Abstract
This study investigated the structure of vegetation communities in Yongneup, a representative montane peatland on Mt. Daeamsan, Korea. It also identified key microenvironmental drivers shaping their distribution. We surveyed 200 quadrats, analyzing herbaceous plant composition alongside peat depth, water level, and soil chemical properties. Multivariate analyses, including cluster analysis and classification tree analysis (CHAID), identified nine distinct vegetation communities. Each community was correlated with specific environmental gradients. Dominant species included Sanguisorba tenuifolia and Carex thunbergii var. appendiculata, with rare species such as Carex chordorrhiza and Drosera rotundifolia present in localized habitats. Peat depth emerged as the primary determinant of vegetation distribution, followed by hydrology and nutrient levels, including phosphorus and cations (Mg2+, Na+, K+). Our results underscored continuous ecological gradients rather than discrete zonation, aligning with ecological continuum theory. These findings provide a robust scientific framework for ecological monitoring and restoration. They also support Korea’s national wetland conservation policies and international commitments such as the Ramsar Convention.
1. Introduction
Although wetlands occupy only about 6% of the Earth’s land surface, they support exceptionally high productivity [1] and host distinctive vegetation with many endemic species. Wetlands also provide essential ecosystem functions, including flood regulation, water purification, and habitat provision for aquatic organisms. Among them, peatlands (typically developed in cool, humid regions) are recognized as major long-term carbon sinks [2]. These systems form through the gradual accumulation of plant material that decomposes slowly under waterlogged and oxygen-poor conditions. Peatlands are characterized by low pH due to organic acids secreted by Sphagnum mosses and are generally oligotrophic environments [3]. Peat refers to partially decomposed organic material containing at least 30% organic matter [4].
Montane peatlands are biodiversity-rich ecosystems that play a vital role in carbon storage and hydrological stability. However, such ecosystems in East Asia, particularly Korea, remain poorly understood, resulting in major gaps in knowledge of their vegetation dynamics and environmental interactions. Yongneup, a high-altitude peatland on Mt. Daeam, is one of Korea’s most important wetlands, notable for its rarity and distinctive boreal flora. However, previous studies show inconsistencies in community classification. Quantitative analyses linking vegetation with microenvironmental variables are rare, leaving the underlying mechanisms largely unresolved.
Peat deposits accumulate over long periods, typically at a rate of about 1 mm per year [5]. Peatlands are generally classified into bogs, fens, swamps, and marshes according to their hydrological regime, pH, and nutrient status. However, this classification remains debated [2,6]. Previous studies proposed various criteria: bogs and fens have been distinguished by indicator species, pH, and base cation content [7], or by the concentrations of total calcium and nitrogen in the soil [8]. Vitt and Chee [9] divided fens into three subtypes based on Ca2+, Mg2+, and electrical conductivity. Wheeler and Proctor [10] defined bogs as environments with Ca 2+, Cl−, and SO42− and a pH ≤ 5.0, while fens contain Ca2+ and HCO3− have a pH ≥ 6.0. Similarly, Tarnocai [2] and Hájek et al. [6] classified fens as habitats with a pH ≥ 5.5, and bogs as those with pH ≤ 5.5. Swamps and marshes are comparatively nutrient-rich and have higher water levels than bogs and fens. Bridgham et al. [11] further noted that bogs are acidic environments dominated by Sphagnum palustre, whereas fens are slightly less acidic.
Vegetation community patterns in wetlands depend on multiple environmental factors, including water source, peat depth and water level. Microtopography and human disturbance also play important roles [12,13,14,15,16,17,18,19]. Numerous studies have emphasized that hydrological and chemical gradients are key determinants of vegetation patterns in peatlands [7,10,11,20]. Wheeler and Proctor [10] and Bragazza et al. [21] reported that vegetation could be categorized by pH and water-level differences, while Glaser et al. [20] suggested that vegetation types could be distinguished by range of pH and Ca2+ concentration. Navrátilová et al. [22] indicated that groundwater levels, water pH, and the availability of ions such as Ca2+, K+, and N–NO3− influence community composition. Branson et al. [23] found that vegetation growth varies with soil moisture, and subsequent studies showed that factors such as electrical conductivity [24], bedrock characteristics [25], soil pH, and Ca content [26] also affect vegetation distribution. Water level and peat bulk density have likewise been proposed as major factors influencing plant community patterns [27].
Peatlands exhibit pronounced microenvironmental heterogeneity, especially in microtopography, water table depth, and peat accumulation. Recent studies have shown that even subtle differences in surface elevation or hydrological regime can lead to substantial changes in vegetation composition [28]. Incorporating fine-scale physical variability into ecological models has further underscored its critical role in regulating plant community structure and ecosystem function [29,30].
Micro-scale chemical variability, such as differences in soil pH, redox potential, and trace metal concentrations, also plays a crucial role in shaping peatland plant and microbial communities. Soil pH serves as a primary driver of species richness and community composition [31,32], while redox gradients influence both the chemical and taxonomic structure of peat soils [33]. Moreover, trace metals such as Fe, Ca, and Al help stabilize peat C-–N pools, with hydrologic conditions controlling metal-mediated nutrient dynamics (2024 Metal C–N study). Under accelerating climate change, montane peatlands are increasingly vulnerable to hydrological instability and degradation, highlighting the urgency of detailed vegetation–environment analyses.
Despite their ecological and hydrological importance, East Asia peatlands remain poorly studied with respect to their microenvironmental heterogeneity and functional dynamics [34]. In Korea, peat-dominated wetlands are inconsistently defined and lack systematic monitoring. This has led to major data gaps in soil characteristics, carbon fluxes, and long-term vegetation changes.
Peatland formation in Korea is limited by climatic and geological constraints, making such habitats rare. Representative peatlands and wetlands with peat-forming potential include Yongneup (Mt. Daeamsan, Inje-gun) [35,36], Moojechineup (Ulju-gun, Ulsan) [37], and Sowhangbyungsan-neup (Mt. Odaesan, Pyeongchang-gun) [38]. Other notable sites are Jilmoe-neup [38], Sajapyeong Alpine Wetland (Mt. Jaeyaksan, Miryang, Gyeongsangnam-do) [39], Hwaeom-neup [40], and the 1100 Highland Ecological Swamp (Jeju-Island) [41]. In addition, wetlands such as those on Mt. Sinbulsan [42], Mt. Chilbosan [43], Mt. Jeombongsan (Neoreunjae-neup, Sum-eungol-neup) [38,44], Simjeok-ri-neup (Seohwa-myeon, Inje) [38], and Wangdeungjae Wetland [44,45] have also been identified. These mountain wetlands have undergone environmental changes over thousands of years and represent key areas requiring ecological investigation for effective restoration and conservation [46]. However, compared to peatlands in other countries, Korean mountain wetlands are generally smaller and spatially isolated, making them particularly vulnerable to external disturbances. Many are being lost to reclamation and unregulated development before their biodiversity and distribution can be fully recorded [47,48].
Yongneup, a high moor on Mt. Daeamsan, is recognized as Korea’s representative acidic peatland of high academic and ecological value. Floristic studies on Yongneup include those by [49,50,51,52,53,54], but the number and identification of recorded species have varied depending on the researcher. Kang [35] classified plant communities into six groups, Park [55] into eight groups, and Ryou [44] into twenty-eight groups. These variations largely reflect differing survey scopes and classification criteria. Yongneup thus represents an ecologically and academically important site, characterized by its unique flora and plant communities, including the common presence of northern (boreal) plant species.
However, taxonomic interpretations have not always been consistent, and the number of communities identified has differed due to classifications based mainly on external physiognomy. Comprehensive, site-wide analyses that quantitatively assess the relationships between microenvironmental factors and vegetation structure remain limited.
Therefore, this study aimed to classify the vegetation communities of Yongneup and to examine how hydrological conditions, peat depth, and soil chemistry drive small-scale variations in vegetation patterns. We further examined Yongneup’s peatland classification to identify its ecological type and provide a scientific foundation for long-term monitoring and conservation planning. This study was guided by the hypothesis that plant community structure in Yongneup peatland is primarily determined by the interactions among peat depth, water flow, and soil chemistry, and that distinct vegetation clusters correspond to identifiable environmental thresholds.
2. Materials and Methods
2.1. Study Site
This study focused on Yongneup, a peatland located on Mt. Daeam in Inje-gun, Korea. Yongneup is located at an elevation of 1200 m a.s.l. Its geographical range extends from 38°12′48″–38°13′32″ N and from 128°07′05″–128°08′00″ E [56]. The wetland covers an area of 3.15 ha [57]. The temperature at Yongneup, calculated while accounting for the altitude based on the method described by [58], was 3.7 °C. The geological features of Mt. Daeam include the granite gneiss system, and biotite gneiss and injection gneiss mostly occur inside the wetland. The rock is coarse and consists of quartz, microcline, perthite, biotite, and garnet, and has been formed by mechanical weathering [56]. The formation of Yongneup is estimated to have begun approximately 4500 years ago. The surface of the wetland consists of Sphagnum peat, with Carex peat deposited beneath [59]. In addition, soil inflow occurred about 1900 years ago [60].
