1. Introduction
Global climate change is profoundly altering key ecological processes and the conditions necessary for the survival of living organisms [
1]. Traditional concepts of environmental factors, developed throughout ecological history, must be reconsidered, as the patterns of their formation and interaction under contemporary changes differ significantly from those underpinning conventional interpretations [
2]. Anthropogenic pressure has reached such a magnitude that even factors and complexes previously regarded as natural, such as climate, soil-forming rocks, topography, biota, and the progression of ecological time, have transformed [
3]. Today, virtually no regions on the planet remain free from human influence. This situation imposes new demands on our understanding of the role of biodiversity reserves: they should not only be strictly protected areas but also include any regions capable of ensuring the conservation of rapidly vanishing biodiversity. Traditional concepts of environmental factors must be reconsidered, as their interactions have been altered by human activity. In this context, reliable tools are essential for assessing the combined impacts of natural and anthropogenic changes on ecosystems. Phytoindication offers such a tool, allowing the evaluation of these integrated environmental effects through the responses of plant communities.
Ecology conceptualises abiotic factors as distinct components of the physical and chemical environment that influence organisms’ distribution, abundance, and survival within an ecosystem [
4]. The attribute “distinct” implies a conceptual interpretation of factors as theoretically independent phenomena. However, in real ecosystems, these factors often interact or are interrelated, and complete independence is seldom observed. Therefore, in practical research, environmental gradients are used as proxies for environmental factors [
5]. An environmental gradient represents the continuous variation of an environmental factor across space or time, reflecting differences in its intensity along a measurable continuum [
6]. This idea was reflected in the concept of the vegetation continuum represented along an environmental gradient, which formed the basis for gradient analysis [
7]. In turn, gradient analysis of vegetation is a development of the ideas of the factorial approach for explaining soil differentiation [
8]. Soil properties are a function of five environmental factors: parent material, regional climate, topography, biota, and time [
9]. These five soil-forming factors are clearly the same factors that determine vegetation. Vegetation distribution corresponds to regional climatic patterns, varies with changes in soil parent material, differs on slopes with opposite aspects, changes according to geographic differences between floras, and evolves [
10]. Environmental gradients are classified into indirect (complex), direct, and resource gradients [
11]. Indirect gradients, such as elevation above sea level, influence plant growth indirectly by correlating with other environmental factors like precipitation, wind, and temperature. Direct gradients exert a direct physiological effect on plants, while resource gradients consist of factors organisms directly use as resources for growth. There is no clear boundary between direct factors and resources. Under certain conditions, a resource may function as a direct factor [
12]. Plant growth performance can be represented as a function of four main environmental factors: nutrients, water, temperature, and light [
13], which correspond to the primary indicator values in virtually all indicator systems.
Plants can serve as indicators of environmental factors [
14,
15]. The conceptual foundation for applying bioindication is the theory of species’ ecological niches [
16]. This theory posits that the species composition of a given community allows for the assessment of environmental conditions [
17]. The bioindication process relies on indicator values that represent the ecological optimum of a species along the gradient of a specific environmental factor [
18]. These values are incorporated into a community-weighted mean calculation based on cover-abundance scores for the relevant plant community [
19,
20]. This calculation is then used to indicate the level of the corresponding environmental factor under which the community has developed [
21]. Phytoindication enables the assessment of various physical abiotic environmental factors, including site illumination, temperature regime, climate continentality, soil or substrate moisture levels, acidity (pH), nutrient availability, and, in some cases, salinity [
22]. Gradient analysis and biological indication share a common theoretical foundation and can be effectively integrated. Indicator scales can be used to interpret the results of ordination analysis, as they enable the identified gradients in species composition to be linked to the abiotic environmental factors influencing them [
23]. Weighted average indicator values calculated for sample plots can be overlaid onto the ordination space as passive variables or used to construct isolines representing the direction and intensity of environmental gradients [
24,
25]. Phytoindication estimates of environmental properties serve as auxiliary variables in ordination analyses, facilitating the ecological interpretation of the primary gradients shaping plant community structure [
26]. This approach enables the quantification of the positions of taxa under study along gradients of moisture, soil pH, trophic status, and light availability, eliminating the need for direct measurement of the abiotic factors themselves. The current methodology allows for statistical analyses aimed at identifying any differences that may exist between specific plant communities [
22].
Phytoindication integrates environmental conditions over extended periods, eliminating the need for repeated and costly measurements, and can be applied in situations where direct assessments are impossible [
20,
27]. It enables the retrospective reconstruction of environmental parameters based on species composition, while species response models allow for the calibration of abiotic indicators [
21]. In addition to natural factors, levels of anthropogenic transformation are indicated using scales of naturalness and hemeroby [
28,
29]. Ellenberg indicator scales assign numerical values to plant species according to their ecological preferences for key environmental factors such as light, temperature, moisture, soil reaction, and nutrient availability. Standardised systems, such as the Ellenberg scales, ensure comparability of results across regions and studies by relying on data from standardised geobotanical relevés [
30]. When combined with geostatistical methods and remote sensing, indicator values facilitate the creation of spatial models of environmental factors, which is especially valuable in large-scale studies where direct measurements at each site are technically or financially unfeasible [
31]. The capacity for spatial modelling of indicator values based on remote sensing data is predicated on the premise that the composition of plant species, which determines the weighted mean indicator value for a given site, is associated with the spectral characteristics of the vegetation cover [
32]. The biomass structure, leaf area, chlorophyll and water content, seasonal dynamics and spatial heterogeneity of different species assemblages are all subject to variation, shaped by specific abiotic conditions [
33]. These variations can be reflected in spectral indices derived from satellite or aerial imagery [
34]. When these spectral indicators and other spatial predictors, such as elevation, distance to water, and climatic data, are statistically linked to known field estimates of indicator values, it becomes possible to develop a model that predicts these values across an entire region [
35,
36]. Remote sensing facilitates the extrapolation of bioindication data from specific points to broader areas, thereby ensuring the acquisition of high-resolution spatial information [
37].
A fundamental challenge in interpreting which indicator values represent specific environmental factors arises because these values do not directly measure them. Instead, they reflect the responses of individual plant species and plant communities to those factors [
38,
39]. Biotic systems can integrate external influences [
40]. The complex filtering mechanism underpinning the formation of diverse plant assemblages in nature is a fundamental factor in the subsequent variation in indicator scores of species both within and between plots [
41]. The law of the limiting factor [
42,
43] posits that a biotic system responds primarily to factors that are either deficient or in excess, exhibiting little or no response when a factor is within the species’ optimal range. This explains why nominal indicator scales often show cross-sensitivity to multiple factors. For instance, the indicator scale for available nitrogen in the Ellenberg system has been demonstrated to correlate with the productivity of plant communities [
44], as it reflects not only soil nitrogen content but also other fertility factors such as phosphorus [
18,
45]. Consequently, in recent adaptations of the Ellenberg indicator system, this scale has been renamed the nutrient availability scale [
18]. Similarly, the indicator values for soil reaction reflect changes in pH and the quantity or saturation of exchangeable Ca
2+ [
46]. Amplitude indicators of water regime dynamics show a stronger correlation with soil moisture indicator values than average water regime indicators [
46]. The accuracy of these indicator scores depends on the contrast of environmental conditions, often referred to as the length of the gradient. In cases of short gradients, indicator values may be influenced more by random fluctuations in species composition than by the actual environmental gradient [
21]. A significant conceptual inconsistency exists within the framework of phytoindication. This inconsistency pertains to the mechanistic transfer of autecological properties of individual plant species, as indicated by indicators of their optimal zones along environmental gradient factors, to the community level for environmental factor assessment [
47]. Consequently, estimates of a plant species’ ecological niche are used as scales to indicate environmental properties at the community level. The presence of a species in a description can result from numerous factors, including environmental conditions, interspecific interactions, or neutral influences [
48]. Nonetheless, it is generally accepted that ecological indicator values for a site represent an integrated signal of species-environment relationships at the community level and provide reliable information about the long-term environmental conditions characterising that site [
49]. Comparisons with empirical measurements of environmental factors reveal shifts in indicator scores across different phytosociological vegetation classes [
50], which may also reflect significant changes in the informational value of plant species depending on the context within different communities. Indicator-based evaluations inherently depend on the structure of plant communities. As demonstrated by Zelený and Schaffers [
51], mean indicator values (such as Ellenberg indices) contain information on compositional similarity among plots, which may introduce bias in analyses linking phytoindication estimates to vegetation composition. Therefore, in our study, we explicitly emphasise that information derived from indicator scales is not independent of community structure and may lead to biassed results if misinterpreted.
