F.; de Freitas, M.A.; Ramalho, W.P.; Bernarde, P.S. Diversidade Local e Influência Da Sazonalidade Sobre Taxocenoses de Anfíbios e Répteis Na Reserva Extrativista Chico Mendes, Acre, Brasil. Iheringia Série Zool. 2017, 107, 1–12. [Google Scholar] [CrossRef]
- Haghtalab, N.; Moore, N.; Heerspink, B.P.; Hyndman, D.W. Evaluating Spatial Patterns in Precipitation Trends across the Amazon Basin Driven by Land Cover and Global Scale Forcings. Theor. Appl. Clim. 2020, 140, 411–427. [Google Scholar] [CrossRef]
- Landeiro, V.L.; Waldez, F.; Menin, M. Spatial and Environmental Patterns of Amazonian Anurans: Differences between Assemblages with Aquatic and Terrestrial Reproduction, and Implications for Conservation Management. Nat. Conserv. 2014, 12, 42–46. [Google Scholar] [CrossRef][Green Version]
- Venâncio, N.M.; Lima, A.P.; de Souza, M.B.; Magnusson, W.E. Between-Year Consistency of Anuran Assemblages in Published Temporary Ponds in a Deforested Area in Western Amazonia. Herpetol. J. 2014, I, 155–160. [Google Scholar][Green Version]
- Böning, P.; Wolf, S.; Upton, K.; Menin, M.; Venegas, P.J.; Lötters, S. Amphibian Diversity and Its Turnover in Floating Meadows along the Amazon River. Salamandra 2017, 53, 379–388. [Google Scholar][Green Version]
- Silva, M.E.S.; Pereira, G.; da Rocha, R.P. Local and Remote Climatic Impacts Due to Land Use Degradation in the Amazon “Arc of Deforestation”. Theor. Appl. Climatol. 2016, 125, 609–623. [Google Scholar] [CrossRef]
- Neckel-Oliveira, S. Effects of Forest Disturbance on Breeding Habitat Availability for Two Species of Anurans in the Amazon. Copeia 2007, 2007, 186–192. [Google Scholar] [CrossRef][Green Version]
- Neckel-Oliveira, S.; Gascon, C. Abundance, Body Size and Movement Patterns of a Tropical Treefrog in Continuous and Fragmented Forests in the Brazilian Amazon. Biol. Conserv. 2006, 128, 308–315. [Google Scholar] [CrossRef]
- Pearman, P.B. Correlates of Amphibian Diversity in an Altered Landscape of Amazonian Ecuador. Conserv. Biol. 1997, 11, 1211–1225. [Google Scholar] [CrossRef]
- Hof, C.; Araújo, M.B.; Jetz, W.; Rahbek, C. Additive Threats from Pathogens, Climate and Land-Use Change for Global Amphibian Diversity. Nature 2011, 480, 516–519. [Google Scholar] [CrossRef]
- Dayrell, J.S.; Magnusson, W.E.; Bobrowiec, P.E.D.; Lima, A.P. Impacts of an Amazonian Hydroelectric Dam on Frog Assemblages. PLoS ONE 2021, 16, e0244580. [Google Scholar] [CrossRef]
- Murcia, C. Edge_effects_in_fragmented_forests_impli. Trends Ecol. Evol. 1995, 10, 58–62. [Google Scholar] [CrossRef]
- Laurance, W.F.; Lovejoy, T.E.; Vasconcelos, H.L.; Bruna, E.M.; Didham, R.K.; Stouffer, P.C.; Gascon, C.; Bierregaard, R.O.; Laurance, S.G.; Sampaio, E. Ecosystem Decay of Amazonian Forest Fragments: A 22-Year Investigation. Conserv. Biol. 2002, 16, 605–618. [Google Scholar] [CrossRef]
- Both, C.; Melo, A.S.; Cechin, S.Z.; Hartz, S.M. Tadpole Co-Occurrence in Ponds: When Do Guilds and Time Matter? Acta Oecologica 2011, 37, 140–145. [Google Scholar] [CrossRef]
- Thompson, P.L.; Guzman, L.M.; De Meester, L.; Horváth, Z.; Ptacnik, R.; Vanschoenwinkel, B.; Viana, D.S.; Chase, J.M. A Process--based Metacommunity Framework Linking Local and Regional Scale Community Ecology. Ecol. Lett. 2020, 23, 1314–1329. [Google Scholar] [CrossRef]
- Xia, Z.; Heino, J.; Wang, J.; Chang, T.; Li, M. Niche Position Accounts for the Positive Occupancy–Abundance Relationship of Lake Fishes. J. Biogeogr. 2024, 51, 1374–1386. [Google Scholar] [CrossRef]
