Abstract
Cirratulid polychaetes are abundant, ecologically relevant, and widely used as bioindicators, but systematic studies worldwide remain scarce due to systematic challenges. Chaetozone Malmgren, 1867, is the most diverse genus of Cirratulidae. The first taxonomic study of Chaetozone in Brazil was carried out on the southeastern and southern coast in a region of sedimentary basins under oil exploration. Four new species are described: C. beneditae sp. nov., C. bidentata sp. nov., C. lesliae sp. nov., and C. lutzae sp. nov. We also provide the first Brazilian record of C. larae (Elias, Rivero & Orensanz, 2017), expanding the distribution from Argentina to Brazil. A taxonomic key to Brazilian Chaetozone species is presented, contributing to cirratulid systematics and South Atlantic polychaete diversity.
1. Introduction
Chaetozone Malmgren, 1867 [1], is a genus of Cirratulidae, a family of sedentary polychaetes with about 380 valid species in 16 genera [2]. Cirratulids are widespread deposit feeders from intertidal to abyssal depths, inhabiting both consolidated and unconsolidated sediments [3]. Several species are recognized as bioindicators of organic enrichment due to their ecological tolerance [4]. Despite advances in their biology and ecology, the systematics of the family remain problematic, with species identification hindered by the difficulty in distinguishing synapomorphies from homoplasies, ontogenetic variation, and strong regenerative capacity [5,6,7].
With 81 valid species currently recognized, Chaetozone is the largest genus with a cosmopolitan distribution [2]. Most research has mainly focused on the North Atlantic and Northeast Pacific. Diagnostic characters include posterior acicular spines forming conspicuous cinctures, typically with unidentate and alternating with capillaries, although bidentate spines may occasionally occur [8].
In the Southwestern Atlantic, knowledge of Chaetozone remains scarce. To date, only Chaetozone corona Berkeley & Berkeley, 1941 [9], has been formally reported from Brazil, near Rio de Janeiro [10], while Chaetozone larae Elias, Rivero & Orensanz, 2017 [11], was described from Argentina. Other cirratulid genera, such as Timarete Kinberg, 1866, Kirkegaardia Blake, 2016, and Dodecaceria Örsted, 1843, have recently received systematic attention [12,13,14]. Along the Brazilian coast, 38 cirratulid species are reported in 11 genera [15], although many records lack formal revision or voucher confirmation.
Herein, we describe four new Chaetozone species from the eastern and southern Brazilian continental shelf. We also provide the first record of C. larae in Brazil, extending its distribution, and present an identification key to Brazilian Chaetozone species.
2. Materials and Methods
2.1. Study Site
The study was conducted in the Campos and Santos Basins, two of the most important sedimentary basins along the southeastern Brazilian margin, between the states of Espírito Santo and Santa Catarina. The Campos Basin covers ~100,000 km2, extending from the coast between Vitória, Espírito Santo, and Cabo Frio, Rio de Janeiro, to ~3400 m depth, and was once Brazil’s main oil-bearing basin [16]. The Santos Basin, covering ~350,000 km2 from Cabo Frio, Rio de Janeiro, to Florianópolis, Santa Catarina, is currently the largest and most significant offshore sedimentary basin in the Southwestern Atlantic, mainly due to port activities and pre-salt oil exploration [17]. Oceanographically, the transition between the two basins is marked by the Cabo Frio Upwelling System, which brings nutrient-rich South Atlantic Central Water onto the shelf, enhancing primary productivity in the region [18,19].
2.2. Sample Collection
Samples were obtained between 2008 and 2021 by three independent projects in southeastern Brazil, coordinated by the Petrobras Research Center (CENPES/PETROBRAS): HABITATS—Environmental Heterogeneity in the Campos Basin (400–3000 m), AMBES—Environmental Heterogeneity in the Espírito Santo Basin and northern Campos Basin (50–3000 m), and the SANTOS Project—Environmental Characterization of the Santos Basin (25–2400 m). Macrobenthic samples were collected with a USNEL box corer (0.25 m2) and sieved through 0.3–0.5 mm meshes. Specimens were fixed in 10% sea water–formalin, rinsed with fresh water, and preserved in 70% ethanol. All sampling permits were held by CENPES/PETROBRAS.
2.3. Systematic Analysis
Specimens were examined and measured at the TaxoN Laboratory, Federal University of Rio de Janeiro (UFRJ), using a Zeiss Stemi SV 11 stereomicroscope and a Zeiss Primo Star optical microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with digital imaging (ZEN 2012 Blue). Some specimens were stained with Shirlastain A (Direct Red 80; Sigma-Aldrich, St. Louis, MO, USA) to enhance body surface structures. Selected specimens were prepared for scanning electron microscopy (SEM) at the Precision Medicine Research Center (Centro de Pesquisa em Medicina de Precisão—CPMP-UFRJ). Specimens were dehydrated through a graded ethanol series (70–100%), critical-point-dried, and coated with ~25 nm of gold. SEM observations were conducted at the National Center of Structural Biology and Bioimaging (Centro Nacional de Biologia Estrutural e Bioimagem—CENABIO/UFRJ), using a FEI model Quanta 250 (Thermo Fisher Scientific, Waltham, MA, USA) and Zeiss model EVO 10. Holotypes and paratypes were deposited in the Annelida Collection of the National Museum (Museu Nacional do Rio de Janeiro—MNRJ/UFRJ), Rio de Janeiro, Brazil.
3. Results
3.1. Systematics
- Family Cirratulidae Ryckholt, 1851 [20]
Description. Body elongated, cylindrical; anterior and/or posterior segments sometimes expanded. Prostomium narrow and conical or broad, without appendages, eyespots present or absent; paired nuchal organs present dorsolaterally. Peristomium achaetous, smooth or with rings (annuli). Grooved dorsal palps one or multiple pairs on posterior margin of peristomium or one or more anterior chaetigers. Branchiae elongated, usually occurring over numerous chaetigers. Pharynx ventral, unarmed. Parapodia biramous with rudimentary lobes. Chaetae simple, including capillaries, acicular spines or bidentate. Pygidium a simple lobe, sometimes with sub-anal disk, or terminal cirri [8,21].
- Genus Chaetozone Malmgren, 1867
- Type species: Chaetozone setosa Malmgren, 1867 [1]
- Type locality. Arctic Ocean, Norway.
