Abstract
The Costa Rican mid-elevation forests have been found to include some of the richest sites in the world for biodiversity per unit area. We used DNA barcodes to study 28,773 phorid fly specimens that were Malaise-trapped in the Nectandra Cloud Forest Reserve, north of the city of San Ramón, Alajuela, Costa Rica. This survey yielded 1964 BINs (Barcode Index Numbers), with a projected total of 2809, the largest known world phorid fauna. The diversity patterns of phorid flies collected at Nectandra were compared to 133,705 phorid flies collected at four other sites in Área de Conservacíon de Guanacaste in northwestern Costa Rica. All sites were highly diverse but differed significantly in their similarity to Nectandra, with low overlap among sites. Together, the number of BINs in northwestern Costa Rica is projected to exceed the entire described world fauna of Phoridae.
1. Introduction
The Phoridae (Diptera: Phoridae) is one of the most diverse yet poorly known families of insects. With only about 4500 currently described species worldwide, they promise to be one of the largest families, with numbers that might exceed 10–100 times the currently known fauna. They are especially diverse in humid tropical forests, although it is difficult to obtain numbers for these flies, owing to their diverse body forms, small size, and cryptic life histories [1,2]. Their life histories and immature forms are even less studied, but from what we know, phorids are scavengers, predators, parasitoids, true parasites, herbivores, and fungivores [1,2].
Over the last 50 years, the study of phorid flies in tropical regions has progressed significantly. The work of Thomas Borgmeier (summarized in [3,4,5]) was the cornerstone of this body of knowledge, but he was highly limited in the tools, funding, and specimens available to him. Given these limitations, the amount of work and knowledge he generated was amazing.
In Borgmeier’s time, collections from Central America were sparse, limited largely to small numbers of phorids within the United States National Museum (Smithsonian Institution). Since then, the ease of travel and use of Malaise traps have revolutionized the study of insects. Hanson and Gauld, during their collecting efforts (including a tremendous amount of Malaise trapping) for their Hymenoptera of Costa Rica (HCR) book [6], collected thousands of specimens of phorids and other insects that they sent to various specialists. Within Costa Rica, the best collections of insects were at the privately funded, and now unfortunately dissolved Instituto Nacional de Biodiversidad (INBio). Since then, Janzen and Hallwachs have Malaise-sampled extensively in Área de Conservación de Guanacaste (ACG) and have sent the specimens to the Centre for Biodiversity Genomics in the University of Guelph, Canada, where they were DNA barcoded and entered into the Barcode of Life Database (BOLD) (www.boldsystems.org, accessed on 10 July 2025). All of this activity has made Costa Rica by far the most intensively sampled country for New World tropical insect biodiversity.
Cloud forests in Costa Rica have an important place in Diptera studies. Janzen’s [7] pioneering work on insect diversity using sweep net samples throughout the country showed that intermediate elevations were the most species-rich. Therefore, when the worldwide dipterological community decided to start a joint inventory project in the tropics, the choice of sites was clear: Zurquí de Moravia, one of Hanson and Gauld’s HCR sites, known for extremely interesting species, and, importantly, its close proximity to San José, so that a staff of local and foreign scientists at INBio could easily visit and make collections [8]. This project, the Zurquí All—Diptera Biodiversity Inventory (ZADBI), set out to conduct a baseline study of all fly families to the species level. The only downside was that the funding did not allow for the extremely timesaving but still (at that time) expensive DNA barcoding. Therefore, only a small fraction of the most species-rich (and specimen-rich) groups of flies, namely the Cecidomyiidae and Phoridae, were processed. The numbers of species present for these families could only be estimated (800 and 400, respectively [9]), but these could only be lower bounds for these two families, as they were known to be hyperdiverse at other sites in New World tropical forests.
