Lethal Heat Exchange—Short-Term Thermoregulation in Two Triturus Species During Abrupt Changes in Living Media (Water vs. Air)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Physiological Remarks
4.2. Methodological Considerations
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| t A | ambient temperature |
| t TI | temperature of the newts Triturus ivanbureschi |
| t TD | temperature of the newts Triturus dobrogicus |
| t TD (TC) T. dobrogicus | average body temperature measured by thermometer with a thermocouple |
| t TI (TC) T. ivanbureschi | average body temperature measured by thermometer with thermocouple |
| t TD (Flir) T. dobrogicus | average body temperature measured by thermal camera |
| t TI (Flir) T. ivanureschi | average body temperature measured by thermal camera |
References
- Matthes, E. Bau und Funktion der Lippensäume Wasserlebender Urodelen. Z. Morphol. Ökol. Tiere 1934, 28, 155–169. [Google Scholar] [CrossRef]
- Denoël, M. Terrestrial versus Aquatic Foraging in Juvenile Alpine Newts (Triturus alpestris). Écoscience 2004, 11, 404–409. [Google Scholar] [CrossRef]
- Heiss, E.; Aerts, P.; Van Wassenbergh, S. Flexibility Is Everything: Prey Capture throughout the Seasonal Habitat Switches in the Smooth Newt Lissotriton Vulgaris. Org. Divers. Evol. 2015, 15, 127–142. [Google Scholar] [CrossRef]
- Griffiths, R.A. Newts and Salamanders of Europe; Academic Press: London, UK, 1996. [Google Scholar]
- Thiesmeier, B.; Schulte, U. Der Bergmolch—Im Flachland Wie Im Hochgebirge Zu Hause; Laurenti-Verlag: Bielefeld, Germany, 2010. [Google Scholar]
- Heiss, E.; Aerts, P.; Van Wassenbergh, S. Aquatic–Terrestrial Transitions of Feeding Systems in Vertebrates: A Mechanical Perspective. J. Exp. Biol. 2018, 221, jeb154427. [Google Scholar] [CrossRef] [PubMed]
- Dennert, W. Über Den Bau Und Die Rückbildung Des Flossensaums Bei Den Urodelen. Anat. Embryol. 1924, 72, 407–462. [Google Scholar] [CrossRef]
- Brossman, K.H.; Carlson, B.E.; Swierk, L.; Langkilde, T. Aquatic Tail Size Carries over to the Terrestrial Phase without Impairing Locomotion in Adult Eastern Red-Spotted Newts (Notophthalmus Viridescens Viridescens). Can. J. Zool. 2013, 91, 7–12. [Google Scholar] [CrossRef]