Description. Prostomium conical to pointed, short, without annulations; eyespots absent or present; posterior margin with nuchal slits or depressions, sometimes pigmented. Peristomium long, cylindrical, annulated or not; with one pair of grooved dorsal palps from posterior margin, rarely from achaetous segment or anterior chaetiger. First branchiae near palps; on achaetous segment or first chaetiger; sometimes first two pairs on single anterior segment. Body usually expanded anteriorly, tapering posteriorly, middle or posterior segments sometimes moniliform, posterior end often expanded. Capillary chaetae on most chaetigers; acicular spines in noto- and neuropodia usually forming armature on cinctured posterior segments with elevated membranes. Cinctures with few to many spines and with or without alternating capillaries sometimes encircling posterior end; bidentate spines absent or present. Some species with long, natatory-like notochaetae, sometimes restricted to mature individuals. Pygidium variable: simple lobe, disk-like, with long terminal cirrus or few short lobes [22].
Remarks. The taxonomy of Chaetozone has become increasingly complex with the description of new species and recognition of additional diagnostic characters [21,23,24]. Traditional characters, such as body shape, peristomial rings, eyes, origin of noto- and neuropodial spines, posterior cinctures, and pygidial form, remain useful but often insufficient for species description [24]. More recent studies have emphasized the presence of an achaetous segment, position of dorsal palps and branchiae, dorsal and ventral grooves, pigmentation, and acicular spine morphology [25,26,27,28]. In this study, bidentate spines are defined as spines bearing two well-developed teeth of similar or nearly similar size, whereas sub-bidentate spines are defined as spines with one tooth less developed and distinctly smaller than the other. Examples of this diversity include the enlarged anterior region of Chaetozone gibber Woodham & Chambers, 1994 [29], gut enlargement in Chaetozone brunnea Blake, 2015 [24], and Chaetozone ronaldi Magalhães & Bailey-Brock, 2013 [28], and the posterior cincture in Chaetozone palaea Blake, 2006 [23]. The presence of eyes remains problematic, since pigmented nuchal organs may be misinterpreted and require ultrastructural confirmation [24]. Pigmentation and methyl green (MG) staining provide additional, though limited, diagnostic value. The recognition of consistent morphological assemblages has led to the proposal of distinct species groups [22,24]. These include the Chaetozone setosa group, characterized by an enlarged lobe or crest overlying the peristomium; the Chaetozone bansei group, with dorsal tentacles shifted posteriorly over a chaetigerous segment; the Chaetozone curvata group, including species with acicular spines that possess a fine tip which curves back and fuses with the shaft to form a superficial blunt tip; the Chaetozone gracilis group, which comprises species with a reduced number of acicular spines that are not formed into the distinctive cinctures or the posterior armature; and a group of species with body pigmentation. In this study, five Chaetozone species are described from Brazil. Four are new to science, all with bi- or sub-bidentate spines, a character that may indicate the emergence of a previously unrecognized species group within Chaetozone.
- Chaetozone larae Elias, Rivero & Orensanz, 2017 [11]
- Chaetozone sp.: Faget, 1983: t.4
- Material examined. BRAZIL. Espírito Santo: −21.673550°, −40.974442°, 17 m, one ind., 3 November 2009, (MNRJP 008584); −19.600992°, −39.176011°, 143 m, three ind., 29 June 2013, (MNRJP 008861); −19.434722°, −39.294144°, 50 m, one ind., 19 January 2012, (MNRJP 008862); −19.163406°, −39.488831°, 26 m, one ind., 18 January 2012, (MNRJP 008863); −21.067025°, −40.313919°, 49 m, 4 ind., 22 January 2012, (MNRJP 008864); −21.067989°, −40.237261°, 153 m, two ind., 23 January 2012, (MNRJP 008865); −19.720647°, −39.559661°, 50 m, six ind., 14 July 2013, (MNRJP 008866).
Description. Complete specimens with 80–120 chaetigers. Anterior region dorsally inflated, with 30 narrow chaetigers, pear-shaped in dorsal view, first 13 chaetigers not expanded, remaining 17 laterally expanded, with narrow, weakly developed dorsal groove visible with light microscopy. Prostomium elongate, conical (Figure 1A and Figure 2A); eyespots absent, nuchal organs present. Peristomium elongate, with two lateral annulations, and low peristomial crest (Figure 2A). Dorsal palps inserted on peristomium; first pair of branchiae postero-lateral to palps in the final portion of peristomium; second pair inserted on first chaetiger (Figure 1A and Figure 2A). Anterior parapodia with noto- and neuropodial capillary chaetae in both rami, 8–10 per fascicle, numbers gradually decreasing along the body. Notopodial acicular spines on chaetigers 46–77, and neuropodial acicular spines on chaetigers 28–52. Notopodial acicular spines 1–7 per fascicle (Figure 2B), increasing in number gradually along body, accompanied by 5–6 capillary chaetae. Neuropodial acicular spines 1–9 per fascicle, increasing in number gradually along body, accompanied by 6–7 capillary chaetae. Chaetae and acicular spines golden-brown (Figure 1,C). Incomplete cinctures with elevated parapodial membranes present from posterior region (Figure 1C). Notopodial acicular spines separated by small dorsal gap, and neuropodial acicular spines with broad ventral gap between opposite parapodia. Pygidium with a simple lobe (Figure 1C).
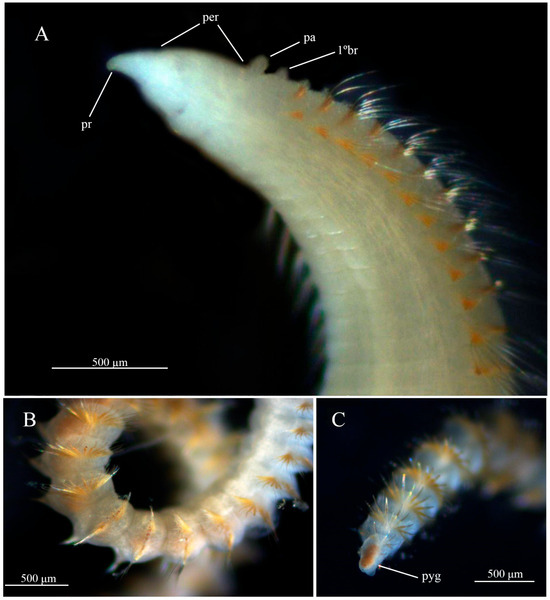
Figure 1.
Chaetozone larae. (A) anterior end, ventrolateral view; (B) segments of the middle region; (C) posterior end, dorsolateral view, (A–C) (MNRJP 008584).
