Abstract
This study explored the relationship between endophytic bacterial communities and the accumulation of the bioactive lignan, magnolin, in the flower buds of three important species: Magnolia biondii, Magnolia denudata, and Magnolia liliiflora. We employed 16S rRNA gene sequencing to characterize the diversity and composition of endophytic bacteria and used high-performance liquid chromatography Mass Spectrometry (LC-MS) to quantify magnolin content. Our results revealed significant differences in bacterial diversity and community structure among the three host species, with Proteobacteria and Actinobacteria being the dominant phyla. Notably, the abundance of specific genera, such as Pseudomonas and Bacillus, showed a significant positive correlation with magnolin concentrations. These findings suggest a potential link between specific endophytic taxa and the biosynthesis of magnolin, providing novel insights for improving the medicinal value of Magnolia plants through microbial regulation. This research lays a foundation for future studies on harnessing endophytic microorganisms to enhance the production of valuable secondary metabolites in medicinal plants.
1. Introduction
Endophytic microorganisms, commonly referred to as endophytes, are a diverse group of fungi and bacteria that reside within the internal tissues and organs of plants without causing disease [1]. Instead, they establish a dynamic and mutually beneficial symbiotic relationship with their host plants [2], maintaining a delicate equilibrium through mechanisms including (1) suppression of host defense responses via microbial secretion of effector proteins [3]; (2) bidirectional nutrient exchange; and (3) modulation of phytohormone levels [4]. Due to their co-evolution with plants, endophytes have developed intricate interactions with their hosts, contributing significantly to plant health. In this partnership, plants provide endophytes with essential nutrients, while the endophytes, in turn, enhance plant resilience and physiological functions [5]. Endophytes are ubiquitously distributed across various plant tissues, including roots, stems, leaves, flowers, and fruits [1,5]. Their beneficial roles are pivotal to plant physiology, directly enhancing host fitness through multiple mechanisms. For instance, they can bolster host defense by outcompeting pathogenic microorganisms for nutrients and space, or by inducing systemic resistance in the plant’s immune system [6,7]. Furthermore, many endophytes are known to produce a diverse arsenal of bioactive metabolites that can deter pathogens or help the plant tolerate environmental pressures, thereby directly contributing to its physiological resilience and metabolic functions [8,9].
Investigations into Panax notoginseng have shown that root-associated endophytes are significantly more abundant than those found in stems and leaves. Similarly, studies on Taxus chinensis (Chinese yew) have identified dominant fungal genera with considerable potential for enhancing the accumulation of paclitaxel [10,11]. These findings highlight the crucial role of endophytes in plant physiology, from facilitating growth and stress tolerance to boosting the production of valuable secondary metabolites [9,12]. Their diverse functions make them promising candidates for applications in agriculture and biotechnology [6].
Previous studies indicate that endophytic fungi are present in Magnolia species [13]. The dried flower buds of these Magnolia species, known in traditional Chinese medicine as Xin Yi, have been widely used for their therapeutic properties, including dispelling common colds and unblocking nasal passages [14]. These medicinal effects are primarily attributed to the presence of magnolin (CAS 31008-18-1; Figure 1), the key bioactive lignan in Xin Yi, with its structural characterization and pharmacological properties well documented in recent studies [15,16,17].

Figure 1.
The chemical structure of magnolin (CAS 31008-18-1).
Magnolin has been officially recognized as a quality control marker for Xin Yi medicinal materials in the Chinese Pharmacopoeia (2020 Edition), with a required minimum content of 0.4% [18]. Beyond its traditional application in treating nasal conditions, modern pharmacological research has demonstrated its anti-inflammatory [19], anti-allergic [14], and skin-whitening properties [20]. However, magnolin content varies significantly among different Magnolia species [21] and their geographical locations [22]. Phytochemical analyses confirm that M. biondii from different provinces contains significantly different magnolin content, often exceeding pharmacopoeial requirements [22]. This variation often correlates with factors like soil properties and climatic factors [23,24]. Additionally, age-dependent variations in secondary metabolite content have been observed in other medicinal plants [25]. These findings highlight the complex interplay between species, geography, and plant age in determining the medicinal value of Magnolia species.
Prior research has identified key enzymes involved in the lignan biosynthesis pathways of various medicinal plants [26,27]. Despite these advances, the diversity and functional roles of endophytic bacteria specifically within the flower buds of these Magnolia species, and their potential influence on characteristic lignan biosynthesis like magnolin, remain largely underexplored. This is particularly true for plants grown in specific urban horticultural environments, where local conditions may shape unique microbial communities [23].
Therefore, the objective of this study was to investigate the endophytic bacterial communities in the flower buds of three Magnolia species (M. biondii, M. denudata, and M. liliiflora) cultivated in Shanghai, China. By employing 16S rRNA gene sequencing and LC-MS, we aimed to characterize the community composition and diversity and to explore associations by systematically comparing these microbial findings with quantified magnolin content. This research provides foundational insights into plant -microbe interactions in a specific locale, paving the way for targeted strategies to enhance the medicinal quality of Xin Yi.
2. Materials and Methods
2.1. Plant Material Collection and Site Description
All plant materials were collected from Magnolia specimens cultivated in a consistent garden environment within Shanghai, China (approximately 31°13′ N, 121°28′ E). This region features a northern subtropical monsoon climate, with an average annual temperature of around 17.6 °C and annual precipitation of approximately 1173 mm. The soil is classified as yellow-brown loam, characterized by good drainage and moderate fertility. All selected trees were of similar age (approximately 15–20 years old) and received standard cultivation practices.
