Abstract
Freshwater mussels in the genera Fusconaia, Pleurobema, and Pleuronaia are similar in their external shell morphology, which has made the identification and classification of species within these genera difficult and led to many taxonomic revisions. Large samples (N = 464) of select mussel species in these genera were collected from 2012 through 2014, primarily in the upper Tennessee River basin of Tennessee and Virginia, USA. Mitochondrial ND1 and nuclear ITS1 DNA sequences were analyzed to assess phylogenetic relationships among taxa. Ten species were verified as phylogenetically distinct at ND1, two of which were cryptic and previously unrecognized species. Described herein as Pleurobema parmaleei and Pleuronaia estabrookianus, each species clade was diverged at this gene region by ~3.0% from the respective closest congener. The nuclear ITS1 gene region’s nucleotide-site insertion/deletion (indel) patterns were analyzed as single mutational events rather than as fifth character states or missing data. Most species, including these two, were phylogenetically distinct at the ITS1 region when incorporating indels into analyses, but some estimated interspecific pairwise distances were lower than corresponding intraspecific estimates. Among morphological traits assessed for each species, differences in foot color and gravidity characteristics illustrated differences between phylogenetically recognized species and their closest congeners. Due to the limited known geographical distributions of these two cryptic species, each may require protection under the U.S. Endangered Species Act. While this study collected large sample sizes for each species, many streams in the basin remain unsampled and could potentially contain populations of these species or additional cryptic species.
1. Introduction
Freshwater mussels (hereafter referred to as “mussels”) are considered the most imperiled taxonomic group in North America [1,2,3]. Of the 298 recognized species in the families Unionidae and Margaritiferidae in the United States and Canada, approximately 70% of these species are considered endangered, threatened, or of special concern at the state and federal levels [3]. Extinction rates for freshwater taxa are five times greater than those for terrestrial fauna and similar to rates estimated for tropical rainforest communities [4]. Mussel habitat has been lost, fragmented, and degraded due to anthropogenic effects from dam construction, sedimentation, and water pollution [1,5,6,7]. The sedentary nature of adult unionid mussels and their general reliance on fish hosts to disperse their larvae make recolonization of isolated stream reaches difficult, especially reaches blocked by dams. Translocation and propagation efforts for mussels are underway to restore mussels to rivers with suitable water and habitat quality [8,9,10,11,12,13]. Protection and restoration of habitat is important for freshwater mussels, not only to address their imperiled status, but also because they serve valuable roles in stream ecosystems [14,15].
Since the late 17th century, taxonomic classifications of mussels have been based primarily upon shell morphology [16]. Approaches to classification have changed as authors have incorporated additional characters, including soft anatomy, larval morphology, and life history traits [17,18,19]. With the recent advent of molecular genetic techniques, mussel taxonomy has undergone further reorganization at the species, genus, and family levels [20], leading to a number of recent taxonomic revisions [21,22,23,24,25,26,27].
The Tennessee and Cumberland River basins, major tributary watersheds of the Ohio River, collectively hold the highest diversity of mussel species in North America [7]. Several species of interest in this study, Fusconaia cor (Conrad, 1834) [28], Fusconaia cuneolus (Lea, 1840) [29], and Pleuronaia barnesiana (Lea, 1838) [30], are endemic to the Tennessee River basin, with Pleuronaia gibber (Lea, 1838) [30] endemic to the Cumberland River basin; Pleurobema oviforme (Conrad, 1834) [28] and Pleuronaia dolabelloides (Lea, 1840) [29] are endemic to the Tennessee and Cumberland River basins, and Fusconaia subrotunda (Lea, 1831) [31] occurs broadly throughout the Ohio River basin [32,33]. The United States Fish and Wildlife Service (USFWS) listed F. cor and F. cuneolus as endangered in 1975 [34], P. gibber as endangered in 1991 [35], and P. dolabelloides as endangered in 2013 [36]. P. oviforme and P. barnesiana are currently proposed as endangered [37].
Historically, P. barnesiana, P. dolabelloides, and P. gibber were classified in the genera Fusconaia, Lexingtonia, and Pleurobema, respectively, but were revised taxonomically by Campbell et al. [38] based on results of a phylogenetic assessment using mitochondrial DNA (mtDNA) sequences, with these three species grouped together within a clade separate from species in the genera Fusconaia and Pleurobema. Using existing taxonomic nomenclature and type specimens, Williams et al. [16] recognized P. barnesiana as the type species for the resurrected genus Pleuronaia based on the earlier type species designation made by Frierson [39], which now includes P. barnesiana, P. dolabelloides, and P. gibber. Additionally, Ortmann [40] considered P. barnesiana to be “distinguished from the other (Fusconaia) species by very shallow beak cavities.” However, no obvious shared morphological traits are currently known to uniquely distinguish these three species from the other species in the genera Fusconaia and Pleurobema. For example, no single trait or set of traditional morphological characteristics, such as shell shape, periostracum color and ray pattern, number of gills in female mussels that brood larvae (four in Fusconaia and two in Pleurobema), size and shape of larvae (glochidia), and foot color, defines Pleuronaia. Hence, using a combination of molecular DNA and morphological traits may be necessary to characterize and describe species in these three genera.
The geographical distribution of species in the genera Fusconaia, Pleurobema, and Pleuronaia varies depending on the inclusion or exclusion of certain species in each genus. Fusconaia is recognized to occur in the Mississippi, Gulf Coast, and Atlantic Slope drainages. However, the suite of species that belong to the genus Pleurobema is more contentious, with current species’ distributions listed for the Mississippi and Gulf Coast drainages, with one species, previously recognized as Pleurobema collina, occurring in Atlantic Slope drainages; however, a recent genetic study [27] concluded that P. collina does not belong in the genus Pleurobema, and placed it in the genus Parvaspina (Perkins, Johnson, Gangloff, 2017) [27]. Molecular genetic studies also have suggested that numerous Pleurobema species may not belong to that genus, but concluded that future work was needed to determine the correct genus or genera for these species [24,41,42]. However, species in the genus Pleuronaia are currently thought to be restricted to the Tennessee and Cumberland drainages [16].
Thus, the purpose of this study was to conduct a phylogenetic and morphological assessment of mussels in the genera Fusconaia, Pleurobema, and Pleuronaia occurring primarily in the upper Tennessee River basin (UTRB). While previous studies typically have relied on small sample sizes to infer phylogenetic relationships within and among freshwater mussel species, this study surveyed numerous collection sites and utilized larger sample sizes per site and per species to detect genetic and morphological variation and potential cryptic biodiversity among taxa.
2. Materials and Methods
2.1. Mussel Collections
Mussels were collected from 2012 through 2014 in the UTRB, primarily in the upper Clinch, Holston, and Powell River watersheds, and in selected tributaries of the Tennessee River downstream of that region, to include the Paint Rock, Alabama, and Duck Rivers, TN (Figure 1, Table S1). Mussels were also collected from Collins River, TN, in the upper Cumberland River basin. Sites were selected based on knowledge of the results of previous field sampling efforts to represent each species’ geographical distribution in the UTRB. Mussels representing the respective genera and species were hand-collected via snorkel search or using view scopes. Once individuals were identified to species with genetic markers, they were then analyzed for morphometric characters. Using genetically identified individuals ensured that misidentified individuals would not affect morphology-based analyses.
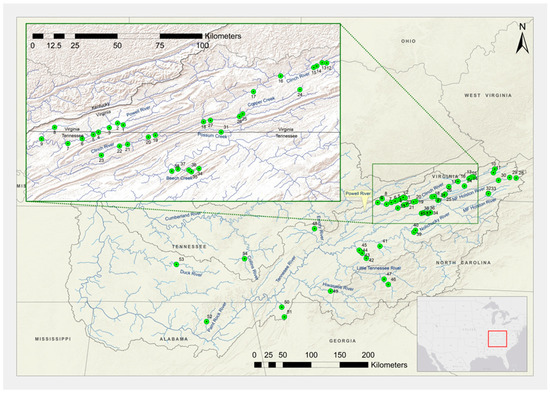
Figure 1.
Sampling localities and site numbers for freshwater mussels collected primarily in the Tennessee River basin from 2012 through 2014. Site numbers correspond to locality information in Table S1.
2.2. DNA Extraction
Mussels were gently opened to a maximum width of 6–8 mm to non-lethally obtain a tissue sample using an Isohelix (Harrietsham, UK) SK-2 buccal swab [43]. The foot was swabbed vigorously with four to six strokes to obtain tissue for DNA extraction [44]. The tissue samples were transported to the Integrated Life Sciences Building at Virginia Tech University, where the samples were chemically stabilized, and DNA extractions were performed using the Isohelix DDK Isolation Kit according to the manufacturer’s instructions.
2.3. Polymerase Chain Reaction
The mitochondrial first subunit of the NADH dehydrogenase (ND1) region and the nuclear ribosomal Internal Transcribed Spacer region (ITS1) were amplified by polymerase chain reaction (PCR). The ND1 gene is one of the more variable mitochondrial DNA regions and typically provides numerous polymorphic sites for assessing interspecific divergence; while ITS1 is less variable, it is a nuclear DNA sequence that contains polymorphic sites and indels that have been used successfully for phylogenetic assessments with mussels [45,46]. Sequences from several species for each respective genus were amplified using primers and conditions reported by Serb et al. [45] for ND1 and King et al. [46] for ITS1. Primers reported by Serb et al. [45] did not consistently amplify target DNA for all species in this study, and thus primers for ND1 were modified; they were used in a multiplex to include one forward primer for all genera and two reverse primers, one to amplify species in the genera Fusconaia and Pleuronaia and the other to amplify species in the genus Pleurobema, as detailed below. Primer sequences used to amplify ND1 sequences for Fusconaia and Pleuronaia species were as follows: forward: 5′-GAAAAGTGCATCAGATTAAAGCTCT-3′; reverse: 5′-CCTGCTTGGAAGGCAAGTGTACT-3′. The forward ND1 primer for Pleurobema species was the same, but the reverse primer was 5′–AGATTTTCAGGCTATTGCTATTAG-3′. Primers for ITS1 were modified to exclude a poly-adenine region thought to influence primer annealing and were as follows: forward: 5′-GGTGAACCTGCGGAAGGATCATTACC-3′; reverse: 5′-TGCGTTCTTCATCGACCCACGAGCCG-3′. The ND1 and ITS1 PCR reaction mixtures consisted of 1 uL of unquantified genomic DNA, 2.2X PCR buffer, 3.96 mM of MgCl2, 0.36 mM each of dNTP, 0.36 μM of each primer, 0.36 mg/mL of BSA, 0.5U of GoTaq DNA polymerase, and ddH2O, added to a total volume of 22 μL. Touchdown PCR protocols were used instead of traditional PCR protocols to increase the amplification success rates. The thermal cycling profile consisted of an initial 95 °C for 3 minutes (min); followed by a touchdown PCR protocol that consisted of 10 cycles of denaturation at 95 °C for 30 seconds (s), annealing at 62 °C for 45 s, and extension at 72 °C for 60 s, with the annealing temperature decreased by 0.5 °C per cycle; followed by 25 cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 45 s, and extension at 72 °C for 60 s, with the annealing temperature decreased by 0.3 °C per cycle and extension time increased by 5 s per cycle; with a final extension step at 72 °C for 2 min; and a final hold at 4 °C.
After PCR reactions, DNA concentration was quantified using a DyNA Quant 200 fluorometer (Hoefer Pharmacia Biotech, San Francisco, CA, USA), diluted to 10 ng/mL, and sent to the Virginia Bioinformatics Institute, where samples were prepared using an Applied Biosystems (Thermo Fisher Scientific, Waltham, MA, USA) Big Dye Terminator 3.1 Cycle Sequencing Kit and then sequenced on an Applied Biosystems 3730 DNA Analyzer with Pop-7 polymer (Thermo Fisher Scientific, Waltham, MA, USA).
2.4. Data Analyses
Forward and reverse ND1 and ITS1 DNA sequences were assembled and edited using Geneious version 7.1.5 (Biomatters, San Francisco, CA, USA). Mitochondrial ND1 and nuclear ITS1 sequences were aligned using the default settings in the program Clustal W [47] embedded in MEGA version 5.05 [48]. Since some individuals in this study contained multiple ITS1 sequences of different lengths within an individual and proved difficult to resolve unambiguously, DNA sequences from these individuals were excluded from the analyses [49]. Data from heterozygous individuals with nuclear ITS1 sequences containing single-nucleotide polymorphisms that were not insertions or deletions were coded and reported using standard International Union of Biochemistry codes. DNA sequences were queried using the Basic Local Alignment Search Tool, also known as BLAST [50], against the National Center for Biotechnology Information database to verify gene identity and species-level assignment. For each ITS1 alignment, indels were coded using binary characters to represent gaps as either present, absent, or unknown [51,52] using the program FastGap version 1.2 [53]. The method for coding gaps created by Simmons and Ochoterena [51] encodes each indel event as a single evolutionary step rather than treating each nucleotide indel as a fifth character state.
Haplotypes were recognized using the program DnaSP version 5, with intraspecific and interspecific genetic divergence assessed for each species by calculating the percent mean number of nucleotide differences among sequences [54]. The program jModelTest version 2.1.6 [55] was used to determine the best nucleotide substitution model for ND1 and ITS1 sequences, separately. The number of substitution schemes analyzed in jModelTest was reduced from the default of eleven schemes to three schemes to reflect the substitution models available for coding in the program MrBayes version 3.2.1 [56]. To test the validity of combining data, ND1 and ITS1 sequences were combined and analyzed in Phylogenetic Analysis Using Parsimony and other Methods (PAUP) version 4 [57] for incongruent length differences in tree topologies using the homogeneity partition test [58]; congruence of sequences is generally recognized at p > 0.05 [58]. Results from the homogeneity partition test were significant (p = 0.01), indicating incongruence between nuclear and mitochondrial trees, and thus, DNA sequences were not combined for subsequent phylogenetic analyses.
Phylogenetic trees were constructed using Bayesian inference in MrBayes using two runs, each with three cold chains and one hot chain, and allowed to run until split frequencies, or the difference in standard deviations between the two runs, consistently stayed below 0.01. Results from jModelTest indicated that the general time-reversible model with invariable sites and a gamma-shaped distribution (GTR + I + G) was the best nucleotide substitution model for ND1. Phylogenetic analysis of ND1 was run in MrBayes for 10 million Markov chain Monte Carlo generations, with a burn-in of 2.5 million generations, tree search temperature set at 0.05, and sampling every 1000 generations, resulting in split frequencies of 0.0036. Results from jModelTest indicated that the Jukes–Cantor model was the best nucleotide substitution model for ITS1. Aligned haplotype sequences with coded gaps from Clustal W were run in MrBayes for 2 million generations, with a burn-in of 0.5 million generations, default tree search temperature of 0.10, and sampling every 1000 generations, resulting in split frequencies of 0.0063. The program FigTree version 1.4.0 [59] was used to view and modify phylogenetic trees created by MrBayes. Phylogenetically based species were identified by reciprocal monophyletic clades of haplotypes within phylogenetic trees [60]. Pairwise genetic distances between putative phylogenetic species were estimated in PAUP. Arbogast et al. [61] recommended incorporating the best-fitting nucleotide substitution model when assessing divergence between species; hence, pairwise genetic distances for ND1 were analyzed using the substitution model GTR + G instead of GTR + I + G because the program could not accept invariable sites for the analysis. Thus, the next highest Bayesian information criterion (BIC)-supported model was implemented. Because nucleotide substitution models in PAUP cannot incorporate binary characters, mean uncorrected p-distances between species were estimated for ITS1.
2.5. Morphology
Morphological characters, such as external and internal shell characters, foot color, and gravidity (female mussel brooding larvae, i.e., glochidia in the gills), were recorded for each individual, with sample sizes for genetically identified individuals and respective morphological traits analyzed reported in Table 1. When gravid individuals were encountered, the number of charged gills, the location of conglutinates in the gills, and the color of charged gills were recorded. Additionally, gravid individuals were transported to and held in the laboratory at the Freshwater Mollusk Conservation Center (FMCC, Blacksburg, VA, USA) until conglutinates were discharged. Morphological characteristics of the conglutinates and glochidia were measured, such as color, shape, and length. Mussels were measured to the nearest tenth of a millimeter (mm) with digital calipers. A total of five measurements were made, including (1) maximum length, (2) maximum height perpendicular to maximum length, (3) height posterior to umbo perpendicular to maximum length, (4) maximum width, and (5) hinge length (Figure 2). Traditional morphological characteristics, such as foot color and gravidity, were recorded.

