Seventies Rule for the Establishment of Non-Native Marine Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
- Non-native species that expanded their range without human assistance, also known as range expanders. It turned out that no species needed to be excluded solely because they were range expanders. Those that were excluded were excluded because they were also considered cryptogenic or of unknown origin (see below);
- Species of which no settled individuals or colonies were observed on artificial structures, like pilings or docks, or “settled” in a natural environment. Thus, organisms that were recorded solely from hull fouling communities, in their pelagic larval stages, or washed ashore on beaches were excluded. Of the 27 datasets, only the New Zealand dataset contained these species. A total of 86 species were omitted as they were only recorded from vessel hulls in that dataset;
- Species with an unknown origin and/or those considered to be cryptogenic. Approximately 4% of the species (86 out of 1959) across the 27 datasets were excluded;
- Species that prefer to establish themselves in waters with salinities < 5 ppt. Approximately 2% of the species (34 out of 1959) across the 27 datasets were excluded due solely to their preference for low-salinity environments (<5 ppt). In many of the datasets, these species (e.g., the Quagga mussel Dreissena bugensis) were already excluded. To ensure consistency, they were removed from all datasets prior to calculating establishment percentages;
- Microorganisms (<2 mm), including planktonic species and pathogens like bacteria, viruses, and fungi. Approximately 10% of the species (202 out of 1959) across the 27 datasets were excluded. The inclusion of these species varied considerably across the datasets. For example, six datasets did not contain any planktonic species while four datasets only had one planktonic species recorded and one Mediterranean dataset had sixty-four planktonic species included. To ensure consistency across datasets, all these species were excluded;
- Endo-parasites. Just 8 of the 1959 species reported across the 27 datasets were excluded as they were endo-parasites. To maintain consistency, endo-parasites were omitted because they were not considered for inclusion in most datasets;
- Vascular plants. Just 9 of the 1959 species reported across the 27 datasets were excluded as they concerned vascular plants. To maintain consistency, vascular plants were omitted because they were not considered for inclusion in most datasets;
- Fish. This was mainly implemented to eliminate bias in the establishment percentages assessed for the Mediterranean Seas. The Suez Canal greatly impacts this region as an introduction pathway that resulted in relatively higher numbers of reported non-native fish species than any other geographical area, where much lower numbers of non-native marine fish are introduced and establish themselves. Of the total 130 non-native fish species that were reported as established across the 27 datasets, 110 were reported from the Mediterranean, 15 from the Atlantic regions, and only 5 additional ones from other seas (Table S2). Regarding the 190 fish species that were reported in at least one of the datasets as non-established, 126 concerned records in the Mediterranean, 41 in Atlantic regions, and only 23 from elsewhere (Table S2). Because the Suez Canal provides a unique type of introduction pathway for fish in the Mediterranean only, unlike any pathway close by other seas included in our analyses, all 275 fish species were excluded across the 27 datasets. Since most of these fish came through the Suez Canal, including them would have strongly biased the calculations.
2.2. Calculations and Statistical Analyses
2.3. Geographical Distribution of the Regions Included in the Analyses
3. Results and Discussion
3.1. Region-Specific Establishment Rates of Non-Native Marine Species
3.2. Region-Specific Establishment Rate Variation over Time
3.3. Seventies Rule for the Establishment of Non-Native Marine Species
