Marine Mammals’ Fauna Detection via eDNA Methodology in Pagasitikos Gulf (Greece)
Abstract
1. Introduction
2. Materials and Methods
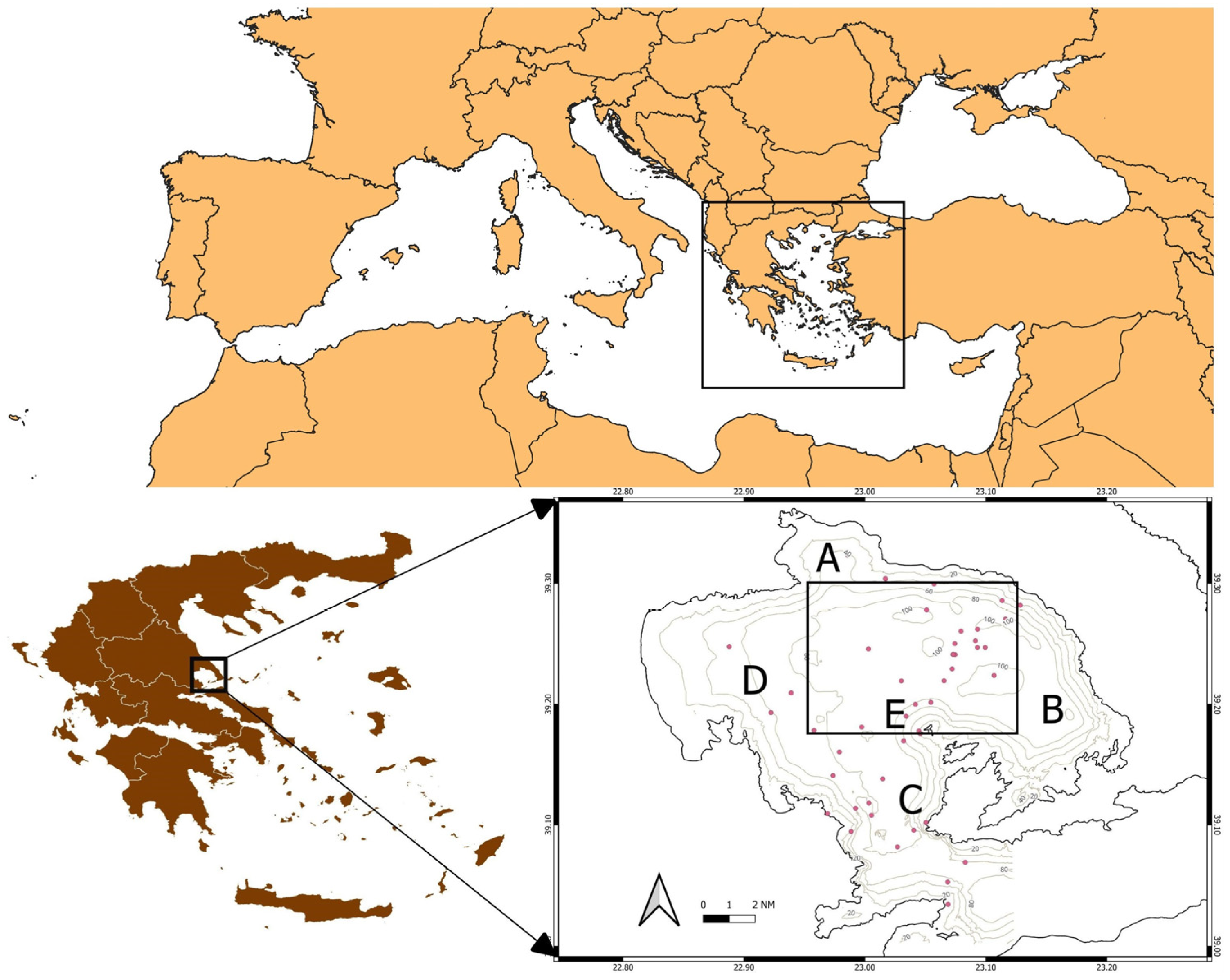
2.1. Water Sampling
2.2. DNA Extraction and PCR Amplification
2.3. Bioinformatics
2.4. Biotic and Abiotic Parameters Assessment
2.5. Statistical Analysis
3. Results
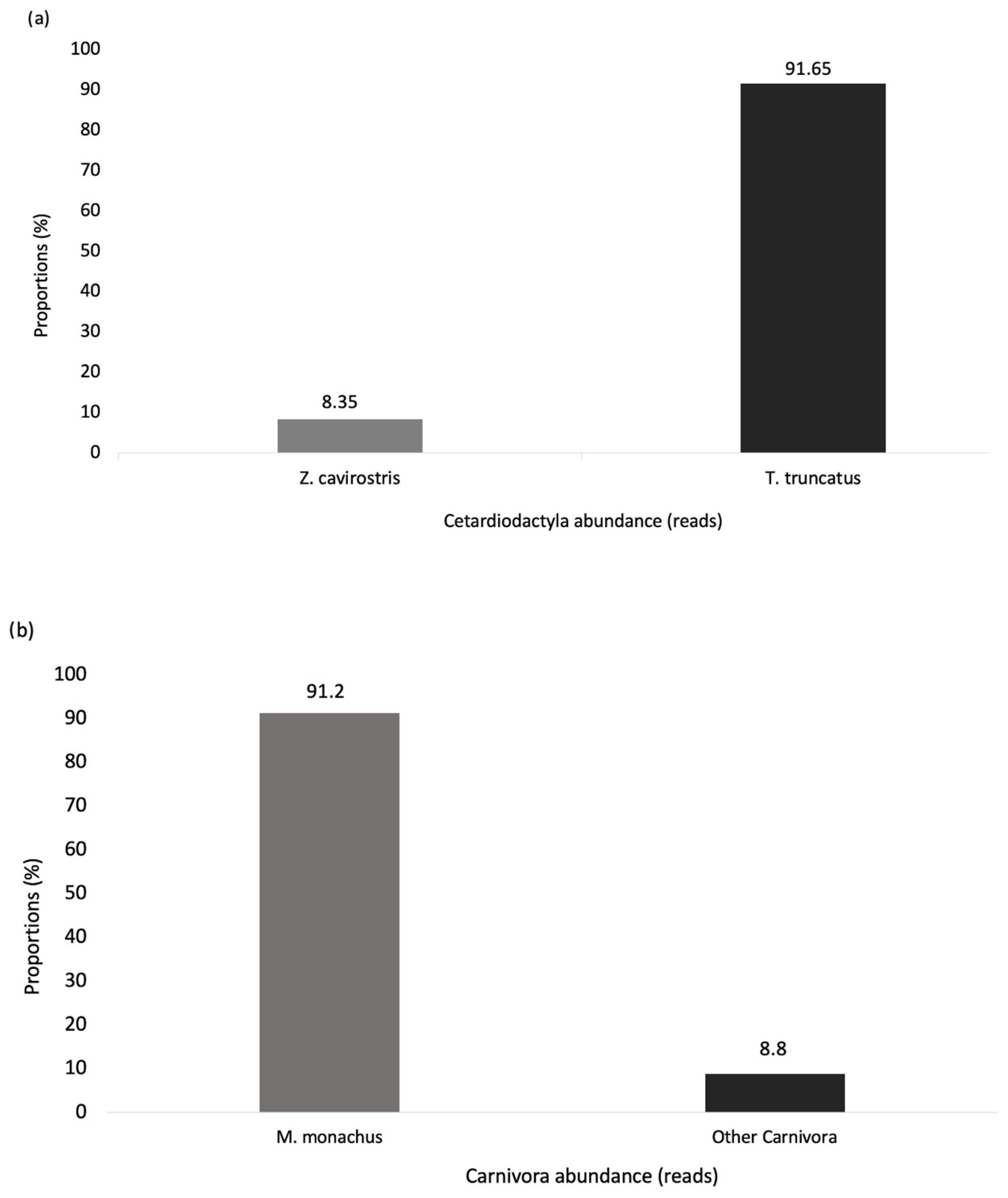
3.1. Overview and Taxa Detected
3.2. Data Categorisation and Statistics
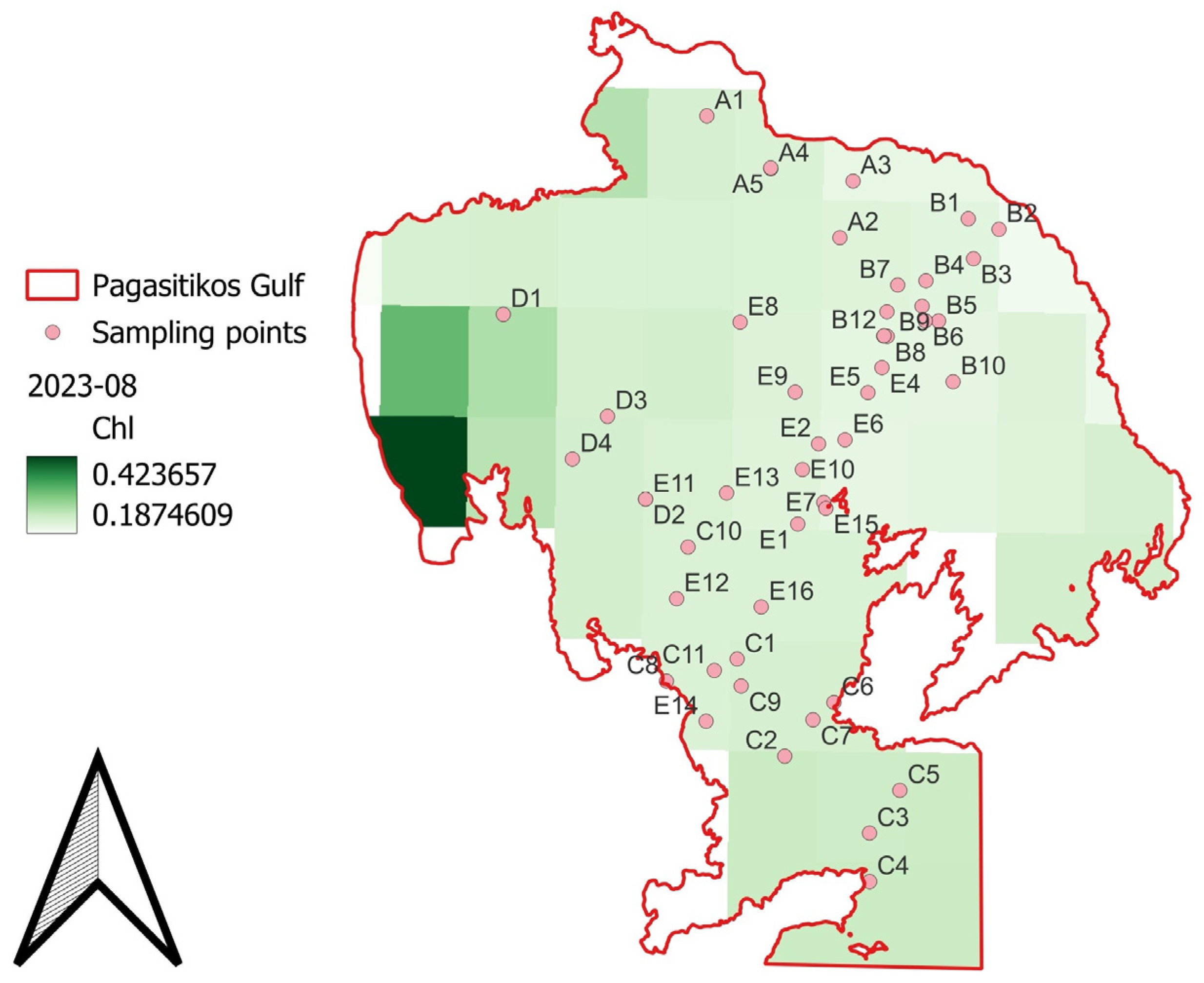
3.3. Biotic and Abiotic Factors
4. Discussion
4.1. Marine Mammals’ Species Identification and Distribution
- (i)
- Distribution of Ziphius cavirostris
- (ii)
- Distribution of Tursiops truncantus
- (iii)
- Correlations between marine mammals’ distribution
4.2. Biotic and Abiotic Factors’ Spatial Distribution
4.3. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plön, S.; Andra, K.; Auditore, L.; Gegout, C.; Hale, P.J.; Hampe, O.; Ramilo-Henry, M.; Burkhardt-Holm, P.; Jaigirdar, A.M.; Klein, L.; et al. Marine mammals as indicators of Anthropocene Ocean Health. NPJ Biodiverse 2024, 3, 24. [Google Scholar] [CrossRef]
- Estes, J.A.; Terborgh, J.; Brashares, J.S.; Power, M.E.; Berger, J.; Bond, W.J.; Carpenter, S.R.; Essington, T.E.; Holt, R.D.; Jackson, J.B.; et al. Trophic downgrading of planet Earth. Science 2011, 333, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Estes, J.A. Ecology. In Encyclopedia of Marine Mammals, 3rd ed.; Würsig, B., Thewissen, J.G.M., Kovacs Kit, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 299–303. [Google Scholar] [CrossRef]
- Roman, J.; McCarthy, J.J. The Whale Pump: Marine mammals enhance primary productivity in a coastal basin. PLoS ONE 2010, 5, e13255. [Google Scholar] [CrossRef]
- Roman, J.; Abraham, A.J.; Kiszka, J.J.; Costa, D.P.; Doughty, C.E.; Friedlaender, A.S.; Hückstädt, L.A.; Marcondes, M.; Wetsel, E.; Pershing, A.J. Migrating baleen whales transport high-latitude nutrients to tropical and subtropical ecosystems. Nat. Commun. 2025, 16, 2125. [Google Scholar] [CrossRef]
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.B.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as marine ecosystem engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef]
- Smith, C.R.; Baco, A.R. Ecology of whale falls at the deep-sea floor. Oceanogr. Mar. Biol. 2003, 41, 311–354. [Google Scholar]
- Pershing, A.J.; Christensen, L.B.; Record, N.R.; Sherwood, G.D.; Stetson, P.B. The impact of whaling on the ocean carbon cycle: Why bigger was better. PLoS ONE 2010, 5, e12444. [Google Scholar] [CrossRef]
