Phyto- and Zooplankton Diversity Under Land Use and Water Quality Dynamics in the Jialing River, China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plankton Sample Collection and Processing
2.3. Environmental Characteristics
2.4. Data Analysis
3. Results
3.1. Environmental Characteristics
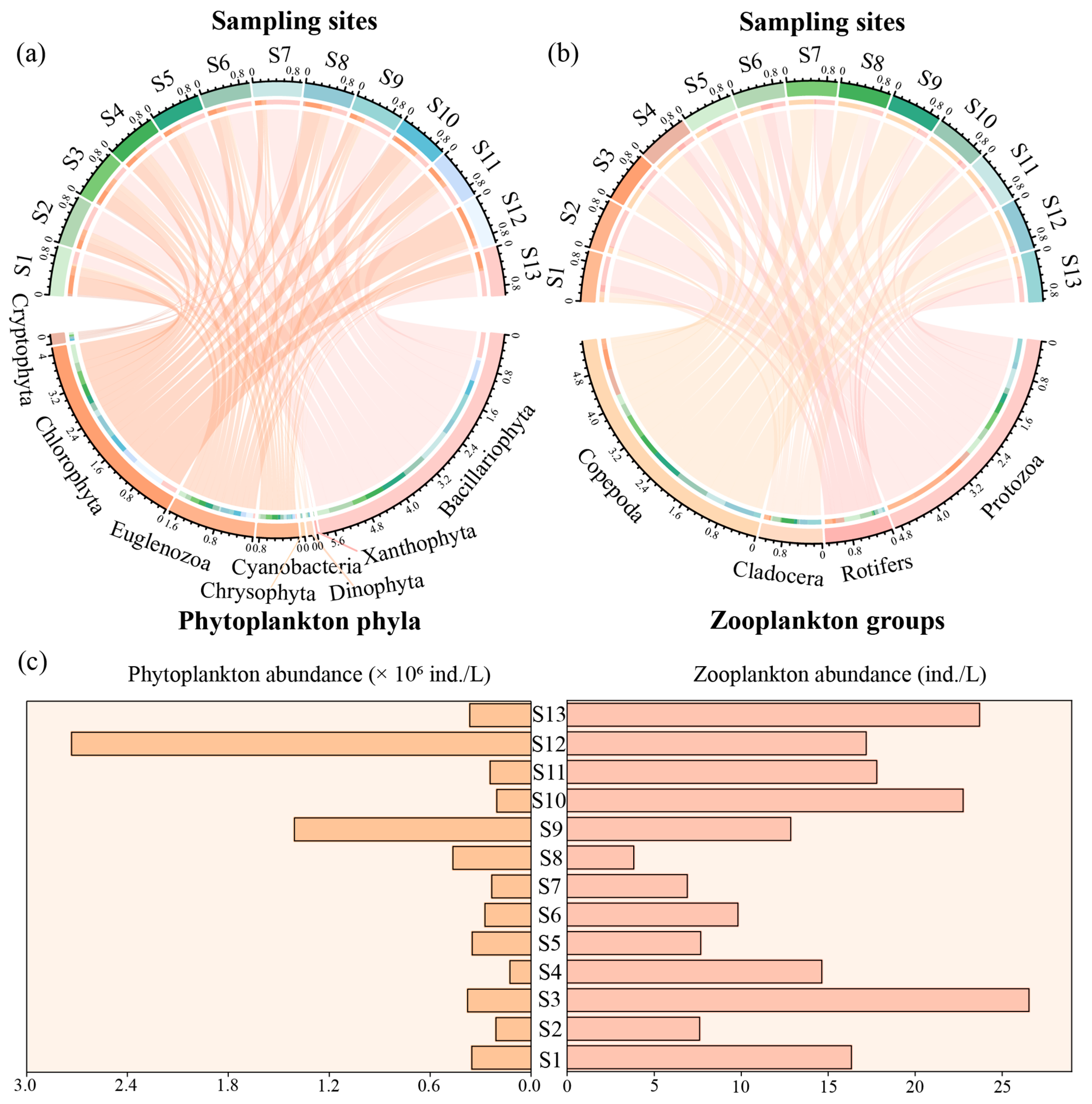
3.2. Plankton Community Structure
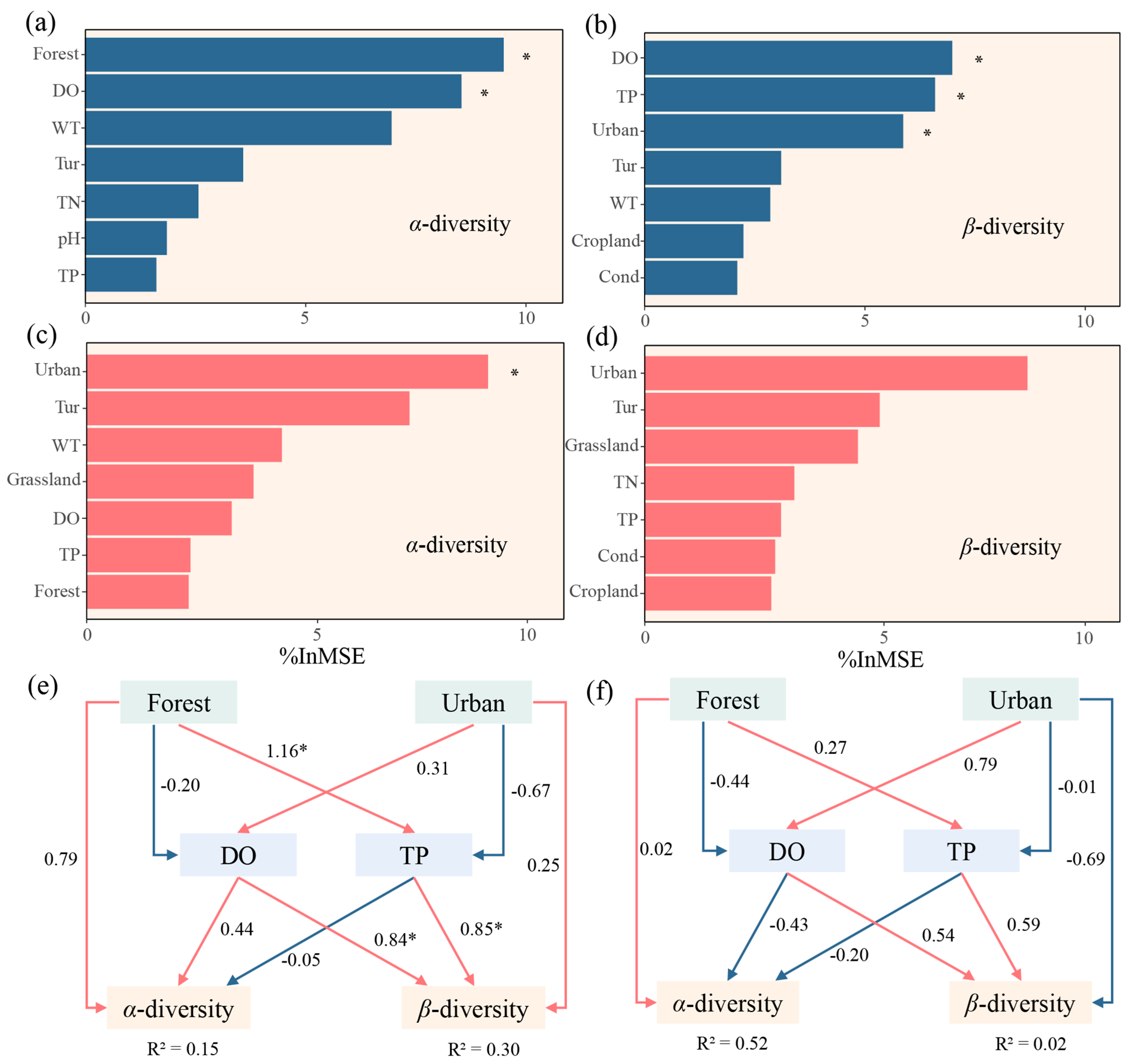
3.3. Drivers of Communities
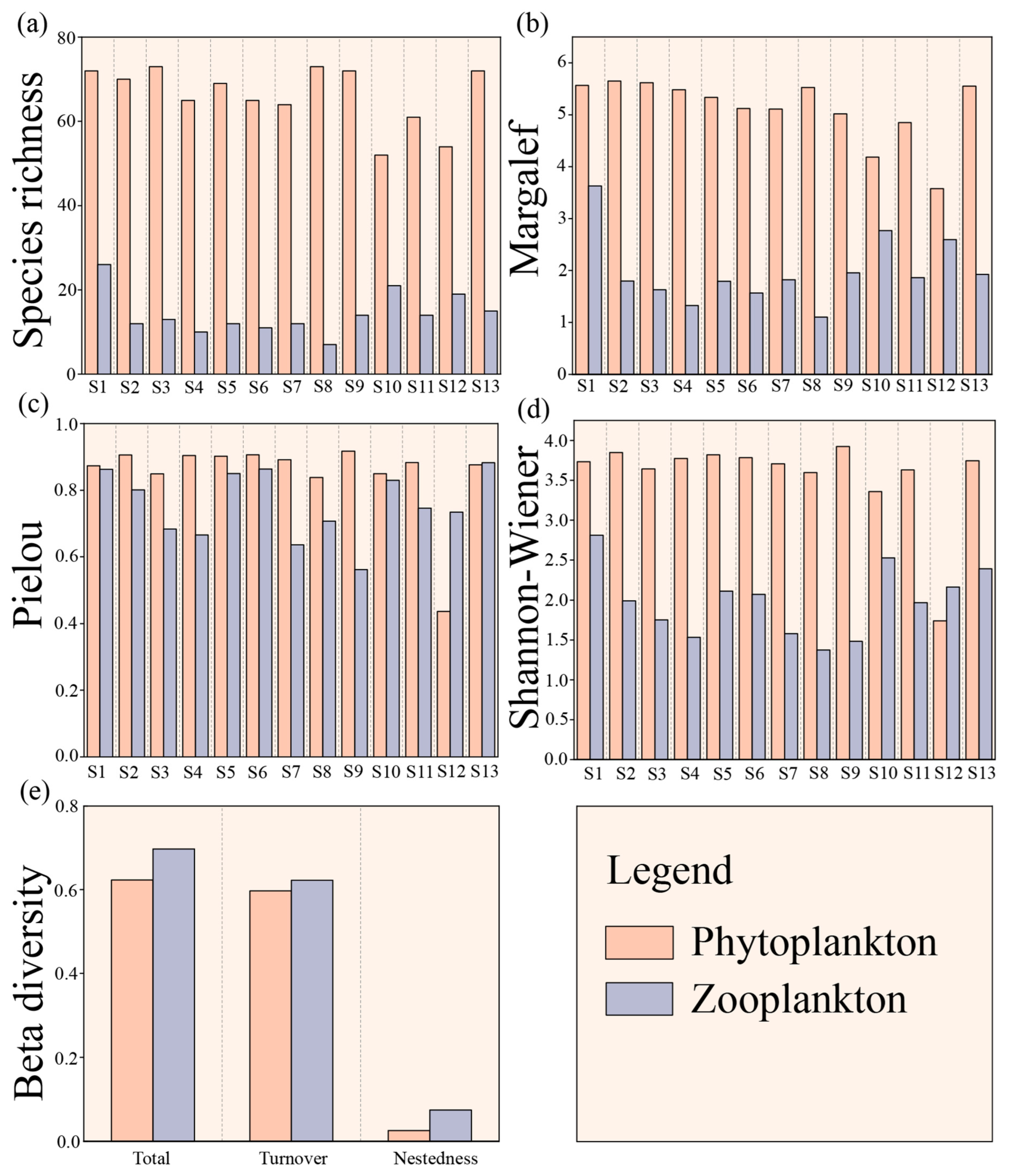
3.4. Biodiversity Indices
3.5. Multiple Factors Affecting Diversity
4. Discussion
4.1. Analysis of Plankton Community Structure
4.2. Drivers of Plankton Community Dynamics
4.3. Response of Plankton Community Diversity to Land Use Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, F.; Altermatt, F.; Yang, J.; An, S.; Li, A.; Zhang, X. Human activities’ fingerprint on multitrophic biodiversity and ecosystem functions across a major river catchment in China. Glob. Change Biol. 2020, 26, 6867–6879. [Google Scholar] [CrossRef]
- Zheng, P.; Jiang, X.; Cao, L.; Mao, J.; Zhang, K.; Zhang, F.; Girón, J.G.; Alahuhta, J.; Heino, J. Ecological uniqueness of fish assemblages and species contributions to beta diversity are affected by river-lake disconnection. Rev. Fish Biol. Fish. 2025, 35, 549–563. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, Y.; Xie, H.; Du, C.; Zhan, A.; Xu, J.; Giesy, J.P.; Wu, F.; Jin, X. Spatial distribution of benthic taxonomic and functional diversity in the Yellow River Basin: From ecological processes to associated determinant factors. Environ. Int. 2024, 188, 108745. [Google Scholar] [CrossRef]
- Li, Z.; Xie, H.; Peng, Z.; Heino, J.; Ma, Y.; Xiong, F.; Gao, W.; Xin, W.; Kong, C.; Li, L.; et al. Hydrology and water quality drive multiple biological indicators in a dam-modified large river. Water Res. X 2024, 25, 100251. [Google Scholar] [CrossRef]
- Bomfim, F.F.; Fares, A.L.B.; Melo, D.G.L.; Vieira, E.; Michelan, T.S. Land use increases macrophytes beta diversity in Amazon streams by favoring amphibious life forms species. Community Ecol. 2023, 24, 159–170. [Google Scholar] [CrossRef]
- Faria, A.P.J.; Ligeiro, R.; Calvão, L.B.; Giam, X.; Leibold, M.A.; Juen, L. Land use types determine environmental heterogeneity and aquatic insect diversity in Amazonian streams. Hydrobiologia 2024, 851, 281–298. [Google Scholar] [CrossRef]