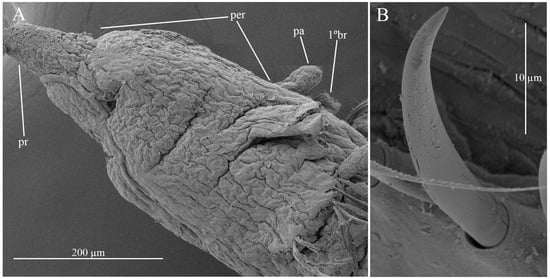
Figure 2.
Chaetozone larae. (A) anterior end, lateral view; (B) acicular spines, (A,B).
- Type locality. Atlantic Ocean: Comodoro Rivadavia, San Jorge Gulf (Argentina) [11].
- Habitat. Subtidal muddy gray bottoms at 70 m depth [11]. In this study, predominantly sandy to muddy bottoms, with or without biodebris, on silt–clay, 15–160 m depth.
- Distribution. Atlantic Ocean: San Jorge Gulf (Argentina) [11]; Espírito Santo; Rio de Janeiro; Paraná (Brazil).
Remarks. Specimens from the southeastern coast of Brazil conform closely to the original description of C. larae. This is related to the dorsally inflated anterior region with the presence of a weakly developed narrow dorsal groove, the insertion of dorsal palps and the first pair of branchiae on the peristomium, and the morphology of capillary and acicular spines. However, some variation was observed in the number of chaetigers (80–120 vs. 87–99 in the type species) and in the appearance of acicular spines and partial cincture. In the Brazilian specimens, notopodial spines begin at chaetiger 14 and neuropodial spines begin at chaetiger 12, whereas in the type material, they occur from chaetigers 43 and 40, respectively. The partial cincture in Brazilian specimens also begins slightly later (chaetiger 89 vs. 84 in holotype, 66–77 in paratypes); however, Brazilian specimens are larger. Additionally, the prostomium is more elongate and conical compared to the short, acuminate prostomium described in the type series. An additional relevant character in the Brazilian specimens is the presence of golden-brown pigmentation at the bases of chaetae and spines, which was not mentioned in the original description of C. larae. This coloration, visible with light microscopy, is a character shared with Chaetozone spinosa Moore, 1903 [30], Chaetozone commonalis Blake, 1996 [21], C. setosa Malmgren, 1867 [1], and Chaetozone dimorphosetosa (Hutchings & Murray, 1984) [31], representing an additional diagnostic character for the species. Despite these minor differences, the diagnostic characters remain consistent, and the Brazilian specimens are herein identified as C. larae, representing the first record of the species outside its type locality and the first occurrence in the Southwestern Atlantic north of Patagonia. This finding significantly extends the known distribution of C. larae northward, suggesting a broader range along the continental shelf of the western South Atlantic.
- Chaetozone bidentata sp. nov.
- Material examined. BRAZIL, Espírito Santo: Holotype—−21.83965155° S −40.52808278°, 27 m, 13 March 2009, (MNRJP 008560); Paratypes—−21.74577967° S −40.71942077° W, 21 m, three ind., 11 March 2009, (MNRJP 008561); −21.56563642° S −40.71590216° W, 22 m, two ind., 10 March 2009, (MNRJP 008562); −21.57071932° S −40.42585121° W, 29 m, one ind., 13 March 2009, (MNRJP 008563); −21.47320549° S −40.81033752° W, 22 m, five ind., 10 March 2009, (MNRJP 008564); −21.47325716° S −40.80998644° W, 21 m, three ind., 10 March 2009, (MNRJP 008565); −21.74575475° S −40.71965482° W, 22 m, five ind., 11 March 2009, (MNRJP 008566); −21.83395538° S −40.81839878° W, 22 m, two ind., 11 March 2009, (MNRJP 008567); −21.3848632° S −40.71165682° W, 28 m, two ind., 7 March 2009, (MEV); −21.41262648° S −40.42227081° W, 33 m, one ind., 7 March 2009, (MNRJP 008568); −21.83965155° S −40.52808278° W, 27 m, eight ind., 13 March 2009, (MNRJP 008569); −19.673900° S, −39.605458° W, 40 m, two ind., 19 January 2012, (MNRJP 008570); −21.8396499° S −40.52813452° W, 27 m, one ind., 13 March 2009, (MNRJP 008571); −21.56511314° S −40.71540714° W, 22 m, one ind., 10 March 2009, (MNRJP 008572); −21.29320893° S −40.80600605° W, 24 m, one ind., 7 March 2009, (MNRJP 008573); −21.56525646° S −40.71588214° W, 21 m, six ind., 10 March 2009, (MNRJP 008574); −21.92420534° S −40.82052809° W, 20 m, one ind., 12 March 2009, (MNRJP 008575); −21.65898830° S −40.52462420° W, 28 m, one ind., 13 March 2009, (MNRJP 008576). Rio de Janeiro: −22.19278258° S −40.92381137° W, 45 m, four ind., 12 March 2009, (MNRJP 008577); −22.20573497° S −40.24472478° W, 97 m, four ind., 15 March 2009, (MNRJP 008578); −22.10641626° S −40.72803811° W, 47 m, one ind., 12 March 2009, (MNRJP 008579); −23.0790° S −43.0517° W, 50 m, two ind., 1 June 2021, (MNRJP 008580).
Description. Holotype complete, with 80 chaetigers, measuring 9.5 mm total length. Anterior chaetigers 1.25 mm in length and 0.4 mm wide; posterior chaetigers with 8.25 mm long and 0.3 mm wide. Anterior region wider than long. Complete paratypes with 50–111 chaetigers. Ventral groove present along entire body. Prostomium conical and elongate (Figure 3A); eyespots absent; nuchal organs not observed. Peristomium elongate, with two lateral annulations and peristomial crest along its length (Figure 3A). Dorsal palps inserted at posterior margin of peristomium; first pair of branchiae postero-lateral to palps; second pair on chaetiger 1 (Figure 3A). Anterior parapodia with 7–10 long and robust capillaries per fascicle, decreasing in number and length posteriorly. Median and posterior chaetigers with acicular and sub-bidentate spines (Figure 3B). Notopodial acicular spines appearing from chaetiger 28 (in paratypes from 12 to 42), 3–8 per fascicle throughout the body, accompanied by 2–4 capillary chaetae in anterior chaetigers, gradually increasing to 6 capillary chaetae in posterior region. Neuropodial acicular spines present from chaetiger 28 (in paratypes from 6 to 26), 4–5 per fascicle, accompanied by 2–3 capillary chaetae throughout body. Sub-bidentate spines restricted to anterior body and present in neuropodial chaetigers from chaetiger 15–40. Incomplete cinctures with wide dorsal and ventral gaps beginning at approximately chaetiger 60. Pygidium simple, rounded lobe (Figure 3C).