- Gilbert, L.; Jeanniard-du-Dot, T.; Authier, M.; Chouvelon, T.; Spitz, J. Composition of cetacean communities worldwide shapes their contribution to ocean nutrient cycling. Nat Commun. 2023, 14, 5823. [Google Scholar] [CrossRef]
- European Commission. Directorate-General for Environment, EU Biodiversity Strategy for 2030: Bringing Nature Back into Our Lives. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:52020DC0380 (accessed on 10 May 2025).
- Papazekou, M.; Dimitriadis, C.; Dalla, D.; Comis, C.M.; Spinos, E.; Vavasis, C.; Kapellaki, K.; Michalopoulou, A.; Valli, A.T.; Barelos, D.; et al. The Ionian Sea in the eastern Mediterranean: Critical year-round habitats for sea turtles and diverse marine megafauna, spanning all life stages and genders. Ocean Coast. Manag. 2024, 251, 107054. [Google Scholar] [CrossRef]
- Williams, R.; Thomas, L.; Ashe, E.; Clark, C.W.; Hammond, P.S. Gauging allowable harm limits to cumulative, sub-lethal effects of human activities on wildlife: A case-study approach using two whale populations. Mar. Policy 2016, 70, 58–64. [Google Scholar] [CrossRef]
- Pirotta, E.; Thomas, L.; Costa, D.P.; Hall, A.J.; Harris, C.M.; Harwood, J.; Kraus, S.D.; Miller, P.J.O.; Moore, M.J.; Photopoulou, T.; et al. Understanding the combined effects of multiple stressors: A new perspective on a longstanding challenge. Sci. Total Environ. 2022, 821, 153322. [Google Scholar] [CrossRef]
- Tett, P.; Gowen, R.J.; Painting, S.J.; Elliott, M.; Forster, R.; Mills, D.K.; Bresnan, E.; Capuzzo, E.; Fernandes, T.F.; Foden, J.; et al. Framework for understanding marine ecosystem health. Mar. Ecol. Prog. Ser. 2013, 494, 1–27. [Google Scholar] [CrossRef]
- Zerbini, A.N.; Waite, J.M.; Durban, J.W.; LeDuc, R.; Dahlheim, M.E.; Wade, P.R. Estimating abundance of killer whales in the nearshore waters of the gulf of Alaska and Aleutian islands using line-transect sampling. Mar. Biol. 2007, 150, 1033–1045. [Google Scholar] [CrossRef]
- Dick, D.M.; Hines, E.M. Using distance sampling techniques to estimate bottlenose dolphin (Tursiops truncatus) abundance at Turneffe Atoll, Belize. Mar. Mammal Sci. 2011, 27, 606–621. [Google Scholar] [CrossRef]
- Hammond, P.S.; Macleod, K.; Berggren, P.; Borchers, D.L.; Burt, L.; Cañadas, A.; Desportes, G.; Donovan, G.P.; Gilles, A.; Gillespie, D.; et al. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013, 164, 107–122. [Google Scholar] [CrossRef]
