Reference Ecosystem Condition-Based Syntaxonomic Study for Ecological Restoration and Protection of Temperate Forests in South Korea
Abstract
1. Introduction
2. Materials and Methods
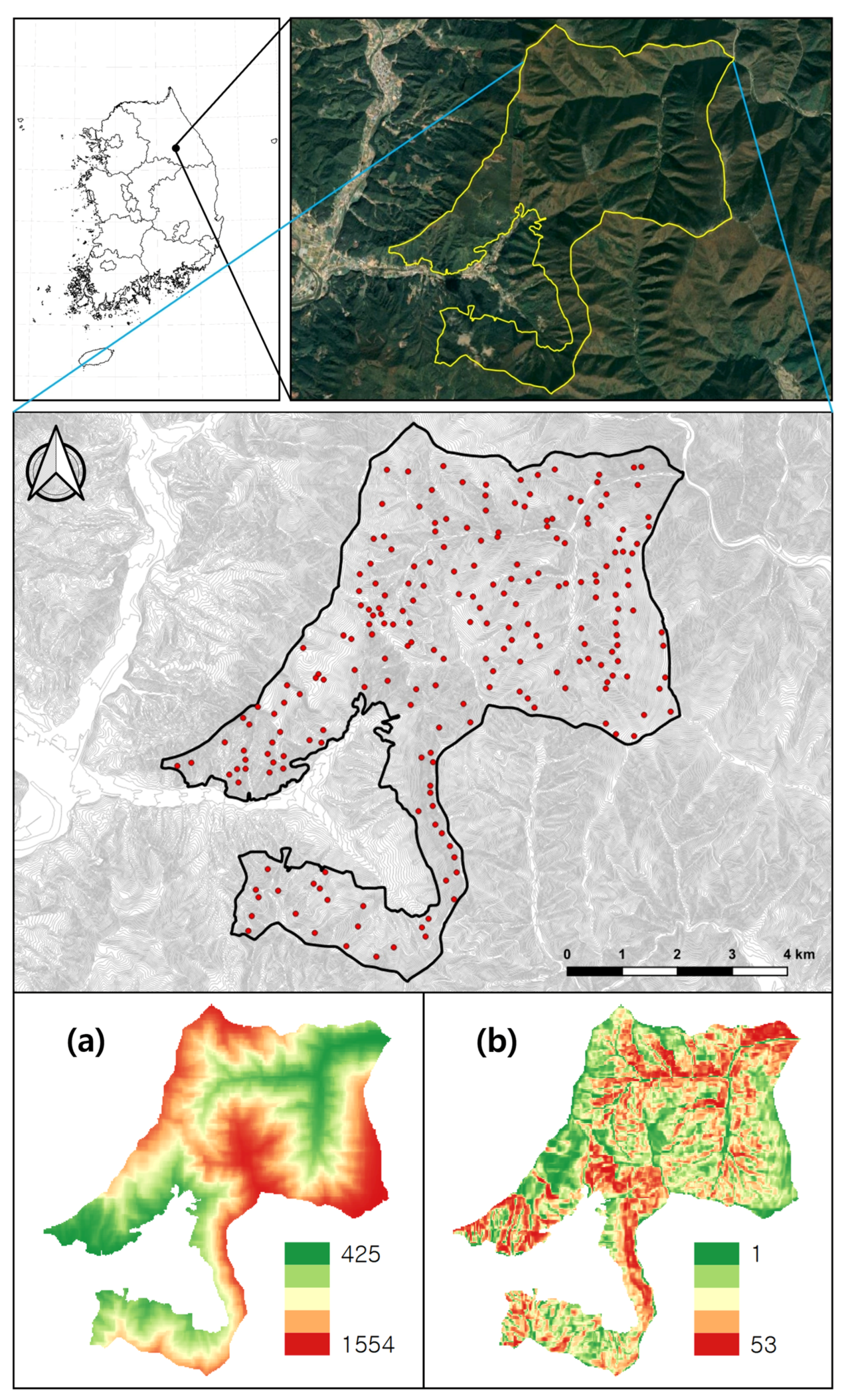
2.1. Study Area
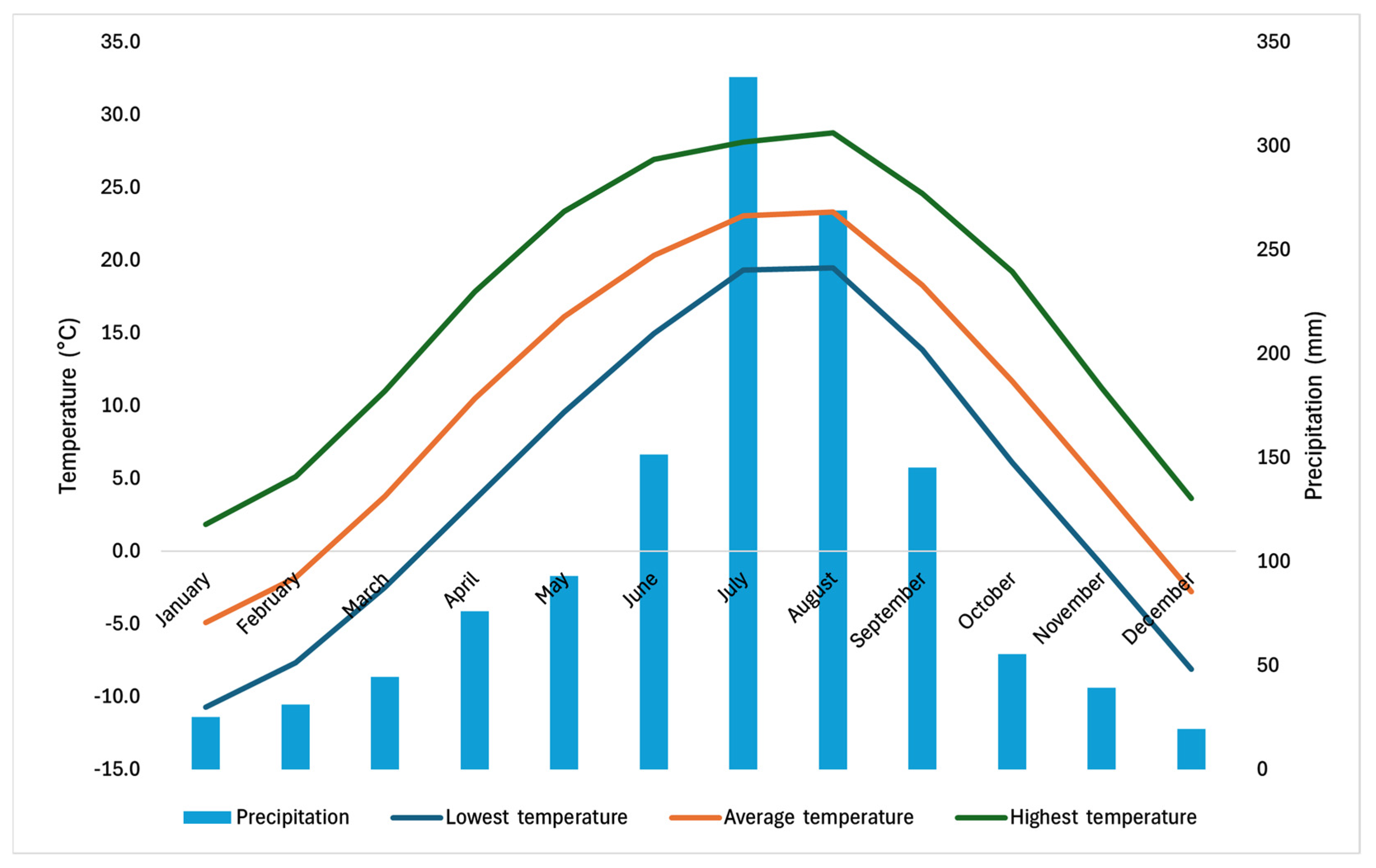
2.2. Climatic Characteristics
2.3. Vegetation Sampling
2.4. Data and Statistical Analysis
3. Results
3.1. Floristic Diversity and Phytogeography
3.2. Vegetation Structure
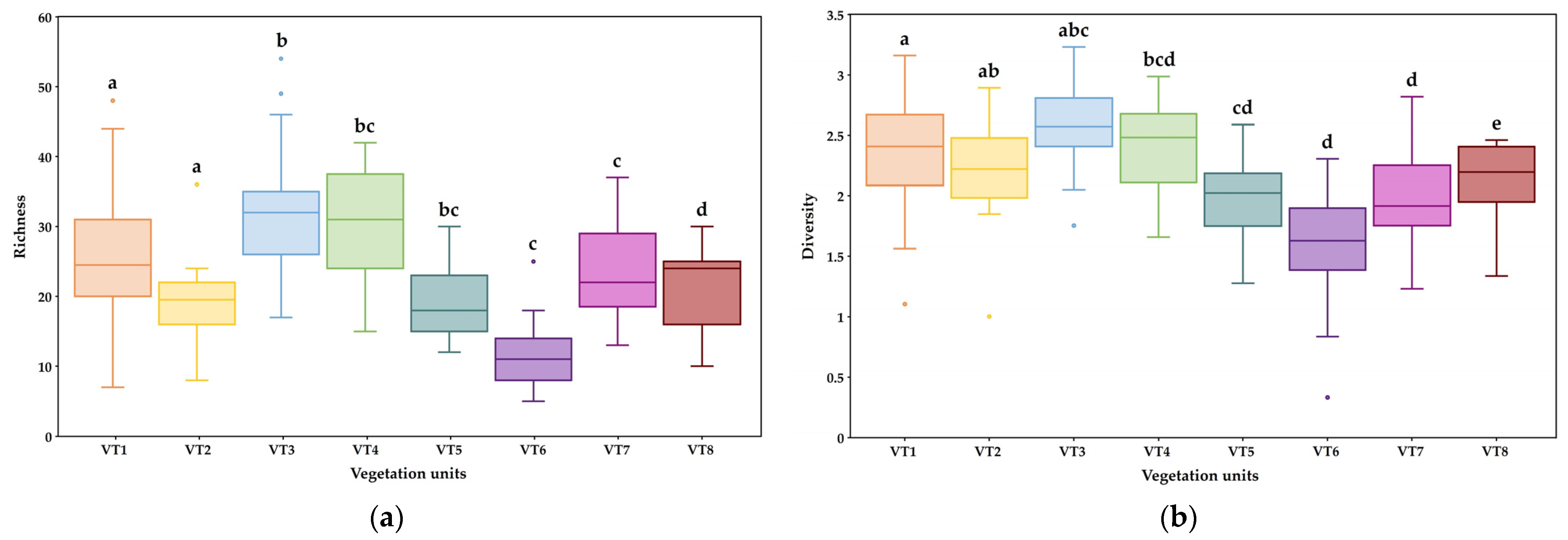
3.3. Species Diversity Index
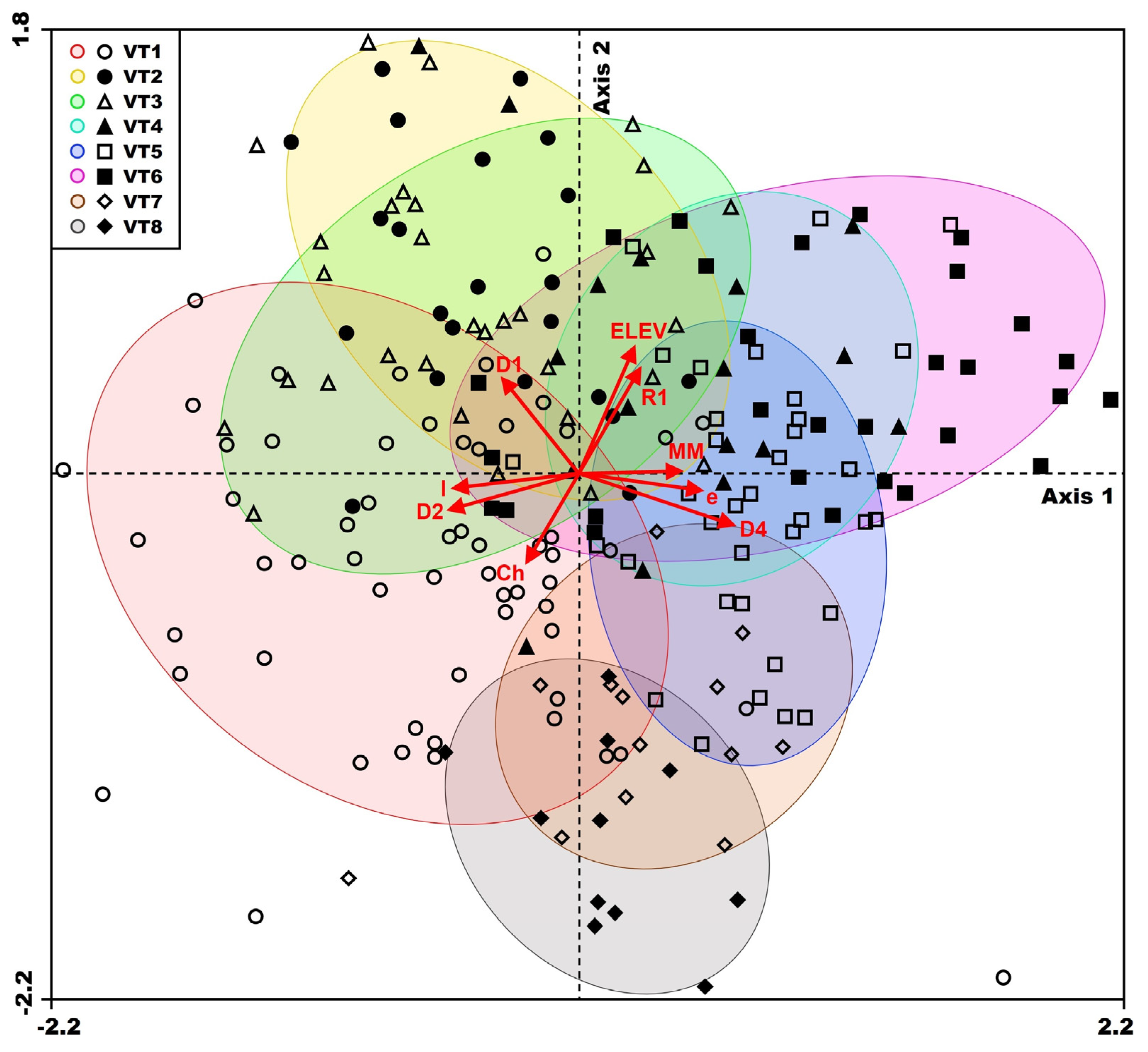
3.4. Relationships Between Vegetation and Environment
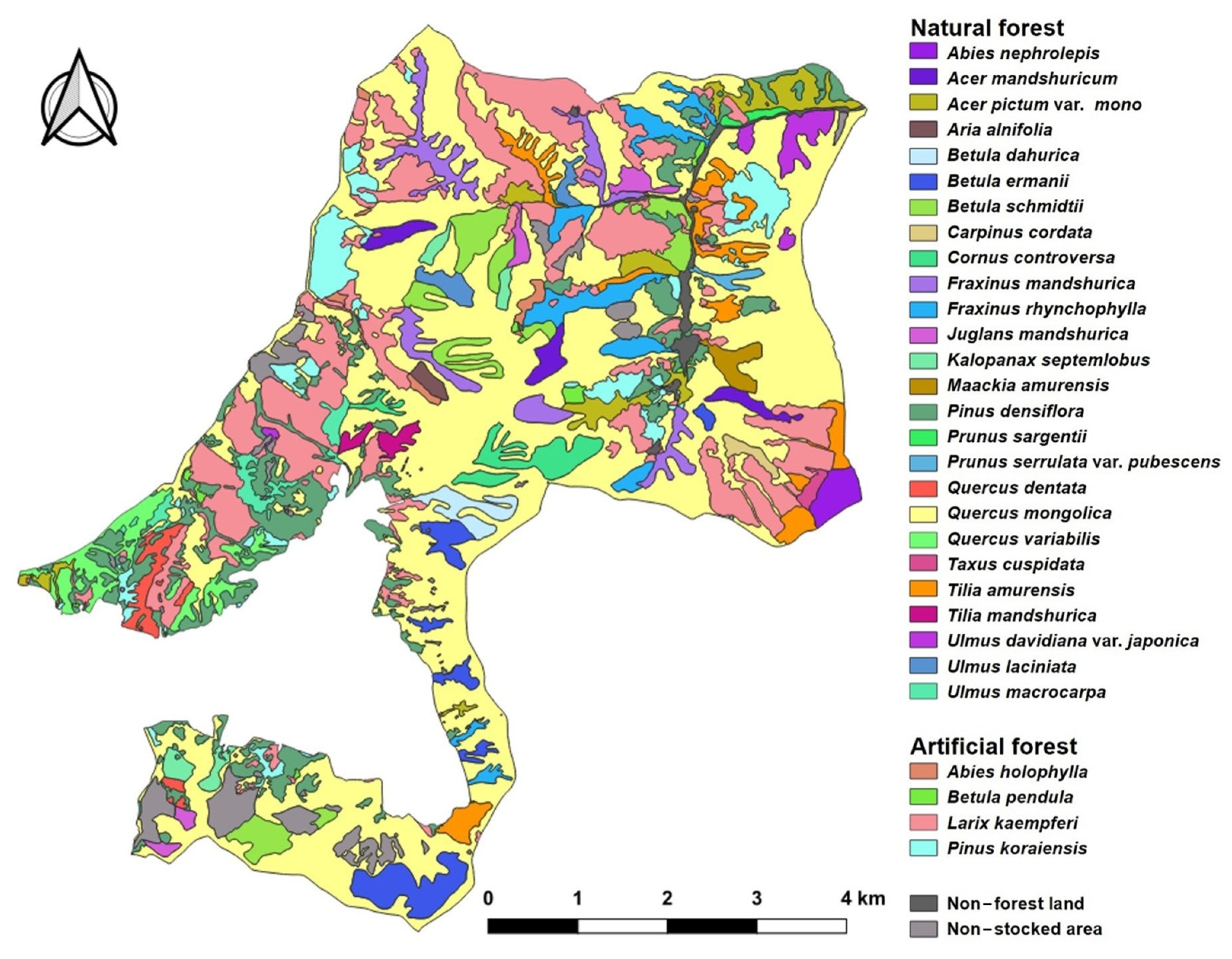
3.5. Physiognomic Vegetation Map
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Aerts, R.; Honnay, O. Forest restoration, biodiversity and ecosystem functioning. BMC Ecol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.A.; Knoke, T. Sustainable development and sustainable forestry: Analogies, differences, and the role of flexibility. Eur. J. For. Res. 2010, 129, 787–801. [Google Scholar] [CrossRef]
- Butchart, S.H.M.; Walpole, M.; Collen, B.; Strien, A.V.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global Biodiversity: Indicators of Recent Declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef]
- Fortunel, C.; Lasky, J.R.; Uriarte, M.; Valencia, R.; Wright, S.J.; Garwood, N.C.; Kraft, N.J.B. Topography and neighborhood crowding can interact to shape species growth and distribution in a diverse Amazonian forest. Ecology 2018, 99, 2272–2283. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Ding, Y.; Xu, H.; Deng, F.; Yao, L.; Ai, X.; Zang, R. Patterns of diversity change for forest vegetation across different climatic regions—A compound habitat gradient analysis approach. Glob. Ecol. Conserv. 2020, 23, e01106. [Google Scholar] [CrossRef]
- Gaston, K.J. Global patterns in biodiversity. Nature 2000, 405, 220–227. [Google Scholar] [CrossRef]
- Roslin, T.; Hardwick, B.; Novotny, V.; Petry, W.K.; Andrew, N.R.; Asmus, A.; Barrio, I.C.; Basset, Y.; Boesing, A.L.; Bonebrake, T.C.; et al. Higher predation risk for insect prey at low latitudes and elevations. Science 2017, 356, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.I.; McEwan, R.W. The Role of Environmental Filtering in Structuring Appalachian Tree Communities: Topographic Influences on Functional Diversity Are Mediated through Soil Characteristics. Forests 2018, 9, 19. [Google Scholar] [CrossRef]
- Herben, T.; Goldberg, D.E. Community assembly by limiting similarity vs. competitive hierarchies: Testing the consequences of dispersion of individual traits. J. Ecol. 2014, 102, 156–166. [Google Scholar] [CrossRef]
- Leps, J.; de Bello, F.; Lavorel, S.; Berman, S. Quantifying and interpreting functional diversity of natural communities: Practical considerations matter. Preslia 2006, 78, 481–501. [Google Scholar]