The accuracy of the phytoindication method depends on the number of species within the plot and their ecological characteristics. Naturally, the number of species increases proportionally with the size of the area surveyed [
52,
53]. Therefore, it can be said that the resolution of this method is subject to an ‘uncertainty principle in ecology’: for an accurate assessment, a larger survey area is required, but then it becomes unclear to which specific point within this area the assessment refers. Ellenberg’s point scales also do not account for species tolerance, which significantly affects the method’s accuracy. It is also simplistically assumed that species’ responses to environmental factors are unimodal and symmetrical [
46,
54], which is not always the case [
55,
56]. Conversely, Didukh’s range scales consider only species tolerance, but not the optimum zone [
57]. However, this important feature is lost in calculations, as range values are converted into point values [
27], with corresponding disadvantages. The range character of Didukh’s scales is incorporated in the ideal indicator method [
58]. The best indicator is a species with zero tolerance; however, such species do not exist in nature. A method was proposed to calculate such a hypothetical indicator based on the floristic composition of the community. Obviously, this hypothetical plant species could occur within only under the conditions of a given community, and the ecological properties of such a species best characterise those conditions: as soon as the conditions change, the species with zero tolerance would disappear. Comparisons of different approaches have shown that the ideal indicator method is more accurate [
20]. However, if phytoindication is considered a surrogate for technical methods of environmental measurement, these features represent critical drawbacks. If, instead, phytoindication is regarded as a method for assessing the response of a plant community to environmental factors, then these shortcomings become important features that enable its correct application. There are no universal measurement methods; each method has its own accuracy range and conditions of application that must be known and understood.
Protected areas are used as convenient natural laboratories in which to assess the ecological consequences of global environmental change [
59]. The naturalness value is a measure of the extent to which plant communities resemble their pristine, undisturbed state [
60] and is therefore traditionally suitable for use in natural reserves [
61]. Nevertheless, the profound impact of anthropogenic activities has had a pervasive effect on virtually every region of the planet [
62]. Within the steppe zone of Ukraine, in particular, natural ecosystems represent exceptional remnants within a landscape that has been extensively transformed by human activity [
63]. Consequently, the application of hemeroby, an indicator of anthropogenic impact, within a protected area can offer both scientific and practical benefits, as it facilitates the detection of subtle gradients of human influence and assists in evaluating ecosystem integrity under current conditions [
64].
The history of phytoindication development raises several unresolved questions: Is phytoindication merely a surrogate for measuring environmental factors, or is it a unique approach to assessing their effects on plant communities? To what extent is it methodologically justifiable to convert environmental drivers of plant growth into indicator values within standardised systems? What precisely does phytoindication measure, and to what extent can it be used to assess ecosystem specificity and facilitate comparisons between ecosystems?
In this study, we aimed to evaluate the response of vegetation cover to the combined influence of natural and anthropogenic factors within a protected area situated in the heart of an industrial region, which is subject to considerable pressure on biodiversity. By applying ecological indicator values alongside assessments of naturalness and hemeroby, we examined how plant communities integrate the effects of multiple drivers. Specifically, we addressed the following research questions: (1) How do natural and anthropogenic gradients jointly shape vegetation structure within the reserve? (2) Which indicator systems most effectively capture these patterns? (3) To what extent can phytoindication differentiate the contributions of natural versus anthropogenic factors? The findings of this study demonstrate that the application of hemeroby evaluation, a traditional approach used in urban and ruderal ecosystems, is also relevant and informative for protected areas, where biodiversity is increasingly influenced by pervasive human impact. This study extends the application of hemeroby beyond its conventional scope and provides new evidence that even conservation landscapes exhibit measurable gradients of anthropogenic influence. The integration of hemeroby with phytoindication has been shown to reveal the functionality of protected areas as sensitive indicators of regional socio-ecological dynamics. This novel framework offers a means to assess ecosystem integrity in the context of global environmental change.
3. Results
3.1. Spatial Pattern of Sampling Plots
The plot pattern departed strongly from CSR (quadrat test: χ2 = 2906.2, df = 24, p < 2.2 × 10−16), with Ripley’s L exceeding CSR envelopes at multiple spatial scales (max |L(r) − r| ≈ 556 m). The point process model with a linear trend in x and y fitted significantly better than CSR (ΔDeviance = 899.8, p < 2.2 × 10−16), indicating a systematic, gradient-oriented (catena-based) sampling layout rather than random placement. Nearest-neighbour distances corroborated a non-CSR structure with numerous short inter-plot distances and a long tail.
3.2. Vegetation Composition and Ecological Gradients
A total of 297 plant species were identified within the study area. The recorded plant communities represent 24 plant associations belonging to 14 vegetation classes. In this study, plant associations were analysed as structural references that may influence or bias phytoindication estimates, rather than as objects of successional interpretation. Therefore, our focus was on the statistical relationships among ecological indicator scales rather than on vegetation dynamics per se. A Canonical Correspondence Analysis (CCA) revealed the effect of association type, ecological factors, naturalness, and hemeroby on species composition. The global permutation test showed these predictors’ highly significant impact on species composition (χ2 = 6.2, F = 13.6, p < 0.001). The adjusted R2, which accounts for the number of predictors and sample size, was 0.26, indicating a moderate proportion of explainable variation in plant species composition. A partial Canonical Correspondence Analysis (CCA) was performed to isolate the unique effect of associations on species composition, while controlling for Ellenberg indicator values, naturalness, and hemeroby. The model showed a significant independent effect of association type (χ2 = 3.2, F = 9.37, p < 0.001). After accounting for the other covariates, the adjusted R2 was 0.13ч’, indicating that approximately 13.2% of the explainable inertia in species composition could be attributed to plant association alone. The environmental factors represented by Ellenberg indicator values, naturalness, hemeroby, and association types included as conditional variables, explained 5.2% of the total inertia in vegetation composition (adjusted R2 = 0.052, F = 10.2, p < 0.001). A one-way MANOVA was conducted to examine the effect of plant associations on a set of Ellenberg indicator values (light, temperature, continentality, moisture, reaction, and nutrient availability). The results revealed a statistically significant multivariate effect of association on the combined set of environmental indicators (Pillai’s trace = 2.7, F(138, 6330) = 36.7, p < 0.001). This reveals that the association is connected with significant differences in the overall ecological conditions inferred from the Ellenberg indicator system. The high value of Pillai’s trace (2.7) suggests a large multivariate effect size, meaning the variation in indicator values among associations is statistically significant and ecologically meaningful.
