First Investigation of the Marine Gastrotrich Fauna from the Waters of North Tunisia, with the Description of a New Species of Halichaetonotus (Gastrotricha, Chaetonotida) †
Abstract
1. Introduction
2. Materials and Methods
2.1. New Samples
2.2. Old Samples
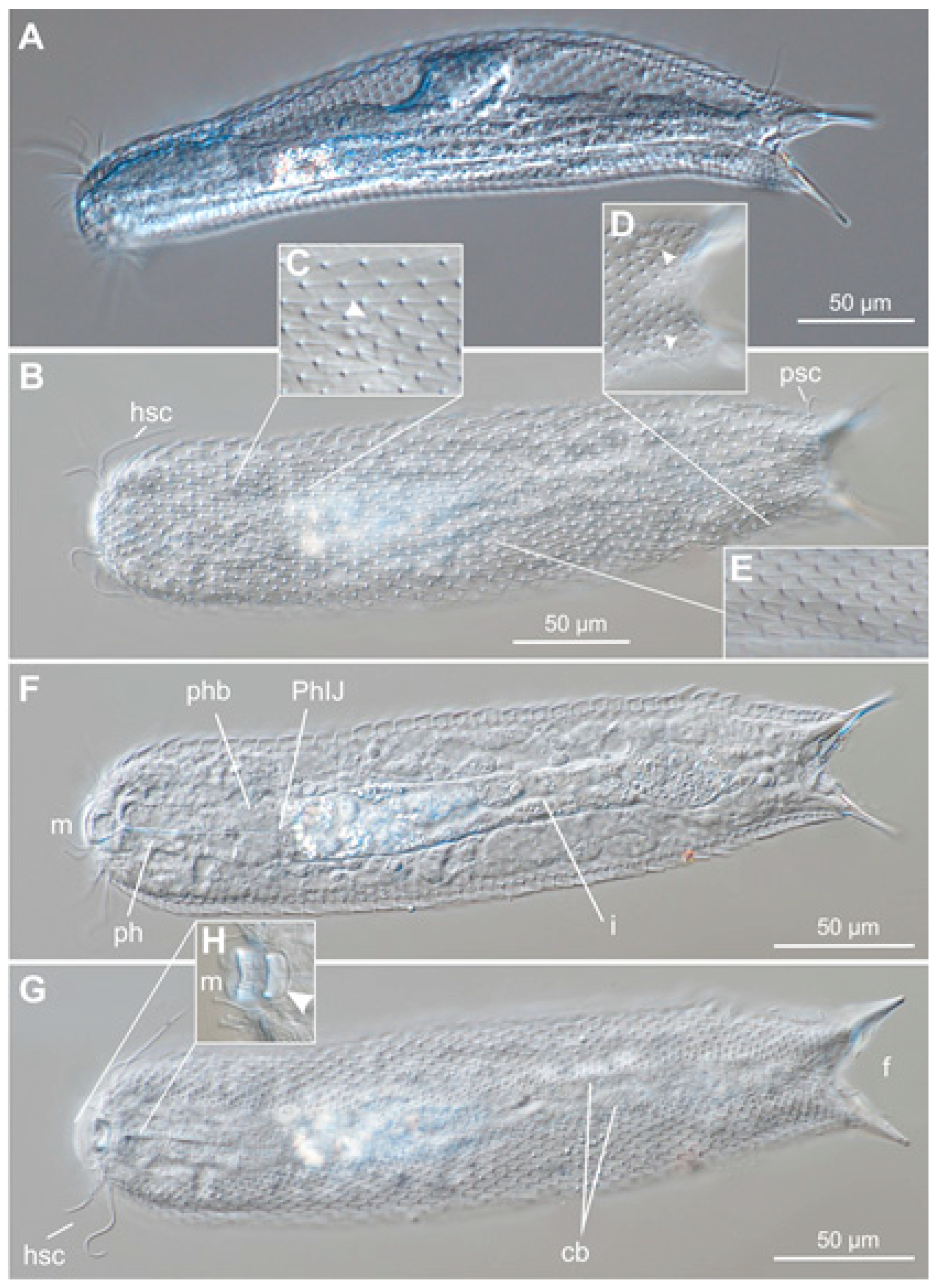
2.3. Species Description
2.4. Granulometric Analysis
3. Results and Discussion
3.1. Sampling Stations
3.2. Granulometric Analysis
3.3. Gastrotrich Fauna
3.4. Taxonomic Account
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gammuto, L.; Serra, V.; Petroni, G.; Todaro, M.A. Molecular phylogenetic position and description of a new genus and species of freshwater Chaetonotidae (Gastrotricha: Chaetonotida: Paucitubulatina), and the annotation of its mitochondrial genome. Invertebr. Syst. 2024, 38, IS23059. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Sibaja-Cordero, J.A.; Segura-Bermúdez, O.A.; Coto-Delgado, G.; Goebel-Otárola, N.; Barquero, J.D.; Cullell-Delgado, M.; Dal Zotto, M. An introduction to the study of Gastrotricha, with a taxonomic key to families and genera of the Group. Diversity 2019, 11, 117. [Google Scholar] [CrossRef]
- Saponi, F.; Todaro, M.A. Status of the Italian freshwater Gastrotricha biodiversity, with the creation of an interactive GIS-based web map. Diversity 2023, 16, 17. [Google Scholar] [CrossRef]
- Coull, B. Long-term variability of estuarine meiobenthos: An 11 Year Study. Mar. Ecol. Prog. Ser. 1985, 24, 205–218. [Google Scholar] [CrossRef]
- Todaro, M.A.; Fleeger, J.W.; Hummon, W.D. Marine Gastrotrichs from the sand beaches of the northern gulf of Mexico: Species list and distribution. Hydrobiologia 1995, 310, 107–117. [Google Scholar] [CrossRef]
- Hochberg, R. Spatiotemporal size-class distribution of Turbanella mustela (Gastrotricha: Macrodasyida) on a northern California beach and its effect on tidal suspension 1. Pac. Sci. 1999, 53, 50–60. [Google Scholar]
- Balsamo, M.; Artois, T.; Smith, J.P.S.; Todaro, M.A.; Guidi, L.; Leander, B.S.; Van Steenkiste, N.W.L. The curious and neglected soft-bodied meiofauna: Rouphozoa (Gastrotricha and Platyhelminthes). Hydrobiologia 2020, 847, 2613–2644. [Google Scholar] [CrossRef] [PubMed]
- Kieneke, A.; Schmidt-Rhaesa, A. 1. Gastrotricha. In Gastrotricha and Gnathifera; Schmidt-Rhaesa, A., Ed.; De Gruyter: Berlin, Germany, 2014; pp. 1–134. ISBN 978-3-11-027381-6. [Google Scholar]
- Balsamo, M.; Todaro, M.A. Gastrotricha. In Freshwater Meiofauna: Biology and Ecology; Bachhuys Publishers: Oegstgeest, The Netherlands, 2002; pp. 45–61. [Google Scholar]
- Todaro, M.A.; Luporini, P. Not too big for its mouth: Direct evidence of a Macrodasyidan Gastrotrich preyed in nature by a dileptid ciliate. Eur. Zool. J. 2022, 89, 785–790. [Google Scholar] [CrossRef]
- Ruppert, E.E. Gastrotricha. In Microscopic Anatomy of Invertebrates: Aschelminthes; Wiley-Blackwell: Hoboken, NJ, USA, 1991; pp. 44–109. [Google Scholar]