- Mason, N.W.H.; de Bello, F.; Mouillot, D.; Pavoine, S.; Dray, S. A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J. Veg. Sci. 2013, 24, 794–806. [Google Scholar] [CrossRef]
- Mcgill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Spasojevic, M.J.; Suding, K.N. Inferring community assembly mechanisms from functional diversity patterns: The importance of multiple assembly processes. J. Ecol. 2012, 100, 652–661. [Google Scholar] [CrossRef]
- Pott, R. Plant Biosystems—An International Journal Dealing with All Aspects of Plant Biology; Taylor & Francis: Oxfordshire, UK, 2011; pp. 9–18. [Google Scholar]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance, 3rd ed.; Harper & Row: New York, NY, USA, 1985. [Google Scholar]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Fitzpatrick, M.C.; Gove, A.D.; Sanders, N.J.; Dunn, R.R. Climate change, plant migration, and range collapse in a global biodiversity hotspot: The Banksia (Proteaceae) of Western Australia. Glob. Change Biol. 2008, 14, 1337–1352. [Google Scholar] [CrossRef]
- Lawler, J.J.; Shafer, S.L.; White, D.; Kareiva, P.; Maurer, E.P.; Blaustein, A.R.; Bartlein, P.J. Projected climate-induced faunal change in the Western Hemisphere. Ecology 2009, 90, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Mateo, R.G.; Felicísimo, Á.M.; Pottier, J.; Guisan, A.; Muñoz, J. Do Stacked Species Distribution Models Reflect Altitudinal Diversity Patterns? PLoS ONE 2012, 7, e32586. [Google Scholar] [CrossRef]
- Pottier, J.; Dubuis, A.; Pellissier, L.; Maiorano, L.; Rossier, L.; Randin, C.F.; Vittoz, P.; Guisan, A. The accuracy of plant assemblage prediction from species distribution models varies along environmental gradients. Glob. Ecol. Biogeogr. 2013, 22, 52–63. [Google Scholar] [CrossRef]
- Svenning, J.C.; Fitzpatrick, M.C.; Normand, S.; Graham, C.H.; Pearman, P.B.; Iverson, L.R.; Skov, F. Geography, topography, and history affect realized-to-potential tree species richness patterns in Europe. Ecography 2010, 33, 1070–1080. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Kumar, P. Assessment of impact of climate change on Rhododendrons in Sikkim Himalayas using Maxent modelling: Limitations and challenges. Biodivers. Conserv. 2012, 21, 1251–1266. [Google Scholar] [CrossRef]
- Alard, D.; Poudevigne, I. Biodiversity in changing landscapes: From species or patch assemblages to system organisation. In Application of Geographic Information Systems and Remote Sensing in River Studies; Leuven, R.S.E.W., Poudevigne, I., Teeuw, R.M., Eds.; Backhuys: Leiden, NL, USA, 2002; pp. 9–24. [Google Scholar]
- Sasaki, T.; Ishii, H.; Morimoto, Y. Evaluating restoration success of a 40-year-old urban forest in reference to mature natural forest. Urban For. Urban Green. 2018, 32, 123–132. [Google Scholar] [CrossRef]
- Ruiz-Jaen, M.C.; Aide, T.M. Restoration Success: How Is It Being Measured? Restor. Ecol. 2005, 13, 569–577. [Google Scholar] [CrossRef]