Yongneup was first introduced to the academic community in 1968. It was designated as a Natural Ecosystem Specially Protected Area in 1989 and as a Wetland Protection Area in 1999. After Korea joined the Ramsar Convention, Yongneup became the first registered Ramsar site in the country, highlighting its high academic significance and rarity value [61].
Yongneup consists of two parts: Large Yongneup and Small Yongneup. Small Yongneup was destroyed and converted to land due to wastewater inflow from military bases and soil from forest trails.
The wetland has a flat topography with few trees. As a result, skating rinks were once constructed at its center [62], and peat deposits were removed, exposing the weathered bedrock layer [63]. Following these disturbances, trampling, wild activity, and soil inflow from surrounding areas continued. However, public access is now restricted by the Wonju Regional Environmental Agency and the military unit, ensuring that the site is well preserved and protected from further disturbance.
2.2. Vegetation and Environmental Factors Survey
Vegetation survey was conducted systematically in Yongneup in June 2009, excluding peripheral forest and shrub areas. A total of 200 quadrats (1 m × 1 m) were positioned within peatland and transitional zones (Figure 1). Within the peatlands, except the transition zones, the quadrats were installed at similar intervals and arranged in a north–south direction, and the SE points were recorded using a GPS unit to enable future monitoring. In each quadrat, the number of species, species composition, absolute cover per species, and the height of dominant species were recorded.
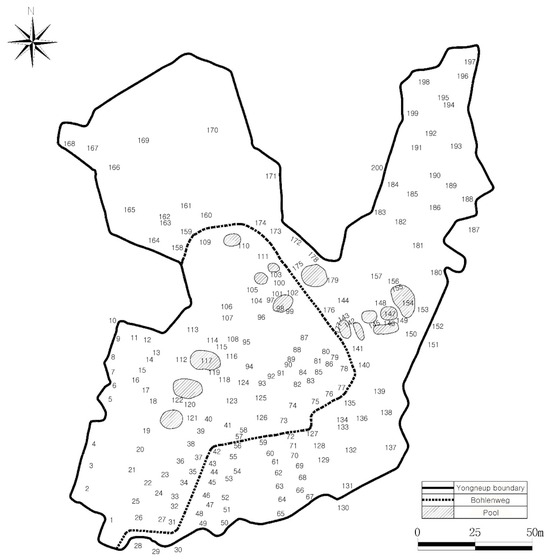
Figure 1.
Distribution of 200 survey plots within Yongneup (plot numbers indicate the order of sampling). A wooden boardwalk (“Bohlenweg” in German) crossing the peatland minimizes visitor-induced disturbance to the surface and vegetation.
Environmental parameters including peat depth, water level, and soil chemistry were measured individually at each quadrat. Peat samples underwent laboratory analyses for moisture content, available phosphorus, total nitrogen, organic matter, and pH. Water chemistry parameters, such as conductivity and ion concentrations (Ca2+, Mg2+, K+, Na+, NH4+, Fe2+, Zn2+, Cl−, NO3−, SO42−, and P), were also analyzed.
Peat and hydrological characteristics were investigated and analyzed at the SE point of the quadrat where the vegetation survey was conducted.
2.2.1. Peat Characteristics
The depth of the peat deposits was measured using a manufactured peat rod (1.8 m length and 1 cm diameter). Depths exceeding 1.8 m were recorded as 1.8 m. Peat samples were collected using a cylindrical soil sampler (15 cm length, 10 cm diameter) for chemical analysis.
A portion of the collected soil samples was used to measure moisture content, while the remaining samples were air-dried in the shade at 20–25 °C, sieved through a 2 mm mesh, and used for further analyses. Soil moisture content was determined by drying the samples in an oven at 105 °C and calculating the percentage based on the difference between the wet soil weight and the dry soil weight.
To extract the available phosphate, 14 ㎖ 0.03 M NH4F was added to 2 g soil dried in the shade, and the mixture was shaken for 1 min. After filtering the extract using filter paper (Whatman #42), 1 ㎖ of the extract was developed using 4% (NH4)6Mo7O24·4H2O. The absorbance of the sample was measured at 882 nm using a spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan) and quantified based on the Bray I method [64].
The total nitrogen content was decomposed by adding 4.4 ㎖ H2SO4 and decomposition accelerating agents (1.5 g K2SO4, 7.5 mg Se) to the dried soil sample (0.4 g) and heating the sample in a digester (2000 Digestion System, Tecator, Höganäs, Sweden). The decomposed solution was distilled using a distiller (1002 Kjeltec System, Distiling Unit, Tecator, Höganäs, Sweden), developed with a mixed indicator (Bromocresol green 0.5 g + methyl red + 95% ethanol 100 ㎖), and titrated with 0.05 N sulfuric acid solution to determine the nitrogen concentration.
To measure the pH, 7 g soil dried in the shade at room temperature was mixed with 35 ㎖ distilled water, and the mixture was shaken for 1 h in a shaker. The shaken solution was filtered through two layers of filter paper (Whatman #1) to measure the pH.
2.2.2. Wetland Water Characteristics
Water levels were measured during the field survey to characterize local hydrological conditions. At sites where the water surface was above the ground, measurements were taken directly using a folding ruler. Where the water table was below the surface, a 70 cm steel pipe was inserted into the soil, and the water level was recorded after it stabilized. When the water table was too low to be detected even with the pipe installed, the value was recorded as 70 cm below the surface.
A total of 165 wetland water samples collected from each quadrat, except the quadrats where samples could not be collected owing to low water levels, were used to measure the pH and conductivity of the water using a pH meter (CH-8603, Mettler Toledo, Greifensee, Switzerland) and a conductivity meter (YSI model 85, Yellow Springs Instrument Inc., OH, USA), respectively. Wetland water samples were filtered through a membrane filter (pore size 0.45 μm).
Cations (Ca2+, Mg2+, K+, Na+, NH4+, Fe 2+, Zn2+) and anions (Cl−, NO3−, SO42−) were quantified using ion chromatography (DX-320, Dionex, Sunnyvale, CA, USA). Phosphorus (P) concentrations were measured using an inductively coupled plasma spectrometer (OPTIMA7300 DV) at the Joint Laboratory Practice Hall, Kangwon National University.
2.3. Statistical Analysis
Vegetation community data were analyzed using a combination of cluster analysis, indicator species analysis (ISA), nonmetric multidimensional scaling (NMS), and CHAID tree analysis to identify significant environmental thresholds. The multivariate statistical software PC-Ord was used to perform the cluster analysis. Vegetation communities within the study area were classified through cluster analysis, and the optimal number of clusters was determined using indicator species analysis (ISA). The ISA was applied to identify characteristic species for each cluster. The Indicator Value (IV) for each species was calculated as the product of its relative abundance and frequency within each group, ranging from 0 (no indication) to 100 (perfect indication). The statistical significance of IVs was assessed using a Monte Carlo permutation test (p < 0.05).
Vegetation cover data were transformed using arcsine square root transformation to meet the assumptions of normality. Nonmetric multidimensional scaling (NMS) ordination was performed to analyze the spatial distribution of the clusters. We performed MRPP (Multi-Response Permutation Procedures) to test the differences among groups classified by cluster analysis. The indicator species and indicator values for each of the nine communities were analyzed using ISA. The relationship between vegetation communities and environmental factors was further explored using TREE analysis (CHAID: Chi-squared Automatic Interaction Detection) in SPSS (version 18; SPSS Inc., Chicago, IL, USA). The CHAID method was selected for its interpretability and ability to identify hierarchical environmental thresholds relevant to wetland management. The splitting criterion was set to p < 0.05 with Bonferroni adjustment, and a minimum node size of five was applied.
3. Results
3.1. Species Composition and Spatial Distribution
A total of 130 taxa of herbaceous species were found in Yongneup, and 93 species were recorded in the 200 quadrats surveyed. Sanguisorba tenuifolia Fisch. ex Link, and Carex thunbergii var. appendiculata (Trautv.) Ohwi were overwhelmingly dominant in Yongneup. S. tenuifolia had a frequency of 90% and an average cover of 36.7%, showing the highest importance value. C. thunbergii var. appendiculata also had a frequency of 82% and an average cover of 29.3%. Molinia japonica Hack. occurred at a high frequency of 64%, and the frequency of Sphagnum palustre L., a key indicator species in peatlands, was 35%. Furthermore, the endangered class II plant Trientalis europaea subsp. arctica (Fisch. ex Hook.) Hultén was widely distributed, with a frequency of 30%. In contrast, Menyanthes trifoliata L. showed a frequency of only 3%. This is because M. trifoliata was distributed only around puddles. The frequencies of insectivorous plants, such as Drosera rotundifolia L. and Utricularia intermedia Hayne, were 11% and 5%, respectively. U. intermedia is distributed around shallow waters, and as quadrats were rarely installed around those areas, the actual frequency in their preferred habitat would be higher than 5%. Endemic species, such as Iris ensata Thunb. and Scrophularia koraiensis Nakai, were also recorded. S. koraiensis was not observed in the quadrats surveyed. In addition, the naturalized plants Bidens frondosa L. and Erigeron annuus (L.) Pers. were found to invade the wetland.