3.3. Composite Natural and Anthropogenic Gradients in Vegetation Composition
A partial CCA model, considering plant association type, naturalness, and hemeroby as constrained variables, revealed that Ellenberg indicator values could explain 3.9% of the plant community inertia (adjusted
R2 = 0.039,
F = 10.3,
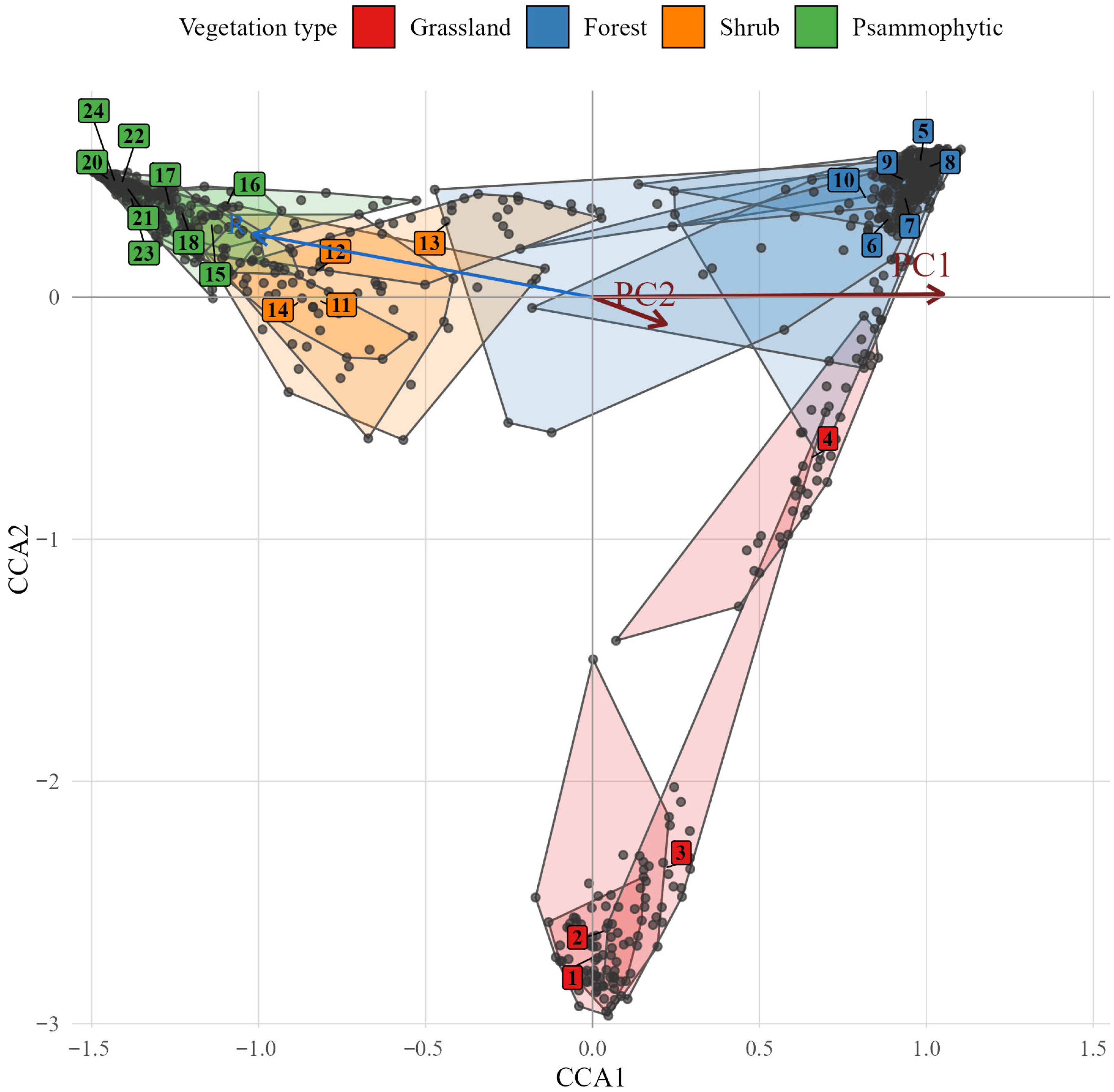
p < 0.001). The influence of environmental factors was primarily directed along the first canonical axis (
Figure 2), which represents the main ecological gradient in the study area.
CCA1 was positively correlated with soil moisture and nutrient availability, and negatively correlated with light availability, temperature, soil pH, and continentality, thereby reflecting a transition from dry, nutrient-poor sandy habitats to moist, fertile sites with denser vegetation. The principal component analysis (PCA) of the environmental factors extracted two principal components with eigenvalues greater than one (
Table 1), which together summarised the dominant ecological gradients identified in the study. Principal component 1 closely corresponded to the direction of CCA axis 1, while principal component 2 aligned with CCA axis 2. Principal component 1 represents a composite gradient characterised by increasing soil moisture and nutrient availability and decreasing light availability, temperature, continentality, and soil reaction (i.e., increasing acidity). Principal component 1 after accounting for the effect of association types included as conditional variables explained 5.2% of the total inertia in vegetation composition (adjusted
R2 = 0.052,
F = 8.0,
p < 0.001). This component reflects a transition from dry, nutrient-poor sandy habitats to more humid and fertile sites with denser vegetation. Such a gradient is typical of sandy river terraces across Europe, where the complex of ecological conditions changes gradually from the elevated dune tops to the interdune or floodplain depressions.
Principal component 2 captures a gradient of increasing naturalness associated with decreasing hemeroby. Principal component 2, after accounting for the effect of association types included as conditional variables, explained 5.3% of the total inertia in vegetation composition (adjusted R2 = 0.053, F = 8.2, p < 0.001). An important finding is that the degree of anthropogenic impact appears to be largely independent of the configuration of natural environmental factors, since the second principal component specifically accounts for variation in naturalness and hemeroby by definition of the orthogonality of principal components.
3.4. Effect of Light Availability on Plant Community Composition
Light availability was able to explain 4.0% of the inertia in the plant species matrix (F = 46.5, p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of light availability explained 0.56% of the total inertia in species composition (F = 8.3, p < 0.001). This represented 14.4% of the total inertia explained by the set of ecological predictors derived from the Ellenberg system, highlighting the relative importance of light availability in shaping plant community composition.