Table 1.
Sample sizes for live individuals identified to species using mitochondrial DNA (mtDNA), and then used for morphological trait analyses. Mussels were sampled in the upper Tennessee River basin in Virginia and Tennessee from 2012 to 2014. Sample sizes of non-genetically identified shells from the FMCC collection are also included. Shells of Pleurobema sp. cf. oviforme in the FMCC collection were later transferred to the McClung Museum of Natural History and Culture, University of Tennessee, Knoxville, where they currently reside.
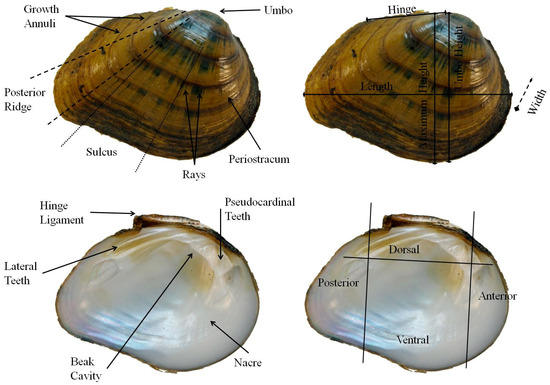
Figure 2.
External (top) and internal (bottom left) shell characteristics investigated in this study, including anatomical regions (bottom right) of the shell of Fusconaia cor.
Traditional categorical and quantitative characteristics were assessed for sacrificed, genetically identified individuals of non-listed species, with respective sample sizes per trait reported in Table 2. Categorical characteristics recorded included the following: shell outline (elongate, quadrate, or round); umbo position (anterior or posterior), periostracum color (yellow, light brown, brown, or dark brown); periostracum texture (dull, satiny, or shiny); ray pattern (no rays, continuous, or discontinuous, i.e., rays broken or interrupted); ray length (extending to shell margin or cessation before shell margin); ray width (narrow < 1 mm or wide > 1 mm); shape of posterior ridge (angular or rounded); presence of sulcus (present or absent); sulcus length (short, extending < 2/3 of shell length from ventral margin toward umbo, or long, extending > 2/3 of shell length from ventral margin toward umbo). Due to small sample sizes of shells for rare and endangered species, non-genetically identified specimens were selected from the FMCC shell collection based on characteristics generally observed in genetically verified individuals (i.e., for F. cor, F. cuneolus, F. subrotunda, P. sp. cf. oviforme, and P. dolebelloides) (Table 1). Umbo elevation was measured using digital calipers to the nearest 0.1 mm. For many of the sacrificed individuals, we possessed data for foot color; therefore, this data was concatenated into the database for shells. Photographs were taken of all sacrificed mussels and those used from the FMCC collection as voucher pictures and for use in geometric morphometric programs and analyses.

Table 2.
Categorical variables for shell traits used in the study and respective sample sizes per species.
A Pentax (Tokyo, Japan) Optio WG1 compact camera was used with settings at macro-focus and with a two-second photograph delay. The delay ensured that vibrations caused by focusing the camera would not result in poor image quality. Bias can be introduced into photographs in various ways, such as inconsistent lighting, focal length, tilt, and distance between lens and specimen [62]. Hence, a light box was used to ensure consistent light, distance between the camera lens and the specimen, stabilization of the camera, and leveling of the specimens. Calipers located 220 mm below the camera lens in the light box were used to hold the specimens; the calipers held the individuals at the posterior and anterior intersections of the left and right valves. Calipers held individuals so that the shell valve was parallel to the camera lens and provided a measurement reference if digital re-measuring was needed.
Glochidia were measured from discharged conglutinates using an ocular micrometer in a compound microscope. Grains of salt were added to the water near glochidia to close them for more accurate measurements; this also ensured that the glochidia were viable. A total of ten glochidia per mussel were measured for height, length, and hinge length. Tukey’s comparisons from analysis of variance (ANOVA) were conducted on each measurement to determine if significant differences occurred among species. Photographs were taken of conglutinates and glochidia, and their general shapes and colors were recorded.
Classification and Regression Tree Analysis of Morphological Data—Both categorical (Table 2) and continuous (Figure 2) morphological variables from live individuals and their shells were analyzed using a classification and regression tree (CART) procedure in the graphic user interface package Rattle version 2.6.7 [63], which summons rpart [64] implemented in the program R version 2.14.1 [65]; data were not partitioned due to low sample sizes for endangered species. Correlations between variables were analyzed to determine whether CART could use categorical and continuous morphological characteristics to consistently separate species. Three CART analyses were conducted: (1) using data collected from live mussels, including traditional continuously distributed variables of shell length, maximum height, height at umbo, width, and hinge length, plus one categorical variable, foot color, which were analyzed together; (2) using data collected from shells of the genetically identified, sacrificed non-endangered mussels and from the subset of shells of endangered mussels maintained in the FMCC shell collection, including the whole suite of continuous and categorical variables previously mentioned, but lacking beak cavity depth; and (3) from the same shell data and variable set as in analysis two, but with beak cavity depth included and minus foot color. CART analysis 1 represented easy-to-measure continuously distributed variables, as shown in Figure 2, with one easy-to-assess categorical variable, i.e., foot color. Our intention was to have a character set that could be assessed by novices in the field to identify live individuals. CART analysis 2 represented a character set of all continuous variables in Figure 2, plus all of the categorical variables in Table 2. Our intention was also application to live mussels in the field, but it is a character set that would require more training and experience to implement. CART analysis 3 was intended for shell-only identifications.
Data were not scaled or transformed, as combinations of variables in subsequent steps should adjust for differing mussel sizes. The package rattle can accommodate missing values in the data set but assigns the modal value observed from all species; thus, the modal value for each species was used to address missing values for foot color. Trees were built using a minimum split and minimum bucket of 12 and 4, respectively, to accommodate small sample sizes observed in endangered species; setting the minimum bucket too low may over-fit the data, with each bucket representing an individual mussel. The minimum split is the minimum number of observations necessary to create a split or node in the decision tree; the minimum bucket is the minimum number of observations necessary to create a group after a split that is either terminal or non-terminal. Overall tree accuracy was determined as the percentage of correct classifications of species on terminal nodes. A confusion matrix was created for each of the three CART trees to show species predictions based on morphological characteristics. A confusion matrix illustrates the true identity of the species in the rows and the predicted classification in the columns. The matrix allows for comparison of correct classification, false negatives or type two errors (the species in question labeled as different species), and false positives or type one errors (other species labeled as the species in question).
Geometric Morphometrics—A transparent radial graph with lines at 15-degree increments was overlain onto photographs of mussel shells. Two homologous locations, the posterior termination of the hinge ligament and the anterior intersection of the hinge ligament and umbo, were used to align the radial graph; an additional nine semi-sliding landmarks were used for analysis. Photographs were loaded into the program tpsdig2 [66] to digitize points; the two homologous points were used to align the 15° overlay grid, and the remaining nine points were digitized where the radial grid intersects the margin of the shell (Figure S1). Digitized points were resized using one of several superimposition methods to eliminate size and orientation bias, but the shape of the digitization remained the same. The most fundamental superimposition method uses Bookstein shape coordinates, also referred to as the two-point registration [62]. This method uses two homologous points shared between individuals as the baseline for superimposition. These points were digitized as 0,0 and 0,1 so that the baseline was consistent between all individuals; thus, only shape differences remained. Another, more favored approach to eliminating size and orientation bias is the generalized least squares Procrustes superimposition [62]. Rather than using a baseline, Procrustes superimposition uses the summed squared distances between analogous landmarks to minimize differences. The advantage of using Procrustes superimposition is that the combination of translation, scaling, and rotation removes all information that is not related to shape [62]. Data were then exported into the program CoordGen6f [67], and the digitized points were translated into Procrustes coordinate systems. Shape variation was analyzed using canonical variates analysis (CVA) [62]. The CVA used priors, such as species identification, to analyze morphological differences that consistently reproduced variability between the species [68]. The CVA determined axes that maximize differences between group means (i.e., species) relative to within-group mean variation [62]. Differences among species were tested using Goodall’s F-test using Procrustes superimposition; Goodall’s F-test analyzes the difference in mean shape between two species relative to shape variation within all samples of each species. Geometric morphometric data produced from CoordGen6f were analyzed using CVA in the program CVAGen6k [67]. The program TwoGroup6h [67] was used to conduct Goodall’s F-test between pairs of species and to illustrate differences between species using vector grids; deformations illustrated the movement of landmarks to highlight key areas of shell variation between species.
3. Results
3.1. Mussel Collections
A total of 464 mussels were collected from 54 sites in 22 streams in the UTRB for phylogenetic assessment of nucleotide variation at the mitochondrial ND1 gene and nuclear ITS1 gene regions. At most collection sites, a maximum of 20 individuals were sampled and analyzed to reduce overweighting in the results of analyses of any single population, although additional individuals were sampled at some sites in order to obtain gravid individuals or because individuals of the species were not abundant (<5 individuals) at other sites.
3.2. Phylogenetic Analyses of ND1
The ND1 amplification resulted in approximately 900 base pairs (bp) of DNA sequences for individuals in the genera Fusconaia and Pleuronaia and approximately 825 bp for individuals in the genus Pleurobema. Within and among these species and genera, 146 haplotypes were observed among 464 individuals (Table 3), excluding the outgroups Lampsilis fasciola and Lampsilis ovata; many of the haplotypes observed for each species were shared among sampling sites (Table S2). GenBank accession numbers and counts for each haplotype are listed in Table S3.

Table 3.
Number (N) of sampled individuals and haplotypes for each species used in the mitochondrial DNA (ND1) and nuclear DNA (ITS1) analyses. 1 Indicates previously unrecognized species. 2 Indicates a potentially unrecognized species pending further genetic and morphological investigation.
The phylogram of ND1 DNA sequences showed that the genera Fusconaia, Pleurobema, and Pleuronaia each formed monophyletic clades (Figure 3); however, P. gibber was not closely associated with other Pleuronaia species. Individuals of P. gibber were phylogenetically distinct from the other Pleuronaia species, and pairwise genetic distances between P. gibber and the other members of the genus were higher than pairwise genetic distances observed among the other Pleuronaia species. Intrageneric variation estimates were 6.5%, 6.3%, and 9.1% for Fusconaia, Pleurobema, and Pleuronaia, respectively. Removal of P. gibber haplotypes from the other sequences of Pleuronaia reduced intrageneric variation to 6.6% (Table 4).
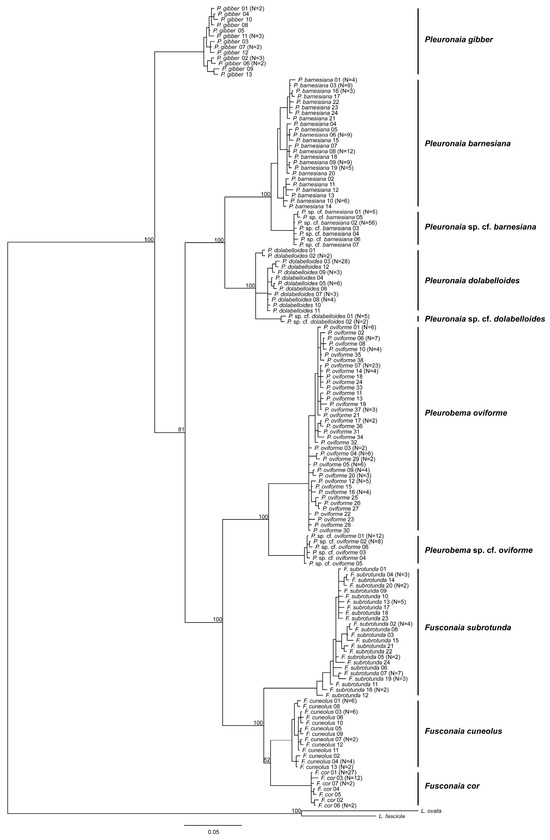
Figure 3.
Bayesian consensus tree showing phylogenetic relationships of freshwater mussels inferred from the mitochondrial gene region ND1. Numbers on branches are posterior probabilities of tree topology. The analysis was run for 10 million generations with split frequencies of 0.0036, with the most likely tree possessing a -ln likelihood of −5677.8262 and a mean -ln likelihood of −5728.8538.