- Van Wassenbergh, S.; Heiss, E. Phenotypic Flexibility of Gape Anatomy Fine-Tunes the Aquatic Prey-Capture System of Newts. Sci. Rep. 2016, 6, 29277. [Google Scholar] [CrossRef]
- Heiss, E.; Handschuh, S.; Aerts, P.; Van Wassenbergh, S. A Tongue for All Seasons: Extreme Phenotypic Flexibility in Salamandrid Newts. Sci. Rep. 2017, 7, 1006. [Google Scholar] [CrossRef]
- Lukanov, S.; Tzankov, N.; Handschuh, S.; Heiss, E.; Naumov, B.; Natchev, N. On the Amphibious Food Uptake and Prey Manipulation Behavior in the Balkan-Anatolian Crested Newt (Triturus ivanbureschi, Arntzen and Wielstra, 2013). Zoology 2016, 119, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Warburg, M.R.; Rosenberg, M. Structure of Gill Epithelium in Triturus Vittatus Larvae. Ann. Anat.-Anat. Anz. 1997, 179, 57–64. [Google Scholar] [CrossRef]
- Erskine, D.J.; Spotila, J.R. Heat-Energy-Budget Analysis and Heat Transfer in the Largemouth Blackbass (Micropterus salmoides). Physiol. Zool. 1977, 50, 157–169. [Google Scholar] [CrossRef]
- Huey, R.B.; Slatkin, M. Cost and Benefits of Lizard Thermoregulation. Q. Rev. Biol. 1976, 51, 363–384. [Google Scholar] [CrossRef]
- Brattstrom, B.H. Amphibian Temperature Regulation Studies in the Field and Laboratory. Am. Zool. 1979, 19, 345–356. [Google Scholar] [CrossRef]
- Bovo, R.P.; Navas, C.A.; Tejedo, M.; Valença, S.E.S.; Gouveia, S.F. Ecophysiology of Amphibians: Information for Best Mechanistic Models. Diversity 2018, 10, 118. [Google Scholar] [CrossRef]
- Giacometti, D.; Tattersall, G.J. Seasonal Variation of Behavioural Thermoregulation in a Fossorial Salamander (Ambystoma maculatum). R. Soc. Open Sci. 2024, 11, 240537. [Google Scholar] [CrossRef] [PubMed]
- Riddell, E.A.; Sears, M.W. Geographic Variation of Resistance to Water Loss within Two Species of Lungless Salamanders: Implications for Activity. Ecosphere 2015, 6, 1–16. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Wallis, G.P. Geographic Variation and Taxonomy of Crested Newts (Triturus cristatus superspecies): Morphological and Mitochondrial DNA Data. Contrib. Zool. 1999, 68, 181–203. [Google Scholar] [CrossRef]