- Urian, K.; Gorgone, A.; Read, A.; Balmer, B.; Wells, R.S.; Berggren, P.; Durban, J.; Eguchi, T.; Rayment, W.; Hammond, P.S. Recommendations for photo-identification methods used in capture-recapture models with cetaceans. Mar. Mammal Sci. 2015, 31, 298–321. [Google Scholar] [CrossRef]
- Barlow, J.; Ferguson, M.C.; Perrin, W.F.; Ballance, L.; Gerrodette, T.; Joyce, G.; Waring, G. Abundance and densities of beaked and bottlenose whales (family Ziphiidae). J. Cetacean Res. Manag. 2005, 7, 263–270. [Google Scholar] [CrossRef]
- Booth, C.G.; Sinclair, R.R.; Harwood, J. Methods for monitoring for the population consequences of disturbance in marine mammals: A review. Front. Mar. Sci. 2020, 7, 115. [Google Scholar] [CrossRef]
- Suarez-Bregua, P.; Alvarez-González, M.; Parsons, K.M.; Rotllant, J.; Pierce, G.J.; Saavedra, C. Environmental DNA (eDNA) for monitoring marine mammals: Challenges and opportunities. Front. Mar. Sci. 2022, 9, 987774. [Google Scholar] [CrossRef]
- Moura, A.E.; Nielsen, S.C.A.; Vilstrup, J.T.; Moreno-Mayar, J.V.; Gilbert, M.T.P.; Gray, H.W.I.; Natoli, A.; Möller, L.; Hoelzel, A.R. Recent diversification of a marine genus (Tursiops spp.) tracks habitat preference and environmental change. Syst. Biol. 2013, 62, 865–877. [Google Scholar] [CrossRef]
- Moura, A.E.; van Rensburg, C.J.; Pilot, M.; Tehrani, A.; Best, P.B.; Thornton, M.; Plön, S.; de Bruyn, P.J.N.; Worley, K.C.; Gibbs, R.A.; et al. Killer Whale Nuclear Genome and mtDNA Reveal Widespread Population Bottleneck during the Last Glacial Maximum. Mol. Biol. Evol. 2014, 31, 1121–1131. [Google Scholar] [CrossRef]
- Moura, A.E.; Shreves, K.; Pilot, M.; Andrews, K.R.; Moore, D.M.; Kishida, T.; Möller, L.; Natoli, A.; Gaspari, S.; McGowen, M.; et al. Phylogenomics of the genus Tursiops and closely related Delphininae reveals extensive reticulation among lineages and provides inference about eco-evolutionary drivers. Mol. Phylogenet. Evol. 2020, 146, 106756. [Google Scholar] [CrossRef]
- Hoelzel, A.R.; Gkafas, G.A.; Kang, H.; Sarigol, F.; Le Boeuf, B.; Costa, D.P.; Beltran, R.S.; Reiter, J.; Robinson, P.W.; McInerney, N.; et al. Genomics of post-bottleneck recovery in the northern elephant seal. Nat. Ecol. Evol. 2024, 8, 686–694. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Exadactylos, A.; Rogan, E.; Raga, J.A.; Reid, R.; Hoelzel, A.R. Biogeography and temporal progression during the evolution of striped dolphin population structure in European waters. J. Biogeogr. 2017, 44, 2681–2691. [Google Scholar] [CrossRef]