Flower buds from three Magnolia species—M. biondii, M. denudata, and M. liliiflora—were collected for this study at two distinct time points: November 2023 (dormant winter bud stage) and February 2024 (pre-flowering stage, corresponding to the traditional “full bloom” harvest time). For each species, three independent biological replicates were sampled from distinct, healthy trees at each time point. Sample identification is detailed in Table 1. To ensure clarity and traceability, this study adopted a systematic sample encoding rule. Each sample is assigned a unique code, such as ‘W1.1’. Among them, the first letter represents plant species (W, E: M. biondii; B: M. denudata; Z: M. liliiflora). The number following the letter represents the collection batch (such as 1, 2, or 3), and the number after the decimal point represents the biological replicates of the collected plants (such as 1, 2, or 3). For example, W1.1 represents the first biological replicate sample from the first batch of collected Magnolia biondii. The February 2024 samples (Batch 1) were used for all analyses described in this study. Upon collection, these flower buds were divided into three portions: one portion was stored at 4 °C for culture-based isolation of endophytes within 24 h; a second portion was immediately snap-frozen in liquid nitrogen for DNA extraction and 16S rRNA gene sequencing; and a third portion was prepared for LC-MS quantification of magnolin.

Table 1.
Experimental sample number.
The November 2023 samples (Batch 2) were used exclusively for a comparative LC-MS quantification of magnolin to assess seasonal variation.
2.2. Isolation and Identification of Culturable Endophytic Bacteria
This section describes a culture-based approach, which serves as a complement to the high-throughput sequencing analysis (Section 2.4). The primary goal of this method was not to perform a quantitative community comparison, but to isolate living endophytic bacteria. These isolates represent a valuable resource for future functional studies, such as testing their direct effects on host plant physiology or secondary metabolite production.
Endophytic bacteria were isolated from the internal tissues of Magnolia flower buds. Buds were rinsed under running tap water for 2 h to remove surface contaminants. This was followed by surface sterilization using 99% ethanol for 60 s (s) and 1% sodium hypochlorite (NaOCl) for 2 min (min). After surface sterilization, the samples were rinsed five times with sterile physiological saline and dried using sterile absorbent paper.
Under aseptic conditions, the sterilized flower buds were cut into 0.2 cm × 0.2 cm segments. These segments (tissue blocks) were directly inoculated onto various culture media, rather than macerating the tissue. This direct plating method was chosen to preferentially isolate true endophytes tightly associated with plant tissues and to minimize potential inhibition from plant secondary metabolites that might be released during maceration, as well as to reduce interference from loosely adhering surface microorganisms or laboratory environmental contaminants. The culture media used were broad-spectrum or semi-selective, including LB agar and Tryptic Soy Agar (TSA) for general heterotrophic bacteria, and R2A agar. Morphologically distinct colonies were selected for purification and identification.
Pure cultures of isolated endophytic bacteria were identified by 16S rRNA gene sequencing. Genomic DNA was extracted from pure strains, and the 16S rRNA gene was amplified using universal bacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), which amplify nearly the full-length gene [28]. Purified PCR products were subjected to Sanger sequencing, and the resulting sequences were identified by BLASTn analysis against the NCBI database. These primers (27F/1492R) are different from those used for high-throughput community sequencing (described in Section 2.4), as they are designed to amplify longer fragments for more accurate species identification of isolates, whereas HTS primers target specific variable regions suitable for shorter read lengths.
2.3. DNA Extraction and Quality Assessment
Genomic DNA was extracted from Magnolia flower bud samples using the CTAB (cetyltrimethylammonium bromide) method. After grinding the samples in liquid nitrogen, CTAB buffer was added, and the mixture was incubated at 65 °C for 30 min. DNA was then extracted using chloroform isoamyl alcohol phase separation. DNA integrity was assessed by 1% agarose gel electrophoresis was performed. DNA concentration was measured using a NanoDrop and diluted to a working concentration of 1 ng/µL for subsequent analyses.
2.4. PCR Amplification and Sequencing
PCR amplification targeted the V4 region of the 16S rRNA gene using primers 515F (5′-AACMGGATTAGATACCCKG-3′) and 806R (5′-ACGTCATCCCCACCTTCC-3′) [29]. The reaction system included Phusion® High-Fidelity PCR Master Mix, specific primers, and template DNA (1 ng/µL). Each DNA sample underwent PCR amplification in three technical replicates, and the PCR products were then pooled for sequencing library construction. The PCR program included an initial denaturation step at 98 °C for 30 s, followed by 30 cycles of 98 °C for 10 s, 55 °C for 30 s, and 72 °C for 30 s. Following amplification, PCR products were purified and sent to amplicon sequencing on an Illumina platform.
Paired-end reads were processed using the DADA2 plugin [30] within the QIIME 2 (v2023.9) platform [31] to generate Amplicon Sequence Variants (ASVs). For taxonomic classification, a pre-trained Naïve Bayes classifier based on the SILVA (v138) 16S rRNA database was used [32]. The analysis focused on the genus-level composition. Prior to downstream analysis, any sequences identified as chloroplasts or mitochondria were removed from the dataset. Alpha diversity (e.g., Observed ASVs, Shannon index) and beta diversity (based on dissimilarity) analyses were conducted using software packages like Picante [33] and vegan [34] in R. Graphics were generated using ggplot2 [35].
2.5. Extraction and Analysis of Magnolin by LC-MS
Approximately 2 g of dried flower bud samples was ground into a fine powder and extracted with 10 mL of methanol via ultrasonic-assisted extraction for 3 h. The resulting extract had a final concentration of 0.1 g/mL (raw material equivalent).
The analysis of magnolin was performed on an Agilent 6545 Q-TOF Liquid Chromatography-Mass Spectrometry (LC-MS) system. Chromatographic separation was achieved on a Phenomenex Luna C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase consisted of 0.1% (v/v) formic acid aqueous solution and acetonitrile (70:30, v/v) at a flow rate of 0.8 mL/min. The column temperature was maintained at 30 °C.
Quantification of magnolin was performed by mass spectrometry. The content was determined by integrating the peak area from the Extracted Ion Chromatogram (EIC) of its protonated molecular ion [M+H]+ at m/z 417.1912. An external standard method was used, comparing against a 0.4 mg/mL pure magnolin standard. The injection volume was 3 µL.