- Lin, Z.; Liu, G.; Guo, K.; Wang, K.; Wijewardene, L.; Wu, N. Scales matter: Regional environment factors affect α diversity but local factors affect β diversity of macroinvertebrates in Thousand Islands Lake catchment area. Ecol. Indic. 2024, 158, 111561. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, X.; Zhang, Y.; Zhang, J.; Dong, X.; Huang, J.; Shen, Y. The impact of anthropic activity based on dam construction and land-use-type in plankton on taxonomic and functional groups. Glob. Ecol. Conserv. 2025, 58, e03494. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, D.; Jin, X.; Li, L.; Wang, C.; Wang, Y.; Pellissier, L.; Johnson, A.C.; Wu, F.; Zhang, X. Long-term wetland biomonitoring highlights the differential impact of land use on macroinvertebrate diversity in Dongting Lake in China. Commun. Earth Environ. 2024, 5, 32. [Google Scholar] [CrossRef]
- Ma, B.; Zhou, R.; Zhang, F.; Ru, H.; Li, Y.; Xu, B.; Lin, P. Relative influence of local habitat and land use/cover on the taxonomic and functional organizations of fish assemblages in the Anning River, Southwest China. Ecol. Indic. 2024, 159, 111673. [Google Scholar] [CrossRef]
- Lucena, M.D.L.D.; Ribeiro-Martins, A.; Casatti, L.; Vieira, T.B.; Brejão, G.L.; Carvalho, F.R.; Michelan, T.S.; Juen, L.; Montag, L.F.D.A.D. Assessing Site and Species Associations with Beta Diversity of Fish Assemblages in Amazonian Streams. Freshw. Biol. 2025, 70, e70001. [Google Scholar] [CrossRef]
- Gomes, J.P.; Stedille, L.I.B.; Milani, J.E.D.F.; Montibeller-Silva, K.; Mantovani, A.; Bortoluzzi, R.L.D.C. Beta diversity as an indicator of priority areas for Myrtaceae assemblage conservation in Subtropical Araucaria Forest. Biodivers. Conserv. 2020, 29, 1361–1379. [Google Scholar] [CrossRef]
- Teichert, N.; Lepage, M.; Chevillot, X.; Lobry, J. Environmental drivers of taxonomic, functional and phylogenetic diversity (alpha, beta and gamma components) in estuarine fish communities. J. Biogeogr. 2018, 45, 406–417. [Google Scholar] [CrossRef]
- Taurozzi, D.; Cesarini, G.; Santo, C.D.; Scalici, M. Beyond the flow: Ecological insights from diatom communities of a Mediterranean intermittent river. Environ. Res. 2025, 285, 122284. [Google Scholar] [CrossRef]
- Laliberté, E.; Schweiger, A.K.; Legendre, P. Partitioning plant spectral diversity into alpha and beta components. Ecol. Lett. 2020, 23, 370–380. [Google Scholar] [CrossRef] [PubMed]