- Vieira, T.B.; Tejerina-Garro, F.L. Relationships Between Environmental Conditions and Fish Assemblages in Tropical Savanna Headwater Streams. Sci. Rep. 2020, 10, 2174. [Google Scholar] [CrossRef]
- Kopp, K.; Signorelli, L.; Bastos, R.P. Distribuição Temporal e Diversidade de Modos Reprodutivos de Anfíbios Anuros No Parque Nacional Das Emas e Entorno, Estado de Goiás, Brasil. Iheringia Série Zool. 2010, 100, 192–200. [Google Scholar] [CrossRef]
- Ries, L.; Fletcher, R.J.; Battin, J.; Sisk, T.D. Ecological Responses to Habitat Edges: Mechanisms, Models, and Variability Explained. Copyrigh 2004, 35, 491–522. [Google Scholar] [CrossRef]
- Gascon, C.; Lovejoy, T.E.; Bierregaard, R.O.J.; Malcolm, J.R.; Stouffer, P.C.; Vasconcelos, H.L.; Laurance, W.F.; Zimmerman, B.; Tocher, M.; Broges, S. Matrix Habitat and Species Persistence in Tropical Forest Remnants. Biol. Conserv. 1999, 91, 223–229. [Google Scholar] [CrossRef]
- da Silva, F.R.; Almeida-Neto, M.; do Prado, V.H.M.; Haddad, C.F.B.; Rossa-Feres, D.d.C. Humidity Levels Drive Reproductive Modes and Phylogenetic Diversity of Amphibians in the Brazilian Atlantic Forest. J. Biogeogr. 2012, 39, 1720–1732. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; de Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- MapBiomas. Coleção 4 Da Série Anual de Mapas de Cobertura e Uso de Solo Do Brasil. Projeto MapBiomas 2019. Available online: https://mapbiomas.org (accessed on 21 October 2025).
- Pupim, A.C.; Morais, M.d.J. Pressão Urbana na Área de Proteção Ambiental Raimundo Irineu Serra (APARIS), Cidade de Rio Branco, Acre, Brasil. Acta Geográfica 2021, 15, 318–337. [Google Scholar] [CrossRef]
- Zamaletdinov, R.; Khamidullina, R.; Pichugin, A.; Kornilov, P.; Fayzulin, A. The Development of the Structural Heterogeneity of the Territory of a Large City as Conditions for the Formation of Urban Ecosystems on the Example of Kazan. Urban Sci. 2025, 9, 354. [Google Scholar] [CrossRef]
- Justino, S.T.P.; da Silva, R.B.G.; Silva, R.B.; Simões, D. Land Use Change and Its Climatic and Vegetation Impacts in the Brazilian Amazon. Sustainability 2025, 17, 7099. [Google Scholar] [CrossRef]
- Cechin, S.Z.; Martins, M. Eficiência de Armadilhas de Queda (Pitfall Traps) Em Amostragens de Anfíbios e Répteis No Brasil. Rev. Bras. Zool. 2000, 17, 729–740. [Google Scholar] [CrossRef]
- Heyer, W.R.; Donnelly, M.A.; McDiarmid, R.W.; Hayek, L.-A.C.; Foster, M.S. Measuring and Monitoring Biological Diversity: Standard Methods for Amphibians; Smithsonian Institution Press: Washington, DC, USA, 1994. [Google Scholar]
- Bartlett, R.D.; Bartlett, P.P. Reptiles and Amphibians of the Amazon: An Ecotourist s Guide; University Press of Florida: Gainesville, FL, USA, 2003; Volume 79, ISBN 978-0813026237. [Google Scholar]
- Buxton, V.L.; Sperry, J.H. Reproductive Decisions in Anurans: A Review of How Predation and Competition Affects the Deposition of Eggs and Tadpoles. Bioscience 2017, 67, 26–38. [Google Scholar] [CrossRef]
- Vaz-Silva, W.; Maciel, N.M.; Nomura, F.; de Morais, A.R.; Batista, V.G.; Santos, D.L.; Andrade, S.P.; de Oliveira, A.Â.B.; Brandão, R.A.; Bastos, R.P. Guia de Identificação Das Espécies de Anfíbios (Anura e Gymnophiona) Do Estado de Goiás e Do Distrito Federal, Brasil Central; Sociedade Brasileira de Zoologia: Brazil, 2020; ISBN 9786587590011. Available online: https://books.scielo.org/id/9qfsp (accessed on 21 October 2025).