The identity of magnolin was confirmed by comparing the retention time (RT) and high-resolution mass spectrometry (HRMS) data of the samples with that of the standard. Detailed identification data, including retention time, molecular formula, adduct, theoretical and observed m/z, and mass error, are presented in Table 2. Quantification was performed by an external standard method, using the peak areas recorded for each sample against a certified standard.

Table 2.
LC-MS identification data for Magnolin.
2.6. Statistical Analysis
To assess differences in magnolin content and alpha diversity among the three Magnolia species, analysis of variance (ANOVA) was conducted. Tukey’s post-hoc test was applied to identify significant differences between treatment groups, with statistical significance set at p < 0.05. All statistical analyses were performed using IBM SPSS software (version 31) (IBM Corp., Armonk, NY, USA).
2.7. Data Availability Statement
The 16S rRNA gene sequencing raw data presented in this study are available in the NCBI Sequence Read Archive (SRA) under the submission ID SUB15608671. The raw LC-MS data supporting the conclusions of this article have been deposited in the Zenodo repository and are accessible via DOI: 10.5281/zenodo.17158991.
3. Results
3.1. Isolation and Identification of Culturable Endophytic Bacteria
Our culture-based investigation aimed to complement the sequencing data by providing a collection of living isolates for potential downstream applications. We acknowledged from the outset the inherent limitations of this approach, famously known as the “Great Plate Count Anomaly,” where only a very small fraction of the microbial community is culturable under standard laboratory conditions. Indeed, a low recovery rate was observed, with no culturable bacteria obtained from six of the twelve samples (B1.1, B1.2, W1.1, W1.2, E1.1, E1.2), underscoring this limitation. From the remaining six samples, a total of seven distinct bacterial taxa were isolated and identified via Sanger sequencing of the 16S rRNA gene. These included species belonging to the genera Bacillus, Pseudomonas, Stenotrophomonas, and Sphingomonas. These results should be interpreted not as a comprehensive community profile, but as a characterization of the limited, yet potentially functional, culturable component of the endophytic microbiome.
3.2. Taxonomic Composition of Endophytic Bacterial Communities Revealed by High-Throughput Sequencing
To obtain a comprehensive profile of the endophytic bacterial communities, we employed high-throughput 16S rRNA gene sequencing. The analysis of relative abundance at the phylum level revealed striking differences in the dominant bacterial lineages across the Magnolia species (Figure 2). The communities were primarily composed of three major phyla: Proteobacteria, Actinobacteriota, and Firmicutes.
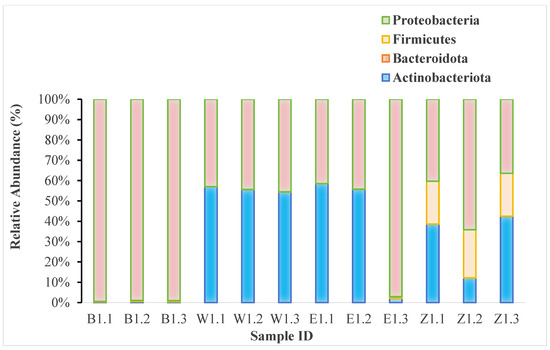
Figure 2.
Relative abundance of the most dominant endophytic bacterial phyla in Magnolia samples, based on 16S rRNA gene sequencing data. This visualization displays the major phyla based on overall abundance across all samples; taxa with lower relative abundance are aggregated into the “Others” category. For a complete breakdown of all taxa down to the genus level, please refer to Supplementary Table S1.
Specifically, samples from M. denudata (B group) were overwhelmingly dominated by Proteobacteria, which accounted for a substantial portion of the community in all three replicates. In sharp contrast, M. biondii samples (W and E groups) displayed a different profile, characterized by a high relative abundance of Actinobacteriota, although Proteobacteria also constituted a significant fraction. The community in M. liliiflora (Z group) appeared to be more mixed, with Proteobacteria, Actinobacteriota, and Firmicutes all present in considerable proportions. These distinct phylum-level signatures strongly suggest that host species is a primary driver shaping the structure of the endophytic bacterial communities. The complete ASV count data and detailed taxonomic assignments down to the genus level are provided in Supplementary Table S1.
The genus-level analysis showed that Sphingomonas was a dominant taxon in M. denudata samples (B1.1, B1.2, B1.3), with its highest relative abundance in B1.3. In contrast, samples from M. biondii (W and E groups) were characterized by a high abundance of Corynebacterium and Vibrio. Cutibacterium was notably present in M. biondii samples E1.1 and E1.2, as well as in M. liliiflora sample Z1.1. while in M. liliiflora sample Z1.1, it was also abundant, although Corynebacterium remained the most dominant genus. The genus Methylobacterium showed a high relative abundance in sample E1.3 but was less prominent in other samples. Genera such as Burkholderia were detected at lower abundances across multiple samples. These findings highlight substantial variability in the composition of endophytic bacterial communities among the different Magnolia species, suggesting that host species is a strong driver of community structure. The complete ASV count data and taxonomic assignments for all samples are provided in Supplementary Table S1.
3.3. Alpha Diversity and Richness of Endophytic Bacteria in Three Magnolia Species
Analysis of the alpha diversity rarefaction curves (based on Observed ASVs) for endophytic bacteria in the three Magnolia species (Figure 3) revealed notable differences in community richness.

Figure 3.
Rarefaction curves of endophytic bacterial communities in Magnolia (Observed ASVs).
Among all samples, B1.1 exhibited the highest ASV richness, with approximately 42 observed ASVs at a sequencing depth of 40,000 reads. Specifically, the high richness in B1.1 was contributed by a diverse array of genera, including a high abundance of Sphingomonas alongside other prominent genera such as Burkholderia, Methylobacterium, and Pseudomonas, reflecting a complex community structure. B1.3 ranked second, with an observed ASV count of approximately 30 at the same sequencing depth. W1.3 followed closely, with around 28 taxa detected at 40,000 sequences. Samples Z1.1, Z1.2, and Z1.3 displayed relatively similar species richness, with ASV counts ranging between 20 and 30. In contrast, W1.1, E1.1, and W1.2 showed lower species richness, with ASV numbers falling below 25. These results suggested that bacterial diversity varied significantly among the Magnolia species and sampling locations, potentially reflecting differences in environmental factors, host-specific microbial associations, or the physiological state of the plant tissues at the time of collection.