Table 4.
Pairwise nucleotide distances between species’ haplotypes at the mitochondrial gene ND1. Pairwise differences were calculated using the general time-reversible model with rates gamma (GTR + G). Bold numerals indicate intraspecific variation. 1 Indicates previously unrecognized species. 2 Indicates a potentially unrecognized species pending further genetic and morphological investigation.
The ND1 sequences of F. cor, F. cuneolus, F. subrotunda, P. oviforme, P. dolabelloides, P. barnesiana, and P. gibber formed species-specific monophyletic clades (Figure 3). Within the respective clades for P. oviforme, P. barnesiana, and P. dolabelloides, previously unrecognized, phylogenetically distinct sub-clades with 100% posterior probability values were identified. The estimated interspecific distance between P. oviforme and P. sp. cf. oviforme was 6.3%, between P. barnesiana and P. sp. cf. barnesiana was 2.9%, and between P. dolabelloides and P. sp. cf. dolabelloides was 3.2%. Estimated interspecific pairwise genetic mean distances among all species ranged from 2.9% to 17.2% (Table 4). The highest observed interspecific distance was between F. cor and P. sp. cf. barnesiana at 17.2%. Estimated interspecific pairwise genetic variation within a freshwater mussel genus is typically recognized at around 3% for ND1; however, studies have recognized species that are morphologically distinct with pairwise genetic distances less than 1% [19,22,69].
Considering variation across the species studied, mean intraspecific distances among haplotypes within species ranged from a low of 0.2% to a high of 1.1% (Table 4). Mean intraspecific pairwise genetic distance within P. barnesiana averaged 1.1%, and the inferred phylogeny comprised three distinct subclades separated by genetic distances of 1.4% to 1.5%. These three distinct subclades did not reflect geographic distributions, as each subclade contained haplotypes sampled from different drainages. Fusconaia subrotunda exhibited a mean intraspecific distance of 1.0%, which can be viewed by the various clades nested within one another within the main clade for the species (Figure 3). A mean intraspecific distance of 0.8% was observed in P. dolabelloides, with two haplotypes separated by approximately 1.7% from the main clade (Figure 3). Grobler et al. [70] obtained similar genetic distance results for this species, with one haplotype that was collected from approximately the same location in Clinch River at Cleveland Islands; removal of the two haplotypes from the pairwise genetic analyses reduced intraspecific variation to 0.4%.
3.3. Phylogenetic Analyses of ITS1
Approximately 520 bp of the nuclear ITS1 gene was sequenced and analyzed for 97 individuals, a subset of those sequenced for ND1; due to limitations in funding and time, select individuals were randomly chosen from sample sites across the distribution of the species within the UTRB and used for analysis of ITS1 (Table S4). Twenty-six haplotypes were observed within this sample, excluding the outgroup sequence from L. fasciola (Table 3). GenBank accession numbers and counts for each haplotype are listed in Table S5. Analysis using the program Clustal W produced a sequence alignment that contained 103 polymorphic nucleotide sites, including indels; use of FastGap encoded 34 gap positions using these indels (Table S6).
Phylogenetic analysis of ITS1 DNA sequences revealed low to moderate separation among species, e.g., by one or two nucleotide changes, but separated many of the respective genera (Figure 4). The undescribed P. sp. cf. oviforme sequences formed a phylogenetically distinct clade from P. oviforme with 100% posterior probability. The undescribed P. sp. cf. barnesiana was not placed in the same clade as P. barnesiana and was separated by 2.66% from this species. Haplotypes for P. dolabelloides were not monophyletic, with two haplotypes being separated by 1.33%; the second haplotype occurred in two individuals, the same individuals that were separated at ND1; the two haplotypes of P. sp. cf. dolabelloides formed a distinct subclade with 95.8% posterior probability from the first P. dolabelloides haplotype.
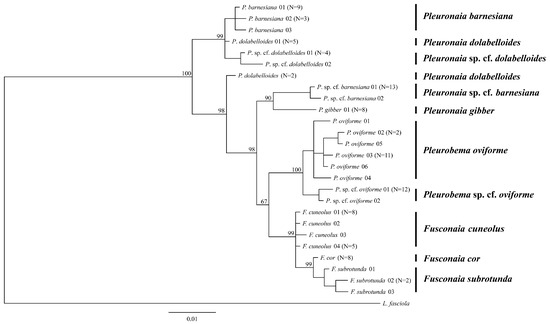
Figure 4.
Bayesian consensus tree showing phylogenetic relationships of freshwater mussels inferred from the nuclear gene ITS1. Numbers on branches are posterior probabilities of tree topology. Haplotype numbers do not correspond with those for mitochondrial DNA data. The analysis was run for 2 million generations with split frequencies of 0.0063, with the most likely tree possessing a -ln likelihood of −1350.1719 and a mean -ln likelihood of −1371.1794.
Intrageneric variation estimates were 0.006%, 0.011%, and 0.208% for Fusconaia, Pleurobema, and Pleuronaia, respectively (Table 5). Removal of P. gibber haplotypes from the other sequences of Pleuronaia reduced intrageneric variation to 0.017%. Estimated pairwise genetic distances among species’ haplotypes ranged from 0.4% to 3.4% for F. cor and F. cuneolus and for P. sp. cf. oviforme vs. P. sp. cf. dolabelloides, respectively, and intraspecific variation ranged from 0.2% to 0.7% (Table 5). In two cases (i.e., P. dolabelloides vs. P. barnesiana and P. dolabelloides vs. P. sp. cf. dolabelloides), intraspecific variation was equal to or greater than interspecific variation; however, intraspecific variation was not always observed, as F. cor and P. gibber were represented by only one haplotype. The single haplotype of P. gibber had lower pairwise differences in relation to Fusconaia and Pleurobema, 0.0155% and 0.0272%, respectively, then it did with other Pleuronaia species at 0.267%. The haplotypes of P. sp. cf. barnesiana also exhibited similar low pairwise genetic difference patterns, but to a lesser degree.

Table 5.
Pairwise nucleotide distances between species’ haplotypes at the nuclear gene ITS1. Pairwise distances were calculated using uncorrected p-distances. Bold numerals indicate intraspecific variation; N = 1 indicates that only one haplotype was recovered for the species; thus, intraspecific variation could not be computed. 1 Indicates previously unrecognized species. 2 Indicates a potentially unrecognized species pending further genetic and morphological investigation.
3.4. Morphology
A total of 414 individuals representing eight morphologically similar and thus difficult-to-identify mussel species distributed in the UTRB were photographed. Traditional shell measurements for live mussels were recorded for 384 individuals across the eight species, and foot color was recorded for 377 of these individuals (Table 6). Additional characters for sacrificed individuals were recorded for 160 individuals of these eight species. Of these, a total of 39 individuals were non-genetically identified shells from the FMCC collection, including nine F. cor, eight F. cuneolus, four F. subrotunda, nine P. dolabelloides, and nine sacrificed individuals of P. sp. cf. oviforme from Little River, TN (Table 1). Due to discovering P. c.f. dolabelloides relatively late during the study and the small sample sizes, morphological comparisons were not performed for this potentially unrecognized species.

Table 6.
Sample sizes of foot color observations for live mussels of each species collected in the upper Tennessee River basin from 2012 to 2014.
Gravid condition was recorded for 50 individuals of seven species (Table 7), and conglutinates and glochidia were photographed to illustrate size and color differences between species (Figure 5).

Table 7.
Number of charged gills and their color for gravid mussels sampled in the upper Tennessee River basin from 2012 to 2014. * No specimens were observed gravid during the study; thus, the number of charged gills is based on observations reported in the literature. NA indicates no specimens were observed gravid, and no information is available in the scientific literature.

Figure 5.
Conglutinates and glochidia of (A,B) Pleurobema oviforme from North Fork Holston River (rkm 175.2); (C,D) Pleurobema sp. cf. oviforme from Little River (rkm 47.6); (E,F) Pleuronaia barnesiana from Copper Creek (rkm 21.7) and Possum Creek (rkm 12.2), respectively; (G,H) Pleuronaia sp. cf. barnesiana from Clinch River (rkm 441.9) and Copper Creek (rkm 21.7), respectively. Conglutinate length listed in mm.
Mean measurements of glochidia for gravid mussels collected in this study are reported in Table 8. Tukey’s pairwise comparisons of height, length, and hinge length revealed significant differences among species. At α = 0.05, the following comparisons between species were significantly different at all three measurements: F. cor and P. sp. cf. oviforme; F. cor and P. sp. cf. barnesiana; F. subrotunda and P. sp. cf. oviforme; F. subrotunda and P. sp. cf. barnesiana; P. oviforme and P. sp. cf. barnesiana; P. barnesiana and P. sp. cf. oviforme; and P. barnesiana and P. sp. cf. barnesiana.

Table 8.
Mean height, length, and hinge length (µm) of glochidia for species observed gravid. No individuals of Fusconaia cuneolus were observed gravid during this study.
Classification and Regression Tree (CART) Analysis of Morphological Data
The CART analysis of live individuals using traditional morphological measurements (continuous variables) plus foot color produced a decision tree with 22 splits, 23 terminal nodes, and an overall classification accuracy of 62.0% on terminal nodes (Figure 6).
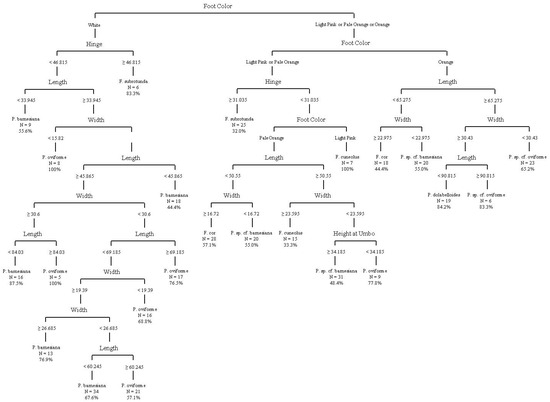
Figure 6.
Decision tree from classification and regression tree analysis using traditional morphometric and foot color data. Overall classification accuracy on terminal nodes was 61.98%. Measurements illustrated are in mm.
All measurements were utilized in the decision tree, except for the maximum height perpendicular to the maximum length. The classification accuracy on terminal nodes ranged from 32.0% to 100%, with three nodes achieving 100% accuracy, including F. cuneolus and two groups of P. oviforme; due to the CART analysis attempting to classify species based on the best way to separate species, some species have multiple terminal nodes. However, terminal nodes with 100% accuracy do not reflect the species’ overall classification accuracy. A confusion matrix (Table 9) provides the tree’s misidentification rates for each species and shows that F. subrotunda was most likely to be confused as another species (31.0% correct or 69.0% error), and other species were most likely to be confused as F. subrotunda (41.9% correct or 58.1% error). Pleuronaia barnesiana was least likely to be confused with another species (84.5% correct), and other species were least likely to be confused with Pleuronaia dolabelloides (84.2% correct).

Table 9.
Confusion matrix of species predictions (N = 384) using classification and regression tree analysis of traditional shell measurements and foot color data. Actual species identifications are reported in the rows, and predictions made by the classification and regression tree analysis (CART) are reported in the columns.
The CART analysis incorporating continuous and categorical variables for “live” individuals from sacrificed individuals and FMCC shells produced a decision tree with 15 splits, 16 terminal nodes, and an overall accuracy of 77.5% on the terminal nodes (Figure 7).
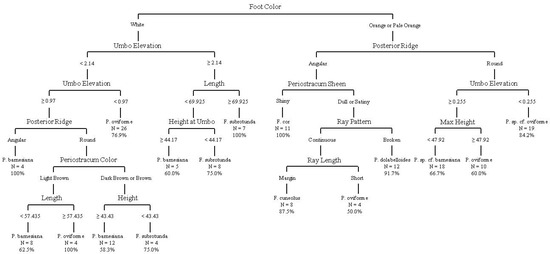
Figure 7.
Decision tree from classification and regression tree analysis using quantitative shell measurements, foot color, and categorical variables from sacrificed live individuals. This analysis includes data from non-genetically identified individuals from the FMCC shell collection. Overall classification accuracy on terminal nodes was 77.5%. Measurements illustrated are in mm.
The analysis utilized the following variables to construct the decision tree: foot color; maximum height perpendicular to maximum length; maximum height at umbo perpendicular to maximum length, maximum length, umbo elevation, periostracum color, periostracum sheen, posterior ridge, ray length, and ray pattern. The classification accuracy on terminal nodes ranged from 50.0% to 100%, with four nodes achieving 100% accuracy, including nodes for F. cor, F. subrotunda, P. oviforme, and P. barnesiana. A confusion matrix (Table 10) gives the tree’s misidentification rates for each species and shows that Pleurobema oviforme was the most likely to be confused with another species (65.3% correct or 34.7% error), and other species were most likely to be confused with Pleuronaia barnesiana (65.5% correct or 34.5% error). Pleurobema sp. cf. oviforme was the least likely to be confused with another species (100% correct), and other species were least likely to be confused with Fusconaia cor (100% correct).

Table 10.
Confusion matrix of species predictions (N = 160) using classification and regression tree analysis of quantitative and categorical variables and foot color from sacrificed live individuals. This analysis includes non-genetically identified individuals from the FMCC shell collection. Actual species identifications are reported in the rows, and predictions made by the classification and regression tree analysis (CART) are reported in the columns.
The CART analysis of continuous and categorical variables for shell-only material from sacrificed individuals and FMCC shells produced a decision tree with 13 splits, 14 terminal nodes, and an overall accuracy of 80.6% on the terminal nodes (Figure 8).
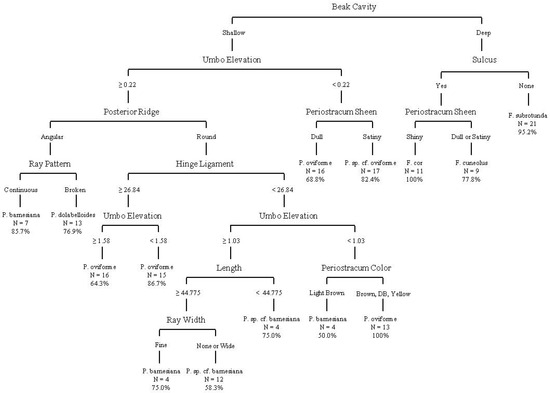
Figure 8.
Decision tree from classification and regression tree analysis using quantitative and categorical variables from sacrificed individuals to represent shell-only individuals. This analysis includes data from non-genetically identified individuals from the FMCC shell collection. Overall classification accuracy on terminal nodes was 80.6%. Measurements illustrated are in mm.
The analysis utilized the following variables: beak cavity, hinge length, maximum length, umbo elevation, periostracum color, periostracum sheen, posterior ridge, ray pattern, ray width, and presence of sulcus. Classification accuracy on terminal nodes ranged from 50% to 100%, with two nodes achieving 100% accuracy, for F. cor and P. oviforme. A confusion matrix (Table 11) provides the tree’s misidentification rates for each species and shows that Pleuronaia sp. cf. barnesiana was the most likely to be confused as another species (71.4% correct or 28.6% error), and other species were most likely to be confused with Pleuronaia sp. cf. barnesiana (62.5% correct or 37.5% error). Fusconaia subrotunda was least likely to be confused with another species (95.2% correct), and other species were least likely to be confused with Fusconaia cor (100% correct).