- Ribeiro, R.d.S.; do Egito, G.T.B.T.; Haddad, C.F.B. Chave de Identificação: Anfíbios Anuros Da Vertente de Jundiaí Da Serra Do Japi, Estado de São Paulo. Biota Neotrop. 2005, 5, 235–247. [Google Scholar] [CrossRef][Green Version]
- Marimon-Junior, B.H.; Hay, J.D. A New Instrument for Measurement and Collection of Quantitative Samples of the Litter Layer in Forests. For. Ecol. Manag. 2008, 255, 2244–2250. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INext3D: Interpolation and Extrapolation for Diversity Analysis of Three-Dimensional Data, Version 2020; National Tsing Hua University: Hsinchu, Taiwan, 2020.
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package, R Package Version 2.7-0; CRAN: Vienna, Austria, 2022.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Siqueira, C.C.; Vrcibradic, D.; Nogueira-Costa, P.; Martins, A.R.; Dantas, L.; Gomes, V.L.R.; Bergallo, H.G.; Rocha, C.F.D. Environmental Parameters Affecting the Structure of Leaf-Litter Frog (Amphibia: Anura) Communities in Tropical Forests: A Case Study from an Atlantic Rainforest Area in Southeastern Brazil. Zoologia 2014, 31, 147–152. [Google Scholar] [CrossRef][Green Version]
- Caldas, F.L.S.; da Silva, B.D.; dos Santos, R.A.; de-Carvalho, C.B.; Santana, D.O.; Gomes, F.F.A.; Faria, R.G. Autoecology of Phyllomedusa Nordestina (Anura: Hylidae) in Areas of the Caatinga and Atlantic Forest in the State of Sergipe, Brazil. North West J. Zool. 2016, 12, 271–285. [Google Scholar][Green Version]
- de Freitas, E.B.; De-Carvalho, C.B.; Faria, R.G.; Batista, R.d.C.; Batista, C.d.C.; Coelho, W.A.; Bocchiglieri, A. Nicho Ecológico e Aspectos Da História Natural de Phyllomedusa Azurea (Anura: Hylidae, Phyllomedusinae) No Cerrado Do Brasil Central. Biota Neotrop. 2008, 8, 101–110. [Google Scholar] [CrossRef]
- Ferrão, M.; Hanken, J.; Oda, F.H.; Campião, K.M.; Penhacek, M.; Anjos, S.; Rodrigues, D.J. A New Snouted Treefrog (Anura, Hylidae, Scinax) from Fluvial Islands of the Juruena River, Southern Brazilian Amazonia. PLoS ONE 2024, 19, e0292441. [Google Scholar] [CrossRef]
- Vieira, T.B.; Casatti, L.; Romero, R.M.; Tejerina-Garro, F.L.; Uchôa de Aquino, P.D.P.; Pompeu, P.S.; Correia, L.L.; Dias, S.V.; De Marco, P. Partitioning Niche and Neutral Drivers of Metacommunity Patterns in Fish from Brazilian Cerrado Streams. Ecohydrol. Hydrobiol. 2025, 100654. [Google Scholar] [CrossRef]
- Ferreira-Silva, S.K.; Alexandre, R.J.R.; Pena, S.A.; Lucena, M.D.L.; Vieira, T.B.; Gomes, F.B.R. Associations between Morphological Attributes and Food Resources in Anurans from the Middle Xingu Region, Brazil. Braz. J. Biol. 2025, 85, 1–13. [Google Scholar] [CrossRef]
- Alexandre, R.J.R.; Bergamini, F.M.; Spigoloni, Z.A.; Dias-Silva, K.; Vieira, R.R.S.; Guerra, V.; Bastos, R.P.; Vieira, T.B. Squamate Reptiles as Indicators in Fragments of Brazilian Cerrado. Stud. Neotrop. Fauna Environ. 2023, 59, 870–879. [Google Scholar] [CrossRef]
- da Silva, Z.D.; Gurgel, E.S.C.; Correia, L.L.; Vieira, T.B. Seed Dispersal by Bats (Chiroptera: Phyllostomidae) and Mutualistic Networks in a Landscape Dominated by Cocoa in the Brazilian Amazon. Glob. Ecol. Conserv. 2024, 55, e03252. [Google Scholar] [CrossRef]