- Schnier, J.; Ahlrichs, W.H.; Gruhl, A.; Schulbert, C.; Teichert, S.; Kieneke, A. Ultrastructure of the epidermal gland system of Tetranchyroderma Suecicum Boaden, 1960 (Gastrotricha: Macrodasyida) indicates a defensive function of its exudate. Zoomorphology 2019, 138, 443–462. [Google Scholar] [CrossRef]
- Kånneby, T.; Todaro, M.A. The phylogenetic position of Neogosseidae (Gastrotricha: Chaetonotida) and the origin of planktonic Gastrotricha. Org. Divers. Evol. 2015, 15, 459–469. [Google Scholar] [CrossRef]
- Todaro, M.A.; Leasi, F.; Hochberg, R. A new species, genus and family of marine Gastrotricha from Jamaica, with a phylogenetic analysis of Macrodasyida based on molecular data. Syst. Biodivers. 2014, 12, 473–488. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Leasi, F. An integrated morphological and molecular approach to the description and systematisation of a novel genus and species of Macrodasyida (Gastrotricha). PLoS ONE 2015, 10, e0130278. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Kånneby, T.; Hochberg, R. Integrated data analysis allows the establishment of a new, cosmopolitan genus of marine Macrodasyida (Gastrotricha). Sci. Rep. 2019, 9, 7989. [Google Scholar] [CrossRef]
- Todaro, M.A.; Cesaretti, A.; Dal Zotto, M. Marine Gastrotrichs from Lanzarote, with a description of a phylogenetically relevant species of Urodasys (Gastrotricha, Macrodasyida). Mar. Biodivers. 2019, 49, 2109–2123. [Google Scholar] [CrossRef]
- Leasi, F.; Todaro, M.A. The muscular system of Musellifer delamarei (Renaud-Mornant, 1968) and other chaetonotidans with implications for the phylogeny and systematization of the Paucitubulatina (Gastrotricha). Biol. J. Linn. Soc. 2008, 94, 379–398. [Google Scholar] [CrossRef]
- Schuster, J.; Atherton, S.; Todaro, M.A.; Schmidt-Rhaesa, A.; Hochberg, R. Redescription of Xenodasys riedli (Gastrotricha: Macrodasyida) based on SEM analysis, with first report of population density data. Mar. Biodivers. 2018, 48, 259–271. [Google Scholar] [CrossRef]
- Fromm, B.; Tosar, J.P.; Aguilera, F.; Friedländer, M.R.; Bachmann, L.; Hejnol, A. Evolutionary implications of the microRNA- and piRNA complement of Lepidodermella squamata (Gastrotricha). Non-Coding RNA 2019, 5, 19. [Google Scholar] [CrossRef]
- Garraffoni, A.R.S.; Araújo, T.Q.; Lourenço, A.P.; Guidi, L.; Balsamo, M. Integrative taxonomy of a new Redudasys species (Gastrotricha: Macrodasyida) sheds light on the invasion of fresh water habitats by Macrodasyids. Sci. Rep. 2019, 9, 2067. [Google Scholar] [CrossRef]
- Bosco, I.; Lourenço, A.P.; Guidi, L.; Balsamo, M.; Hochberg, R.; Garraffoni, A.R.S. Integrative description of a new species of Acanthodasys Remane, 1927 (Gastrotricha, Macrodasyida, Thaumastodermatidae) based on four distinct morphological techniques and molecular data. Zool. Anz. 2020, 286, 31–42. [Google Scholar] [CrossRef]
- Campos, A.; Todaro, M.A.; Garraffoni, A.R.S. A New species of Paraturbanella Remane, 1927 (Gastrotricha, Macrodasyida) from the Brazilian coast, and the molecular phylogeny of Turbanellidae Remane, 1926. Diversity 2020, 12, 42. [Google Scholar] [CrossRef]