Table 11.
Confusion matrix of species predictions (N = 160) using classification and regression tree analysis of shell-only quantitative and categorical variables from sacrificed live individuals. This analysis includes non-genetically identified individuals from the FMCC shell collection. Actual species identifications are reported in the rows, and predictions made by the classification and regression tree analysis (CART) are reported in the columns.
Geometric Morphometrics—Photographs for 414 individuals of eight species (Table 1) were digitized for geometric morphometric analyses. The CVA yielded four distinct canonical variates, but the plot illustrates overlaps of individuals between species (Figure 9).
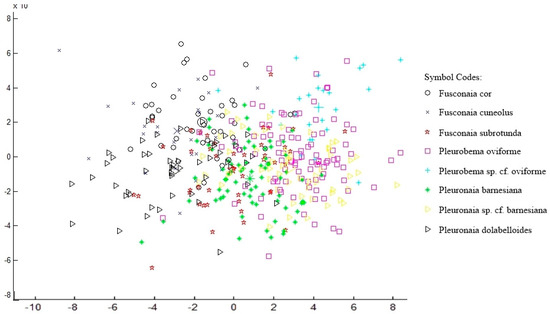
Figure 9.
Canonical variates analysis (CVA) plot using geometric morphometric data depicting canonical variates one and two as X- and Y-axes, respectively. Larger symbols indicate species means.
Groupings by CVA produced 44.7% accuracy in assigning individuals to their respective species (Table 12). Pleurobema sp. cf. oviforme was least likely to be confused with another species (87.5% correct), and other species were least likely to be confused with Pleurobema oviforme (57.8% correct). Goodall’s F-tests produced significant differences between all groups of species except when analyzing Pleurobema oviforme vs. Pleuronaia sp. cf. barnesiana (Table 11); while there were significant differences between mean shapes of species, overlap of shell shape between individuals of different species was observed. Mean shapes of each species were visually compared to determine the extent of differences, but similarity was too great to make meaningful distinctions among species (Figure 9).

Table 12.
Confusion matrix of species predictions using canonical variates analysis (CVA) of geometric morphometric data.
Fusconaia cor had foot colors of pale orange to orange in large individuals and white in smaller individuals <50 mm. Only one individual was collected gravid, with all four gills charged and appearing pink in color; conglutinates also were pink, appearing like a “+” symbol looking down the longest axis [71]. Fusconaia cuneolus had predominantly pale orange foot colors but varied from orange to white, occasionally appearing light pink. No individuals were collected gravid, but Ortmann [72] and Williams et al. [16] listed all four gills as charged. Fusconaia subrotunda had foot colors predominantly white, but varied from orange to white. Only one individual was observed gravid; all four gills were charged and red in color. Conglutinates were red, elongate, slender, and conical, sometimes being bifurcate, trifurcate, or multi-furcate [71]. Pleurobema oviforme typically had foot colors of white and occasionally pale-orange to orange. In gravid individuals, only the outer two gills were charged and were white to pale orange in color. The conglutinates were white to pale orange, sometimes bifurcate, but were flat in the cross-section and elliptical in the side-view (Figure 5A). The foot color of Pleurobema sp. cf. oviforme was orange. In gravid individuals, only the outer two gills were charged and orange in color. The conglutinates were orange, sometimes bifurcate, but when flat, they appeared elliptical in outline (Figure 5C). The foot color of Pleuronaia barnesiana was typically white, with one individual having a pale-orange-colored foot. In gravid individuals, all four gills were charged, but the conglutinates were small, slender, and conical and light tan in color (Figure 5E), making them initially difficult to see when inspecting gravid individuals. The foot color of Pleuronaia sp. cf. barnesiana was orange to pale orange. In gravid individuals, all four gills were charged and orange in color. The conglutinates were pale orange, sometimes bifurcate, but when flat, appeared elliptical in outline (Figure 5G). The foot color of Pleuronaia dolabelloides was typically orange, varying from orange to pale orange, and rarely white. In gravid individuals, only the outer two gills were charged and pink in color. The conglutinates were elongate, slender, and conical, sometimes being bifurcate, trifurcate, or multi-furcate [71]. The foot color of Pleuronaia sp. cf. dolabelloides varied from white to orange. No individuals were collected gravid, and regarding the number and location of charged gills, no research is available, nor have recent observations been made by biologists.
3.5. Taxonomic Accounts
On the basis of the molecular and morphological data reported above, we describe Pleurobema sp. cf. oviforme and Pleuronaia sp. cf. barnesiana as a new species and elevate a previously synonymized species of mussels, respectively. While Pleuronaia cf. sp. dolabelloides may warrant recognition as a distinct species, we defer reporting it as such until further specimens are studied both genetically and morphologically. All shell and soft-part material examined for this study is deposited and maintained in the McClung Museum of Natural History and Culture (MMNHC) at the University of Tennessee, Knoxville.
3.5.1. Pleurobema parmaleei sp. nov. Schilling, Jones, Hallerman, Phipps, and Dinkins, 2025 [42]
Common name: Orangefoot Clubshell
ZooBank LSID registration number:
urn:lsid:zoobank.org:pub:B3C28463-09C7-4AB9-A8F3-816BFF077E30
Material examined: We compiled a partial synonymy for Pleurobema oviforme to assess the best available name based on year published, location of collection, and shell morphology, with an emphasis on type specimens collected from the upper Tennessee River basin (Table 13); a complete synonymy for the species can be found in Parmalee and Bogan 1998 [32]. Based on our assessment, we found no suitable name for the species. None of the available type specimens that we examined are from Little River, TN, and the shells of the potentially available types are not morphologically similar to the ones that we examined from this river.
Holotype: Designated herein as MMNHC 26957, collected by D.E. Schilling from type locality, 1 July 2012 (Figure 10). Type Locality: Little River, upstream of Kinzel Springs, Blount Co., TN. 35.68228 -83.78775. Soft body is preserved in alcohol at MMNHC.

Figure 10.
Photograph of holotype MMNHC 26957 Pleurobema parmaleei located at the McClung Museum of Natural History and Culture, University of Tennessee, Knoxville, TN.
Paratypes: MMNHC 26956, collected by D.E. Schilling from type locality, 1 July 2012 (N = 4); MMNHC 26958, collected by D.E. Schilling from type locality, 20 May 2013 (N = 2); MMNHC 26801, collected by D.E. Schilling, A.T. Phipps, Little River upstream of Webb Road Crossing, Blount Co., TN, 20 May 2013 (N = 9). All of the above holotype and paratype specimen soft bodies are preserved in alcohol at MMNHC and have been genetically identified.
Etymology: The species name honors Dr. Paul W. Parmalee (1926–2006), former head curator and assistant director of the Illinois State Museum and former director and curator of malacology at the University of Tennessee’s McClung Museum of Natural History and Culture. He published over 100 peer-reviewed papers on a wide range of topics, including zoology, zooarchaeology, paleontology, and folk art. He co-authored The Freshwater Mussels of Tennessee, the first state mussel reference book to include color photographs, synonymy, and distribution maps for each species, along with notes on distribution, shell description, life history, and ecology.
Description: The key external characters include a shell outline that is predominantly ovate and elongate, even in smaller individuals <50 mm, but becomes sub-rhomboid in larger individuals, and the periostracum color is brown to dark brown, but it can vary from light to dark brown (Figure 11 and Figure 12). Maximum shell length is up to 104 mm, height (perpendicular to maximum length) up to 72 mm, and width up to 34 mm. Variations in size, external shell shape, and periostracum color and ray pattern are shown in Figure 12. The sheen of the periostracum is typically very satiny, but occasionally dull. Periostracum rays are rarely present in larger individuals, but smaller individuals (e.g., <50 mm) may have narrow, faintly green rays, typically 1 mm or less. The rays typically extend continuously to the shell margin, but occasionally are very faint or absent. The posterior ridge of the shell is rounded, and the shell disk lacks a sulcus. The height of the umbo is typically very low, 1 mm or less, occasionally flush with or below the shell margin. The position of the umbo is predominantly anteriorly located and is typically eroded in individuals from the Little River, TN, population.
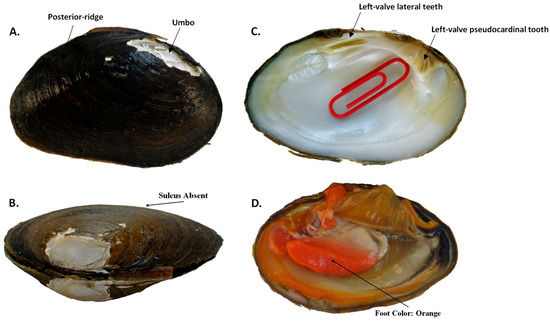
Figure 11.
Pleurobema parmaleei sp. nov. external and internal shell morphology and foot color, demonstrating the following traits: (A) rounded posterior ridge and low umbo that typically is either flush with or does not extend past the dorsal shell margin; (B) compressed shell with no sulcus; (C) thick lateral teeth of left-valve with deep interdentum, large triangular and serrated pseudocardinal tooth, and very shallow umbo cavity, as indicated by red paper clip that can only be barely inserted into cavity; and (D) brightly colored orange foot.
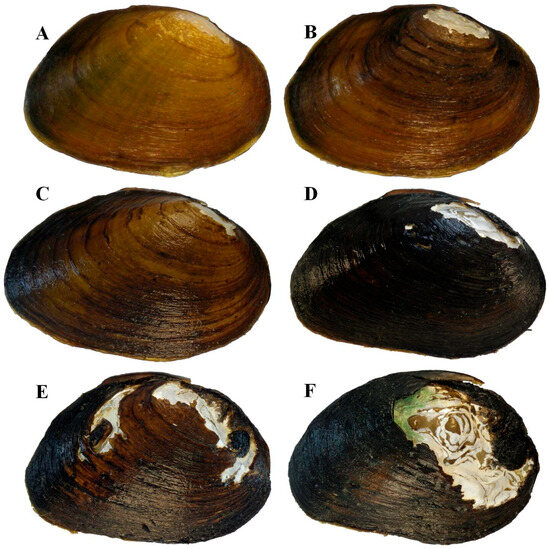
Figure 12.
Individuals of Pleurobema parmaleei sp. nov. depicting size classes and variation in periostracum color and ray patterns: (A) 37 mm; (B) 44 mm; (C) 65 mm; (D) 79 mm from Little River, km 33.2; (E) 89 mm; (F) 104 mm. All other specimens were collected from Little River, TN, kilometer 47.6.
The key interior characteristics include beak cavities that are shallow to very shallow and rounded. Nacre is white to dull bronze. The shell disks are moderately thick but thickest anteriorly (2–3 mm) and thinnest posteriorly (1–2 mm). The left valve has two thick and short (<20 mm) lateral teeth projecting slightly past the hinge ligament, with a deep interdentum between them, and two moderately thick pseudocardinal teeth are ventrally oriented anterior to the umbo and sometimes oriented toward the umbo. The right valve has one thick and short lateral tooth (<20 mm) and one large triangular serrated pseudocardinal tooth, usually with a smaller tooth anteriorly located. The beak cavities are shallow to very shallow (typically <1–2 mm deep) and rounded. The foot color is orange. Mantle color is orange anteriorly, occasionally becoming cream white to white posteriorly toward the incurrent aperture. Aperture margins are primarily purple to black, occasionally with orange. Incurrent papillae are single, bifurcate, or multifurcate; excurrent papillae are single and fused. In gravid individuals, only the outer two gills are charged and orange in color. The conglutinates are orange and narrow, and when flat, elliptical in outline, and sometimes bifurcate to multifurcate (Figure 5C). Glochidia are orange and rounded in shape (Figure 5D). Gravid individuals were observed during May and June with water temperatures ranging from 16 °C to 23 °C, suggesting a tachytictic short-term brooder strategy.
Similar sympatric species and diagnosis: Pleurobema parmaleei can be differentiated from P. oviforme using morphological and molecular characteristics. Whereas P. parmaleei typically has an elongate, more compressed shell with a dark satiny periostracum and an orange foot, P. oviforme typically has a white foot; it is sometimes pale orange, but rarely orange. Gravid female P. parmaleei have orange conglutinates (Figure 5C) with orange glochidia (Figure 5D), while female P. oviforme commonly have white to pink conglutinates (Figure 5A) with white to blueish colored glochidia (Figure 5B). Pleurobema parmaleei is distinguished from Pleuronaia barnesiana by being more elongate, having an orange foot, and having orange charged gills; it is further distinguished from P. barnesiana by only the two outer gills being charged, versus all four gills being charged in P. barnesiana. Furthermore, conglutinates of P. parmaleei are distinguished from P. oviforme and P. barnesiana by being orange and elliptical in shape. Diagnostic DNA markers include mitochondrial DNA ND1 DNA sequence haplotypes and nuclear ITS1 DNA sequences.
Distribution: Pleurobema parmaleei has been collected at multiple sites in Little River, TN [73], but appears to be endemic to this tributary of the Tennessee River in eastern Tennessee. We sampled over 50 sites throughout the UTRB, and in total, hundreds of individual mussels of the various study species were examined genetically and morphologically; we documented this species only in Little River.
Remarks: Prior to this study, Pleurobema parmaleei was a completely unrecognized cryptic species with no formal description, type specimen, or valid name ever applied to it. Specifically, no shell or set of shells from Little River, TN, representing this species has had a formal description, name, or type specimen applied to it; therefore, no available synonym exists for this species and population. Nominally, it has been hiding under the umbrella of P. oviforme, a taxon that has been assigned numerous names since the species’ earliest description by Timothy Conrad in 1834. In that initial description, the type locality was given simply as Tennessee, and the type specimen has been lost [28]. The range of P. oviforme is now known to include the Cumberland River drainage downstream of Cumberland Falls in Kentucky and Tennessee and the Tennessee River drainage in Virginia, North Carolina, Tennessee, Georgia, and Alabama [16,32]. Between 1834 and 1897, four authors described 18 different “species” that have, over time, been synonymized under P. oviforme. Further, all descriptions were based on shell morphology, which was likely due to the species’ conchological variability within any given stream or river reach, as well as the species exhibiting traits conforming to “Ortmann’s Law of Stream Position”. Ortmann’s Law posits there is a clear gradation in obesity between individuals in the headwaters (more compressed or flat) and those downstream in the larger rivers (more convex and swollen). Since none of these descriptions included information on soft part anatomy (e.g., foot color, number of gravid gills, or color of gravid gills), for any of the “species” having a type locality in the upper Tennessee River basin, it is impossible to discern if any of the available names (e.g., Unio holstonensis, U. argenteus, U. tesserulae, U. clinchensis, U. lawii, U. acuens, U. pattinoides, U. conasaugaensis, U brevis, U. bellulus, or U. swordianus) belong to P. oviforme, other taxa in the genus Pleuronaia, or what is now understood to be P. parmaleei. Further, none of the specimens representing these synonyms of P. oviforme were collected directly from Little River, TN. Therefore, since no genetic or soft part anatomical information exists for any of these names, and none of the names represent the type location and population in Little River, a unique name is required for the description of this new species.