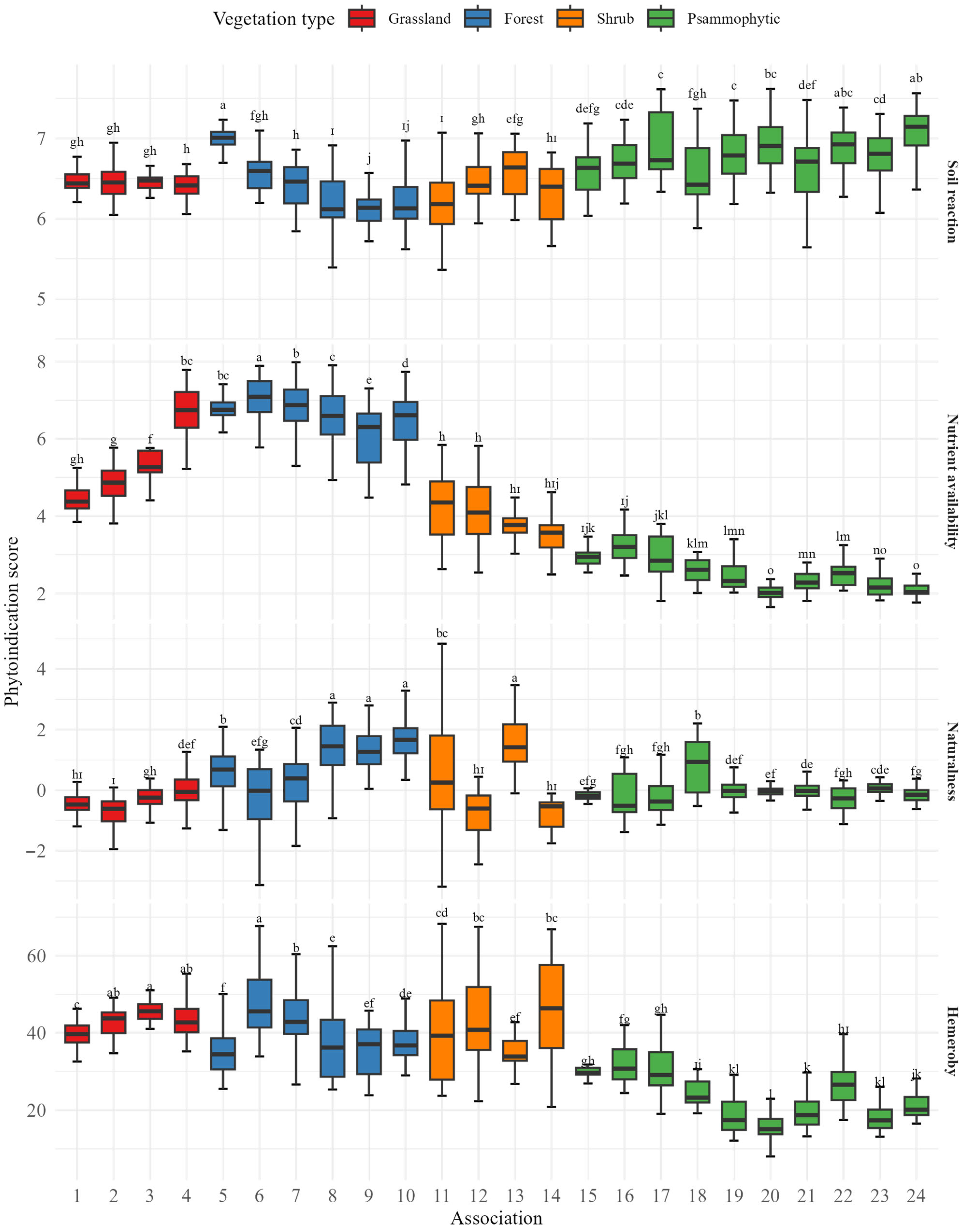
The phytoindication estimates of light availability ranged from 5.1 to 8.5 in 95% of cases. Community affiliation based on plant association accounted for 94.1% of the variation in light availability (
F = 753.9,
p < 0.001). Psammophytic communities exhibited the highest levels of light availability, with phytoindication scores consistently exceeding 7.4 (
Table 1). Forest communities had light availability values below 6.0. Grassland communities and shrublands occupied an intermediate position in terms of light availability.
3.5. Temperature Gradient and Its Influence on Vegetation Structure
Temperature was found to explain 2.7% of the inertia in the plant species matrix (F = 31.4, p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of temperature explained 0.68% of the total inertia in species composition (F = 9.9, p < 0.001). This represented 17.4% of the total inertia explained by the ecological predictors derived from the Ellenberg system. The phytoindication estimates of the temperature regime ranged from 4.2 to 6.1 in 95% of cases. The association membership of a plant community accounted for 66.2% of the variation in the temperature regime (F = 93.1, p < 0.001). Grassland communities exhibited the lowest temperature regime values, forest communities had intermediate values, and the highest values were observed in psammophytic communities.
3.6. Continentality Reveals Consistent Ecological Patterns
Continentality was able to explain 3.8% of the inertia in the plant species matrix (F = 43.1, p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of continentality explained 0.78% of the total inertia in species composition (F = 11.5, p < 0.001). This represents 20.0% of the total inertia explained by the ecological predictors derived from the Ellenberg system. Continentality scores ranged from 7.0 to 13.4 in 95% of cases. The association membership of a plant community explained 86.5% of the variation in continentality (F = 302.0, p < 0.001). The association Limonio meyeri–Festucetum pseudodalmaticae and other associations occurring on elevated terrace sites, including both psammophytic communities and the shrub association Aceri tatarici–Quercetum roboris, were characterised by continentality values generally exceeding 10. Grassland and forest communities typically exhibited continentality values below 10.
3.7. Soil Moisture Gradient and the Ecological Structuring of Plant Communities
Soil moisture was able to explain 3.8% of the inertia in the plant species matrix (
F = 44.1,
p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of soil moisture explained 0.71% of the total inertia in species composition (
F = 10.5,
p < 0.001). This represents 18.2% of the total inertia explained by the ecological predictors derived from the Ellenberg system. Phytoindication estimates of soil moisture ranged from 1.8 to 5.5 in 95% of cases. The membership of a community in a plant association explained 89.9% of the variation in soil moisture (
F = 417.5,
p < 0.001). Grassland communities formed a consistent sequence ordered by increasing soil moisture:
Limonio meyeri–Festucetum pseudodalmaticae →
Poëtum pratensis →
Junco gerardii–Agrostietum stoloniferae →
Caricetum gracilis. Forest ecosystems had intermediate soil moisture values ranging from 4.5 to 5.2, while psammophytic communities exhibited the lowest values. These relationships are clearly illustrated in
Figure 3, which visualises the gradual shift in moisture conditions across vegetation types and highlights the distinct hydrological positions of grassland, forest, and psammophytic associations. Communities on elevated sandy dune areas formed a clear decreasing sequence of soil moisture from
Salici rosmarinifoliae–Holoschoenetum vulgaris, usually occurring in interdune depressions, to
Secali sylvestri–Caricetum colchicae and
Secaletum sylvestre, which typically occupy the driest dune tops. As shown in
Figure 4, this pattern illustrates the transition from mesic shrub communities in sheltered microrelief depressions to xeric grasslands adapted to extreme drought and nutrient-poor sandy substrates.
3.8. Effect of Soil Reaction on Vegetation Composition
Soil reaction explained 1.9% of the inertia in the plant species matrix (
F = 21.8,
p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of soil reaction explained 0.66% of the total inertia in species composition (
F = 9.6,
p < 0.001). This represents 16.9% of the total inertia explained by the ecological predictors derived from the Ellenberg system. The phytoindication estimates of soil reaction ranged from 5.8 to 7.4 in 95% of cases. The community membership in a plant association explained 49.5% of the variation in soil reaction (
F = 46.9,
p < 0.001). The highest soil reaction values were characteristic of associations such as
Populetum nigro-albae,
Artemisio dniproicae–Salicetum acutifoliae, and
Secaletum sylvestre. Although these associations differ substantially in floristic composition, they share a common occurrence on elevated, well-drained landforms, where carbonate accumulation and reduced leaching promote higher pH levels. In contrast, the lowest soil reaction values were observed in
Ficario–Ulmetum minoris,
Melico nutantis–Quercetum roboris,
Symphyto officinalis–Anthriscetum sylvestris, and
Salici rosmarinifoliae–Holoschoenetum vulgaris, which typically occupy low-lying, periodically waterlogged areas with more acidic soils. This pattern, illustrated in
Figure 3, highlights the close link between topographic position and soil chemical environment across the studied vegetation types.