A comparison of rarefaction curves across different Magnolia species revealed distinct patterns in endophytic bacterial diversity. All three samples from M. denudata exhibited higher rarefaction curve trends, suggesting a generally greater abundance of endophytic bacteria within this species. In contrast, samples from Group 2 of M. biondii exhibited lower rarefaction curve trends, indicating a relatively limited bacterial diversity in this subgroup. These findings exhibited the significant influence of Magnolia species on the overall microbial diversity trends.
Further analysis of rarefaction curves within the same Magnolia species exhibited substantial variations in bacterial diversity among individual samples. Within M. denudata (B1.1, B1.2, B1.3), B1.1 and B1.3 exhibited high species richness, whereas B1.2 exhibited a markedly lower diversity, suggesting a significant difference in microbial composition even within the same species.
These results indicated that species identity strongly influenced endophytic bacterial diversity, while individual sample differences within the same species can also significantly impact microbial community composition. For instance, M. denudata (B samples) generally supported richer communities characterized by genera like Sphingomonas, while M. biondii (W and E samples) harbored less diverse communities often dominated by Corynebacterium and Vibrio. Such variations may stem from factors such as environmental conditions, plant physiological state, or localized microbial interactions, emphasizing the complex nature of endophyte-host relationships in Magnolia species.
The rarefaction curves indicated that sequencing depth was sufficient, as most samples plateaued after 20,000 sequences, suggesting that the sequencing adequately captured the diversity of endophytic bacterial communities. Among the samples, B1.1 exhibited the highest species diversity, indicating that its endophytic bacterial population was the most abundant. M. liliiflora (Z1.1, Z1.2, Z1.3) also demonstrated relatively high species diversity, reflecting a substantial presence of endophytic bacteria, albeit with some variation among individual samples. In contrast, M. biondii had the lowest species diversity, characterized by a more restricted community dominated by a few taxa like Corynebacterium and Vibrio, suggesting a more restricted and less diverse endophytic bacterial community.
3.4. Correlation Between Species Diversity and Dominance
Analysis of species dominance in relation to sequencing depth and bacterial relative abundance revealed an inverse relationship: higher species diversity was associated with lower dominance values, whereas lower diversity corresponded to higher dominance (Figure 4). M. liliiflora (Z1.1, Z1.2, Z1.3) exhibited high species diversity in the relative abundance analysis, yet in the dominance analysis, its dominance index ranged from 0.75 to 0.85, indicating that no single bacterial species dominated the community. Instead, sample Z1.1, with the lowest dominance, featured a more balanced composition with co-dominant genera including Cutibacterium, Sphingomonas, and Burkholderia. Similarly, M. denudata (B1.1, B1.2, B1.3) displayed notable variation in species diversity and dominance. B1.1 had the highest diversity and a lower dominance index (~0.85), aligning with its broad microbial community. Conversely, B1.2 and B1.3 exhibited higher dominance values (~0.90), driven primarily by the high relative abundance of Sphingomonas, suggesting that a few bacterial species had a pronounced presence in these samples, despite the overall microbial diversity being lower than in B1.1. M. biondii Group 1 (W1.1, W1.2, W1.3) showed a similar trend: W1.1 had higher species diversity and lower dominance (~0.85), indicating a more balanced microbial composition. In contrast, W1.2 and W1.3 exhibited lower diversity but higher dominance (~0.90), suggesting that only a few bacterial species were predominant in these samples. M. biondii Group 2 (E1.1, E1.2, E1.3) followed the same pattern, where E1.2 had greater species diversity and lower dominance (~0.85), while E1.1 and E1.3 had higher dominance (~0.90), implying that fewer bacterial species were responsible for the majority of the microbial community in these samples.
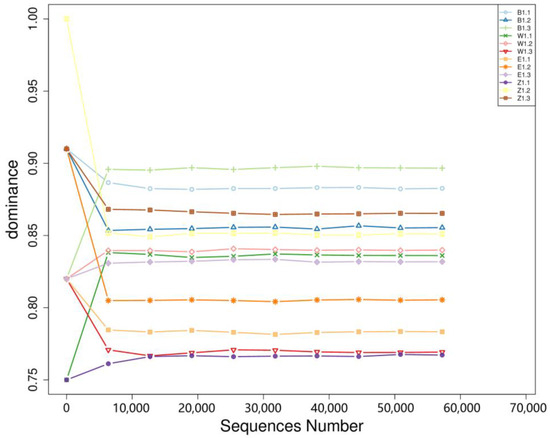
Figure 4.
Dominance curves of three endophytic bacteria in Magnolia.
3.5. Implications of Diversity and Dominance Trend
These findings demonstrated that endophytic bacterial diversity varied significantly across different Magnolia species and even among individual samples of the same species. M. denudata consistently harbored the richest microbial communities, whereas M. biondii exhibited the most restricted bacterial diversity, with certain samples dominated by only a few species. The observed inverse correlation between species richness and dominance suggested that environments with high microbial diversity tended to support more balanced microbial ecosystems, whereas lower diversity led to the predominance of a few bacterial taxa. These variations may stem from species-specific microbial associations, environmental factors, or physiological differences among the Magnolia species, further emphasizing the need for deeper exploration into the ecological interactions between endophytic bacteria and their host plants.
An analysis of the relationship between species diversity and dominance across samples revealed a clear inverse correlation. Samples with higher species diversity—including B1.1, W1.1, E1.2, and Z1.1—exhibited lower dominance values, indicating that microbial communities in these samples were more evenly distributed, with no single bacterial species overwhelmingly dominating the population. This suggested a more balanced microbial ecosystem, where multiple species coexisted without one becoming overly dominant. Conversely, samples with lower species diversity—such as B1.2, B1.3, W1.2, W1.3, E1.1, and E1.3—displayed higher dominance values, meaning that only a few bacterial species were predominant, while the rest of the microbial community was less represented. This pattern suggested that in environments with reduced microbial diversity, certain species tended to outcompete others, establishing dominance within the community.
