Abstract
This study presents the first comprehensive IUCN-based assessment for all 88 Limonium species occurring in Greece, aiming to close a critical conservation gap for this highly diverse and important genus in the country. To identify patterns of extinction risk, we applied the IUCN Red List Categories and Criteria, integrating data on endemism, ploidy, and anthropogenic threats. Moreover, we employed spatial analysis to identify conservation hotspots, and we statistically analyzed how threat status changes across geographic space. Our results show that 51 species (58.0%) are threatened, with endemics (62.3%) exhibiting a significantly higher risk than non-endemics. A greater proportion of diploid species were also found to be threatened compared to their polyploid counterparts. Longitude was identified as a key spatial predictor of threat, with risk concentrated in southern and western coastal zones. The most prevalent threats are coastal development (56.9% of threatened species) and invasive species (33.3%). This work provides a vital baseline for Limonium conservation, highlighting the urgent need for a dual conservation strategy that combines efficient in situ actions with ex situ measures for the most imperiled species.
1. Introduction
The Mediterranean Basin, recognized as a global biodiversity hotspot [1], supports exceptional species richness, including numerous narrowly endemic taxa increasingly threatened by anthropogenic activities, habitat degradation, and climate change [2,3]. The genus Limonium exemplifies this pattern, with its highest diversity occurring within the Mediterranean region. Many of its species are local endemics, highly adapted to the harsh environmental conditions of coastal habitats such as rocky shores, sandy beaches, and salt marshes [4,5]. Their specialized physiological traits, coupled with often limited dispersal capabilities, render them particularly vulnerable to escalating threats such as habitat loss, urbanization, and salinization, underscoring the need for targeted conservation efforts [6,7].
Despite comprising approximately 650 species worldwide [8] and playing a significant ecological role in coastal ecosystems, Limonium remains underrepresented in global conservation assessments. This is evidenced by the fact that only 30 species have been evaluated by the IUCN to date [9], with two-thirds (20) of them classified as threatened. This disproportionate representation highlights a major conservation gap for a genus characterized by high levels of diversity and endemism.
Greece represents one of the most significant centers of Limonium diversity in the Mediterranean, currently hosting 88 accepted species [10,11,12,13,14], ranking second only to Spain [4]. Notably, 77 of these species are endemic to Greece, corresponding to an exceptionally high endemism rate of approximately 88%, with the majority exhibiting highly restricted distributions and small population sizes, factors that heighten their extinction risk [13]. Ploidy levels are also essential to consider, as polyploidy may influence species’ adaptability, ecological amplitude, and distribution range, thereby playing a crucial role in shaping extinction risk and informing conservation strategies [15].
The International Union for Conservation of Nature (IUCN) Red List system, established in 1964, serves as the globally recognized standard for evaluating species’ extinction risk [16]. This comprehensive framework assesses factors such as population size, geographic distribution, and the severity of threats to both species and their habitats, providing essential data for conservation prioritization [17]. However, despite its global importance, only about 17% of all described plant species have been assessed to date, revealing a significant gap in plant conservation. Of the 380,221 described plant species [18], only 73,558 have been evaluated, with 28,114 classified as threatened [9].
While the global Red List offers overarching insights into extinction risk, national Red Lists provide vital information for regional conservation planning. In Greece, previous relevant assessments have identified 14 Limonium species as threatened [19,20], including 13 species endemic to the country and Limonium glomeratum (formerly referred to as L. densiflorum in Phitos et al. [19]), an endemic to the broader Mediterranean region. These threatened species show distinct regional distribution patterns: the Peloponnese hosts four of them (L. aphroditae, L. cythereum, L. kardamylii, L. messeniacum), Crete supports five (L. calliopsium, L. cornarianum, L. creticum, L. cythereum, L. elaphonisicum), while another four are restricted to the Ionian Islands (L. damboldtianum, L. ithacense, L. phitosianum, L. zacynthium). Limonium corinthiacum is confined to Sterea Ellas [19,20]. Notably, L. cythereum is the only species recorded in both the Peloponnese and Crete [11], highlighting patterns of regional endemism within the genus and the need for geographically tailored conservation strategies.
As part of the project “Compilation of the Red List of Threatened Species of Plants of Greece”, coordinated by the Natural Environment and Climate Change Agency (NECCA, Greece) in collaboration with the IUCN, we conducted a complete assessment of the conservation status of all Limonium species in Greece. Building on this work, the present study investigates the relationship between conservation status and key biological and ecological traits of Greek Limonium taxa. By analyzing an extensive dataset, including information on endemism, geographic distribution, population size, threat categories, and ploidy levels, we aim to identify patterns of extinction risk and species vulnerability. Specifically, our study seeks to: (i) compare the conservation status of endemic versus non-endemic species, (ii) analyze the relationship between conservation status and ploidy levels, (iii) assess the impact of various threat factors on Limonium species, (iv) examine geographic variations in conservation status and threats across Greek regions, and (v) propose targeted conservation strategies customized to the needs of Greek Limonium species.
2. Materials and Methods
2.1. Source of Data
Distribution data were compiled from extensive field surveys covering 49% of the studied species (43 out of 88 Greek Limonium species), published records (e.g., floristic accounts, monographic treatments, and specialized journal articles), taxonomic databases (e.g., Global Biodiversity Information Facility [21], Euro+Med PlantBase, PhytoBank), and records from public agencies such as the Management Units of Protected Areas under the Natural Environment and Climate Change Agency (NECCA). The Extent of Occurrence (EOO) and Area of Occupancy (AOO) were calculated using the GeoCAT online tool [22], applying a 2 × 2 km grid cell size in line with IUCN recommendations. Geographic coordinates were analyzed in GeoCAT to determine the spatial extent of the georeferenced data. The use of GeoCAT, an open-source tool developed by Bachman et al. [23], ensured standardized and reproducible calculations of EOO and AOO.
Data on population size and habitat types were collected through field surveys and an extensive review of the literature, although published population estimates remain limited. Conservation measures, encompassing legal protection status, in situ management efforts, and ex situ conservation programs, were also documented. Direct threats to habitats and individuals were recorded through in situ observations and literature sources, with threats classified according to the IUCN Threats Classification Scheme [24]. Ploidy levels were determined from existing scientific literature [10,11,12,13,14], to correlate with species vulnerability and distribution patterns.