3.4. Similarities Between Established Non-Native Marine Species Across Climate Zones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katsanevakis, S.; Olenin, S.; Puntila-Dodd, R.; Rilov, G.; Stæhr, P.A.U.; Teixeira, H.; Tsirintanis, K.; Birchenough, S.N.R.; Jakobsen, H.H.; Knudsen, S.W.; et al. Marine Invasive Alien Species in Europe: 9 Years after the IAS Regulation. Front. Mar. Sci. 2023, 10, 1271755. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A. Impacts of Invasive Alien Marine Species on Ecosystem Services and Biodiversity: A Pan-European Review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Grosholz, E. Ecological and Evolutionary Consequences of Coastal Invasions. Trends Ecol. Evol. 2002, 17, 22–27. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Essl, F.; Evans, T.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Marková, Z.; Mrugała, A.; Nentwig, W.; et al. A Unified Classification of Alien Species Based on the Magnitude of Their Environmental Impacts. PLoS Biol. 2014, 12, e1001850. [Google Scholar] [CrossRef]
- Coppis, S.; Gittenberger, A. Climate Zone Related Establishment Chances of Marine Alien Species. In Proceedings of the Programs and Abstracts 22nd International Conference on Aquatic Invasive Species, Oostend, Belgium, 18–22 April 2022; p. 33. [Google Scholar]
- Crooks, J.A. Lag Times and Exotic Species: The Ecology and Management of Biological invasions in Slow-Motion11. Écoscience 2005, 12, 316–329. [Google Scholar] [CrossRef]
- Culver, C.; Kuris, A. The Apparent Eradication of a Locally Established Introduced Marine Pest. Biol. Invasions 2000, 2, 245–253. [Google Scholar] [CrossRef]
- Hopkins, G.; Forrest, B.; Jiang, W.; Garner, J. Successful eradication of a non-indigenous marine bivalve from a subtidal soft-sediment environment. J. Appl. Ecol. 2011, 48, 424–431. [Google Scholar] [CrossRef]
- Wotton, D.; O’Brien, C.; Stuart, M.; Fergus, D. Eradication success down under: Heat treatment of a sunken trawler to kill the invasive seaweed Undaria pinnatifida. Mar. Pollut. Bull. 2004, 49, 844–849. [Google Scholar] [CrossRef]
- Keeler-May, G. Early-detection and rapid response to a novel wakame (Undaria pinnatifida) incursion in Broad Bay, Rakiura/Stewart Island. In Proceedings of the Programs and Abstracts 22nd International Conference on Aquatic Invasive Species, Oostend, Belgium, 18–22 April 2022; ICAIS: Oostend, Belgium, 2022; p. 64. [Google Scholar]
- Robinson, T.B.; Alexander, M.E.; Simon, C.A.; Griffiths, C.L.; Peters, K.; Sibanda, S.; Miza, S.; Groenewald, B.; Majiedt, P.; Sink, K.J. Lost in Translation? Standardising the Terminology Used in Marine Invasion Biology and Updating South African Alien Species Lists. Afr. J. Mar. Sci. 2016, 38, 129–140. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Progress in Invasion Biology: Predicting Invaders. Trends Ecol. Evol. 2001, 16, 199–204. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management Priorities for Marine Invasive Species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Zenetos, A.; Albano, P.; López, E.; Stern, N.; Tsiamis, K.; Galanidi, M. Established Non-Indigenous Species Increased by 40% in 11 Years in the Mediterranean Sea. Mediterr. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Williamson, M. Invaders, Weeds and the Risk from Genetically Manipulated Organisms. Experientia 1993, 49, 219–224. [Google Scholar] [CrossRef]
- Williamson, M.; Fitter, A. The Varying Success of Invaders. Ecology 1996, 77, 1661–1666. [Google Scholar] [CrossRef]
- Williamson, M.H.; Brown, K.C.; Holdgate, M.W.; Kornberg, H.; Southwood, R.; Mollison, D. The Analysis and Modelling of British Invasions [and Discussion]. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986, 314, 505–522. [Google Scholar]
- Jeschke, J.M.; Pyšek, P. Tens Rule. In Invasion Biology: Hypotheses and Evidence; Jeschke, J.M., Heger, T., Eds.; CABI: Oxfordshire, UK, 2018. [Google Scholar]
- Galil, B.S. The Alien Crustaceans in the Mediterranean Sea: An Historical Review. In the Wrong Place—Alien Marine Crustaceans: Distribution, Biology and Impacts; Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 377–401. ISBN 978-94-007-0591-3. [Google Scholar]
- Minchin, D.; Cottier-Cook, E.; Clark, P. Alien Species in British Brackish and Marine Waters. Aquat. Invasions 2013, 8, 3–19. [Google Scholar] [CrossRef]
- Gittenberger, A.; Rensing, M.; Faasse, M.; van Walraven, L.; Smolders, S.; Perez, H.; Gittenberger, E. Non-Indigenous Species Dynamics in Time and Space within the Coastal Waters of The Netherlands. Diversity 2023, 15, 719. [Google Scholar] [CrossRef]
- Gittenberger, A.; Mirimin, L.; Boyd, J.; O’Beirn, F.; Devine, G.; O’Brien, M.; Rensing, M.; O’Dwyer, K.; Gittenberger, E. Marine Non-Indigenous Species Dynamics in Time and Space within the Coastal Waters of the Republic of Ireland. Diversity 2023, 15, 1019. [Google Scholar] [CrossRef]
- Massé, C.; Viard, F.; Humbert, S.; Antajan, E.; Auby, I.; Bachelet, G.; Bernard, G.; Bouchet, V.M.P.; Burel, T.; Dauvin, J.-C.; et al. An Overview of Marine Non-Indigenous Species Found in Three Contrasting Biogeographic Metropolitan French Regions: Insights on Distribution, Origins and Pathways of Introduction. Diversity 2023, 15, 161. [Google Scholar] [CrossRef]