- Hohenlohe, P.A.; Funk, W.C.; Rajora, O.P. Population genomics for wildlife conservation and management. Mol. Ecol. 2021, 30, 62–82. [Google Scholar] [CrossRef]
- van Oppen, M.J.H.; Coleman, M.A. Advancing the protection of marine life through genomics. PLoS Biol. 2022, 20, e3001801. [Google Scholar] [CrossRef]
- Charlton-Robb, K.; Gershwin, L.; Thompson, R.; Austin, J.; Owen, K.; McKechnie, S. A new dolphin species, the Burrunan dolphin Tursiops australis sp. nov., endemic to southern Australian coastal waters. PLoS ONE 2011, 6, e24047. [Google Scholar] [CrossRef]
- Akritopoulou, E.; Exadactylos, A.; Komnenou, A.; Drougas, A.; Sarantopoulou, J.; Gkafas, G.A. Validation of eDNA methodology in marine mammals’ detection: Novel detection of Mediterranean monk seal (Monachus monachus) in Amvrakikos gulf (preliminary results). In Proceedings of the 5th International Congress on Applied Ichthyology, Oceanography, and Aquatic Environment Mytilene, Lesvos, Greece, 30 May–2 June 2024. [Google Scholar]
- Funk, W.C.; Mckay, J.K.; Hohenlohe, P.A.; Allendorf, F.W. Harnessing genomics for delineating conservation units. Trends Ecol. Evol. 2012, 683, 489–496. [Google Scholar] [CrossRef]
- Hostetler, J.A.; Martin, J.; Kosempa, M.; Edwards, H.H.; Rood, K.A.; Barton, S.L.; Runge, M.C. Reconstructing population dynamics of a threatened marine mammal using multiple data sets. Sci. Rep. 2021, 11, 2702. [Google Scholar] [CrossRef]
- Fan, G.; Zhang, Y.; Liu, X.; Wang, J.; Sun, Z.; Sun, S.; Zhang, H.; Chen, J.; Lv, M.; Han, K.; et al. The first chromosome-level genome for a marine mammal as a resource to study ecology and evolution. Mol. Ecol. Resour. 2019, 19, 944–956. [Google Scholar] [CrossRef]
- Stat, M.; Huggett, M.J.; Bernasconi, R.; DiBattista, J.D.; Stephen, B.; Newman, J.; Harvey, S.E.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef]
- Gkafas, G.A.; de Jong, M.; Exadactylos, A.; Raga, J.A.; Aznar, F.J.; Hoelzel, A.R. Sex-specific impact of inbreeding on pathogen load in the striped dolphin. Proc. R. Soc. B 2020, 287, 20200195. [Google Scholar] [CrossRef]
- Hammond, P.S.; Lacey, C.; Gilles, A.; Viquerat, S.; Börjesson, P.; Herr, H.; Macleod, K.; Ridoux, V.; Santos, M.B.; Scheidat, M.; et al. Estimates of Cetacean Abundance in European Atlantic Waters in Summer 2016 from the SCANS-III Aerial and Shipboard Surveys. SCANS-III Project Report 1, 39p. Available online: https://scans3.wp.st-andrews.ac.uk/files/2021/06/SCANS-III_design-based_estimates_final_report_revised_June_2021.pdf (accessed on 14 August 2023).