3.2. Vegetation Community
The results of ISA and cluster analysis suggested that the vegetation in Yongneup could be divided into nine communities (Table 1). Each cluster was named based on the name of the species with an indicator value of 60 or higher. However, the species to be named were limited to one or two species, and communities wherein the indicator value of species did not exceed 60 were named based on the species with the highest indicator value. Although some indicator species showed relatively low indicator values (e.g., IV = 28), these were still statistically significant (p < 0.05). This result can be attributed to the low species diversity within those communities, where even a single species with consistent and exclusive occurrence can serve as a meaningful indicator of community characteristics.

Table 1.
Indicator species and indicator values in nine communities.
The vegetation in Yongneup was classified into nine distinct communities: Lycopus lucidus Turcz. ex Benth.-Geranium koreanum var. hirsutum Nakai, Caltha palustris L., S. tenuifolia, C. thunbergii var. appendiculata, Juncus papillosus Franch. & Sav., M. japonica, Sparganium hyperboreum Laest. ex Beurl.-Carex canescens L., Carex chordorrhiza L. f.-Rhynchospora fujiiana Makino, and Calamagrostis purpurea (Trin.) Trin. (Table 1). Other communities within the peatland community were distributed according to microtopographic features, such as puddles. Communities were classified using NMS (Figure 2). Axis 1 explained 50.0% of the variance, and Axis 2 explained 32.0% of the variance, explaining a total of 82.0% of the variance.
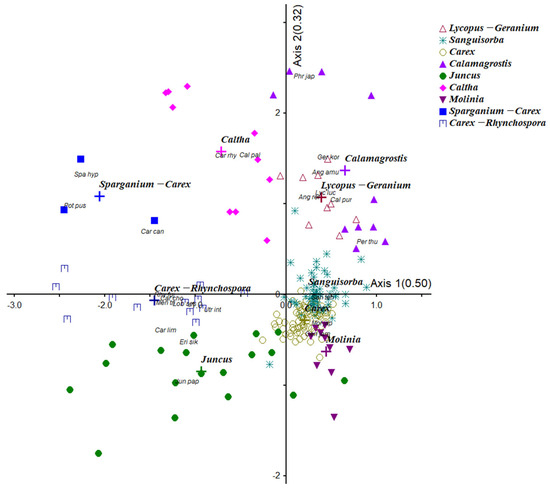
Figure 2.
Community analysis by Nonmetric Multidimensional Scaling in Yongneup. Lycopus-Geranium: Lycopus lucidus-Geranium koreanum var. hirsutum, Sanguisorba: Sanguisorba tenuifolia, Carex: Carex thunbergii var. appendiculata, Calamagrostis: Calamagrostis purpurea, Juncus: Juncus papillosus, Caltha: Caltha palustris, Molinia: Molinia japonica, Sparganium-Carex: Sparganium hyperboreum-Carex canescens, Carex-Rhynchospora: Carex chordorhiza-Rhynchospora fujiiana. A cross (+) symbol represents the centroid of each community type or group in ordination space.
The NMS ordination clarified the primary gradients: Axis 1 was related to peat depth and water level, while Axis 2 corresponded to nutrient concentrations (especially Mg2+ and phosphorus). CHAID analysis also showed that peat depth was the strongest predictor of vegetation distribution. Dominant species exhibited clear preferences along these environmental gradients, reflecting nuanced ecological sorting rather than distinct vegetation zones.
The main indicator species for Axis 1 were as follows: S. tenuifolia on the right, and C. chordorhiza and R. fujiiana on the left. Axis 1 in the NMS ordination reflected a peat depth and water level gradient, while Axis 2 was associated with Mg2+ and available phosphorus concentration, suggesting hydrological and geochemical sorting of communities. The peatland community was located on the right side of Axis 1, and the transition zone and disturbance zone communities were located on the left side. In addition, M. japonica was located in the lower part of Axis 2, whereas C. palustris, C. purpurea, G. koreanum var. hirsutum were positioned in the upper part. The upper section mainly included communities distributed around puddles (Figure 2).
3.2.1. Lycopus lucidus-Geranium koreanum var. hirsutum Community
The Lycopus lucidus Turcz. ex Benth.-G. koreanum var. hirsutum community had the following indicator species, with the largest number of indicator species recorded among the nine communities: L. lucidus, G. koreanum var. hirsutum, Angelica amurensis Schischk., Artemisia stolonifera (Maxim.) Kom., Viola acuminata Ledeb., Potentilla fragarioides L., Lychnis cognata Maxim., etc. L. lucidus had the highest indicator value of 81, followed by G. koreanum var. hirsutum (68), A. amurensis (60), A. stolonifera (51), and V. acuminata (48) (Table 1). The L. lucidus-G. koreanum var. hirsutum community was found in a total of nine survey quadrats and included 44 species. Shannon’s diversity (H′) value was 2.27, and Evenness (J′) was 0.86 (Table 2). This community was distributed in an area adjacent to the mountain area to the left of the entrance. This area is a transition zone in contact with the mountains and is an area that experiences disturbance and receives soil inflow.
3.2.2. Caltha palustris Community
The C. palustris community had the following indicator species: C. palustris (the highest indicator value of 81), C. rhynchophysa (58), and C. dickinsii (20) (Table 1). It covered 10 survey quadrats, and included 35 species. The H′ value was 1.62, and J′ was 0.89 (Table 2). The C. palustris community was distributed in transition zones and the intersection of mountain areas and wetlands, and specifically, was distributed in the area to the right of the entrance to Yongneup (Figure 3).
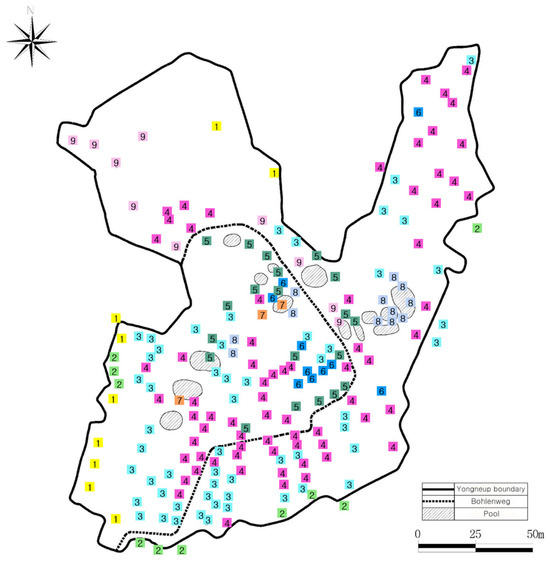
Figure 3.
Distribution of nine plant communities in Yongneup. 1. Lycopus lucidus-Geranium koreanum var. hirsutum, 2. Caltha palustris, 3. Sanguisorba tenuifolia, 4. Carex thunbergii var. appendiculata, 5 Juncus papillosus, 6. Molinia japonica, 7. Sparganium hyperboreum-Carex canescens, 8. Carex chordorhiza-Rhynchospora fujiiana, 9. Calamagrostis purpurea. Distribution of nine plant communities in Yongneup. “Bohlenweg” in the legend indicates a wooden boardwalk crossing the peatland.
3.2.3. Sanguisorba tenuifolia Community
S. tenuifolia served as the indicator species for the S. tenuifolia community but was also distributed throughout the entire study area, showing the lowest indicator value (28). This community had only one indicator species, representing the lowest number among all communities (Table 1). This community was found in 57 survey quadrats and included the largest number of species (44 species). The H’ value was 1.39, and J′ was 0.77 (Table 2). This community was distributed throughout the interior part of the wetland (Figure 3).
3.2.4. Carex thunbergii var. appendiculata Community
The indicator species in the C. thunbergii var. appendiculata community included C. thunbergii var. appendiculata and their indicator values were 38 (Table 1). This community had the largest number of survey quadrats (a total of 71 areas), and included 34 species. The H’ and J’ values were 1.51 and 0.76, respectively (Table 2). The C. thunbergii var. appendiculata community was generally distributed within Yongneup, along with the S. tenuifolia community. Particularly, it was mainly distributed along the plank trail or in the northeastern part where the waterway drained out, while it was scarcely found within the area of the former ice rink (Figure 3).
3.2.5. Juncus papillosus Community
The indicator species of the J. papillosus community were J. papillosus (the value of 58), Eriocaulon sikokianum Maxim. (34), Eleocharis congesta var. japonica (Miq.) T. Koyama (26), and C. hakonensis (25) (Table 1). This community was found in 18 survey quadrats, and the total number of species was 23. The H′ value was 1.44, and J′ was 0.82 (Table 2). The J. papillosus community was mainly distributed in the central part of the wetland (Figure 3).
3.2.6. Molinia japonica Community
The indicator value M. japonica was the highest (57) in the M. japonica community, followed by Gentiana jamesii Hemsl. (36), and Athyrium niponicum (Mett.) Hance (29) (Table 1). This community covered a total of 10 survey quadrats and included 16 species. The H′ and J′ were 1.45 and 0.82, respectively (Table 2). This community was mainly located in an area that was formerly part of the former ice-rink area, specifically in sections where peat had accumulated and no water channels were present (Figure 3).
3.2.7. Sparganium hyperboreum-Carex canescens Community
The indicator value S. hyperboreum was the highest (67) in the S. hyperboreum-C. canescens L. community. The indicator values of C. canescens and Potamogeton pusillus L. were 62 and 33, respectively (Table 1). The number of survey quadrats where this species occurred was three, which was the least among communities, and the number of species found in this community was also the lowest (five species). H′ was the lowest at 0.60, and J′ was 0.84 (Table 2). The S. hyperboreum-C. canescens community was distributed in deep puddles (Figure 3).