In contrast, using morphology on Drosophilidae, Grimaldi and Richenbacher [10] declared Zurquí de Moravia to be the richest site in the entire world for that family, documenting 352 species from one year of Malaise trapping. They also hypothesized that the cloud forests of the Andes mountains would be even richer if sampled in a manner similar to ZADBI. In another group of flies, Agromyzidae, the cloud forest fauna of Costa Rica was effectively doubled by the ZADBI survey, mostly conducted at Zurquí. Unfortunately, permission challenges and cost makes it unlikely that the phorids and cecidomyiids from the Zurquí collections will ever be barcoded, so their status will remain unassessed.
Meanwhile, Janzen and colleagues (e.g., ref. [11]) are still surveying throughout Costa Rica and sending material to Guelph for sequence analysis, so a thorough comparison of cloud forest sites is possible in the future. We take this opportunity, however, to make a preliminary report on the phorid fauna of a private reserve, namely Nectandra Cloud Forest Reserve. We have been collecting and sequencing in a fashion similar to that of Janzen and others and are making note of the truly exceptional diversity there. The goal of this study is to compare Nectandra’s phorid fauna with other nearby sites in the ACG, to augment the almost non-existent knowledge of phorid diversity patterns in Costa Rica.
2. Materials and Methods
Specimens were captured by three Malaise traps (Townes lightweight design [12]) purchased from Sante traps (santetraps.com). They were operated continuously for two years, from April 2023–May 2025 (although we have processed only part of this material) in the 158 ha Nectandra Cloud Forest Reserve on the Atlantic slope in Alajuela province, Costa Rica, 15 km north of San Ramon, 10.183° N, 84.517° W, at an elevation of 1100—1200 m. Samples were collected (i.e., the collecting bottle was changed) every two weeks. Trap 1 was located in a 50 m2 clearing surrounded by forest; Trap 2 was on the edge of the hilltop forest exposed to the moderate to strong Caribbean trade winds, and Trap 3 was in the dense forest understory (Figure 1). The weather statistics were obtained from the La Balsa weather station, 2 km south of Nectandra (Figure 2).

Figure 1.
Approximate sites of three Malaise traps at Nectandra. (a)—Trap 1, open parking area surrounded by forest. (b)—Trap 2, just within the boundary of windswept forest edge (white dot indicates position of trap). (c)—Trap 3, closed forest interior.
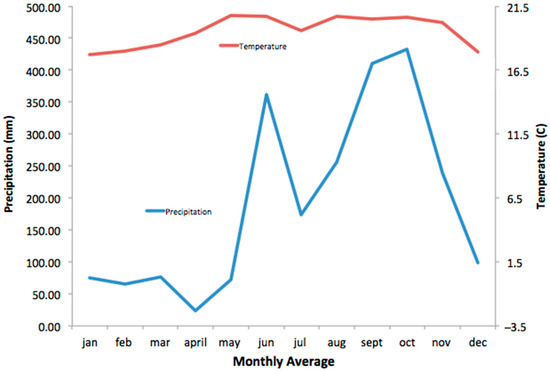
Figure 2.
Weather station data for Nectandra.
All adult phorid flies of both sexes were removed from the samples and sent preserved in 96% ETOH to the Center for Biodiversity Genomics (CBG) at the University of Guelph, where they were sequenced for the standard 658 bp COI barcode region. All specimens were classified into BINs (barcode index numbers) [13,14,15], which are not necessarily equivalent to species, but we use them as proxies for species, or as unevaluated species hypotheses. As part of the regular workflow, each specimen was photographed at the CBG using a Keyence V7000 imaging system. The Keyence is programmable and automatically photographs specimens in their 96-well plates in buffer solutions (i.e., “wet”) after DNA extraction. The resulting images are posted on the BOLD website at a resolution that is adequate for genus-level identification. Because there are few phorid taxonomists and taxonomic difficulties, most BINs were not identified beyond the family level as “Phoridae” at CBG. All BINs were, therefore, identified to the genus level by BVB, who is the world’s foremost expert on Neotropical phorids. It should be noted that some genus-level identifications are tentative, as some males (such as Calamiscus Borgmeier) cannot be identified at this level of magnification. Furthermore, the Metopina Macquart group of genera includes many specimens that can only be determined to genus based on females, which are relatively rare in Malaise trap samples. Most of these females are brachypterous or wingless and have to be carried by males in copula to reach the collection bottle (e.g., [16]). When recognizable females were present, however, the BINs of Metopina-group genera were identified. All information about the samples and specimens, including photographs, are available on BOLD.