- Amaral, D.C.; Bomfim, F.D.F.; Lansac-Tôha, F.A. Drivers of zooplankton functional and taxonomic β-diversity in two neotropical floodplains: Implications for conservation. Biodivers. Conserv. 2024, 33, 3905–3922. [Google Scholar] [CrossRef]
- Wu, N.; Liu, G.; Qi, X.; Lin, Z.; Wang, Y.; Wang, Y.; Li, Y.; Oduro, C.; Khan, S.; Zhou, S.; et al. Different facets of alpha and beta diversity of benthic diatoms along stream watercourse in a large near—Natural catchment. Ecol. Evol. 2024, 14, e11577. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010, 19, 134–143. [Google Scholar] [CrossRef]
- Dey, D.; Shruti, M.; De, K.; Adhikari, B.S.; Hussain, Y.A. Local and species contribution of beta diversity of macrophytes in perspective of conservation and restoration of Ganga River, India. Hydrobiologia 2024, 851, 2053–2070. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, Q.; Lu, X.; Fan, Y.; Liu, Y.; Tan, X. Local environmental variables outperform spatial and land use pattern in the maintenance and assembly of phytoplankton communities in the wetland cluster. J. Clean. Prod. 2023, 419, 138275. [Google Scholar] [CrossRef]
- Li, Z.; Heino, J.; Liu, Z.; Meng, X.; Chen, X.; Ge, Y.; Xie, Z. The drivers of multiple dimensions of stream macroinvertebrate beta diversity across a large montane landscape. Limnol. Oceanogr. 2021, 66, 226–236. [Google Scholar] [CrossRef]
- Lin, P.; Fujiwara, M.; Ma, B.; Xia, Z.; Wu, X.; Wang, C.; Chang, T.; Gao, X. Ecological drivers shaping mainstem and tributary fish communities in the upper Jinsha River, southeastern Qinghai-Tibet Plateau. Ecol. Process. 2025, 14, 8. [Google Scholar] [CrossRef]
- Tang, X.; He, H.; Qin, Q.; Xu, F.; Liu, F.; Zhang, F. Seasonal variation in the β—Diversity of periphytic algae and its response to landscape patterns in the Chishui River, a naturally flowing tributary of the upper Yangtze River. Ecol. Evol. 2025, 15, e70976. [Google Scholar] [CrossRef]
- Chang, C.; Hu, E.; Xue, X.; Li, J.; Du, D.; Yang, F.; Li, M. Hydro-morphology and water quality jointly shape the structure and network stability of the plankton community in multi-tributary river basins. J. Hydrol. 2024, 643, 131945. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Y.; Shen, Y.; Liu, Y.; Gao, H.; Guo, P. Driving mechanism of land use and landscape pattern to phytoplankton and zooplankton community and their trophic interactions in river ecosystems. J. Environ. Manag. 2024, 370, 122691. [Google Scholar] [CrossRef]