- Herrera-Lopera, J.M.; Solé, M.; Cultid-Medina, C.A. Mapping the Missing: Assessing Amphibian Sampling Completeness and Overlap With Global Protected Areas. Ecol. Evol. 2025, 15, e71137. [Google Scholar] [CrossRef]
- Bailey, L.L.; Jones, P.; Thompson, K.G.; Foutz, H.P.; Logan, J.M.; Wright, F.B.; Crockett, H.J. Determining Presence of Rare Amphibian Species: Testing and Combining Novel Survey Methods. J. Herpetol. 2019, 53, 115. [Google Scholar] [CrossRef]
- Palmeirim, A.F.; Vieira, M.V.; Peres, C.A. Herpetofaunal Responses to Anthropogenic Forest Habitat Modification across the Neotropics: Insights from Partitioning β-Diversity. Biodivers. Conserv. 2017, 26, 2877–2891. [Google Scholar] [CrossRef]
- Russildi, G.; Arroyo-Rodríguez, V.; Hernández-Ordóñez, O.; Pineda, E.; Reynoso, V.H. Species- and Community-Level Responses to Habitat Spatial Changes in Fragmented Rainforests: Assessing Compensatory Dynamics in Amphibians and Reptiles. Biodivers. Conserv. 2016, 25, 375–392. [Google Scholar] [CrossRef]
- Gardner, T. Declining Amphibian Populations: A Global Phenomenon in Conservation Biology. Anim. Biodivers. Conserv. 2001, 24, 25–44. [Google Scholar] [CrossRef]
- Heyer, W.R. The Adaptive Ecology of the Species Groups of the Genus Leptodactylus (Amphibia, Leptodactylidae). Evolution 1969, 23, 421. [Google Scholar] [CrossRef]
- Medina, R.G.; Ponssa, M.L.; Aráoz, E. Environmental, Land Cover and Land Use Constraints on the Distributional Patterns of Anurans: Leptodacylus Species (Anura, Leptodactylidae) from Dry Chaco. PeerJ 2016, 4, e2605. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Demaynadier, P.G.; Hunter, M.L. Effects of Silvicultural Edges on the Distribution and Abundance of Amphibians in Maine. Conserv. Biol. 1998, 12, 340–352. [Google Scholar] [CrossRef]
- Dixo, M.; Martins, M. Are Leaf-Litter Frogs and Lizards Affected by Edge Effects Due to Forest Fragmentation in Brazilian Atlantic Forest? J. Trop. Ecol. 2008, 24, 551–554. [Google Scholar] [CrossRef]
- Schlaepfer, M.A.; Gavin, T.A. Edge Effects on Lizards and Frogs in Tropical Forest Fragments. Conserv. Biol. 2001, 15, 1079–1090. [Google Scholar] [CrossRef]
- Andriatsitohaina, B.; Ramsay, M.S.; Kiene, F.; Lehman, S.M.; Rasoloharijaona, S.; Rakotondravony, R.; Radespiel, U. Ecological Fragmentation Effects in Mouse Lemurs and Small Mammals in Northwestern Madagascar. Am. J. Primatol. 2020, 82, e23059. [Google Scholar] [CrossRef] [PubMed]
- Blanco-torres, A.; Duré, M.; Argenis, M.; Gómez, B. Trophic Ecology of Scinax rostratus (Peters, 1863) and Scinax ruber (Laurenti, 1768) (Anura: Hylidae) in Tropical Dry Forest of Northern Colombia. Herpetol. Notes 2017, 10, 405–409. [Google Scholar]
- Piatti, L.; de Amaro, P.M.O.; Araújo, J.F.d.J.; Sanches, V.Q.A.; Bernarde, P.S. Anurans of a Disturbed Area in Jarú, Rondônia, Brazil. Check List 2012, 8, 83–87. [Google Scholar] [CrossRef]