3.9. Effect of Nutrient Availability on Vegetation Composition
Nutrient availability was able to explain 3.9% of the inertia in the plant species matrix (F = 44.9, p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of nutrient availability explained 0.75% of the total inertia in species composition (F = 11.0, p < 0.001). This accounts for 19.2% of the total inertia explained by ecological predictors derived from the Ellenberg system. The phytoindication estimates of nutrient availability ranged from 1.9 to 7.7 in 95% of cases. Association membership accounted for 90.2% of the variation in nutrient availability (F = 434.5, p < 0.001). Forest communities were found under relatively high nutrient availability conditions, typically exceeding 6. Psammophytic communities occupied areas with low nutrient availability, generally below 4.5. Grassland communities occupied an intermediate position.
3.10. Anthropogenic Gradient Reflected by Naturalness and Hemeroby
Naturalness was able to explain 2.2% of the inertia in the plant species matrix (F = 23.0, p < 0.001). After accounting for the influence of association types and other environmental factors included as conditional variables, the pure effect of naturalness explained 0.51% of the total inertia in species composition (F = 8.3, p < 0.001). The naturalness scores of plant communities ranged from 0.5 to 5.3 in 95% of cases. Association membership accounted for 51.6% of the variation in naturalness (F = 51.3, p < 0.001). Psammophytic communities generally exhibited the highest levels of naturalness.
Hemeroby was able to explain 2.7% of the inertia in the plant species matrix (F = 30.7, p < 0.001). The pure effect of hemeroby, after accounting for the influence of association types and other environmental factors included as conditional variables, explained 0.56% of the total inertia in species composition (F = 8.3, p < 0.001). The hemeroby scores of plant communities ranged from 14.0 to 56.5 in 95% of cases. Association membership accounted for 67.6% of the variation in hemeroby (F = 98.8, p < 0.001). The highest hemeroby levels were observed in associations such as Salicetum albae and Melico transsilvanicae–Agropyretum.
The proportion of variation explained by association membership was considered a marker of indicator specificity, i.e., the extent to which an indicator is aligned with the syntaxonomic structure of vegetation. Indicators of natural environmental factors typically showed high specificity, with more than 84% of their variation explained by association membership. In contrast, indicators of the level of anthropogenic transformation, such as naturalness and hemeroby, exhibited lower values (<68%), indicating that they are less specific in this sense and reflect processes that operate partly independently of vegetation type composition.
3.11. Relationships Between Environmental Gradients and Structural Characteristics of Plant Communities
The species richness of plant communities showed significant correlations with phytoindication estimates of environmental factors (
Table 2). Species richness was positively correlated with light availability, continentality, soil pH, and hemeroby. Conversely, an increase in species richness was associated with decreased soil moisture, nutrient availability, and naturalness.
The relationships among the components of the horizontal structure of plant communities also correlated with phytoindication estimates of environmental factors. Increases in the canopy and shrub layers were primarily linked to higher soil moisture and nutrient availability. In contrast, an increase in herb layer density was mainly associated with greater light availability and continentality. Shrub layer dominance increased with higher hemeroby and lower naturalness of plant communities, increased light availability and decreased temperature and continentality. Additionally, coordinated variation in the canopy and herb layers was sensitive to higher hemeroby and lower naturalness of plant communities.
The correlations between the pure effects of environmental factors and the characteristics of plant communities were more selective. The pure effects of temperature, soil reaction, and naturalness exhibited a statistically significant negative correlation with plant community species richness. The ratio of the canopy and shrub layers to the herb layer was positively correlated with the pure effect of naturalness. The pure effects of light availability and continentality were significantly positively correlated only with shrub layer dominance. In contrast, the pure effects of soil reaction and hemeroby were negatively correlated with this aspect of the horizontal structure of plant communities. Additionally, the pure impact of soil moisture was positively correlated with coordinated variation in the canopy and herb layers.