2.2. Implementation of the IUCN Criteria and Categories
To assess the conservation status of each endemic taxon, we applied the IUCN Red List Categories and Criteria (version 3.1) following the global application guidelines [17]. For non-endemic taxa, national assessments followed adapted IUCN Red List Criteria to reflect regional contexts [25]. Species were assigned to standard categories: Extinct (EX), Extinct in the Wild (EW), Critically Endangered (CR), Endangered (EN), Vulnerable (VU), Near Threatened (NT), Least Concern (LC), and Data Deficient (DD) (Table A1). For this study, species falling under the categories CR, EN, or VU are collectively referred to as “threatened”. According to the IUCN methodology, classification was based on five quantitative criteria: (A) reduction in population size over time, (B) geographic range size and fragmentation, decline or fluctuations, (C) small population size and ongoing decline, (D) very small or restricted populations, and (E) quantitative analysis of extinction risk. The application of these criteria provided a robust framework for assessing species conservation status and prioritizing conservation actions.
All collected data, including distribution, population size and mature individuals, habitat and ecology, threats, utilization, and conservation measures, were entered into the IUCN Species Information Service (SIS) database. Red List assessments were conducted through SIS, which automatically determines a preliminary category based on the input data. This categorization was then subject to expert review and confirmation to ensure accuracy and contextual relevance. The terminology used, including mature individual, population, subpopulation, population size, and location, follows IUCN [9]. All data analyses, including conservation status breakdowns, spatial analyses, and visualizations, were carried out in RStudio 2025.05.1+513 [26] within an R environment [27].
2.3. Spatial and Statistical Analysis
To analyze the spatial distribution of threatened Limonium species and identify areas of concentrated extinction risk, a combined dataset of species’ populations occurrences was constructed. For each occurrence point, geographic coordinates were recorded, along with the species’ IUCN threat status. The three-tiered threat status was numerically weighted from lowest to highest risk: 1 for VU, 2 for EN, and 3 for CR.
To investigate spatial patterns in extinction risk among threatened Limonium species, an ordinal regression model was employed. This generalized linear model was used to predict how the cumulative probability of a species’ assignment to a higher threat category changes across geographic space [28]. The model’s response variable was the three-tiered IUCN threat status, ordered from lowest to highest risk: VU, EN, and CR. Geographic coordinates (latitude and longitude) served as the sole predictor variables. The objective of this analysis was to identify significant spatial patterns in threat.
Second, a Kernel Density Estimation (KDE) analysis was conducted in ArcMap 10.4 (Esri Inc., Redlands, CA, USA), using the Spatial Analyst Toolbox under the WGS 1984 coordinate system. This non-parametric spatial technique was used to estimate the probability density function of spatial point patterns, with species occurrence points weighted according to their assigned numerical threat status [29,30]. The output cell size was set between 500 and 1000 m, and a fixed search radius ranging from 1 to 5 km was used, reflecting the spatial resolution of available data and the restricted distribution of most Limonium species. The resulting raster surface provides a continuous estimate of threat-weighted species density, allowing the identification of potential conservation hotspots. This approach follows established applications of KDE in conservation planning (e.g., [30]).
3. Results
3.1. Overall Conservation Status
The assessment of all 88 Limonium species occurring in Greece determined their current IUCN conservation status (Figure 1). Of the assessed taxa, 6 species (6.8%) were classified as CR, 18 species (20.5%) as EN, and 27 species (30.7%) as VU. Overall, 51 species (58.0%) of Greek Limonium are currently categorized as threatened (CR, EN, or VU). An additional 4 species (4.5%) were assessed as NT, while 33 species (37.5%) were classified as LC. No species were found to be EX, EW, or DD in this assessment.
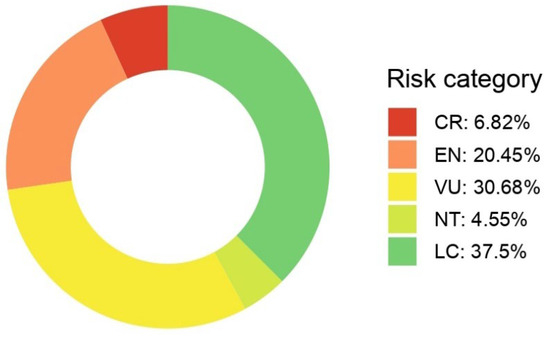
Figure 1.
The distribution of IUCN Red List threat categories for all 88 Limonium species assessed in Greece. CR: Critically Endangered; EN: Endangered; VU: Vulnerable; NT: Near Threatened; LC: Least Concern.
3.2. IUCN Criteria Applied
An analysis of the IUCN criteria used to assess the 51 threatened Limonium species revealed the primary factors driving their classification. The most frequently applied criterion was Criterion B (geographic range size and fragmentation), which was met by 30 species (58.8%). Criterion D (very small or restricted populations) was met by 22 species (43.1%), whereas Criterion C (small population size and ongoing decline) was met by 2 species (3.9%). No threatened species were assessed under Criterion A (reduction in population size) or Criterion E (quantitative analysis of extinction risk).
3.3. Conservation Status of Endemic vs. Non-Endemic Species
The assessment of Greek Limonium species, based on their endemism status, revealed distinct patterns of conservation risk (Figure 2). Among the 77 endemic species, 5 (6.5%) were classified as CR, 17 (22.1%) as EN, and 26 (33.8%) as VU. In total, 48 endemic species (62.3%) are currently considered as threatened (CR, EN, or VU). An additional 4 (5.2%) were assessed as NT, while 25 (32.5%) were classified as LC.
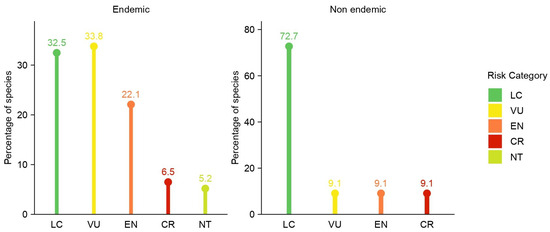
Figure 2.
The distribution of IUCN Red List categories for endemic (left) and non-endemic (right) Limonium species in Greece. CR: Critically Endangered; EN: Endangered; VU: Vulnerable; NT: Near Threatened; LC: Least Concern.
In contrast, among the 11 non-endemic species, 1 (9.1%) was classified as CR, 1 (9.1%) as EN, and 1 (9.1%) as VU, totaling 3 species (27.3%) categorized as threatened. None of the non-endemic species were assessed as NT, while the remaining 8 (72.7%) were classified as LC.
3.4. Conservation Status and Ploidy Levels
Ploidy level was also considered in the assessment of the Limonium species, revealing notable differences in conservation status between polyploid and diploid taxa (Figure 3). Among the 67 polyploid species, 5 (7.5%) were classified as CR, 13 (19.4%) as EN, and 19 (28.3%) as VU. In total, 37 polyploid species (55.2%) were categorized as threatened. One additional polyploid species (1.5%) was assessed as NT, while 29 species (43.3%) were categorized as LC.