- Santos, D.L.; de Andrade, S.P.; Victor, E.P., Jr.; Vaz-Silva, W. Amphibians and Reptiles from Southeastern Goiás, Central Brazil. Check List 2014, 10, 131–148. [Google Scholar] [CrossRef][Green Version]
- Lambertini, C.; Ernetti, J.; Missassi, A.; Jorge, R.; da Silva Leite, D.; Lima, A.; Toledo, L. Chytrid Fungus in Amphibians from the Lowland Brazilian Amazon. Dis. Aquat. Organ. 2022, 152, 115–125. [Google Scholar] [CrossRef] [PubMed]

| Family Specie | IUCN | IUCN * | APA | FEC | PZ | RFH | Total |
|---|---|---|---|---|---|---|---|
| Aromobatidae | 4 | 1 | 8 | 8 | 21 | ||
| Allobates femoralis (Boulenger, 1884) | LC | LC | 4 | 4 | |||
| Allobates hodli Simões, Lima & Farias, 2010 | LC | LC | 1 | 1 | |||
| Allobates trilineatus (Boulenger, 1884) | LC | LC | 4 | 8 | 4 | 16 | |
| Bufonidae | 13 | 7 | 26 | 18 | 64 | ||
| Rhinella castaneotica (Caldwell, 1991) | LC | LC | 12 | 6 | 23 | 5 | 46 |
| Rhinella major (Müller & Hellmich, 1936) | LC | LC | 3 | 3 | |||
| Rhinella marina (Linnaeus, 1758) | LC | LC | 1 | 1 | 13 | 15 | |
| Craugastoridae | 0 | 8 | 0 | 9 | 17 | ||
| Oreobates quixensis Jiménez de la Espada, 1872 | LC | LC | 3 | 3 | |||
| Pristimantis fenestratus (Steindachner, 1864) | LC | LC | 8 | 4 | 12 | ||
| Pristimantis skydmainos (Flores & Rodríguez, 1997) | LC | LC | 2 | 2 | |||
| Dendrobatidae | 2 | 0 | 1 | 4 | 7 | ||
| Ameerega hahneli (Boulenger, 1884) | LC | LC | 1 | 1 | |||
| Ameerega trivittata (Spix, 1824) | LC | LC | 2 | 4 | 6 | ||
| Hylidae | 37 | 12 | 50 | 8 | 107 | ||
| Dendropsophus acreanus (Bokermann, 1964) | LC | LC | 1 | 1 | |||
| Dendropsophus leucophyllatus (Beireis, 1783) | LC | LC | 1 | 1 | 2 | ||
| Dendropsophus minutus (Peters, 1872) | LC | LC | 2 | 2 | 4 | ||
| Osteocephalus castaneicola Moravec, Aparicio, Guerrero-Reinhard, Calderón, Jungfer & Gvozdík, 2009 | LC | LC | 1 | 1 | |||
| Osteocephalus taurinus Steindachner, 1862 | LC | LC | 1 | 1 | |||
| Scinax funereus (Cope, 1874) | LC | LC | 1 | 1 | |||
| Scinax garbei (Miranda-Ribeiro, 1926) | LC | LC | 3 | 16 | 1 | 20 | |
| Scinax ruber (Laurenti, 1768) | LC | LC | 21 | 8 | 30 | 4 | 63 |
| Scinax sp. | 1 | 1 | 2 | ||||
| Trachycephalus typhonius (Linnaeus, 1758) | LC | LC | 10 | 1 | 1 | 12 | |
| Leptodactylidae | 50 | 58 | 98 | 21 | 227 | ||
| Adenomera andreae (Müller, 1923) | LC | LC | 29 | 33 | 47 | 5 | 114 |
| Adenomera hylaedactyla (Cope, 1868) | LC | LC | 10 | 15 | 12 | 3 | 40 |
| Engystomops freibergi (Donoso-Barros, 1969) | LC | LC | 27 | 9 | 36 | ||
| Leptodactylus bolivianus Boulenger, 1898 | LC | LC | 1 | 1 | |||
| Leptodactylus didymus Heyer, García-Lopez & Cardoso, 1996 | LC | LC | 4 | 2 | 1 | 7 | |
| Leptodactylus knudseni Heyer, 1972 | LC | LC | 1 | 1 | |||
| Leptodactylus leptodactyloides (Andersson, 1945) | LC | LC | 4 | 4 | 6 | 14 | |
| Leptodactylus pentadactylus (Laurenti, 1768) | LC | LC | 1 | 1 | 2 | ||
| Leptodactylus petersii (Steindachner, 1864) | LC | LC | 1 | 1 | |||
| Leptodactylus rhodonotus (Günther, 1869) | LC | LC | 2 | 2 | |||
| Lithodytes lineatus (Schneider, 1799) | LC | 5 | 2 | 2 | 9 | ||
| Microhylidae | 26 | 74 | 20 | 26 | 146 | ||