3.12. Spatial Patterns of Environmental Factors and Landscape Differentiation
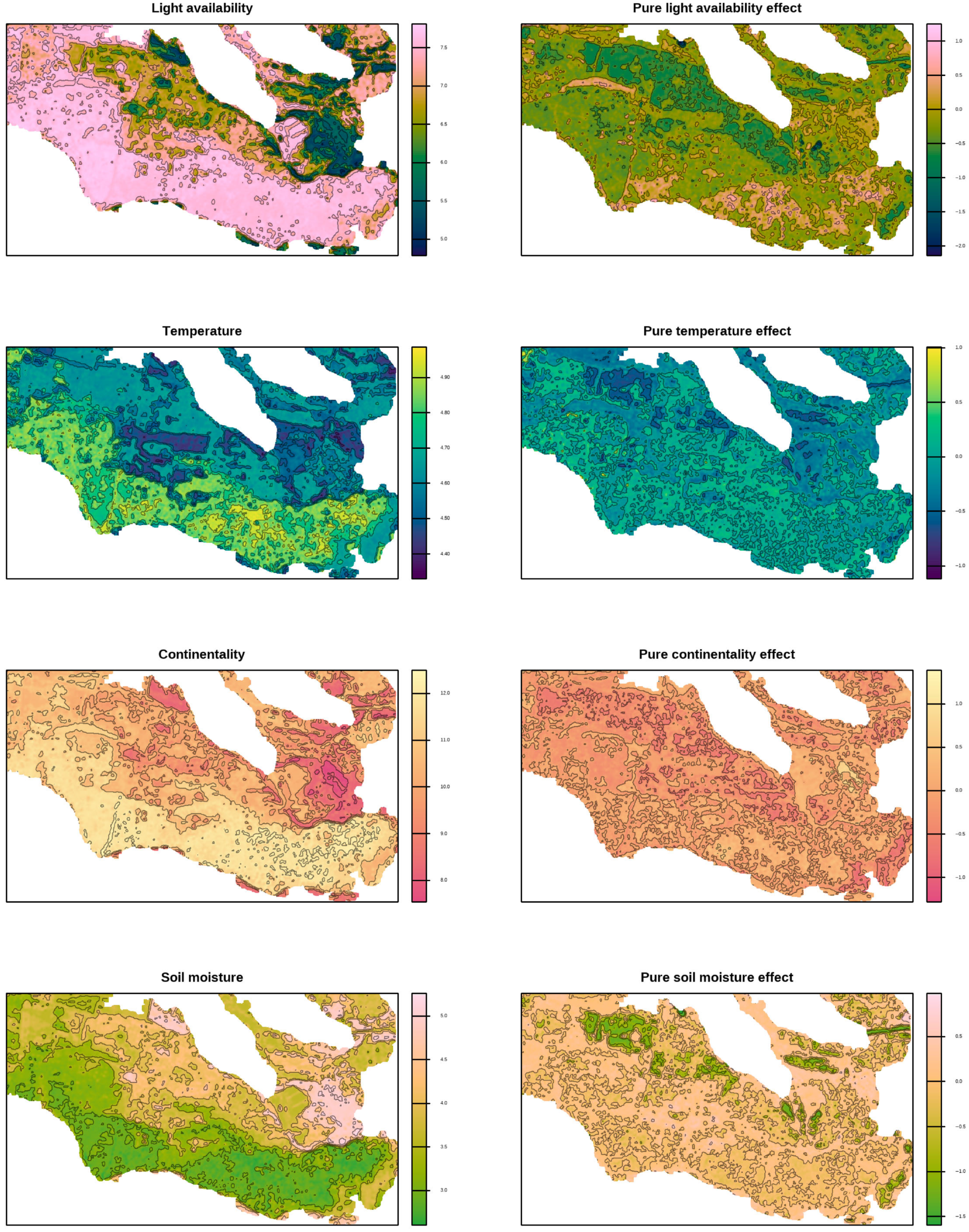
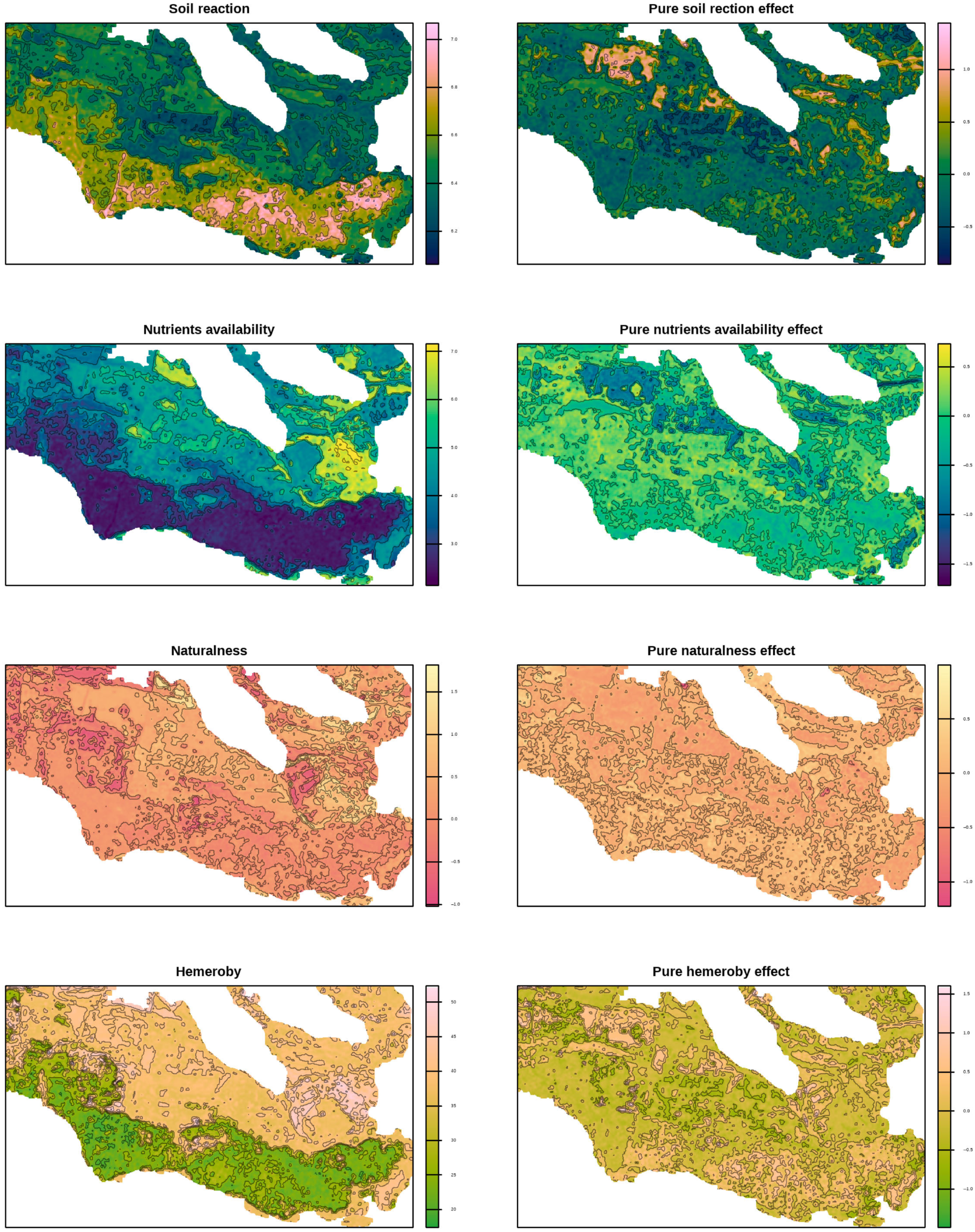
The spatial variability patterns of environmental factors distinctly divided the study area into two zones: the northern and northeastern parts, and the southern and eastern parts (
Figure 5 and
Figure 6). This differentiation is interpreted as a large-scale (regional) pattern reflecting the principal ecological division of the floodplain-terrace system. Forests and grasslands predominantly characterise the northern and northeastern zone, while the south and eastern zone is dominated by psammophytic grasslands or shrublands. The spatial patterns of the various factors were quite similar, showing a clear correspondence to these zones. The pure effects of environmental factors were unique, resulting in spatial variability patterns of pure effects that were largely distinct.
The full and pure impact of the factors differed substantially in the scale of their spatial patterns. Fine-scale spatial patterns were spatially restricted and revealed a more detailed picture of local variability, capturing subtle environmental differences within vegetation types and microtopographic units. The full effects of factors generally manifested at a large scale, clearly dividing the territory into two markedly different regions, whereas the pure effects of factors were more sensitive to fine-scale spatial variations.
Figure 5.
Spatial variability of environmental factors based on the Ellenberg indicator system predicted by a random forest using Sentinel-derived spectral indices (left panel) and their pure effects (right panel).
Figure 5.
Spatial variability of environmental factors based on the Ellenberg indicator system predicted by a random forest using Sentinel-derived spectral indices (left panel) and their pure effects (right panel).
Figure 6.
Spatial variability of environmental factors based on the Ellenberg indicator system, naturalness, and hemeroby predicted by a random forest using Sentinel-derived spectral indices (left panel) and their pure effects (right panel).
Figure 6.
Spatial variability of environmental factors based on the Ellenberg indicator system, naturalness, and hemeroby predicted by a random forest using Sentinel-derived spectral indices (left panel) and their pure effects (right panel).
4. Discussion
4.1. Why Instrumental Measurements Do Not Replace Phytoindication
Environmental factors influencing the biotic components of ecological systems are discussed from the earliest stages of ecology as a science and appear intuitively understandable, as this perception is rooted in everyday experience. However, the nonlinearity of biotic responses, formulated in Liebig’s law of the minimum and Shelford’s law of tolerance, makes their behaviour often counterintuitive and complex, forming the basis for modern interpretations of ecological gradients [
89]. In their implicit form, these laws assign particular importance to context when explaining the influence of environmental factors on biotic systems. The effect of one factor depends on the quantitative parameters of other factors, which should be understood as the response of a specific species to their combined action [
90].
The bell-shaped model of a species’ response to an environmental factor [
91,
92] suggests that the species’ sensitivity to the factor also varies with its intensity: it is minimal both at the extremes and at the optimum. The highest sensitivity occurs in the zone corresponding to the steepest slope of the species’ bell-shaped response curve. However, the effect can be qualitatively positive (on the left branch of the curve) or negative (on the right branch). The complexity of response patterns increases when the asymmetry of the response curve and its polymodality are considered [
93,
94], particularly as a consequence of competitive interactions among species.
This complexity indicates that “simple” measurements of environmental factors by physical or chemical methods provide only an initial pool of information. In this context, physical and chemical processes of measuring environmental properties can compete with one another, whereas their biological equivalents cannot, in principle, match them in terms of accuracy [
90]. From an ecological perspective, the significance lies not so much in the physical value of an environmental factor
per se, but in the response of the living system to its influence [
95].