- Png-Gonzalez, L.; Comas-González, R.; Calvo-Manazza, M.; Follana-Berná, G.; Ballesteros, E.; Díaz-Tapia, P.; Falcón, J.M.; García Raso, J.E.; Gofas, S.; González-Porto, M.; et al. Updating the National Baseline of Non-Indigenous Species in Spanish Marine Waters. Diversity 2023, 15, 630. [Google Scholar] [CrossRef]
- Mghili, B.; Lamine, I.; Rami Laamraoui, M.; Aksissou, M.; Galanidi, M. Updating the National List of Marine Alien Species in Morocco. Mediterr. Mar. Sci. 2024, 25, 231–249. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.; Corsini-Foka, M.; Gerovasileiou, V.; SimbouraI, N.; Xentidis, N.J.; Tsiamis, K. Is the Trend in New Introductions of Marine Non-Indigenous Species a Reliable Criterion for Assessing Good Environmental Status? Τhe Case Study of Greece. Mediterr. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Çinar, M.E.; Bilecenoğlu, M.; Yokeş, M.B.; Öztürk, B.; Taşkin, E.; Bakir, K.; Doğan, A.; Açik, Ş. Current Status (as of End of 2020) of Marine Alien Species in Turkey. PLoS ONE 2021, 16, e0251086. [Google Scholar] [CrossRef]
- Chainho, P.; Fernandes, A.; Amorim, A.; Ávila, S.P.; Canning-Clode, J.; Castro, J.J.; Costa, A.C.; Costa, J.L.; Cruz, T.; Gollasch, S.; et al. Non-Indigenous Species in Portuguese Coastal Areas, Coastal Lagoons, Estuaries and Islands. Estuar. Coast. Shelf Sci. 2015, 167, 199–211. [Google Scholar] [CrossRef]
- Ojaveer, H.; Olenin, S.; Narščius, A.; Florin, A.-B.; Ezhova, E.; Gollasch, S.; Jensen, K.R.; Lehtiniemi, M.; Minchin, D.; Normant-Saremba, M.; et al. Dynamics of Biological Invasions and Pathways over Time: A Case Study of a Temperate Coastal Sea. Biol. Invasions 2017, 19, 799–813. [Google Scholar] [CrossRef]
- Marine Non-Indigenous Species: Data to 2022. Version: 27 March 2024. Available online: https://www.stats.govt.nz/indicators/marine-non-indigenous-species-data-to-2022/ (accessed on 10 October 2024).
- Bazairi, H.; Sghaier, Y.R.; Benamer, I.; Langar, H.; Pergent, G.; Bouras, E.; Verlaque, M.; Soussi, J.B.; Zenetos, A. Alien Marine Species of Libya: First Inventory and New Records in El-Kouf National Park (Cyrenaica) and the Neighbouring Areas. Mediterr. Mar. Sci. 2013, 14, 451–462. [Google Scholar] [CrossRef]
- Teixeira, L.M.P.; Creed, J.C. A Decade on: An Updated Assessment of the Status of Marine Non-Indigenous Species in Brazil. Aquat. Invasions 2020, 15, 30–43. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsiamis, K.; Ioannou, G.; Michailidis, N.; Zenetos, A. Inventory of Alien Marine Species of Cyprus (2009). Mediterr. Mar. Sci. 2009, 10, 109–134. [Google Scholar] [CrossRef]
- Gittenberger, A.; van der Veer, H.W.; Philippart, C.J.M.; van der Hoorn, B.; D’Hont, A.; Wesdorp, K.H.; Schrieken, N.; Klunder, L.; Kleine-Schaars, L.; Holthuijsen, S.; et al. Native and Non-Native Species of the Dutch Wadden Sea in 2018; Commissioned by Office for Risk Assessment and Research, The Netherlands Food and Customer Product Safety Authority of the Ministry of Agriculture, Nature and Food Quality; GiMaRIS: Sassenheim, The Netherlands, 2019; GiMaRIS report 2019_09; pp. 1–124. [Google Scholar]
- Wolff, W.J. Non-Indigenous Marine and Estuarine Species in The Netherlands. Zool. Meded. 2005, 79, 1–116. [Google Scholar]
- Faasse, M.A. Faunistisch Overzicht van de Nederlandse Kelkwormen (Entoprocta). Ned. Faun. Meded. 2006, 24, 7–11. [Google Scholar]
- Waser, A.M.; Lackschewitz, D.; Knol, J.; Reise, K.; Wegner, K.M.; Thieltges, D.W. Spread of the Invasive Shell-Boring Annelid Polydora websteri (Polychaeta, Spionidae) into Naturalised Oyster Reefs in the European Wadden Sea. Mar. Biodivers. 2020, 50, 63. [Google Scholar] [CrossRef]
- Jha, U.; Jetter, A.; Lindley, J.A.; Postel, L.; Wootton, M. Extension of Distribution of Pseudodiaptomus marinus, an Introduced Copepod, in the North Sea. Mar. Biodivers. Rec. 2013, 6, e53. [Google Scholar] [CrossRef]
- Stegenga, H.; Mol, I.; Prud’homme an Reine, W.F.; Lokhorst, G.M. Checklist of the Marine Algae of the Netherlands. Gorteria Dutch Bot. Arch. 1997, 4, 1–60. [Google Scholar]
- Devine, G.; Gaffney, J.; Gittenberger, A.; Rensing, M. Alien Species and Aquaculture. A Summary of Work to Support the Aquaculture Sector in Understanding, Preventing and Managing IAS Risks; BIM: Dublin, Ireland, 2023. [Google Scholar]
- WoRMS Editorial Board World Register of Marine Species. Available online: https://www.marinespecies.org (accessed on 9 December 2024).