3.2.8. Carex chordorrhiza-Rhynchospora fujiiana Community
The indicator species of the C. chordorrhiza-Rhynchospora fujiiana Makino community was C. chordorhiza, and its value was the highest at 97, followed by R. fujiiana (67). Additionally, Lobelia sessilifolia Lamb., and Carex limosa L. were also indicator species (Table 1). This community was recorded in 12 survey quadrats, where 22 species were found. The H′ and J′ values were 1.61 and 0.80, respectively (Table 2). This community was mainly distributed around puddles (Figure 3).
3.2.9. Calamagrostis purpurea Community
The indicator species of the C. purpurea community were as follows: C. purpurea (indicator value of 60), Persicaria thunbergii (Siebold & Zucc.) H. Gross (54), Angelica reflexa B. Y. Lee (31), Phragmites japonica Steud. (30), Pseudostellaria palibiniana (Takeda) Ohwi (20), Isodon excisus (Maxim.) Kudô (20), and Symplocarpus nipponicus Makino (Table 1). The C. purpurea community was found in 10 survey quadrats and included 30 species. The H′ value was 1.73 and J′ was 0.86 (Table 2). The C. purpurea community was mainly distributed in areas with relatively deep peat inside the reclaimed section (former rink area) (Figure 3).
3.3. Effect of Microenvironment on Vegetation Communities
A total of 20 microenvironment factors were measured. The water level was the highest in the S. hyperboreum-C. canescens community, and the peat depth the highest in the C. thunbergii var. appendiculata community. The organic matter content was 66.73 ± 3.27%, and was the highest in the C. chordorhiza-R. fujiiana community (Table 2). To identify the key environmental factors influencing the distribution of plant communities, a classification tree analysis was performed using the CHAID (Chi-squared Automatic Interaction Detector) algorithm in SPSS (Figure 4). The results indicated that peat depth was the most influential variable in differentiating vegetation types, followed by water level, available phosphorus (P), concentrations of waterborne Mg2+, Na+, and K+, and organic matter content. When peat depth was ≤22.0 cm, vegetation communities were distinguished based on water level: L. lucidus-G. koreanum var. hirsutum communities occurred at water levels ≤−13.0 cm, whereas C. palustris communities appeared when the water level exceeded −13.0 cm. For peat depths ranging from 22.0 to 48.3 cm, available phosphorus was the next most significant factor. Communities dominated by S. tenuifolia were found when P concentration exceeded 8.93 mg kg−1, while sites with lower phosphorus levels (≤8.93 mg kg−1) were further split based on waterborne Mg2+ concentration. In this case, M. japonica, S. tenuifolia were associated with Mg2+ levels ≤0.41 ppm, and J. papillosus communities with Mg2+ > 0.41 ppm. In the peat depth range of 48.3 to 74.0 cm, Na+ concentration influenced community structure. At Na+ ≤ 1.43 ppm, C. chordorrhiza-R. fujiiana communities were predominant, while Na+ > 1.43 ppm led to further division by K+ concentration. Carex communities occurred at K+ ≤ 0.48 ppm, C. chordorrhiza-R. fujiiana at 0.48–0.58 ppm, and mixed communities of S. tenuifolia, C. thunbergii var. appendiculata, and C. purpurea at K+ > 0.58 ppm. Finally, in sites with peat depth > 74.0 cm, organic matter content served as the main splitting factor. S. tenuifolia and C. thunbergii var. appendiculata communities were associated with OM ≤ 64.5%, whereas C. thunbergii var. appendiculata, S. tenuifolia communities were more common when OM exceeded 64.5%. These findings serve as an empirical foundation for understanding the mechanisms driving plant community differentiation and provide key environmental thresholds for monitoring and management strategies in high-altitude peatland ecosystems.
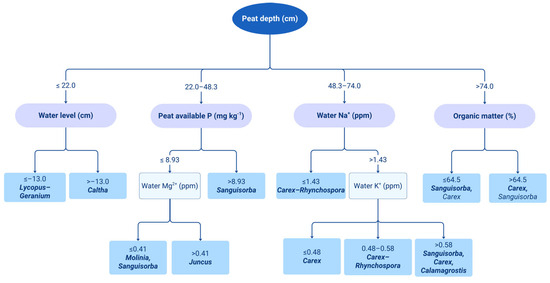
Figure 4.
Classification tree (CHAID) showing the influence of environmental variables on vegetation community types in Yongneup. The primary splitting variable was peat depth, followed by water level, available phosphorus, cation concentrations (Mg2+, Na+, K+), and organic matter content. Terminal nodes are labeled with dominant species representing each community type. Lycopus-Geranium: Lycopus lucidus-Geranium koreanum var. hirsutum, Sanguisorba: Sanguisorba tenuifolia, Carex: Carex thunbergii var. appendiculata, Calamagrostis: Calamagrostis purpurea, Juncus: Juncus papillosus, Caltha: Caltha palustris, Molinia: Molinia japonica, Carex-Rhynchospora: Carex chordorhiza-Rhynchospora fujiiana.

Table 2.
Environmental characteristics of the nine vegetation communities in Yongneup (mean values).
Table 2.
Environmental characteristics of the nine vegetation communities in Yongneup (mean values).
| Community | L. lucidus -G. koreanum var. hirsutum | C. palustris | S. tenuifolia | C. thunbergii var. appendiculata | J. papillosus | M. japonica | S. hyperboreum -C. canescens | C. chordorhiza -R. fujiiana | C. purpurea | |
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | ||||||||||
| Number of plots | 9 | 10 | 57 | 71 | 18 | 10 | 3 | 12 | 10 | |
| Number of species | 44 | 35 | 44 | 34 | 23 | 16 | 5 | 22 | 30 | |
| Shannon’s diversity (H′) | 2.275 | 1.615 | 1.390 | 1.510 | 1.443 | 1.453 | 0.598 | 1.605 | 1.726 | |
| Evenness (J′) | 0.861 | 0.887 | 0.774 | 0.760 | 0.816 | 0.817 | 0.836 | 0.795 | 0.857 | |
| * OBW (%) | 13.6 | 20.0 | 34.1 | 32.4 | 60.9 | 18.8 | 80.0 | 59.1 | 20.0 | |
| * OBU (%) | 47.7 | 40.0 | 31.8 | 32.4 | 13.0 | 37.5 | 0.0 | 9.1 | 40.0 | |
| Water level (cm) | −54.78 | −0.90 | −20.19 | −16.21 | −14.56 | −40.60 | 19.33 | −0.00 | −55.90 | |
| Peat depth (cm) | 11.67 | 26.60 | 83.26 | 87.55 | 43.96 | 49.80 | 43.52 | 65.83 | 37.00 | |
| Peat moisture (%) | 39.11 | 64.59 | 70.18 | 80.18 | 62.46 | 71.05 | 62.95 | 84.10 | 57.85 | |
| Peat available P (mg kg−1) | 7.96 | 12.07 | 12.47 | 17.78 | 6.64 | 8.55 | 7.94 | 12.47 | 18.91 | |
| Peat pH | 4.79 | 4.77 | 4.60 | 4.55 | 4.92 | 4.41 | 4.96 | 4.78 | 4.54 | |
| Organic matter (%) | 15.11 | 40.23 | 47.04 | 61.80 | 51.91 | 61.72 | 49.60 | 66.69 | 26.72 | |
| Water pH | 5.11 | 5.29 | 5.00 | 4.95 | 4.88 | 4.62 | 5.33 | 4.84 | 5.07 | |
| Conductivity (μs cm−1) | 88.40 | 33.42 | 23.70 | 22.45 | 29.95 | 25.10 | 19.97 | 17.58 | 33.43 | |
| Major cations (ppm) ** | Na+ | 2.92 | 2.23 | 1.99 | 2.00 | 1.81 | 2.01 | 1.85 | 1.43 | 1.92 |
| Ca2+ | 3.17 | 3.15 | 2.28 | 2.33 | 2.61 | 2.07 | 2.61 | 2.03 | 2.58 | |
| Mg2+ | 0.54 | 0.51 | 0.39 | 0.41 | 0.55 | 0.38 | 0.34 | 0.45 | 0.46 | |
| K+ | 1.84 | 1.36 | 0.89 | 0.96 | 0.92 | 0.80 | 0.66 | 0.72 | 1.31 | |
* OBW: Obligate wetland species, OBU: Obligate upland species. ** Cation concentrations are summarized in Appendix A Table A1.
4. Discussion
4.1. Interpretation of Species Composition and Distribution
A total of 130 species of flora were recorded in this study (including seven bryophyte species). Kang [65] reported 164 species, Kang and Kwak [49] reported 191 species, Kim et al. [50] reported 63 species, and Seo [57] reported 68 species. These differences in species numbers are likely due to partial surveys within Yongneup or to surveys conducted at the wetland margins and along road slopes near both the Small and Large Yongneup area. Furthermore, this study only targeted herbaceous species in wetlands, transition zones, and disturbance zones, excluding woody species, which resulted in the observed differences in the number of species recorded. Overall, S. tenuifolia was distributed throughout the area, while C. thunbergii var. appendiculata was found within the interior of the wetland. Inside the reclaimed section (former rink area), M. japonica, C. chordorhiza, and C. limosa were distributed around puddles. C. purpurea occurred mainly along the embankment and in the northern part of this section. L. lucidus and G. koreanum var. hirsutum were found to the left of the entrance, whereas C. palustris, C. rhynchophysa, and C. dickinsii were distributed to the right, forming a transition zone. Drosera rotundifolia L. was observed together with M. japonica inside the reclaimed section but also appeared sporadically in other areas. S. hyperboreum and P. pusillus were found in puddles, particularly in deeper ones.