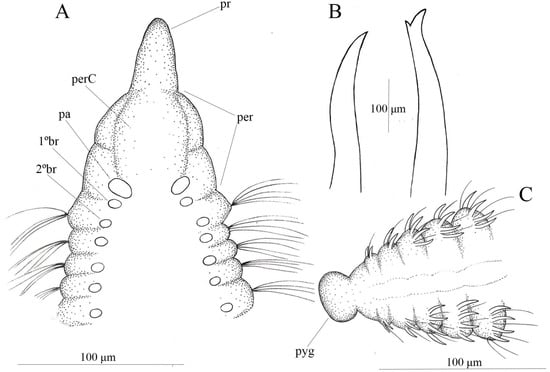
Figure 3.
Chaetozone bidentata sp. nov. (A) anterior end, dorsal view; (B) acicular spines and sub-bidentate spines; (C) posterior end, dorsal view, (A–C) (MNRJP 008560).
- Type locality. Atlantic Ocean: Espírito Santo (Brazil).
- Habitat. Sandy to muddy bottoms with silt–clay and biodebris, 20–97 m depth.
- Distribution. Atlantic Ocean: Espírito Santo and Rio de Janeiro, Brazil.
Remarks. Chaetozone bidentata sp. nov. belongs to a subset of Chaetozone species characterized by the presence of simple (unidentate) spines in addition to bidentate or sub-bidentate acicular spines distributed along the body. The occurrence of bidentate spines within the genus Chaetozone is highly unusual, but has been previously reported in juveniles of C. setosa [32] and C. zetlandica [29], as well as in adult specimens of Chaetozone diodonta Doner & Blake, 2006 [25] and Chaetozone lunula Blake, 1996 [21]. In Chaetozone bidentata sp. nov., sub-bidentate spines are present in all adult specimens. Based on the present study, C. beneditae sp. nov., C. lesliae sp. nov., and C. lutzae sp. nov., are also herein recognized as members of this group. Chaetozone bidentata sp. nov. differs from C. diodonta, in which acicular notopodial spines appear from chaetiger 65 and sub-bidentate spines are restricted to chaetigers posterior to 90, with the last ten parapodial fascicles containing only one capillary chaetae and one acicular spine. It also differs from C. lunula, which exhibits a complete cincture and a rounded pygidium bearing a long terminal anal cirrus. Chaetozone bidentata sp. nov. is further distinguished from other congeners described from the Brazilian coast in this study: C. lutzae sp. nov. exhibits sub-bidentate spines throughout the entire body, a moniliform posterior region, and a complete cincture; C. lesliae sp. nov. is characterized by dorsal palps inserted on the second achaetous segment, the first pair of branchiae arising from chaetiger 1, and sub-bidentate spines along the body; C. beneditae sp. nov. presents a moniliform posterior region, and bidentate spines distributed throughout the body.
- Etymology. The specific epithet bidentata derives from the Latin bi- (two) and dentatus (toothed), meaning “two-toothed.” It refers to the presence of sub-bidentate spines, which are restricted to the neuropodia.
- Chaetozone lutzae sp. nov.
- Material examined. BRAZIL, Espírito Santo: Holotype—−21.65358373°, −40.81429678°, 21 m, 11 March 2009, (MNRJP 008581). Paratypes—−18.682028°, −38.928189°, 55 m, one ind., 17 January 2012, (MNRJP 008582). Rio de Janeiro: −22.19278258°, −40.92381137°, 45 m, one ind., 12 March 2009, (MNRJP 008583).
Description. Holotype complete, with 41 chaetigers, 2.5 mm long. Anterior region 0.62 mm long and 0.25 mm wide. Posterior region moniliform. Complete paratypes with 32 to 40 chaetigers. Ventral groove present along entire body. Prostomium short, conical (Figure 4A); eyespots absent, nuchal organs not observed. Peristomium elongate, with two lateral annulations with visible peristomial crest (Figure 4A). Dorsal palps inserted on posterior margin of peristomium; first pair of branchiae positioned postero-lateral to palps; second pair inserted on first chaetiger (Figure 4A). Neuropodial capillary chaetae approximately twice as long as notopodial. Anterior parapodia with only capillary chaetae in both rami, 4–7 per fascicle, numbers gradually decreasing along body. Acicular spines and sub-bidentate spines present (Figure 4B). Acicular spines arising from notopodium at chaetiger 15 (in paratypes from chaetigers 11–13), and from neuropodium at chaetiger 5 (in paratypes from chaetigers 5–6). Sub-bidentate spines arising from neuropodium at chaetiger 6. Notopodial acicular spines 3–4 per fascicle along the body, accompanied by 4–5 capillary chaetae. Neuropodial acicular spines 1–3 per fascicle, accompanied by 2–3 capillary chaetae. Sub-bidentate neuropodial spines 1–4 per fascicle along body, accompanied by 3–4 capillary chaetae. Incomplete cinctures present from chaetiger 24 (Figure 4C). Pygidium a simple lobe surrounding the anal opening on the ventral anus (Figure 4C).
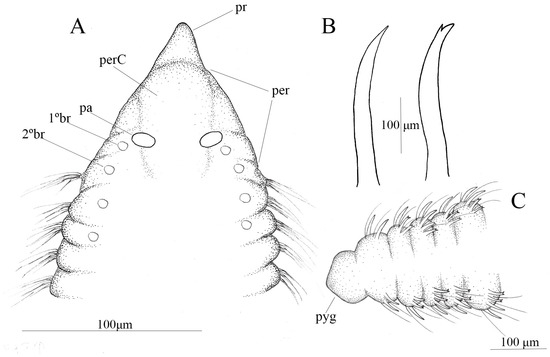
Figure 4.
Chaetozone lutzae sp. nov. (A) anterior end, dorsal view; (B) spine and sub-bidentate spine; (C) posterior end, dorsal view, (A–C) (MNRJP 008581).
- Type locality. Atlantic Ocean: Espírito Santo (Brazil).