- Gao, W.; Xiong, F.; Lu, Y.; Xin, W.; Wang, H.; Feng, G.; Kong, C.; Fang, L.; Gao, X.; Chen, Y. Water quality and habitat drive phytoplankton taxonomic and functional group patterns in the Yangtze River. Ecol. Process. 2024, 13, 11. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, J.; Xie, H.; Liang, E.; Cai, H. Anthropogenic activities disturb phytoplankton taxa and functional groups in an urban river. Environ. Res. 2025, 265, 120411. [Google Scholar] [CrossRef]
- Hou, X.; Wang, D.; Hu, X.; Li, Y.; Zhang, H.; Niu, L.; Huang, R.; Xu, J. Unraveling long-term health dynamics of impounded lakes integrating water diversion-adapted planktonic index of biotic integrity and machine learning. Environ. Res. 2025, 285, 122366. [Google Scholar] [CrossRef]
- Tang, X.; Liu, L.; Wang, Y.; Xu, J.; Huang, Y.; Qin, Q.; Xu, F.; Zhang, F. Characteristics of plankton community structure and applicability of biomanipulation in midstream of the Jialing River—The biggest tributary of the Yangtze River. J. Freshw. Ecol. 2024, 39, 2378835. [Google Scholar] [CrossRef]
- Wang, X.; Ding, L.; Wu, Y.; Bol, R. Combined effects of flood, drought and land use dominate water quality and nutrient exports in Jialing River basin, SW China. Sci. Total Environ. 2024, 954, 176733. [Google Scholar] [CrossRef]
- Zhang, S.; Li, W.; An, W.; Hou, J.; Hou, X.; Tang, C.; Gan, Z. Temporal and spatial evolutionary trends of regional extreme precipitation under different emission scenarios: Case study of the Jialing River Basin, China. J. Hydrol. 2023, 617, 129156. [Google Scholar] [CrossRef]
- Wang, Y.; Junaid, M.; Deng, J.; Tang, Q.; Luo, L.; Xie, Z.; Pei, D. Effects of land-use patterns on seasonal water quality at multiple spatial scales in the Jialing River, Chongqing, China. CATENA 2024, 234, 107646. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, S.; Ruan, Q.; Tang, C. Synergetic impact of climate and vegetation cover on runoff, sediment, and nitrogen and phosphorus losses in the Jialing River Basin, China. J. Clean. Prod. 2022, 361, 132141. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Huang, X.; Liu, C.; Yang, Y. Effects of land use and nutrients on the characteristics of dissolved organic matter in the Nanchong Section of Jialing River, China in December 2019. Water Supply 2022, 22, 1863–1875. [Google Scholar] [CrossRef]