These findings highlighted the dynamic nature of endophytic bacterial communities in Magnolia species, where diversity-rich environments promote microbial equilibrium, while low-diversity conditions allow specific taxa to dominate. The observed trends may be influenced by host plant factors, environmental conditions, or selective microbial interactions, warranting further investigation into the ecological mechanisms shaping endophyte distributions in Magnolia species.
3.6. Analysis of Dominance and Evenness of Endophytic Bacteria in Three Magnolia Species
Alpha diversity metrics, including species richness estimation (Chao_1 index), species dominance (Dominance index), sequencing coverage (Goods_coverage), species evenness (Pielou_e index), and diversity index (Shannon index), were analyzed to evaluate the structural characteristics of endophytic bacterial communities across different samples (Table 3). A comparative analysis revealed that B1.1 exhibited the highest species richness, with a Chao_1 index of 43.0, whereas W1.2 and E1.1 had the lowest richness values, at 20.0 and 21.0, respectively. This indicated that B1.1 harbored a more diverse endophytic bacterial community, while W1.2 and E1.1 had a more limited bacterial repertoire. Regarding species dominance, B1.3 and Z1.3 exhibited the highest dominance values, at 0.897 and 0.865, respectively, suggesting that only a few bacterial species were highly prevalent in these samples. namely Sphingomonas in B1.3 and Corynebacterium in Z1.3. In contrast, W1.3 and Z1.1 had the lowest dominance values, at 0.769 and 0.767, respectively, indicating a more balanced microbial distribution, with no single species overwhelmingly dominating the community.

Table 3.
Alpha diversity detection of three endophytic bacteria in Magnolia species.
Species evenness, which reflects how uniformly microbial species are distributed within a sample, was highest in E1.1 (0.170) and lowest in B1.3 (0.094). This suggested that E1.1 maintained a more balanced distribution of bacterial species, whereas B1.3 was characterized by a skewed distribution, where certain species were significantly more dominant than others. The Shannon diversity index, which accounts for both species richness and evenness, was highest in E1.1 (0.745) and lowest in B1.3 (0.467). This aligned with the observed trends in evenness and richness, confirming that E1.1 exhibited the most diverse and well-balanced bacterial community, whereas B1.3 had a more restricted and uneven microbial composition.
These findings reinforced the notion that endophytic bacterial diversity varied significantly across different Magnolia species and sample conditions, with certain samples supporting a more even and diverse microbial community, while others are characterized by a few dominant species. The interplay between species richness, dominance, and evenness provides valuable insights into the ecological balance of endophytic bacteria in Magnolia, potentially influencing plant health, metabolic activity, and medicinal compound biosynthesis.
The sequencing coverage for all Magnolia samples reached 1.000, indicating that the sequencing depth was sufficient to comprehensively capture the diversity of endophytic bacterial communities.
Variations in species richness estimation (Chao_1 index) and diversity index (Shannon index) across different samples highlighted the distinct structural characteristics of endophytic bacterial communities among different Magnolia species. B1.1 exhibited the highest species richness, suggesting a more diverse microbial community. Meanwhile, W1.3 and Z1.1 had relatively high diversity index values, indicating that their endophytic bacterial communities were not only diverse but also more evenly distributed.
These findings suggested that different Magnolia species harbored distinct microbial compositions, where some samples supported a highly diverse but unevenly distributed community, while others maintained a more balanced and evenly structured microbiome. Such differences may be influenced by factors such as host plant species, environmental conditions, or specific microbial interactions, which collectively shape the diversity and uniformity of endophytic bacterial communities within Magnolia species.
3.7. Similarity Analysis of Endophytic Bacterial Community Structures in Three Magnolia Species
Beta diversity analysis was conducted to compare the microbial community structures across the three Magnolia species (Figure 5). The results revealed high similarity within individual species, as indicated by darker colors representing greater resemblance in microbial composition. Specifically, the endophytic bacterial communities in M. denudata (B1.1, B1.2, B1.3) exhibited strong internal similarity, suggesting a relatively consistent microbiome structure within this species. Similarly, high similarity was observed within M. biondii (W1.1, W1.2, W1.3), M. biondii Group 2 (E1.1, E1.2, E1.3), and M. liliiflora (Z1.1, Z1.2, Z1.3), reinforcing the notion that samples from the same species share comparable microbial compositions.

Figure 5.
Beta diversity analysis of endophytic bacterial communities based on dissimilarity. The plot displays a correlation matrix where both the size and color intensity of each circle represent the degree of similarity between sample pairs. Larger, darker circles indicate higher similarity (lower dissimilarity), while smaller, lighter circles indicate lower similarity (higher dissimilarity).
When comparing samples across different species, W1 and E1 showed high similarity, likely due to their shared classification under M. biondii. This suggests that geographical or genetic proximity within the same species may contribute to similar endophytic bacterial communities. In contrast, B1 samples (M. denudata) exhibited lower similarity with W1, E1, and Z1 samples, as indicated by lighter colors, reflecting a distinct microbial community structure in M. denudata compared to the other species.
These results indicated that endophytic bacterial communities tend to be more consistent within the same Magnolia species, whereas significant structural differences exist between species from different subgenera. The observed divergence in microbial communities across Magnolia species may be attributed to host-specific factors such as genetic lineage, physiological traits, or environmental adaptation, highlighting the complex interplay between plant species and their associated microbiomes.