These data were compared with collections from four nearby sites (within 200 km, but on the Pacific slope), including those obtained from the PL12 site [11], a geothermal development in the forest of Parque Nacional Rincon de la Vieja (ACG). Accumulation curves were derived from Chao’s shiny R app iNext [17,18], with a doubling of collection effort for each site indicated by the dashed line. Similarity was based on Jaccard statistics on downloaded data from BOLD. The observed BIN richness, extrapolation of BIN richness, and similarity among and within site were made with SpadeR [19] using the iChao1 index, an improved version of the Chao1 index that uses the number of singleton and doubletons to calculate the number of unencountered species.
3. Results
The deployment of the 3 Malaise traps in Nectandra over six months led to the collection of 28,773 specimens which included members of 1964 BINs (Table 1; Supplementary Information (SI), Table S1). Clearly, this is a much greater species richness than at the other sites. The iChao1 projection is shown only for Trap 2, but all three Nectandra traps were individually more BIN-rich than those of the other sites (Figure 3—other Nectandra sites not shown). The Pailas 12 traps, especially PL12-3, were the second most diverse traps, but are projected to have almost 500 BINs fewer than Nectandra (Table 1).

Table 1.
Alpha diversity of Phoridae form Nectandra and the sites in Área de Conservacíon de Guanacaste. PL-Pailas Dos site (transitional forest), DER (cloud forest), BSE (dry tropical forest), ESG (wet tropical forest), and NECT (cloud forest). Abbreviation: # = number of. For further characterization of sites, see [20].
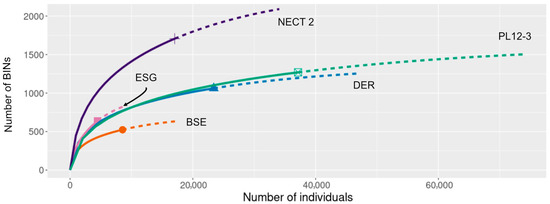
Figure 3.
Sample size-based rarefaction and extrapolation sampling curve for four sites in ACG and Nectandra. The differing line lengths are due to the unequal sampling efforts per site.
As expected, the BINs are dominated by the huge genus Megaselia Rondani with 1459 BINs (SI Table S2). In second place are the Metopina-group genera, represented by at least 155 BINs, including 133 for which females are needed to identify. The third most BIN-rich genus is Apocephalus Coquillett, with 92. A more detailed analysis of the genus-level composition of the various sites will have to await identification of the many BINs from sites other than Nectandra [20].
Furthermore, most Nectandra BINs have a low number of specimens, once the few dominant BINs are excluded (Figure 4 and Figure 5). Other sites are highly dominated by one or more BINs, except for PL12, which is most similar to Nectandra. Strikingly, 611 of the BINs are represented at Nectandra by a single specimen (Figure 4), including 234 BINs known only from Nectandra in all of BOLD (as of August 2025).
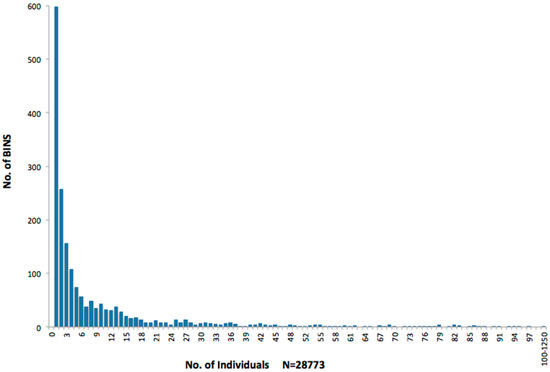
Figure 4.