- Martínez, A.; Eckert, E.M.; Artois, T.; Careddu, G.; Casu, M.; Curini-Galletti, M.; Gazale, V.; Gobert, S.; Ivanenko, V.N.; Jondelius, U.; et al. Human access impacts biodiversity of microscopic animals in sandy beaches. Commun. Biol. 2020, 3, 175. [Google Scholar] [CrossRef]
- Kieneke, A.; Todaro, M.A. Discovery of two ‘chimeric’ Gastrotricha and their systematic placement based on an integrative approach. Zool. J. Linn. Soc. 2021, 192, 710–735. [Google Scholar] [CrossRef]
- Kolicka, M. Gastrotricha—Not only in sediments: New epiphytic species of Chaetonotida from the Jubilee greenhouse of the botanical garden in Kraków. Eur. J. Taxon. 2019, 511, 1–100. [Google Scholar] [CrossRef]
- Kolicka, M. From saline to salty? Heterolepidoderma sinus (Chaetonotida, Chaetonotidae) from subsaline coal mine settling ponds. Zootaxa 2019, 4559, 568–572. [Google Scholar] [CrossRef]
- Kolicka, M.; Dabert, M.; Olszanowski, Z.; Dabert, J. Sweet or salty? The origin of freshwater Gastrotrichs (Gastrotricha, Chaetonotida) revealed by molecular phylogenetic analysis. Cladistics 2020, 36, 458–480. [Google Scholar] [CrossRef] [PubMed]
- Minowa, A.K.; Garraffoni, A.R.S. New data on Brazilian semiplanktonic Gastrotrichs (Gastrotricha: Chaetonotida). Zootaxa 2022, 5209, 45–68. [Google Scholar] [CrossRef]
- Rataj Križanová, F.; Vďačný, P. A huge undescribed diversity of the subgenus Hystricochaetonotus (Gastrotricha, Chaetonotidae, Chaetonotus) in central Europe. Eur. J. Taxon. 2022, 840, 1–93. [Google Scholar] [CrossRef]
- Rataj Križanová, F.; Vďačný, P. A Heterolepidoderma and Halichaetoderma gen. nov. (Gastrotricha: Chaetonotidae) riddle: Integrative taxonomy and phylogeny of six new freshwater species from Central Europe. Zool. J. Linn. Soc. 2023, 200, zlad079. [Google Scholar] [CrossRef]
- Salgado, K.F.A.; Minowa, A.K.; Garraffoni, A.R.S. New records, neotype designation and DNA sequences of three species of Chaetonotus (Gastrotricha: Chaetonotidae) from Brazil. Zootaxa 2022, 5213, 101–129. [Google Scholar] [CrossRef]
- Araújo, T.Q. A description of a new species of Cephalodasys (Macrodasyida: Gastrotricha) from Florida, USA using an integrative morphological approach. Zootaxa 2024, 5463, 581–597. [Google Scholar] [CrossRef]
- Saponi, F.; Kosakyan, A.; Cesaretti, A.; Todaro, M.A. A contribution to the taxonomy and phylogeny of the genus Chaetonotus (Gastrotricha, Paucitubulatina, Chaetonotidae), with the description of a new species from Italian inland waters. Eur. Zool. J. 2024, 91, 1078–1092. [Google Scholar] [CrossRef]
- Hochberg, R.; Litvaitis, M.K. A muscular double helix in Gastrotricha. Zoologischer 2001, 240, 61–68. [Google Scholar] [CrossRef]
- Hochberg, R.; Litvaitis, M. The muscular system of Dactylopodola Baltica and other Macrodasyidan Gastrotrichs in a functional and phylogenetic perspective. Zool. Scr. 2001, 30, 325–336. [Google Scholar] [CrossRef]
- Hochberg, R.; Litvaitis, M.K. Organization of muscles in Chaetonotida Paucitubulatina (Gastrotricha). Meiofauna Mar. 2003, 12, 47–58. [Google Scholar]