- Duan, X.; Chen, B.; Zhang, T.; Guan, Y.; Zeng, K. Habitat Quality Evolution and Multi-Scenario Simulation Based on Land Use Change in the Jialing River Basin. Sustainability 2024, 16, 6968. [Google Scholar] [CrossRef]
- Hu, H.; Wei, Y. The Freshwater Algae of China-Systematic, Taxonomy and Ecology; Science Press: Beijing, China, 2006; pp. 79–285. [Google Scholar]
- Jiang, Y.; Du, N. Chinese Fauna-Freshwater Cladocera; Science Press: Beijing, China, 1979; pp. 79–273. [Google Scholar]
- Shen, Y. New Technology of Micro-Biological Monitoring; China Architecture and Building Press: Beijing, China, 1990. [Google Scholar]
- Yang, J.; Huang, X. The 30 m annual land cover datasets and its dynamics in China from 1985 to 2023. Earth Syst. Sci. Data 2024, 13, 3907–3925. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, D.; Villeger, S.; De Bortoli, J.; Leprieur, F.; Logez, M.; Martinez-Santalla, S.; Martin-Devasa, R.; Gomez-Rodriguez, C.; Crujeiras, R. Betapart: Partitioning Beta Diversity into Turnover and Nestedness Components, R Package Version 1.6; 2023. Available online: https://CRAN.R-project.org/package=betapart (accessed on 10 October 2025).
- Hou, L.; Xiong, W.; Chen, M.; Xu, J.; Johnson, A.C.; Zhan, A.; Jin, X. Pesticide pollution reduces the functional diversity of macroinvertebrates in urban aquatic ecosystems. Environ. Sci. Technol. 2025, 59, 8568–8577. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xu, F.; Xia, J.; Yang, X.; Zhang, F.; Liu, S.; Zhang, T. Evaluation of the current status and risks of aquatic ecology in the Jialing River Basin based on the characteristics and succession trends of phytoplankton communities. Ecol. Indic. 2025, 170, 113121. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Rosseel, Y. lavaan: An R package for structural equation modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Lin, Y.; Ma, H.; Ding, J.; Sun, H.; Chen, Q. Spatial distribution patterns of phytoplankton community during different water periods along cascade reservoirs in the Lancang River. Acta Sci. Circum. 2022, 42, 392–401. [Google Scholar]