Abundance distribution of BINs from Nectandra.
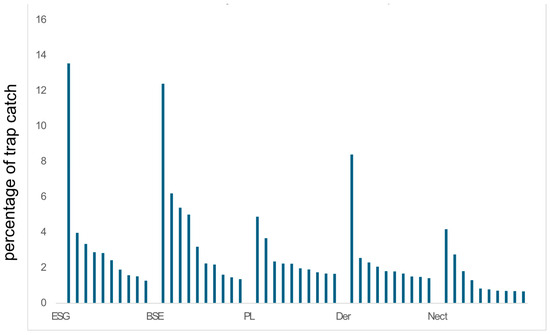
Figure 5.
Percentage of total number of BINs at a site made up by specimens of the 10 most abundant phorid fly BINs. From four sites from ACG and Nectandra. See also SI—Table S4. Abbreviations: ESG (wet tropical forest), BSE (dry tropical forest), PL (transitional forest), Der—Derrumbe (cloud forest), and Nect—Nectandra (cloud forest).
The amount of overlap among the common species of the Nectandra site with the four ACG sites is extremely low, with Jaccard indices in the range of 30–40% among the three Nectandra sites (Table 2) but sinking as low as 3.42% with BSE, 13—20% with Derrumbe, PL12, and ESG. These data roughly correspond with the distance from Trap 1 at Nectandra (Figure 6) and demonstrate that these data could be used to evaluate species turnover with greater accuracy in larger studies.

Table 2.
Jaccard indices for Malaise traps catches of Phoridae from three Nectandra sites, the sites in Area de Conservación de Guanacaste [11], and three other sites. Abbreviations: Nect 1-3—Nectandra sites 1-3, PL—Pailas 12-3, PL12-5 (transitional forest), DER (cloud forest), BSE (dry tropical forest), and ESG (wet tropical forest).
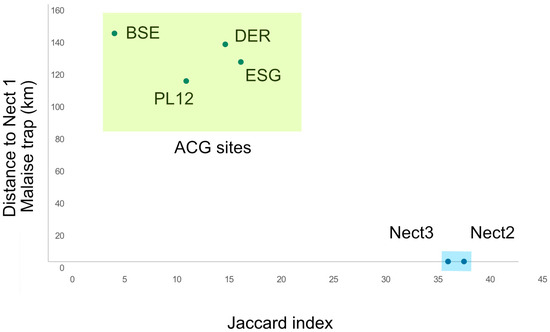
Figure 6.
Jaccard index versus distance from Nectandra Malaise trap site 1. Abbreviations: PL—Pailas 12 site (transitional forest), DER (cloud forest), BSE (dry tropical forest), and ESG (wet tropical forest).
The top 10 most abundant BINs for each of the 5 sites showed no overlap (SI, Table S3).
The three most common BINs from Nectandra were a species of undescribed genus related to Myriophora Brown (1238 individuals BOLD:ADA8408), a Puliciphora Dahl species (one of the Metopina group of genera—BOLD:AGC5698 with 834 individuals), and a Megaselia (BOLD:ACW4518 with 514 individuals). Within the Nectandra site, July showed peak density for all three of the most abundant BINs at all trap sites (Figure 7). The specimens of the undescribed genus were observed in higher numbers in Trap 3 (understory forest) than in Trap 2 (partially exposed forest) and in Trap 1 in a clearing. The 834 Puliciphora specimens were present in all three traps but persisted for a longer duration at Trap 3. In contrast, the 513 Megaselia BOLD:ACW4518 were caught by Trap 2 throughout the entire 6-month period. The significance of the different patterns is unclear but warrants further elucidation.