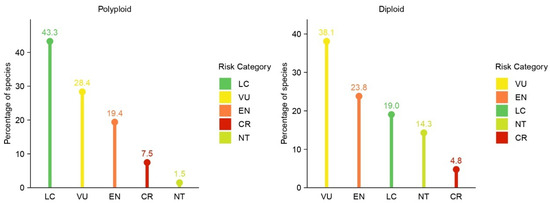
Figure 3.
The distribution of IUCN Red List categories for polyploid (left) and diploid (right) Limonium species in Greece. CR: Critically Endangered; EN: Endangered; VU: Vulnerable; NT: Near Threatened; LC: Least Concern.
In comparison, the 21 diploid species exhibited a slightly higher overall proportion of threatened taxa, with 1 species (4.8%) classified as CR, 5 (23.8%) as EN, and 8 (38.1%) as VU. Altogether, 14 diploid species (66.7%) were categorized as threatened, 3 (14.3%) as NT, and 4 (19%) as LC.
3.5. Main Threats to Threatened Species
The analysis of threats affecting the 51 Greek Limonium species categorized as threatened showed distinct and consistent patterns (Figure 4). The most prevalent threat was Residential and Commercial Development, impacting 29 species (56.9%), often observed directly along the coastal Limonium habitats (Figure 5). Invasive Species represented the second most common threat, affecting 17 species (33.3%), with mat-forming species of the Aizoaceae family contributing to habitat degradation and competition (Figure 5). Human Intrusions and Disturbance impacted 9 species (17.6%). Additional threats included Pollution (6 species; 11.8%) and Natural System Modifications (5 species; 9.8%). The least frequent threat was Transportation and Service Corridors, recorded for only 2 species (3.9%).
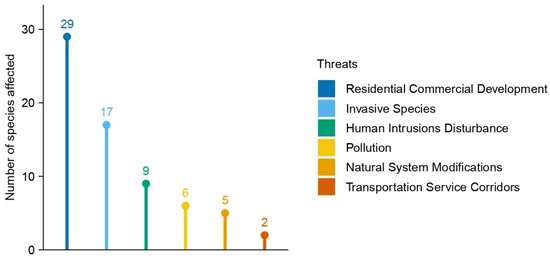
Figure 4.
The distribution of the main threats impacting Limonium species in Greece. The numbers reflect the total count of threatened species affected by each threat category.

Figure 5.
Visual evidence of the primary anthropogenic threats impacting Limonium habitats. (Left): Residential and Commercial Development often coincides with the spread of invasive, mat-forming Aizoaceae species, leading to habitat loss and fragmentation. (Right): Close-up view of the proliferation of invasive Carpobrotus edulis actively outcompeting native coastal vegetation.
3.6. Spatial Patterns of Conservation Priority in Limonium
The ordinal regression analysis confirmed that longitude was the only statistically significant predictor of threat category (Estimate: −0.136, p = 0.0238). Latitude, with a p-value of 0.6389, was not found to be a significant predictor of extinction risk in this model. The negative estimate for longitude indicates that an increase in longitude (moving eastward) is associated with a lower probability of a species being in a higher threat category, a trend visually supported by the model plot (Figure 6).
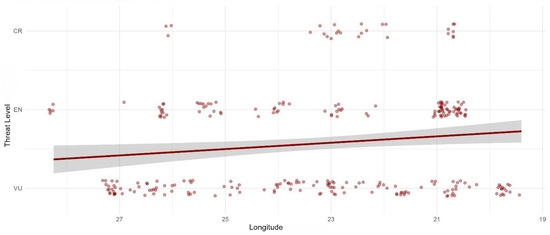
Figure 6.
The threat level of Limonium species in Greece as a function of longitude. The plot illustrates the negative correlation between longitude and threat level, with each point representing a single population occurrence.
The Kernel Density Estimation (KDE) analysis of threatened Limonium species in Greece (Figure 7) revealed distinct spatial patterns of extinction risk. Areas of elevated conservation priority were primarily concentrated along the southern and western coasts of the Peloponnese, in parts of western Crete, and on select islands of the southern and central Aegean. In particular, regions in the Ionian Islands and the southwestern Peloponnese emerged as hotspots, exhibiting the highest threat-weighted species densities.
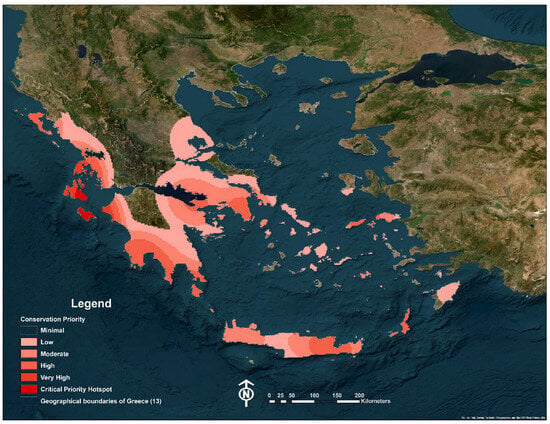
Figure 7.
Conservation priority density map of Limonium species (Kernel density weighted by IUCN threat category: CR = 3, EN = 2, VU = 1).
3.7. Ex Situ and In Situ Conservation Actions
An analysis of global collections reveals that 37 Greek Limonium species (42%) are represented in international and national ex situ collections, including botanical gardens and seed banks [31,32,33,34,35]. Of these, 29 are endemic taxa (37.7% of all Greek endemics). Although this represents significant progress, conservation efforts remain fragmented.
More specifically in Greece, the Seed Bank of the National and Kapodistrian University of Athens (NKUA) holds two collections of L. creticum and one collection of L. sinuatum. The Seed Bank of the Mediterranean Agronomic Institute of Chania (MAICH) holds collections of 10 species, including 12 collections of L. cornarianum, 6 of L. creticum, 3 of L. elaphonisicum, 3 of L. sougiae, and single collections of L. calliopsium, L. hierapetrae, L. grabusae, L. stenotatum, and L. sinuatum. Knowledge transfer protocols remain limited, though germination protocols exist for all species stored in the MAICH Seed Bank [36,37]. The Institute of Plant Breeding and Genetic Resources of the Hellenic Agricultural Organization DEMETER maintains a broader collection of 37 Limonium species, represented by 87 unique accession numbers. Within this collection, L. hierapetrae is represented by ten accessions, and L. sieberi by six. Three species, namely L. cornarianum, L. creticum and L. recticaule, are each represented by five accessions, while L. aegaeum, L. artelariae and L. stenotatum are represented by four. Five species (L. calliopsium, L. chersonesum, L. damboldtianum, L. elaphonisicum and L. sitiacum) are represented by three accessions each. Another five species are represented by two accessions, namely L. fragile, L. frederici, L. proliferum, L. sinuatum and L. sougiae. Finally, 18 species are represented by a single accession each, namely L. ammophilon, L. antipaxorum, L. arcuatum, L. compactum, L. crateriforme, L. cythereum, L. echioides, L. glomeratum, L. grabusae, L. korakonisicum, L. narbonense, L. ocymifolium, L. oligotrichum, L. phitosianum, L. roridum, L. virgatum, L. xerocamposicum and L. zacynthium [35]. In addition, an asexual propagation protocol was published for L. chersonesum [38].