- Fengler, F.H.; Bressane, A.; Carvalho, M.M.; Longo, R.M.; de Medeiros, G.A.; de Melo, W.J.; Jakovac, C.C.; Ribeiro, A.I. Forest restoration assessment in Brazilian Amazonia: A new clustering-based methodology considering the reference ecosystem. Ecol. Eng. 2017, 108, 93–99. [Google Scholar] [CrossRef]
- Jeon, D.U.; Chon, J.H. System Thinking in the Resilience of the Ecosystem and Ecotourism of Mt. Gariwang Based on the Controversy around the Venue Construction for PyeongChang 2018 Olympic. Korean Syst. Dyn. Rev. 2014, 15, 61–79. [Google Scholar]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Braun−Blanquet, J. Plant Sociology: The Study of Plant Communities; McGraw−Hill: New York, NY, USA, 1932. [Google Scholar]
- Lee, T.B. Coloured Flora of Korea; HyangMoonSa: Seoul, Republic of Korea, 2003. [Google Scholar]
- Lee, Y.N. Flora of Korea; KyoHakSa: Seoul, Republic of Korea, 2006. [Google Scholar]
- Lee, D.H. Knowing Right Trees of Korea; EBeeLak: Seoul, Republic of Korea, 2014.
- Lee, D.H. Knowing Right Wildflowers of Korea; EBeeLak: Seoul, Republic of Korea, 2016.
- The World Flora Online. Available online: https://www.worldfloraonline.org/ (accessed on 18 October 2023).
- Hill, M.O. Twinspan: A Fortran Program for Arranging Multivariate Data in an Ordered Two-Way Table by Classification of the Individuals and Attributes; Cornell University Section of Ecology and Systematics: New York, NY, USA, 1979.
- Ellenberg, H. Grundlagen der Vegetationsgliederung. I. Teil Aufgaben und Methoden der Vegetationskunde. In Einführung in die Phytologie; Walter, H., Ed.; Eugen Ulmer: Stuttgart, Germany, 1956; pp. 1–136. [Google Scholar]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Oxford University Press: Oxford, UK, 1934. [Google Scholar]
- Numata, M. Illustrated Plant Ecology; Ashakura Book Company: Tokyo, Japan, 1970; pp. 33–43. [Google Scholar]
- Kim, M.S.; Cho, H.J.; Kim, J.S.; Bae, K.H.; Chun, J.H. The Classification of Forest Vegetation Types and Species Composition in the Sector between Danmoknyeong and Guryongnyeong of Baekdudaegan. Korean J. Environ. Ecol. 2018, 32, 176–184. [Google Scholar] [CrossRef]
- Song, J.H.; Kwon, J.N.; Yun, C.W. Forest Vegetation Structure in Maruguem(the Ridge Line) Area of Dakmokryeong to Daetjae, the Baekdudaegan. Korean J. Environ. Ecol. 2019, 33, 28–51. [Google Scholar] [CrossRef]
- Cheon, K.I.; Byun, J.G.; Jung, S.C.; Sung, J.H. Community Structure of Quercus mongolica Stand in Hyangrobong Area, Baekdudaegan. J. Agric. Life Sci. 2014, 48, 1–13. [Google Scholar] [CrossRef][Green Version]
- Haq, F.; Ahmad, H.; Alam, M.; Ahmad, I.; Ullah, R. Species diversity of vascular plants of Nandiar Valley Western Himalaya, Pakistan. Pak. J. Bot. 2010, 42, 213–229. [Google Scholar]