3.8. Principal Component Analysis (PCA) of Endophytic Bacterial Communities
Principal Component Analysis (PCA) was performed on the genus-level relative abundance data of the endophytic bacterial community data from the three Magnolia species (see Figure 6). The first principal component (PC1) accounted for 26.16% of the variation, while the second principal component (PC2) explained 13.09% of the variation. Samples from groups W and E clustered tightly in the upper left quadrant, indicating that these two groups share highly similar endophytic bacterial community structures that are distinctly separate from those of the other species. In contrast, the Z group samples were distributed across the central to lower left portions of the plot. Within this group, one cluster in the middle left was positioned adjacent to the B group—albeit with some separation—while another formed a more loosely organized cluster. This distribution suggested that, although the Z group samples exhibit a degree of internal similarity, their overall consistency is somewhat lower compared to the more tightly clustered W and E groups. Notably, the B group samples formed an independent cluster on the right side of the plot. This distinct grouping indicated that while the B group shares similar community structures among its own samples, its endophytic bacterial profile is markedly different from those of the W, E, and Z groups. Overall, these results demonstrated that endophytic bacterial community structures are relatively consistent within individual Magnolia species, yet significant differences exist between species—particularly with the B group exhibiting a uniquely distinct microbial profile compared to the other groups.
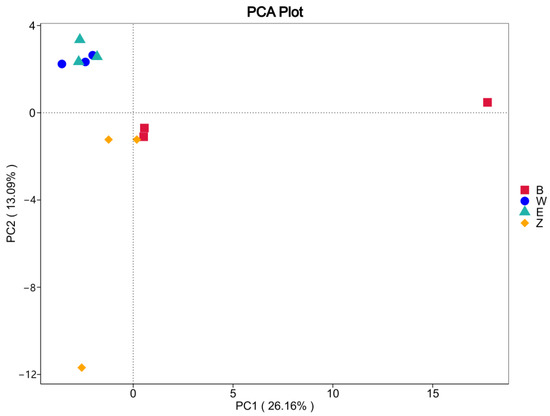
Figure 6.
PCA analysis of three endophytic bacteria in Magnolia.
3.9. Quantification of Magnolin Content in Three Magnolia Species
The magnolin content in the methanolic extracts of flower buds from the three Magnolia species was quantified at two time points—November 2023 and February of the following year—using a validated Liquid Chromatography-Mass Spectrometry (LC-MS) method. The Extracted Ion Chromatogram (EIC) for magnolin (Figure 7) showed a major peak at 15.26 min. This peak was unequivocally identified as magnolin by comparing its retention time and accurate mass-to-charge ratio with an authentic standard. In addition to the target analyte, several minor peaks were observed between 1 and 14 min. While full structural elucidation of these compounds was beyond the scope of this study, they likely correspond to structurally related lignan derivatives, isomers, or matrix interferences commonly found in plant extracts. Importantly, these minor peaks were chromatographically well-separated from the magnolin peak, ensuring they did not interfere with its accurate quantification. The resulting concentrations, expressed as a percentage (%) of dry weight, are detailed in Table 4. The results revealed significant variations in magnolin concentration among different Magnolia species, highlighting species-specific differences in lignan accumulation.
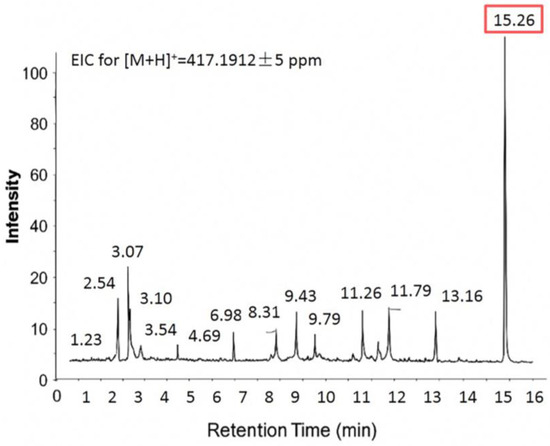
Figure 7.
Extracted ion chromatogram (EIC) at m/z 417.1912 ± 5 ppm corresponding to protonated magnolin ([M+H]+), detected by LC-MS in positive electrospray ionization (ESI+) mode. The peak of interest at 15.26 min is highlighted with a shaded box.

Table 4.
Magnolin content (% of dry weight) in the flower buds of three Magnolia species at different collection times.
Among the three species, M. biondii exhibited the highest magnolin content, with four recorded measurements of 1.08%, 0.73%, 0.75%, and 0.61%. This consistently high concentration suggested that M. biondii serves as a superior natural source of magnolin, reinforcing its medicinal value in traditional herbal applications. In contrast, M. denudata displayed significantly lower magnolin levels, with two recorded measurements of 0.01% and 0.04%. The minimal accumulation of magnolin in this species suggested that it may have limited pharmacological potential concerning magnolin-derived medicinal properties. M. liliiflora exhibited the lowest magnolin content, with recorded values of 0.02% and 0.00%, indicating negligible magnolin biosynthesis within this species.
These findings suggested that magnolin biosynthesis is strongly species-dependent, with M. biondii demonstrating a significantly higher capacity for magnolin production compared to M. denudata and M. liliiflora. The stark contrast in magnolin levels across species may be attributed to genetic differences in lignan biosynthetic pathways, variations in endophytic microbial interactions, or environmental influences affecting secondary metabolite accumulation.
4. Discussion
Our study, conducted on three Magnolia species from the Shanghai region, revealed marked differences in the taxonomic composition and diversity of their endophytic bacterial communities. Using a suite of analytical methods including relative abundance analysis, species diversity curves, alpha and beta diversity metrics, and PCA, we were able to suggest potential associations between correlate microbial community characteristics with magnolin content measurements, thereby elucidating potential impacts of endophytes on lignan biosynthesis. It is important to note that all correlations discussed herein are based on a comparison between the endophytic community data and the magnolin content data from samples collected in February. These findings align with growing evidence that plant-associated microbiomes play a critical role in modulating host plant metabolism and secondary metabolite production.
These species-specific microbial profiles likely reflect ecological adaptations driven by host genetics, environmental conditions, and seasonal variations. Similar observations of host-specific microbial communities have been reported in other plant species, where genetic variations in host plants are a primary determinant of endophytic community structure [36,37]. This suggests a co-evolutionary relationship and fine-tuned interactions between Magnolia species and their associated endophytes.
