Abstract
Body condition is a standard measure of the individual fitness and health status in many animal species and is typically estimated by calculating the body condition indices (BCIs). The present study used capture/recapture data and the BCIs to test whether the activity (number of times an individual has been recaptured) of adult T. ivanbureschi was related to individual body condition. For three consecutive seasons, we set funnel traps in a temporary pond near Sofia, Bulgaria. A ventral pattern was used for individual identification, and the linear regression of lnMass/lnSVL was used for BCI calculation. The overall recapture rate for the population was 52.52%, with males recaptured more often than females. Activity and estimated population size varied across seasons. Body condition generally decreased towards the end of the aquatic phase in all years, with females consistently maintaining higher BCIs than males. There was no relationship between mean BCI per session and population activity for either sex, but individual BCI scores were correlated with individual activity, and this relationship was independent of both sex and temperature. The results suggest that winter activity may carry energetic costs later in the season and highlight potential sex-based differences in aquatic behavior.
1. Introduction
More than 40% of all known amphibian species are currently in decline [1]. Due to their ectothermic nature and complex life cycles, amphibians are strongly dependent on the ambient temperature, moisture and precipitation—factors that are fundamentally affected by climate change [2]. Although warming of the cooler areas might create new opportunities for colonization, most species are expected to shrink their range as climate change effects become more pronounced [3]. Assessing the implications of climate change-driven shifts in phenology is crucial for understanding the consequences of climate change, but it is often challenging to link that to the fitness of the individuals [4]. There still remains a major gap in understanding how climate-induced shifts in environmental conditions during winter affect the physiological state and activity levels of amphibians, particularly through changes in body condition.
Body condition is an important relevant measure that has been commonly used in wildlife conservation biology for the past few decades [5]. It is often used to examine the effects of different biotic and abiotic factors on the health and fitness at an individual or population level, and it is related to aspects such as survival and reproductive success [6]. Since the actual energy reserves of an individual are difficult to estimate without very invasive techniques or the outright sacrifice of the animal [7], a common approach is to use the relationship between body mass (M) and length (L) to calculate the body condition indices (BCIs) [8].
There is a wide variety of different BCIs, but two commonly and widely used approaches are the ratio and residual BCIs. The ratio-based approach has the advantage of simplicity and easier calculation, but it is strongly correlated to body size, limiting the comparison to individuals of similar size. The residuals approach calculates BCI from the linear regression of mass on length, minimizing the effect of body size [9]; it requires data to be linear, homoscedastic and with normal distribution [10]. In amphibians, the body condition is known to reflect environmental changes, particularly with the onset of the breeding season, which may affect both survival and fecundity [11]. Maintaining a good body condition is of fundamental importance to population persistence [12], and although non-hibernating individuals may benefit from limited exposure to harsh conditions [2], warmer winters could lead to increased metabolism and body fat depletion [13]. As shifts in temperature and seasonal timing alter the duration and intensity of activity periods, body condition may serve as a physiological indicator of how individuals are coping with these rapid environmental changes.
Understanding these dynamics in a species with distinct aquatic and terrestrial phases that also exhibits unusual winter activity offers a unique opportunity to study the relationship between seasonal changes, body condition and behavior. Recent data have demonstrated persistent winter activity and ceasing hibernation in the Buresch crested newt, Triturus ivanbureschi Arntzen and Wielstra, 2013 [14]. This behavioral shift deviates from the typical hibernation behavior seen in most temperate amphibians and presents a valuable opportunity to examine whether continuous winter activity is related to body condition. In the present study, we documented the change in the body condition of captured active newts from the onset of winter until the desiccation of the pond, and we investigated whether it has any correlation to newt activity on both a population and individual level. We expected that newts with higher BCI would display a higher level of activity, as well as having more recaptures during the trapping sessions.
2. Materials and Methods
The Buresch crested newt belongs to the T. karelinii group and has a large, sturdy body, a relatively short aquatic phase, and its range spreads from the Southeastern Balkan peninsula to Western Anatolia [15]. In Bulgaria, it is distributed from 0 to 1700 m a.s.l. across most of the country and occurs in various types of stagnant or slow-flowing water bodies; it preys on both aquatic and terrestrial invertebrates and small vertebrates [16]. The breeding season is March–May, although breeding activity has been recorded as early as January [14].