- Habitat. Sandy to muddy bottoms with silt–clay and biodebris, 21–55 m depth.
- Distribution. Atlantic Ocean: Espírito Santo and Rio de Janeiro, Brazil.
Remarks. Chaetozone lutzae sp. nov. is assigned to the same species group as C. bidentata; see comments above. In C. lutzae sp. nov., sub-bidentate spines are present in all adult specimens examined. This species differs from C. diodonta, in which notopodial spines appear from chaetiger 65 and notopodial bidentate spines are restricted to chaetigers posterior to 90, with the last ten parapodial fascicles containing only one capillary and one acicular spine. The new species with a simple pygidium differs from C. lunula, which has a rounded pygidium with a long terminal anal cirrus. Among the Brazilian species described herein, C. lutzae sp. nov. is distinguished by sub-bidentate spines distributed throughout the body, and complete cinctures from chaetiger 24. It also differs from C. bidentata sp. nov., which has sub-bidentate neuropodial spines restricted to anterior body; C. lesliae sp. nov., which has dorsal palps inserted on the second achaetous segment and the first pair of branchiae on chaetiger 1; and C. beneditae sp. nov., characterized by the absence of peristomial annulations and by the presence of bidentate spines from chaetiger 16.
- Etymology. This species is named in honor of Bertha Maria Júlia Lutz (1894–1976), a Brazilian biologist, politician, and leading feminist. In 1919, she became secretary and researcher at the National Museum of Rio de Janeiro, being the second woman to join Brazil’s civil service. She played a decisive role in securing women’s suffrage in Brazil in 1932 and, in 1945, was one of only four women delegates at the San Francisco Conference that founded the United Nations, where she was instrumental in including the principle of gender equality in the UN Charter.
- Chaetozone lesliae sp. nov.
- Material examined. BRAZIL. Espírito Santo: Holotype—−20.205628°, −39.999917°, 44 m, 20 January 2012, (MNRJP 008867). Paratypes—−20.205628°, −39.999917°, 44 m, seven ind., 20 January 2012, (MNRJP 008868); −20.581506°, −40.107619°, 45 m, seven ind., 21 January 2012, (MNRJP 008869); −20.579383°, −40.191872°, 41 m, four ind., 21 January 2012, (MNRJP 008870); −20.579758°, −40.191972°, 41 m, four ind., 12 July 2013, (MNRJP 008871); −20.576214°, −40.347436°, 26 m, two ind., 12 July 2013, (MNRJP 008872); −20.581403°, −40.107689°, 49 m, two ind., 12 July 2013, (MNRJP 008873).
Description. Holotype complete, with 63 chaetigers, 6.1 mm total length. Anterior region 2.5 mm long and 0.25–0.425 mm wide; posterior region 3.6 mm long and 0.25 mm wide. Complete paratypes with 32–72 chaetigers. Ventral groove present along entire body. Prostomium conical and rounded (Figure 5A); eyespots absent; nuchal organ pigmented. Peristomium shorter than prostomium, with two peristomial annulations, the first twice the length of the second; peristomial crest present along peristomium (Figure 5A). Achaetous segment present between peristomium and first chaetiger, bearing palps inserted dorsolaterally and first pair of branchiae inserted postero-laterally to palps; second pair of branchiae inserted on first chaetiger (Figure 5A). Anterior parapodia with fibrillated notopodial and neuropodial capillary chaetae, 3–6 per fascicle. Acicular spines and sub-bidentate spines present (Figure 5B). Notopodial acicular spines from chaetiger 48 (in paratypes from chaetigers 22–56), 2–4 per fascicle alternating with capillaries along the body. Neuropodial acicular spines from chaetiger 10 (from chaetigers 16–49 in paratypes). Sub-bidentate neuropodial spines arising from chaetiger 13. Neuropodial spines 2–4 per fascicle, accompanied by 3–4 capillary chaetae. Incomplete cinctures with elevated parapodial membranes present from chaetiger 52 (Figure 5C). Pygidium formed by a simple conical lobe (Figure 5C).
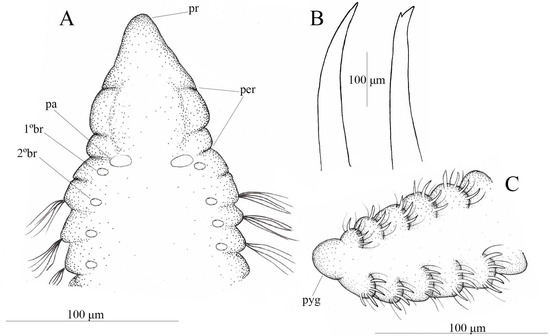
Figure 5.
Chaetozone lesliae sp. nov. (A) anterior end, dorsal view; (B) acicular and sub-bidentate spines; (C) posterior end, dorsal view (A–C) (MNRJP 008867).
- Type locality. Atlantic Ocean: Espírito Santo (Brazil).
- Habitat. Sand, mud and biodebris, 26 to 49 m depth.
- Distribution. Presently known only from Atlantic Ocean: Espírito Santo (Brazil).
Remarks. Chaetozone lesliae sp. nov. is assigned to same species group as C. bidentata and C. lutzae; see comments above. In Chaetozone lesliae sp. nov., sub-bidentate spines are present in adults, first appearing in the neuropodia from chaetiger 13, while notopodial acicular spines are observed from chaetiger 48. This species differs from C. diodonta, in which notopodial acicular spines arise from chaetiger 65, and bidentate notopodial spines are restricted to posterior region from chaetiger 90. The new species also differs from C. lunula, which presents complete cinctures and a rounded pygidium bearing a long terminal anal cirrus. Among the Brazilian species described herein, C. lesliae sp. nov. is distinguished by the insertion of the dorsal palps and the first pair of branchiae on the achaetous segment. This contrasts with C. bidentata sp. nov., and C. lutzae sp. nov., in which the palps and first branchiae arise from the peristomium and first chaetiger, respectively. Chaetozone lesliae sp. nov. is separated by its incomplete cinctures, which begin at chaetiger 52 versus chaetiger 24 in C. lutzae sp. nov., and by the more posterior insertion of branchiae and palps. Finally, it differs from C. beneditae sp. nov. by having the dorsal palps on the achaetous segment, and bidentate spines starting in the posterior region.
