Integrative Taxonomic Assessment of Two Atractus (Serpentes: Dipsadidae) from Mérida Andes, Venezuela
Abstract
1. Resumen
2. Introduction
3. Materials and Methods
3.1. DNA Extraction, Amplification, and Sequencing
3.2. Phylogenetic Analyses
3.3. Ecological Niche Modeling
3.4. Input Data (Records and Environmental Variables)
3.5. Approach to Predictive Models (Habitat Suitability)
3.6. Evaluation Index and Visualization
3.7. Sample Source
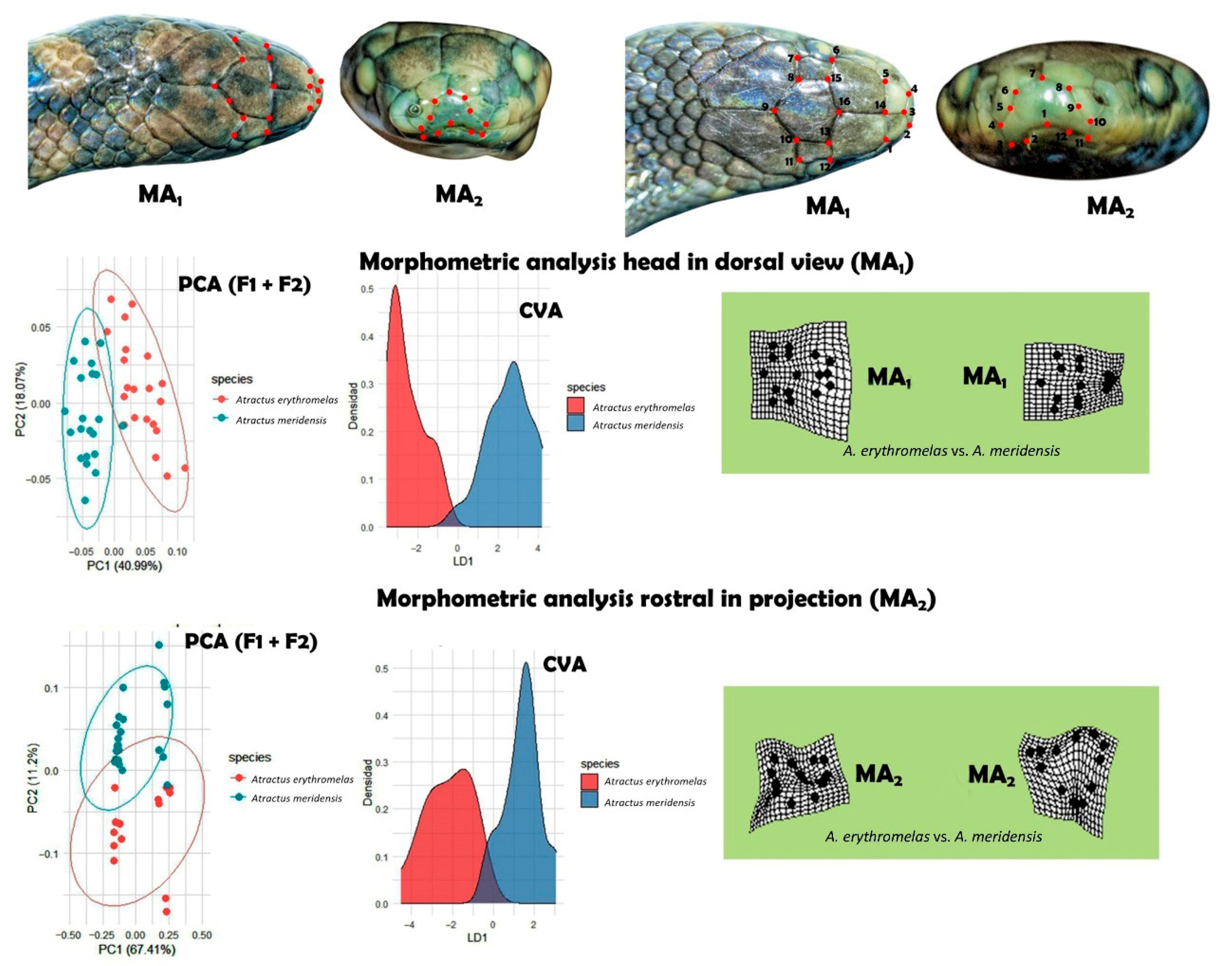
3.8. Morphometric and Morphological Data
3.9. Statistical Processing (Comparative Data and Geometric Morphometrics)
4. Results
4.1. Phylogenetic Approach
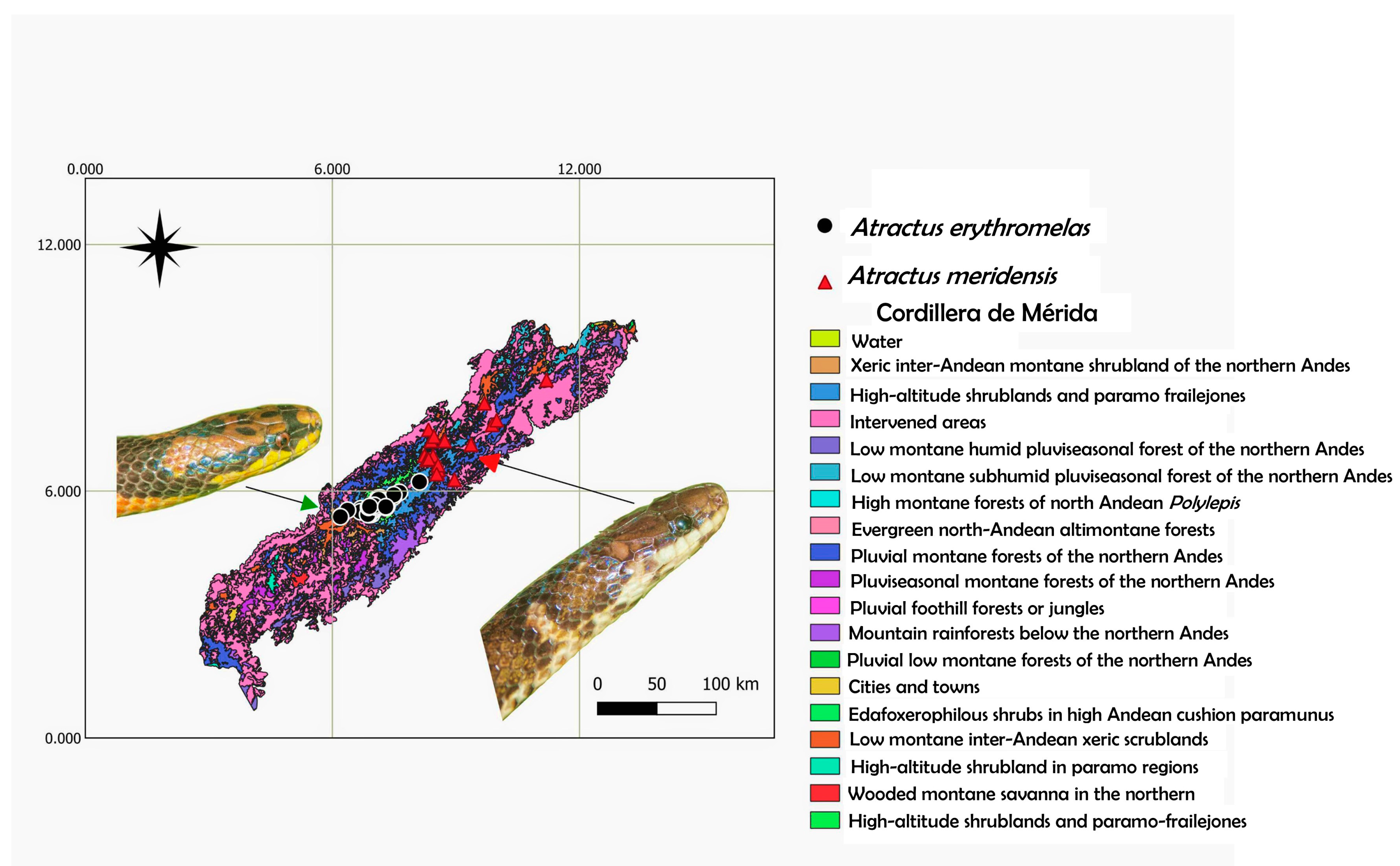
4.2. Ecological Approach
4.3. Morphological–Statistical Approach
4.3.1. Spatial Morphometry
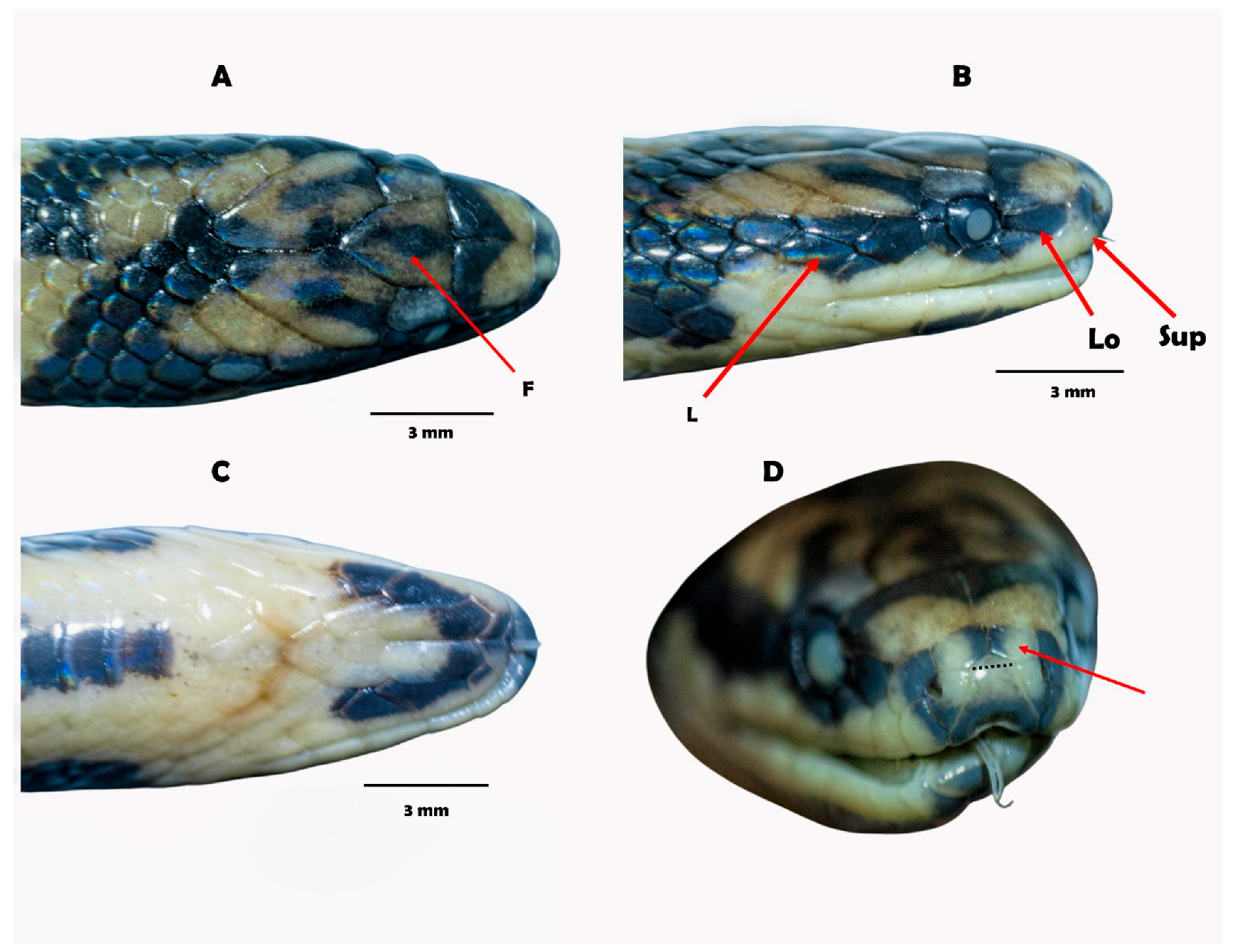
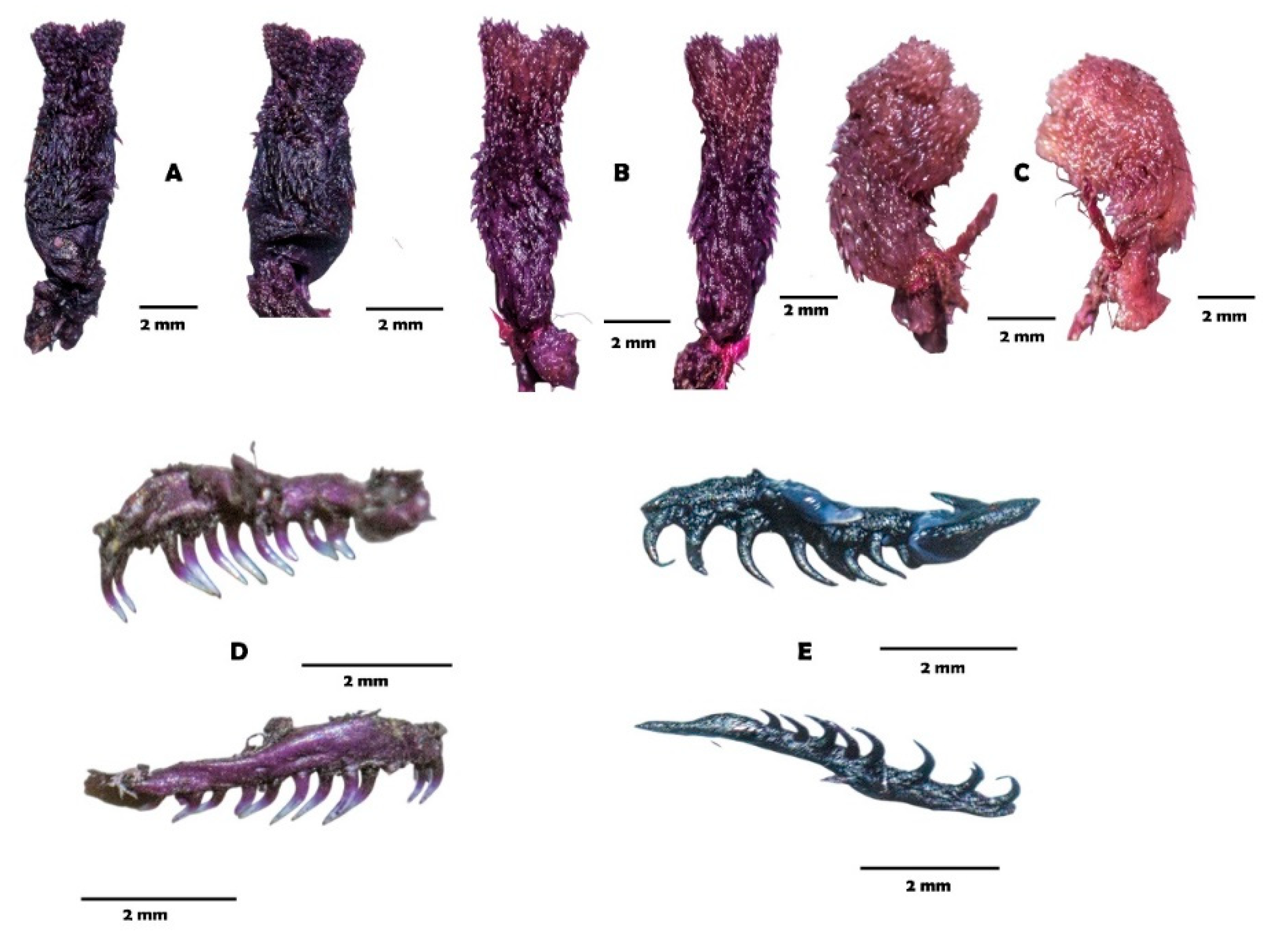
4.3.2. Systematic Overview
5. Discussion
5.1. Species Limits and Taxonomy
5.2. Biogeographical Interpretation and Conservation Status
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaher, H.; Murphy, R.W.; Arredondo, J.C.; Graboski, R.; Machado-Filho, P.R.; Mahlow, K.; Montingelli, G.G.; Quadros, A.B.; Orlov, N.L.; Wilkinson, M.; et al. Large-scale molecular phylogeny, morphology, divergence-time estimation, and the fossil record of advanced caenophidian snakes Squamata: Serpentes. PLoS ONE 2019, 14, e0216148. [Google Scholar] [CrossRef]
- Pandelis, G.G.; Grundler, M.C.; Rabosky, D.L. Ecological correlates of cranial evolution in the megaradiation of dipsadine snakes. BMC Ecol. Evol. 2023, 23, 48. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Hosek, J. (Eds.) The Reptile Database. 2024. Available online: http://www.reptile-database.org (accessed on 1 May 2022).
- Rivas, G.A.; Molina, C.R.; Ugüeto, G.N.; Barros, T.R.; Barrio-Amorós, C.L.; Kok, J.R.P. Reptiles of Venezuela: An updated and commented checklist. Zootaxa 2012, 3211, 1–64. [Google Scholar] [CrossRef]
- Natera-Mumaw, M.; Esqueda-González, L.F.; Castelaín-Fernández, M. Atlas Serpientes de Venezuela; Dimacofi Negocios Avanzados S.A.: Santiago de Chile, Chile, 2015. [Google Scholar]
- Passos, P.; Meneses-Pelayo, E.; Ramos, L.O.; Martins, A.R.; Machado, A.R.; Lopes, R.T.; Barrio-Amorós, C.; Lynch, J.D. Taxonomy without borders: Revision of the genus Atractus Serpentes: Dipsadidae from the Andes between Colombia and Venezuela. South Am. J. Herpetol. 2024, 32, 1–123. [Google Scholar] [CrossRef]
- Padial, J.M.; Miralles, A.; De la Riva, I.; Vences, M. The integrative future of taxonomy. Front. Zool. 2010, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Esqueda, L.F.; La Marca, E. Revisión taxonómica y biogeográfica (con descripción de cinco nuevas especies) del género Atractus (Colubridae: Dipsadinae) en los Andes de Venezuela. Herpetotropicos 2005, 2, 1–32. [Google Scholar]
- Esqueda, L.F.; La Marca, E.; Bazó, S. Un nuevo colúbrido semifosorial del género Atractus (Dipsadinae) de la vertiente lacustre de los Andes de Venezuela. Herpetotropicos 2007, 2, 87–93. [Google Scholar]
- Esqueda, L.F.; Rojas-Runjaic, F.J.M.; Prudente, A.; Bazó, S.; Navarrete, L.F.; Carmargo-Sillet, E.; Ortiz, J.C.; Correa, C.; Guerrero, P.; Urra, F. A first phylogenetic and taxonomic approach to sleepyhead snakes from Venezuela (Dipsadidae: Atractus), with the description of two new Andean species. Org. Divers. Evol. 2025, 25, 1–37. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. Available online: https://www.academia.edu/2034992/BioEdit_a_user_friendly_biological_sequence_alignment_editor_and_analysis_program_for_Windows_95_98_NT (accessed on 16 September 2025).