The study took place in a small temporary pond (less than 20 m in diameter) located near the village of Bistritsa, Bulgaria (42.595 N, 23.368 E; 800 m a.s.l.). For the period 2021–2024, funnel traps were set periodically from December to August; traps were set in the evening and collected the following morning. We began our study in December because, at this time, the pond fills with water and newts are expected to return to the site after the autumn drought (August–November). Trapping sessions depended on researchers’ availability and weather conditions, so they were not equally spaced and time between consecutive sessions varied between one and two weeks. The number of traps depended on the water level and varied between 4 (in December, water depth around 30 cm) and 30 (during peak breeding season, water depth of more than 100 cm). In the presence of an ice sheet covering the pond (during the winter months), we used a pickaxe to break the ice and set up the traps in the resulting holes (Figure 1). We photographed the unique ventral pattern of all captured individuals against a uniform background and measured the snout–vent length (SVL) to the nearest 0.5 mm and body mass with accuracy of 0.01 g using a digital scale; when the number of captured newts exceeded 40, only the first 20 males and females were measured. We calculated the size dimorphism index (SDI) following the formula from [17], as follows: [(larger sex/smaller sex) − 1], arbitrarily set to positive when males are larger and negative when females are larger. After photographs and measurements were taken, all collected newts were released at the site of capture. The HotSpotter software v. 1.0 [18] was used to compare photographs of the ventral patterns of individuals and identify recaptures. This method has been demonstrated to work well for amphibians and newt species in particular [19,20], and unlike other marking techniques, such as toe-clipping, our method is non-invasive [21].

Figure 1.
(A) Trap session on 11 February 2022; a total of 19 traps were set, with 2–4 traps in each hole under the ice—one directly under the hole and the others in opposite directions at about 2 m from the first (pushed with a long pole), tied on long cords ending with a float (a plastic bottle); on the left—a female, one of the 11 adult newts captured in the session. (B) Trap session on 26 May 2022; a total of 30 traps were set, using floats (plastic bottles) to prevent the drowning of frogs, toads, turtles and snakes, which were also active at that time; on the left—a male, one of 18 adult newts captured in this session.
To investigate changes in BCI over time, we used a linear mixed model (LMM). We included sex and time (in days after the start of the study period) as predictors and individual BCI as the response variable. All BCI scores were calculated using the residuals from the regression line of ln body mass versus ln SVL. Since we hypothesized that there may be a non-linear relationship between BCI and time, we included a polynomial term for time in the model. Additionally, we controlled for individual differences by including newt identity as a random term in the model, which accounts for repeated measures on the same individuals over time.
To test for a difference in BCI between males and females, we included an interaction term between sex and time, as it was expected that the relationship between BCI and time might differ between sexes. Statistical significance for the fixed effects was assessed using the lmerTest package (v. 3.1-3, available at https://github.com/runehaubo/lmerTestR, accessed on 5 April 2025).
We estimated the recapture rates for males and females in the different study periods and tested for significant differences between the sexes using a Chi-square test.
To examine the relationship between BCI and newt activity, we created the following two models: one looking at individual activity (estimated as the total number of times an individual has been captured) and the second looking at population activity (measured by the number of captured newts per trap in each sampling session). The practice of using individuals per trap (or trap-hour) for relative abundance estimates has been regularly applied in studies on newt population traits [22], and comparing these numbers across different sessions is a relatively robust way of understanding population activity. For the individual activity model, the response variable was the number of captures of a focal individual, and the predictors were its relative BCI (rBCI) and sex. The rBCI was calculated by extracting the variation explained by the random term (newt identity) in the aforementioned LMM, which reflects how an individual’s BCI deviates from the predicted population BCI. For the population activity model, we created a Negative Binomial Model (GLM) fitting the mean BCI per each session and sex as the predictor and capture success as the response.
To explore whether water temperature influenced capture success, we included it as a predictor in the Negative Binomial GLM. We did not include air temperature in the model because it was strongly correlated with water temperature (Pearson’s correlation, correlation estimate = 0.87, p < 0.001).
To assess seasonal activity patterns, we used a Generalized Linear Model (GLM) to compare the number of captures per trap throughout the year. We also tested whether the activity patterns differed by sex and whether the female-to-male ratio changed during the season using linear regression.
The individual recognition allowed us to estimate the active population size for each season during the study period. We used the Jolly–Seber model for open population as it accounts for the probability of a newt entering the pond during the study [23]. The lowest value for the Akaike’s Information Criterion (AIC) was used to determine the model’s fit and the model notations were as follows: survival rate (Phi), capture probability (p), probability of entrance (b) and population size (N).
All analyses were performed in R v. 4.2.1 [24], using various statistical models to explore how different variables influenced the BCI and recapture rates. All model assumptions were checked using residual plots.
3. Results
3.1. Population Statistics
In this study, we captured a total of 1765 individuals and measured 1458 of them. The recapture rate for the whole population was 52.52% (see the Supplementary File). The total number of captures and recapture rate of males and females in the different study periods are presented in Table 1. Overall, males were recaptured more often than females, with a recapture rate of 40.5% for females and 59% for males (χ2(1) = 54.06, p < 0.00001). The population estimates varied across seasons, with a low of 449 for the first year, a peak of 635 for the second, followed by a slight decline to 586 for the third year of the study (numbers of males and females combined); capture probability was markedly higher for males, while the probability of entrance and survival rate were approximately equal for both sexes across the three years (Table 1). There was a pronounced sexual dimorphism, with females being slightly larger and heavier (Table 1).

Table 1.