Considering the evaluation of in situ conservation effectiveness, a distributional check of the 51 threatened Limonium species against the Greek Natura 2000 network was performed. This analysis reveals varying degrees of protection (see Supplementary Table S1). Specifically, 9 species are distributed exclusively inside Natura 2000 areas, while 17 species have populations both inside and outside these protected areas. The rest 25 species (49.0% of all threatened taxa) are located entirely outside of the Natura 2000 network. It is important to note that since no Limonium species is listed in the annexes of the Habitats Directive (Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora), their presence within a Natura 2000 site does not guarantee species-specific legal protection under European law.
In parallel, publicly available knowledge regarding past and ongoing in situ conservation actions remains limited, primarily consisting of fragmentary information found in gray literature (e.g., conference papers, dissertations, and project deliverables). Documented actions include the eradication of invasive species like Carpobrotus edulis, population reinforcement using ex situ-grown seedlings, and the installation of light fencing to protect threatened Cretan endemics such as Limonium elaphonisicum and L. creticum [39]. Moreover, as a first step in management and protection, the Management Unit of the Protected Areas of the Ionian Islands installed signs to inform the public about rare and threatened Limonium species (L. korakonisicum, L. phitosianum, and L. zacynthium), emphasizing that uprooting them is prohibited [40].
4. Discussion
This study presents the first comprehensive, IUCN-based assessment of the conservation status of all 88 Limonium species in Greece, a recognized center of diversity for the genus. The results underscore the urgent conservation priority of Greek Limonium, with 51 species (58.0%) classified as threatened. This proportion is substantially higher than the global average of approximately 38% for assessed plant species [9], highlighting the heightened vulnerability of Limonium within the biodiversity hotspot of Greece. Our assessment has led to a significant increase in the total number of Limonium species evaluated by the IUCN, with the addition of 71 Greek endemics bringing the total from 30 to 101 species. This contribution directly addresses the conservation gap identified in the introduction for a genus characterized by high levels of diversity and endemism.
The necessity of periodic IUCN reassessment is confirmed by observing the changes in the conservation status for the 14 Limonium species previously evaluated in national Red Data Books [19,20]. Our comparison reveals a clear acceleration in extinction risk for several taxa (Appendix A, Table A1). Specifically, comparing the 2009 assessment [20] to our current findings, three taxa were uplisted to a higher threat category, including L. aphroditae (from EN to CR) and L. cornarianum (from VU to CR). Furthermore, species categorized as “Rare” in the 1995 assessment [19] have now been formally classified as Endangered (EN), validating a substantial elevation of their risk status based on current IUCN criteria. This direct comparison highlights the critical value of recurring assessments in tracking conservation deterioration and prioritizing resources.
A key finding of our study is the noticeable difference in extinction risk between endemic and non-endemic taxa. Greek endemics exhibit a considerably higher proportion of threatened species (62.3%) compared to non-endemic taxa (27.3%). This pattern aligns with well-established ecological principles: species with restricted geographic distributions and small population sizes, common among narrow endemics, are inherently more vulnerable to extinction drivers [41]. The high degree of regional localization observed in areas such as the Peloponnese, Crete, and the Ionian Islands further emphasizes the need for spatially explicit conservation strategies.
The relationship between ploidy levels and conservation status also revealed distinct patterns. While polyploid species constitute the majority of Greek Limonium (67 species), diploid species show a higher proportion of threatened taxa (66.7% of diploids) compared to polyploids (55.2% of polyploids). These results suggest that diploid Limonium species, despite being fewer in number, exhibit a greater conservation concern compared to their polyploid counterparts, potentially reflecting narrower distribution or greater ecological sensitivity. Further investigation is warranted into the ecological and genetic factors underlying these differences, as polyploidy can influence adaptive capacity and range dynamics [42].
The assessment criteria applied in this study also shed light on the main factors contributing to the extinction risk for Greek Limonium. A large proportion of species were assessed under Criterion B (58.8%) and Criterion D (43.1%), underlining that limited distribution and small population size are the dominant causes of vulnerability. In contrast, no species were evaluated under Criterion A (population decline) or Criterion E (quantitative extinction risk), which indicates a major gap in demographic and long-term monitoring data. This overreliance on spatial parameters rather than population-based metrics reveals a fundamental limitation in current conservation assessments. Nevertheless, recent efforts have begun to address this gap. A Population Viability Analysis (PVA) was recently applied to three Limonium endemics on Zakynthos Island, providing insights into extinction probabilities and demographic sensitivity under various threat scenarios [40].
The ordinal regression analysis, which identified longitude as the only statistically significant predictor, provides a powerful quantitative confirmation of the spatial patterns observed. While latitude was not found to be a statistically significant predictor, this limitation in the model’s output is consistent with the genus’s distribution: the northern parts of Greece contain few Limonium species, and those that do appear (e.g., L. narbonense, L. virgatum) are common and classified as Least Concern. Consequently, the model was constrained from detecting a strong latitudinal gradient because threatened populations are absent from the northern range of the genus. The model’s negative estimate for longitude suggests that extinction risk for Limonium is not randomly distributed across Greece but rather increases significantly as one moves westward. This finding aligns with the KDE analysis, which visually pinpointed specific coastal zones in the Ionian Islands and southwestern Peloponnese as conservation hotspots for the genus. These spatial clusters emphasize the localized nature of extinction risk for Greek Limonium and target specific coastal zones that warrant immediate conservation attention.
Our threat analysis for the 51 threatened Limonium species highlights direct anthropogenic pressures as principal drivers of decline. Residential and Commercial Development emerged as the most pervasive threat, impacting over half (56.9%) of these species. This is consistent with the coastal habitat preference of many Limonium taxa, where urbanization and expansion of tourist infrastructure cause habitat loss and fragmentation [6,7]. The significant impact of Invasive Species (33.3%) and Human Intrusions and Disturbance (17.6%) also contribute substantially to habitat degradation [6,41].
These external pressures are compounded by the species’ inherent biological vulnerabilities. Experimental studies on Mediterranean endemics such as Limonium mansanetianum, for instance, show that physiological traits and reproductive biology play a vital role in their survival. Its seed germination is severely inhibited by elevated salinity levels, which further underscores the importance of integrating in situ population reinforcement with ex situ conservation strategies [2].