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 387, 3022–3027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Librado, P.; Rozas, J. DnaSP v6: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D. MAFFT multiple sequence alignment software version 7, Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 259, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, A.; Gribaldo, S. BMGE (Block Mapping and Gathering with Entropy): A new software for selection of phylogenetic informative regions from multiple sequence alignments. BMC Evol. Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Lemoine, F.; Correia, D.; Lefort, V.; Doppelt-Azeroual, O.; Mareuil, F.; Cohen-Boulakia, S.; Gascuel, O. NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Res. 2019, 47, W260–W265. [Google Scholar] [CrossRef] [PubMed]
- Maddison, W.P.; Maddison, D.R. Mesquite: A Modular System for Evolutionary Analysis. Version 3.70. 2021. Available online: http://www.mesquiteproject.org (accessed on 10 March 2022).
- Triant, D.A.; Dewoody, J.A. The occurrence, detection, and avoidance of mitochondrial DNA translocations in mammalian systematics and phylogeography. J. Mammal. 2007, 88, 908–920. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Anisimova, M.; Gil, M.; Dufayard, J.F.; Dessimoz, C.; Gascuel, O. Survey of branch support methods demonstrates accuracy, power, and robustness of fast likelihood-based approximation schemes. Syst. Biol. 2011, 60, 685–699. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree Version 1.4.2. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 March 2022).
- Bouckaert, R.; Drummond, A.J. bModelTest: Bayesian phylogenetic site model averaging and model comparison. BMC Evol. Biol. 2017, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Atchadé, Y.F.; Roberts, G.O.; Rosenthal, J.S. Towards optimal scaling of metropolis-coupled Markov chain Monte Carlo. Stat. Comput. 2011, 21, 555–568. [Google Scholar] [CrossRef]
- Maturana-Russel, P.; Brewer, B.J.; Klaere, S.; Bouckaert, R.R. Model selection and parameter inference in phylogenetics using nested sampling. Syst. Biol. 2018, 682, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Müller, N.F.; Bouckaert, R. Coupled MCMC in BEAST 2. bioRxviv 2019. [Google Scholar] [CrossRef]
- Rambaut, A.; Suchard, M.; Drummond, A.J. Tracer Version 1.7.1. 2022. Available online: http://www.beast.community (accessed on 10 July 2022).
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J. TreeAnnotator Version 1.8.3. 2016. Available online: http://beast.bio.ed.ac.uk (accessed on 10 March 2022).
- Miller, M.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for 1100 inference of large phylogenetic trees. In Gateway Computing Environments; 1101 Workshop GCE; IEEE: New Orleans, LA, USA, 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA XI: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2, New 1km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Amatulli, G.; Domisch, S.; Tuanmu, M.N.; Parmentier, B.; Ranipeta, A.; Malczyk, J.; Jetz, W. Un conjunto de variables topográficas globales a escala cruzada para el modelado ambiental y de biodiversidad. Datos Científicos 2018, 5, 180040. [Google Scholar] [CrossRef]
- Huber, O.; Oliveira-Miranda, M.A. Ambientes Terrestres. In Libro Rojo de los Ecosistemas Terrestres de Venezuela; Rodríguez, J.P., Rojas-Suárez, F., Giraldo Hernández, D., Eds.; Provita, Shell Venezuela, Lenovo Venezuela: Caracas, Venezuela, 2010; pp. 29–89. [Google Scholar]
- QGIS.org. QGIS Geographic Information System. QGIS Association. 2024. Available online: http://www.qgis.org (accessed on 10 July 2024).