- Faisal, S.; Haq, F.; Iqbal, Z. Statistical analysis for the classification and ordination of the vegetation of Chour valley. A multivariate approach. Acta Ecol. Sin. 2022, 42, 446–452. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Brouillet, L. Are many plant species paraphyletic? Taxon 1994, 43, 21–23. [Google Scholar] [CrossRef]
- Archibold, O.W. Ecology of World Vegetation; Chapman & Hall: London, UK, 1995. [Google Scholar]
- El-Sheikh, M.A.; Al-Shehri, M.A.; Alfarhan, A.H.; Alatar, A.A.; Rajakrishnan, R.; Al-Rowaily, S.L. Threatened Prunus arabica in an ancient volcanic protected area of Saudi Arabia: Floristic diversity and plant associations. Saudi J. Biol. Sci. 2019, 26, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Alatar, A.A.; El-Sheikh, M.A.R.; Thomas, J.; Hegazy, A.K.; El Adawy, H.A. Vegetation, Floristic Diversity, and Size-Classes of Acacia gerrardii in an Arid Wadi Ecosystem. Arid. Land Res. Manag. 2015, 29, 335–359. [Google Scholar] [CrossRef]
- El-Sheikh, M.A. Population structure of woody plants in the arid cloud forests of Dhofar, southern Oman. Acta Bot. Croat. 2013, 72, 97–111. [Google Scholar] [CrossRef]
- Song, J.S. A Comparative Phytosociological Study of Cool Temperate Forests (Quercus forest and Fagus forest) in Korea and Germany. Korean J. Ecol. 1999, 22, 219–225. [Google Scholar]
- Stupar, V.; Brujić, J.; Škvorc, Ž.; Črni, A. Vegetation types of thermophilous deciduous forests (Quercetea pubescentis) in the Western Balkans. Phytocoenologia 2016, 46, 49–68. [Google Scholar] [CrossRef]
- Egerton, F.N. Ecological Studies and Observations Before 1900; Taylor, B.J., White, T.J., Eds.; University of Oklahoma Press: Norman, OK, USA, 1976; pp. 311–351. [Google Scholar]
- Baroni, C.; Armiraglio, S.; Gentili, R.; Carton, A. Landform–vegetation units for investigating the dynamics and geomorphologic evolution of alpine composite debris cones (Valle dell’Avio, Adamello Group, Italy). Geomorphology 2007, 84, 59–79. [Google Scholar] [CrossRef]
- Choo, G.C.; Kim, G.T.; Kim, J.O. Studies on the Structure of Forest Community at the Ridge from Gittaebong to Cheongoksan. Korean J. Environ. Ecol. 2002, 15, 354–360. [Google Scholar]
- Jeong, B.K.; Oh, C.H. Analysis on the Community Structure of Quercus mongolica Fisch. ex Ledeb. in the Baekdudaegan Mountains by Elevation—Between Hyangnobong and Gitdaebaggybong. Korean J. Environ. Ecol. 2013, 27, 449–461. [Google Scholar]
- De Cáceres, M.; Chytrý, M.; Agrillo, E.; Attorre, F.; Botta-Dukát, Z.; Capelo, J.; Czúcz, B.; Dengler, J.; Ewald, J.; Faber-Langendoen, D.; et al. A comparative framework for broad-scale plot-based vegetation classification. Appl. Veg. Sci. 2015, 18, 543–560. [Google Scholar] [CrossRef]