- Stoyanov, A.; Tzankov, N.; Naumov, B. Die Amphiben Und Reptilien Bulgariens; Chimaira: Frankfurt am Main, Germany, 2011. [Google Scholar]
- Lukanov, S.; Tzankov, N. Life History, Age and Normal Development of the Balkan-Anatolian Crested Newt (Triturus ivanbureschi Arntzen and Wielstra, 2013) from Sofia District. North-West. J. Zool. 2016, 12, 22–32. [Google Scholar]
- Rowley, J.J.L.; Alford, R.A. Non-Contact Infrared Thermometers Can Accurately Measure Amphibian Body Temperatures. Herpetol. Rev. 2007, 38, 308–311. [Google Scholar]
- Herczeg, G.; Gonda, A.; Saarikivi, J.; Merilä, J. Experimental Support for the Cost–Benefit Model of Lizard Thermoregulation. Behav. Ecol. Sociobiol. 2006, 60, 405–414. [Google Scholar] [CrossRef]
- Vickers, M.; Manicom, C.; Schwarzkopf, L. Extending the Cost-Benefit Model of Thermoregulation: High-Temperature Environments. Am. Nat. 2011, 177, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-Safety Margins and the Necessity of Thermoregulatory Behavior across Latitude and Elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef]
- Gvoždík, L.; Puky, M.; Šugerková, M. Acclimation Is Beneficial at Extreme Test Temperatures in the Danube Crested Newt, Triturus dobrogicus (Caudata, Salamandridae): Thermal Acclimation in Newts. Biol. J. Linn. Soc. 2007, 90, 627–636. [Google Scholar] [CrossRef]
- Marek, V.; Gvoždík, L. The Insensitivity of Thermal Preferences to Various Thermal Gradient Profiles in Newts. J. Ethol. 2012, 30, 35–41. [Google Scholar] [CrossRef]
- Balogová, M.; Gvoždík, L. Can Newts Cope with the Heat? Disparate Thermoregulatory Strategies of Two Sympatric Species in Water. PLoS ONE 2015, 10, e0130918. [Google Scholar]
- Fahrbach, M.; Gerlach, U. The Genus Triturus; Edition Chimaira: Frankfurt am Main, Germany, 2018; ISSN 1613-2327. [Google Scholar]
- Kearney, M.; Shine, R.; Porter, W.P. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA 2009, 106, 3835–3840. [Google Scholar] [CrossRef]
- Iturra-Cid, M.; Vidal, M.; Labra, A.; Ortiz, J.C. Thermal ecology of Pleurodema thaul (Amphibia: Leptodactylidae). Gayana 2014, 78, 25–30. [Google Scholar] [CrossRef]