- Hernández, P.A.; Graham, C.H.; Maestro, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 295, 773–785. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.S.; Phillips, J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifer, S.N. Ecological niche modeling in MaxEnt: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 212, 335–342. [Google Scholar] [CrossRef]
- Pearson, K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. Philos. Mag. Ser. 1900, 5, 157–175. [Google Scholar] [CrossRef]
- Warren, D.L.; Matzke, N.J.; Cardillo, M.; Baumgartner, J.B.; Beaumont, L.J.; Turelli, M.; Glor, R.E.; Huron, N.A.; Simões, M.; Iglesias, T.L.; et al. ENMTools 1.0: An R package for comparative ecological biogeography. Ecography 2021, 444, 504–511. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Brown, H.; Dolan, M.; Haywood, C. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Nat. Sci. Data 2018, 5, 180254. [Google Scholar] [CrossRef]
- Dowsett, H.J.; Robinson, M.M.; Haywood, A.M.; Salzmann, U.; Hill, D.J.; Sohl, L.E.; Chandler, M.; Williams, M.; Foley, K.; Stoll, D.K. The PRISM3D paleoenvironmental reconstruction. Stratigraphy 2010, 7, 123–139. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the Earth land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Thuiller, W.; Araujo, M.B.; Lavorel, S. Generalized models vs. classification tree analysis: Predicting spatial distributions of plant species at different scales. J. Veg. Sci. 2003, 14, 669–680. [Google Scholar] [CrossRef]
- Peterson, A.T.; Nakazawa, Y. Environmental data sets matter in ecological niche modelling: An example with Solenopsis invicta and Solenopsis richteri. Glob. Ecol. Biogeogr. 2008, 171, 135–144. [Google Scholar] [CrossRef]
- Manly, B.F.J. Randomization, Bootstrap and Monte Carlo Methods in Biology; Chapman & Hall/CRC: New York, NY, USA, 2006; p. 358. [Google Scholar]
- Allouche, O.; Tsoaraw, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic. J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for MaxEnt ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2020. Available online: https://www.R-project.org/ (accessed on 10 March 2022).
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; d’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Schoener, T. The Anolis Lizards of Bimini: Resource Partitioning in a Complex Fauna. Ecology 1968, 494, 704–726. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 6111, 2868–2883. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Chacón-Moreno, E.; Moral, P.S. Mapa bioclimático de la cordillera de Mérida. Ecotrópicos 2020, 32, e0010. [Google Scholar] [CrossRef]
- IUCN. Mapping Standards and Data Quality for the IUCN Red List Spatial Data Version 1.19. 2022. Available online: https://www.iucnredlist.org/resources/mappingstandards (accessed on 1 July 2023).
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting Red List threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List Categories and Criteria: Version 3.1, 2nd ed.; IUCN Species Survival Commission: Gland, Switzerland; Cambridge, UK, 2012; p. 32. [Google Scholar]
- Boulenger, G.A. On some batrachians and reptiles from Venezuela. Ann. Mag. Nat. Hist. 1903, 11, 481–484. [Google Scholar] [CrossRef]
- Roze, J.A. El género Atractus Serpentes: Colubridae en Venezuela. Acta Biológica Venez. 1961, 3, 103–119. [Google Scholar]
- Roze, J.A. La Taxonomía y Zoogeografía de los Ofidios de Venezuela; Ediciones de la Biblioteca; Universidad Central de Venezuela: Caracas, Venezuela, 1966; 362p. [Google Scholar]
- Lancini, A.R. Atractus mariselae, una nueva especie de serpiente minadora de los Andes de Venezuela Serpentes: Colubridae. Publicaciones Ocas. Científicas Nat. 1969, 15, 1–6. [Google Scholar]
- González-Sponga, M.A. Atractus emigdioi Serpentes: Colubridae nueva especie para los Andes de Venezuela. In Monografía Científica “Augusto Pi Suner”; Instituto Pedagógico: Caracas, Venezuela, 1971; Volume 3, pp. 1–11. [Google Scholar]
- Barros, T.R. Una nueva especie de Atractus (Serpentes: Colubridae) de la Sierra de Perijá, Estado Zulia, Venezuela. Anartia 2000, 11, 1–10. [Google Scholar]
- Schargel, W.E.; García-Pérez, J.E. A new species and a new record of Atractus Serpentes: Colubridae from the Andes of Venezuela. J. Herpetol. 2002, 36, 398–402. [Google Scholar] [CrossRef]
- Markezich, A.L.; Barrio-Amorós, C.L. A new species of Atractus Serpentes: Colubridae from northeastern Venezuela. Bull. Md. Herpetol. Soc. 2004, 40, 111–121. [Google Scholar]
- Sánchez, D.; de Sousa, L.; Esqueda, L.F.; Manzanilla, J. Especie nueva de Atractus Serpentes: Colubridae del macizo del Turimiquire, tramo oriental de la cordillera de la costa, Venezuela. Saber 2004, 162, 89–95. [Google Scholar]
- Kok, P.J.R. A new snake of the genus Atractus Wagler, 1828 Reptilia: Squamata: Colubridae from Kaieteur National Park, Guyana, northeastern South America. Zootaxa 2006, 1378, 19–35. [Google Scholar] [CrossRef]
- Myers, C.W.; Schargel, W.E. Morphological extremes-two new snakes of the genus Atractus from northwestern South America Colubridae: Dipsadinae. Am. Mus. Novit. 2006, 35321, 1–13. [Google Scholar] [CrossRef]
- Passos, P.; Fuenmayor, G.R.; Barrio-Amorós, C. Description of two new species from Venezuela in the highly diverse dipsadine genus Atractus Serpentes: Colubridae. Amphib. Reptil. 2009, 30, 233–243. [Google Scholar] [CrossRef]
- Passos, P.; Kok, P.J.R.; Albuquerque, N.R.; Rivas, G. Ground snakes of the Lost World: A review of Atractus Serpentes: Dipsadidae from the Pantepui region, northern South America. Herpetol. Monogr. 2013, 27, 52–86. [Google Scholar] [CrossRef]
- Esqueda, L.F. A new semifossorial snake species (Dipsadidae: Atractus Wagler, 1828) from the Lara-Falcón mountainous system, northwestern Venezuela. Herpetotropicos 2011, 6, 35–41. [Google Scholar]
- Montes-Correa, A.C.; Arévalo-Páez, M.; Rada-Vargas, E.; Portillo-Mozo, A.D.; Granda-Rodríguez, H.D.; Rivero-Blanco, C.A. First record of Atractus turikensis Squamata: Colubridae: Dipsadinae from the Colombian Perijá highlands. Herpetol. Bull. 2017, 141, 35–39. [Google Scholar]
- Pérez-Santos, C.; Moreno, A.G. Ofidios de Colombia. Boll. Del Mus. Reg. Di Sci. Nat. Di Torino 1988, 71, 15–31. [Google Scholar]
- Passos, P.; Lynch, J.D.; Fernandes, R. Taxonomic status of Atractus sanctaemartae and A. nebularis, and description of a new species of Atractus from Atlantic coast of Colombia. Herpetol. J. 2009, 18, 175–186. [Google Scholar]
- Passos, P.; Arredondo, J.C.; Fernandes, R.; Lynch, J.D. Three new Atractus Serpentes: Dipsadidae from the Andes of Colombia. Copeia 2009, 3, 425–436. [Google Scholar] [CrossRef]
- Passos, P.; Lynch, J.D. Revision of Atractus Serpentes: Dipsadidae from Middle and Upper Magdalena Drainage of Colombia. Herpetol. Monogr. 2010, 24, 149–173. [Google Scholar] [CrossRef]