- Peng, X.; Yi, K.; Lin, Q.; Zhang, L.; Zhang, Y.; Liu, B.; Wu, Z. Annual changes in periphyton communities and their diatom indicator species, in the littoral zone of a subtropical urban lake restored by submerged plants. Ecol. Eng. 2020, 155, 105958. [Google Scholar] [CrossRef]
- Ailifeire, A.; Ma, J.; Wu, Z.; Su, Y. Seasonal dynamics and influencing factors in plankton communities of ecologically restored and unrestored zones in Lake Xuanwu, Nanjing. J. Lake Sci. 2025, 37, 798–811. [Google Scholar] [CrossRef]
- Qian, X.; Li, J.; Ao, W.; Pang, B.; Bao, S.; Wang, Q.; Liu, B.; Wang, Z. Seasonal dynamics of phytoplankton and its relationship with environmental factors in Lake Hulun. J. Lake Sci. 2022, 34, 1814–1827. [Google Scholar] [CrossRef]
- Ciros-Pérez, J.; Ortega-Mayagoitia, E.; Alcocer, J. The role of ecophysiological and behavioral traits in structuring the zooplankton assemblage in a deep, oligotrophic, tropical lake. Limnol. Oceanogr. 2015, 60, 2158–2172. [Google Scholar] [CrossRef]
- Zhao, K.; Song, K.; Pan, Y.; Wang, L.; Da, L.; Wang, Q. Metacommunity structure of zooplankton in river networks: Roles of environmental and spatial factors. Ecol. Indic. 2017, 73, 96–104. [Google Scholar] [CrossRef]
- Ersoy, Z.; Brucet, S.; Bartrons, M.; Mehner, T. Short-term fish predation destroys resilience of zooplankton communities and prevents recovery of phytoplankton control by zooplankton grazing. PLoS ONE 2019, 14, e0212351. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, M.; Yu, R.; Sun, H.; Zhang, L.; Sun, L.; Lv, C.; Xu, J. Response of planktonic diversity and stability to environmental drivers in a shallow eutrophic lake. Ecol. Indic. 2022, 144, 109560. [Google Scholar] [CrossRef]
- Sun, C.; Xia, L.; Zhang, M.; He, Q.; Yu, N.; Xiang, H.; Yang, H. The impacts of different seasons on macroinvertebrate community structure and functional diversity in the Jingui River, China. Glob. Ecol. Conserv. 2024, 51, e02876. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Song, J.; Kong, F.; Zhang, X.; Li, Q. Characteristics of Plankton Community Structure and Its Relation to Environmental Factors in Weihe River, China. Ecol. Environ. Sci. 2022, 31, 117. [Google Scholar]