- Feder, M.E.; Londos, P.L. Hydric Constraints upon Foraging in a Terrestrial Salamander, Desmognathus ochrophaeus (Amphibia: Plethodontidae). Oecologia 1984, 64, 413–418. [Google Scholar] [CrossRef]
- Titon, B.; Gomes, F.R. Relation between Water Balance and Climatic Variables Associated with the Geographical Distribution of Anurans. PLoS ONE 2015, 10, e0140761. [Google Scholar] [CrossRef]
- Roznik, E.A.; Rodriguez-Barbosa, C.A.; Johnson, S.A. Hydric Balance and Locomotor Performance of Native and Invasive Frogs. Front. Ecol. Evol. 2018, 6, 159. [Google Scholar] [CrossRef]
- Vučić, T.; Ivanović, A.; Ajduković, M.; Bajler, N.; Cvijanović, M. The Reproductive Success of Triturus ivanbureschi × T. macedonicus F1 Hybrid Females (Amphibia: Salamandridae). Animals 2022, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Natchev, N.; Koynova, T.; Tachev, K.; Doichev, D.; Marinova, P.; Velkova, V.; Jablonski, D. Temperature Regulation in the Balkan Spadefoot (Pelobates balcanicus Karaman, 1928) at the Beginning of Nocturnal Activity. PeerJ 2022, 10, e13647. [Google Scholar] [CrossRef]
- Blais, B.R.; Velasco, D.E.; Frackiewicz, M.E.; Low, A.Q.; Koprowski, J.L. Assessing Thermal Ecology of Herpetofauna across a Heterogeneous Microhabitat Mosaic in a Changing Aridland Riparian System. Environ. Res. Ecol. 2023, 2, 035001. [Google Scholar] [CrossRef]
- Luna, S.; Font, E. Use of an Infrared Thermographic Camera to Measure Field Body Temperatures of Small Lacertid Lizards. Herpetol. Rev. 2013, 44, 59–62. [Google Scholar]
- Khozatskii, L. Body surface temperature in some amphibians and reptiles. J. Leningr. Univ. 1959, 14, 92–105. [Google Scholar]
- Chukwuka, C.O.; Virens, E.; Cree, A. Accuracy of an Inexpensive, Compact Infrared Thermometer for Measuring Skin Surface Temperature of Small Lizards. J. Therm. Biol. 2019, 84, 285–291. [Google Scholar] [CrossRef] [PubMed]
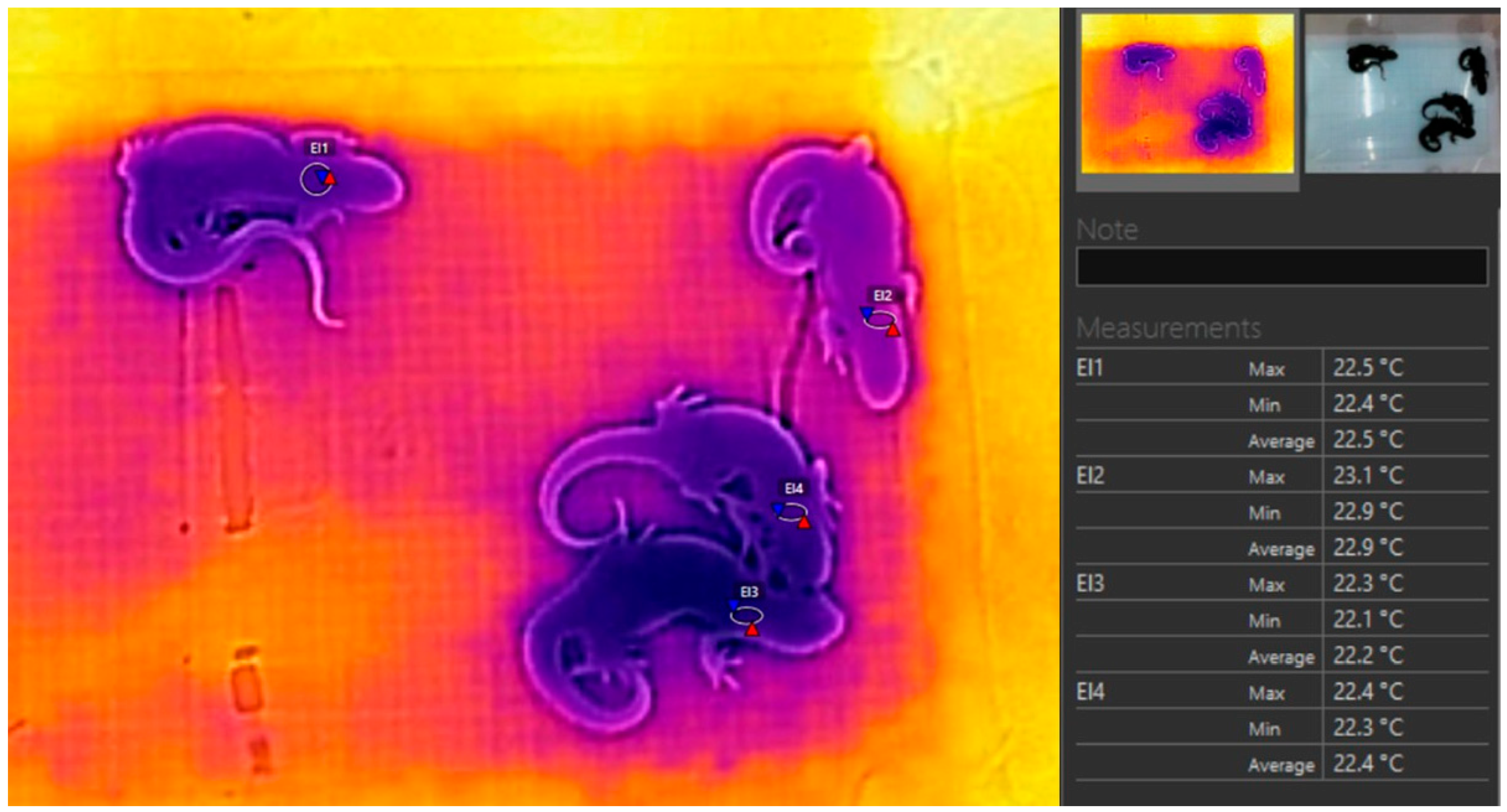
| Day | t A | t TD (TC) | t TI (TC) | t A—t TD (TC) | t A—t TI (TC) |
|---|---|---|---|---|---|
| mean | 23.19 | 21.13 | 21.22 | 2.06 | 2.06 |
| min | 17.00 | 15.58 | 15.58 | 1.42 | 1.43 |
| max | 25.90 | 23.71 | 23.90 | 2.38 | 2.40 |
| SD | 3.08 | 2.84 | 2.84 | 0.33 | 0.39 |