Table 13.
Partial synonymy for Pleurobema oviforme focusing on type specimens collected from UTRB with specimen name, the year published, available collection location information from original description, and the author who published the description.
Table 13.
Partial synonymy for Pleurobema oviforme focusing on type specimens collected from UTRB with specimen name, the year published, available collection location information from original description, and the author who published the description.
| Name | Year Published | Type Locality Information from Publication | Author |
|---|---|---|---|
| Unio oviformis | 1834 | Rivers in Tennessee | T. A. Conrad [28] |
| Unio ravenelianus | 1834 | French Broad River, near Asheville, North Carolina | I. Lea [31] |
| Unio rudis | 1837 | French Broad River, near Asheville, North Carolina | T. A. Conrad [74] |
| Unio holstonensis | 1840 | Holston River, Tennessee | I. Lea [29] |
| Unio argenteus | 1841 | Holston River, Tennessee | I. Lea [75] |
| Unio mundus | 1857 | Tennessee River, Tuscumbia, Alabama | I. Lea [76] |
| Unio lesleyi | 1860 | Kentucky, Tennessee | I. Lea [77] |
| Unio tesserulae | 1861 | Nolichucky River, Tennessee | I. Lea [78] |
| Unio striatissimus | 1865 | Tennessee | J.G. Anthony [79] |
| Unio clinchensis | 1867 | Clinch River, Tennessee, and French Broad River | I. Lea [80] |
| Unio planior | 1868 | Holston River, Washington County, Virginia | I. Lea [81] |
| Unio lawii | 1871 | Tennessee River, Tuscumbia, Alabama | I. Lea [82] |
| Unio acuens | 1871 | Holston River near Concord, East Tennessee | I. Lea [82] |
| Unio pattinoides | 1871 | Clinch River, Holston River | I. Lea [82] |
| Unio conasaugaensis | 1872 | Conasauga Creek, Monroe County, Tennessee | I. Lea [83] |
| Unio brevis | 1872 | Conasauga Creek, Monroe County, Tennessee | I. Lea [83] |
| Unio bellulus | 1872 | Holston River, Tennessee River, Tennessee River (Muscle Shoals), | I. Lea [83] |
| Unio swordianus | 1897 | Powell’s Creek, Lee Co., VA | S.H. Wright [84] |
3.5.2. Pleuronaia estabrookianus, Species Elevation, Lea 1845 [85]
Common name: Appalachia Pearlymussel. (Note: We applied a unique common name to this species since none existed previously.)
Material examined: We compiled a partial synonymy for Pleuronaia barnesiana to assess the best available name based on year published, collection location, and shell morphology, with an emphasis on type specimens collected from the upper Tennessee River basin (Table 14); a complete synonymy for the species can be found in Parmalee and Bogan 1998 [32]. However, based on our assessment of available names, Unio estabrookianus (shaded in Table 14) was the best available name based on the year published, collection location, and similarity of shell morphology; the genus Unio has been replaced by Pleuronaia for this group of mussels.

Table 14.
Partial synonymy for Pleuronaia barnesiana, focusing on type specimens collected from the upper Tennessee River basin, with type specimen name, the year published, available collection location information from the original description, and the author who published the description. Gray-shaded row highlights Unio estabrookianus as the best available name for the species elevation and description.
Lectotype: United States National Museum, Smithsonian Institute, Washington, D.C., specimen collection number 84389, collected from Clinch River, Tennessee (Figure 13).