Total number of captures, recapture rate, mean SVL and mass, calculated SDI and estimated population size by sex and season. TC—total count; R—recaptured; NR—not recaptured; RR—recapture rate; N—population size; p—capture probability; b—probability of entrance; Phi—survival rate; measurements and estimates are presented as the Mean ± SE (Min–Max).
3.2. BCI Change
The body condition index (BCI) changed during the periods of activity in the seasons of 2021–2022, 2022–2023 and 2023–2024. The pattern differed between seasons (Likelihood Ratio, χ2(2) = 32.03, p < 0.001) and for males and females (Likelihood Ratio, χ2(1) = 127.37, p < 0.001), but the BCI generally decreased towards the end of the season (Figure 2). Females tend to have higher BCIs than males (LMM, t = −12.66, p < 0.001). We also found that the mean BCI per session for both females and males decreased with rising water temperature (linear regression, t = −8.76, p < 0.001). The effect was less pronounced for males than it was for females (linear regression, t = 4.45, p < 0.001).
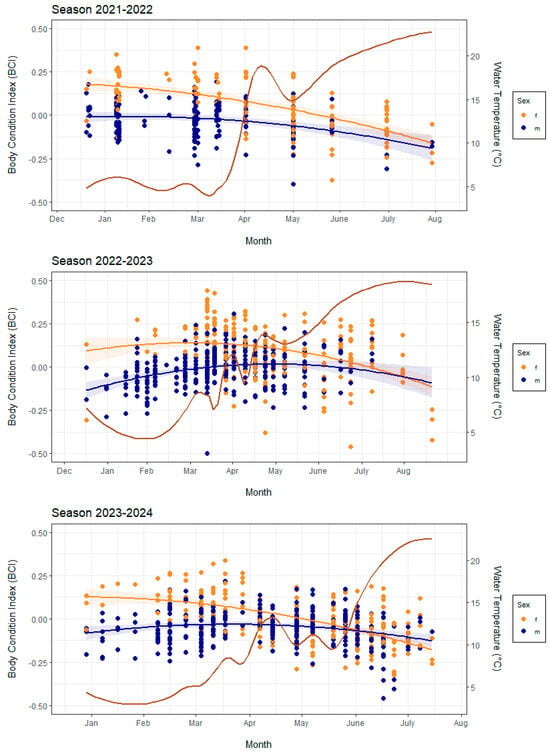
Figure 2.
Annual BCI change for males and females for each season. Points indicate individual newts, with fitted trend lines from the GLM for each sex (blue—males, orange—females). The brown line indicates water temperature variation across the respective seasons. The shaded area indicates 95% confidence intervals.
3.3. Population Activity
Population activity, estimated as the number of newts captured per trap, changed non-linearly during the season (Likelihood Ratio, χ2(2) = 62.29, p < 0.001; Figure 3). Activity levels differed for males and females (Likelihood Ratio, χ2(2) = 41.56, p < 0.001) and between study periods (Likelihood Ratio, χ2(2) = 47.89, p < 0.001), but the overall trend remained similar across seasons (month × season interaction: Likelihood Ratio, χ2(4) = 6.19, p = 0.186). Generally, males were most active during the breeding season and less active at the beginning and end of the aquatic phase, while female activity remained more consistent throughout the season. However, females seemed to be leaving the water later than males, evidenced by the fact that the female-to-male ratio increased towards the end of the season in all study periods (LM, t = 2.35, df = 67, p = 0.022; Figure 4). The sex ratio varied within seasons and showed clear inter-annual differences (LM, t = 2.35, df = 67, p = 0.022). In all study periods, the female-to-male ratio increased toward the end of the season (Figure 4), suggesting that females remained in the water longer than males. While the overall seasonal trend did not differ significantly between years (time of the year × season interaction: Likelihood Ratio, χ2(2) = 0.171, p = 0.940), the sex ratios themselves varied considerably across years (Likelihood Ratio, χ2(2) = 38.87, p < 0.001).
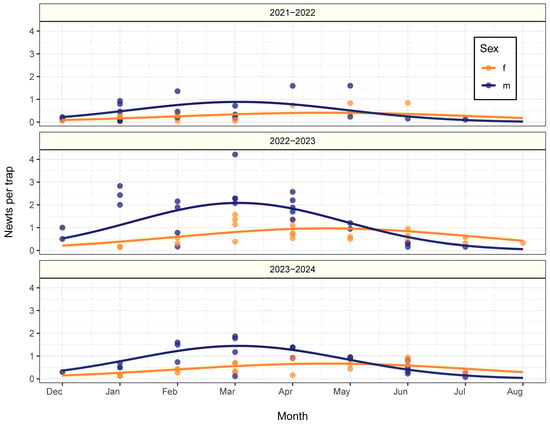
Figure 3.
Seasonal patterns of newt activity by sex and study period. Points represent the average number of captured newts per trap for males (blue) and females (orange) for each month, and solid lines show the predicted activity levels based on a Negative Binomial GLM, with sex-specific trends across the three study periods.