- Lasco, R.D.; Delfino, R.J.P.; Espaldon, M.L.O. Agroforestry systems: Helping smallholders adapt to climate risks while mitigating climate change. Wiley Interdiscip. Rev. Clim. Chang. 2014, 5, 825–833. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Fanfarillo, E.; Zangari, G.; Küzmič, F.; Fiaschi, T.; Bonari, G.; Angiolini, C. Summer roadside vegetation dominated by Sorghum halepense in peninsular Italy: Survey and classification. Rend. Lincei Sci. Fis. Nat. 2022, 33, 93–104. [Google Scholar] [CrossRef]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Catford, J.A.; Daehler, C.C.; Murphy, H.T.; Sheppard, A.W.; Hardesty, B.D.; Westcott, D.A.; Rejmánek, M.; Bellingham, P.J.; Pergl, J.; Horvitz, C.C.; et al. The intermediate disturbance hypothesis and plant invasions: Implications for species richness and management. Perspect. Plant Ecol. Evol. Syst. 2012, 14, 231–241. [Google Scholar] [CrossRef]
- Boch, S.; Prati, D.; Müller, J.; Socher, S.; Baumbach, H.; Buscot, F.; Gockel, S.; Hemp, A.; Hessenmöller, D.; Kalko, E.K.V.; et al. High plant species richness indicates management-related disturbances rather than the conservation status of forests. Basic Appl. Ecol. 2013, 14, 496–505. [Google Scholar] [CrossRef]
- Gauch, H.G. Multivariate Analysis in Community Ecology; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Saima, S.; Dasti, A.A.; Hussain, F.; Wazir, S.M.; Malik, S.A. Floristic compositions along an 18-km long transect in Ayubia National Park district Abbottabad, Pakistan. Pak. J. Bot. 2009, 41, 2115–2127. [Google Scholar]
- Xia, K.; Daws, M.I.; Peng, L.L. Climate drives patterns of seed traits in Quercus species across China. New Phytol. 2022, 234, 1628–1638. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.K.; Chettri, B.; Vijayan, L. Distribution pattern of trees along an elevation gradient of Eastern Himalaya, India. Acta Oecol. 2011, 37, 329–336. [Google Scholar] [CrossRef]
- Souza, A.A.; Galvão, L.S.; Santos, J.R. Relationships between Hyperion-derived vegetation indices, biophysical parameters, and elevation data in a Brazilian savannah environment. Remote Sens. Lett. 2010, 1, 55–64. [Google Scholar] [CrossRef]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and mapping of the ecoregions of Italy. Plant Biosyst. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M.; Goldewijk, K.K. Habitat conversion and global avian biodiversity loss. Proc. R. Soc. B Biol. Sci. 2003, 270, 1293–1300. [Google Scholar] [CrossRef]
- Guo, Y.; Schöb, C.; Ma, W.; Mohammat, A.; Liu, H.; Yu, S.; Jiang, Y.; Schmid, B.; Tang, Z. Increasing water availability and facilitation weaken biodiversity–biomass relationships in shrublands. Ecology 2019, 100, e02624. [Google Scholar] [CrossRef]
- Lai, L.; Huang, X.; Yang, H.; Chuai, X.; Zhang, M.; Zhong, T.; Chen, Z.; Chen, Y.; Wang, X.; Thompson, J.R. Carbon emissions from land-use change and management in China between 1990 and 2010. Sci. Adv. 2016, 2, e1601063. [Google Scholar] [CrossRef] [PubMed]