- Cochran, W.G. Some methods of strengthening the common x2 tests. Biometrics 1954, 10, 417–451. [Google Scholar] [CrossRef]
- Fisher, R.A. Statistical Methods for Research Workers, 13th ed.; Hafner: New York, NY, USA, 1958. [Google Scholar]
- Camilli, G.; Hopkins, K. Applicability of the Chi-Square to 2 × 2 Contingency Tables with Small Expected Cell Frequencies. Psychol. Bull. 1978, 85, 163–167. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E: Plymouth, UK, 2006; 192p. [Google Scholar]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Froglia, C.; Scanu, M. Notes on the Spreading of Penaeus aztecus Ives 1891 (Decapoda, Penaeidae) in the Mediterranean Sea and on Its Repeated Misidentifications in the Region. Biology 2023, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.J.; Stehlíková, J.; Beveridge, C.M.; Burrows, M.T.; De Blauwe, H.; Faasse, M. Distribution of the invasive bryozoan Tricellaria inopinata in Scotland and a review of its European expansion. Aquat. Invasions 2013, 8, 281–288. [Google Scholar] [CrossRef]
- Zenetos, A.; Gratsia, E.; Cardoso, A.-C.; Tsiamis, K. Time Lags in Reporting of Biological Invasions: The Case of Mediterranean Sea. Mediterr. Mar. Sci. 2019, 20, 469–475. [Google Scholar] [CrossRef]
- Etheringron, T.R. Geographical isolation and invasion ecology. Prog. Phys. Geogr. 2015, 39, 1–14. [Google Scholar] [CrossRef]
- Jeschke, J.; Gómez Aparicio, L.; Haider, S.; Heger, T.; Lortie, C.; Pyšek, P.; Strayer, D. Support for Major Hypotheses in Invasion Biology Is Uneven and Declining. NeoBiota 2014, 14, 1–20. [Google Scholar] [CrossRef]
- García-Berthou, E.; Alcaraz, C.; Pou-Rovira, Q.; Zamora, L.; Coenders, G.; Feo, C. Introduction pathways and establishment rates of invasive aquatic species in Europe. Can. J. Fish. Aquat. Sci. 2005, 62, 453–463. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Stefanova, K.; Gladan, Ž.N.; Skejić, S.; Despalatović, M.; Cvitković, I.; Dragičević, B.; Dulčić, J.; Vidjak, O.; et al. Non-Indigenous Species Refined National