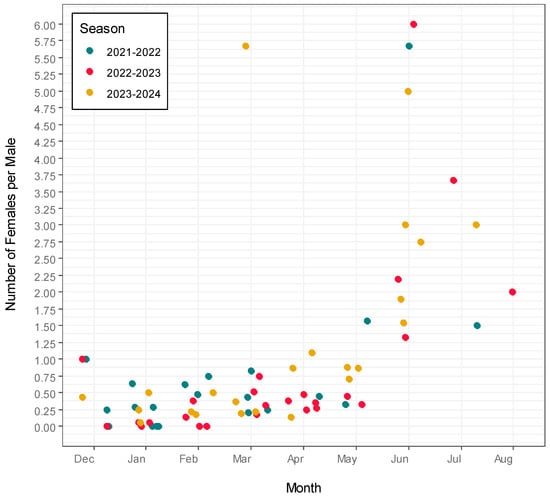
Figure 4.
Female-to-male ratio change throughout the season. The graph displays how the ratio of females versus males is changing during the year in all study periods. Blue points show the ratios in season 2021–2022, red points show the ratios in season 2022–2023 and yellow points show the ratios in season 2023–2024.
There was no relationship between mean BCI per session and population activity for either sex (Negative Binomial GLM, z = −1.49, p = 0.135). On the contrary, individual BCI scores (rBCI) (relative to the population baseline) were correlated with individual activity, measured as the total number of times an individual has been captured (Negative Binomial GLM, z = −2.07, p = 0.038). This relationship was not dependent on sex (Negative Binomial GLM, z = 1.52, p = 0.129). Water temperature did not influence capture success (Negative Binomial GLM, z = 1.47, p = 0.143). There was a non-linear relationship between newt activity and water temperature (Likelihood Ratio, χ2(2) = 34.467, p < 0.001). Activity increased as temperatures rose up to a certain point and then decreased at higher temperatures, following a U-shape. This suggests that newts were more active at moderate temperatures, with reduced activity at both low and high extremes of temperature.
4. Discussion
Since body condition can vary significantly over time and with seasons [25,26], it is important that annual body condition trends are followed over a number of years before drawing any conclusions. We investigated the changes in body condition in adult Buresch’s crested newts for three consecutive years, expecting that animals which remained active during the winter might rapidly use stored energy resources and show a decrease in body condition during the winter period. However, we found no change in BCI from December to March in all three years, but we observed a decrease during the breeding season and towards the end of the active period (i.e., the desiccation of the pond). The trend differed between females and males, but expectedly for this species, females had higher BCI scores than males and SDI was female-biased [27]. This is somewhat contrary to what has been reported for the closely related great crested newt, Triturus cristatus (Laurenti, 1768), as a long-term study by [28] observed an increasing trend throughout the breeding season (March–May) for males and no variation in the BCI for females. It is possible that, by feeding during the winter, Buresch’s crested newts are compensating for the increased metabolic rate associated with remaining active. Despite the existence of reliable and safe techniques for establishing the diet of Triturus newts via stomach flushing [29,30], these remove the ingested food from the stomach, and we felt that they pose a starvation risk for the animals, considering the scarcity of available prey during the winter. Future studies could test this by performing laboratory experiments in which individuals are prevented from hibernation and the body condition of newts that are fed is compared to the body condition of newts that are not.
The increase in body mass, and therefore BCI, in T. cristatus males has been attributed to the development of the crest—an important predictor of reproductive success [31]. Throughout the three seasons of our study, males had prominently developed crests shortly after the onset of winter, and this was also the case in previous seasons (see [15]). Since tail height depends on food provisioning and decreases rapidly when individuals are not fed [32], maintaining a prominently developed crest outside of the breeding season might be costly. Consistent with this hypothesis, the persistent winter activity may have a delayed effect, which explains the decline in BCI during the breeding season and towards the end of the study. However, it is also possible that newts’ feeding during the winter simply allows males to grow their crests early on, without any carry-over effects. In this case, the decline in body condition might result from increased activity during the breeding season. In females, the decrease in body condition might also be explained by individuals depositing their eggs as the breeding season progresses [33,34].
Finally, the rapid decline in BCI observed after the end of the breeding season might be associated with the advance of the summer drought. Since the number of captured newts becomes increasingly lower, individuals in a better condition might be leaving the pond early, while individuals with a worse body condition stay longer in the water to accumulate energy reserves for a successful migration on land [35]. Residual BCIs are not comparable across populations in time and space [8], and for this reason, we analyzed the results for each season separately. Previous research on climate change-associated changes in amphibians showed that milder winters can be associated with lower body condition [36]; however, laboratory experiments suggest a positive effect on body mass when individuals are subjected to a shorter hibernation length in milder conditions [2]. Our results appear to be in line with the latter study, as BCI displayed a consistent trend in all three years.