- Etymology. This species is named in honor of Leslie Harris, collection manager of polychaetes at the Natural History Museum of Los Angeles County (NHMLA). Recognized as an authority on the marine fauna and flora of California, she plays an important role in maintaining and expanding the museum’s polychaete collection, contributes to the advancement of polychaete systematics, and is a supporter of new generations of researchers in the field, including many Brazilian scientists who have benefited from her generous collaboration.
- Chaetozone beneditae sp. nov.
- Material examined. BRAZIL. Espírito Santo: Holotype—−19.601436°, −39.175814°, 153 m, 24 January 2012, (MNRJP 008874); Paratypes—−19.720650°, −39.559683°, 50 m, three ind., 19 January 2012, (MEV); −21.067989°, −40.237261°, 153 m, one ind., 23 January 2012, (MNRJP 008875). Paraná: −25.5744°, −45.1574°, 150 m, one ind., 21 June 2021, (MNRJP 008876).
Description. Holotype complete, with 32 chaetigers, 1.75 mm long. Anterior region 0.37 mm long and 0.17 mm wide. Complete paratypes with 24–50 chaetigers. Prostomium short, conical and rounded (Figure 6A); eyespots absent; nuchal organs not observed under light microscopy. Peristomium elongate, without annulations, and with visible peristomial crest (Figure 6A). Dorsal palps inserted on posterior margin of peristomium; first pair of branchiae postero-lateral to palps; second pair inserted on first chaetiger (Figure 6A). Anterior parapodia poorly differentiated, bearing only noto- and neuropodial fibrillated capillary chaetae, 5–7 per fascicle, decreasing gradually in number along body. Acicular spines and bidentate spines present (Figure 6B). Acicular noto- and neuropodial spines from chaetiger 15 and 16, respectively. Bidentate neuropodial spines from chaetiger 6, 1–4 per fascicle. Notopodial acicular spines 2–4 per fascicle along body, accompanied by 2–3 capillary chaetae. Incomplete cinctures with spines separated by small dorsal and ventral gap, present from chaetiger 20 (Figure 6C). Pygidium formed by a simple lobe surrounding anus (Figure 6C).
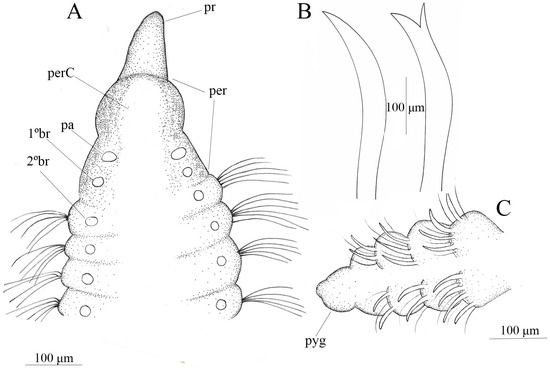
Figure 6.
Chaetozone beneditae sp. nov. (A) anterior end, dorsal view; (B) spine and bidentate spine (C) posterior end, dorsal view. (A–C) (MNRJP 008874).
- Type locality. Atlantic Ocean: Espírito Santo (Brazil).
- Habitat. Predominately sand, mud, 50–153 m depth.
- Distribution. Atlantic Ocean: Espírito Santo; São Paulo; Paraná (Brazil).
Remarks. Chaetozone beneditae sp. nov. assigned to same species group as C. bidentata and C. lutzae and C. leslie see comments above. In Chaetozone beneditae sp. nov., bidentate neuropodial spines arise from chaetiger 6 and present in adult specimens; acicular notopodial and neuropodial spines from chaetiger 15 and 16, respectively. The species differs from C. diodonta, in which notopodial acicular spines first appear from chaetiger 65 and notopodial bidentate spines are restricted to chaetigers posterior to 90. The new species also differs from C. lunula, which has complete cinctures and a rounded pygidium with a long terminal anal cirrus, while C. beneditae sp. nov. has cinctures from chaetiger 20 and a simple pygidial lobe. Among species described from the Brazilian coast in this study, C. beneditae sp. nov. is distinguished from all congeners because it is the only one with t bidentate, not sub-bidentate spines. Chaetozone beneditae sp. nov. is distinguished from C. bidentata sp. nov. by the presence of a moniliform posterior region, peristomium without annulations, and by the more posterior origin of spines in both noto- and neuropodia in posterior chaetigers. Chaetozone beneditae sp. nov. differs from C. lutzae sp. nov. by its incomplete cinctures beginning from chaetiger 20, while C. lutzae sp. nov. has complete cinctures from chaetiger 24 and bidentate neuropodial spines along the body. Chaetozone beneditae sp. nov. is further separated from C. lesliae sp. nov. by the insertion of dorsal palps on the posterior margin of the peristomium and the origin of the first pair of branchiae postero-lateral to the palps, whereas in C. lesliae sp. nov. the dorsal palps are inserted on the achaetous segment, and the first pair of branchiae arises from chaetiger 1.
- Etymology. The species is named in honor of Benedita Souza da Silva Sampaio, known as “Bené”. Born in a favela in Rio de Janeiro, she was the first Black woman to reach the Chamber of Deputies and to serve as both senator and governor in Brazil. A distinguished political leader, her work has been fundamental in defending democracy and advancing the rights and visibility of Black people. This dedication both reflects and reinforces the ongoing transformation within Brazilian universities, as this study was conducted by two Black women students and their professor, a strong advocate for inclusion, equity, and social change.
3.2. Key to Species of Chaetozone Found in Brazilian Waters
1. Bidentate or sub-bidentate spines present ........................................................................... 2
1. Only unidentate (simple) spines present .............................................................................. 5
2. Dorsal palps and first pair of branchiae inserted on an achaetous segment................................................................................. C. lesliae sp. nov.
2. Dorsal palps and first pair of branchiae inserted on the peristomium............................. 3
3. Posterior region moniliform; neuropodial bidentate spines from chaetiger 6 .................................................................................................................... C. beneditae sp. nov.
3. Peristomium elongated; posterior region not moniliform ................................................. 4
4. Sub-bidentate spines present throughout the body; incomplete cinctures from chaetiger 24. ........................................................................................................... C. lutzae sp. nov.
4. Sub-bidentate spines restricted to anterior region; incomplete cinctures from chaetiger 60 ................................................................................................................. C. bidentata sp. nov.