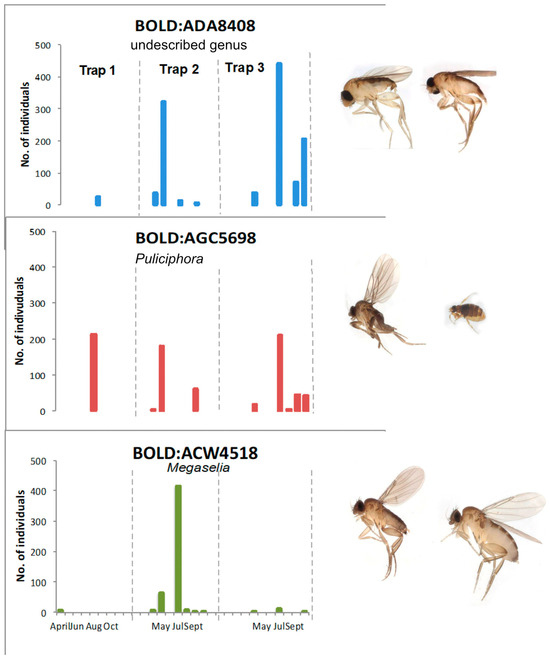
Figure 7.
Comparison of three most abundant BINs in Nectandra material and their occurrence. Images are male (left) and female (right), from BOLD.
Our data have some important gaps that we hope to fill within the near future. We cannot determine much about phenology at this time, as our dataset is incomplete, with only six months of trapping (SI, Table S4).
4. Discussion
The forests of ACG and Nectandra have yielded 3753 phorid BINs, with 4798 predicted. This is more than the described fauna for the entire world (4500 species). This ratio is true for many of the constituent Malaise trappable phorid genera as well: for instance, 31 BINs of the phorid genus Conicera Meigen compared to the 39 species described worldwide [21]. Furthermore, although there were only four BINs of Conicera from ACG, two of these were not found at Nectandra.
Flies are highly under-sampled given what we know of other sites. Having only four Melaloncha Brues BINs so far from Nectandra is the best example of this, as these phorids are relatively well-known bee parasitoids for which we have recent revisions [22,23]. There are 11 species of Melaloncha recorded from Zurquí in PCAT, and we expect there will be more eventually found at Nectandra. Another example is Apocephalus, the ant-decapitating flies. This last genus is likely underrepresented in our data, based on our study of Apocephalus at La Selva Biological Station in the Caribbean lowlands [24]. La Selva is a better ant habitat and has over 150 species of Apocephalus, but we expect Nectandra’s 92 BINs to rise drastically as more habitats are sampled along warmer, drier forest edges.
In most cases, however, we have no idea of how many species (or BINs) to expect per site because no sites have been comprehensibly sampled with barcodes and identified by a taxonomist. Since the phorids from the ZADBI project were not fully evaluated, the Nectandra site has become the most comprehensively examined site yet for the New World tropics, as analyzed by a phorid specialist.
There are other phorid inventory projects using DNA barcodes taking place in the world, but none in the tropics. The current ecological work conducted by Hartop et al. [25] suggests that the rare phorid species do not matter in their large-scale analyses of biodiversity patterns. In the abstract of their paper, they state that “Under sampling of rare taxa and choice of operational taxonomic units do not alter the main ecological inferences.” They made their collections in Sweden, however, and have not yet applied their methods to megadiverse tropical sites like Nectandra, where almost one-third of the taxa are known from only one specimen.
Meier et al. [26], in their study of fungus gnats (Mycetophilidae) in Singapore, argued that relativity few samples will be necessary to allow the mobilization of large amounts of data for biodiversity studies. Their process was to take two groups of samples and compare them to look at overlap. The first group of samples were from 1554 specimens from Malaise traps (12) around the island of Singapore. Once the initial sampling was completed, the second set of samples was analyzed. This second set doubled the number of specimens, but only increased the number of distinct barcodes by 3%. Thus, the first sample had 97% of the barcodes from that area, and the area was declared complete. They stated that once the “important” species were found, this would allow researchers to make great inroads in groups that are typically avoided because they are so diverse.