Figure 13.
Photograph of lectotype 84389 Pleuronaia estabrookianus located at the United States National Museum, Washington, D.C.
Voucher specimens: MMNHC 26980, collected by D.E. Schilling, Copper Creek, Williams Mill, Scott County, Virginia, 9 August 2012; MMNHC 26981, collected by D.E. Schilling and A.T. Phipps, Indian Creek, Tazewell County, Virginia, 20 May 2013 (N = 1); MMNHC 26982, collected by D.E. Schilling and A.T. Phipps, Clinch River at Artrip, Russell County, Virginia, 29 May 2012 (N = 1); MMNHC 26983, collected by D.E. Schilling and A.T. Phipps, approximately one mile upstream from Williams Mill, Scott County, Virginia, 22 February 2013 (N = 1); MMNHC 26984, collected by D.E. Schilling and A.T. Phipps, Clinch River at Kyles Ford, Hancock County, Tennessee, no date (N = 1); MMNHC 26985, collected by D.E. Schilling and A.T. Phipps, Clinch River at Artrip, Scott County, Virginia, 5 June 2013 (N = 1); MMHNC 226986, collected by D.E. Schilling and A.T. Phipps, Copper Creek at Williams Mill, Scott County, Virginia, 23 June 2013 (N = 2); MMNHC 26987, collected by D.E. Schilling and A.T. Phipps, Clinch River at Kyles Ford, Hancock County, Tennessee, no date (N = 1); MMNHC 26988, collected by D.E. Schilling, A.T. Phipps, and H. Faust, East Fork Chickamauga Creek, Whitfield County, Georgia, 5 August 2014 (N = 1); MMNHC 26989, collected by D.E. Schilling and A.T. Phipps, Clinch River at Cleveland, Russell County, Virginia, 23 June 2013 (N = 1); MMNHC 26990, collected by D.E. Schilling and A.T. Phipps, Clinch River at Cleveland, Russell County, Virginia, 5 June 2013 (N = 1); MMNHC 26991, collected by D.E. Schilling and A.T. Phipps, Clinch River at Artrip, Russell County, Virginia, 13 June 2012 (N = 1); MMNHC 26992, collected by D.E. Schilling and A.T. Phipps, Clinch River at Cleveland, Russell County, Virginia, 13 June 2012 (N = 1); MMNHC 26993, collected by D.E. Schilling and A.T. Phipps, Clinch River at Cleveland, Russell County, Virginia, 18 June 2012 (N = 1). All of the above voucher specimen soft bodies are preserved in alcohol at MMNHC and have been genetically identified.
Etymology: The species name honors Joseph Estabrook, fifth president of the University of Tennessee from 1834 to 1850. Under his leadership, the university instituted regular classes for instruction, produced its first college catalog, and built its first on-campus dormitories. He moved the university’s curriculum away from a dominance of classical studies and toward subjects such as chemistry, mineralogy, geology, trigonometry, and civil engineering. Estabrook was an astute observer of natural history and collected numerous specimens of mollusks from East Tennessee, many of which he sent to Isaac Lea at the Philadelphia Academy of Science.
Description: Key external characters include a shell outline that is predominantly ovate and elongate (Figure 14A), becoming subtriangular in larger individuals. Maximum shell length from 74 mm, height (perpendicular to maximum length) to 55 mm, and width up to 30 mm. The periostracum color varies from light to dark brown but occasionally yellow (Figure 14 and Figure 15). Variations in size, external shell shape, and periostracum color and ray pattern are shown in Figure 15. The periostracum sheen may be satiny or dull. Periostracum rays are typically present, being irregularly spaced, varying from fine to wide, and typically fading to indistinct toward the ventral margin. The posterior ridge of the shell is rounded (Figure 14A). The shell lacks a sulcus (Figure 14B). The height of the umbo is typically elevated 1–2 mm above the dorsal shell margin, rarely flush with or below the shell margin. The position of the umbo is predominantly anteriorly located, especially in elongated individuals. The shell disk is moderately thick, thickest anteriorly and thinnest posteriorly. The nacre is white, becoming iridescent posteriorly.
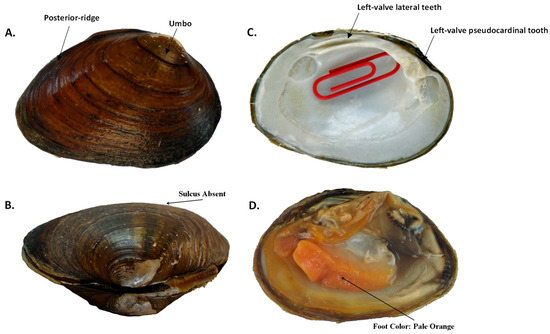
Figure 14.
Pleuronaia estabrookianus, species elevation, external and internal shell morphology, and foot color, demonstrating the following traits: (A) rounded posterior ridge and low umbo that typically is either flush with or does not extend past the dorsal shell margin; (B) compressed shell with no sulcus; (C) thick lateral teeth of left valve with deep interdentum, large triangular and serrated lateral tooth, and very shallow umbo cavity, as indicated by red paper clip that can only be barely inserted into cavity; and (D) orange-colored foot.
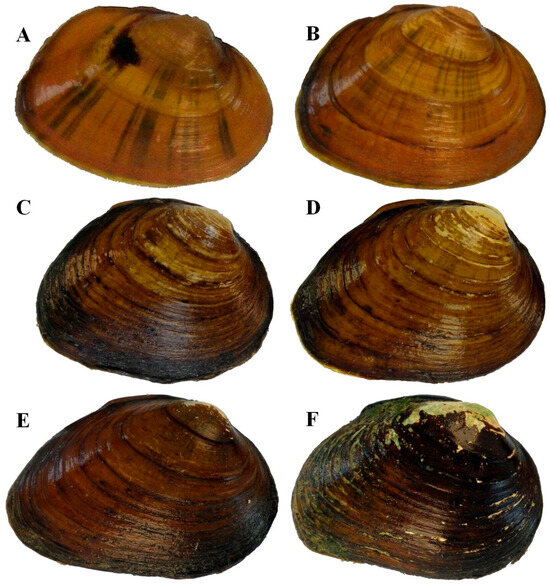
Figure 15.
Individuals of Pleuronaia estabrookianus depicting size classes and variation in periostracum color and ray patterns: (A) 28 mm from Clinch River, km 441.9; (B) 35 mm from Clinch River, km 435.8; (C) 47 mm from Copper Creek, km 24.1; (D) 55 mm from Indian Creek (Powell Drainage), km 0.3; (E) 60 mm from Clinch River, km 435.8; (F) 71 mm from Clinch River, km 435.8.
Key internal characters include beak cavities that are moderately deep (2–3 mm) and rounded. Left valve with two thick, moderately long, slightly curved lateral teeth projecting slightly past the hinge ligament; two low, moderately thick pseudocardinal teeth ventrally oriented towards the umbo (Figure 14C). Right valve with one thick, double-edged, moderately long, slightly curved lateral tooth, projecting slightly past the hinge ligament; one large, erect pseudocardinal tooth, usually with a smaller tooth anteriorly located. Foot color is orange to pale orange (Figure 14D). Mantle color is orange. Gravid individuals were observed during June with water temperatures of 19 °C to 25 °C, suggesting a tachytictic, short-term brooding strategy. In gravid individuals, all four gills are charged and orange in color. The conglutinates are pale orange, sometimes bifurcate, but when flat, they appear ovular in outline (Figure 5G). Glochidia were yellow to orange in color and rounded in shape (Figure 5H). Aperture margins vary from tan or brown to black. Incurrent papillae are single, bifurcate, or multifurcate; excurrent papillae are single and fused.
Similar sympatric species and diagnosis: The species is most similar in appearance to Pleuronaia barnesiana, as the shell traits are nearly indistinguishable, but Pleuronaia estabrookianus can be diagnosed from P. barnesiana using morphological and molecular characters. For P. estabrookianus, the foot is orange (Figure 14D), while for P. barnesiana, the foot is typically pale white to sometimes pale orange. In the gravid female of P. estabrookianus, the charged gills are orange, the conglutinates are orange and ovular shaped in outline when lying flat (Figure 5G), and the glochidia are yellow-orange to orange (Figure 5H), while for P. barnesiana, the charged gills are tan, the conglutinates are light tan and narrow (Figure 5E), and the glochidia color is clearish-white (Figure 5F). P. barnesiana conglutinates are typically tan to brown and long and conical, and P. oviforme are typically white and elliptical. Diagnostic DNA markers include mitochondrial DNA ND1 sequence haplotypes and nuclear ITS1 DNA sequences.
Distribution: Pleuronaia estabrookianus appears to be endemic to the upper Tennessee River basin but is now restricted to the Clinch and Powell River drainages of Virginia and Tennessee and the South Chickamauga Creek drainage of Georgia and Tennessee. We sampled over 50 sites throughout the UTRB, and in total, hundreds of individual mussels of our study species were examined genetically and morphologically; we only documented this species in the Clinch, Powell, and South Chickamauga Creek watersheds.
Remarks: Pleuronaia estabrookianus was originally described by Lea in 1845 from specimens collected from the Clinch River in Tennessee, but the exact collection location is unknown. Over the next 36 years, Lea introduced a series of names that have, over time, been synonymized into the single taxon of P. barnesiana. Ortmann [40] eventually placed P. estabrookianus and many of the other names in synonymy under P. barnesiana, largely based on the similarities of their shell morphology and aspects of soft body reproductive anatomy. Hence, for 180 years, P. estabrookianus has been hiding in synonymy as a cryptic but distinct species. In the initial description of P. barnesiana, the type locality was given as the Cumberland River, Tennessee; however, this species has never been reported outside of the Tennessee River drainage, indicating the reported type locality was in error [16]. Conchologically, P. barnesiana also typifies “Ortmann’s Law of Stream Position”, in that there is a clinal variation in the degree of shell obesity, with headwater individuals more compressed or flat compared to those downstream in the larger rivers. This may explain the profusion of names that Lea assigned to this taxon. However, Ortmann [92] recognized variation in the shell and soft part coloration within and among the various forms of P. barnesiana occurring throughout the Tennessee River basin, stating the following:
“The soft-parts are often uniformly pale, whitish, but may shade to orange, and the orange is most prominent on foot, adductors, and mantle-margin; but the paler tints prevail, and often the orange is replaced by yellowish or brown. The gills are pale, but are generally suffused with blackish. The gonads are brown to red, mostly of a peculiar dull lavender color in the female, and the latter color, or purplish brown, is the prevailing color of the eggs and placentae. The charged gills become thus a rather dark purple, or purple-brown, shading sometimes to dull red or blackish, in other cases to brownish, brownish pink, brick-red, or even pale brown. These are very peculiar tints, by which this species is easily recognized in the field: four marsupial gills of this blackish-purple color are not known in any other Nayad”.
Ortmann observed and documented considerable color variation in the foot, mantle, gills, and gravid gills of this species throughout its range. His observations in part agree with ours, in that we genetically identified individuals with the pale whitish soft parts as typical P. barnesiana, and we genetically identified those with orange soft parts as P. estabrookianus. Further, Lea’s figured specimen of Unio estabrookianus agrees morphologically with the shells of the species that we genetically identified; to us, Lea’s figured specimen is indistinguishable from specimens examined in our study (Figure 13). Ecologically, these two species occupy the same habitat and are sympatric in distribution. However, based on our study, P. estabrookianus appears to be restricted to the upper Tennessee River basin, whereas P. barnesiana has a greater distributional range throughout the basin. We considered using an earlier synonym of P. barnesiana—Unio bigbyensis (Lea 1841) [75]—but Lea’s specimens of U. bigbyensis were collected from Bigby Creek, a tributary to the Duck River, which is much further downstream in the Tennessee River system. All of our molecular DNA sequence data from the Duck River were identified as P. barnesiana, and morphologically, the individuals we examined from the Duck River drainage looked very different compared to P. estabrookianus. In Lea’s full description of Unio estabrookianus, he reported the species was in his cabinet and in the cabinet of President Estabrook, and he described differences in the periostracum between young and mature individuals, thus implying there were multiple specimens involved in the original description. In the U.S. National Museum (USNM), there are two lots of U. estabrookianus: USNM 84389 (two paired valves) and 84389a (three paired valves) [93]. The two paired valves in USNM 84389 closely match the general features (outline, spacing of external annuli, and placement and shape of posterior ridge) and measurements (3.0 × 2.2 inches vs. 3.1 × 2.2 inches) of the figured specimen for Unio estabrookianus. We hereby designate the specimen in USNM 84389 as the lectotype for Pleuronaia estabrookianus, and we designate the two specimens in USNM 84389a as paralectotypes.
4. Discussion
4.1. Phylogenetic Analyses of ND1
Phylogenetic and pairwise genetic distance analyses indicated that the six study species (F. cor, F. cuneolus, F. subrotunda, P. oviforme, P. barnesiana, and P. dolabelloides) each formed a distinct monophyletic clade. Although individuals of F. cor and F. cuneolus are morphologically similar, the phylogenetic distinctiveness of each species was confirmed using DNA sequences. The species are sympatric in the Clinch River, and the ND1 sequences from both species each formed distinct monophyletic clades, which were separated by genetic distances of 5.6%. Our phylogenetic analyses also revealed two cryptic species within the genera Pleurobema and Pleuronaia. Pleurobema parmaleei collected from Little River in Blount County, TN, formed a monophyletic clade distinct from P. oviforme. Importantly, in this study, these two species were collected sympatrically in Little River and were separated by a genetic distance of 6.3%. In addition, Morrison et al. [94] used our ND1 DNA sequences deposited on GenBank and further showed that P. parmaleei is genetically distinct from P. clava, a close congener of P. oviforme, and numerous other Pleurobema species from the Mobile River basin. The Pleuronaia estabrookianus sequences sampled in the UTRB, including an individual collected in Georgia, formed a monophyletic clade distinct from P. barnesiana; further, these two species were also collected sympatrically in the Clinch and Powell drainages and were separated by a genetic distance of 2.9%. The Pleuronaia sp. cf. dolabelloides sequences observed in individuals collected from Georgia formed a monophyletic clade distinct from P. dolabelloides; these species were not collected sympatrically, but were separated by a genetic distance of 3.2%. Pleurobema parmaleei and Pleuronaia estabrookianus were collected in sympatry with their closest respective congeners, P. oviforme and P. barnesiana. Further, due to distinct ND1 haplotypes and distinct color and morphological differences in their shells and conglutinates of individuals occurring sympatrically, these species likely are reproductively isolated and can be recognized as distinct under the biological species concept [95]. While Pleuronaia sp. cf. dolabelloides was not collected in sympatry with its closest congener, based on genetic distance, it may also be reproductively isolated from P. dolabelloides, and on this basis could be considered as a species using the phylogenetic species concept [88]. The number of individuals of Pleuronaia sp. cf. dolabelloides examined is small, however, and analysis of more samples would be appropriate before diagnosing this population in South Chickamauga Creek as a new species. In addition to genetic distinctiveness, various morphological differences were observed between this potentially new species and its closest congener, P. dolabelloides [71].
The DNA sequences of P. gibber grouped distinctly apart from the other congeners in Pleuronaia; therefore, noting the genetic distinctiveness, geographic isolation, and morphologically smaller size of P. gibber, other characteristics such as life history traits, glochidial morphology, and soft anatomy should be explored in order to determine definitively whether this species belongs in a genus other than Pleuronaia.
4.2. Phylogenetic Analyses of ITS1
Estimated pairwise genetic distances among taxa in this study were not directly comparable to those of other studies due to the contrasting approach for encoding gaps in DNA sequence alignments. For example, other studies coded gaps as missing data [22] or as a fifth character state [49]. Simmons and Ochoterena’s method [51] encodes each indel event as a single evolutionary step. Coding each gap as a fifth character state is incorrect because it is unlikely that multiple one-nucleotide deletions occur; rather, one deletion of multiple nucleotides likely occurs [67,96]. Nagy et al. [52] illustrated the usefulness of gaps for phylogenetic inference and recommended that such coding be incorporated into future studies; however, indel evolution in DNA sequences is poorly understood, so the best approach for incorporating them is still unresolved.
Against this background, the results of the phylogenetic analyses of ITS1 sequences in this study revealed a slight separation of species and were less definitive regarding the delineation of species and genera than phylogenetic analyses of ND1. Within genera, species typically diverged by one or more indels. We did run the phylogenetic analyses without the addition of these binary-coded characteristics, added by FastGap [53]; basically, indels were treated as missing data. Thus, phylogenetic trees and pairwise differences did not recognize gap differences between species; this outcome is similar to that of Campbell et al. [24]. The ITS1 phylogram indicated a paraphyletic lineage of P. dolabelloides; the haplotype (two individuals) that was not monophyletic with the other P. dolabelloides haplotype (five individuals) was also unique in the mitochondrial ND1 gene. This paraphyletic lineage is also the reason that intraspecific variation in ITS1 sequences for P. dolabelloides was greater than interspecific variation between P. dolabelloides and other species.
4.3. Molecular Genetic Marker Comparison