- Meneses-Pelayo, E.; Passos, P. New polychromatic species of Atractus Serpentes: Dipsadidae from the eastern portion of the Colombian Andes. Copeia 2019, 1072, 250–261. [Google Scholar] [CrossRef]
- Passos, P.; Dobiey, M.; Venegas, P.J. Variation and natural history notes on giant ground snake, Atractus gigas Serpentes: Dipsadidae. S. Am. J. Herpetol. 2010, 52, 73–82. [Google Scholar] [CrossRef]
- Schargel, W.E.; Lamar, W.W.; Passos, P.; Valencia, J.H.; Cisneros-Heredia, D.F.; Campbell, J.A. A new giant Atractus Serpentes: Dipsadidae from Ecuador, with notes on some other large Amazonian congeners. Zootaxa 2013, 37215, 455–474. [Google Scholar] [CrossRef]
- Salazar-Valenzuela, D.; Torres-Carvajal, O.; Passos, P. A new species of Atractus (Serpentes: Dipsadidae) from the Andes of Ecuador. Herpetologica 2014, 703, 350–363. [Google Scholar] [CrossRef]
- Arteaga, A.; Mebert, K.; Valencia, J.H.; Cisneros-Heredia, D.F.; Peñafiel, N.; Reyes-Puig, C.; Vieira-Fernandes, J.L.; Guayasamin, J.M. Molecular phylogeny of Atractus (Serpentes, Dipsadidae), with emphasis on Ecuadorian species and the description of three new taxa. ZooKeys 2017, 661, 91–123. [Google Scholar] [CrossRef]
- Arteaga, A.; Quezada, A.; Vieira, J.; Guayasamin, J.M. Leaving no stone unturned: Three additional new species of Atractus ground snakes (Serpentes, Colubridae) from Ecuador discovered using a biogeographical approach. ZooKeys 2022, 1121, 175–210. [Google Scholar] [CrossRef] [PubMed]
- Cunha, O.R.; Nascimento, F.P. Ofídios da Amazônia XX: As espécies de Atractus Wagler, 1828, na Amazônia oriental e Maranhão (Ophidia, Colubridae). Bol. Do Mus. Para. Emílio Goeldi 1983, 123, 1–38. [Google Scholar]
- Martins, M.; Oliveira, M.E. The snakes of the genus Atractus Wagler Reptilia: Squamata: Colubridae from the Manaus region, central Amazonia, Brazil. Zool. Meded. 1993, 69, 21–40. [Google Scholar]
- Passos, P.; Fernandes, R.; Zanella, N. A new species of Atractus Serpentes: Colubridae from Southern Brazil. Herpetologica 2005, 61, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Passos, P.; Melo-Sampaio, P.R.; Ramos, L.O.; Grazziotin, F.G.; Fouquet, A.; Torres-Carvajal, O. When the tail shakes the snake: Phylogenetic affinities and morphology of Atractus badius Serpentes: Dipsadidae, reveals some current pitfalls on the snake’s genomic age. Ann. Acad. Bras. Cienc. 2022, 94, e20191254. [Google Scholar] [CrossRef] [PubMed]
- Dowling, H.G. A proposed standard system of counting ventrals in snakes. Br. J. Herpetol. 1951, 1, 97–99. [Google Scholar]
- Savage, J.M. A revision of the Ecuadorian snakes of the colubrid genus Atractus. Misc. Publ. Mus. Zoology. Univ. Mich. 1960, 112, 1–184. [Google Scholar]
- Hoogmoed, M.S. Revision of the genus Atractus in Surinam, with the resurrection of two species Colubridae, Reptilia. Notes on the Herpetofauna of Surinam VII. Zool. Verh. 1980, 175, 1–47. [Google Scholar]
- Myers, C.W. Rare snakes—Five new species from eastern Panama: Reviews of northern Atractus and southern Geophis Colubridae: Dipsadinae. Am. Mus. Novit. 2003, 3391, 1–47. [Google Scholar] [CrossRef]
- Almeida, P.C.; Feitosa, D.T.; Passos, P.; Prudente, A.L.C. Morphological variation and taxonomy of Atractus latifrons (Günther, 1868) (Serpentes: Dipsadidae). Zootaxa 2014, 3860, 064–080. [Google Scholar] [CrossRef] [PubMed]
- Downs, F.L. Intrageneric Relationships Among Colubrid Snakes of the Genus Geophis Wagler; University of Michigan, Museum of Zoology: Ann Arbor, MI, USA, 1967; Volume 373. [Google Scholar]
- Melo-Sampaio, P.R.; Venegas, P.J. A new species of ground snake genus Atractus Wagler, 1828 Serpentes, Dipsadidae from the Peruvian Andes revealed by unequivocal morphological characters. Evol. Syst. 2023, 72, 257–266. [Google Scholar] [CrossRef]
- Manzani, P.R.; Abe, A. Sobre dois novos métodos de preparação de hemipênis de serpentes. Memórias Inst. Butantan 1988, 501, 15–20. [Google Scholar]
- Pesantes, O. A method for preparing hemipenis of preserved snakes. J. Herpetol. 1994, 28, 93–95. [Google Scholar] [CrossRef]
- Zaher, H. Hemipenial morphology of the South American xenodontine snakes, with a proposal for a monophyletic Xenodontinae and a reappraisal of colubroid hemipenes. Bull. Am. Mus. Nat. Hist. 1999, 240, 1–168. [Google Scholar]
- Passos, P.; Prudente, A.L.C.; Lynch, J.D. Redescription of Atractus punctiventris and description of two new Atractus Serpentes: Dipsadidae from Brazilian Amazonia. Herpetol. Monogr. 2016, 30, 1–20. [Google Scholar] [CrossRef]
- Uzzell, T. A revision of lizards of the genus Prionodactylus, with a new genus for P. leucostictus and notes on the genus Euspondylus Sauria, Teiidae. Postilla 1973, 159, 1–67. [Google Scholar] [CrossRef]
- Harvey, M.B.; Embert, D. Review of Bolivian Dipsas Serpentes: Colubridae, with comments on other South American species. Herpetol. Monogr. 2008, 22, 54–105. [Google Scholar] [CrossRef]
- Dowling, H.G.; Savage, J.M. A guide to the snake hemipenis: A survey of basic structure and systematic characteristics. Zoologica 1960, 45, 17–28. [Google Scholar] [CrossRef]
- Schargel, W.E.; Castoe, T.A. The hemipenes of some snakes of the semifossorial genus Atractus, with comments on variation in the genus. J. Herpetol. 2003, 37, 718–721. [Google Scholar] [CrossRef]
- Myers, C.W.; Cadle, J.E. On the snake hemipenis, with notes on Psomophis and techniques of eversion: A response to Dowling. Herpetol. Rev. 2003, 344, 295–302. [Google Scholar]
- Zaher, H.; Prudente, A.L. Hemipenes of Siphlophis Serpentes, Xenodontinae and techniques of hemipenial preparation in snakes: A response to Dowling. Herpetol. Rev. 2003, 34, 295–302. [Google Scholar]
- Myers, C.W.; McDowell, S.B. New taxa and cryptic species of Neotropical Snakes Xenodontinae, with commentary on hemipenes as generic and specific characters. Bull. Am. Mus. Nat. Hist. 2014, 385, 1–112. [Google Scholar] [CrossRef]
- Oliveira, L.; Grazziotin, F.G.; Sánchez-Martínez, P.M.; Sasa, M.; Flores-Villela, O.; Prudente, A.L.C.; Zaher, H. Phylogenetic and morphological evidence reveals the association between diet and the evolution of the venom delivery system in Neotropical goo-eating snakes. Syst. Biodivers. 2023, 21, 2153944. [Google Scholar] [CrossRef]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution. Boston, USA. 2025. Available online: https://www.xlstat.com (accessed on 10 March 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2023. Available online: https://dplyr.tidyverse.org (accessed on 10 March 2024).
- Zar, J.H. Biostatistical Analisys; Prentice–Hall, Inc.: Hoboken, NJ, USA, 1999; 123p. [Google Scholar]
- Fisher, R.A. The use of multiple measurements in taxonomic problems. Ann. Eugen. 1936, 72, 179–188. [Google Scholar] [CrossRef]