We are skeptical that such inventory work can be so quickly conducted in Costa Rica with the currently available tools. Extreme species diversity, like that found in Costa Rican phorids, will require other paradigms. Species descriptions will have to dramatically accelerate with an emphasis on molecular data. The rarity of species will need to be investigated more thoroughly as well, since some of the singletons at Nectandra are extremely common elsewhere.
Further study of rarity and ecological processes will be needed so that we can answer the following questions:
- -
- How local are the sampling ranges of Malaise traps for phorid flies? We have found many instances where a species suddenly occurs in large numbers in one Malaise trap. Is this because a parasitized host or an ephemeral resource is suddenly available nearby? If so, how close does the resource need to be to generate this spike?
- -
- The top 10 species among the four sites in ACG and Nectandra (SI Table S3) show no overlap. Will intervening areas have intermediate amounts of overlap? If so, can these data be used to develop a general model for species turnover in phorids?
- -
- What are the ecological or phylogenetic characteristics of BINs that are unique to Nectandra? Are they small offshoots of more widespread lineages, or groups that occur more widely that are awaiting more intensive collection and barcoding elsewhere?
- -
- Work in Brazil has discovered a whole new source of rare species in the canopy [27]. Over 60% of the species of Diptera in the Brazilian inventory were found only at 8 m and higher above the forest floor. What will sampling in the Nectandra canopy and other sites reveal about species diversity?
Janzen and Hallwachs [11] proposed that the number of common species of insects found at forest edges, such as at our Nectandra site 2 and the Zurquí Malaise trap 1, might be heavily augmented by the transport of individuals by wind from areas at a greater distance, or by the movement of species from the local canopy down to the ground level. One other possibility is that the edge habitat, or that of the associated clearing, might be the home for many more species that have specialized needs that are not met in either the forest, such as at our Nectandra site 3, or a small, within-forest clearing, such as at our Nectandra site 1.
Like all tropical forests, the cloud forests of Costa Rica are endangered by human activities [28]. Monitoring such sites over the long term is essential to determine the extent of this impact. Certainly, if Janzen and Hallwach’s “transporation by wind” is a strong effect, then this will have profound effects on conservation in general. We cannot just save the forest at Nectandra, because the sources of species, presumably from neighboring areas will have to be maintained farther upwind as well. Also, the effects of insecticides used on agricultural areas at lower elevations will become an even more widespread problem. Therefore, we need to continue researching the source of Nectandra’s extreme diversity to try to prevent its loss.
5. Conclusions
The Nectandra site is the most diverse site for phorid flies yet documented in the world, with nearly 2000 BINs. Together with the ACG BINs (1118 BINs), they predict that northern Costa Rica will have a total of about 4800, more than the 4500 species described worldwide. Much of this diversity is found in the nearly 30% of species known from single specimens, giving pause to ideas of the completeness of surveys for this group in tropical New World forests.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17100717/s1, Supplementary Information: Table S1. Abundance of all BINs known from Nectandra. Table S2. Number of BINs per genus from Nectandra. Groupings are highest monophyletic taxon. Table S3. Megaselia species shared by urban Los Angeles, ACD and Nectandra, Costa Rica. Table S4. Ten most abundant BINs at ACG and Nectandra. Table S5. Trap samples that have been sorted and sequenced so far.
Author Contributions
Conceptualization, B.V.B. and E.T.L.; writing—original draft preparation, B.V.B.; writing—review and editing, B.V.B. and E.T.L.; formal analysis, B.V.B. and E.T.L., supervision, E.T.L.; project administration, E.T.L. All authors have read and agreed to the published version of the manuscript.
Funding
The David A. and Evelyne T. Lennette Trust provided the funding for the DNA barcoding. The ZADBI project was funded by National Science Foundation Research Grant DEB-1145890 to B. Brown and A. Borkent.
Data Availability Statement
All of our data are available on the BOLD web site.