| n | 9 | 4 | |||
| number of sets | 9 | 9 | 9 | 9 | 9 |
| 4 June 2020 | |||||
| mean | 23.36 | 21.10 | 21.34 | 2.26 | 2.02 |
| min | 17.00 | 16.31 | 16.35 | 0.69 | 0.65 |
| max | 26.30 | 24.48 | 24.60 | 4.10 | 3.38 |
| SD | 3.03 | 3.07 | 3.02 | 0.95 | 0.78 |
| n | 9 | 4 | |||
| number of sets | 10 | 10 | 9 | 10 | 9 |
| 5 June 2020 | |||||
| mean | 23.38 | 21.20 | 21.18 | 2.18 | 2.19 |
| min | 17.00 | 16.66 | 16.73 | 0.34 | 0.27 |
| max | 26.80 | 24.53 | 24.35 | 2.80 | 2.78 |
| SD | 3.34 | 2.86 | 2.66 | 0.76 | 0.81 |
| n | 9 | 4 | |||
| number of sets | 8 | 8 | 8 | 8 | 8 |
| 6 June 2020 | |||||
| mean | 22.98 | 20.48 | 20.35 | 2.50 | 2.63 |
| min | 16.60 | 16.04 | 16.43 | −0.33 | 0.18 |
| max | 26.60 | 23.49 | 23.43 | 4.50 | 4.38 |
| SD | 3.34 | 2.69 | 2.60 | 1.22 | 1.17 |
| n | 9 | 4 | |||
| number of sets | 10 | 10 | 10 | 10 | 10 |
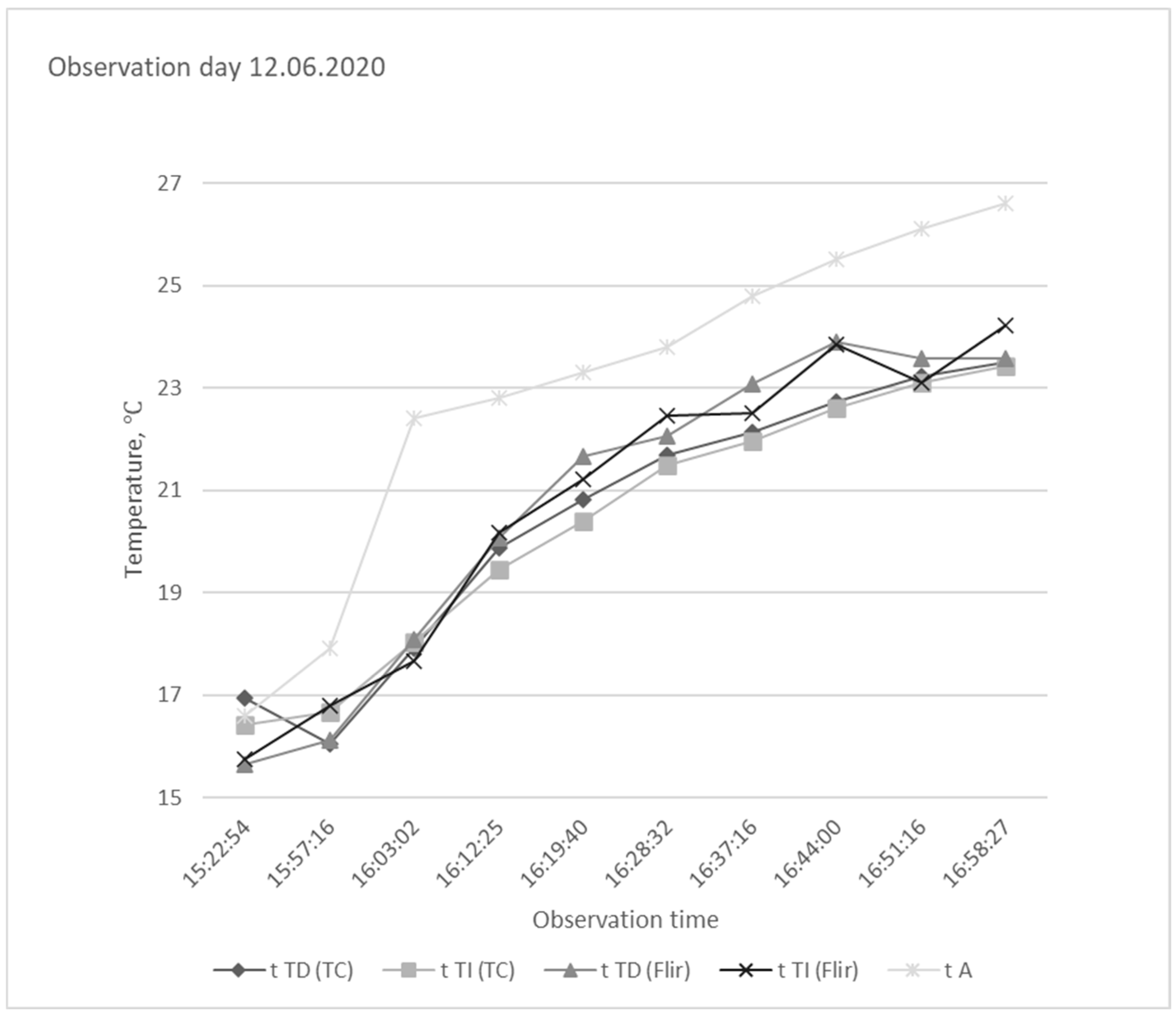
| 12 June 2020 |
| Day | Predictor | β | SE | t | p | 95% CI | Effect |
|---|---|---|---|---|---|---|---|
| 4 June2020 | Ambient temperature | 0.92 | 0.01 | 70.29 | p < 0.001 | 0.89–0.94 | + |
| 4 June2020 | Group dummy | 0.11 | 0.11 | 0.2 | n.s. | −0.98–1.20 | |
| 4 June2020 | Ambient temperature*Group dummy | <0.001 | 0.02 | −0.04 | n.s. | −0.05–0.05 | |
| Model | F(3, 113) = 2378 | p < 0.001 | R2= 0.98 | ||||
| 5 June 2020 | Ambient temperature | 0.96 | 0.03 | 29.09 | p < 0.001 | 0.9–1.03 | + |
| 5 June 2020 | Group dummy | 0.21 | 1.41 | 0.15 | n.s. | 2.99–−2.57 | |
| 5 June 2020 | Ambient temperature*Group dummy | <0.001 | 0.06 | 0.02 | n.s. | −0.12–0.12 | |
| Model | F(3, 126) = 408 | p < 0.001 | R2= 0.91 | ||||
| 6 June 2020 | Ambient temperature | 0.84 | 0.02 | 42.01 | p < 0.001 | 0.8–0.88 | + |
| 6 June 2020 | Group dummy | 1.21 | 0.85 | 1.42 | n.s. | −0.48–2.89 | |
| 6 June 2020 | Ambient temperature*Group dummy | −0.05 | 0.04 | −1.45 | n.s. | −0.12–0.02 | |
| Model | F(3, 100) = 818 | p < 0.001 | R2= 0.96 | ||||
| 12 June 2020 | Ambient temperature | 0.76 | 0.03 | 25.8 | p < 0.001 | 0.7–0.82 | + |
| 12 June 2020 | Group dummy | 0.22 | 1.23 | 0.18 | n.s. | −2.21–2.66 | |
| 12 June 2020 | Ambient temperature*Group dummy | −0.02 | 0.05 | −0.29 | n.s. | −0.12–0.09 | |