- Mahalanobis, P.C. Sobre la distancia generalizada en estadística. Actas Inst. Nac. Cienc. India 1936, 2, 49–55. [Google Scholar]
- Adams, D.C.; Otárola-Castillo, E. geomorph: An r package for the collection and analysis of geometric morphometric shape data. Methods Ecol. Evol. 2013, 4, 393–399. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. Geometric morphometrics: Ten years of progress following the “revolution”. Ital. J. Zool. 2004, 71, 5–16. [Google Scholar] [CrossRef]
- Adams, D.C.; Rohlf, F.J.; Slice, D.E. A field comes of age: Geometric morphometrics in the 21st century. Hystrix 2013, 24, 7–14. [Google Scholar] [CrossRef]
- Rohlf, F.J. Rotational fit Procrustes methods. In Proceedings Michigan Morphometrics Workshop; Special publication n8 2. Museum of Zoology; Rohl, F.J., Bookstein, F.L., Eds.; University of Michigan: Ann Arbor, MI, USA, 1990; pp. 227–236. [Google Scholar]
- Rohlf, F.J.; Slice, D. Extensions of the Procrustes method for the optimal superimposition of landmarks. Syst. Zool. 1990, 391, 40–59. [Google Scholar] [CrossRef]
- Klingenber, C.P.; McIntyre, G.S. Geometric morphometrics of developmental instability: Analyzing patterns of fluctuating asymmetry with Procrustes methods. Evolution 1998, 52, 1363–1375. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Marugán-Lobón, J. Evolutionary covariation in geometric morphometric data: Analyzing integration, modularity, and allometry in a phylogenetic context. Syst. Biol. 2013, 62, 591–610. [Google Scholar] [CrossRef]
- Klingenberg, C.P.; Barluenga, M.; Meyer, A.; Wainwright, P. Shape analysis of symmetric structures: Quantifying variation among individuals and asymmetry. Evolution 2002, 56, 1909–1920. [Google Scholar] [CrossRef] [PubMed]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D. Geometric Morphometrics for Biologists: A Primer, 2nd ed.; Elsevier Academic Press: London, UK, 2012; 443p. [Google Scholar]
- Zelditch, M.L.; Swiderski, D.L.; Sheets, H.D.; Fink, W.L. Landmarks. In Geometric Morphometrics for Biologists: A Primer; Elsevier Academic Press: Cambridge, UK, 2004; 437p. [Google Scholar]
- Rohlf, F.J. Geometric morphometrics and phylogeny. In Morphology, Shape and Phylogeny: Taylor & Francis: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2002; 318p. [Google Scholar]
- Claude, J. Morphometrics with R; Springer: New York, NY, USA, 2008; 317p. [Google Scholar]
- Bookstein, F.L. Morphometric Tools for Landmark Data: Geometry and Biology; Cambridge University Press: Cambridge, UK, 1991. [Google Scholar]
- Alfaro, M.E.; Zoller, S.; Lutzoni, F. Bayes or Bootstrap? A simulation study comparing the performance of Bayesian Markov Chain Monte Carlo sampling and bootstrapping in assessing phylogenetic confidence. Mol. Biol. Evol. 2003, 20, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Holder, M.; Lewis, P.O. Phylogeny estimation: Traditional and Bayesian approaches. Nat. Rev. Genet. 2003, 44, 275–284. [Google Scholar] [CrossRef]
- Yang, Z.; Rannala, B. Molecular phylogenetics: Principles and practice. Nat. Rev. Genet. 2012, 135, 303–314. [Google Scholar] [CrossRef]
- ICZN. International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- Peters, J.A.; Orejas-Miranda, B.R. Catalogue of the Neotropical Squamata: Part 1. Snakes. Bull. United States Natl. Mus. 1970, 297, 1–347. [Google Scholar]
- INE. División Político Territorial de la República Bolivariana de Venezuela; Consultado el 25 de octubre de 2024; Instituto Nacional de Estadística de Venezuela: Caracas, Venezuela, 2013. [Google Scholar]
- Monzón-Briceño, C.A. La evolución político administrativa de Mérida y el dominio y jurisdicción del sur del lago. Boletín Acad. Nac. Hist. 2005, 352, 119–155. [Google Scholar]
- Ataroff, M.; Sarmiento, L. Las Unidades Ecológicas de los Andes de Venezuela; La Marca, E., Soriano, P., Eds.; Reptiles de Los Andes de Venezuela; Fundación Polar, Codepre-ULA, Fundacite-Mérida, Biogeos: Mérida, Mexico, 2004; pp. 9–26. [Google Scholar]
- Ellis, E.C.; Goldewijk, K.K.; Siebert, S.; Lightman, D.; Ramankutty, N. Anthropogenic transformation of the biomes. Glob. Ecol. Biogeogr. 2010, 19, 589–606. [Google Scholar] [CrossRef]
- Johnston, A.S.A.; Sibly, R.M.; Hodson, M.E.; Alvarez, T.; Thorbek, P. Effects of agricultural management practices on earthworm populations and crop yield: Validation and application of a mechanistic modelling approach. J. Appl. Ecol. 2015, 525, 1334–1342. [Google Scholar] [CrossRef]
- Maggi, F.; Tang, F.H.M. Estimated decline in global earthworm population size caused by pesticide residue in soil. Soil Secur. 2021, 5, 100014. [Google Scholar] [CrossRef]
- Cruz-Arroyave, C.A.; Toro-Cardona, F.A.; Parra, J.L. Integrating niche and occupancy models to infer the distribution of an endemic fossorial snake (Atractus lasallei). PLoS ONE 2024, 19, e0308931. [Google Scholar] [CrossRef]
- Esqueda, L.F.; La Marca, E. Patrones Ecogeográficos y Estatus de Conservación. In Atlas Serpientes de Venezuela; Natera-Mumaw, M., Esqueda-González, L.F., Castelaín-Fernández, M., Eds.; Dimacofi Negocios Avanzados S.A.: Santiago de Chile, Chile, 2015. [Google Scholar]
- Osorio, M.R.A.; Lozano, E.; Graterol, G.S. Cartografía de la cobertura y uso de la tierra en la cuenca alta del rio Santo Domingo, estado Mérida, Venezuela. Rev. For. Venez. 2009, 532, 183–190. [Google Scholar]
- Ataroff, M. Bosques Nublados del Neotrópico: Capítulo Venezuela; Kappelle, M., Brown, A.D., Eds.; Editorial IMBIO: Heredia, Costa Rica, 2001; pp. 397–442. [Google Scholar]
- Ataroff, M.; Rada, F. Deforestation impact on water dynamics in a Venezuelan Andean cloud forest. Ambio 2000, 29, 438–442. [Google Scholar] [CrossRef]
- Cavelier, J.; Lizcaíno, D.; Pulido, M.T. Bosques Nublados del Neotrópico: Capítulo Colombia; Kappelle, M., Brown, A.D., Eds.; Editorial IMBIO: Heredia, Costa Rica, 2001; pp. 443–496. [Google Scholar]
- Esqueda, L.F.; Rojas-Runjaic, F.J.M.; Correa, C.; Ortiz, J.C.; Guerrero, P.; Jiménez, J.D.; Bazó, S.; Moreno-Pérez, A.; Aguilar, M.; Urra, F. Morphological and molecular analyses of mountain centipede snake Serpentes: Tantilla reveal a new species from Venezuelan Andes. Acad. Biol. 2025, 3. [Google Scholar] [CrossRef]
- Gray, B.G. Distribution of native and exotic earthworms in the Eastern United States: Implications for the ecology of vermivorous snakes. Bull. Chic. Herpetol. Soc. 2010, 45, 73–86. [Google Scholar]
- Silva, L.J.; Valdez, J.; Ojasti, J. Algunos aspectos de una comunidad de ofidios del norte de Venezuela. Biotropica 1985, 172, 112–125. [Google Scholar] [CrossRef]
- Martins, M.; Oliveira, M.E. Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetol. Nat. Hist. 1999, 6, 78–150. [Google Scholar]