- Hochberg, R.; Litvaitis, M.K. Ultrastructural and immunocytochemical observations of the nervous systems of three macrodasyidan gastrotrichs. Acta Zool. 2003, 84, 171–178. [Google Scholar] [CrossRef]
- Leasi, F.; Todaro, M.A. Meiofaunal cryptic species revealed by confocal microscopy: The case of Xenotrichula Intermedia (Gastrotricha). Mar. Biol. 2009, 156, 1335–1346. [Google Scholar] [CrossRef]
- Leasi, F.; Rothe, B.H.; Schmidt-Rhaesa, A.; Todaro, M.A. The musculature of three species of gastrotrichs surveyed with confocal laser scanning microscopy (CLSM). Acta Zool. 2006, 87, 171–180. [Google Scholar] [CrossRef]
- Rothe, B.H.; Kieneke, A.; Schmidt-Rhaesa, A. The nervous system of Xenotrichula intermedia and X. velox (Gastrotricha: Paucitubulatina) by means of immunohistochemistry (IHC) and TEM. Meiofauna Mar. 2011, 19, 71–88. [Google Scholar]
- Rothe, B.H.; Schmidt-Rhaesa, A.; Kieneke, A. The nervous system of Neodasys chaetonotoideus (Gastrotricha: Neodasys) revealed by combining confocal laserscanning and transmission electron microscopy: Evolutionary comparison of neuroanatomy within the Gastrotricha and basal protostomia. Zoomorphology 2011, 130, 51–84. [Google Scholar] [CrossRef]
- Kieneke, A.; Ostmann, A. Structure, function and evolution of somatic musculature in Dasydytidae (Paucitubulatina, Gastrotricha). Zoomorphology 2012, 131, 95–114. [Google Scholar] [CrossRef]
- Münter, L.; Kieneke, A. Novel myo-anatomical insights to the Xenotrichula Intermedia species complex (Gastrotricha: Paucitubulatina): Implications for a pan-European species and reconsideration of muscle homology among Paucitubulatina. Proc. Biol. Soc. Wash. 2017, 130, 166–186. [Google Scholar] [CrossRef]
- Guidi, L.; Todaro, M.A.; Ferraguti, M.; Balsamo, M. Reproductive system and spermatozoa ultrastructure support the phylogenetic proximity of Megadasys and Crasiella (Gastrotricha, Macrodasyida). Contrib. Zool. 2014, 83, 119–131. [Google Scholar] [CrossRef]
- Guidi, L.; Garraffoni, A.R.S.; Semprucci, F.; Balsamo, M. Spermatozoa ultrastructure, spermatogenesis and reproductive system of Acanthodasys australis (Gastrotricha, Macrodasyida). Zool. Anz. 2020, 286, 108–116. [Google Scholar] [CrossRef]
- Guidi, L.; Balsamo, M.; Ferraguti, M.; Todaro, M.A. Reproductive organs and spermatogenesis of the peculiar spermatozoa of the genus Kryptodasys (Gastrotricha, Macrodasyida), with an appraisal of the occurrence and origin of the tail-less spermatozoa in Gastrotricha. J. Zool. Syst. Evol. Res. 2021, 59, 1673–1688. [Google Scholar] [CrossRef]
- Guidi, L.; Balsamo, M.; Grassi, E.; Semprucci, F.; Todaro, M.A. New data on reproductive system and spermatozoa confirm Macrodasys as a model in comparative reproductive analysis in Macrodasyida (Gastrotricha). Water 2022, 14, 3085. [Google Scholar] [CrossRef]