5. Anterior region dorsally expanded, posterior segments moniliform ................ C. larae
5. Body cylindrical in anterior two-thirds, dorsoventrally compressed posteriorly ................................................................................................................................... C. corona
4. Discussion
The present study represents the first formal contribution to the systematics of Chaetozone from Brazilian waters, with the description of four new species and the first record of C. larae outside its type locality in Argentina. These findings significantly expand the known diversity of the genus in the Southwestern Atlantic and highlight Brazil as an area harboring a still underexplored cirratulid fauna.
The recognition of C. larae on the southeastern coast of Brazil extends its distribution northward from San Jorge Gulf into subtropical latitudes, suggesting a broader and possibly continuous distribution along the continental shelf of the western South Atlantic. Although minor morphological differences were observed between Brazilian specimens and the type material, these were interpreted as intraspecific variation. The presence of golden-brown pigmentation at the bases of chaetae and spines, a character not previously noted for this species, may represent an additional diagnostic feature of C. larae.
The new species described herein contributes to the growing evidence that Chaetozone is more morphologically diverse than traditionally recognized. The occurrence of bidentate or sub-bidentate spines in four of the species described is remarkable, as this character has historically been considered rare within the genus, with bidentate spines occasionally reported in the ventral-most position of neuropodial fascicles and the dorsal-most position of notopodial fascicles containing unidentate spines. Although previously documented in juveniles of C. setosa and C. zetlandica, as well as adults of C. diodonta and C. lunula, the consistent presence of sub-bidentate or bidentate neuropodial spines in adult specimens from Brazil indicates that this trait may be more widespread than assumed. These results reinforce earlier observations that traditional diagnostic characters, such as the position of palps and branchiae, and development of posterior cinctures, must be complemented with additional characters, such as pigmentation patterns and spine morphology, for reliable species identification.
Globally, Chaetozone has undergone extensive systematic revision in the past decades, with numerous new species described from the Pacific, Atlantic, and polar regions [21,23,25,27,28]. The discovery of four new species in a relatively restricted geographic area suggests that the diversity of Chaetozone in Brazil is still underestimated. Considering the ecological importance of cirratulids in benthic communities and the morphological complexity of Chaetozone, further integrative approaches combining morphology with molecular data will be essential to resolve species limits and to test hypotheses regarding species groups within the genus.
This study increases the number of valid species of Chaetozone worldwide from 81 to 85, with the description of four new species and the first record of C. larae in Brazilian waters. Prior to this study, only C. larae and C. corona had been reported from the Southwestern Atlantic Ocean [10,11]. These findings expand the known distribution of the genus in the region and confirm Brazil as an area of underestimated cirratulid diversity.
Author Contributions
Conceptualization, R.F., C.M. and C.R.; methodology, R.F. and C.M.; data curation, R.F. and C.M.; investigation, R.F., C.M. and C.R.; resources, C.R.; supervision, C.R.; validation, C.R.; visualization, R.F., C.M. and C.R.; project administration, C.R.; writing—original draft preparation, R.F.; writing—review and editing, R.F., C.M. and C.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research and APC received no external funding.
Data Availability Statement
The examined material has been deposited in the reference collection of the Rio de Janeiro National Museum—MNRJ/UFRJ, RJ, Brazil.
Acknowledgments
We thank CENPES/PETROBRAS for providing study material and the staff of CENABIO/UFRJ and CPMP-UFRJ for support with SEM and MO imaging. C.M. was supported by undergraduate and Master’s fellowships from CNPq, PIBIC/PR2-UFRJ, and CAPES, under the supervision of C.R. R.F. received a PhD scholarship from CAPES, also supervised by C.R.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CENABIO | Centro Nacional de Biologia Estrutural e Bioimagem |
| CENPES | Centro de Pesquisas, Petrobras |
| MNRJP | Museu Nacional do Rio de Janeiro–Polychaeta |
| MO | Optical microscopy |
| PETROBRAS | Petróleo Brasileiro S.A. |
| PR2 | Pró-reitoria de graduação e pesquisa |
| SEM | Scanning Electron Microscopy |
| UFRJ | Universidade Federal do Rio de Janeiro |
| br | Branchia |
| Cr | dorsal crest |
| dGr | dorsal groove |
| neP | neuropodium |
| noP | Notopodium |
| nuO | nuchal organ |
| per | peristomium |
| perC | peristomial crest |
| pr | Prostomium |
| pyg | Pygidium |
| aGr | anterior groove |
| pa | Palps |
| vGr | ventral groove |
References
- Malmgren, A.J. Annulata Polychaeta Spetsbergiæ, Grœnlandiæ, Islandiæ et Scandinaviæ. Hactenus Cognita; Ex Officina Frenckelliana: Helsingforslæ, Finland, 1867; 127p. [Google Scholar]
- Read, G.; Fauchald, K. (Eds.) World Polychaeta Database. Chaetozone Malmgren, 1867. 2025. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=129242 (accessed on 11 September 2025).
- Jumars, P.A. Target species for deep-sea studies in ecology, genetics and physiology. Zool. J. Linn. Soc. 1975, 57, 341–348. [Google Scholar] [CrossRef]
- Dean, H.K. The use of polychaetes (Annelida) as indicator species of marine pollution: A review. Rev. Biol. Trop. 2008, 56, 11–38. [Google Scholar]
- Petersen, M.E. Reproduction and development in Cirratulidae (Annelida: Polychaeta). Hydrobiologia 1999, 402, 107–128. [Google Scholar] [CrossRef]
- Weidhase, M.; Helm, C.; Bleidorn, C. Morphological investigations of posttraumatic regeneration in Timarete cf. punctata (Annelida: Cirratulidae). Zool. Lett. 2015, 1, 20. [Google Scholar]
- Rouse, G.W.; Pleijel, F.; Tilic, E. Annelida; Oxford University Press: Oxford, UK, 2022; 418p. [Google Scholar]
- Blake, J.A.; Magalhães, W.F. Cirratulidae Ryckholt, 1851. In Handbook of Zoology Annelida Volume 1: Annelida Basal Groups and Pleistoannelida, Sedentaria I; Purschke, G., Böggemann, M., Westheide, W., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 339–397. [Google Scholar]
- Berkeley, E.; Berkeley, C. On a collection of Polychaeta from Southern California. Bull. South. Calif. Acad. Sci. 1941, 40, 16–60. Available online: https://www.biodiversitylibrary.org/page/34209137 (accessed on 16 September 2025).