The results of the incongruent length differences test indicated that the mtDNA and nuclear gene trees should not be concatenated because the gene phylograms differed too greatly in branch lengths or placements of individuals within the tree. Nonetheless, the phylogenetic analyses of these two markers gave similar results. Each gene tree generally resulted in distinct clades for Fusconaia, Pleurobema, and Pleuronaia, but with different placements of P. gibber. Phylogenetic analysis of ND1 indicated clades supporting species identifications for F. cor, F. cuneolus, F. subrotunda, P. oviforme, P. parmaleeii, P. barnesiana, P. estabrookianus, P. dolabelloides, P. sp. cf. dolabelloides, and P. gibber. Both genetic markers indicated slight divergence within P. dolabelloides, but due to the low sample sizes for the disparate haplotypes observed within P. dolabelloides, further studies should explore whether this phylogenetic clade comprises more than one species in Clinch River. Based on the discovery of this divergent clade, analysis of additional molecular markers and phenotypic traits could determine whether a cryptic species that is phenotypically similar to P. dolabelloides exists in the upper Clinch River.
4.4. Taxonomic Assessment and Key Morphological Traits
Molecular genetics and morphological analyses of the freshwater mussels investigated in this study support recognition of at least two new taxa. While these two species are morphologically very similar to other species, especially in the appearance of their shells, some general traits can help correctly identify them. Currently, Pleurobema parmaleeii is known to occur only in Little River, Blount County, Tennessee, where it co-occurs with P. oviforme and P. barnesiana. Typically, P. oviforme and P. barnesiana have a white-colored foot, with the former occasionally having a pale-orange foot; all individuals of P. parmaleei had an orange-colored foot. Additionally, these species can easily be distinguished when in gravid condition, with P. barnesiana having all four gills charged and tan in color; P. oviforme having the outer two gills charged, typically white and occasionally pale-orange in color; and P. parmaleei having the outer two gills charged and orange in color. These morphological characteristics can be used to identify P. parmaleei from congeners within its known range in Little River, TN.
The known distribution of Pleuronaia estabrookianus is wider and more sporadic. It has been found in one location each in the Powell River and South Chickamauga Creek drainages, and it occurs throughout the Clinch River and its tributaries. Thus, it could occur in additional streams of the Upper Tennessee River Basin. It was found to co-occur with F. cor, F. cuneolus, F. subrotunda, P. oviforme, P. barnesiana, P. dolabelloides, and P. sp. cf. dolabelloides. Leaving aside molecular genetic markers, P. estabrookianus is typically distinguished from the similar-appearing sympatric P. barnesiana and P. oviforme by its pale-orange foot. Additionally, P. estabrookianus is distinguished from P. barnesiana by having gills charged orange in color, and P. estabrookianus is distinguished from P. oviforme by having all four gills charged and orange in color.
The known distribution of P. sp. cf. dolabelloides is limited to the South Chickamauga Creek drainage. Analyses of the genetic markers used in this study indicate that this potentially undescribed species is different from its closest congener, P. dolabelloides. Conchological characteristics for P. sp. cf. dolabelloides were not congruent with characteristics for P. dolabelloides; individual mussels collected in the South Chickamauga drainage were initially thought to be those of P. parmaleei based upon external conchology, but foot color was not congruent with P. parmaleei. Further molecular and morphological sampling of this putative species is needed to characterize its distribution and morphological attributes.
4.5. Classification and Regression Tree Analysis of Morphological Data
In this study, quantitative variables were easy to measure, while categorical variables were more difficult to measure, and individuals often possessed characteristics that were intermediate between two categories. Importantly, an incorrect assessment of categories such as beak cavity depth, shape of posterior ridge, length and pattern of periostracum rays, periostracum color, and presence or absence of sulcus variables could affect any species’ assignment. Thus, adequate training is required to score categorical variables as accurately as possible. However, a benefit of the CART approach is the ability of the program to use variables from multiple categories (e.g., continuous and categorical variables) to identify species. A drawback is that CART uses a “greedy” algorithm; i.e., this algorithm makes categorical splits that best discriminate species early on in the identification process, but does not find the overall best algorithm that reduces error on terminal nodes.
The best-performing CART analysis used shell-only quantitative and categorical variables from sacrificed individuals and achieved an overall accuracy of 80.6% on terminal nodes. Beak cavity depth was the root, which varied minimally among species and was deeper for species of Fusconaia compared to species of Pleurobema and Pleuronaia. Terminal nodes were reached by combinations of quantitative and categorical variables. The increased accuracy of this tree is due to the use of categorical variables, namely, beak cavity. Categorical variables improved the accuracy of decision trees and are often used by field biologists. While categorical traits proved useful for identifying species, they can overlap among species and categories, leading to judgment decisions being made by the practitioner. Further, such judgment decisions can vary among biologists, making the transmission of species identification knowledge difficult.
The second-best-performing CART analysis used quantitative and categorical variables from sacrificed live individuals and achieved an overall accuracy of 77.5% on terminal nodes. Foot color was the root and varied less in this analysis than in the previous one due to using the modal value of this trait, which was orange. The modal value was applied to all individuals used from the FMCC collection, which resulted in specimens of F. cor, F. cuneolus, P. parmaleei, and P. dolabelloides from the collection being coded with the same foot color. Since these species generally have an orange foot, applying the modal value for foot color likely did not greatly affect the analysis and classification rates for these species. Terminal nodes were determined by combinations of quantitative and categorical variables. The lower accuracy of this analysis was due to morphological overlap between species, but the categorical variables improved the accuracy of species identifications.
The least accurate CART analysis used traditional quantitative morphometric and foot color data and achieved an overall accuracy of 62.0% on terminal nodes. Foot color was the root, or first split, but varied within species; only for P. parmaleei did all individuals have the same foot color. Eight individuals of F. cuneolus were identified with 100% accuracy, as these were the only mussels to have pink-colored feet. Terminal nodes were reached by combinations of foot color and quantitative variables. The lower accuracy of this analysis was due to an overlap of traditional quantitative measurements among species.
4.6. Geometric Morphometrics
The CVA using geometric morphometric data exhibited a high amount of morphological overlap among species, and thus, consistent patterns to separate species were not found when all species were analyzed together. Analyses for pairs of species using Goodall’s F-test demonstrated that statistically significant morphometric differences occurred between all species pairs but one; however, these morphometric traits overlapped between individuals and among species. Significant differences were not observed between P. oviforme and P. estabrookianus, which is consistent with difficulties in identifying these two species in the field without knowledge of foot color. While most of these pairwise comparisons are statistically significant, the morphological differences cannot be applied by field biologists due to the high morphological overlap among species.
We chose to use only 11 shell landmarks for geometric morphometric analyses under the assumption that fewer landmarks could illustrate more visually obvious changes that could be incorporated into field-level identification characteristics. However, future studies should explore more landmarks, as they have the benefit of recognizing differences in curvature of external margins, especially where the landmarks are not close together (e.g., the posterior margin). Even if statistical differences occur between species with minimal morphological overlap, geometric morphometrics are still not that useful for field biologists; direct comparison would require precise photographs and digitization of the specimens in question. Geometric morphometrics should be used to determine if significant differences exist between species and then used to determine the best solution (e.g., measurements or traits) that will help field biologists separate the species in question.
5. Conclusions
We collected 464 individuals representing the genera Fusconaia, Pleurobema, and Pleuronaia from streams primarily located throughout the upper Tennessee River basin, a biodiversity hotspot for mussels. Examination of morphological and molecular characteristics showed ten distinct lineages, including three apparent cryptic species. We provide descriptions of one new species, Pleurobema parmaleei, and a previously synonymized species, Pleuronaia estabrookianus, and recommend further investigation of a possible third, Pleuronaia sp. cf. dolabelloides. Observation of numerous shared intraspecific ND1 haplotypes across drainages suggests that populations of each species at these localities were once part of larger regional populations and that genetic exchange between these drainages occurred historically. Many of these drainages are now separated by large hydroelectric dams and reservoirs that inhibit or prevent gene flow between populations. Shared haplotypes indicate that mussel translocations likely would not adversely affect the fitness of receiving populations, but assessments including population genetic analyses at nuclear DNA microsatellite and single-nucleotide polymorphism (SNP) loci, and variation in life history traits should be explored before translocations are implemented. If such nuclear loci indicate recent genetic exchange and life history traits, such as spawning and gravidity periods, and host fish usage are similar among populations, then mussel translocations could be recommended if the receiving population needs assistance through either genetic diversity or population size augmentation.
The two cryptic species in the genera Pleurobema and Pleuronaia discovered in this study have limited known geographical distributions and ultimately could warrant protection under the U.S. Endangered Species Act. Pleurobema parmaleei was collected only in Little River, TN. Pleuronaia estabrookianus was collected primarily in the upper Clinch drainage in Virginia, with one individual each collected from the Powell and South Chickamauga drainages. Pleuronaia sp. cf. dolabelloides was collected only in the South Chickamauga drainage. Interestingly, P. estabrookianus was not collected in the Holston River system during our study. Hence, further survey work should be conducted to locate additional populations of these species to define their distributions. However, it is likely that anthropogenic impacts have eliminated much of the suitable habitat for these species in many streams and therefore constricted their ranges.
Classification schemes that utilize only morphology to classify freshwater mussel taxa have the potential to overlook cryptic species. Using molecular phylogenetic approaches and the phylogenetic species concept, this study discovered two previously unrecognized freshwater mussel taxa on the basis of reciprocal monophyly of mtDNA and nuclear sequences. Because of the large sample sizes utilized in this project, while focusing on relatively few streams among the many in the UTRB, we believe it is possible that additional cryptic species may occur in this region. Molecular genetic approaches to clarify the phylogenetic affinity of cryptic taxa are useful, but more surveys and genetic analyses are needed for characterizing similar-looking species that may occur in the numerous small rivers and creeks of the UTRB. In addition, population genetic and phylogenetic analyses of these taxa utilizing multi-locus nuclear markers such as single-nucleotide polymorphisms (SNPs) or whole-genome sequencing could also help to further test the validity of the cryptic species discovered in this study.
Supplementary Materials
The following supporting materials can be downloaded at: https://www.mdpi.com/article/10.3390/d17100739/s1. Table S1: Site numbers and locality information for sites sampled for freshwater mussels primarily in the Tennessee River basin from 2012 through 2014. NA = information not available. Table S2: Site numbers and counts of haplotypes analyzed for the mitochondrial gene region ND1. Table S3: Counts of individuals per haplotype and GenBank accession numbers for ND1. Table S4: Site numbers and counts of haplotypes analyzed for the nuclear gene region ITS1. Haplotype numbers do not correspond with those for mitochondrial DNA data. Table S5: Counts of individuals per haplotype and GenBank accession numbers for ITS1. Haplotype numbers do not correspond with those for mitochondrial DNA data. Table S6: Variable nucleotide sites for haplotypes at the nuclear gene region ITS1. A blank at a nucleotide site indicates an identical nucleotide as the first sequence. Haplotype numbers do not correspond with those for mitochondrial DNA data. Insertions or gaps are indicated by “-”; identical nucleotide positions at the first sequence are blank. Figure S1: Geometric morphometric measurements of freshwater mussel shells.
Author Contributions
Conceptualization: J.W.J.; Methodology: D.E.S., J.W.J., E.M.H. and G.R.D.; Resources: J.W.J., E.M.H. and G.R.D.; Field work: D.E.S., J.W.J. and A.T.P.; Formal analysis: D.E.S. and J.W.J.; Visualization: D.E.S. and J.W.J.; Writing—original draft: D.E.S., J.W.J., E.M.H. and G.R.D.; Writing—review and editing: D.E.S., J.W.J., E.M.H., A.T.P. and G.R.D.; Project administration: J.W.J.; Funding acquisition: J.W.J. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Virginia Department of Wildlife Resources, the Tennessee Wildlife Resources Agency, the Tennessee Wildlife Foundation, and the Department of Fish and Wildlife Conservation at Virginia Polytechnic Institute and State University. The participation of author Eric Hallerman in this project was supported in part by the U.S. Department of Agriculture Hatch Program.
Institutional Review Board Statement
This study was not subject to approval by the Institutional Animal Care and Use Committee because it did not involve vertebrate animals.
Data Availability Statement
The authors state that the data supporting the results of this study are available in the article and the Supplementary Information files.
Acknowledgments
We thank Hugh Faust and Jon Mollish for their willingness to travel to sites and assist in field collections. We also thank Bob Butler, Don Hubbs, Bobby Brown, Allen Pyburn, Steve Fraley, Chuck Howard, Tim Lane, and Jen Rogers for their assistance in field collections. We thank Ryan England of the McClung Museum, University of Tennessee, Knoxville, for his assistance in cataloging specimens for this project. We further thank the McClung Museum and the Etnier Fish Collection at the University of Tennessee, Knoxville, for housing the shells and mussel type specimens and soft parts obtained during this study. We thank Ellen Strong at the National Museum of Natural History, Smithsonian Institute, for helpful discussions on nomenclature issues of our study species. We acknowledge Steve Ahlstedt and Jason Wisniewski for their valuable knowledge needed to complete collections. We would like to extend a special acknowledgment to James Williams for his expertise in examining a photograph of a live individual of Pleurobema parmaleei and his comments on how unique the papillae of this species were, which led to specimens being collected years later. We thank Michael Pinder at the Virginia Department of Wildlife Resources for initiating the primary funding for this project and providing key conceptual ideas for only using genetically identified mussels for morphological assessment.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. The views expressed in this article are the authors’ and do not necessarily represent those of the U.S. Fish and Wildlife Service.
Abbreviations
The following abbreviations are used in this manuscript:
| BSA | Bovine serum albumin |
| ITS1 | Nuclear ribosomal internal transcribed spacer region 1 |
| MtDNA | Mitochondrial DNA |
| ND1 | NADH dehydrogenase-encoding region of the mitochondrial DNA molecule |
| PCR | Polymerase chain reaction |
| UTRB | Upper Tennessee River basin |
| USFWS | U.S. Fish and Wildlife Service |
References
- Williams, J.D.; Warren, M.L., Jr.; Cummings, K.S.; Harris, J.L.; Neves, R.J. Conservation status of the freshwater mussels of the United States and Canada. Fisheries 1993, 18, 6–22. [Google Scholar] [CrossRef]
- Neves, R.J.; Bogan, A.E.; Williams, J.D.; Ahlstedt, S.A.; Hartfield, P.W. Status of aquatic mollusks in the southeastern United States: A downward spiral of diversity. In Aquatic Fauna in Peril: The Southeastern Perspective; Benz, G.W., Collins, D.E., Eds.; Special Publication 1; Southeast Aquatic Research Institute. Lenz Design and Communications: Decatur, GA, USA, 1997; pp. 43–86. [Google Scholar]