- Cesaretti, A.; Leasi, F.; Todaro, M.A. Confocal laser scanning microscopy applied to a new species helps understand the functioning of the reproductive apparatus in stylet-bearing Urodasys (Gastrotricha: Macrodasyida). Water 2023, 15, 1106. [Google Scholar] [CrossRef]
- Curini-Galletti, M.; Artois, T.; Delogu, V.; Smet, W.H.D.; Fontaneto, D.; Jondelius, U.; Leasi, F.; Martínez, A.; Meyer-Wachsmuth, I.; Nilsson, K.S.; et al. Patterns of diversity in soft-bodied meiofauna: Dispersal ability and body size matter. PLoS ONE 2012, 7, e33801. [Google Scholar] [CrossRef]
- Curini-Galletti, M.; Artois, T.; Di Domenico, M.; Fontaneto, D.; Jondelius, U.; Jörger, K.M.; Leasi, F.; Martínez, A.; Norenburg, J.L.; Sterrer, W.; et al. Contribution of soft-bodied meiofaunal taxa to Italian marine biodiversity. Eur. Zool. J. 2020, 87, 369–384. [Google Scholar] [CrossRef]
- Kieneke, A.; Martínez Arbizu, P.M.; Fontaneto, D. Spatially structured populations with a low level of cryptic diversity in European marine Gastrotricha. Mol. Ecol. 2012, 21, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Magpali, L.; Machado, D.R.P.; Araújo, T.Q.; Garraffoni, A.R.S. Long distance dispersal and pseudo-cryptic species in Gastrotricha: First description of a new species (Chaetonotida, Chaetonotidae, Polymerurus) from an oceanic island with volcanic rocks. Eur. J. Taxon. 2021, 746, 62–93. [Google Scholar] [CrossRef]
- Macher, J.-N.; Martínez, A.; Çakir, S.; Cholley, P.-E.; Christoforou, E.; Curini Galletti, M.; van Galen, L.; García-Cobo, M.; Jondelius, U.; de Jong, D.; et al. Enhancing metabarcoding efficiency and ecological insights through integrated taxonomy and DNA reference barcoding: A case study on beach meiofauna. Mol. Ecol. Resour. 2024, 24, e13997. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Ben Rais Lasram, F.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T.; et al. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.A.; Matinato, L.; Balsamo, M.; Tongiorgi, P. Faunistics and zoogeographical overview of the Mediterranean and Black Sea marine Gastrotricha. Biogeographia 2003, 24, 131–160. [Google Scholar] [CrossRef]
- Todaro, M.A.; Leasi, F.; Bizzarri, N.; Tongiorgi, P. Meiofauna densities and gastrotrich community composition in a Mediterranean Sea cave. Mar. Biol. 2006, 149, 1079–1091. [Google Scholar] [CrossRef]
- Todaro, M.A.; Guidi, L.; Leasi, F.; Tongiorgi, P. Morphology of Xenodasys (Gastrotricha): The first species from the Mediterranean sea and the establishment of Chordodasiopsis gen. nov. and Xenodasyidae fam. nov. J. Mar. Biol. Assoc. U. K. 2006, 86, 1005–1015. [Google Scholar] [CrossRef]
- Marotta, R.; Todaro, M.A.; Ferraguti, M. The unique gravireceptor organs of Pleurodasys helgolandicus (Gastrotricha: Macrodasyida). Zoomorphology 2008, 127, 111–119. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A. Italian marine Gastrotricha: VI. seven new species of Macrodasyida. Zootaxa 2009, 2278, 47–68. [Google Scholar] [CrossRef]
- Hummon, W.D. Global Distribution of Marine Gastrotricha. Available online: http://www.gastrotricha.unimore.it/checklist.htm (accessed on 18 October 2024).