- Lenihan, J.M.; Drapek, R.; Bachelet, D.; Neilson, R.P. Climate change effects on vegetation distribution, carbon, and fire in California. Ecol. Appl. 2003, 13, 1667–1681. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, X.; Bai, Y.; Tang, Z.; Wang, W.; Zhao, Y.; Wan, H.; Xie, Z.; Shi, X.; Wu, B.; et al. Carbon pools in China’s terrestrial ecosystems: New estimates based on an intensive field survey. Proc. Natl. Acad. Sci. USA 2018, 115, 4021–4026. [Google Scholar] [CrossRef]
- Spribille, T.; Stroh, H.G.; Triepke, F.J. Are habitat types compatible with floristically-derived associations? J. Veg. Sci. 2001, 12, 791–796. [Google Scholar] [CrossRef]

| Vegetation units | A | ||||||||
| a | b | ||||||||
| I | II | III | IV | ||||||
| i | ii | iii | iv | ||||||
| 1 | 2 | ||||||||
| Vegetation types | VT1 | VT2 | VT3 | VT4 | VT5 | VT6 | VT7 | VT8 | |
| Total number of species | 258 | 126 | 221 | 161 | 145 | 96 | 110 | 87 | |
| Average number of species (/100 m2) | 22.5 | 17.5 | 14.0 | 10.6 | 24.1 | 30.2 | 11.8 | 12.6 | |
| Number of plots | 58 | 22 | 31 | 17 | 35 | 29 | 13 | 11 | |
| --Species group 1_Character and differential species of Quercus mongolica-Fraxinus rhynchophylla community group | |||||||||
| Quercus mongolica | III5r | II5r | III5r | V54 | V51 | V5r | V5+ | ||
| Fraxinus rhynchophylla | V5r | III4r | IV4r | III2r | III2r | III2r | V3r | V3+ | |
| --Species group 2_Character and differential species of Acer pictum var. mono-Acer pseudosieboldianum community | |||||||||
| Acer pictum var. mono | III5r | V2r | IV5r | IV2r | II1r | III2r | II2+ | II3r | |
| Acer pseudosieboldianum | II3+ | III3+ | III3+ | IV3+ | IV4+ | III4+ | I2r | ||
| Schisandra chinensis | IV4+ | IV2r | IV3r | III1+ | II++ | I3r | |||
| --Species group 3_Character and differential species of Kalopanax septemlobus-Actinidia arguta subcommunity | |||||||||
| Kalopanax septemlobus | III1r | I5r | II4r | II3r | I2r | IIrr | |||
| Actinidia arguta | III3r | III3r | I2+ | I++ | I++ | I++ | |||
| Morus bombycis | III3r | II3+ | II1r | I1r | (r)11 | I++ | |||
| --Species group 4_Character and differential species of Cornus controversa-Staphylea bumalda group | |||||||||
| Cornus controversa | II3r | I11 | III3r | I22 | I11 | I1r | I++ | ||
| Staphylea bumalda | III3r | I2r | I1r | (r)++ | I++ | Irr | |||
| Rubus pungens | II4+ | I+r | I33 | I++ | (r)++ | I32 | I++ | ||
| Aralia elata | II3r | (r)++ | (r)++ | Irr | (r)++ | I1+ | |||
| --Species group 5_Character and differential species of Dryopteris crassirhizoma-Polystichum tripteron group | |||||||||
| Dryopteris crassirhizoma | II3+ | V4+ | III3+ | II11 | I1+ | ||||
| Polystichum tripteron | (r)++ | IV2r | II1r | I1r | I+r | Irr | |||
| Philadelphus schrenkii | II3+ | IV3r | II2r | II+r | I11 | ||||
| Deutzia glabrata | I2+ | II4+ | III3+ | I1r | |||||
| Magnolia sieboldii | I1+ | III4r | II2+ | I++ | II2+ | II3+ | |||
| --Species group 6_Character and differential species of Acer mandshuricum-Ulmus laciniata subgroup | |||||||||
| Acer mandshuricum | I+r | I21 | III5+ | I++ | I+r | ||||