- Balestrin, R.L.; Di-Bernardo, M.; Moreno, A.G. Feeding ecology of the neotropical worm snakes Atractus reticulatus in southern Brazil. Herpetol. J. 2007, 17, 62–64. [Google Scholar]
- Ataroff, M.; Naranjo, M.E. Interception of water by pastures of Pennisetum clandestinum Hochst. ex Chiov. and Melinis minutiflora Beauv. Agric. For. Meteorol. 2009, 149, 1616–1620. [Google Scholar] [CrossRef][Green Version]
- Hendrix, F.P.; Callaham, M.A.; Drake, J.M.; Huang, C.H.; James, S.W.; Snyder, B.A.; Zhang, W. Pandora’s box contained bait: The global problem of introduced earthworms. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 593–613. [Google Scholar] [CrossRef]
- Lavelle, P.; Lapied, E. Endangered earthworms of Amazonia: An homage to Gilberto Righi: The 7th international symposium on earthworm ecology · Cardiff · Wales · 2002. Pedobiología 2003, 475, 419–427. [Google Scholar] [CrossRef]
- Serrano, F.C.; Ponte-Nogueira, M.; Sawaya, R.J.; Alencar, L.R.V.; Nogueira, C.C.; Grazziotin, F. There and back again: When and how the world’s richest snake family Dipsadidae dispersed and speciated across the Neotropical region. J. Biogeogr. 2024, 515, 878–893. [Google Scholar] [CrossRef]
- Prudente, A.L.C.; Passos, P. Morphological variation, polymorphism, and Taxonomy of the Atractus torquatus complex Serpentes: Dipsadidae. Zootaxa 2012, 3407, 1–21. [Google Scholar] [CrossRef]
- Passos, P.; Mueses-Cisneros, J.J.; Lynch, J.D.; Fernandes, R. Pacific lowland snakes of the genus Atractus Serpentes: Dipsadidae, with description of three new species. Zootaxa 2009, 22931, 1–34. [Google Scholar] [CrossRef]
- Melo-Sampaio, P.R.; Passos, P.; Prudente, A.L.C.; Venegas, P.J.; Torres-Carvajal, O. Systematic review of the polychromatic ground snakes Atractus snethlageae complex reveals four new species from threatened environments. J. Zool. Syst. Evol. Res. 2021, 59, 718–747. [Google Scholar] [CrossRef]
- Murphy, J.C.; Salvi, D.; Braswell, A.L.; Jowers, M.J. Phylogenetic position and biogeography of three-lined snakes Atractus trilineatus: Squamata, Dipsadidae in the Eastern Caribbean. Herpetologica 2019, 753, 247–253. [Google Scholar] [CrossRef]
- Melo-Sampaio, P.R.; Passos, P.; Fouquet, A.; Prudente, A.L.C. Systematic review of Atractus schach Serpentes: Dipsadidae species complex from the Guiana Shield with description of three new species. Syst. Biodivers. 2019, 173, 207–229. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; de Lima, L.C.B.; Passos, P.; Silva, M.J.J. The good, the bad and the boa: An unexpected new species of a true boa revealed by morphological and molecular evidence. PLoS ONE 2024, 194, E0298159. [Google Scholar] [CrossRef]
- Arteaga, A.; Pyron, R.A.; Batista, A.; Vieira, J.; Pelayo, E.M.; Smith, E.N.; Barrio-Amorós, C.L.; Koch, C.; Agne, S.; Valencia, J.H.; et al. Systematic revision of the Eyelash Palm-Pitviper Bothriechis schlegelii (Serpentes, Viperidae), with the description of five new species and revalidation of three. Evol. Syst. 2024, 8, 15–64. [Google Scholar] [CrossRef]
- Reyes-Velasco, J. A revision of recent taxonomic changes to the eyelash palm pitviper, Bothriechis schlegelii Serpentes, Viperidae. Herpetozoa 2024, 37, 305–319. [Google Scholar] [CrossRef]
- Cartens, B.C.; Pelletier, T.A.; Reid, N.M.; Satler, J.D. How to fail at species delimitation. Mol. Ecol. 2013, 22, 4369–4383. [Google Scholar] [CrossRef]
- Taylor, S.A.; Larson, E.L. Insights from genomes into the evolutionary importance and prevalence of hybridization in nature. Nat. Ecol. Evol. 2019, 3, 170–177. [Google Scholar] [CrossRef]
- Mason, A.J.; Grazziotin, F.G.; Zaher, H.; Lemmon, A.R.; Lemmon, E.M.; Parkison, C.L. Reticulate evolution in nuclear Middle America causes discordance in the phylogeny of palm-pitvipers Viperidae: Bothriechis. J. Biogeogr. 2019, 465, 833–844. [Google Scholar] [CrossRef]
- Myers, E.M. Genome-wide data reveal extensive gene flow during the diversification of the western rattlesnakes Viperidae: Crotalinae: Crotalus. Mol. Phylogenet. Evol. 2021, 165, 107313. [Google Scholar] [CrossRef]
- Schield, D.R.; Adams, R.H.; Card, D.C.; Perry, B.W.; Pasquesi, G.M.; Jezkova, T.; Portik, D.M.; Andrew, A.L.; Spencer, C.L.; Sanchez, E.E.; et al. Insight into the roles of selection in speciation from genomic patterns of divergence and introgression in secondary contact in venomous rattlesnakes. Ecol. Evol. 2017, 711, 3951–3966. [Google Scholar] [CrossRef] [PubMed]
- Schield, D.R.; Perry, B.W.; Adams, R.H.; Card, D.C.; Jezkova, T.; Pasquesi, G.I.; Nikolakis, Z.L.; Row, K.; Meik, J.M.; Smith, C.F.; et al. Allopatric divergence and secondary contact with gene flow: A recurring theme in rattlesnake speciation. Biol. J. Linn. Soc. 2019, 128, 149–169. [Google Scholar] [CrossRef]
- Wiens, J.J. Speciation and ecology revisited: Phylogenetic niche conservatism and the origin of species. Evolution 2004, 581, 193–197. [Google Scholar] [CrossRef]
- Kozak, K.H.; Wiens, J.J. Does niche conservatism promote speciation? A case study in North American salamanders. Evolution 2006, 6012, 2604–2621. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberon, J.; Sánchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef]
- Martínez-Meyer, E.; Townsend, P.A.; Hargrove, W.W. Ecological niches as stable distributional constraints on mammal species, with implications for Pleistocene extinctions and climate change projections for biodiversity. Glob. Ecol. Biogeogr. 2004, 134, 305–314. [Google Scholar] [CrossRef]
- Cadena, C.D.; Kozak, K.H.; Gómez, J.P.; Parra, J.L.; McCain, C.M.; Bowie, R.C.; Carnaval, A.C.; Moritz, C.; Rahbek, C.; Roberts, T.E.; et al. Latitude, elevational climatic zonation and speciation in New World vertebrates. Proc. R Soc. B Biol. Sci. 2012, 279, 194–201. [Google Scholar] [CrossRef]
- Jetz, W.; Rahbek, C. Geographic range size and determinants of avian species richness. Science 2002, 2975586, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Kissling, W.D.; Flantua, S.G.; Bermúdez, M.A.; Mulch, A.; Muellner-Riehl, A.N.; Kreft, H.; Linder, H.P.; Badgley, C.; Fjeldså, J.; et al. Geological and climatic influences on mountain biodiversity. Nat. Geosci. 2018, 11, 718–725. [Google Scholar] [CrossRef]
- Schluter, D. Evidence for ecological speciation and its alternative. Science 2009, 323, 737–741. [Google Scholar] [CrossRef]
- Azevedo, J.A.; Guedes, T.B.; Nogueira, C.D.; Passos, P.; Sawaya, R.J.; Prudente, A.L.; Barbo, F.E.; Strüssmann, C.; Franco, F.L.; Arzamendia, V.; et al. Museums and cradles of diversity are geographically coincident for narrowly distributed Neotropical snakes. Ecography 2020, 43, 328–339. [Google Scholar] [CrossRef]
- Audemard, F.E.; Audemard, F.A. Structure of the Mérida Andes, Venezuela: Relations with the South America–Caribbean geodynamic interaction. Tectonophysics 2002, 345, 1–26. [Google Scholar] [CrossRef]