The high proportion of threatened Greek Limonium species, combined with the dominant anthropogenic pressures, underlines the need for targeted conservation actions. Key priorities should include stricter enforcement of coastal land-use regulations, the establishment and expansion of protected areas, and the implementation of effective management strategies for invasive species. Given the high regional endemism of many taxa, micro-reserves and locally focused conservation initiatives could prove particularly effective [43]. The Plant Micro-Reserve (PMR) system, successfully implemented in the Valencian Community of Spain, represents a suitable approach for conserving highly localized and endangered Limonium species in areas affected by habitat fragmentation [6,43]. In Greece, PMRs have already been deployed in Crete, the Peloponnese and the East Aegean Islands, leading to the development of successful monitoring and conservation actions for several threatened local and regional endemics [39,44,45].
Among the threatened Greek endemics, those assessed as CR, each limited to a single, isolated population with very few mature individuals, as confirmed by our field surveys, require urgent conservation attention (Figure 8):

Figure 8.
Photographic composite of the four Critically Endangered (CR) Limonium species in their natural coastal habitats, presented clockwise from the top left: Limonium korakonisicum, Limonium nichoriense, Limonium albomarginatum and Limonium aphroditae.
- Limonium korakonisicum. Monitoring data from Zakynthos (2014–2018) confirm that L. korakonisicum consists of a single, small population in Korakonisi (Zakynthos, Ionian Islands), with low seedling survival and limited genetic diversity [39]. A dual strategy is therefore recommended: in situ reinforcement of the existing population, coupled with ex situ actions such as seed banking, cultivation trials, and the establishment of safety neopopulations, following Laguna and Ferrer-Gallego [46].
- Limonium nichoriense. This species is endemic to a single coastal site near Petalidi (Messinia, Peloponnese), with fewer than 50 mature individuals [10]. Major threats include habitat degradation and the spread of invasive species (Carpobrotus edulis, Oxalis pes-caprae). Priority actions include manual removal of invasives, population monitoring, and ex situ safeguarding in line with Mediterranean translocation standards [3].
- Limonium albomarginatum. This Greek narrow-endemic species is restricted to a single location on the Mani Peninsula in the southern Peloponnese, with a small population of approximately 500 mature individuals [47]. The primary threat it faces is the expansion of invasive species, Carpobrotus edulis and Opuntia ficus-indica. As no conservation measures are currently in place, a dual strategy is recommended: controlling invasives in the habitat and implementing ex situ measures such as seed banking and living collections. Further research is needed to better understand its ecology and population dynamics, as it is considered a high-conservation-priority taxon [13].
- Limonium aphroditae. This is a narrow-endemic Greek species confined to a single population in Limnaria Bay on the island of Kythira [13]. Recent fieldwork (2023) documented a severe population decline, with the number of mature individuals dropping from approximately 250–300 in 2009 to fewer than 50. This species faces threats from habitat degradation due to pollution, which is likely caused by rainwater runoff from surrounding areas. Given the species’ critically small population size, it is essential to prioritize ex situ conservation efforts, such as establishing a seed bank and living collections, to secure its survival [48].
For such highly imperiled species at imminent risk of extinction, a combined conservation approach is required, including immediate in situ actions complemented by proactive ex situ measures such as seed banking, living collections, and the establishment of new populations [49], drawing on successful experiences from other Mediterranean Limonium species (e.g., [50]). Mitigating the present paucity of available information about the ex situ conservation of these species, focused efforts should be dedicated to assessing and documenting best practices in horticulture and restoration actions through the use of carefully managed living plant collections [51,52,53]. Such actions are already ongoing for these four Critically Endangered endemic species, including the successful establishment and cultivation of living collections in facilities associated with the NKUA’s Botanical Garden (Figure 9), forming the starting material for future plant translocations.

Figure 9.
Living collections of four Critically Endangered Greek endemic Limonium species. (A) Inflorescence of ex situ grown Limonium albomarginatum, (B) Cultivated individuals of Limonium nichoriense, (C) Cultivated individuals of Limonium korakonisicum, (D) Flowering individuals of Limonium aphroditae in cultivation.
Finally, given the high vulnerability of Greek Limonium taxa and their pronounced regional endemism, combined with the fact that they are not included in the annexes of Directive 92/43/EEC and that only two of them (L. carpathum and L. frederici) are currently protected under Presidential Decree 67/1986, it is deemed imperative to update and amend the aforementioned Presidential Decree. This revision should ensure the inclusion of more threatened Limonium taxa, especially the Critically Endangered ones, among the species under formal legal protection, thereby providing the necessary legislative framework for their effective conservation.
5. Conclusions
This study closes a significant knowledge gap by presenting the first comprehensive IUCN-based assessment for all 88 Limonium species in Greece, revealing that 51 species (58.0%) are threatened. The results definitively establish that extinction risk is concentrated among Greek narrow endemics and is highly localized, increasing significantly toward the western coastal zones of the country. This risk is primarily clustered in the southern and western coastal regions identified as hotspots by the KDE analysis. The primary drivers of this threat are direct anthropogenic pressures, with Residential and Commercial Development being the most pervasive factor. The lack of data necessary for applying Criterion A and E highlights a need to shift conservation efforts toward demographic monitoring and quantitative population viability assessment. Ultimately, the findings underscore the imperative for immediate policy action, including updating national legislation (Presidential Decree 67/1986) to secure legal protection for all threatened Limonium taxa, coupled with deploying targeted, dual-approach in situ (e.g., micro-reserves) and ex situ (e.g., seed banking and planned translocations) conservation strategies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100726/s1, Table S1: Conservation status, criteria, and distributional overlap with the Natura 2000 Network for all threatened Greek Limonium species; Table S2: Complete assessment data matrix: conservation status, correlates, and detailed threat scores for all Greek Limonium species.
Author Contributions
Conceptualization, E.A. and A.-T.V.; Methodology, E.A., A.-T.V. and N.G.; Software, A.-T.V. and N.G.; Validation, E.A., A.-T.V., K.K., A.-E.B. and T.C.; Formal Analysis, N.G. and E.A.; Investigation, E.A., A.-T.V. and K.K.; Data Curation, E.A., N.G. and A.-E.B.; Writing—Original Draft Preparation, E.A., A.-T.V. and A.-E.B.; Writing—Review and Editing, T.C. and K.K.; Visualization, N.G.; Supervision, T.C.; Project Administration, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Environment and Climate Change Agency (NECCA, Greece), Project: Compilation of the Red List of Threatened Species of Plants of Greece (MIS code: 5094982).
Data Availability Statement
The data supporting the findings of this study are included within the article in Appendix A (Table A1) and its Supplementary Material (Tables S1 and S2).