| Model | F(3, 126) = 317 | p < 0.001 | R2= 0.88 |
| Animal ID | Group | D1 | D2 | D3 | D4 | Mean 1_3 | Δ |
|---|---|---|---|---|---|---|---|
| TD1 | A | 21.28 | 21.4 | 21.29 | 20.46 | 21.32 | −0.86 |
| TD2 | A | 21.22 | 21 | 21.00 | 20.26 | 21.06 | −0.80 |
| TD3 | A | 21.16 | 21 | 21.20 | 20.81 | 21.13 | −0.32 |
| TD4 | A | 21.07 | 21.1 | 20.90 | 20.42 | 21.02 | −0.60 |
| TD5 | A | 21.01 | 21 | 21.18 | 20.27 | 21.08 | −0.81 |
| TD6 | A | 21.08 | 21.1 | 21.29 | 20.35 | 21.15 | −0.80 |
| TD7 | A | 21.10 | 20.9 | 21.26 | 20.63 | 21.08 | −0.45 |
| TD8 | A | 21.07 | 21.2 | 21.26 | 20.47 | 21.16 | −0.69 |
| TD9 | A | 21.22 | 21.3 | 21.40 | 20.66 | 21.29 | −0.63 |
| TI1 | B | 21.17 | 21.1 | 21.31 | 20.27 | 21.21 | −0.94 |
| TI2 | B | 21.21 | 21.3 | 21.21 | 20.45 | 21.25 | −0.80 |
| TI3 | B | 21.26 | 21.4 | 21.11 | 20.45 | 21.26 | −0.81 |
| TI4 | B | 21.26 | 21.5 | 21.10 | 20.24 | 21.27 | −1.03 |
| Group | n | mean Δ (℃) | SD Δ | t | p |
|---|---|---|---|---|---|
| T. dobrogicus | 9 | −0.66 | 0.18 | −10.99 | p < 0.001 |
| T. ivanbureschi | 4 | −0.90 | 0.11 | −16.35 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihova, D.; Topliceanu, S.; Velkova, V.; Natchev, N. Lethal Heat Exchange—Short-Term Thermoregulation in Two Triturus Species During Abrupt Changes in Living Media (Water vs. Air). Diversity 2025, 17, 691. https://doi.org/10.3390/d17100691
Mihova D, Topliceanu S, Velkova V, Natchev N. Lethal Heat Exchange—Short-Term Thermoregulation in Two Triturus Species During Abrupt Changes in Living Media (Water vs. Air). Diversity. 2025; 17(10):691. https://doi.org/10.3390/d17100691
Chicago/Turabian StyleMihova, Daniela, Sebastian Topliceanu, Valeriya Velkova, and Nikolay Natchev. 2025. "Lethal Heat Exchange—Short-Term Thermoregulation in Two Triturus Species During Abrupt Changes in Living Media (Water vs. Air)" Diversity 17, no. 10: 691. https://doi.org/10.3390/d17100691
APA StyleMihova, D., Topliceanu, S., Velkova, V., & Natchev, N. (2025). Lethal Heat Exchange—Short-Term Thermoregulation in Two Triturus Species During Abrupt Changes in Living Media (Water vs. Air). Diversity, 17(10), 691. https://doi.org/10.3390/d17100691