In areas where species such as C. jaluensis and C. dispalata had been known to exist according to numerous studies, only C. thunbergii var. appendiculata was found. Few references on C. thunbergii var. appendiculata are provided in other studies, except pictures of C. thunbergii var. appendiculata in Yongneup in the data provided in Lee [66] and Oh [67] reports. The considerably morphological difference between C. thunbergii var. appendiculata, and C. jaluensis or C. dispalata can be described as follows: C. thunbergii var. appendiculata has two stigmata and C. jaluensis or C. dispalata has three stigmata. Several studies have identified the species P. japonica as P. japonica or Phragmites australis (Cav.) Trin. ex Steud. The difference between P. japonica and P. australis is as follows: the first glume of P. australis is shorter than half the length of the lowest palea, whereas in P. japonica, the first glume is longer than half the length of the lowest palea.
Furthermore, Sium ninsi L., G. koreanum var. hirsutum, Pedicularis resupinate L., Scabiosa tschiliensis Grüning, Scirpus wichurae Boeckeler, E. sikokianum, M. japonica, Aegopodium alpestre Ledeb., Eleocharis congesta var. japonica (Miq.) T. Koyama, Lychnis wilfordii (Regel) Maxim., and J. papillosus have been identified differently depending on the researchers conducting the study. Except [49,68] reports, moss species have not been investigated in detail. However, this study investigated S. palustre, Polytrichum commune Hedw., Bryonoguchia molkenboeri (Sande Lac.) Z. Iwats. & Inoue, S. fuscum, and Plagiomnium vesicatum (Besch.) T. J. Kop., Carex chordorhiza L. f. and C. limosa, which are rarely mentioned in previous studies, were found in peatlands. Hada [69] stated that the C. thunbergii var. appendiculata community can develop in oligotrophic areas due to accumulated peat. Among the species found in Akasaka-Oike of Okayama and Makura wetlands of Hiroshima, which were investigated by Hada, the common species in Yongneup were C. thunbergii var. appendiculata, S. palustre, S. wichurae, M. japonica, R. fujiiana, Thelypteris palustris (A. Gray) Schott, D. rotundifolia, and J. papillosus.
4.2. Vegetation Community Analysis
In previous studies, depending on the researchers conducting the study, communities were subdivided into six [35], eight [55], eleven [49], or twenty-eight communities [44]. Choi and Koh [70] considered the entire vegetation community a S. tenuifolia community and subdivided it into four communities. In this study, we classified Yongneup into nine communities. The distribution of the communities was nearly similar to that reported in previous studies. However, the community names differed because of variations in species nomenclature and the subdivision of communities. As mentioned above, the difference in community names could be attributed to regional variation. In areas where the C. thunbergii var. appendiculata community had been reported, C. dispalata or C. jaluensis communities were found instead. The C. chordorhiza-R. fujiiana community was not recorded in most previous studies. However, in this study, it was found to be distributed around puddles. Choi and Koh [70] classified the flora in the transition zones as a single community, the G. eriostemon community. The Arundinella hirta (Thunb.) Tanaka-P. australis subcommunity, A. amurensis subcommunity, and the G. koreanum var. hirsutum and C. purpurea communities showed similar species composition. Kang and Kwak [49] reported that the C. dispalata and S. palustre communities were decreasing in size, whereas the M. japonica community was expanding. In contrast, our results showed that C. thunbergii var. appendiculata was widely distributed, while M. japonica was confined mainly to the inner ice rink area. Compared with [44], the overall results were similar, although slight differences were observed due to the subdivision of communities and the inclusion of woody species. In addition, the area dominated by the C. canescens community was found to be extensive, which differs from the findings of the present study. Furthermore, C. thunbergii var. appendiculata was observed in the area previously reported to be inhabited by C. dispalata, as mentioned earlier.
4.3. Effects of Location Factors on Vegetation Communities
The classification tree analysis clearly showed that peat depth is a major determinant of vegetation community composition in this high-altitude peatland. However, this study has a limitation: it represents only a snapshot in time and focuses on herbaceous species, excluding woody or bryophyte dynamics. This finding aligns with previous studies highlighting peat accumulation as a key driver of hydrological and nutrient gradients in mire ecosystems [71,72]. Similar patterns of peat accumulation shaping community composition have been reported in alpine fens of Central Europe [73], suggesting convergent mechanisms across temperate montane peatlands. Shallower peat layers were associated with distinctive water levels, which in turn structured different plant communities such as Lycopus–Geranium and Caltha. These species are known to be sensitive to groundwater fluctuations, supporting the observation that hydrological conditions are crucial for maintaining species assemblages in ombrotrophic and minerotrophic peatlands [74,75]. Multiple environmental factors, including peat depth, nutrient availability, and cation concentrations, jointly influence vegetation patterns. This finding aligns with [10], who argued that mire ecosystems are governed by hydrological and chemical gradients rather than a single environmental threshold. This is especially relevant in the context of highland peatlands, where such interactions create a complex mosaic of microhabitats.
The strong influence of waterborne nutrients, especially phosphorus and cations (Mg2+, Na+, and K+), on community differentiation suggests a gradient in soil fertility and ionic composition, possibly shaped by both peat decomposition processes and inflow characteristics. The presence of Sanguisorba in P-rich sites may reflect its competitive advantage under higher nutrient availability, whereas Juncus and Molinia were more associated with low Mg2+ levels, potentially reflecting adaptation to poor base saturation.
Furthermore, in deeper peat zones, sodium and potassium concentrations became increasingly relevant. This suggests that even within relatively homogeneous peat environments, subtle geochemical variations can exert species-sorting effects. The presence of Carex–Rhynchospora and Sanguisorba–Carex–Calamagrostis communities in overlapping ranges may reflect what Wheeler and Proctor [10] describe as transitional communities formed along diffuse ecotones, rather than discrete habitat types. The presence of mixed communities, such as those containing Sanguisorba, Carex, and Calamagrostis, suggests transitional zones where species with overlapping ecological tolerances coexist. Wheeler and Proctor [10] emphasize that vegetation in peatlands rarely occurs in sharply defined zones but rather along continuous ecological gradients. These findings support the view that the vegetation structure in Yongneup represents not static typologies, but rather fluid assemblages shaped by subtle environmental variations.
Lastly, organic matter content also played a role in the deepest peat layers, influencing the relative dominance of Sanguisorba and Carex. The dominance of C. thunbergii var. appendiculata in deep peat zones suggests its potential use as an indicator species for long-term peatland stability under climate change scenarios. High organic matter may indicate more advanced peat formation and stability, potentially favoring Carex–Sanguisorba communities that are adapted to mature peat substrates.
Overall, this study supported the view that multiple interacting environmental gradients, including peat depth, water level, and soil chemistry, govern the spatial heterogeneity of vegetation communities in highland peatlands. These findings are in line with ecological niche theory and contribute valuable baseline data for long-term vegetation monitoring and peatland conservation in Korea. These findings underline the need for site-specific conservation strategies that account for microenvironmental heterogeneity, particularly in high-altitude peatlands vulnerable to climate change and land-use pressures. The identified environmental thresholds for dominant communities may serve as key indicators for future monitoring, restoration prioritization, and adaptive wetland management under ecological disturbance or climatic shifts. This study was limited by its snapshot temporal scope and focus on herbaceous species, excluding woody or bryophyte dynamics that may influence community structure.
These findings reinforce the ecological continuum theory, indicating that Yongneup’s plant assemblages are shaped by gradual shifts in environmental conditions rather than abrupt habitat boundaries. The identification of threshold values for peat depth, water level, and nutrient concentrations offers practical indicators for long-term ecological monitoring and restoration prioritization in montane peatlands. Furthermore, these insights contribute to establishing ecological reference conditions aligned with national wetland conservation frameworks and international targets such as the Ramsar Convention.
Our findings confirm peat depth as a critical determinant of vegetation composition, aligning with global patterns observed in montane peatlands. However, this study further illustrates that microenvironmental gradients (hydrology and soil nutrients) interactively shape vegetation communities, creating continuous rather than discrete ecological transitions. This nuanced understanding contrasts with previous studies that often classified Yongneup communities into overly simplified or purely physiognomic categories. The identification of critical environmental thresholds, particularly peat depth and phosphorus availability, provides practical benchmarks for long-term monitoring and restoration strategies. Additionally, these results reinforce ecological continuum theory, advocating for adaptive, gradient-based management practices in montane peatland ecosystems facing increasing climate instability and anthropogenic disturbances. Future studies incorporating temporal dynamics and bryophyte communities are recommended to further elucidate long-term ecological processes.