- Williams, R.; Kelly, N.; Boebel, O.; Friedlaender, A.S.; Herr, H.; Kock, K.-H.; Lehnert, L.S.; Maksym, T.; Roberts, J.; Scheidat, M.; et al. Counting whales in a changing world: Challenges and future directions. Sci. Rep. 2014, 4, 4170. [Google Scholar] [CrossRef]
- Foote, A.D.; Thomsen, P.F.; Sveegaard, S.; Wahlberg, M.; Kielgast, J.; Kyhn, L.A.; Salling, A.B.; Galatius, A.; Orlando, L.; Gilbert, M.T.P. Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of marine mammals. PLoS ONE 2012, 7, e41781. [Google Scholar] [CrossRef]
- Baker, C.S.; Steel, D.; Nieukirk, S.; Klinck, H. Environmental DNA (eDNA) from the wake of the whales: Droplet digital PCR for detection and species identification. Front. Mar. Sci. 2018, 5, 133. [Google Scholar] [CrossRef]
- Akritopoulou, E.; Exadactylos, A.; Komnenou, A.; Sarantopoulou, I.; Kofidou, E.; Drougas, A.; Gkafas, G.A. eDNA analysis revealed the presence of Cuvier’s beaked whale (Ziphius cavirostris) in shallow waters in Greece, Εastern Mediterranean Sea. In Proceedings of the 36th European Cetacean Society Conference, Ponta Delgada, Azores, 12–16 May 2025. [Google Scholar]
- Closek, C.J.; Santora, J.A.; Starks, H.A.; Schroeder, I.D.; Andruszkiewicz, E.A.; Sakuma, K.M.; Bograd, S.J.; Hazen, E.L.; Field, J.C.; Boehm, A.B. Marine vertebrate biodiversity and distribution within the central California current using environmental DNA (eDNA) metabarcoding and ecosystem surveys. Front. Mar. Sci. 2019, 6, 732. [Google Scholar] [CrossRef]
- Berta, A.; Sumich, J.L.; Kovacs, K.M. Marine Mammals: Evolutionary Biology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- McGowen, M.R.; Tsagkogeorga, G.; Alvarez-Carretero, A.; dos Reis, M.; Struebig, M.; Deaville, R.; Jepson, P.D.; Jarman, S.; Polanowski, A.; Morin, P.A.; et al. Phylogenomic resolution of the cetacean tree of life using target sequence capture. Syst. Biol. 2020, 69, 479–501. [Google Scholar] [CrossRef]
- Amaral, A.R.; Beheregaray, L.B.; Bilgmann, K.; Boutov, D.; Freitas, L.; Robertson, K.M.; Sequeira, M.; Stockin, K.A.; Coelho, M.M.; Möller, L.M. Seascape genetics of a globally distributed, highly mobile marine mammal: The short-beaked common dolphin (Delphinus delphis). PLoS ONE 2012, 7, e31482. [Google Scholar] [CrossRef]
- Valsecchi, E.; Bylemans, J.; Goodman, S.J.; Lombardi, R.; Carr, I.; Castellano, L.; Galimberti, A.; Galli, P. Novel universal primers for metabarcoding environmental DNA surveys of marine mammals and other marine vertebrates. Environ. DNA 2020, 2, 460–476. [Google Scholar] [CrossRef]
- Baker, C.S.; Steel, D.; Calambokidis, J.; Falcone, E.; Gonzalez-Peral, U.; Barlow, J.; Burdin, A.M.; Clapham, P.J.; Ford, J.K.B.; Gabriele, C.M.; et al. Strong maternal fidelity and natal philopatry shape genetic structure in North Pacific humpback whales. Mar. Ecol. Prog. Ser. 2013, 494, 291–306. [Google Scholar] [CrossRef]
- Buxton, A.; Matechou, E.; Griffin, J. Optimising sampling and analysis protocols in environmental DNA studies. Sci. Rep. 2021, 11, 11637. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Kielgast, J.; Iversen, L.L.; Wiuf, C.; Rasmussen, M.; Gilbert, M.T.P.; Orlando, L.; Willerslev, E. Monitoring endangered freshwater biodiversity using environmental DNA. Mol. Ecol. 2012, 21, 2565–2573. [Google Scholar] [CrossRef]
- Yates, M.C.; Furlan, E.; Thalinger, B.; Yamanaka, H.l; Bernatchez, L. Beyond species detection—leveraging environmental DNA and environmental RNA to push beyond presence/absence applications. Environ. DNA 2023, 5, 829–835. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jacobsen, L. Identification of mustelid species: Otter (Lutra lutra), American mink (Mustela vison) and polecat (Mustela putorius), by analysis of DNA from faecal samples. J. Zool. 1999, 247, 177–181. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Ma, H.; Stewart, K.; Lougheed, S.; Zheng, J.; Wang, Y.; Zhao, J. Characterization, optimization, and validation of environmental DNA (eDNA) markers to detect an endangered aquatic mammal. Conserv. Genet. Resour. 2016, 8, 561–568. [Google Scholar] [CrossRef]
- Valsecchi, E.; Coppola, E.; Pires, R.; Parmegiani, A.; Casiraghi, M.; Galli, P.; Bruno, A. A species-specific qPCR assay provides novel insight into range expansion of the Mediterranean monk seal (Monachus monachus) by means of eDNA analysis. Biodivers. Conserv. 2022, 31, 1175–1196. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: Detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- Moura, A.E.; Sillero, N.; Rodrigues, A.; Natoli, A.; Möller, L.M.; Hoelzel, A.R. Delphinus species delimitation and phylogenetics revisited with genome-wide SNPs and morphology. Syst. Biol. 2020, 69, 910–927. [Google Scholar] [CrossRef]
- Aubrie, B.; Onoufriou, A.; Gaggiotti, O.E.; Aguilar de Soto, N.; McCarthy, M.L.; Morin, P.A.; Rosso, M.; Dalebout, M.; Davison, N.; Baird, R.W.; et al. Biogeography in the deep: Hierarchical population genomic structure of two beaked whale species. Glob. Ecol. Conserv. 2022, 40, e02308. [Google Scholar] [CrossRef]
- Cunha, H.A.; de Castro, R.L.; Secchi, E.R.; Crespo, E.A.; Lailson-Brito, J.; Azevedo, A.F.; Lazoski, C.; Solé-Cava, A.M. Molecular and Morphological Differentiation of Common Dolphins (Delphinus sp.) in the Southwestern Atlantic: Testing the Two Species Hypothesis in Sympatry. PLoS ONE 2015, 10, e0140251. [Google Scholar] [CrossRef]
- Pinfield, R.; Dillane, E.; Runge, A.K.W.; Evans, A.; Mirimin, L.; Niemann, J.; Reed, T.E.; Reid, D.G.; Rogan, E.; Samarra, F.I.P.; et al. False-negative detections from environmental DNA collected in the presence of large numbers of killer whales (Orcinus orca). Environ. DNA 2019, 1, 316–328. [Google Scholar] [CrossRef]
- Nybakken, J.W. Marine Biology: An Ecological Approach, 4th ed.; Addison-Wesley Educational Publishers Inc.: Menlo Park, CA, USA, 1997; pp. 1–481. [Google Scholar]
- Raitsos, D.E.; Korres, G.; Triantafyllou, G.; Petihakis, G.; Pantazi, M.; Tsiaras, K.; Pollani, A. Assessing chlorophyll variability in relation to the environmental regime in Pagasitikos Gulf, Greece. J. Mar. Syst. 2012, 94, 16–22. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Keramidas, I.; Tsagarakis, K.; Tsikliras, A.C. Ecosystem Models and Effort Simulations of an Untrawled Gulf in the Central Aegean Sea. Front. Mar. Sci. 2019, 6, 648. [Google Scholar] [CrossRef]
- Pardalis, S.V.; Komnenou, A.; Exadactylos, A.; Gkafas, G.A. Small Scale Fisheries, Dolphins and Societal Challenges: A Case Study in the City of Volos, Greece. Conservation 2021, 1, 7. [Google Scholar] [CrossRef]
- Fabinyi, M.; Dressier, W.; Pido, M. Fish, Trade and Food Security: Moving beyond ‘Availability’ Discourse in Marine Conservation. Hum. Ecol. 2017, 45, 177–188. [Google Scholar] [CrossRef]
- ELSTAT. Available online: https://www.statistics.gr/el/statistics/-/publication/SPA03/- (accessed on 10 May 2025).