Research on another temperate amphibian—the Common toad, Bufo bufo (L., 1758)—found that individuals who were experimentally prevented from hibernation had decreased BCI [37], while hibernating individuals showed a negative correlation between body mass and hibernation length [2]. As in both of those studies, hibernation was experimentally manipulated, the study population may provide the first opportunity to investigate the effects of changes in overwintering behavior in the wild and evaluate laboratory findings. We also tested whether individuals with higher BCI scores were more active than individuals with lower scores, with “activity” represented by the number of times an individual has been recaptured. This assumes that more active individuals will move about more and might be recaptured more often as a result. However, if T. ivanbureschi exhibits underwater territorial behavior, the number of captures might not reflect activity, but which parts of the pond we have sampled more frequently. Although we tried to spread traps as evenly across the pond as possible, we could not keep track of whether a particular newt was usually recaptured in a certain area of the pond. Our results on the effects of water temperature are in line with what has previously been reported for the species [38]; the same study also reports negligible effects of dissolved oxygen levels, oxidative reduction potential and acidity levels on newt activity, highlighting the correlation between stable pond conditions and higher BCI values. Considering the above, we think that the most plausible explanation is that healthier individuals (i.e., with higher BCI) are simply more mobile, as decreased mobility (or even immobility) is a common symptom of adverse health in many amphibian species [39].
Our study demonstrates that female newts were less likely to be recaptured than males. Sex differences in capture probability have been observed in many taxa, incl. reptiles [40] and amphibians [41,42,43], and could be attributed to sex-related differences in behavior [44,45] and mortality rates [43]. Such difference in the recapture rate might occur if (1) female mortality is higher than male mortality, so the females are recaptured less often; (2) the females are more numerous than the males, so the chance to capture each individual more than once is lower; (3) males are more active than females (e.g., when searching for mates) and are captured more often as a result or (4) females spend less time in the water and as consequence are less likely to be recaptured. Considering our population estimates, we can exclude the first two reasons, as mortality rates were equal and, in all years, and males had a higher estimated population size. The study area is also relatively isolated, consisting of several small ponds with low established migration levels between them and a stable population size in each pond [20]. At the same time, there are data that suggest male crested newts are more vagile than females, especially during the breeding season. For T. cristatus, [46] established that males with larger body size and higher survival rate had lower breeding site fidelity, and [47] found a positive correlation between newt density and pond departure rate. For T. ivanbureschi, the only available data are for male migrations between ponds [20]. The results of our study also seem to corroborate data from [48], which reports a gradual change in the sex ratio from male-biased at the onset of breeding season to female-biased towards the end of the aquatic phase for several newt species from Western Europe. The underlying reasons for that might be reproductive timing and behavior (i.e., female newts deposit eggs individually and wrap them in water vegetation), energy accumulation (female newts are more exhausted after egg deposition) or external environmental factors.
For the T. ivanbureschi population in the present study, the overall recapture rate between the years was 20%. This relatively low percentage may be due to several factors: (1) although individually specific, the ventral pattern in Triturus newts could change significantly with time [19], so between years comparisons may miss some recaptures; (2) the inter-pond migration within the newts metapopulation in the area might be considerably higher than the previously reported by [20] (again, this would be related to the limitations of the image recognition, as well as the inability to sample all ponds at the same time) and (3) the actual yearly survival for individuals from the study population may be significantly lower than the seasonal survival rate for yet undiscovered reasons. In their study on Triturus dorbrogicus, the authors in [19] point out that during a five year period, black spots gradually expanded and merged at the expense of the orange background area, considerably changing the ventral pattern; however, the authors also suggest that the process may have been influenced by the rearing conditions of the animals in the laboratory. The available data on migration patterns for T. ivanbureschi in the area indicates that newts were almost always recaptured in the pond they were first photographed, but only 76 out of 501 adult newts were identified as recaptures between years based on their ventral pattern [20]. Considering that the local population has existed for many decades, the most probable explanation would be a combination of factors (1) and (2). However, this could only be established by an invasive marking technique such as Visual Implant Elastomers, which have been demonstrated by [49] to be reliable in long-term studies on the Italian cave salamander, Hydromantes italicus (Dunn, 1923). This species has a total length of up to 12 cm (including the tail), so it is very likely that a similar method would also work on the larger Buresch crested newt.
Like many other temperate amphibians, the Buresch crested newt is expected to lose a significant proportion of its habitats across Europe and the Near East (approximately 74%) due to climate change [50]. More generally, research predicts climate change-associated decline in body condition [51,52], fecundity [4,52] and survival [36]. Coupled with the increases in frequency and magnitude of droughts across Europe, this threatens the persistence of local populations [53]. Interestingly, the authors in [52] find no relation between drought and adult survival in the North American crawfish frog (Lithobates areolatus); however, the authors attribute this to the burrowing lifestyle of the frogs, which largely protects them from short-term climate variations. Considering that newts are much more dependent on water for their survival [54,55], the consequences of droughts could be much more severe. Still, if the causes of amphibian decline are identified sooner rather than later, conservationists might be able to save species from extinction by translocating endangered populations to habitats with more suitable climatic conditions [3,56].