- Zhang, W.; Han, S.; Zhang, D.; Shan, B.; Wei, D. Variations in dissolved oxygen and aquatic biological responses in China’s coastal seas. Environ. Res. 2023, 223, 115418. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Shi, X.; Zhao, S.; Sun, B.; Liu, Y.; Arvola, L.; Li, G.; Wang, Y.; Pan, X.; Wu, R.; et al. Primary productivity of phytoplankton and its influencing factors in cold and arid regions: A case study of Wuliangsuhai Lake, China. Ecol. Indic. 2022, 144, 109545. [Google Scholar] [CrossRef]
- Mello, K.D.; Valente, R.A.; Randhir, T.O.; Santos, A.C.A.D.; Vettorazzi, C.A. Effects of land use and land cover on water quality of low-order streams in Southeastern Brazil: Watershed versus riparian zone. CATENA 2018, 167, 130–138. [Google Scholar] [CrossRef]
- Ni, L.; Li, H.; Zhou, L.; Shi, J.; Nie, Y.; Zhao, F.; Li, S. Structural characteristics of zooplankton communities in Hongze Lake driven by water environmental factors from 2016 to 2020. Environ. Monit. Assess. 2023, 195, 1503. [Google Scholar] [CrossRef]
- Roman, M.R.; Brandt, S.B.; Houde, E.D.; Pierson, J.J. Interactive effects of hypoxia and temperature on coastal pelagic zooplankton and fish. Front. Mar. Sci. 2019, 6, 139. [Google Scholar] [CrossRef]
- Suikkanen, S.; Uusitalo, L.; Lehtinen, S.; Lehtiniemi, M.; Kauppila, P.; Mäkinen, K.; Kuosa, H. Diazotrophic cyanobacteria in planktonic food webs. Food Webs 2021, 28, e00202. [Google Scholar] [CrossRef]
- Yunev, O.A.; Carstensen, J.; Stelmakh, L.V.; Belokopytov, V.N.; Suslin, V.V. Reconsideration of the phytoplankton seasonality in the open Black Sea. Limnol. Oceanogr. Lett. 2021, 6, 51–59. [Google Scholar] [CrossRef]
- Ellis, E.C. Land use and ecological change: A 12,000-year history. Annu. Rev. Environ. Resour. 2021, 46, 1–33. [Google Scholar] [CrossRef]
- Coffey, R.; Paul, M.J.; Stamp, J.; Hamilton, A.; Johnson, T. A review of water quality responses to air temperature and precipitation changes 2: Nutrients, algal blooms, sediment, pathogens. JAWRA J. Am. Water Resour. Assoc. 2019, 55, 844–868. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, L.; Li, Y.; Lin, Q.; He, C.; Huang, S.; Li, H.; Zhang, X.; Liu, B.; Ge, F.; et al. The changing characteristics of phytoplankton community and biomass in subtropical shallow lakes: Coupling effects of land use patterns and lake morphology. Water Res. 2021, 200, 117235. [Google Scholar] [CrossRef]
- Xie, H.; Jin, X.; Li, W.; Cai, K.; Yang, G.; Chen, K.; Xu, J.; Johnson, A.C. Identifying critical land use thresholds for biodiversity conservation in China’s lake ecosystems. Environ. Sci. Technol. 2025, 59, 5431–5442. [Google Scholar] [CrossRef] [PubMed]