Acknowledgments
The authors gratefully acknowledge C.A. Thanos (Seed Bank of the National and Kapodistrian University of Athens), C. Fournaraki (Seed Bank of the Mediterranean Agronomic Institute of Chania), and N. Krigas (Hellenic Agricultural Organization DEMETER, Institute of Plant Breeding and Genetic Resources, Balkan Botanic Garden of Kroussia) for kindly providing information on the Limonium collections conserved at the NKUA, MAICH, and Hellenic Agricultural Organization DEMETER Seed Banks, respectively.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AOO | Area of Occupancy |
| CR | Critically Endangered |
| DD | Data Deficient |
| EN | Endangered |
| EOO | Extent of Occurrence |
| EX | Extinct |
| EW | Extinct in the Wild |
| IUCN | International Union for Conservation of Nature |
| KDE | Kernel Density Estimation |
| LC | Least Concern |
| MAICH | Mediterranean Agronomic Institute of Chania |
| NECCA | Natural Environment and Climate Change Agency |
| NKUA | National and Kapodistrian University of Athens |
| NT | Near Threatened |
| PMR | Plant Micro-Reserve |
| PVA | Population Viability Analysis |
| VU | Vulnerable |
Appendix A

Table A1.
Revisions and additions to the list of Limonium species occurring in Greece, according to their IUCN categorization.
Table A1.
Revisions and additions to the list of Limonium species occurring in Greece, according to their IUCN categorization.
| S. No | Species | Endemic (Yes/No) | Presidential Decree | Red Data Book 1995 | Red Data Book 2009 | IUCN 2025 |
|---|---|---|---|---|---|---|
| 1 | Limonium artelariae | Yes | - | - | - | VU |
| 2 | Limonium aegaeum | Yes | - | - | - | LC |
| 3 | Limonium albomarginatum | Yes | - | - | - | CR |
| 4 | Limonium ammophilon | Yes | - | - | - | LC |
| 5 | Limonium amopicum | Yes | - | - | - | LC |
| 6 | Limonium antipaxorum | Yes | - | - | - | NT |
| 7 | Limonium aphroditae | Yes | - | - | EN | CR |
| 8 | Limonium archaeothirae | Yes | - | - | - | LC |
| 9 | Limonium arcuatum | Yes | - | - | - | VU |
| 10 | Limonium astypaleanum | Yes | - | - | - | VU |
| 11 | Limonium athinense | Yes | - | - | - | EN |
| 12 | Limonium atticum | Yes | - | - | - | LC |
| 13 | Limonium aucheri | No | - | - | - | LC |
| 14 | Limonium bellidifolium | No | - | - | - | VU |
| 15 | Limonium brevipetiolatum | Yes | - | - | - | NT |
| 16 | Limonium calliopsium | Yes | - | - | EN | EN |
| 17 | Limonium carpathum | Yes | Yes | - | - | VU |
| 18 | Limonium cephalonicum | Yes | - | - | - | NT |
| 19 | Limonium chersonesum | Yes | - | - | - | EN |
| 20 | Limonium compactum | Yes | - | - | - | LC |
| 21 | Limonium contractum | Yes | - | - | - | VU |
| 22 | Limonium corinthiacum | Yes | - | - | EN | VU |
| 23 | Limonium cornarianum | Yes | - | - | VU | CR |
| 24 | Limonium coronense | Yes | - | - | - | VU |
| 25 | Limonium crateriforme | Yes | - | - | - | LC |
| 26 | Limonium creticum | Yes | - | Rare | - | VU |
| 27 | Limonium cythereum | Yes | - | VU | VU | |
| 28 | Limonium damboldtianum | Yes | - | Rare | - | EN |
| 29 | Limonium doerfleri | Yes | - | - | - | LC |
| 30 | Limonium dolihiense | Yes | - | - | - | EN |
| 31 | Limonium echioides | No | - | - | - | LC |
| 32 | Limonium elaphonisicum | Yes | - | - | VU | VU |
| 33 | Limonium epiroticum | Yes | - | - | - | VU |
| 34 | Limonium fragile | Yes | - | - | - | VU |
| 35 | Limonium frederici | Yes | Yes | - | - | LC |
| 36 | Limonium glomeratum | No | - | Vulnerable | - | CR |
| 37 | Limonium grabusae | Yes | - | - | - | LC |
| 38 | Limonium graecum | No | - | - | - | LC |
| 39 | Limonium helenae | Yes | - | - | - | EN |
| 40 | Limonium heraionense | Yes | - | - | - | EN |
| 41 | Limonium hierapetrae | Yes | - | - | - | VU |
| 42 | Limonium hirsuticalyx | Yes | - | - | - | LC |
| 43 | Limonium ikaricum | Yes | - | - | - | EN |
| 44 | Limonium isidorum | Yes | - | - | - | VU |
| 45 | Limonium ithacense | Yes | - | Rare | - | EN |
| 46 | Limonium kardamylii | Yes | - | - | VU | EN |
| 47 | Limonium kirikosicum | Yes | - | - | - | EN |
| 48 | Limonium korakonisicum | Yes | - | - | - | CR |
| 49 | Limonium lobatum | No | - | - | - | EN |
| 50 | Limonium meandrinum | Yes | - | - | - | VU |
| 51 | Limonium messeniacum | Yes | - | - | EN | VU |
| 52 | Limonium microcycladicum | Yes | - | - | - | LC |
| 53 | Limonium monolithicum | Yes | - | - | - | LC |
| 54 | Limonium nichoriense | Yes | - | - | - | CR |
| 55 | Limonium narbonense | No | - | - | - | LC |
| 56 | Limonium ocymifolium | Yes | - | - | - | LC |
| 57 | Limonium ophioides | Yes | - | - | - | VU |
| 58 | Limonium oligotrichum | Yes | - | - | - | VU |
| 59 | Limonium pagasaeum | Yes | - | - | - | EN |
| 60 | Limonium palmare | Yes | - | - | - | LC |
| 61 | Limonium parosicum | Yes | - | - | - | EN |
| 62 | Limonium phitosianum | Yes | - | Rare | - | NT |
| 63 | Limonium pigadiense | Yes | - | - | - | LC |
| 64 | Limonium proliferum | Yes | - | - | - | LC |
| 65 | Limonium pusillum | Yes | - | - | - | VU |
| 66 | Limonium pylium | Yes | - | - | - | VU |
| 67 | Limonium quinnii | Yes | - | - | - | EN |
| 68 | Limonium recticaule | Yes | - | - | - | EN |
| 69 | Limonium roridum | No | - | - | - | LC |
| 70 | Limonium samium | Yes | - | - | - | VU |
| 71 | Limonium saracinatum | Yes | - | - | - | VU |
| 72 | Limonium sartorianum | Yes | - | - | - | VU |
| 73 | Limonium schinousae | Yes | - | - | - | LC |
| 74 | Limonium sieberi | No | - | - | - | LC |
| 75 | Limonium sinuatum | No | - | - | - | LC |
| 76 | Limonium sirinicum | Yes | - | - | - | LC |