Acknowledgments
BVB is greatly indebted to many of those who have worked with him on his inventory projects over the last 30 years, but none more than Wendy Porras, who has been his colleague and field assistant during the last 20 years. She sorted all of the phorid flies out of the Malaise traps, arranged collection permits, and exported the specimens. We thank Freddy Castillo for his capable field assistance and maintenance of the traps at Nectandra. Travel in Costa Rica was supported by donations from the Natural History Museum of Los Angeles County by Esther Chao and Victoria Dean and was further facilitated by Luis Chiappe. Technical help from Giar-Ann Kung, Austin Baker, Estella Hernandez, Lisa Gonzalez, and Weiping Xie is also gratefully acknowledged. Paul Hebert, Daniel Janzen, and Winnie Hallwachs are among the pioneers in the use of DNA barcodes to support biological surveys, and we thank them, as well as Valerie Levesque-Beaudin, Dirk Steinke, and Chris Ho from the Centre for Biodiversity Genomics for their support. The capacity of the CBG to provide sequencing support and to sustain BOLD was enabled by grants from the Canada Foundation for Innovation and from the New Frontiers in Research Fund to Paul Hebert, with partial matching resources from the Walder Foundation of Chicago. Finally, BVB thanks colleagues Dalton Amorim, Mike Sharkey, Emily Hartop, Rudolph Meier, and Steve Marshall for conversations about tropical biodiversity.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Brown, B.V. Phoridae (Hump-backed flies, Scuttle flies). In Manual of Central American Diptera; Brown, B.V., Borkent, A., Cumming, J.M., Wood, D.M., Woodley, N.E., Zumbado, M.A., Eds.; NRC Research Press: Ottawa, ON, Canada, 2010; Volume 2, pp. 725–761. [Google Scholar]
- Disney, R.H.L. Scuttle flies: The Phoridae; Chapman and Hall: London, UK, 1994; pp. xii + 467. [Google Scholar]
- Borgmeier, T. A catalogue of the Phoridae of the World (Diptera, Phoridae). Stud. Entomol. 1968, 11, 1–367. [Google Scholar]
- Borgmeier, T. Supplement to a catalogue of the Phoridae of the World. Stud. Entomol. 1971, 14, 177–224. [Google Scholar]
- Kempf, W.W. Father Thomas Borgmeier, O.F.M. (1892-1975). Stud. Entomol. 1976, 19, 1–37. [Google Scholar]
- Hanson, P.E.; Gauld, I.D. The Hymenoptera of Costa Rica; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1995; pp. xvii + 893. [Google Scholar]
- Janzen, D.H. Sweep samples of tropical foliage insects: Effects of seasons, vegetation types, elevation, time of day, and insularity. Ecology 1973, 54, 687–708. [Google Scholar] [CrossRef]
- Borkent, A.; Brown, B.V. How to inventory tropical flies (Diptera)-One of the megadiverse orders of insects. Zootaxa 2015, 3949, 301–322. [Google Scholar] [CrossRef]
- Brown, B.V.; Borkent, A.; Adler, P.H.; De Souza Amorim, D.; Barber, K.; Bickel, D.; Boucher, S.; Brooks, S.E.; Burger, J.; Burington, Z.L.; et al. Comprehensive inventory of true flies (Diptera) at a tropical site. Commun. Biol. 2018, 1, 21. [Google Scholar] [CrossRef]
- Grimaldi, D.A.; Richenbacher, C. Exceptional species diversity of Drosophilidae (Diptera) in a Neotropical forest. Am. Mus. Novit. 2023, 2023, 1–28. [Google Scholar] [CrossRef]
- Janzen, D.H.; Hallwachs, W. Using DNA barcoded Malaise trap samples to measure impact of a geothermal energy project on the biodiversity of a Costa Rican old-growth rain forest. Genome 2020, 63, 407–436. [Google Scholar] [CrossRef] [PubMed]
- Townes, H. A light-weight Malaise trap. Entomol. News 1972, 83, 239–247. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. The Barcode of Life Data System (www.barcodinglife.org). Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. A DNA-based registry for all animal species: The barcode index number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Wei, C.; Chan, D.; Agda, J.; Agda, J.; Ballesteros-Mejia, L.; Boutou, H.A.; El Bastami, Z.M.; Ma, E.; Manjunath, R.; et al. OLD v4: A centralized bioinformatics platform for DNA-based biodiversity data. Methods Mol Biol. 2024, 2744, 403–441. [Google Scholar] [CrossRef]
- Miller, P.L. Alternative reproductive routines in a small fly, Puliciphora borinquenensis (Diptera: Phoridae). Ecol. Entomol. 1984, 9, 293–302. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.; Hseih, T.; Sander, E.; Ma, K.; Colwell, R.; Ellison, A. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Chao, A.; Ma, K.; Hseih, T. iNEXT (iNterpolation and EXTrapolation). Online: Software for Interpolation and Extrapolation of Species Diversity. Program and User’s Guide. Published. Available online: https://chao.shinyapps.io/iNEXTOnline/ (accessed on 8 October 2025).