Furthermore, the CHAID tree analysis (Figure 4) provides a hierarchical perspective on how peat depth interacts with hydrological and chemical variables to shape dominant species assemblages. This functional interpretation extends beyond a traditional Braun-Blanquet-type classification by revealing the mechanistic linkages between environmental gradients and vegetation structure. In line with the findings of [76] for the Banhine wetland, where soil and water chemistry explained the dominance of sweet grasses under fluctuating hydrological regimes, our results highlight that water-level dynamics, ion concentrations, and nutrient availability jointly drive species dominance and compositional turnover in high-altitude peatlands. Integrating such ecological process-based perspectives into vegetation classification enhances our ability to interpret wetland functioning and supports more effective conservation and management planning.
In the context of ongoing degradation and climate change, further shifts in Yongneup’s vegetation composition are anticipated. Communities dominated by S. tenuifolia and C. thunbergii var. appendiculata may expand under progressively drier conditions and increased sediment inflow, reflecting their tolerance to nutrient enrichment and fluctuating water levels. In contrast, peat-forming and hydrophilous species such as Sphagnum palustre and M. trifoliata are likely to decline with continued lowering of the water table and surface desiccation, reducing the peat accumulation potential. Over time, these changes may lead to a transition from peat-forming wetland vegetation toward meadow-like assemblages dominated by graminoids.
Since 2012, several restoration and conservation measures have been implemented in Yongneup, including the relocation of a nearby military base, improvement of the water balance, removal of alien plant species, and visitor access control. These efforts are expected to stabilize hydrological conditions and reduce external disturbances, which may have altered the vegetation composition compared with the 2009 survey. Long-term monitoring is essential to evaluate restoration effectiveness and vegetation responses to climate variability. Maintaining stable hydrological regimes and minimizing sediment inflow remain critical for sustaining the resilience of Yongneup’s peatland vegetation and preventing further degradation.
Author Contributions
K.L. and J.L. contributed equally to this work. J.L. conducted the investigation, drafted the original manuscript, and contributed to the review and editing of the manuscript. K.L. was responsible for the investigation, statistical analysis, data visualization, and also participated in reviewing and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Environment (MOE) of the Republic of Korea (NIE-B-2025-35).
Data Availability Statement
The data contain information on endangered species and therefore cannot be made publicly available. Data are, however, available from the corresponding author upon reasonable request.
Acknowledgments
We thank Hyeongsoo Seo and Anna Seo for their assistance in the field. We are deeply grateful to Choung YS for his guidance and support. This study was conducted with permission for access to Yongneup granted by the Wonju Regional Environmental Office in 2008 and 2009. The authors would like to thank the Ministry of Environment (MOE) of the Republic of Korea for its financial support (NIE-B-2025-35).
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Detailed ion concentrations and minor chemical parameters for nine vegetation communities in Yongneup (mean ± standard error).
Table A1.
Detailed ion concentrations and minor chemical parameters for nine vegetation communities in Yongneup (mean ± standard error).
| Community | L. lucidus -G. koreanum var. hirsutum | C. palustris | S. tenuifolia | C. thunbergii var. appendiculata | J. papillosus | M. japonica | S. hyperboreum -C. canescens | C. chordorhiza -R. fujiiana | C. purpurea | |
|---|---|---|---|---|---|---|---|---|---|---|
| Factors | ||||||||||
| Number of plots | 9 | 10 | 57 | 71 | 18 | 10 | 3 | 12 | 10 | |
| Number of species | 44 | 35 | 44 | 34 | 23 | 16 | 5 | 22 | 30 | |
| Shannon’s diversity (H′) | 2.275 | 1.615 | 1.390 | 1.510 | 1.443 | 1.453 | 0.598 | 1.605 | 1.726 | |
| Evenness (J′) | 0.861 | 0.887 | 0.774 | 0.760 | 0.816 | 0.817 | 0.836 | 0.795 | 0.857 | |
| Obligate wetland species (%) | 13.6 | 20.0 | 34.1 | 32.4 | 60.9 | 18.8 | 80.0 | 59.1 | 20.0 | |
| Obligate upland species (%) | 47.7 | 40.0 | 31.8 | 32.4 | 13.0 | 37.5 | 0.0 | 9.1 | 40.0 | |
| Water level (cm) | −54.78 ± 7.34 | −0.90 ± 1.30 | −20.19 ± 3.49 | −16.21 ± 2.77 | −14.56 ± 6.16 | −40.60 ± 10.05 | 19.33 ± 8.29 | −0.00 ± 0.00 | −55.90 ± 9.00 | |
| Peat depth (cm) | 11.67 ± 7.82 | 26.60 ± 9.61 | 83.26 ± 4.85 | 87.55 ± 3.89 | 43.96 ± 5.26 | 49.80 ± 3.25 | 43.52 ± 13.91 | 65.83 ± 4.62 | 37.00 ± 10.25 | |
| Peat moisture contents (%) | 39.11 ± 4.34 | 64.59 ± 6.53 | 70.18 ± 2.21 | 80.18 ± 0.88 | 62.46 ± 5.40 | 71.05 ± 5.21 | 62.95 ± 0.18 | 84.10 ± 2.16 | 57.85 ± 4.39 | |
| Peat total N (mg g−1) | 4.65 ± 0.79 | 11.29 ± 2.05 | 13.97 ± 0.87 | 16.81 ± 0.58 | 12.25 ± 1.61 | 14.98 ± 1.12 | 12.57 ± 1.34 | 15.31 ± 0.96 | 7.06 ± 1.84 | |
| Peat available P (mg kg−1) | 7.96 ± 2.00 | 12.07 ± 2.16 | 12.47 ± 1.29 | 17.78 ± 1.08 | 6.64 ± 1.52 | 8.55 ± 1.13 | 7.94 ± 0.72 | 12.47 ± 2.01 | 18.91 ± 2.51 | |
| Peat pH | 4.79 ± 0.05 | 4.77 ± 0.02 | 4.60 ± 0.03 | 4.55 ± 0.02 | 4.92 ± 0.08 | 4.41 ± 0.05 | 4.96 ± 0.24 | 4.78 ± 0.07 | 4.54 ± 0.11 | |
| Organic matter (%) | 15.11 ± 2.68 | 40.23 ± 8.00 | 47.04 ± 2.78 | 61.80 ± 1.79 | 51.91 ± 6.24 | 61.72 ± 4.99 | 49.60 ± 3.21 | 66.69 ± 3.71 | 26.72 ± 6.03 | |
| Water pH | 5.11 ± 0.13 | 5.29 ± 0.06 | 5.00 ± 0.03 | 4.95 ± 0.04 | 4.88 ± 0.11 | 4.62 ± 0.02 | 5.33 ± 0.03 | 4.84 ± 0.14 | 5.07 ± 0.12 | |
| Conductivity (μs cm−1) | 88.40 ± 13.70 | 33.42 ± 3.93 | 23.70 ± 0.96 | 22.45 ± 1.10 | 29.95 ± 4.75 | 25.10 ± 3.86 | 19.97 ± 3.36 | 17.58 ± 1.41 | 33.43 ± 1.53 | |
| Na+ (ppm) | 2.92 ± 0.81 | 2.23 ± 0.26 | 1.99 ± 0.09 | 2.00 ± 0.10 | 1.81 ± 0.14 | 2.01 ± 0.23 | 1.85 ± 0.23 | 1.43 ± 0.12 | 1.92 ± 0.08 | |
| Ca2+ (ppm) | 3.17 ± 0.50 | 3.15 ± 0.22 | 2.28 ± 0.08 | 2.33 ± 0.10 | 2.61 ± 0.22 | 2.07 ± 0.11 | 2.61 ± 0.25 | 2.03 ± 0.15 | 2.58 ± 0.23 | |
| Mg2+ (ppm) | 0.54 ± 0.08 | 0.51 ± 0.05 | 0.39 ± 0.02 | 0.41 ± 0.02 | 0.55 ± 0.05 | 0.38 ± 0.03 | 0.34 ± 0.01 | 0.45 ± 0.03 | 0.46 ± 0.04 | |
| K+ (ppm) | 1.84 ± 0.80 | 1.36 ± 0.37 | 0.89 ± 0.10 | 0.96 ± 0.11 | 0.92 ± 0.12 | 0.80 ± 0.15 | 0.66 ± 0.27 | 0.72 ± 0.14 | 1.31 ± 0.39 | |
| NH4+ (ppm) | 0.14 ± 0.02 | 0.74 ± 0.27 | 0.39 ± 0.05 | 0.31 ± 0.05 | 0.20 ± 0.06 | 0.27 ± 0.09 | 0.05 ± 0.02 | 0.07 ± 0.01 | 0.24 ± 0.06 | |
| Fe2+ (ppm) | 0.23 ± 0.05 | 0.45 ± 0.22 | 0.43 ± 0.06 | 0.43 ± 0.04 | 1.37 ± 0.88 | 0.49 ± 0.11 | 0.64 ± 0.34 | 0.42 ± 0.04 | 0.34 ± 0.01 | |
| Zn2+ (ppm) | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.00 | 0.05 ± 0.01 | |
| Cl− (ppm) | 6.17 ± 2.96 | 2.31 ± 0.42 | 1.61 ± 0.14 | 1.57 ± 0.13 | 1.92 ± 0.32 | 1.87 ± 0.45 | 1.25 ± 0.45 | 1.18 ± 0.19 | 1.99 ± 0.42 | |
| NO3− (ppm) | 3.11 ± 1.30 | 2.87 ± 0.99 | 1.16 ± 0.23 | 0.66 ± 0.09 | 0.43 ± 0.06 | 0.48 ± 0.08 | 1.15 ± 0.98 | 0.48 ± 0.08 | 0.42 ± 0.02 | |
| SO42− (ppm) | 4.12 ± 1.48 | 2.51 ± 0.29 | 3.23 ± 0.26 | 3.01 ± 0.28 | 4.04 ± 0.89 | 3.54 ± 0.79 | 1.71 ± 0.09 | 3.13 ± 0.43 | 3.21 ± 0.56 | |