5. Conclusions
This study presents the first investigation of BCI change in the Buresch crested newt across seasons for consecutive years, and it tries to link that to population and individual activity. Body condition declined during the breeding season and towards the end of the aquatic phase, suggesting the delayed cost of winter activity or increased energy demands during reproduction. Female newts had higher body conditions than males and tended to leave the pond later, while males had higher capture probability, highlighting potential sex-based differences in energy allocation and aquatic behavior. These trends remained consistent throughout the study period, and the obtained results could be used to form a better understanding of how this species might respond to ongoing habitat changes and climatic shifts. Future studies should address more specific questions about body condition maintenance mechanisms by precise laboratory experiments and more robust marking techniques that allow for terrestrial as well as aquatic tracking.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d17050350/s1, Table S1: Raw_data.xlsx.
Author Contributions
Conceptualization, S.L.; methodology, S.L. and I.A.; validation, S.L. and I.A.; formal analysis, S.L. and I.A.; investigation, S.L.; resources, S.L.; data curation, S.L.; writing—original draft preparation, S.L. and I.A.; writing—review and editing, S.L. and I.A.; visualization, I.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All research was carried out in accordance with the Bulgarian Ministry of Environment and Water, permit No. 861/13.01.2021.
Data Availability Statement
The raw data from the CMR sessions used for the present study are available as a Supplementary File in .csv format.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- The IUCN Red List of Threatened Species. Version 2024-3. Available online: https://www.iucnredlist.org (accessed on 21 February 2025).
- Üveges, B.; Mahr, K.; Szederkényi, M.; Bókony, V.; Hoi, H.; Hettyey, A. Experimental evidence for beneficial effects of projected climate change on hibernating amphibians. Sci. Rep. 2016, 6, 26754. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Thuiller, W.; Pearson, R.G. Climate warming and the decline of amphibians and reptiles in Europe. J. Biogeogr. 2006, 33, 1712–1728. [Google Scholar] [CrossRef]
- Benard, M.F. Warmer winters reduce frog fecundity and shift breeding phenology, which consequently alters larval development and metamorphic timing. Glob. Change Biol. 2015, 21, 1058–1065. [Google Scholar] [CrossRef]
- Hayes, J.P.; Shonkwiler, J.S. Morphometric indicators of body condition: Worthwhile or wishful thinking? In Body Composition Analysis of Animals: A Handbook of Non-Destructive Methods; Speakman, J.R., Ed.; Cambridge University Press: Cambridge, UK, 2001; pp. 8–38. [Google Scholar]
- Stevenson, R.D.; Woods, W.A. Condition indices for conservation: New uses for evolving tools. Integr. Comp. Biol. 2006, 46, 1169–1190. [Google Scholar] [CrossRef]
- McCaffrey, K.R.; Balaguera-Reina, S.A.; Falk, B.G.; Gati, E.V.; Cole, J.M.; Mazzotti, F.J. How to estimate body condition in large lizards? Argentine black and white tegu (Salvator merianae, Dumeril and Bibron, 1839) as a case study. PLoS ONE 2023, 18, e0282093. [Google Scholar] [CrossRef] [PubMed]
- Jakob, E.M.; Marshall, S.D.; Uetz, G.W. Estimating fitness: A comparison of body condition indices. Oikos 1996, 77, 61–67. [Google Scholar] [CrossRef]
- Green, A.J. Mass/length residuals: Measures of body condition or generators of spurious results? Ecology 2001, 82, 1473–1483. [Google Scholar] [CrossRef]
- Labocha, M.K.; Schutz, H.; Hayes, J.P. Which body condition index is best? Oikos 2014, 123, 111–119. [Google Scholar] [CrossRef]
- Bucciarelli, G.M.; Clark, M.A.; Delaney, K.S.; Riley, S.P.D.; Shaffer, H.B.; Fisher, R.N.; Honeycutt, R.L.; Kats, L.B. Amphibian responses in the aftermath of extreme climate events. Sci. Rep. 2020, 10, 3409. [Google Scholar] [CrossRef]
- McCaffery, R.M.; Maxell, B.A. Decreased winter severity increases viability of a montane frog population. Proc. Natl. Acad. Sci. USA 2010, 107, 8644–8649. [Google Scholar] [CrossRef]