| 77 | Limonium sitiacum | Yes | - | - | - | LC |
| 78 | Limonium sougiae | Yes | - | - | - | VU |
| 79 | Limonium spreitzenhoferi | Yes | - | - | - | VU |
| 80 | Limonium stenotatum | Yes | - | - | - | LC |
| 81 | Limonium taenari | Yes | - | - | - | LC |
| 82 | Limonium thirae | Yes | - | - | - | LC |
| 83 | Limonium vanandense | Yes | - | - | - | LC |
| 84 | Limonium virgatum | No | - | - | - | LC |
| 85 | Limonium vravronense | Yes | - | - | - | EN |
| 86 | Limonium xerocamposicum | Yes | - | - | - | LC |
| 87 | Limonium xiliense | Yes | - | - | - | VU |
| 88 | Limonium zacynthium | Yes | - | Rare | - | EN |
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Fos, M.; Alfonso, L.; Ferrer-Gallego, P.P.; Laguna, E. Effect of salinity, temperature and hypersaline conditions on the seed germination in Limonium mansanetianum, an endemic and threatened Mediterranean species. Plant Biosyst. 2021, 155, 165–171. [Google Scholar] [CrossRef]
- Fenu, G.; Calderisi, G.; Boršić, I.; Bou Dagher Kharrat, M.; García Fernández, A.; Kahale, R.; Panitsa, M.; Cogoni, D. Translocations of threatened plants in the Mediterranean Basin: Current status and future directions. Plant Ecol. 2023, 224, 765–775. [Google Scholar] [CrossRef]
- Erben, M. Limonium Mill. In Flora iberica; Castroviejo, S., Aedo, C., Cirujano, S., Laínz, M., Montserrat, P., Morales, R., Muñoz Garmendia, F., Navarro, C., Paiva, J., Soriano, C., Eds.; Real Jardín Botánico, CSIC: Madrid, Spain, 1993; Volume 3, pp. 2–143. [Google Scholar]
- Caperta, A.S.; Espírito-Santo, M.D.; Silva, V.; Ferreira, A.; Paes, A.P.; Róis, A.S.; Costa, J.C.; Arsénio, P. Habitat specificity of a threatened and endemic, cliff-dwelling halophyte. AoB Plants 2014, 6, plu032. [Google Scholar] [CrossRef]
- Heywood, V.H. An overview of in situ conservation of plant species in the Mediterranean. Flora Mediterr. 2014, 24, 5–24. [Google Scholar] [CrossRef]
- Laguna, E.; Fos, S.; Ferrando-Pardo, I.; Ferrer-Gallego, P.P. Endangered Halophytes and Their Conservation. In Handbook of Halophytes; Grigore, M.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–25. [Google Scholar] [CrossRef]
- Malekmohammadi, S.; Koutroumpa, K.; Crespo, M.B.; Domina, G.; Korotkova, N.; Akhani, H.; von Mering, S.; Borsch, T.; Berendsohn, W.G. A taxonomic backbone for the Plumbaginaceae (Caryophyllales). PhytoKeys 2024, 243, 67–103. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species; Version 2024-2. 2024. Available online: http://www.iucnredlist.org (accessed on 7 January 2025).
- Apostolopoulos, E.; Constantinidis, T. Limonium ophioides and L. nichoriense (Plumbaginaceae), two new diploid species from Peloponnisos, Greece. Phytotaxa 2024, 655, 159–172. [Google Scholar] [CrossRef]
- Koutroumpa, K. Limonium artelariae (Plumbaginaceae), a new endemic species and further taxonomic and floristic notes on the genus in the island of Crete. Willdenowia 2024, 54, 65–79. [Google Scholar] [CrossRef]
- Brullo, S.; Brullo, C.; Cambria, S.; Giusso del Galdo, G.; Minissale, P. Phytosociological investigation on the class Crithmomaritimi-Limonietea in Greece. Plant Sociol. 2017, 54, 3–57. [Google Scholar] [CrossRef]
- Brullo, S.; Erben, M. The genus Limonium (Plumbaginaceae) in Greece. Phytotaxa 2016, 240, 1–212. [Google Scholar] [CrossRef]
- Valli, A.-T.; Artelari, R. Limonium korakonisicum (Plumbaginaceae), a new species from Zakynthos Island (Ionian Islands, Greece). Phytotaxa 2015, 217, 63–72. [Google Scholar] [CrossRef]
- Naghiloo, S.; Vamosi, J. Are Polyploid Species Less Vulnerable to Climate Change? A Simulation Study in North American Crataegus. Am. J. Clim. Change 2023, 12, 359–375. [Google Scholar] [CrossRef]
- Rodrigues, A.S.L.; Pilgrim, J.; Lamoreux, J.F.; Hoffmann, M.; Brooks, T.M. The value of the IUCN Red List for conservation. Trends Ecol. Evol. 2006, 21, 71–76. [Google Scholar] [CrossRef] [PubMed]
- IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 16. 2024. Available online: https://www.iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 10 March 2025).
- WFO. World Flora Online. 2025. Available online: http://www.worldfloraonline.org/ (accessed on 7 January 2025).
- Phitos, D.; Strid, A.; Snogerup, S.; Greuter, W. The Red Data Book of Rare and Threatened Plants of Greece; WWF: Gland, Switzerland, 1995. [Google Scholar]
- Phitos, D.; Constantinidis, T.; Kamari, G. The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Botanical Society: Athens, Greece, 2009; Volume II (E-Z). [Google Scholar]
- GBIF: The Global Biodiversity Information Facility. 2020. What is GBIF? Available online: https://www.gbif.org/what-is-gbif (accessed on 13 January 2025).
- GeoCAT. Geospatial Conservation Assessment Tool. 2025. Available online: https://geocat.iucnredlist.org/ (accessed on 20 February 2024).
- Bachman, S.; Moat, J.; Hill, A.W.; de Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. Zookeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Threats Classification Scheme. Version 3.3. 2022. Available online: https://www.iucnredlist.org/resources/threat-classification-scheme (accessed on 15 March 2025).
- IUCN. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels, Version 4.0; IUCN: Gland, Switzerland; Cambridge, UK, 2012.
- Posit Team. RStudio: Integrated Development Environment for R. 2025. Available online: http://www.posit.co/ (accessed on 25 July 2025).
- R Core Team. R: A language and Environment for Statistical Computing. 2023. Available online: https://www.R-project.org/ (accessed on 25 July 2025).