| Chiasmocleis bassleri Dunn, 1949 | LC | LC | 15 | 3 | 18 | ||
| Ctenophryne geayi Mocquard, 1904 | LC | LC | 2 | 2 | |||
| Elachistocleis muiraquitan Nunes-de-Almeida & Toledo, 2012 | LC | LC | 16 | 59 | 10 | 85 | |
| Hamptophryne boliviana (Parker, 1927) | LC | LC | 10 | 20 | 11 | 41 | |
| Phyllomedusidae | 0 | 0 | 0 | 1 | 1 | ||
| Phyllomedusa camba De la Riva, 1999 | LC | LC | 1 | 1 | |||
| Abundance | 132 | 160 | 203 | 95 | 590 | ||
| Richeness | 16 | 16 | 18 | 25 | 37 |
| Assemblage | Index | Observed | Estimated | SE | |
|---|---|---|---|---|---|
| Total | Species richness | 40.00 | 47.63 | 13.86 | |
| Shannon diversity | 15.84 | 16.55 | 0.79 | ||
| Simpson diversity * | 10.68 | 10.86 | 0.65 | ||
| Fragments | APA | Species richness | 16.00 | 16.66 | 2.95 |
| Shannon diversity | 10.72 | 11.42 | 0.85 | ||
| Simpson diversity * | 8.45 | 8.96 | 1.01 | ||
| FEC | Species richness | 17.00 | 41.35 | 15.15 | |
| Shannon diversity | 7.44 | 8.29 | 0.88 | ||
| Simpson diversity | 4.91 | 5.03 | 0.59 | ||
| PZ | Species richness | 19.00 | 22.11 | 5.58 | |
| Shannon diversity | 10.17 | 10.78 | 0.76 | ||
| Simpson diversity * | 7.84 | 8.12 | 0.65 | ||
| RFH | Species richness | 25.00 | 45.04 | 20.80 | |
| Shannon diversity | 17.82 | 22.43 | 2.50 | ||
| Simpson diversity * | 13.99 | 16.24 | 2.05 | ||
| Edge Distance | ed1 | Species richness | 25.00 | 27.56 | 6.22 |
| Shannon diversity | 10.93 | 11.93 | 0.95 | ||
| Simpson diversity * | 6.71 | 6.94 | 0.64 | ||
| ed2 | Species richness | 21.00 | 23.48 | 7.09 | |
| Shannon diversity | 12.09 | 13.25 | 1.07 | ||
| Simpson diversity * | 8.68 | 9.21 | 0.81 | ||
| ed3 | Species richness | 28.00 | 42.29 | 21.24 | |
| Shannon diversity | 16.47 | 19.66 | 1.97 | ||
| Simpson diversity * | 12.03 | 13.17 | 1.33 | ||
| ed4 | Species richness | 20.00 | 21.12 | 4.75 | |
| Shannon diversity | 12.23 | 13.12 | 0.89 | ||
| Simpson diversity * | 8.55 | 8.99 | 1.00 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, Y.A.; Guerra, V.; Correia, L.L.; Vieira, T.B.; de Souza, M.B. Environmental Drivers and Edge Effects on Anuran Diversity in Fragmented Forests of the Southwestern Brazilian Amazon. Diversity 2025, 17, 764. https://doi.org/10.3390/d17110764
Pereira YA, Guerra V, Correia LL, Vieira TB, de Souza MB. Environmental Drivers and Edge Effects on Anuran Diversity in Fragmented Forests of the Southwestern Brazilian Amazon. Diversity. 2025; 17(11):764. https://doi.org/10.3390/d17110764
Chicago/Turabian StylePereira, Yara Araújo, Vinicius Guerra, Letícia Lima Correia, Thiago Bernardi Vieira, and Moisés Barbosa de Souza. 2025. "Environmental Drivers and Edge Effects on Anuran Diversity in Fragmented Forests of the Southwestern Brazilian Amazon" Diversity 17, no. 11: 764. https://doi.org/10.3390/d17110764
APA StylePereira, Y. A., Guerra, V., Correia, L. L., Vieira, T. B., & de Souza, M. B. (2025). Environmental Drivers and Edge Effects on Anuran Diversity in Fragmented Forests of the Southwestern Brazilian Amazon. Diversity, 17(11), 764. https://doi.org/10.3390/d17110764