| P (ppm) | 0.06 ± 0.00 | 0.09 ± 0.01 | 0.06 ± 0.00 | 0.13 ± 0.07 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.01 | 0.06 ± 0.00 | 0.06 ± 0.00 | |
References
- Mitsch, W.J.; Gosselink, J.G. Wetlands; Van Nostrand Reinhold Company: New York, NY, USA, 1993. [Google Scholar]
- Tarnocai, C. The effect of climate change on carbon in Canadian peatlands. Glob. Planet. Change 2006, 53, 222–232. [Google Scholar] [CrossRef]
- Kang, S.J. Phytosociological Study of the Dae-am mountain Raised Bog. In Studies on Nature in the DMZ Area; Kangwon National University Press: Chuncheon, Republic of Korea, 1987; pp. 169–201. [Google Scholar]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands—Background and Principles Including a Framework for Decision-Making; International Mire Conservation Group and International Peat Society: Jyväskylä, Finland, 2002. [Google Scholar]
- Cho, K.-S. Limnological and Ecological Study of the Mt. Daeam High Moor. In Studies on Nature in the DMZ Area; Kangwon National University Press: Chuncheon, Republic of Korea, 1987; pp. 143–167. [Google Scholar]
- Hájek, M.; Horsák, M.; Hájková, P.; Dítě, D. Habitat diversity of central European fens in relation to environmental gradients and an effort to standardise fen terminology. Perspect. Plant Ecol. Evol. Syst. 2006, 8, 97–114. [Google Scholar] [CrossRef]
- Zoltai, S.C.; Vitt, D.H. Canadian wetlands: Environmental gradients and classification. Vegetatio 1995, 118, 131–137. [Google Scholar]
- Wells, E.D. Classification of peatland vegetation in Atlantic Canada. J. Veg. Sci. 1996, 7, 847–878. [Google Scholar] [CrossRef]
- Vitt, D.H.; Chee, W.L. The relationships of vegetation to surface water chemistry and peat chemistry in fens of Alberta, Canada. Vegetatio 1990, 89, 87–106. [Google Scholar]
- Wheeler, B.D.; Proctor, M.C.F. Ecological gradients, subdivisions and terminology of north-west European mires. J. Ecol. 2000, 88, 187–203. [Google Scholar] [CrossRef]
- Bridgham, S.D.; Pastor, J.; Janssens, J.A.; Chapin, C.; Malterer, T.J. Multiple limiting gradients in peatlands: A call for a new paradigm. Wetlands 1996, 16, 45–65. [Google Scholar] [CrossRef]
- Anderson, D.S.; Davis, R.B. The vegetation and its environments in Maine peatlands. Can. J. Bot. 1997, 75, 1785–1805. [Google Scholar] [CrossRef]
- Gerdol, R. Community and species-performance patterns along an alpine poor-rich mire gradient. J. Veg. Sci. 1995, 6, 175–184. [Google Scholar]
- Gerdol, R.; Bragazza, L. Syntaxonomy and community ecology of mires in the Rhaetian Alps (Italy). Phytocoenologia 2001, 31, 271–300. [Google Scholar] [CrossRef]
- Hájek, M.; Hekera, P.; Hájková, P. Spring fen vegetation and water chemistry in the Western Carpathian flysch zone. Folia Geobot. 2002, 37, 205–224. [Google Scholar] [CrossRef]
- Hájková, P.; Hájek, M.; Apostolova, I. Diversity of wetland vegetation in the Bulgarian high mountains, main gradients and context-dependence of the pH role. Plant Ecol. 2006, 184, 111–130. [Google Scholar] [CrossRef]
- Malmer, N. Vegetational gradients in relation to environmental conditions in northwestern European mires. Can. J. Bot. 1986, 64, 375–383. [Google Scholar] [CrossRef]
- Vitt, D.H.; Bayley, S.E.; Jin, T.L. Seasonal variation in water chemistry over a bog-rich fen gradient in continental western Canada. Can. J. Fish. Aquat. Sci. 1995, 52, 587–606. [Google Scholar] [CrossRef]
- Welch, B.A.; Davis, C.B.; Gates, R.J. Dominant environmental factors in wetland plant communities invaded by Phragmites australis in East Harbor, Ohio, USA. Wetl. Ecol. Manag. 2006, 14, 511–525. [Google Scholar] [CrossRef]
- Glaser, P.H.; Janssens, J.A.; Siegel, D.I. The response of vegetation to chemical and hydrological gradients in the Lost River peatland, northern Minnesota. J. Ecol. 1990, 78, 1021–1048. [Google Scholar] [CrossRef]
- Bragazza, L.; Rydin, H.; Gerdol, R. Multiple gradients in mire vegetation—A comparison of a Swedish and an Italian bog. Plant Ecol. 2005, 177, 223–236. [Google Scholar] [CrossRef]
- Navrátilová, J.; Navrátil, J.; Hájek, M. Relationships between environmental factors and vegetation in nutrient-enriched fens at fishpond margins. Folia Geobot. 2006, 41, 353–376. [Google Scholar] [CrossRef][Green Version]
- Branson, F.A.; Miller, R.F.; McQueen, I.S. Plant communities and associated soil and water factors on shale-derived soils in northeastern Montana. Ecology 1970, 51, 391–407. [Google Scholar] [CrossRef]
- Asada, T. Vegetation gradients in relation to temporal fluctuation of environmental factors in Bekanbeushi peatland, Hokkaido, Japan. Ecol. Res. 2002, 17, 505–518. [Google Scholar] [CrossRef]
- Klinger, L.F. Coupling of soils and vegetation in peatland succession. Arct. Alp. Res. 1996, 28, 380–387. [Google Scholar] [CrossRef]
- Kutnar, L.; Martinčič, A. Ecological relationships between vegetation and soil-related variables along the mire margin–mire expanse gradient in the eastern Julian Alps. Ann. Bot. Fenn. 2003, 40, 177–189. [Google Scholar]
- Paratley, R.D.; Fahey, T.J. Vegetation–environment relations in a conifer swamp in central New York. Bull. Torrey Bot. Club 1986, 113, 357–371. [Google Scholar] [CrossRef]
- Graham, J.D.; Glenn, N.F.; Spaete, L.P.; Hanson, P.J. Characterizing peatland microtopography using gradient and microform-based approaches. Ecosystems 2020, 23, 1464–1480. [Google Scholar] [CrossRef]
- Shi, X.; Thornton, P.E.; Ricciuto, D.M.; Hanson, P.J.; Mao, J.; Sebestyen, S.D.; Griffiths, N.A.; Bisht, G. Representing northern peatland microtopography and hydrology within the Community Land Model. Biogeosciences 2015, 12, 6463–6477. [Google Scholar] [CrossRef]
- Cresto Aleina, F.; Runkle, B.R.K.; Kleinen, T.; Kutzbach, L.; Schneider, J.; Brovkin, V. Modeling micro-topographic controls on boreal peatland hydrology and methane fluxes. Biogeosciences 2015, 12, 5689–5704. [Google Scholar] [CrossRef]
- Barrett, S.E.; Watmough, S.A. Factors controlling peat chemistry and vegetation composition in Sudbury peatlands after 30 years of pollution emission reductions. Environ. Pollut. 2015, 206, 122–132. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, G.; Wang, M.; Zhao, M.; Yuan, Y.; Meng, J.; Zhao, Y.; Hu, N.; Zhang, T.; Liu, B. Variations in soil seed banks in sedge peatlands across an altitude gradient. Diversity 2024, 16, 571. [Google Scholar] [CrossRef]
- Milesi, V.P. Redox gradient shapes the chemical and taxonomic composition of peatland microbial communities. Geobiology 2024, 22, e70001. [Google Scholar] [CrossRef]
- Zhang, M.; Bu, Z.-J.; Liu, S.-S.; Chen, J.; Xing, W. Mid-late Holocene peatland vegetation and hydrological variations in Northeast Asia and their responses to solar and ENSO activity. Catena 2021, 203, 105339. [Google Scholar] [CrossRef]
- Kang, S.J. Ecological studies of the raised bog in the Dae-am mountain adjacent to DMZ in Korea (II). J. Plant Biol. 1970, 13, 20–24. [Google Scholar]
- Chang, N.K.; Kim, Y.P.; Oh, I.H.; Son, Y.H. Past vegetation of moor in Mt. Daeam in terms of the pollen analysis. J. Ecol. Environ. 1987, 10, 195–204. [Google Scholar]
- Bae, J.-J.; Choo, Y.-S.; Song, S.-D. The patterns of inorganic cations, nitrogen and phosphorus of plants in Moojechi Moor on Mt. Jeongjok. Korean J. Ecol. 2003, 26, 109–114. [Google Scholar] [CrossRef][Green Version]