- Petihakis, G.; Triantafyllou, G.; Pollani, A.; Koliou, A.; Theodorou, A. Field data analysis and application of a complex water column biogeochemical model in different areas of a semi-enclosed basin: Towards the development of an ecosystem management tool. Mar. Environ. Res. 2005, 59, 493–518. [Google Scholar] [CrossRef]
- Bousbouras, G.; Angelidis, P. Hydrodynamic simulation of the Pagasitikos gulf, Greece. CWEEE 2024, 13, 58–85. [Google Scholar] [CrossRef]
- Voulgaris, K.; Vafidis, D. Specific oceanographic conditions reflect meiofaunal communities: The case of a semi-enclosed gulf (Pagasitikos Gulf, Eastern Mediterranean). Cont. Shelf Res. 2025, 289, 105470. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W. The Basic Polymerase Chain Reaction. In Cold Spring Harbor Protocols; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC 2009, 10, 421. [Google Scholar] [CrossRef]
- Copernicus Marine Services. Available online: https://marine.copernicus.eu/access-data (accessed on 20 February 2025).
- Bilgmann, K.; Parra, G.J.; Holmes, L.; Peters, K.J.; Jonsen, I.D.; Möller, L.C. Abundance estimates and habitat preferences of bottlenose dolphins reveal the importance of two gulfs in South Australia. Sci. Rep. 2019, 9, 8044. [Google Scholar] [CrossRef]
- Evans, K.; Thresher, R.; Warneke, R.M.; Bradshaw, C.J.A.; Pook, M.; Thiele, D.; Hindell, M.A. Periodic variability in cetacean strandings: Links to large-scale climate events. Biol. Lett. 2005, 1, 147–150. [Google Scholar] [CrossRef]
- Simmonds, M.P.; Isaac, S.J. The impacts of climate change on marine mammals: Early signs of significant problems. Oryx 2007, 41, 19–26. [Google Scholar] [CrossRef]
- Evans, P.G.H.; Bjørge, A. Impacts of climate change on marine mammals. MCCIP Sci. Rev. 2013, 2013, 134–148. [Google Scholar]
- Brakes, P.; Dall, S.R.X. Marine Mammal Behavior: A Review of Conservation Implications. Front. Mar. Sci. 2016, 3, 87. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi. (Version 2.6) [Computer Software]. 2024. Available online: https://www.jamovi.org (accessed on 27 June 2024).
- R Core Team. R: A Language and Environment for Statistical Computing. (Version 4.4) [Computer Software]. 2024. Available online: https://cran.r-project.org (accessed on 25 June 2024).
- Klaoudatos, D.; Vardali, S.; Apostologamvrou, C.; Lolas, A.; Neofitou, N.; Conides, A.; Gkafas, G.A.; Sarantopoulou, J.; Kolindrini, D.; Roditi, K.; et al. Ecological Assessment of Fishery Communities in an Otter-Trawl-Restricted, Semi-Enclosed Gulf in Greece. J. Mar. Sci. Eng. 2023, 11, 1668. [Google Scholar] [CrossRef]
- Klaoudatos, D.; Vlachou, M.; Theocharis, A. From Data to Insight: Machine Learning Approaches fornFish Age Prediction in European Hake. J. Mar. Sci. Eng. 2024, 12, 1466. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Hatziioannou, M.; Malandrakis, E.E.; Tsigenopoulos, C.S.; Karapanagiotidis, I.T.; Mente, E.; Vafidis, D.; Exadactylos, A. Heterozygosity fitness correlations and generation interval of the Norway lobster in the Aegean Sea, eastern Mediterranean. J. Biol. Res. 2019, 26, 14. [Google Scholar] [CrossRef]
- Frantzis, A.; Alexiadou, P.; Paximadis, G.; Politi, E.; Gannier, A.; Corsini-Foka, M. Current knowledge of the cetacean fauna of the Greek Seas. J. Cetacean Res. Manag. 2003, 5, 219–232. [Google Scholar] [CrossRef]
- Baş, A.A.; Erdogan, M.A.; Morris, N.R.C.; Yeoman, K.; Humphrey, O.; Gaggioli, E.; Roland, C. Seasonal encounter rates and residency patterns of an unstudied population of bottlenose dolphin (Tursiops truncatus) in the northwestern Levantine Sea, Turkey. Hyla 2016, 1, 1–13. [Google Scholar]
- Bearzi, G.; Bonizzoni, S.; Santostasi, N.L.; Furey, N.B.; Eddy, L.; Valavanis, V.D.; Gimenez, O. Dolphins in a scaled-down Mediterranean: The Gulf of Corinth’s odontocetes. In Advances in Marine Biology; Notarbartolo di Sciara, G., Podestà, M., Curry, B.E., Eds.; Academic Press: Oxford, UK, 2016; Volume 75, pp. 297–331. [Google Scholar]
- Milani, C.B.; Vella, A.; Vidoris, P.; Christidis, A.; Koutrakis, E.; Sylaios, G.; Kallianiotis, A. Encounter rate and relative abundance of bottlenose dolphins and distribution modelling of main cetacean species in the North Aegean Sea (Greece). J. Black Sea/Medit. Environ. 2017, 23, 101–120. [Google Scholar]
- Arcangeli, A.; Atzori, F.; Azzolin, M.; Babey, L.; Campana, I.; Carosso, L.; Crosti, R.; Garcia-Garin, O.; Gregorietti, M.; Orasi, A.; et al. Testing indicators for trend assessment of range and habitat of low-density cetacean species in the Mediterranean Sea. Front. Mar. Sci. 2023, 10, 1116829. [Google Scholar] [CrossRef]
- Gnone, G.; Bellingeri, M.; Dhermain, F.; Dupraz, F.; Nuti, S.; Bedocchi, D.; Moulins, A.; Rosso, M.; Alessi, J.; Mccrea, R.S.; et al. Distribution, abundance, and movements of the bottlenose dolphin (Tursiops truncatus) in the Pelagos Sanctuary MPA (north-west Mediterranean Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 372–388. [Google Scholar] [CrossRef]