| Ulmus laciniata | I++ | II4r | III3r | Irr | |||||
| Sambucus kamtschatica | II1r | (r)++ | III1r | I+r | (r)++ | ||||
| Prunus padus | I+r | (r)++ | III2+ | I1r | (r)++ | I++ | |||
| --Species group 7_Character and differential species of Tilia amurensis-Tripterygium regelii subcommunity | |||||||||
| Tilia amurensis | I4+ | II5+ | II5r | II1r | III3r | I1+ | I++ | ||
| Tripterygium regelii | II3+ | (r)22 | II1+ | IV3+ | IV3r | III2r | I++ | ||
| Symplocos sawafutagi | II1r | (r)++ | I++ | III3+ | IV2r | II1+ | II1+ | ||
| --Species group 8_Character and differential species of Sasa borealis group | |||||||||
| Sasa borealis | (r)rr | I32 | I+r | I3+ | I51 | V5+ | |||
| --Species group 9_Character and differential species of Ainsliaea acerifolia-Pseudostellaria heterophylla group | |||||||||
| Ainsliaea acerifolia | (r)++ | (r)33 | II2+ | IV4+ | III4+ | ||||
| Pseudostellaria heterophylla | I2+ | I++ | III2+ | IV2+ | II+r | ||||
| Carex siderosticta | I1+ | I2+ | III2+ | III1r | I++ | IV1r | |||
| Stephanandra incisa | II3r | (r)++ | I2+ | II2+ | III3r | I1+ | |||
| Athyrium yokoscense | II1r | I1+ | II++ | III1+ | II1+ | I1r | Irr | ||
| --Species group 10_Character and differential species of Calamagrostis arundinacea-Artemisia stolonifera subgroup | |||||||||
| Calamagrostis arundinacea | I+r | (r)rr | I2+ | IV3+ | I2+ | I1+ | I++ | I++ | |
| Artemisia stolonifera | II++ | II++ | IV1+ | I++ | (r)++ | I+r | |||
| Angelica decursiva | I+r | I+r | II+r | IV+r | I++ | Irr | |||
| Isodon excisus | II3r | III3+ | III4+ | I2r | I+r | I11 | I++ | ||
| --Species group 11_Character and differential species of Lindera obtusiloba-Carex humilis var. nana community | |||||||||
| Lindera obtusiloba | IV4r | III3r | II2+ | I++ | IV4r | IV3r | V5+ | IV3+ | |
| Carex humilis var. nana | (r)++ | I++ | I1+ | I2r | II1r | V2r | V3+ | ||
| Lespedeza bicolor | I+r | I1r | Irr | IV3+ | IV1+ | ||||
| --Species group 12_Character and differential species of Ampelopsis glandulosa var. heterophylla-Artemisia keiskeana subcommunity | |||||||||
| Ampelopsis glandulosa var. heterophylla | II1r | I1+ | I+r | III+r | II+r | (r)++ | IV1+ | II++ | |
| Artemisia keiskeana | I+r | (r)++ | I+r | (r)11 | III1+ | II++ | |||
| Lonicera praeflorens | II1r | I++ | (r)++ | I++ | I+r | III++ | I++ | ||
| Lonicera subsessilis | I1+ | I++ | I3+ | I+r | II+r | I1+ | III2+ | I21 | |
| --Species group 13_Character and differential species of Quercus dentata-Quercus variabilis subcommunity | |||||||||
| Quercus dentata | (r)2r | II5+ | V51 | ||||||
| Quercus variabilis | II5r | V51 | |||||||
| Dioscorea quinqueloba | I+r | I++ | I++ | (r)++ | IV++ | ||||
| Spiraea chinensis | (r)11 | IV1+ | |||||||
| Companions omitted (351 spp.) | |||||||||
| Origin | Physiognomy Vegetation Type Community | Spatial Distribution | Patch | ||
|---|---|---|---|---|---|
| Area (ha) | Ratio (%) | Number (Trees) | Average Area (ha) | ||