- Choung, Y.; Joo, K.Y.; Park, D.S. Distribution, Ecological Characteristics, and Conservation Strategies of Mountain Wetlands in Gangwon Province—Focused on Inje, Yanggu, and Pyeongchang-gun; Gangwon Regional Environmental Technology Development Center: Gangneung, Republic of Korea, 2005. [Google Scholar][Green Version]
- Park, K.; Woo, S.H. Survey areas and watershed characteristics. In Final Report on the Detailed Survey of Inland Wetlands in Korea—Sandeulneup, Hwaponeup, Jangcheokji, and Geumgang Lake; Ministry of Environment: Gwacheon, Republic of Korea, 2006; pp. 23–37. [Google Scholar][Green Version]
- Son, M.W.; Chang, M.G. Formation processes of Hwaeomneup Wetland, Cheonseong Mountain. J. Korean Assoc. Reg. Geogr. 2009, 15, 204–214. [Google Scholar][Green Version]
- Ministry of Environment; National Institute of Environmental Research. Detailed Survey Report on Wetland Protected Areas; Ministry of Environment: Seoul, Republic of Korea, 2007. [Google Scholar][Green Version]
- Shin, Y.-H.; Kim, S.-H.; Park, S.-J. The geochemical roles and properties of mountain wetland in Mt. Shinbulsan. J. Korean Geomorphol. Assoc. 2005, 12, 133–149. [Google Scholar][Green Version]
- Koh, J.K.; Lee, E.B.; Jeon, E.S. Vegetation of the Chilbosan Wetland in Suwon and flora of the wetland and its surroundings. Nat. Conserv. 1995, 89, 85–92. [Google Scholar][Green Version]
- Ryou, S.H. Studies on Vegetation and Successive Dynamics of Moors in Montane Zone, Korea. Ph.D. Thesis, Chungnam National University, Daejeon, Republic of Korea, 2004. [Google Scholar][Green Version]
- Lee, J.-H.; Kim, J.K. Ecological survey of the Wangdeungjae wet meadow threatened by environmental changes. Rural Dev. Stud. 1997, 16, 25–34. [Google Scholar][Green Version]
- Yu, J.M.; Kim, M.S.; Chung, G.Y. A study on the flora and conservation of wetlands and adjacent areas of Mt. Cheongoksan (Gyeongbuk). Res. Rep. Korean Assoc. Conserv. Nat. 1999, 18, 19–39. [Google Scholar][Green Version]
- Lee, S.-D. The study of current status of conservation and management policy on wetlands in Korea. J. Wetl. Res. 2003, 5, 1–13. [Google Scholar][Green Version]
- Park, W.-G.; Yoo, S.-I.; Park, K.-S. Flora of natural marshes in Wondae-ri (Inje-gun, Kangwon-do). J. For. Sci. 2000, 16, 50–68. [Google Scholar][Green Version]
- Kang, S.-J.; Kwak, A.K. Study on the flora and vegetation of the high moor in Mt. Daeam. J. Wetl. Res. 2000, 2, 117–131. [Google Scholar][Green Version]
- Kim, B.-W.; Lee, J.-S.; Oh, Y.-J. A study on the flora in the Mt. Daeam high moor. J. Environ. Sci. 2005, 11, 1–8. [Google Scholar][Green Version]
- Lee, Y.N. Swamp plants on Mt. Daeam in the central part of Korea. Korean J. Plant Taxon. 1969, 1, 7–14. [Google Scholar] [CrossRef]
- Lee, W.T. Flora of the Mt. Daeham high moor. In Survey Report on the Natural Ecosystem of Mt. Daeham; Environmental Administration: Seoul, Republic of Korea, 1988; pp. 47–76. [Google Scholar]
- Lee, W.T.; Kim, Y.S.; Jeon, E.S.; Paik, W.-K. Flora of the Mt. Daeham and Mt. Daewoo natural monuments. In Academic Survey Report on the Mt. Daeham and Mt. Daewoo Natural Monument Areas; Cultural Heritage Administration: Daejeon, Republic of Korea, 2003; pp. 99–149. [Google Scholar]
- Choi, H.-J.; Kweon, H. Detailed Survey Report on Wetland Protected Areas—Flora; Ministry of Environment: Gwacheon, Republic of Korea, 2007; pp. 189–211. [Google Scholar]
- Park, B.K. On the vegetation of high-moor on Mt. Daeam, Kangwon-Do, Korea. J. Korean Res. Inst. Better Living 1973, 11, 25–31. [Google Scholar]
- Choi, T.B.; Roh, H.C.; Han, J.H.; Kim, K.Y. Detailed Survey Report on Wetland Protected Areas—General Overview; Ministry of Environment: Gwacheon, Republic of Korea, 2007; pp. 7–49. [Google Scholar]
- Seo, B. Characteristics of the Vegetation at Some Montane Moors in Gangwon Province, Korea. Master’s Thesis, Kangwon National University, Chuncheon, Republic of Korea, 2007. [Google Scholar]
- Yang, K.C. Classification of Major Habitats Based on the Climatic Conditions and Topographic Features in Korea. Ph.D. Thesis, Chung-Ang University, Seoul, Republic of Korea, 2001. [Google Scholar]
- Kang, S.J. Peat stratigraphy and pollen analysis of the Mt. Daeham high moor. In Daewonsan Natural Ecosystem Survey Report; Environmental Administration: Seoul, Republic of Korea, 1988; pp. 101–146. [Google Scholar]
- Kang, S.J.; Yoshioka, T. Environmental change of high moor in Mt. Daeam of Korean Peninsula. Korean J. Limnol. 2005, 38, 45–53. [Google Scholar]
- Ministry of Environment. Feasibility Study on the Restoration of Yongneup, Mt. Daeham (First Year); Ministry of Environment: Gwacheon, Republic of Korea, 1997. [Google Scholar]
- Yoon, J.-Y. The Geomorphic Development and Artificial Change of Environment at the Moor Yongneup, Mt. Daeam. Master’s Thesis, Kyung Hee University, Seoul, Republic of Korea, 2002. [Google Scholar]
- Park, J.-K. Water table variation of Yongneup located at Daeam-san, Yanggu-gun. J. Korean Geomorphol. Assoc. 2001, 8, 35–49. [Google Scholar]
- Buurman, P.; van Lagen, B.; Velthorst, E.J. Manual for Soil and Water Analysis; Backhuys Publishers: Leiden, The Netherlands, 1996. [Google Scholar]
- Kang, S.J. Ecological studies of the raised bog in Daeam Mountain adjacent to DMZ in Korea. J. Res. Sci. Educ. 1976, 2, 81–104. [Google Scholar]
- Lee, H.H.M. Classification of Wetlands in Korea. Master’s Thesis, Inha University, Incheon, Republic of Korea, 2000. [Google Scholar]
- Oh, Y.C. Korean Caricoideae of Cyperaceae; Sungshin Women’s University Press: Seoul, Republic of Korea, 2006. [Google Scholar]
- Lee, T.B. Illustrated Flora of Korea; Hyangmunsa: Seoul, Republic of Korea, 2003. [Google Scholar]
- Hada, Y. The vegetation of Makura moor in Geihoku Cho, Hiroshima Prefecture, Japan. Bull. Okayama Univ. Sci. 1973, 9, 69–83. [Google Scholar]
- Choi, K.-R.; Koh, J.-K. Studies on moor vegetation of Mt. Daeam, east-central Korea. J. Ecol. Environ. 1989, 12, 237–244. [Google Scholar]
- Rydin, H.; Jeglum, J.K. The Biology of Peatlands, 2nd ed.; Oxford University Press: Oxford, UK, 2013. [Google Scholar]
- Joosten, H.; Tanneberger, F.; Moen, A. (Eds.) Mires and Peatlands of Europe: Status, Distribution and Conservation; Schweizerbart Science Publishers: Stuttgart, Germany, 2017. [Google Scholar]
- Ahmad, S.; Wang, M.; Bates, A.; Martini, F.; Regan, S.; Saunders, M.; Liu, H.; McElwain, J.; Gill, L. Flatlining fens? Small-scale variations in peat properties and microtopography as indicators of ecosystem homogenization. Ecol. Indic. 2025, 172, 113317. [Google Scholar] [CrossRef]
- Bragazza, L.; Freeman, C.; Jones, T.; Rydin, H.; Limpens, J.; Fenner, N.; Ellis, T.; Gerdol, R.; Hájek, M.; Hájek, T. Atmospheric nitrogen deposition promotes carbon loss from peat bogs. Proc. Natl. Acad. Sci. USA 2006, 103, 19386–19389. [Google Scholar] [CrossRef]
- Tahvanainen, T. Water chemistry of mires in relation to the poor–rich vegetation gradient and contrasting geochemical zones of the northeastern Fennoscandian Shield. Folia Geobot. 2004, 39, 353–369. [Google Scholar] [CrossRef]
- Zaplata, M.K.; Nhabanga, A.; Stalmans, M.; Volpers, T.; Burkart, M.; Sperfeld, E. Grasses cope with high-contrast ecosystem conditions in the large outflow of the Banhine wetlands, Mozambique. Afr. J. Ecol. 2021, 59, 190–203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).