- Omena, E.; Creed, J.C. Polychaete fauna of seagrass beds (Halodule wrightii Ascherson) along the coast of Rio de Janeiro (Southeast Brazil). Mar. Ecol. 2004, 25, 273–288. [Google Scholar] [CrossRef]
- Elías, R.; Rivero, M.S.; Orensanz, J.L. New species of Monticellina and Chaetozone (Polychaeta: Cirratulidae) in the SW Atlantic, and a review of Monticellina species. J. Mar. Biol. Assoc. UK 2017, 97, 1553. [Google Scholar] [CrossRef]
- Magalhães, W.F.; Seixas, V.C.; Paiva, P.C.; Elias, R. The multitentaculate cirratulidae of the genera Cirriformia and Timarete (Annelida: Polychaeta) from shallow waters of Brazil. PLoS ONE 2014, 9, e112727. [Google Scholar] [CrossRef]
- Freitas, R.; Ribeiro, R.P.; Ruta, C. Kirkegaardia Blake, 2016 (Annelida: Cirratulidae) from Southeastern Brazil with description of nine new species. PLoS ONE 2022, 17, e0265336. [Google Scholar] [CrossRef]
- Ruta, C.; Mundim, D.M.; Freitas, R.; Ribeiro, P.R. New species and record of Dodecaceria (Annelida: Cirratulidae) from the Biological Reserve of Rocas Atoll, Brazil, the only atoll in the South Atlantic Ocean. PLoS ONE 2023, 18, e0293087. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.C.Z.; Nallin, S.A.H.; Steiner, T.M.; Forroni, T.O.; Gomes-Filho, D.; Araújo, G.R.; Freitas, R.; Costa, C.A.O.; Ruta, C.; Gomes, K.R.E.; et al. Catálogo das Espécies de Annelida “Polychaeta” do Brasil. 2006–2022. Available online: https://intranet.ib.unicamp.br/intranet/polychaeta/_apresentacao.php (accessed on 30 June 2025).
- Mohriak, W.U.; Mello, M.R.; Karner, G.D.; Dewey, J.F.; Maxwell, J.R. Structural and stratigraphic evolution of the Campos Basin, offshore Brazil. In Extensional Tectonics and Stratigraphy of the North Atlantic Margins; Tankard, A.J., Balkwill, H.R., Eds.; AAPG Memoir; American Association of Petroleum Geologists: Tulsa, OK, USA, 1989; Volume 46, pp. 577–598. [Google Scholar]
- Mio, E.; Chang, H.K.; Corrêa, F.S. Integração de métodos geofísicos na modelagem crustal da Bacia de Santos. Rev. Bras. Geofís. 2005, 23, 275–284. [Google Scholar] [CrossRef]
- Valentin, J.L. Analyse des paramètres hydrobiologiques dans la remontée de Cabo Frio (Brésil). Mar. Biol. 1984, 82, 259–276. [Google Scholar] [CrossRef]
- Silveira, I.C.A.D.; Schmidt, A.C.K.; Campos, E.J.D.; Godoi, S.S.D.; Ikeda, Y. A corrente do Brasil ao largo da costa leste brasileira. Rev. Bras. Oceanogr. 2000, 48, 171–183. [Google Scholar] [CrossRef]
- Ryckholt, P. Mélanges paléontologiques. Part 1. Mém. Couronnés Mém. Savants Étrangers Acad. Roy. Sci. Lett. Beaux-Arts Belg. 1851, 24, 1–176. [Google Scholar]
- Blake, J.A. Family Cirratulidae Ryckholt, 1851. Including a revision of the genera and species from the Eastern North Pacific. In Taxonomic Atlas of the Benthic Fauna of the Santa Maria Basin and Western Santa Barbara Channel. The Annelida Part 3—Polychaeta: Orbiniidae to Cossuridae; Blake, J.A., Hilbig, B., Scott, P.H., Eds.; Santa Barbara Museum of Natural History: San Diego, CA, USA, 1996. [Google Scholar]
- Blake, J.A. New species and records of Caulleriella, Chaetocirratulus and Chaetozone (Annelida, Cirratulidae) from continental shelf and slope depths of the Western North Atlantic Ocean. Zootaxa 2022, 5113, 1–89. [Google Scholar] [CrossRef]
- Blake, J.A. New species and records of deep-water Cirratulidae (Polychaeta) from off Northern California. Sci. Mar. 2006, 70 (Suppl. S3), 45–57. [Google Scholar] [CrossRef]
- Blake, J.A. New species of Chaetozone and Tharyx (Polychaeta: Cirratulidae) from the Alaskan and Canadian Arctic and the Northeastern Pacific, including a description of the lectotype of Chaetozone setosa Malmgren from Spitsbergen in the Norwegian Arctic. Zootaxa 2015, 3919, 501–552. [Google Scholar] [CrossRef]
- Doner, S.A.; Blake, J.A. New species of Cirratulidae (Polychaeta) from the northeastern United States. Sci. Mar. 2006, 70 (Suppl. S3), 65–73. [Google Scholar] [CrossRef]
- Doner, S.A.; Blake, J.A. Two new species of Aphelochaeta (Polychaeta: Cirratulidae) from deep water off northern California. Zoosymposia 2009, 2, 127–137. [Google Scholar] [CrossRef]
- Dean, H.K.; Blake, J.A. Chaetozone and Caulleriella (Polychaeta: Cirratulidae) from the Pacific coast of Costa Rica, with description of eight new species. Zootaxa 2007, 1451, 41–68. [Google Scholar] [CrossRef]
- Magalhães, W.F.; Bailey-Brock, J.H. Bitentaculate Cirratulidae (Annelida: Polychaeta) from the northwestern Pacific Islands with description of nine new species. Zootaxa 2013, 3630, 1–80. [Google Scholar] [CrossRef]
- Woodham, A.; Chambers, S. Some taxonomic problems of bi-tentaculate cirratulids. Polych. Res. 1994, 16, 14–15. [Google Scholar]
- Moore, J.P. Polychaeta from the coastal slope of Japan and from Kamchatka and Bering Sea. Proc. Acad. Nat. Sci. USA 1903, 55, 401–490. [Google Scholar]
- Hutchings, P.A.; Murray, A. Taxonomy of polychaetes from the Hawkesbury River and the southern estuaries of New South Wales, Australia. Rec. Aust. Mus. Suppl. 1984, 3, 1–118. [Google Scholar] [CrossRef]
- Christie, G. A comparative study of the reproductive cycles of three Northumberland populations of Chaetozone setosa (Polychaeta: Cirratulidae). J. Mar. Biol. Assoc. UK 1985, 65, 239–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).