Notably, an inverse relationship between species diversity and dominance emerged. Samples with high diversity, such as those from M. denudata, exhibited lower dominance, implying a more equitable distribution among bacterial species. In contrast, the lower-diversity communities in M. biondii were dominated by a few taxa. PCA and distance matrix heatmaps further confirmed that microbial community structures, analyzed at the genus level, are closely associated with host plant characteristics, with M. biondii forming a distinctly separate cluster (PC1 and PC2 explained 39.25% of total variation). These findings underscore the structural stability of these communities and validate the robustness of our sequencing approach. Such distinct clustering based on host species is a common pattern in plant microbiome studies, indicating that even within the same genus, specific host genotypes can exert strong selective pressures on their associated microbial communities [36].
When these findings are considered alongside the endophytic community data, intriguing correlations emerge that allow for the formulation of hypotheses for future research. Our analysis revealed an inverse co-occurrence: M. biondii, the species with the highest magnolin content, also harbored the least diverse and most simplified endophytic bacterial community. which was consistently dominated by the genera Corynebacterium and Vibrio. This leads to the hypothesis that a less complex microbial community might be associated with, or even conducive to, higher magnolin accumulation. It is conceivable that a simplified community reduces microbial competition for metabolic precursors shared with the lignan biosynthesis pathway, or that specific dominant taxa within this simple community play a direct role in promoting lignan production. This counterintuitive concept, where lower diversity correlates with higher functional output, has been observed in other specialized systems. For instance, studies in high-yielding ginseng cultivars have reported less complex microbial consortia compared to their wild relatives, suggesting that specific, highly efficient plant-microbe partnerships can be more important for a particular function than overall community diversity [38].
For instance, our data showed that key bacterial genera like Corynebacterium and Vibrio were more prominent in the high-magnolin M. biondii. whereas the lower-magnolin M. denudata was characterized by a more diverse community rich in Sphingomonas and Burkholderia. This observation raises the possibility that these microbes might act as biological catalysts or regulators, enhancing the expression of enzymes or metabolic pathways involved in lignan production. This idea is supported by findings in Magnolia officinalis, where high magnolol and honokiol content was associated with specific endophytic fungi that regulate key phenylpropanoid pathway genes, such as phenylalanine ammonia-lyase (PAL) and caffeoyl-CoA O-methyltransferase (COMT) [39]. Crucially, our hypothesis is further supported by direct evidence from other lignan-producing plants. A study on Podophyllum hexandrum, for example, found that inoculation with a specific endophytic fungus, Fusarium solani, significantly increased the production of the lignan podophyllotoxin by up-regulating PAL gene expression [40]. Similarly, some Corynebacterium species are known for their metabolic versatility, including involvement in aromatic compound degradation and synthesis, which could potentially extend to lignan precursors.
Conversely, M. denudata and M. liliiflora contained significantly lower magnolin levels and were observed to harbor more diverse and complex endophytic bacterial communities. with genera such as Sphingomonas and Cutibacterium being abundant but not creating the same level of low-diversity dominance seen in M. biondii. This could lead to the alternative hypothesis that higher microbial diversity, or the presence of different dominant taxa, might exert a competitive or even inhibitory effect on magnolin biosynthesis.
It is important to reiterate that the analysis of culturable bacteria, which yielded only seven taxa and had low recovery rates, cannot be used to draw firm conclusions about the overall bacterial community structure or its differences among the Magnolia species. That role is served by the high-throughput sequencing data. However, the true value of this culture-based effort lies not in its quantitative power, but in the generation of a tangible collection of living isolates. They represent invaluable resources for future hypothesis-driven research. For instance, these isolates can now be used in co-culture experiments with Magnolia tissues or in planta inoculation studies to directly test their ability to influence magnolin biosynthesis. This provides a clear path forward to experimentally validate the correlational patterns observed in our broader sequencing study and to mechanistically understand the functional roles of specific endophytes in modulating the production of key medicinal compounds in Magnolia.
It is critical to emphasize that these are hypotheses based on correlational data from this study which was conducted in a single geographical location (Shanghai). The specific soil type, climate, and horticultural practices of this region undoubtedly influence the composition of the endophytic communities we observed. The inverse correlation between diversity and magnolin content observed here is a compelling regional finding that warrants further investigation across different geographical locations to determine if it is a widespread phenomenon or a site-specific interaction. Future research is essential to experimentally validate these potential relationships. Uncovering the mechanistic interactions between specific endophytes and plant metabolic pathways could lead to novel strategies for optimizing lignan biosynthesis in selected Magnolia species.
Despite these promising correlations, our conclusions primarily rely on observational and correlation analyses and direct functional validation through targeted gene knockout experiments remains lacking. Additionally, laboratory-based microbial interactions may not fully replicate natural conditions, potentially affecting the extrapolation of our findings. Therefore, while our data suggest a compelling link, mechanistic proof is still required.
Future studies should integrate multi-omics technologies—such as metagenomics, metabolomics, and transcriptomics—alongside synthetic biology approaches to dissect the metabolic regulatory mechanisms of key endophytic bacteria.
5. Conclusions
This study provides the first systematic characterization of the culturable endophytic bacterial communities in the flower buds of M. biondii, M. denudata, and M. liliiflora from the Shanghai region and correlates them with magnolin content. Our results revealed a significant and novel inverse relationship: M. biondii, which produces the highest levels of magnolin, harbors the least diverse endophytic community, dominated by genera such as Corynebacterium and Vibrio. This suggests that a simplified, host-selected microbiome may be conducive to, or even a promoter of, lignan biosynthesis. These findings establish a crucial foundation for future mechanistic studies and propose a new perspective for enhancing the medicinal quality of Xin Yi through microbial-informed breeding or the application of specific endophytic inoculants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100716/s1, Table S1: Abundance and taxonomic classification of endophytic bacterial ASVs.