- Bermúdez, M.A.; Van Der Beek, P.; Bernet, M. Asynchronous Miocene-Pliocene exhumation of the central Venezuelan Andes. Geology 2011, 39, 139–142. [Google Scholar] [CrossRef]
- Erikson, P.J.; Kelly, S.A.; Osmolovky, P.; Verosub, K.L. Linked basin sedimentation and orogenic uplift: The Neogene Barinas basin sediments derived from the Venezuelan Andes. J. South Am. Earth Sci. 2012, 39, 138–156. [Google Scholar] [CrossRef]
- Boschman, L.M. Andean mountain building and magmatism: Insights from paleogeographic reconstructions. Glob. Planet. Change 2021, 202, 103493. [Google Scholar] [CrossRef]
- Hernández-Hernández, T.; Miller, E.C.; Román-Palacios, C.; Wiens, J.J. Speciation across the Tree of Life. Biol. Rev. 2021, 96, 1205–1242. [Google Scholar] [CrossRef]
- Aguilée, R.; Gascuel, F.; Lambert, A.; Ferriere, R. Clade diversification dynamics and the biotic and abiotic controls of speciation and extinction rates. Nat. Commun. 2018, 9, 3013. [Google Scholar] [CrossRef]
- Anderson, S.A.S.; Weir, J.T. The role of divergent ecological adaptation during allopatric speciation in vertebrates. Science 2022, 378, 1214–1218. [Google Scholar] [CrossRef]
- Navas, C. Patterns of distribution of anurans in high Andean tropical elevations: Insights from integrating biogeography and evolutionary physiology. Integr. Comp. Biol. 2006, 4671, 82–91. [Google Scholar] [CrossRef]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Rahbek, C.N.; Gotelli, J.; Colwell, R.K.; Entsminger, G.L.; Fernando, T.; Rangel, L.V.B.; Graves, G.R. Predicting continental-scale patterns of bird species richness with spatially explicit models. Proc. R. Soc. B Biol. Sci. 2006, 274, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Lagomarsino, L.P.; Condamine, F.L.; Antonelli, A.; Mulch, A.; Davis, C.C. The abiotic and biotic drivers of rapid diversification in Andean bellflowers Campanulaceae. New Phytol. 2016, 210, 1430–1442. [Google Scholar] [CrossRef]
- Quintero, I.; Jetz, W. Global elevational diversity and diversification of birds. Nature 2018, 555, 246–250. [Google Scholar] [CrossRef]
- Thom, G.; Gehara, M.; Smith, B.T.; Miyaki, C.Y.; do Amaral, F.R. Microevolutionary dynamics show tropical valleys are deeper for montane birds of the Atlantic Forest. Nat. Commun. 2021, 12, 62–69. [Google Scholar] [CrossRef]
- Moreira, M.O.; Wiens, J.J.; Fonseca, C.; Rojas, D. Climatic-niche breadth, niche position, and speciation in lizards and snakes. J. Biogeogr. 2024, 516, 969–981. [Google Scholar] [CrossRef]
- Aubret, F.; Shine, R. Causes and consequences of aggregation by neonatal tiger snakes (Notechis scutatus, Elapidae). Austral Ecol. 2009, 34, 210–217. [Google Scholar] [CrossRef]
- Böhm, M.; Kemp, R.; Williams, R.; Davidson, A.D.; Garcia, A.; McMillan, K.M.; Bramhall, H.R.; Collen, B. Rapoport’s rule and determinants of species range size in snakes. Divers. Distrib. 2017, 23, 1472–1481. [Google Scholar] [CrossRef]
- Birskis-Barros, I.; Alencar, L.R.; Prado, P.I.; Böhm, M.; Martins, M. Ecological and Conservation Correlates of Rarity in New World Pitvipers. Diversity 2019, 11, 147. [Google Scholar] [CrossRef]
- Ferreira-Silva, C.; Ribeiro, S.C.; de Alcantara, E.P.; Ávila, R.W. Natural history of the rare and endangered snake Atractus ronnie Serpentes: Colubridae in northeastern Brazil. Phyllomedusa 2019, 181, 77–87. [Google Scholar] [CrossRef]
- Llambí, L.D. Estructura, diversidad y dinámica de la vegetación En el ecotono bosque-páramo: Revisión de la evidencia en la cordillera de Mérida. Acta Biol. Colomb. 2015, 203, 5–19. [Google Scholar] [CrossRef]
- Chacón-Moreno, E.; Rodríguez-Morales, M.; Paredes, D.; Suárez del Moral, P.; Albarrán, A. Impacts of global change on the spatial dynamics of treeline in Venezuelan Andes. Front. Ecol. Evol. 2021, 9, 615223. [Google Scholar] [CrossRef]
- Miglani, R.; Upadhyay, J.; Rana, M.; Bisht, S.S. In vitro effect of insecticide emamectin benzoate on earthworm, Metaphire posthuma, using contact filter paper method. Appl. Biol. Res. 2019, 21, 207–209. [Google Scholar] [CrossRef]
- Singh, J.; Schädler, M.; Demetrio, W.; Brown, G.G.; Eisenhauer, N. Climate change effects on earthworms—A review. Soil Org. 2019, 91, 113–137. [Google Scholar] [CrossRef]
- Perry, G.; Pianka, E.R. Animal foraging: Past, present and future. Trends Ecol. Evol. 1997, 129, 360–369. [Google Scholar] [CrossRef]
- Böhm, M.; Collen, B.; Baillie, J.E.M.; Bowles, P.; Chanson, J.; Cox, N.; Hammerson, G.; Hoffmanng, M.; Livingstone, S.R.; Ram, M.; et al. The conservation status of the world’s reptiles. Biol. Conserv. 2013, 157, 372–385. [Google Scholar] [CrossRef]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, L. Nuestra academia en la penumbra. Saber 2023, 35, 12788969. [Google Scholar] [CrossRef]
- Oliveira-Miranda, M.A.; Huber, O.; Rodríguez, J.P.; Rojas-Suárez, F.; de Oliveira-Miranda, R.; Hernández-Montilla, M.; Zambrano-Martínez, S. Riesgo de Eliminación de los Ecosistemas Terrestres de Venezuela. In Libro Rojo de los Ecosistemas Terrestres de Venezuela; Rodríguez, J.P., Rojas-Suárez, F., Giraldo Hernández, D., Eds.; Provita, Shell Venezuela, Lenovo Venezuela: Caracas, Venezuela, 2010; pp. 107–235. [Google Scholar]
- Arévalo, E.; Davis, S.K.; Sites, J.W. Mitochondrial DNA-sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in Central Mexico. Syst. Biol. 1994, 43, 387–418. [Google Scholar] [CrossRef]
- Chiari, Y.; Vences, M.; Vieites, D.R.; Rabemananjara, F.; Bora, P.; Ravoahangimalala, O.R.; Meyer, A. New evidence for parallel evolution of colour patterns in Malagasy poison frogs (Mantella). Mol. Ecol. 2004, 13, 3763–3774. [Google Scholar] [CrossRef] [PubMed]
- Noonan, B.P.; Chippindale, P.T. Dispersal and vicariance: The complex evolutionary history of bold snakes. Mol. Phylogenet. Evol. 2006, 40, 347–358. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Martin, A.; Romano, S.; McMillan, W.O.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0; University of Hawaii: Honolulu, HI, USA, 1991; 94p. [Google Scholar]
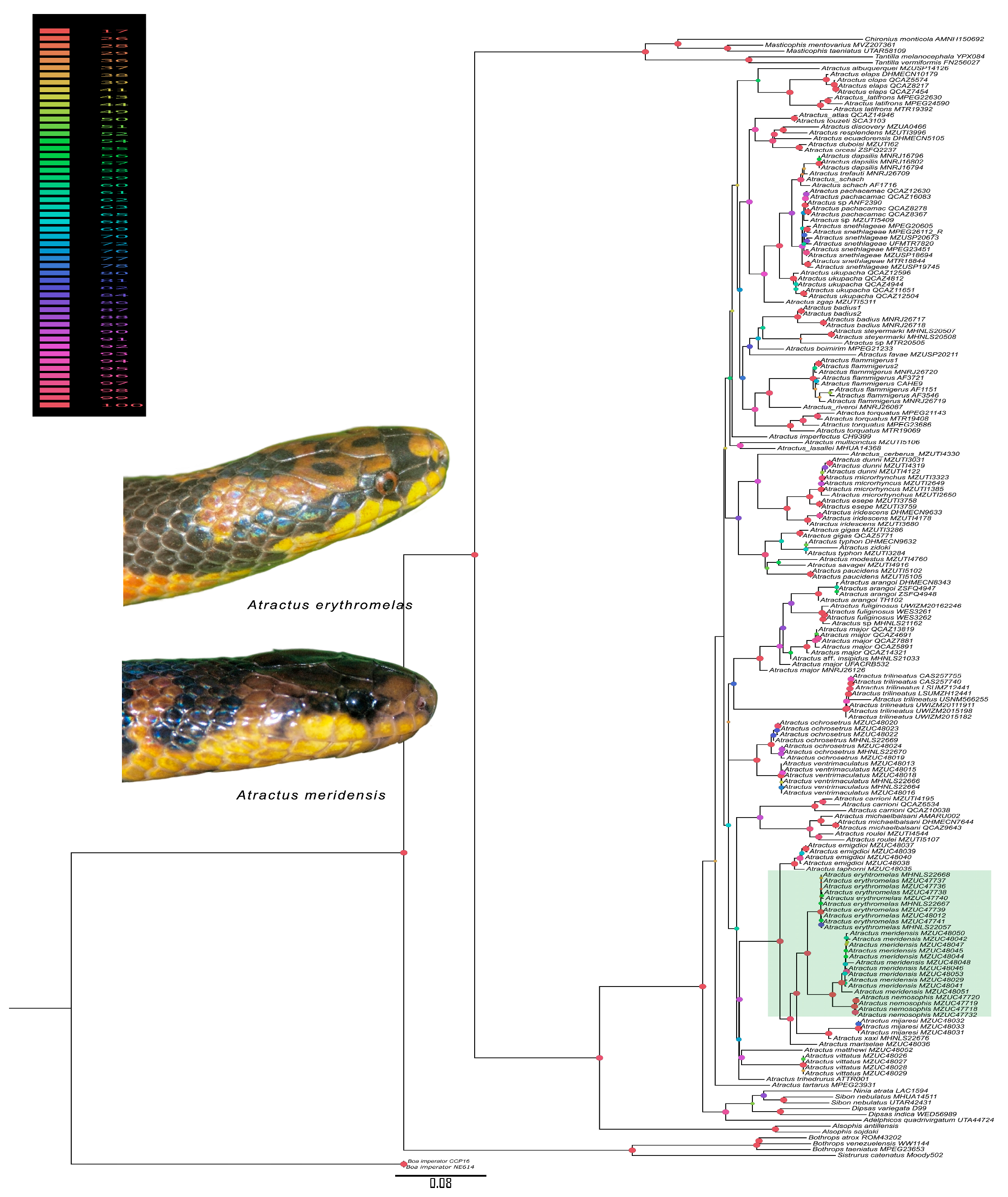
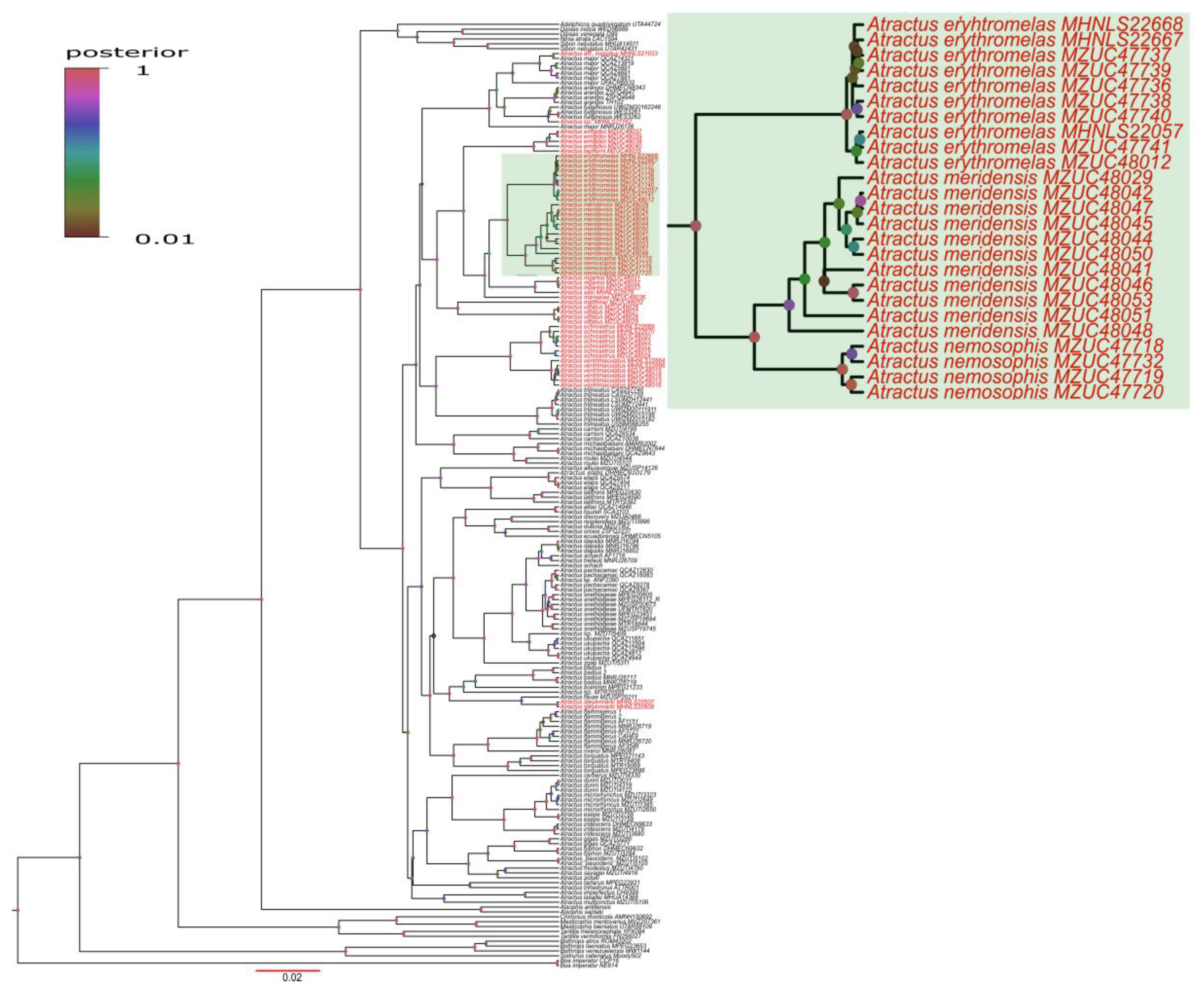
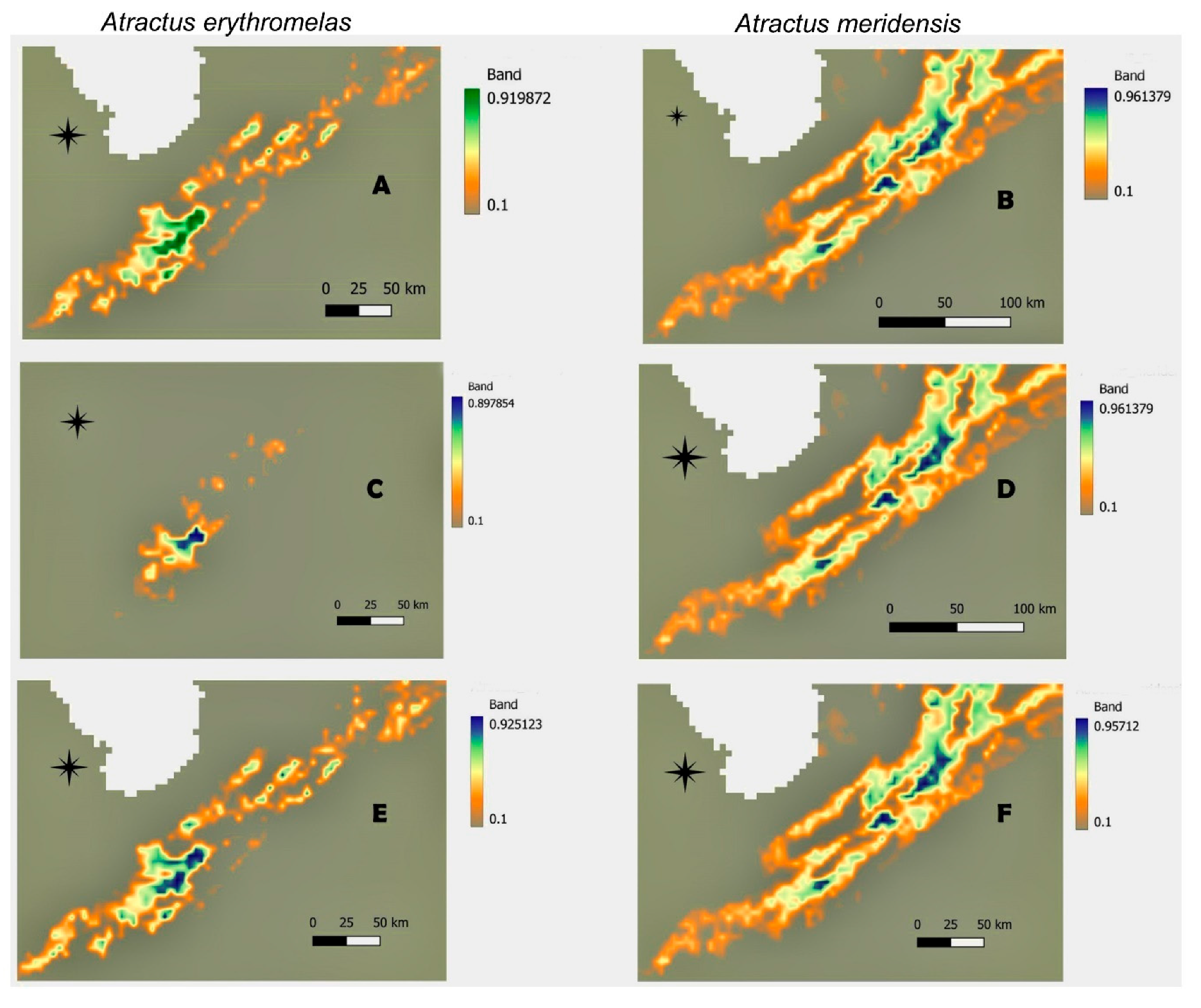

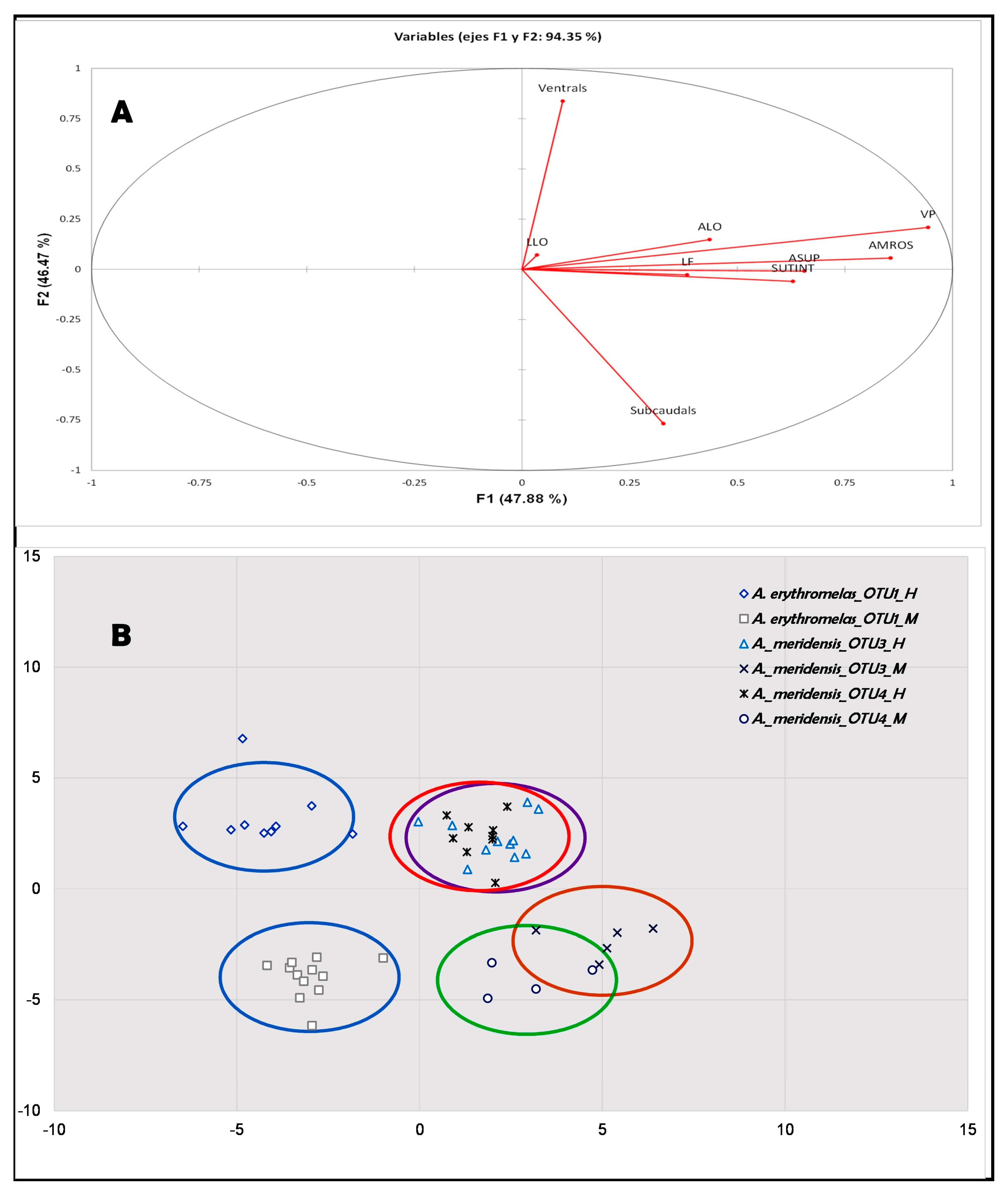


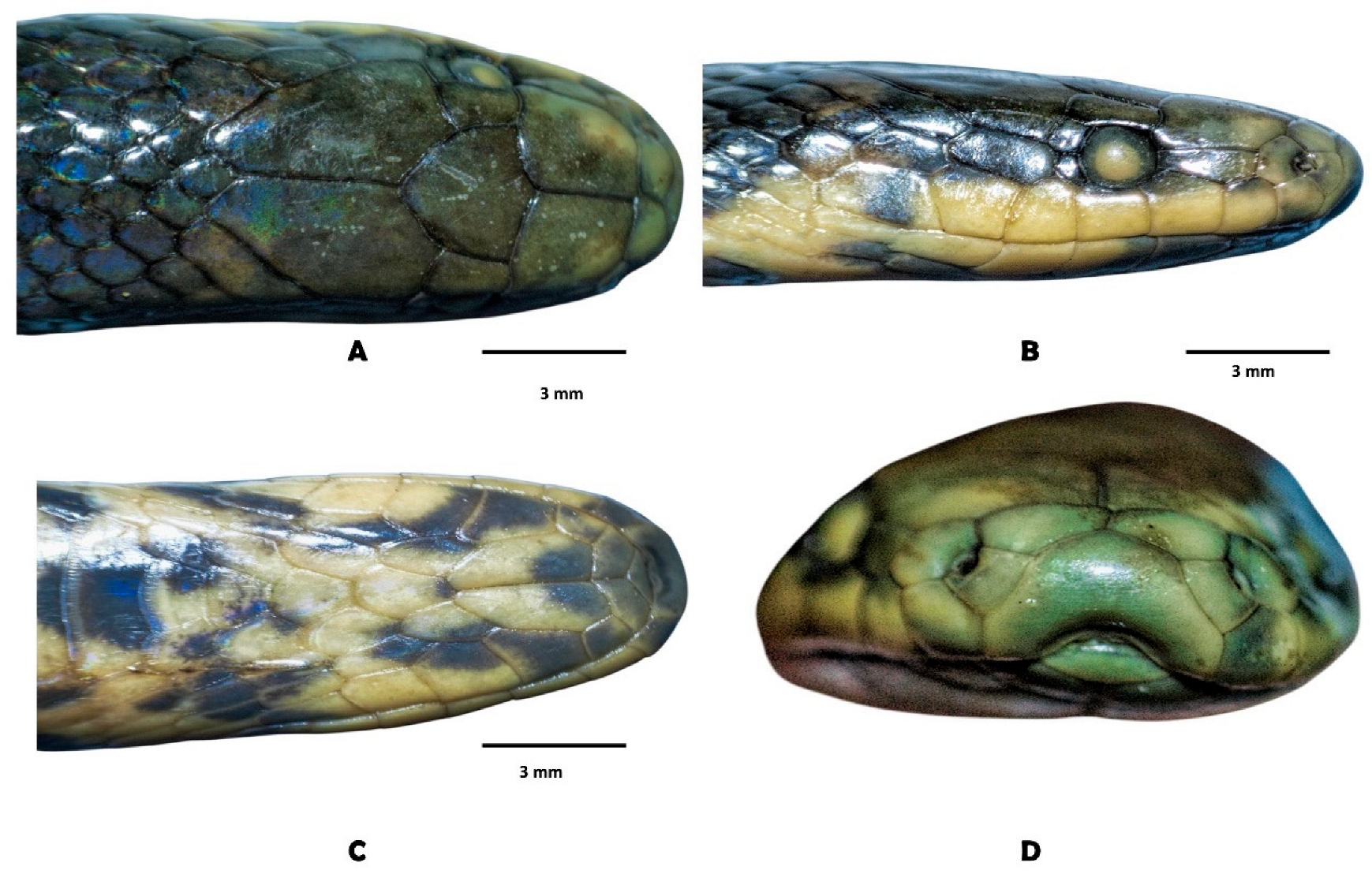

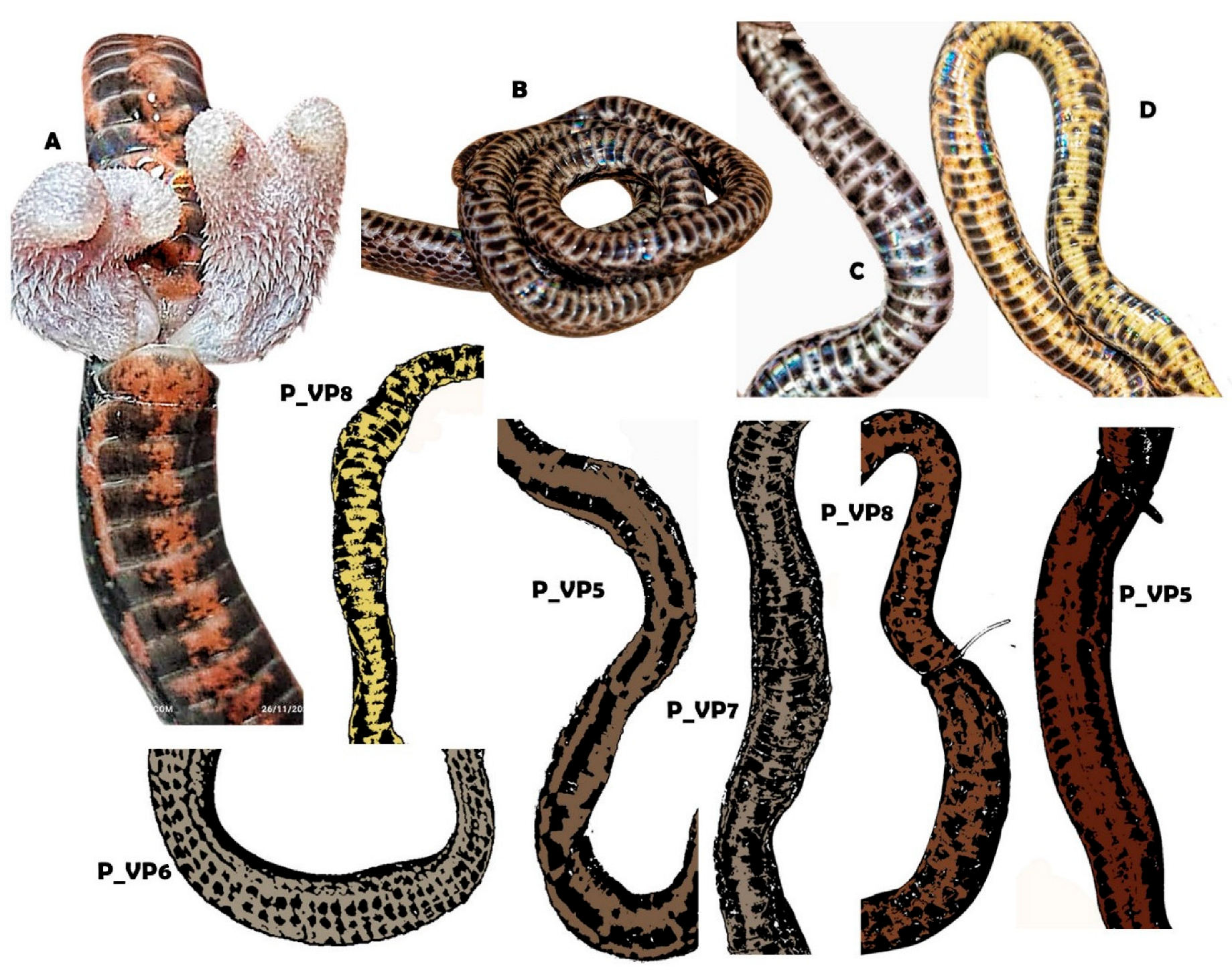


| Morphology, Coloration and Geography | A. meridensis | A. erythromelas | Atractus nemosophis | A. micheleae |
|---|---|---|---|---|
| Geographic Distribution (CM, Venezuela) | Isolated populations to the northeast of the state of Mérida and Trujillo, in the river basins Motatán, Boconó and Santo Domingo | Medium-high basin of the Chama River, from the city of Mérida to beyond Mucurubá, Mérida state | Isolated populations northeast of the Trujillo-Mérida state, in the river basins Motatán, Boconó and Santo Domingo | Southwest isolated populations of the state Táchira and the Boconó River Basin, Trujillo state |