Baseline Inventories: A Synthesis in the Context of the European Union’s Marine Strategy Framework Directive. Mar. Pollut. Bull. 2019, 145, 429–435. [Google Scholar] [CrossRef]
- Ojaveer, H.; Galil, B.S.; Carlton, J.T.; Alleway, H.; Goulletquer, P.; Lehtiniemi, M.; Marchini, A.; Miller, W.; Occhipinti-Ambrogi, A.; Peharda, M.; et al. Historical Baselines in Marine Bioinvasions: Implications for Policy and Management. PLoS ONE 2018, 13, e0202383. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, G.A.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive, Descriptor 2, Non-Indigenous Species: Delivering Solid Recommendations for Setting Threshold Values for Non-Indigenous Species Pressure on European Seas; Publications Office of the European Union: Luxembourg, 2021; p. 36. [Google Scholar]
- Zenetos, A.; Çinar, M.; Crocetta, F.; Golani, D.; Rosso, A.; Servello, G.; Shenkar, N.; Turon, X.; Verlaque, M. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuar. Coast Shelf Sci. 2017, 191, 171–187. [Google Scholar] [CrossRef]
- Tsiamis, K.; Zenetos, A.; Deriu, I.; Gervasini, E.; Cardoso, A. The native distribution range of the European marine non-indigenous species. Aquatic Invasions 2018, 13, 187–198. [Google Scholar] [CrossRef]
- Carlton, J.T. Biological invasions and cryptogenic species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, V.; Simboura, M.; Tsiamis, K.; Pancucci-Papadopoulou, M. Deep cleaning of alien and cryptogenic species records in the Greek Seas (2018 update). Manag. Biol. Invasions 2018, 9, 209–226. [Google Scholar] [CrossRef]
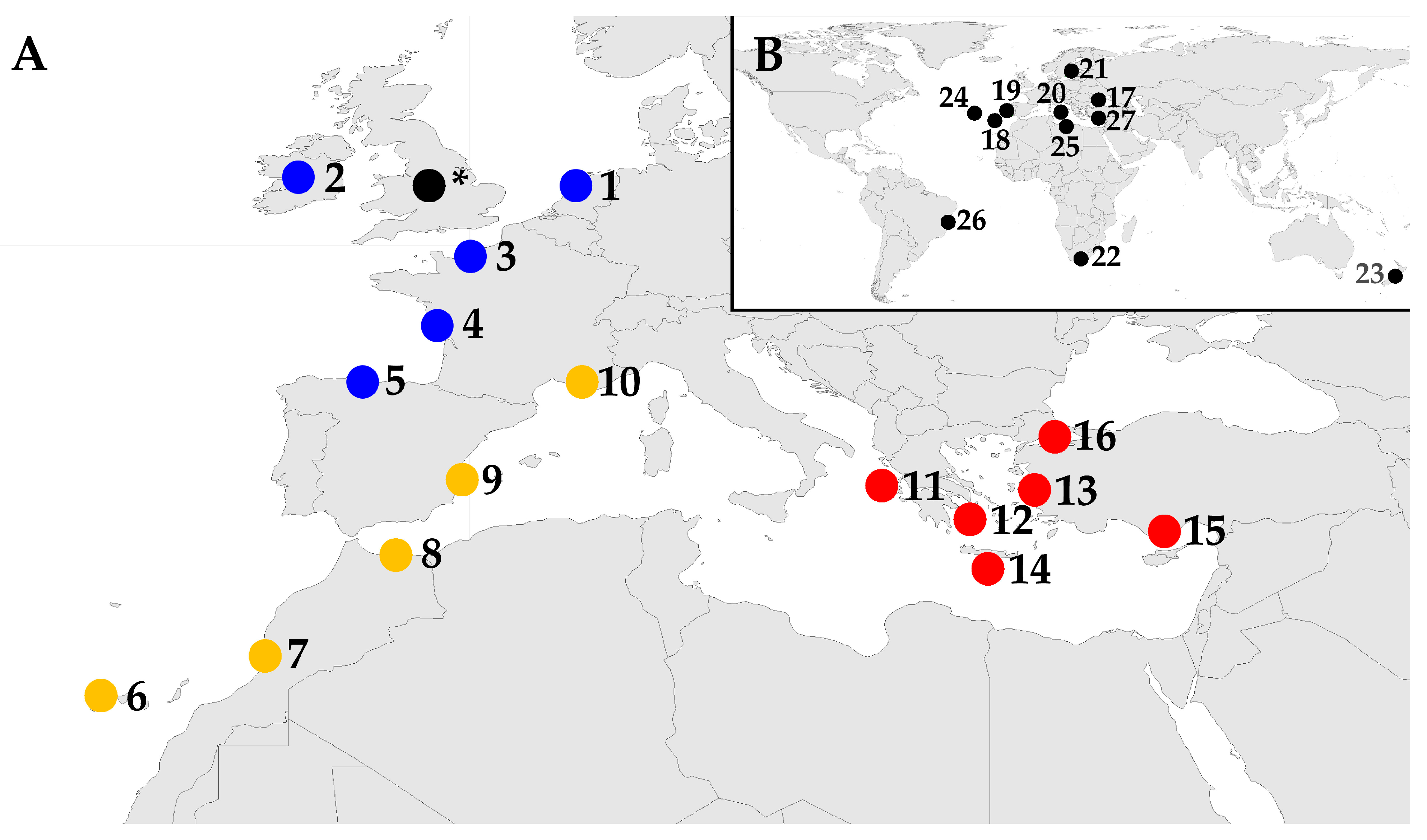
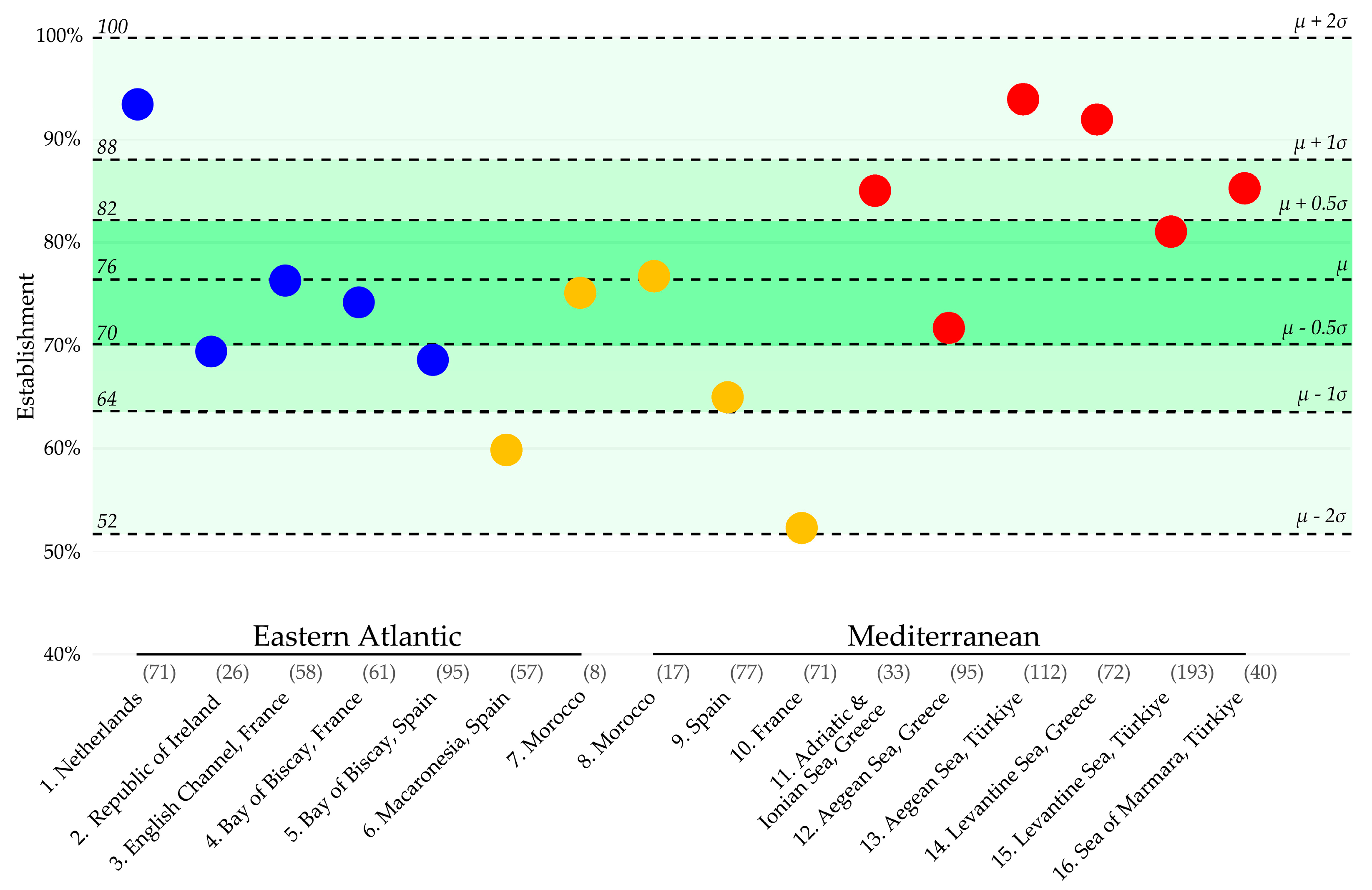
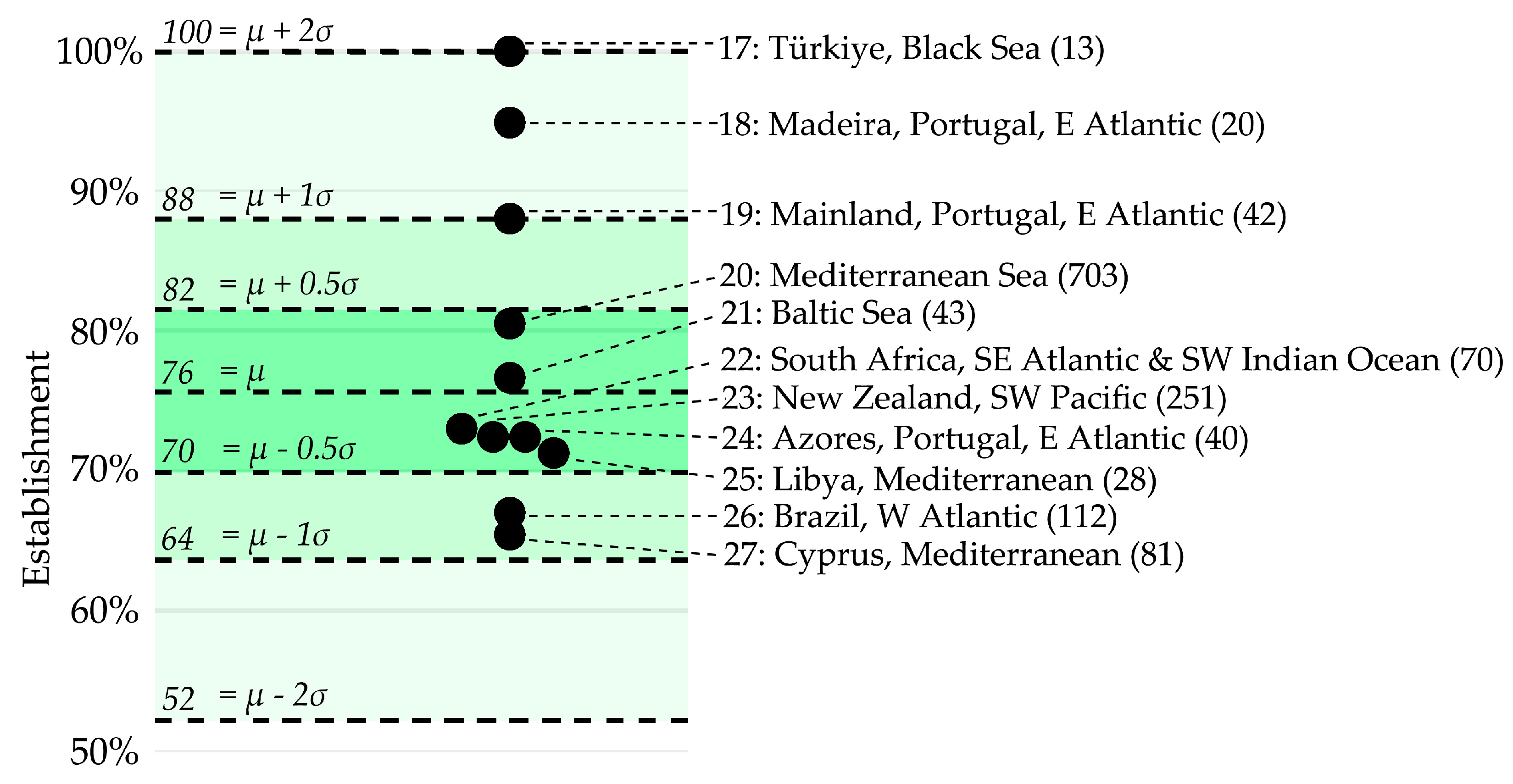
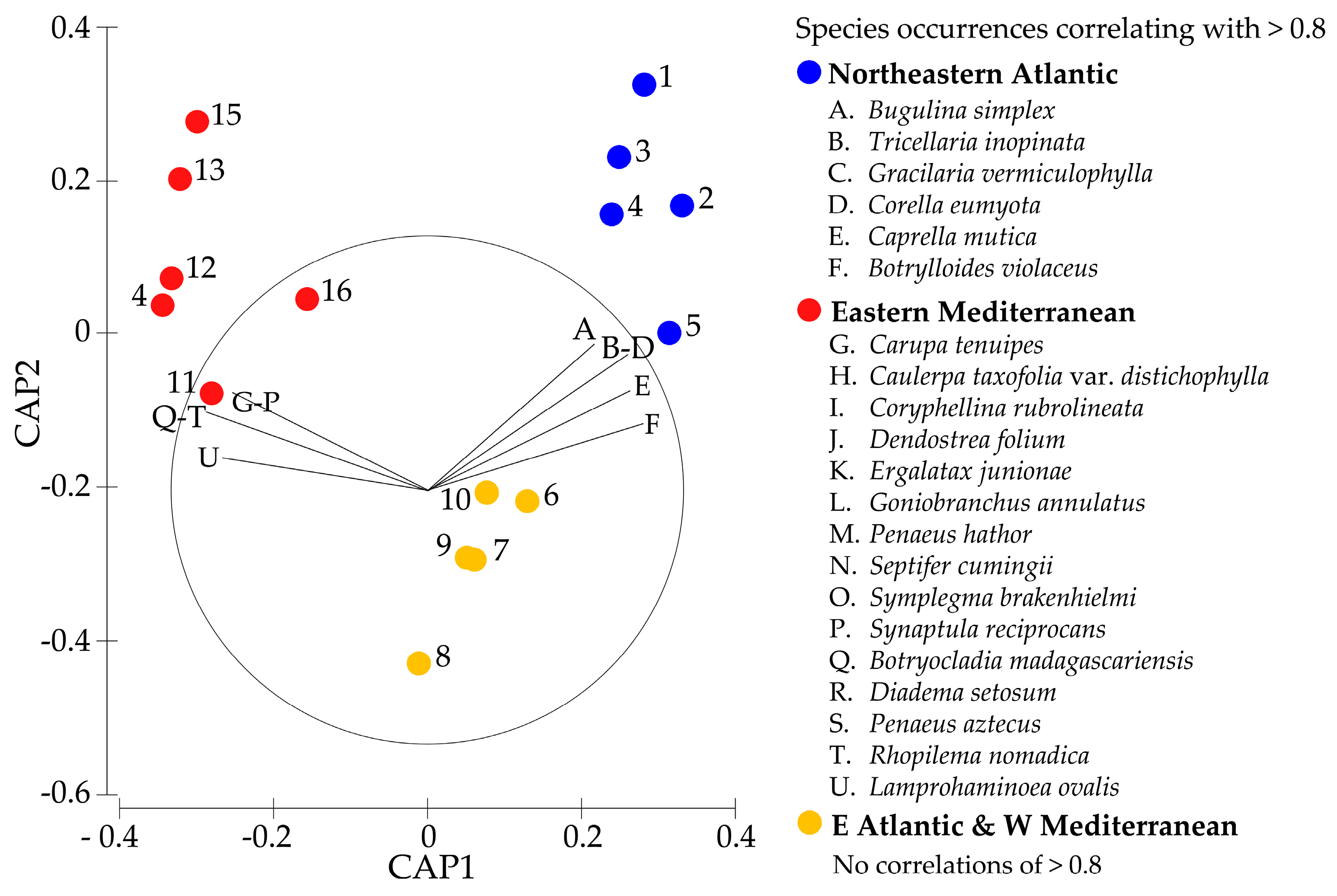
| Group | Minimum | Average | Maximum |
|---|---|---|---|
| NE Atlantic (Regions 1–5) | 68.4% (Region 5) | 76.1% | 93.0% (Region 1) |
| E Atlantic and W Mediterranean (Regions 6–10) | 52.1% (Region 10) | 65.6% | 76.5% (Region 8) |
| E Mediterranean (Regions 11–16) | 71.6% (Region 12) | 84.6% | 93.8% (Region 13) |
| SIMPER Analysis Results | NE Atlantic | E Atlantic and W Med. | E. Mediterranean |
|---|---|---|---|
| Northeastern Atlantic | 34.5% | 10.3% | 4.3% |
| E Atlantic and W Mediterranean | 10.3% | 16.6% | 9.9% |
| Eastern Mediterranean | 4.3% | 9.9% | 28.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppis, S.; Gittenberger, A. Seventies Rule for the Establishment of Non-Native Marine Species. Diversity 2025, 17, 18. https://doi.org/10.3390/d17010018
Coppis S, Gittenberger A. Seventies Rule for the Establishment of Non-Native Marine Species. Diversity. 2025; 17(1):18. https://doi.org/10.3390/d17010018
Chicago/Turabian StyleCoppis, Stan, and Arjan Gittenberger. 2025. "Seventies Rule for the Establishment of Non-Native Marine Species" Diversity 17, no. 1: 18. https://doi.org/10.3390/d17010018
APA StyleCoppis, S., & Gittenberger, A. (2025). Seventies Rule for the Establishment of Non-Native Marine Species. Diversity, 17(1), 18. https://doi.org/10.3390/d17010018