- Chao, A.; Ma, K.H.; Hsieh, T.C.; Chiu, C.H. Online Program SpadeR (Species-Richness Prediction And Diversity Estimation in R). Program and User’s Guide. Available online: https://chao.shinyapps.io/SpadeR/ (accessed on 8 October 2025).
- Brown, B.V. Some remarkably common, but undescribed, Megaselia Rondani (Diptera: Phoridae) from northwestern Costa Rica. Zootaxa 2022, 5120, 373–390. [Google Scholar] [CrossRef]
- Brown, B.V. Phorid Catalog (PCAT)—Online Data for Phorid Flies. Available online: www.phorid.net/pcat (accessed on 9 August 2025).
- Brown, B.V. Revision of the subgenus Udamochiras of Melaloncha bee-killing flies (Diptera: Phoridae). Zool. J. Linn. Soc. 2004, 140, 1–42. [Google Scholar] [CrossRef]
- Brown, B.V. Revision of the untreated taxa of Melaloncha s. s. bee-killing flies (Diptera: Phoridae). Zootaxa 2006, 1280, 1–68. [Google Scholar] [CrossRef]
- Brown, B.V.; Feener, D.H. Efficiency of two mass sampling methods for sampling phorid flies (Diptera: Phoridae) in a tropical biodiversity survey. Contrib. Sci. 1995, 459, 1–10. [Google Scholar] [CrossRef]
- Hartop, E.; Lee, L.; Srivathsan, A.; Jones, M.; Peña-Aguilera, P.; Ovaskainen, O.; Roslin, T.; Meier, R. Resolving biology’s dark matter: Species richness, spatiotemporal distribution, and community composition of a dark taxon. BMC Biol. 2024, 22, 215. [Google Scholar] [CrossRef]
- Meier, R.; Srivathsana, A.; Oliveira, S.; Balbi, M.; Ang, Y.; Yeo, D.; Kjærandsen, J.; Amorim, D. “Dark taxonomy”: A new protocol for overcoming the taxonomic impediments for dark taxa and broadening the taxon base for biodiversity assessment. Cladistics 2025, 41, 223–238. [Google Scholar] [CrossRef]
- Amorim, D.S.; Brown, B.V.; Boscolo, D.; Ale-Rocha, R.; Alvarez-Garcia, D.M.; Balbi, M.I.P.A.; de Marco Barbosa, A.; Capellari, R.S.; de Carvalho, C.J.B.; Couri, M.S.; et al. Vertical stratification of insect abundance and species richness in an Amazonian tropical forest. Sci. Rep. 2022, 12, 1734. [Google Scholar] [CrossRef] [PubMed]
- Janzen, D.H.; Hallwachs, W. To us insectometers, it is clear that insect decline in our Costa Rican tropics is real, so let’s be kind to the survivors. Proc. Natl. Acad. Sci. USA 2021, 118, e2002546117. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).