However, the undeniable advantage of the bioindication method lies in its ability to assess the response of the ecosystem to various environmental factors [
96,
97]. There is some diversity in how phytoindication scales are defined and what they precisely indicate. These scales may be described as ‘primary environmental traits’ [
17], ‘surrogates for measured environmental variables’ [
21], ‘abiotic environmental variables’ [
18], ‘indirect assessment of site conditions’ [
98], ‘local environmental conditions’ or ‘site conditions’ in terms of ecological variables [
23] or ‘dynamic factors’ as opposed to static factors that can be easily measured instrumentally [
99]. Indicator values are regarded as estimates of the position of species optima along gradients of environmental factors [
18,
23]. Therefore, by definition, phytoindication aims to identify the characteristics of species’ responses to environmental factors, rather than to determine the factors themselves. In this context, bioindication should be regarded as a leading methodological approach for measuring environmental factors, precisely the response of biotic systems to physical environmental drivers. This view fundamentally differs from considering bioindication merely as a cheap and rapid proxy for estimating environmental factors. Within an ecological framework, there is no alternative to bioindication. At the same time, physical and chemical methods serve a supplementary function as external correlates for the meaningful interpretation of observed patterns. The intuitive reluctance to accept that “precise” measurements of environmental properties using chemical or physical instruments may be imprecise from an ecological point of view creates a misleading aura of optionality around bioindication methods. Of particular note is the emphasis on evaluating the response of the biotic system within the context of hemeroby, where the difficulty or even the practical impossibility of directly estimating disturbance and human impact is considered a fundamental premise [
99].
Instrument-based (physico-chemical) measurements cannot replace phytoindication, as they record only the values of environmental factors rather than the integrated, context-dependent, and often nonlinear responses of plant communities. Phytoindication captures tolerance, asymmetry, and (poly)modality in species’ responses, integrates spatial heterogeneity, and enables the assessment of naturalness and hemeroby dimensions that cannot, in principle, be measured directly by instruments. Within this framework, instrumental methods serve as useful external correlates rather than as alternatives. At the same time, indicator scales require careful application and regional calibration, because their estimates depend on community structure and may introduce bias.
4.2. Correlation Among Indicator Scales Is an Inherent Property of Phytoindication, Not a Drawback
The application of bioindication necessitates a reconsideration of the nature of environmental factors’ effects. The effect of “primary” factors, which align closely with our intuitive understanding of ecological drivers, can rarely be isolated and, in this respect, acts as a combination of factors. The correlation between indicator values was established for individual species and integrated assessments of environmental conditions [
18]. Our results indicate that the first principal component reflects a coordinated increase in soil moisture and nutrient availability, contrasted with an increase in light availability and temperature. This pattern clearly corresponds to the differentiation of the study area into floodplain sites with lower relief, which favour the accumulation of moisture and nutrients. Conversely, elevated landforms represented by sandy hills create moisture-deficient conditions, as sandy soils have low water-holding capacity but high permeability [
100], leading to rapid water loss from the root zone. The high aeration of sandy soils also enhances the mineralisation of soil organic matter [
101], preventing nutrient accumulation in sandy substrates. The geographic aspect of these relationships is visually summarised in
Figure 2 and
Figure 5, which illustrate the large-scale environmental gradient that shapes the spatial differentiation of vegetation. Predictably, sparse herbaceous cover is associated with high light availability [
102]. Carbonates accumulate [
81] in the relief depressions, whereas sandy soils are entirely devoid of them [
103,
104]. This also explains the coordinated dynamics of soil pH with other phytoindication indices.
Correlation among environmental factors presents a systemic challenge for instrumental measurements because it violates assumptions of independence, complicates the isolation of “pure” effects, and reduces interpretability. In contrast, within phytoindication, such correlation is an inherent property of vegetation as an integrated, hierarchically organised system: communities respond to the combined regime of factors rather than to their isolated values. Accordingly, correlations among indicator scales should be interpreted as an informative ecological signal which, if necessary, can be partitioned by partial ordination rather than regarded as a methodological flaw.
4.3. Continentality Where None Should Exist
The coordinated dynamics of continentality alongside other phytoindication estimates require explanation. By definition, continentality is an ecological phenomenon operating on a much larger scale than the spatial variability of different environmental factors, which are usually site-specific [
27]. Continentality of climate manifests as variation in interannual temperature and precipitation regimes along the west–east gradient across Eurasia [
105]. The spatial distribution of their species determines this ecological characteristic of the plant species as a whole [
106]. The interpretation of continentality values may be ambiguous at the local scale due to potential correlations with various factors, including seasonal variations in temperature and precipitation, diurnal temperature fluctuations, annual minimum temperatures, and drought conditions [
18]. Theoretically, within a single geographic locality and the same physico-geographical zone, continentality should not vary systematically among individual sites. Nevertheless, the observed spatial and environmental patterns were statistically confirmed. We interpret this variability in continentality within the landscape as arising from differences in the geographical (floristic) origins of plant assemblages occupying distinct environmental zones of the first river terrace. These assemblages are adapted to contrasting levels of climatic continentality, which likely explains the spatial and ecological patterns detected in our analysis. Communities on sandy hills are represented predominantly by species of Eurasian origin, which are adapted to a higher level of climatic continentality. In contrast, floodplain ecosystems comprise species with European or Mediterranean distributions, adapted to lower levels of continentality. These features have certain ecological parallels. Existence on sandy hills with sparse herbaceous cover, caused by moisture deficit and nutrient limitation, is accompanied by substantial climatic fluctuations within a single day and over the growing season. Therefore, species preadapted to higher levels of continentality gain a competitive advantage under such conditions.
Continentality is a macro-scale indicator and, in principle, should not vary systematically within a single locality. Therefore, the patterns we detected reflect biogeographic filtering rather than microclimate: species pools adapted to higher continentality dominate the sandy uplands (Eurasian elements), whereas floodplain sites draw more from European–Mediterranean floras adapted to lower continentality. In other words, the ‘local’ signal arises from community composition aligned with the main edaphic gradient (dry, nutrient-poor, open dunes versus moister, richer, denser vegetation), rather than from site-level climate.
4.4. Indicators of Anthropogenic Impact: Hemeroby and Naturalness
It should be noted that the identified composite environmental gradient also correlates with the level of hemeroby and the naturalness of plant communities. This relationship is likely to have geographical causes. Spatially, the floodplain ecosystems are adjacent to the reserve’s ruderal areas. Thus, the floodplain ecosystems function as a buffer zone, protecting the psammophytic steppe at the reserve’s centre. Consequently, a pattern emerges whereby the more humid and nutrient-rich ecosystems are somewhat more hemerobic, whereas ecosystems with opposite characteristics tend to be more natural. This spatial configuration, illustrated in
Figure 1, highlights the functional zonation of the reserve, where peripheral floodplain habitats with higher human influence protect the core areas of natural psammophytic vegetation. In this context, the first principal component represents a composite ecological gradient reflecting the coordinated spatial and temporal dynamics of a set of factors perceived as “primary.” The collection of factors defining the first principal component allows us to interpret it as a gradient prioritising natural drivers influencing vegetation cover. By contrast, the second principal component highlights the predominance of factors associated with the anthropogenic transformation of plant communities. However, it should be emphasised that anthropogenic transformation is not “refined” but represents a correlated complex of variability, including natural factors. Increasing hemeroby is associated with higher light availability, which can be explained by the reduced density of the herbaceous layer in response to anthropogenic transformation. Conversely, greater soil water availability can be regarded as a factor supporting the naturalness of plant communities. In other words, it can be assumed that plant communities in arid conditions are more sensitive to anthropogenic impacts.