| Altitudinal range | 1160–2684 m asl | 1077–3275 m asl | 1864–2404 m asl | 900–1500 m asl |
| Habitat (natural) | Andean montane semicaducifolious forest and cloud forest | Andean montane semicaducifolious forests and cloud forest | Andean montane semicaducifolious forest and cloud forest | Andean submontane forest and Andean montane semicaducifolious forest |
| Maximum Total Lenght (SVL + TaL) | 433.68 mm males and 406.46 mm in females | 338 mm in males and 449.57 mm in females | 419 mm in males and 470 mm in females | 324.74 mm in males and 405 mm females |
| Rows of dorsal scales at midbody | 17 (always) | 15 or 17 | 17 (always) | 17 |
| Ventral scale (count) | 156–173 males and 160–180 females | 151–169 males and 169–179 females | 158–177 males, 173–190 females | 152–159 males and 162–172 females |
| Subcaudal scale (count) | 27–39 males and 21–35 females | 26–35 males and 16–27 females | 36–41 males and 23–31 females | 32–35 males and 23–28 females |
| Supralabials | 7–8 (3,4) | 7 (3,4) | 7 (3,4) | 8(4,5) or 8 (3,4) |
| Infralabials | 6 (3-4) | 6 (3) | 6 (3), rarely four | 7(4) |
| Supralabials in contact with Loreal | Two | Two | Two | Three |
| Eye-Prefrontal contact | Greater than eye-loreal contact | Greater than eye-loreal contact | Usually shorter than eye-loreal contact | Greater than eye-loreal contact |
| Rostral (dorsal view) | Distinctively visible from above (condition C) | Slightly visible or barely visible from above (frequent) | Distinctively visible from above (condition C) | Barely visible from above |
| Prenasal | Longer than tall, small or similar than postnasal | Taller than long, usually smaller than postnasal | Taller than long, smaller than postnasal | Higher than long, slightly larger than postnasal |
| Eye-Loreal contact | Distinctively narrow, less than the anterior margin of the loreal-postnasal | Usually narrow or similar that the anterior margin of the loreal-postnasal | Distinctively narrow, less than the anterior margin of the loreal-postnasal | Distinctively narrow, less than the anterior margin of the loreal-postnasal |
| Supralabial-rostral contact | Ample, supralabial scale pentagonal but does not form an angle, that is, protrusion between postnasal and prenasal. Enlarged supralabial | Short-moderate, usually the supralabial scale is pentagonal, taller than wide, forming an angle, that is, postnasal and prenasal project. Small or moderate supralabial | Ample, supralabial scale pentagonal but does not form an angle, protrusion between postnasal and prenasal. Enlarged supralabial | Short, supralabial scale pentagonal, taller than wide, protrusion between postnasal-prenasal. Small supralabial |
| Posterior supratemporal (elongated) | Absent, less frequent present | Absent, less frequent present | Present (frequent) | Present (frequent) |
| Internasals | Usually longer than wide, moderate-long suture | usually quandragular, short-moderate suture | Longer than wide, moderate-long suture | Usually longer than wide, short-moderate suture |
| Frontal scute | Usually so wide than long or wider | Usually longer than wide | Usually so wide than long or wider | Usually so wide than long or wider |
| Head in lateral view, snout | Forming an oblique angle | Rounded or subacuminate | Forming an oblique angle | variable, rounded or subacuminate |
| Head in view dorsal (forms) | Oblong, snout not compressed | Oblong, snout not compressed | Oblong, snout not compressed | Subtriagular, snout slightly compressed |
| Snout (lateral view) | Slightly truncated | Rounded | Rounded | Rounded |
| Maxillary and lateral process | Well-developed palatine lateral process, located between the fourth and fifth tooth | Well-developed palatine lateral process, located between the six and seven tooth | Well-developed palatine lateral process, located between the third and fourth tooth | Well-developed palatine lateral process, located between the third and fourth tooth |
| Maxillary teeth | 5–6 | 9–10 | 5–6 | 5–8 |
| Hemipenis | Semicapitate, semicalyculate, moderate bilobed or bilobate, subcylindrical lobes and rounded apex | Semicapitate, semicalyculate, slightly bilobed, clavate lobes and rounded apex | Semicapitate, semicalyculate, moderate bilobed, cylindrical lobes and plane apex | Semicapitate, semicalyculate, moderate bilobed, cylindrical lobes and plane apex |
| Dorsal pattern in life | Dichromatic-bicolor, red-white, and black croosbands, interspersed, light bands marginated of back | Polymorphic, there are bicolor individuals with red-white and black bands, interspersed, clear non-marginalized bands; another pattern is brown or yellow brown with irregular black stains (tabby); reddish background, almost immaculate, with few spots that form an occasionally | A pattern frequent is the grayish-brown dorsum, without a vertebral line but with small dark spots scattered irregularly along the body; another reddish or reddish -brown pattern uniform or with a vertebral black (less than one scale) and another observed is grayish brown with irregularly scattered spots, being able to also be uniform | Dichromatic-bicolor, red-white and black-brown crossbands, interspersed, lights croosbands not marginated of black |
| Dark line behind and in front of the eyes | Usually absent, rarely defined | Present both and conspicuous, rarely absent | Absent | Absent |
| Light band behind the parietals | Present, wel defined | Present, well defined | Present, inconspicuous in adults | Absent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esqueda, L.F.; Ortiz, J.C.; Correa, C.; Guerrero, P.C.; Navarrete, L.F.; Urra, F. Integrative Taxonomic Assessment of Two Atractus (Serpentes: Dipsadidae) from Mérida Andes, Venezuela. Diversity 2025, 17, 725. https://doi.org/10.3390/d17100725
Esqueda LF, Ortiz JC, Correa C, Guerrero PC, Navarrete LF, Urra F. Integrative Taxonomic Assessment of Two Atractus (Serpentes: Dipsadidae) from Mérida Andes, Venezuela. Diversity. 2025; 17(10):725. https://doi.org/10.3390/d17100725
Chicago/Turabian StyleEsqueda, Luis Felipe, Juan Carlos Ortiz, Claudio Correa, Pablo C. Guerrero, Luis Fernando Navarrete, and Félix Urra. 2025. "Integrative Taxonomic Assessment of Two Atractus (Serpentes: Dipsadidae) from Mérida Andes, Venezuela" Diversity 17, no. 10: 725. https://doi.org/10.3390/d17100725
APA StyleEsqueda, L. F., Ortiz, J. C., Correa, C., Guerrero, P. C., Navarrete, L. F., & Urra, F. (2025). Integrative Taxonomic Assessment of Two Atractus (Serpentes: Dipsadidae) from Mérida Andes, Venezuela. Diversity, 17(10), 725. https://doi.org/10.3390/d17100725