- Williams, J.D.; Bogan, A.E.; Butler, R.S.; Cummings, K.S.; Garner, J.T.; Harris, J.L.; Johnson, N.A.; Watters, G.T. A revised list of the freshwater mussels (Mollusca: Bivalvia: Unionida) of the United States and Canada. Freshwat. Mollusk Biol. Conserv. 2017, 20, 33–58. [Google Scholar] [CrossRef]
- Ricciardi, A.; Rasmussen, J.B. Extinction rates of North American freshwater fauna. Conserv. Biol. 1999, 13, 1220–1222. [Google Scholar] [CrossRef]
- Watters, G.T. Small dams as barriers to freshwater mussels (Bivalvia, Unionoida) and their hosts. Biol. Conserv. 1996, 75, 79–85. [Google Scholar] [CrossRef]
- Hughes, M.H.; Parmalee, P.W. Prehistoric and modern freshwater mussel (Mollusca: Bivalvia: Unionoidea) faunas of the Tennessee River: Alabama, Kentucky, and Tennessee. Regul. Rivers Res. Manage. 1999, 15, 25–42. [Google Scholar] [CrossRef]
- Haag, W.R. North American Freshwater Mussels: Natural History, Ecology, and Conservation; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Carey, C.S.; Jones, J.W.; Butler, R.S.; Hallerman, E.M. Restoring the endangered oyster mussel (Epioblasma capsaeformis) to the upper Clinch River, Virginia: An evaluation of population restoration techniques. Restor. Ecol. 2015, 23, 447–454. [Google Scholar] [CrossRef]
- Hua, D.; Jiao, Y.; Neves, R.; Jones, J. Use of PIT tags to assess individual heterogeneity of laboratory-reared juveniles of the endangered Cumberlandian combshell (Epioblasma brevidens) in a mark–recapture study. Ecol. Evol. 2015, 5, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Haag, W.; Williams, J. Biodiversity on the brink: An assessment of conservation strategies for North American freshwater mussels. Hydrobiologia 2014, 735, 45–60. [Google Scholar] [CrossRef]
- Smith, D.R.; Butler, R.S.; Jones, J.W.; Gatenby, C.M.; Hylton, R.E.; Parkin, M.J.; Schulz, C.A. Developing a landscape-scale, multi-species, and cost-efficient conservation strategy for imperiled aquatic species in the Upper Tennessee River Basin, USA. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2017, 27, 1224–1239. [Google Scholar] [CrossRef]
- Patterson, M.A.; Mair, R.A.; Eckert, N.; Gatenby, C.M.; Brady, T.; Jones, J.W.; Simmons, B.R.; Devers, J.L. Freshwater Mussel Propagation for Restoration; Cambridge University Press: Cambridge, UK, 2018; 338p. [Google Scholar]
- Hyde, J.M.; Jones, J.W. Propagation and Release of Freshwater Mussels (Bivalvia: Unionidae) for Two Natural Resource Damage Assessment and Restoration Cases in the Clinch and Powell Rivers in Virginia and Tennessee. J. Freshw. Mollusk Biol. Conserv. 2025, 28, 8–21. [Google Scholar] [CrossRef]
- Spooner, D.E.; Vaughn, C.C. Context-dependent effects of freshwater mussels on stream benthic communities. Freshwat. Biol. 2006, 51, 1016–1024. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Nichols, S.J.; Spooner, D.E. Community and food web ecology of freshwater mussels. J. N. Am. Bentholog. Soc. 2008, 27, 409–423. [Google Scholar] [CrossRef]
- Williams, J.D.; Bogan, A.E.; Garner, J.T. Freshwater Mussels of Alabama and the Mobile Basin in Georgia, Mississippi and Tennessee; The University of Alabama Press: Tuscaloosa, AL, USA, 2008. [Google Scholar]
- Simpson, C.T. Synopsis of the naiades, or pearly fresh-water mussels. Proc. U.S. Natl. Mus. 1900, 22, 501–1044. [Google Scholar] [CrossRef]
- Ortmann, A.E. Notes upon the families and genera of the najades. Ann. Carnegie Mus. 1912, 8, 222–365. [Google Scholar] [CrossRef]
- Heard, W.H.; Guckert, R.H. A re-evaluation of the recent Unionacea (Pelecypoda) of North America. Malacologia 1970, 10, 333–355. [Google Scholar]
- Graf, D.L.; Cummings, K.S. Review of the systematics and global diversity of freshwater mussel species (Bivalvia: Unionoida). J. Mollusc. Stud. 2007, 73, 291–314. [Google Scholar] [CrossRef]
- Gangloff, M.M.; Williams, J.D.; Feminella, J.W. A new species of freshwater mussel (Bivalvia: Unionidae), Pleurobema athearni, from the Coosa River drainage of Alabama, USA. Zootaxa 2006, 1118, 43–56. [Google Scholar] [CrossRef]
- Jones, J.W.; Neves, R.J.; Ahlstedt, S.A.; Hallerman, E.M. A holistic approach to taxonomic evaluation of two closely related endangered freshwater mussel species, the oyster mussel Epioblasma capsaeformis and tan riffleshell Epioblasma florentina walkeri (Bivalvia: Unionidae). J. Mollusc. Stud. 2006, 72, 267–283. [Google Scholar] [CrossRef]
- Zanatta, D.T.; Murphy, R.W. Evolution of active host-attraction strategies in the freshwater mussel tribe Lampsilini (Bivalvia: Unionidae). Mol. Phylogenet. Evol. 2006, 41, 195–208. [Google Scholar] [CrossRef]
- Campbell, D.C.; Lydeard, C. The genera of Pleurobemini (Bivalvia: Unionidae: Ambleminae). Am. Malacol. Bull. 2012, 30, 19–38. [Google Scholar] [CrossRef]
- Campbell, D.C.; Lydeard, C. Molecular systematics of Fusconaia (Bivalvia: Unionidae: Ambleminae). Am. Malacol. Bull. 2012, 30, 1–17. [Google Scholar] [CrossRef]
- Lane, T.W.; Hallerman, E.; Jones, J. Phylogenetic and taxonomic assessment of the endangered Cumberland bean, Villosa trabalis and purple bean, Villosa perpurpurea (Bivalvia: Unionidae). Conserv. Genet. 2016, 17, 1109–1124. [Google Scholar] [CrossRef]
- Perkins, M.A.; Johnson, N.A.; Gangloff, M.M. Molecular systematics of the critically-endangered North American spinymussels (Unionidae: Elliptio and Pleurobema) and description of Parvaspina gen. nov. Conserv. Genet. 2017, 18, 745–757. [Google Scholar] [CrossRef]
- Conrad, T.A. New Freshwater Shells of the United States, with Coloured Illustrations; and a Monograph of the Genus Anculotus of Say; Also a Synopsis of the American Naiades; J. Dobson: Philadelphia, PA, USA, 1834; pp. 1–76, 8 plates. [Google Scholar]
- Lea, I. Descriptions of new fresh water and land shells. Proc. Am. Philos. Soc. 1840, 1, 284–289. [Google Scholar]
- Lea, I. Description of new freshwater and land shells. Trans. Am. Philos. Soc. 1838, 6, 1–154. [Google Scholar] [CrossRef]
- Lea, I. Observations on the naiades, and descriptions of new species of that and other families. Trans. Am. Philos. Soc. 1831, 4, 63–121. [Google Scholar] [CrossRef]
- Parmalee, P.W.; Bogan, A.E. The Freshwater Mussels of Tennessee; The University of Tennessee Press: Knoxville, TN, USA, 1998. [Google Scholar]
- Watters, G.T.; Hoggarth, M.A.; Stansbery, D.H. The Freshwater Mussels of Ohio; Ohio State University Press: Columbus, OH, USA, 2009. [Google Scholar]
- USFWS (United States Fish and Wildlife Service). Proposed endangered status for 216 species on Convention on International Trade. Fed. Reg. 1975, 40, 44329–44333. [Google Scholar]
- USFWS (United States Fish and Wildlife Service). Endangered and threatened wildlife and plants; determination of endangered status for the Cumberland pigtoe mussel. Fed. Reg. 1991, 56, 21084–21087. [Google Scholar]
- USFWS (United States Fish and Wildlife Service). Endangered and threatened wildlife and plants; endangered species status for the fluted kidneyshell and slabside pearlymussel and designation of critical habitat. Fed. Reg. 2013, 78, 59269–59287. [Google Scholar]
- USFWS (United States Fish and Wildlife Service). Service Proposes Listing Three Eastern Freshwater Mussels Under the Endangered Species Act. 2023. Available online: https://www.fws.gov/press-release/2023-08/service-proposes-listing-three-eastern-freshwater-mussels-under-endangered (accessed on 5 May 2025).
- Campbell, D.C.; Serb, J.M.; Buhay, J.E.; Roe, K.J.; Minton, R.L.; Lydeard, C. Phylogeny of North American amblemines (Bivalvia, Unionoida): Prodigious polyphyly proves pervasive across genera. Invert. Biol. 2005, 124, 131–164. [Google Scholar] [CrossRef]
- Frierson, L.S. A Classified and Annotated Check List of the North American Naiades; Baylor University Press: Waco, TX, USA, 1927. [Google Scholar]
- Ortmann, A.E. The nayades (freshwater mussels) of the upper Tennessee Drainage. with notes on synonymy and distribution. Proc. Am. Phil. Soc. 1918, 57, 521–626. [Google Scholar]
- Inoue, K.; Hayes, D.M.; Harris, J.L.; Johnson, N.A.; Morrison, C.L.; Eackles, M.S.; King, T.L.; Jones, J.W.; Hallerman, E.M.; Christian, A.D.; et al. The Pleurobemini (Bivalvia: Unionoida) revisited: Molecular species delineation reveals multiple conspecifics and undescribed species. Invertebr. Syst. 2018, 32, 689–702. [Google Scholar] [CrossRef]
- Johnson, N.A.; Henderson, A.R.; Jones, J.W.; Beaver, C.E.; Ahlstedt, S.A.; Dinkins, G.R.; Eckert, N.L.; Endries, M.J.; Garner, J.T.; Harris, J.L.; et al. Glacial vicariance and secondary contact shape demographic histories in a freshwater mussel species complex. J. Hered. 2023, 115, 72–85. [Google Scholar] [CrossRef]
- Moyer, G.R.; Díaz-Ferguson, E. Identification of endangered Alabama lampmussel (Lampsilis virescens) specimens collected in the Emory River, Tennessee, USA via DNA barcoding. Conserv. Genet. 2012, 13, 885–889. [Google Scholar] [CrossRef]
- Henley, W.F.; Grobler, P.J.; Neves, R.J. Non-invasive method to obtain DNA from freshwater mussels (Bivalvia: Unionidae). J. Shellfish Res. 2006, 25, 975–977. [Google Scholar]
- Serb, J.M.; Buhay, J.E.; Lydeard, C. Molecular systematics of the North American freshwater bivalve genus Quadrula (Unionidae: Ambleminae) based on mitochondrial ND1 sequences. Mol. Phylogenet. Evol. 2003, 28, 1–11. [Google Scholar] [CrossRef]
- King, T.L.; Eackles, M.S.; Gjetvaj, B.; Hoeh, W.R. Intraspecific phylogeography of Lasmigona subviridis (Bivalvia: Unionidae): Conservation implications of range discontinuity. Mol. Ecol. 1999, 8, S65–S78. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Campbell, D.C.; Johnson, P.D.; Williams, J.D.; Rindsberg, A.K.; Serb, J.M.; Small, K.K.; Lydeard, C. Identification of ‘extinct’ freshwater mussel species using DNA barcoding. Mol. Ecol. Res. 2008, 8, 711–724. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Simmons, M.P.; Ochoterena, H. Gaps as characters in sequence-based phylogenetic analyses. Syst. Biol. 2000, 49, 369–381. [Google Scholar] [CrossRef]
- Nagy, L.G.; Kocsubé, S.; Csanádi, Z.; Kovács, G.M.; Petkovits, T.; Vágvölgyi, C.; Papp, T. Re-mind the gap! Insertion–deletion data reveal neglected phylogenetic potential of the nuclear ribosomal internal transcribed spacer (ITS) of fungi. PLoS ONE 2012, 7, e49794. [Google Scholar] [CrossRef] [PubMed]
- Borchsenius, F. FastGap 1.2. Department of Biosciences, Aarhus University, Denmark. 2009. Available online: http://www.aubot.dk/FastGap_home.htm (accessed on 1 June 2014).
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Huelsenbeck, J.; Ronquist, F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Swofford, D. PAUP *: Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4.0b10; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002. Available online: http://paup.csit.fsu.edu/ (accessed on 1 June 2014).
- Dowton, M.; Austin, A.D. Increased congruence does not necessarily indicate increased phylogenetic accuracy—The behavior of the incongruence length difference test in mixed-model analyses. Syst. Biol. 2002, 51, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree, a Graphical Viewer of Phylogenetic Trees. 2007. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 1 June 2014).
- Cracraft, J. Species concepts and speciation analysis. In Current Ornithology; Springer: Berlin/Heidelberg, Germany, 1983; pp. 159–187. [Google Scholar]
- Arbogast, B.S.; Edwards, S.V.; Wakeley, J.; Beerli, P.; Slowinski, J.B. Estimating divergence times from molecular data on phylogenetic and population genetic timescales. Ann. Rev. Ecol. Syst. 1992, 33, 707–740. [Google Scholar] [CrossRef]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Williams, G.J. Rattle: A data mining guide for R. R J. 2009, 1, 45–55. [Google Scholar] [CrossRef]
- Therneau, T.M.; Atkinson, B.; Ripley, B. Rpart: Recursive Partitioning. R Package Version 2010, 3, 1–46. Available online: https://cran.r-project.org/web/packages/rpart/rpart.pdf (accessed on 2 May 2025).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 2 May 2025).
- Rohlf, F.J. Tpsdig2, Version 2.0; Department of Ecology and Evolution, State University of New York, Stony Brook: New York, NY, USA, 2005. Available online: https://tpsdig2.software.informer.com/ (accessed on 2 May 2025).
- Sheets, D.H. Integrated Morphometrics Software (IMP)—MathWorks, MATLAB6; The Mathworks: Natick, MA, USA, 2000. Available online: https://www.animal-behaviour.de/imp/ (accessed on 2 May 2025).
- Christian, A.D.; Harris, J.L.; Serb, J.M. Preliminary Analysis for Identification, Distribution, and Conservation Status of Species of Fusconaia and Pleurobema in Arkansas; Report; Arkansas Game and Fish Commission: Perrytown, AR, USA, 2008; p. 40. [Google Scholar]
- Inoue, K.; McQueen, A.L.; Harris, J.L.; Berg, D.J. Molecular phylogenetics and morphological variation reveal recent speciation in freshwater mussels of the genera Arcidens and Arkansia (Bivalvia: Unionidae). Biol. J. Linn. Soc. 2014, 112, 535–545. [Google Scholar] [CrossRef]
- Grobler, P.J.; Jones, J.W.; Johnson, N.A.; Beaty, B.; Struthers, J.; Neves, R.J.; Hallerman, E.M. Patterns of genetic differentiation and conservation of the slabside pearlymussel, Lexingtonia dolabelloides (Lea, 1840) in the Tennessee River drainage. J. Molluscan Stud. 2006, 72, 65–75. [Google Scholar] [CrossRef]
- Schilling, D.E. Assessment of Morphological and Molecular Genetic Variation of Freshwater Mussel Species Belonging to the Genera Fusconaia, Pleurobema, and Pleuronaia in the Upper Tennessee River Basin. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA, 2015; p. 194. Available online: https://vtechworks.lib.vt.edu/server/api/core/bitstreams/3a92b706-3d63-47f7-896e-c756b8a0449a/content (accessed on 2 May 2025).
- Ortmann, A.E. The anatomy of certain mussels from the Upper Tennessee. Nautilus 1921, 34, 81–91. [Google Scholar]
- Schilling, D.E.; Phipps, A.T.; Jones, J.W.; Hallerman, E.M. A Survey of Freshwater Mussels (Unionidae) in Little River, Blount County, Tennessee. Southeast. Nat. 2017, 16, 105–116. [Google Scholar] [CrossRef]
- Conrad, T.A. Monography of the Family Unionidae, or Naiades of Lamarck, (Fresh Water Bivalve Shells) of North America, Illustrated by Figures Drawn on Stone from Nature; J. Dobson: Philadelphia, PA, USA, 1837; Volume 1, pp. 73–80. [Google Scholar]
- Lea, I. Continuation of Mr. Lea’s Paper on fresh water and land snails. Proc. Am. Philos. Soc. 1841, 2, 11–15. [Google Scholar]
- Lea, I. Descriptions of six new species of unions from Alabama. Proc. Natl. Acad. Sci. USA 1857, 9, 83. [Google Scholar]
- Lea, I. New Unionidæ of the United States and northern Mexico. J. Acad. Nat. Sci. 1860, 4, 327–374. [Google Scholar]
- Lea, I. Descriptions of eleven new species of the genus Unio from the United States. Proc. Natl. Acad. Sci. USA 1861, 13, 391–393. [Google Scholar]
- Anthony, J.G. Descriptions of new species of North American Unionidae. Am. J. Conchol. 1865, 1, 155–164. [Google Scholar]
- Lea, I. Descriptions of five new species of Unionidæ and one Paludina of the United States. Proc. Natl. Acad. Sci. USA 1867, 19, 81. [Google Scholar]
- Lea, I. New Unionidæ, Melanidæ, etc., chiefly of the United States. J. Acad. Nat. Sci. Phila. 1868, 6, 303–343. [Google Scholar]
- Lea, I. Descriptions of twenty new species of Uniones of the United States. Proc. Natl. Acad. Sci. USA 1871, 23, 189–193. [Google Scholar]
- Lea, I. Descriptions of twenty-nine species of Unionidae from the United States. Proc. Natl. Acad. Sci. USA 1872, 24, 155–161. [Google Scholar]
- Wright, S.H. Contribution to a knowledge of United States Unionidae. Nautilus 1897, 11, 4–5. [Google Scholar]
- Lea, I. Descriptions of new fresh water and land snails. Proc. Am. Philos. Soc. 1845, 4, 162–168. [Google Scholar]
- Lea, I. Descriptions of new species of Unio, from Tennessee, Alabama and North Carolina. Proc. Natl. Acad. Sci. USA 1858, 10, 40–41. [Google Scholar]
- Lea, I. Descriptions of five new species of uniones from North Alabama. Proc. Natl. Acad. Sci. USA 1860, 12, 92–93. [Google Scholar]
- Lea, I. Descriptions of twenty-five new species of Unionidae from Georgia, Alabama, Mississippi, Tennessee and Florida. Proc. Natl. Acad. Sci. USA 1861, 13, 38–41. [Google Scholar]
- Reeve, L. Monograph of the genus Unio. In Conchologa Iconica: Or Illustrations of the Shells of Molluscous Animals; L. Reeve and Company: London, UK, 1856; Volume 16, pp. 1864–1869, 96 colored plates. [Google Scholar]
- Lea, I. Descriptions of eight new species of Unio of the United States. Proc. Acad. Natl. Sci. USA 1865, 17, 88–89. [Google Scholar]
- Lea, I. Descriptions of sixteen new species of the genus Unio of the United States. Proc. Natl. Acad. Sci. USA 1868, 20, 143–145. [Google Scholar]
- Ortmann, A.E. A new type of the nayad genus Fusconaia group of F. barnesiana Lea. Nautilus 1917, 31, 58–64. [Google Scholar]
- Morrison, C.; Johnson, N.; Jones, J.; Fitzgerald, D.; Aunins, A.; King, T.; Hallerman, E. Genetic and morphological characterization of the clubshell species complex (Pleurobema clava and P. oviforme) to inform conservation planning. Ecol. Evol. 2021, 12, 15325–15350. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.I.; Baker, H.B. The types of Unionacea (Mollusca: Bivalvia) in the Academy of Natural Sciences of Philadelphia. Proc. Acad. Nat. Sci. USA 1973, 12, 145–186. [Google Scholar]
- Mayr, E. The Growth of Biological Thought: Diversity, Evolution, and Inheritance; Harvard University Press: Cambridge, UK, 1982. [Google Scholar]
- Källersjö, M.; Von Proschwitz, T.; Lundberg, S.; Eldenäs, P.; Erséus, C. Evaluation of ITS rDNA as a complement to mitochondrial gene sequences for phylogenetic studies in freshwater mussels: An example using Unionidae from north-western Europe. Zool. Scr. 2005, 34, 415–424. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).