- Caruso, N.M.; Sears, M.W.; Adams, D.C.; Lips, K.R. Widespread rapid reductions in body size of adult salamanders in response to climate change. Glob. Change Biol. 2014, 20, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Lukanov, S.; Lazarkevich, I.; Dimitrova, B. Persistent winter activity in Triturus ivanbureschi Arntzen & Wielstra, 2013 (Amphibia: Caudata). Acta Zool. Bulg. 2022, 74, 281–285. [Google Scholar]
- Wielstra, B.; Litvinchuk, S.N.; Naumov, B.; Tzankov, N.; Arntzen, J.W. A revised taxonomy of crested newts in the Triturus karelinii group (Amphibia: Caudata: Salamandridae), with the description of a new species. Zootaxa 2013, 3682, 441–453. [Google Scholar] [CrossRef]
- Stojanov, A.; Tzankov, N.; Naumov, B. Die Amphibien und Reptilien Bulgariens; Chimaira: Frankfurt am Main, Germany, 2011; p. 582. [Google Scholar]
- Lovich, J.E.; Gibbons, J.W. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 1992, 56, 269–281. [Google Scholar]
- Crall, J.P.; Stewart, C.V.; Berger-Wolf, T.Y.; Rubenstein, D.I.; Sundaresan, S.R. HotSpotter—Patterned species instance recognition. In Proceedings of the 2013 IEEE Workshop on Applications of Computer Vision (WACV), Clearwater Beach, FL, USA, 15–17 January 2013; pp. 230–237. [Google Scholar] [CrossRef]
- Naumov, B.; Lukanov, S. Notes on age-related changes in body size and color pattern in captive Triturus dobrogicus (Kiritzescu, 1903). Herpetozoa 2013, 30, 159–168. [Google Scholar]
- Lukanov, S. Inter-pond migration during the aquatic phase by male Triturus ivanbureschi. Russ. J. Herpetol. 2022, 29, 373–376. [Google Scholar] [CrossRef]
- Parris, K.; McCarthy, M. Identifying effects of toe clipping on anuran return rates: The importance of statistical power. Amphib-Reptilia 2001, 22, 275–289. [Google Scholar]
- Naumov, B.; Popgeorgiev, G.; Kornilev, Y.; Plachiyski, G.; Stojanov, A.; Tzankov, N. Distribution and ecology of the Alpine newt Ichthyosaura alpestris (Laurenti, 1768) (Amphibia: Salamandridae) in Bulgaria. Acta Zool. Bulg. 2020, 72, 83–102. [Google Scholar]
- Schwarz, C.J.; Arnason, A.N. A general methodology for the analysis of open-model capture recapture experiments. Biometrics 1996, 52, 860–873. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 10 October 2024).
- Băncilă, R.I.; Hartel, T.; Plăiaşu, R.; Cogălniceanu, D.; Smets, J. Comparing three body condition indices in amphibians: A case study of yellow-bellied toad Bombina variegata. Amphib-Reptilia 2010, 31, 558–562. [Google Scholar]
- Jarvis, L.E. Terrestrial ecology of juvenile great crested newts (Triturus cristatus) in a woodland area. Herpetol. J. 2016, 26, 287–296. [Google Scholar]
- Lukanov, S.; Tzankov, N. Life history, age and normal development of the Balkan-Anatolian crested newt (Triturus ivanbureschi Arntzen and Wielstra, 2013) from Sofia district. North-West. J. Zool. 2016, 12, 22–32. [Google Scholar]
- Jarvis, L.E. Factors affecting body condition in a great crested newt Triturus cristatus population. Herpetol. Bull. 2015, 134, 1–5. [Google Scholar]
- Berzin, D.L.; Burakova, A.V. Diet features of the Crested newt Triturus cristatus (Laurenti, 1768) at the eastern border of its range. Russ. J. Ecol. 2022, 53, 221–227. [Google Scholar] [CrossRef]
- Naumov, B.; Vacheva, E.; Lukanov, S. Diet and feeding habits of Triturus cristatus (Laurenti, 1768) (Amphibia: Salamandridae) in its southernmost locality. North-West. J. Zool. 2024, 20, 116–125. [Google Scholar]
- Hedlund, L. Factors affecting differential mating success in male crested newts, Triturus cristatus. J. Zool. 1990, 220, 33–40. [Google Scholar] [CrossRef]
- Green, A.J. Large male crests, an honest indicator of condition, are preferred by female smooth, newts, Triturus vulgaris (Salamandridae) at the spermatophore transfer stage. Anim. Behav. 1991, 41, 367–369. [Google Scholar] [CrossRef]
- Arntzen, J.W.; Smithson, A.; Oldham, R.S. Marking and tissue sampling effects on body condition and survival in the newt Triturus cristatus. J. Herpetol. 1999, 33, 567. [Google Scholar] [CrossRef]
- Halliday, T.; Tejedo, M. Intrasexual selection and alternative mating behaviour. In Amphibian Biology; Heatwole, H., Sullivan, B.K., Eds.; Surrey Beatty & Sons Pty Ltd.: Baulkham Hills, Australia, 1995; Volume 2, pp. 419–468. [Google Scholar]
- Brzeziński, M.; Eliava, G.; Żmihorski, M. Road mortality of pond-breeding amphibians during spring migrations in the Mazurian Lakeland, NE Poland. Eur. J. Wildl. Res. 2012, 58, 685–693. [Google Scholar] [CrossRef]