Inter-correlations between indicator values are discussed as a potential source of bias in the results of bioindication [
17,
107]. We regard this phenomenon not as an artefact that undermines the method’s accuracy, but as a regular consequence of the integrated response of biosystems to the combined effects of multiple individual environmental factors. The dimensionality of the space of environmental factors is considerably greater than the potential repertoire of biosystem responses. Consequently, the dimensionality of the reaction space is reduced, which explains the observed correlations between the indicator values. Phytoindication assessments of environmental factors reveal two principal trends of variability, which represent synthetic (composite) ecological gradients that are difficult to interpret in terms of any single primary factor. The composition of primary factors contributing to a given synthetic gradient varies regionally depending on landscape structure, making each combination unique to the specific territory studied. The predominant trend of environmental variability corresponds to the significant gradient of natural conditions within the study area, accounting for 67% of the variation in ecological regimes (principal component 1). The secondary trend of variability reflects the impact of anthropogenic transformation of the vegetation cover, accounting for 15% of the variation in plant communities. This ratio underscores the respective influences of natural and anthropogenic variability in shaping the vegetation cover conditions in the study area. The variability of habitat conditions (assessed via principal component analysis of phytoindication estimates) influences the variability of vegetation structure (evaluated by the contribution of these principal components to explaining community structure, after controlling for the effect of association types included as conditional variables). This comparison yields two significant findings. First, the coordinated dynamics of environmental factors (82%) explained only 10.5% of the inertia of community composition. Second, the contribution of the minor component (hemeroby/naturalness gradient) was greater in explaining the variability of vegetation structure than that of natural factors (5.3% versus 5.2%, respectively). It is evident that the correspondence of plant communities to natural conditions has developed over an extended period of vegetation history and is reflected in the structure of vegetation types. Indeed, the explanatory power of plant associations (13.2%) exceeded that of environmental factors. It should be emphasised that plant associations and higher-rank syntaxa differ in the specific ecological conditions to which they are adapted. Thus, the contribution of natural environmental factors to vegetation variability is closely correlated with plant associations.
4.5. Association Specificity: Natural Drivers vs. Hemeroby
A plant association can be understood within two principal paradigms of ecology. On the one hand, according to the structuralist paradigm, it results from interspecific interactions among plants sharing similar ecological preferences. During succession, the environment transforms, causing plants within an association to exhibit comparable ecological preferences: «The vegetation is both a cause and a product of succession; it alters the habitat and the altered habitat, in turn, determines the vegetation» [
108]. On the other hand, the continuationism paradigm interprets a plant association as the outcome of the spatial co-occurrence of ecologically similar species along environmental gradients: «Every species of plant is a law unto itself, the distribution of which in space depends upon its individual peculiarities of migration and environmental requirement» [
109]. Both paradigms assume the ecological similarity of species within a plant association concerning their preferences for environmental conditions. Therefore, a plant association can capture a substantial proportion of the explanatory power of phytoindication assessments of environmental factors.
An important finding of our study is that the gradients of naturalness and hemeroby are less specific to plant associations than natural environmental gradients, and consequently, plant associations account for a smaller proportion of their explanatory power. This observation supports the notion that, compared with natural drivers, anthropogenic factors operate over much shorter timescales [
110]. As a result, plant communities in regionally natural environments have not developed response patterns that are specific at the level of plant associations. This is likely because anthropogenic pressure is an evolutionarily recent factor, to which neither species nor communities have developed specialised adaptations. Furthermore, the sources of anthropogenic influence are not formally correlated with the spatial distribution of plant associations [
111], meaning that any association may be subject to such impacts. It should be noted that the structuralist paradigm is more effective in explaining this pattern, since anthropogenic disturbance can be regarded as a factor that returns a plant community to earlier stages of successional dynamics, which, by definition, are less specific. In turn, hemeroby can be interpreted as a gradient of anthropogenic influence on plant communities and increasing disturbance that drives communities back towards earlier and less specific stages of successional dynamics.
4.6. Shelford’s Law and the Fate of “Primary” Drivers
But what is the role of the “primary factors,” which are classically well known and intuitively clear to us, and considered universal for comparing ecosystems and landscape ecological structures? The synthetic nature of prevailing environmental gradients cannot be a basis for comparing different landscape systems due to differences in their comparative frameworks. Synthetic factors may be viewed as tools for identifying the specificity of regional conditions. Still, they cannot be used to compare different landscape systems, since only comparable entities can be compared. “Primary” factors, as independent drivers, exist as environmental conditions, but their independence disappears when we consider the consequences of their action. This disappearance of the specific effects of ecological factors is a consequence of Shelford’s law of tolerance. When species occur within their ecological optimum, their sensitivity to variation in environmental factors (which remain within optimal values for that species) is virtually absent. Thus, the specificity of the action of ecological factors simultaneously operating at optimal levels for a species disappears. In fact, the prevailing gradient of environmental conditions represents such a synthetic construct. The pure effect of a given factor can manifest itself when its values deviate from the optimum, falling into excess or deficit. Is the complete correspondence of species and their communities to the prevailing synthetic gradient of environmental conditions, therefore, a form of apparent insensitivity to the environment? The “pure” effects of ecological factors, when controlling for the covariation of other factors and the affiliation of communities to specific associations, were statistically significant. However, they explained only a small fraction of community inertia. The pure influence of factors has a pronounced fine-scale spatial component. In contrast, the action of “primary” factors in the context of synthetic factors operates at broad or meso-scale spatial levels. Thus, local mismatches between the ecological optima of plant species and the prevailing environmental conditions may account for the specific manifestation of the effects of primary ecological factors.
4.7. Implementation of Phytoindication: Conservation, Global Change, and Anthropogenic Pressure
The findings of this study have significant implications for both ecological theory and conservation practice. The identified integration of natural and anthropogenic gradients demonstrates that, even within formally protected areas, vegetation structure and function continue to reflect the legacy and ongoing influence of human activities. This highlights the importance of adopting a dynamic perspective on conservation landscapes, recognising that they function as open socio-ecological systems rather than isolated refuges. Phytoindication offers a robust framework for assessing ecosystem integrity under such conditions. By capturing the integrated response of plant communities to multiple environmental drivers, it enables the detection of subtle degradation processes that may not be apparent through conventional monitoring methods based solely on species richness or instrumental measurements. The capacity to quantify hemeroby and naturalness gradients within protected areas allows conservation managers to evaluate the functional effectiveness of buffer zones, identify areas of ecological vulnerability, and prioritise restoration efforts.
From a broader perspective, the combined use of phytoindication and remote sensing can support large-scale ecological assessments across regions and time periods. This integration facilitates the early detection of shifts in vegetation functioning driven by climate change, land-use change, or other anthropogenic pressures. Such a multi-level approach aligns with the principles of ecosystem-based management and nature-based solutions, thereby contributing to the implementation of the Convention on Biological Diversity and the European Union’s Biodiversity Strategy for 2030. Ultimately, the study reinforces that vegetation responses observed through phytoindication serve as ecological diagnostics and indicators of ecosystem resilience. Understanding these patterns can guide adaptive management, restoration planning, and the long-term monitoring of biodiversity in the context of global environmental change.
4.8. Methodological Constraints on Phytoindication
Despite its conceptual advantages, phytoindication faces several methodological limitations. The strong correlations observed among ecological indicator scales restrict the comparability of their absolute values across different regions or time periods. These interdependencies suggest that indicator systems capture integrated biotic responses rather than isolated environmental factors. Consequently, interpreting phytoindication results requires an explicit examination of the correlation structure among indicators, supported by ordination or variance-partitioning analyses. From this perspective, the “simplicity” often attributed to the method is somewhat illusory, as its reliable application demands a critical evaluation of multicollinearity, gradient length, and the contextual specificity of ecological responses.