- Cañadas, A.; Aguilar de Soto, N.; Aissi, M.; Arcangeli, A.; Azzolin, M.; Nagy, A.B.; Bearzi, G.; Campana, I.; Chicote, C.; Cotte, R.; et al. The challenge of habitat modelling for threatened low density species using heterogeneous data: The case of Cuvier’s beaked whales in the Mediterranean. Ecol. Indic. 2018, 85, 128–136. [Google Scholar] [CrossRef]
- Hostetter, P.; Koroza, A.; Tsimpidis, T.; Pietroluongo, G.; Carlucci, R.; Cipriano, G. Occurrence of Physeter macrocephalus and Ziphius cavirostris in the North Ikaria Basin, Aegean Sea. In Proceedings of the IMEKO TC-19 International Workshop on Metrology for the Sea, Naples, Italy, 5–7 October 2020. [Google Scholar]
- D’Amico, A.; Bergamasco, A.; Zanasca, P.; Carniel, S.; Nacini, E.; Portunato, N.; Teloni, V.; Mori, C.; Barbanti, R. Qualitative correlation of marine mammals with physical and biological parameters in the Ligurian Sea. IEEE J. Ocean. Eng. 2003, 28, 29–43. [Google Scholar] [CrossRef]
- Podesta, M.; Azzellino, A.; Canadas, A.; Frantzis, A.; Moulins, A.; Rosso, M.; Tepsich, P.; Lanfredi, C. Cuvier’s beaked whale, Ziphius cavirostris, distribution and occurrence in the Mediterranean Sea: High-use areas and conservation threats. Adv. Mar. Biol. 2016, 75, 103–140. [Google Scholar] [CrossRef]
- Azzellino, A.; Gaspari, S.; Airoldi, S.; Nani, B. Habitat use and preferences of cetaceans along the continental slope and the adjacent pelagic waters in the western Ligurian Sea. Deep Res. Part I Oceanogr. Res. Pap. 2008, 55, 296–323. [Google Scholar] [CrossRef]
- Gkafas, G.A.; Orfanidis, S.; Vafidis, D.; Panagiotaki, P.; Küpper, F.C.; Exadactylos, A. Genetic diversity and structure of Cymodocea nodosa meadows. Appl. Ecol. Environ. Res. 2015, 14, 145–160. [Google Scholar] [CrossRef]
- Petihakis, G.; Triantafyllou, G.; Koliou, A.; Theodorou, A. Exploring the dynamics of a marine ecosystem (Pagasitikos Gulf, Western Aegean, Greece) through the analysis of temporal and spatial variability of nutrients. In Proceedings of the 6th International Symposium on Coastal Zone Research, Management and Planning, Porto, Portugal, 22–26 September 2002. [Google Scholar]
- Petihakis, G. Hydrodynamic and Ecological Simulation of Pagasitikos Gulf Ecosystem. Ph.D. Thesis, University of Thessaly, Volos, Greece, 2004. [Google Scholar]
- Boldrocchi, G.; Conte, L.; Galli, P.; Bettinetti, R.; Valsecchi, E. Cuvier’s beaked whale (Ziphius cavirostris) detection through surface-sourced eDNA: A promising approach for monitoring deep-diving cetaceans. Ecol. Indic. 2024, 161, 111966. [Google Scholar] [CrossRef]
- Blanco, C.; Raga, J.A. Cephalopod prey of two Ziphius cavirostris (Cetacea) stranded on the western Mediterranean coast. J. Mar. Biol. Assoc. UK 2000, 80, 381–382. [Google Scholar] [CrossRef]
- Valenzuela, L.O.; Sironi, M.; Rowntree, V.J.; Seger, J. Isotopic and genetic evidence for culturally inherited site fidelity to feeding grounds in southern right whales (Eubalaena australis). Mol. Ecol. 2009, 18, 782–791. [Google Scholar] [CrossRef]
- Richard, G.; Titova, O.V.; Fedutin, I.D.; Steel, D.; Meschersky, I.G.; Hautin, M.; Burdin, A.M.; Hoyt, E.; Filatova, O.A.; Jung, J.L. Cultural Transmission of Fine-Scale Fidelity to Feeding Sites May Shape Humpback Whale Genetic Diversity in Russian Pacific Waters. J. Hered. 2018, 109, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Gómez de Segura, A.; Crespo, E.A.; Pedraza, S.N.; Hammond, P.S.; Raga, J.A. Abundance of small cetaceans in waters of the central Spanish Mediterranean. Mar. Biol. 2006, 150, 149–160. [Google Scholar] [CrossRef]
- Miokovic, D.; Kovacic, D.; Pribanic, S. Stomach content analysis of one bottlenose dolphin (Tursiops truncatus, Montague 1821) from the Adriatic Sea. Nat. Croat. 1999, 8, 61–65. [Google Scholar]
- Tsitsika, E.V.; Maravelias, C.D. Factors Affecting Purse Seine Catches: An Observer-Based Analysis. Mediterr. Mar. Sci. 2006, 7, 27–40. [Google Scholar] [CrossRef][Green Version]
- Bearzi, G.; Fortuna, C.M.; Randall, R.R. Ecology and conservation of common bottlenose dolphin Tursiops truncatus in the Mediterranean Sea. Mammal. Rev. 2008, 39, 92–123. [Google Scholar] [CrossRef]
- Borrell, A.; Vighi, M.; Genov, T.; Giovos, I.; Gonzalvo, J. Feeding ecology of the highly threatened common bottlenose dolphin of the Gulf of Ambracia, Greece, through stable isotope analysis. Mar. Mammal Sci. 2021, 37, 98–110. [Google Scholar] [CrossRef]
- Benmessaoud, R.; Chaeib, O.; Cherif, M.; Kouched, W.; Troudi, D.; Rjeibi, M.; Bejaoui, N.; Missaoui, H. Diet of Bottlenose dolphins (Tursiops truncatus) from the Tunisian coasts: Insights from stomach content analyses. In Proceedings of the Sixième Conférence sur la Conservation des Cétacés dans les Pays du Sud de la Méditerranée (CSMC6), Monastir, Tunisie, 13–15 November 2023. [Google Scholar]