- Guisan, A.; Harrell, F. Ordinal response regression models in ecology. J. Veg. Sci. 2000, 11, 617–626. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, F.; Guin, C.; Zhu, H. A spatio-temporal kernel density estimation framework for predictive crime hotspot mapping and evaluation. Appl. Geogr. 2018, 99, 89–97. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kouris, A.D.; Christopoulos, A.; Leros, M.; Loupou, M.; Rammou, D.-L.; Youlatos, D.; Troumbis, A.Y. Where to Protect? Spatial Ecology and Conservation Prioritization of the Persian Squirrel at the Westernmost Edge of Its Distribution. Land 2025, 14, 876. [Google Scholar] [CrossRef]
- Botanic Gardens Conservation International (BGCI). PlantSearch: Richmond, UK. 2025. Available online: https://plantsearch.bgci.org (accessed on 1 September 2025).
- Thanos, C.A. (Seed Bank of the National and Kapodistrian University of Athens, Athens, Greece). Personal communication, 2025.
- Fournaraki, C. (Seed Bank of the Mediterranean Agronomic Institute of Chania, Chania, Crete, Greece). Personal communication, 2025.
- ENSCOBASE. The ENSCONET Virtual Seed Bank. 2009. Available online: http://enscobase.maich.gr/ (accessed on 30 September 2025).
- Krigas, N. (Hellenic Agricultural Organization HAO-DEMETER, Institute of Plant Breeding and Genetic Resources, Balkan Botanic Garden of Kroussia, Laboratory of Protection and Evaluation of Native and Floriculture Species, Thermi, Thessaloniki, Greece). Personal communication, 2025.
- Society for Ecological Restoration (SER); Royal Botanic Gardens Kew (RBGK); International Network for Seed-based Restoration (INSR). Seed Information Database (SID). 2025. Available online: https://ser-sid.org/ (accessed on 1 September 2025).
- Fournaraki, C. Conservation of Threatened Plants of Crete: Seed Ecology, Operation and Management of a Gene Bank. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2010. [Google Scholar]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Vegetative propagation and ex-situ conservation of Acantholimon androsaceum and Limonium chersonesum, two promising local endemics of Crete (Greece) available for floricultural and pharmaceutical sustainable exploitation. Not. Bot. Horti Agrobot. 2021, 49, 12261. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G.; Christodoulou, C.; Fournaraki, C.; Giusso del Galdo, G.P.; Gotsiou, P.; Kyratzis, A.; Piazza, C.; Vicens, M.; Pinna, M.S.; et al. An early evaluation of translocation actions for endangered plant species on Mediterranean islands. Plant Divers. 2019, 41, 94–104. [Google Scholar] [CrossRef]
- Valli, A.-T.; Papaioannou, C.; Liveri, E.; Papasotiropoulos, V.; Trigas, P. Conservation biology of three threatened Limonium species endemic to Zakynthos Island (Ionian Islands, Greece). Oryx 2024, 58, 587–599. [Google Scholar] [CrossRef]
- Işık, K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011, 35, 411–417. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Mizrachi, E.; Marchal, K. The evolutionary significance of polyploidy. Nat. Rev. Genet. 2017, 18, 411–424. [Google Scholar] [CrossRef]
- Laguna, E. The Micro-Reserves as a Tool for Conservation of Threatened Plants in Europe; Council of Europe: Strasbourg, France, 2001. [Google Scholar]
- Panitsa, M.; Trigas, P.; Kokkoris, I.P.; Kougioumoutzis, K.; Tsakiri, M.; Koumoutsou, E.; Topouzidis, N.; Bertsouklis, K.; Tzanoudakis, D.; Iatrou, G. Establishment of a Plant Micro-Reserve Network within the responsibility areas of the Management Unit of Chelmos-Vouraikos National Park and Protected areas of Northern Peloponnese (Greece). In Proceedings of the 4th Mediterranean Plant Conservation Week, Valencia, Spain, 23–27 October 2023. [Google Scholar]
- Panitsa, M.; Fournaraki, C.; Koutsovoulou, K.; Kougioumoutzis, K.; Choreftakis, M.; Thanos, C.A. In situ and ex situ plant conservation in the Aegean Archipelago. In Proceedings of the 5th Mediterranean Plant Conservation Week, Limassol, Cyprus, 7–11 April 2025. [Google Scholar]
- Laguna, E.; Ferrer-Gallego, P.P. Proximity reinforcements and safety neopopulations, new complementary concepts for certain types of in situ plant introductions. Conserv. Veg. 2012, 16, 14. [Google Scholar]
- Apostolopoulos, E. Limonium albomarginatum. The IUCN Red List of Threatened Species 2025: e.T228116508A228119133. 2025. Available online: https://doi.org/10.2305/IUCN.UK.2025-1.RLTS.T228116508A228119133.en (accessed on 19 June 2025).
- Apostolopoulos, E.; Magdalinou, E. Limonium aphroditae. The IUCN Red List of Threatened Species 2025: e.T228116659A228119148. 2025. Available online: https://doi.org/10.2305/IUCN.UK.2025-1.RLTS.T228116659A228119148.en (accessed on 26 June 2025).
- Maschinski, J.; Albrecht, M.A. Center for Plant Conservation’s best practice guidelines for the reintroduction of rare plants. Plant Divers. 2017, 39, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Laguna, E.; Navarro, A.; Pérez-Rovira, P.; Ferrando, I.; Ferrer-Gallego, P.P. Translocation of Limonium perplexum (Plumbaginaceae), a threatened coastal endemic. Plant Ecol. 2016, 217, 1183–1194. [Google Scholar] [CrossRef]
- Godefroid, S.; Piazza, C.; Rossi, G.; Buord, S.; Stevens, A.-D.; Aguraiuja, R.; Cowell, C.; Weekley, C.W.; Vogg, G.; Iriondo, J.M.; et al. How successful are plant species reintroductions? Biol. Conserv. 2011, 144, 672–682. [Google Scholar] [CrossRef]
- Cano, Á.; Powell, J.; Aiello, A.S.; Andersen, H.L.; Arbour, T.; Balzer, A.; Bauer, D.S.; Bugarchich, J.; Cano, F.; Contreras, M.P. Insights from a century of data reveal global trends in ex situ living plant collections. Nat. Ecol. Evol. 2025, 9, 214–224. [Google Scholar] [CrossRef]
- Godefroid, S.; Lacquaye, S.; Ensslin, A.; Dalrymple, S.; Abeli, T.; Branwood, H.; Pardo, I.F.; Gallego, P.P.F.; Zippel, E.; Gouveia, L. Current state of plant conservation translocations across Europe: Motivations, challenges and outcomes. Biodivers. Conserv. 2025, 34, 769–792. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).