| Unit | Min | Max | Medium | Mean | SD | |
|---|---|---|---|---|---|---|
| Wid | m | 345.2 | 794.1 | 478.8 | 513.83 | 148.66 |
| Tur | NTU | 4.1 | 5.4 | 4.8 | 4.85 | 0.36 |
| WT | °C | 20.5 | 23.7 | 22.0 | 21.98 | 0.79 |
| DO | mg·L−1 | 6.52 | 9.09 | 7.38 | 7.60 | 0.77 |
| Cond | μs·cm−1 | 293 | 329 | 299 | 302.54 | 9.52 |
| pH | — | 8.0 | 8.6 | 8.4 | 8.33 | 0.19 |
| NH3-N | mg·L−1 | 0.066 | 0.282 | 0.138 | 0.164 | 0.076 |
| TN | mg·L−1 | 0.731 | 0.743 | 0.733 | 0.735 | 0.004 |
| TP | mg·L−1 | 0.001 | 0.038 | 0.012 | 0.015 | 0.009 |
| Cropland | % | 31.01 | 80.45 | 66.78 | 61.60 | 14.43 |
| Forest | % | 0 | 4.98 | 0.67 | 1.46 | 1.58 |
| Grassland | % | 0 | 0.08 | 0 | 0.01 | 0.02 |
| Water | % | 17.40 | 44.82 | 28.72 | 28.67 | 8.68 |
| Urban | % | 0 | 45.63 | 2.23 | 8.26 | 12.85 |
| Phyla | Dominant Species | Dominance Index |
|---|---|---|
| Phytoplankton | ||
| Bacillariophyta | Cyclotella bodanica | 0.02 |
| Cyclotella sp. | 0.02 | |
| Chlorophyta | Chlorella vulgaris | 0.04 |
| Platymonas incisa | 0.24 | |
| Zooplankton | ||
| Protozoa | Cyclopyxis arcelloides | 0.06 |
| Difflugia corona | 0.05 | |
| Difflugia globulosa | 0.06 | |
| Copepoda | Sinocalanus dorrii | 0.03 |
| Nauplii | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Huang, Y.; Chen, C.; He, H.; Qin, Q.; Xu, F.; Zhang, F. Phyto- and Zooplankton Diversity Under Land Use and Water Quality Dynamics in the Jialing River, China. Diversity 2025, 17, 707. https://doi.org/10.3390/d17100707
Tang X, Huang Y, Chen C, He H, Qin Q, Xu F, Zhang F. Phyto- and Zooplankton Diversity Under Land Use and Water Quality Dynamics in the Jialing River, China. Diversity. 2025; 17(10):707. https://doi.org/10.3390/d17100707
Chicago/Turabian StyleTang, Xiaopeng, Yiling Huang, Chang Chen, Haoyun He, Qiang Qin, Fei Xu, and Fubin Zhang. 2025. "Phyto- and Zooplankton Diversity Under Land Use and Water Quality Dynamics in the Jialing River, China" Diversity 17, no. 10: 707. https://doi.org/10.3390/d17100707
APA StyleTang, X., Huang, Y., Chen, C., He, H., Qin, Q., Xu, F., & Zhang, F. (2025). Phyto- and Zooplankton Diversity Under Land Use and Water Quality Dynamics in the Jialing River, China. Diversity, 17(10), 707. https://doi.org/10.3390/d17100707