| Natural forest | Abies nephrolepis | 23.26 | 0.6 | 1 | 23.26 |
| Acer mandshuricum | 38.51 | 0.9 | 3 | 12.84 | |
| Acer pictum var. mono | 88.18 | 2.2 | 9 | 9.80 | |
| Aria alnifolia | 7.86 | 0.2 | 1 | 7.86 | |
| Betula dahurica | 26.80 | 0.7 | 1 | 26.80 | |
| Betula ermanii | 85.22 | 2.1 | 6 | 14.20 | |
| Betula schmidtii | 112.68 | 2.8 | 11 | 10.24 | |
| Carpinus cordata | 9.34 | 0.2 | 1 | 9.34 | |
| Cornus controversa | 50.73 | 1.3 | 3 | 16.91 | |
| Fraxinus mandshurica | 101.20 | 2.5 | 5 | 20.24 | |
| Fraxinus rhynchophylla | 95.03 | 2.3 | 8 | 11.88 | |
| Juglans mandshurica | 28.13 | 0.7 | 4 | 7.03 | |
| Kalopanax septemlobus | 27.71 | 0.7 | 4 | 6.93 | |
| Maackia amurensis | 18.36 | 0.5 | 1 | 18.36 | |
| Pinus densiflora | 310.70 | 7.7 | 110 | 2.82 | |
| Prunus sargentii | 5.81 | 0.1 | 2 | 2.91 | |
| Prunus serrulate var. pubescens | 7.80 | 0.2 | 1 | 7.80 | |
| Quercus dentata | 30.90 | 0.8 | 5 | 6.18 | |
| Quercus mongolica | 1739.61 | 42.9 | 52 | 33.45 | |
| Quercus variabilis | 69.84 | 1.7 | 11 | 6.35 | |
| Taxus cuspidata | 6.98 | 0.2 | 1 | 6.98 | |
| Tilia amurensis | 98.75 | 2.4 | 10 | 9.87 | |
| Tilia mandshurica | 14.88 | 0.4 | 2 | 7.44 | |
| Ulmus davidiana var. japonica | 32.61 | 0.8 | 4 | 8.15 | |
| Ulmus laciniata | 21.61 | 0.5 | 2 | 10.80 | |
| Ulmus macrocarpa | 32.64 | 0.8 | 5 | 6.53 | |
| Subtotal | 3085.15 | 76.1 | 263 | 11.73 | |
| Artificial forest | Abies holophylla | 16.61 | 0.4 | 7 | 2.37 |
| Betula pendula | 10.99 | 0.3 | 12 | 0.92 | |
| Larix kaempferi | 629.96 | 15.5 | 86 | 7.33 | |
| Pinus koraiensis | 147.03 | 3.6 | 39 | 3.77 | |
| Subtotal | 804.59 | 19.8 | 144 | 5.59 | |
| Non-forest land | 37.77 | 0.9 | 10 | 3.78 | |
| Non-stocked area | 127.77 | 3.2 | 64 | 2.00 | |
| Subtotal | 165.54 | 4.1 | 74 | 2.24 | |
| Total | 4055.28 | 100.0 | 481 | 8.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Koo, N.; Kim, A.R.; Lee, K.; Yun, S.J. Reference Ecosystem Condition-Based Syntaxonomic Study for Ecological Restoration and Protection of Temperate Forests in South Korea. Diversity 2025, 17, 40. https://doi.org/10.3390/d17010040
Kim M, Koo N, Kim AR, Lee K, Yun SJ. Reference Ecosystem Condition-Based Syntaxonomic Study for Ecological Restoration and Protection of Temperate Forests in South Korea. Diversity. 2025; 17(1):40. https://doi.org/10.3390/d17010040
Chicago/Turabian StyleKim, Minsu, Namin Koo, A Reum Kim, Kiwoong Lee, and Soon Jin Yun. 2025. "Reference Ecosystem Condition-Based Syntaxonomic Study for Ecological Restoration and Protection of Temperate Forests in South Korea" Diversity 17, no. 1: 40. https://doi.org/10.3390/d17010040
APA StyleKim, M., Koo, N., Kim, A. R., Lee, K., & Yun, S. J. (2025). Reference Ecosystem Condition-Based Syntaxonomic Study for Ecological Restoration and Protection of Temperate Forests in South Korea. Diversity, 17(1), 40. https://doi.org/10.3390/d17010040