Author Contributions
Conceptualization, R.F. and D.Z.; methodology, D.Z.; software, R.F.; validation, R.F., D.Z. and L.Y.; formal analysis, R.F.; investigation, R.F. and L.Y.; resources, R.F. and L.Y.; data curation, R.F.; writing—original draft preparation, R.F.; writing—review and editing, R.F. and D.Z.; visualization, R.F. and D.Z.; supervision, D.Z.; project administration, D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Project ’Identification and Analysis of Active Components of Michelia alba Essential Oil and Breeding of Improved Varieties’ from the Shanghai Landscaping & City Appearance Administration Bureau (grant number G250201), and the Central Financial Forestry and Grassland Science and Technology Promotion and Demonstration Project ’Standardized Propagation of Michelia alba Seedlings and Demonstration of Cultivation and Maintenance Techniques′ from the National Forestry and Grassland Administration (grant number 沪[2025]TG02号).
Data Availability Statement
The original data presented in the study are openly available in https://doi.org/10.5281/zenodo.17158991.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and prospects. J. Adv. Res. 2019, 17, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Rosenblueth, M.; Martínez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and chemistry of endophytes. Nat. Prod. Rep. 2006, 23, 753–771. [Google Scholar] [CrossRef]
- Strobel, G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and functions of the bacterial microbiota of plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Waqar, S.; Bhat, A.A.; Khan, A.A. Endophytic fungi: Unravelling plant-endophyte interaction and the multifaceted role of fungal endophytes in stress amelioration. Plant Physiol. Biochem. 2024, 206, 108174. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-C.; Tao, W.-Y. Paclitaxel-producing fungal endophyte stimulates the accumulation of taxoids in suspension cultures of Taxus cuspidate. Sci. Hortic. 2009, 121, 97–102. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Chen, T.; Liu, J.; Chen, J.; Zhang, W.; Lu, B.; Zhang, L. The diversity and chemotaxonomic study of endophytic fungi from Magnolia denudata. Mycosystema 2010, 29, 194–200, (In Chinese with English abstract). [Google Scholar]
- Xu, L.; Sun, L.; Chen, Y.; Nie, T.; Zhu, H.; Yin, Z. Magnolia biondii Pamp.: A comprehensive review of the pharmacognosy, phytochemistry, pharmacology, and applications. Ind. Crops Prod. 2024, 222, 119648. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.M.; Lee, C.K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef]
- Shen, Y.; Li, C.; Zhou, S.; Pang, E.; Story, D.; Xue, C. Chemistry and Bioactivity of Flos Magnoliae, A Chinese Herb for Rhinitis and Sinusitis. Curr. Med. Chem. 2008, 15, 1616–1627. [Google Scholar] [CrossRef]
- Kim, H.J.; Nam, Y.R.; Nam, J.H. Flos Magnoliae Inhibits Chloride Secretion via ANO1 Inhibition in Calu-3 Cells. Am. J. Chin. Med. 2018, 46, 1079–1092. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Part I.; China Medical Science and Technology Press: Beijing, China, 2020; p. 195. [Google Scholar]
- Jang, W.Y.; Kim, M.-Y.; Cho, J.Y. Antioxidant, Anti-Inflammatory, Anti-Menopausal, and Anti-Cancer Effects of Lignans and Their Metabolites. Int. J. Mol. Sci. 2022, 23, 15482. [Google Scholar] [CrossRef]
- Uto, T.; Tung, N.H.; Ohta, T.; Shoyama, Y. (+)-Magnolin Enhances Melanogenesis in Melanoma Cells and Three-Dimensional Human Skin Equivalent; Involvement of PKA and p38 MAPK Signaling Pathways. Planta Medica 2022, 88, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, L.; Zhang, D.; Zhao, G.; Liang, Y.; Jia, X.; Guo, L.; Xu, C.; Gao, X. Chemical constituents and bioactivities of the essential oils of Magnolia biondii flower buds from three provinces in China. Flavour Fragr. J. 2024, 39, 302–311. [Google Scholar] [CrossRef]
- Schühly, W.; Skarbina, J.; Kunert, O.; Nandi, O.I.; Bauer, R. Chemical Characterization of Magnolia Biondii (Flos Magnoliae, Xin Yi). Nat. Prod. Commun. 2009, 4, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Carrión, V.J.; Perez-Jaramillo, J.; Cordovez, V.; Tracannna, V.; Hollander, M.D.; Ruiz-buck, D.; Mendes, L.W.; Ijcken, W.F.J.; Gomez-exposito, R.; Elsayed, S.S.; et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science 2019, 366, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Liu, S.-Y.; Yan, Y.; Yin, L.; Di, P.; Liu, H.-M.; Liu, H.-Z. Candidate genes involved in the biosynthesis of lignan in Schisandra chinensis fruit based on transcriptome and metabolomes analysis. Chin. J. Nat. Med. 2020, 18, 684–695. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Chio, C.; Kameshwar, A.K.S.; Ma, T.; Qin, W. Transcriptome analysis to identify genes involved in lignan, sesquiterpenoid and triterpenoid biosynthesis in medicinal plant Kadsura heteroclita. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 1802–1831. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.; Liu, D.; Sun, H.; Wu, J.; Huo, Y.; Chen, X.; Fang, R.; Zhang, L. Designing specific bacterial 16S primers to sequence and quantitate plant endo-bacteriome. Sci. China Life Sci. 2021, 65, 1000–1013. [Google Scholar] [CrossRef]
- Zhao, J.; Rodriguez, J.; Martens-Habbena, W. Fine-scale evaluation of two standard 16S rRNA gene amplicon primer pairs for analysis of total prokaryotes and archaeal nitrifiers in differently managed soils. Front. Microbiol. 2023, 14, 1140487. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Simpson, G.L.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package. R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 March 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Zhao, L.; Wang, M.; Sun, M. Diversity and structure of the rhizosphere microbial communities of wild and cultivated ginseng. BMC Microbiol. 2022, 22, 2. [Google Scholar] [CrossRef] [PubMed]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, D.; Kumar, S. Biotechnological interventions for harnessing podophyllotoxin from plant and fungal species: Current status, challenges, and opportunities for its commercialization. Crit. Rev. Biotechnol. 2016, 37, 739–753. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).