- Bailey, H.; Thompson, P. Effect of oceanographic features on fine-scale foraging movements of bottlenose dolphins. Mar. Ecol. Prog. Ser. 2010, 418, 223. [Google Scholar] [CrossRef]
- Neri, A.; Sartor, P.; Voliani, A.; Mancusi, C.; Marsili, L. Diet of Bottlenose Dolphin, Tursiops truncatus (Montagu, 1821), in the Northwestern Mediterranean Sea. Diversity 2023, 15, 21. [Google Scholar] [CrossRef]
- Akritopoulou, E.; Koitsanou, E.; Dimou, E.; Mpanias, I.; Oikonomidou, Z.; Komnenou, A.; Exadactylos, A.; Gkafas, G.A. A citizen science study on marine mammals in Pagasitikos Gulf (Greece); preliminary results. In Proceedings of the Hellenic Center for Marine Research, Marine and Inland Waters Research Symposium, Porto Heli, Greece, 16–19 September 2022. [Google Scholar]
- Valsecchi, E.; Arcangeli, A.; Lombardi, R.; Boyse, E.; Carr, I.M.; Galli, P.; Goodman, S.J. Ferries and environmental DNA: Underway sampling from commercial vessels provides new opportunities for systematic genetic surveys of marine biodiversity. Front. Mar. Sci. 2021, 8, 704786. [Google Scholar] [CrossRef]
- Szekely, D.; Corfixen, N.L.; Mørch, L.L.; Knudsen, S.W.; McCarthy, M.L.; Teilmann, J.; Heide-Jørgensen, M.P.; Olsen, M.T. Environmental DNA captures the genetic diversity of bowhead whales (Balaena mysticetus) in West Greenland. Environ. DNA 2021, 3, 248–260. [Google Scholar] [CrossRef]
- Dimoudi, A.; Voulgaris, K.; Varkoulis, A.; Georgiou, K.; Klaoudatos, D.; Skordas, K.; Vafidis, D.; Neofitou, N. The impacts of “Daniel” and “Elias” storms on water quality of Pagasitikos Gulf—A first record. In Proceedings of the 5th International Congress on Applied Ichthyology, Oceanography, and Aquatic Environment Mytilene, Lesvos, Greece, 30 May–2 June 2024. [Google Scholar]
- The National Observatory of Athens. Available online: https://www.noa.gr/en/ (accessed on 30 September 2023).
- Henson, S.A.; Cole, H.S.; Hopkins, J.; Martin, A.P.; Yool, A. Detection of climate change–driven trends in phytoplankton phenology. Glob. Change Biol. 2018, 24, e101–e111. [Google Scholar] [CrossRef] [PubMed]
- Siokou, I.; Anagnostou, C.; Catsiki, V.A.; Gotsis-Skretas, O.; Hatzianestis, I.; Kontoyiannis, H.; Krassakopoulou, E.; Panayotidis, P.; Papadopoulos, V.; Pavlidou, A.; et al. The marine ecosystem and the anthropogenic impacts in the South Evoikos Gulf: Central Aegean Sea. In The Handbook of Environmental Chemistry; Barceló, D., Kostianoy, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–32. [Google Scholar] [CrossRef]
- Rey-Iglesia, A.; Gaubert, P.; Themudo, G.E.; Pires, R.; de la Fuente, C.; Freitas, L.; Aguilar, A.; Borrell, A.; Krakhmalnaya, T.; Vasconcelos, R.; et al. Mitogenomics of the endangered Mediterranean monk seal reveals dramatic loss of diversity and supports historical gene-flow between Atlantic and eastern Mediterranean populations. Zool. J. Linn. Soc. 2021, 191, 1147–1159. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R. The ecology of environmental DNA and implications for conservation genetics. Conserv. Genet. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Smith-Root, Vancouver, USA. Available online: https://www.smith-root.com/ (accessed on 12 February 2022).

| YEAR | AREA | READSMon | READSTur | READSZiph | READST | |
|---|---|---|---|---|---|---|
| YEAR | 1.00 | 0.869 | 0.667 | 0.954 | 0.363 | 0.601 |
| AREA | 0.869 | 1.00 | 0.508 | 0.151 | 0.303 | 0.304 |
| READSMon | 0.667 | 0.508 | 1.00 | 0.415 | 0.022 * | 0.039 * |
| READSTur | 0.954 | 0.151 | 0.415 | 1.00 | 0.314 | 0.568 |
| READSZiph | 0.363 | 0.303 | 0.022 * | 0.314 | 1.00 | 0.083 |
| READST | 0.601 | 0.304 | 0.039 * | 0.568 | 0.083 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akritopoulou, E.; Exadactylos, A.; Komnenou, A.; Sarantopoulou, J.; Domenikiotis, C.; Gkafas, G.A. Marine Mammals’ Fauna Detection via eDNA Methodology in Pagasitikos Gulf (Greece). Diversity 2025, 17, 692. https://doi.org/10.3390/d17100692
Akritopoulou E, Exadactylos A, Komnenou A, Sarantopoulou J, Domenikiotis C, Gkafas GA. Marine Mammals’ Fauna Detection via eDNA Methodology in Pagasitikos Gulf (Greece). Diversity. 2025; 17(10):692. https://doi.org/10.3390/d17100692
Chicago/Turabian StyleAkritopoulou, Elena, Athanasios Exadactylos, Anastasia Komnenou, Joanne Sarantopoulou, Christos Domenikiotis, and Georgios A. Gkafas. 2025. "Marine Mammals’ Fauna Detection via eDNA Methodology in Pagasitikos Gulf (Greece)" Diversity 17, no. 10: 692. https://doi.org/10.3390/d17100692
APA StyleAkritopoulou, E., Exadactylos, A., Komnenou, A., Sarantopoulou, J., Domenikiotis, C., & Gkafas, G. A. (2025). Marine Mammals’ Fauna Detection via eDNA Methodology in Pagasitikos Gulf (Greece). Diversity, 17(10), 692. https://doi.org/10.3390/d17100692