- Griffiths, R.A.; Sewell, D.; McCrea, R.S. Dynamics of a declining amphibian metapopulation: Survival, dispersal and the impact of climate. Biol. Conserv. 2010, 143, 485–491. [Google Scholar] [CrossRef]
- Jørgensen, C.B. External and internal control of patterns of feeding, growth and gonadal function in a temperate zone anuran, the toad Bufo bufo. J. Zool. 1986, 210, 211–241. [Google Scholar] [CrossRef]
- Lukanov, S.; Doncheva, T.; Kostova, N.; Naumov, B. Effects of selected environmental parameters on the activity and body condition of the Buresch’s crested newt (Triturus ivanbureschi) with notes on skin secretions. North-West. J. Zool. 2021, 17, 34–38. [Google Scholar]
- Ockleford, C.; Adriaanse, P.; Berny, P.; Brock, T.; Duquesne, S.; Grilli, S.; Hernandez-Jerez, A.F.; Bennekou, S.H.; Klein, M.; Kuhl, T.; et al. Scientific Opinion on the state of the science on pesticide risk assessment for amphibians and reptiles. EFSA J. 2018, 16, e05125. [Google Scholar] [CrossRef]
- Hailey, A.; Willemsen, R.E. Population density and adult sex ratio of the tortoise Testudo hermanni in Greece: Evidence for intrinsic population regulation. J. Zool. 2000, 251, 325–338. [Google Scholar] [CrossRef]
- Pickett, E.J.; Stockwell, M.P.; Pollard, C.J.; Garnham, J.I.; Clulow, J.; Mahony, M.J. Estimates of sex ratio require the incorporation of unequal catchability between sexes. Wildl. Res. 2012, 39, 350–354. [Google Scholar] [CrossRef]
- Berven, K.A. Factors affecting population fluctuations in larval and adult stages of the wood frog (Rana sylvatica). Ecology 1990, 71, 1599–1608. [Google Scholar] [CrossRef]
- Anholt, B.R.; Hotz, H.; Guex, G.D.; Semlitsch, R.D. Overwinter survival of Rana lessonae and its hemiclonal associate Rana esculenta. Ecology 2003, 84, 391–397. [Google Scholar] [CrossRef]
- Amrhein, V.; Scaar, B.; Baumann, M.; Minéry, N.; Binnert, J.P.; Korner-Nievergelt, F. Estimating adult sex ratios from bird mist netting data. Methods Ecol. Evol. 2012, 3, 713–720. [Google Scholar] [CrossRef]
- McKnight, D.T.; Ligon, D.B. Correcting for unequal catchability in sex ratio and population size estimates. PLoS ONE 2017, 12, e0184101. [Google Scholar] [CrossRef]
- Denoël, M.; Dalleur, S.; Langrand, E.; Besnard, A.; Cayuela, H. Dispersal and alternative breeding site fidelity strategies in an amphibian. Ecography 2018, 41, 1543–1555. [Google Scholar] [CrossRef]
- Cayuela, H.; Schmidt, B.R.; Weinbach, A.; Besnard, A.; Joly, P. Multiple density-dependent processes shape the dynamics of a spatially structured amphibian population. J. Anim. Ecol. 2019, 88, 164–177. [Google Scholar] [CrossRef]
- Arntzen, J. Seasonal variation in sex ratio and asynchronous presence at ponds of male and female Triturus newts. J. Herpetol. 2002, 36, 30–35. [Google Scholar] [CrossRef]
- Lunghi, E.; Bruni, G. Long-term reliability of Visual Implant Elastomers in the Italian cave salamander (Hydromantes italicus). Salamandra 2018, 54, 283–286. [Google Scholar]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar] [CrossRef]
- Moldowan, P.D.; Tattersall, G.J.; Rollinson, N. Climate-associated decline of body condition in a fossorial salamander. Glob. Change Biol. 2022, 28, 1725–1739. [Google Scholar] [CrossRef]
- Lannoo, M.; Stiles, R. Effects of short-term climate variation on a long-lived frog. Copeia 2017, 105, 726–733. [Google Scholar] [CrossRef]
- Cayuela, H.; Arsovski, D.; Bonnaire, E.; Duguet, R.; Joly, P.; Besnard, A. The impact of severe drought on survival, fecundity, and population persistence in an endangered amphibian. Ecosphere 2016, 7, e01246. [Google Scholar] [CrossRef]
- Dodd, C.K. Cost of living in an unpredictable environment: The ecology of Striped Newts Notophthalmus perstriatus during a prolonged drought. Copeia 1993, 1993, 605–614. [Google Scholar] [CrossRef]
- Fahrbach, M.; Gerlach, U. The Genus Triturus: History, Biology, Systematics, Captive Breeding; Chimaira Verlag: Frankfurt, Germany, 2018; 550p. [Google Scholar]
- Bickford, D.; Howard, S.D.; Ng, D.J.J.; Sheridan, J.A. Impacts of climate change on the amphibians and reptiles of Southeast Asia. Biodivers. Conser. 2010, 19, 1043–1062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).