- Hummon, W.D. Marine Gastrotricha of the Near East: 1. fourteen new species of Macrodasyida and a redescription of Dactylopodola agadasys Hochberg, 2003. Zookeys 2011, 94, 1–59. [Google Scholar] [CrossRef]
- Dal Zotto, M.; Ghiviriga, S.; Todaro, M.A. A new Tetranchyroderma (Gastrotricha, Thaumastodermatidae) with triancres from the Mediterranean sea. Meiofauna Mar. 2010, 18, 41–48. [Google Scholar]
- Leasi, F.; Todaro, M.A. The gastrotrich community of a North Adriatic sea site, with a redescription of Musellifer profundus (Chaetonotida: Muselliferidae). J. Mar. Biol. Assoc. U. K. 2010, 90, 645–653. [Google Scholar] [CrossRef]
- Sergeeva, N.; Ürkmez, D.; Todaro, M.A. Significant occurrence of Musellifer profundus Vivier, 1974 (Gastrotricha, Chaetonotida) in the Black sea. Check List 2019, 15, 219–224. [Google Scholar] [CrossRef]
- Saponi, F.; Rebecchi, C.; Cesaretti, A.; Souid, A.; Todaro, M.A. Nuovi dati sui Gastrotrichi marini della Sicilia. Biol. Mar. Mediterr. 2024, 28, 133–136. [Google Scholar]
- Todaro, M.A.; Rebecchi, C. Gastrotricha from the gulf of Squillace and the gulf of Sant’Eufemia (Calabria, Italy). In Proceedings of the 52 SIBM Congress, Rome, Italy, 12–15 June 2024. [Google Scholar]
- WoRMS—World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=stats (accessed on 18 October 2024).
- d’Hondt, J.-L. Contribution a l’étude de la microfaune interstitielle des plages de l’ouest Algérien. Vie Milieu 1973, XXIII, 227–241. [Google Scholar]
- Westheide, W. Räumliche und zeitliche differenzierungen im verteilungsmuster der marinen interstitialfauna. Verhandlungsbericht Dtsch. Zool. 1972, 65, 23–32. [Google Scholar]
- Todaro, M.A.; Kånneby, T.; Dal Zotto, M.; Jondelius, U. Phylogeny of Thaumastodermatidae (Gastrotricha: Macrodasyida) inferred from nuclear and mitochondrial sequence data. PLoS ONE 2011, 6, e17892. [Google Scholar] [CrossRef]
- Remane, A. Beitrage zur systematik der susswassergastrotrichen. Zool. Jahrbücher Abt. Syst. Geogr. Und Biol. Tiere 1927, 53, 268–320. [Google Scholar]
- Remane, V.A. Mesodasys, Ein neues genus der Gastrotricha Macrodasyoidea aus der Kieler Bucht. Kiel. Meeresforsch 1951, 8, 102–105. [Google Scholar]
- Tongiorgi, P.; Balsamo, M. A new Tetranchyroderma species (Gastrotricha, Macrodasyoidea) from the Adriatic coast. Boll. Zool. 1984, 51, 335–338. [Google Scholar] [CrossRef]
- Remane, A. Die gastrotrichen des küstengrundwassers von schilksee. Schriften Naturwissenschaftlichen Ver. Schleswig-Holst. 1934, 20, 473–478. [Google Scholar]
- Hummon, W.D.; Todaro, M.A.; Tongiorgi, P. Italian marine Gastrotricha: II. one new genus and ten new species of Macrodasyida. Boll. Zool. 1993, 60, 109–127. [Google Scholar] [CrossRef]
- Todaro, M.A. Contribution to the study of the Mediterranean meiofauna: Gastrotricha from the island of Ponza, Italy. Boll. Zool. 1992, 59, 321–333. [Google Scholar] [CrossRef]
- Seward-Thompson, B.L.; Hails, J.R. An Appraisal of the computation of statistical parameters in grain size analysis. Sedimentology 1973, 20, 161–169. [Google Scholar] [CrossRef]
- Todaro, M.A.D.; Hummon, W.D.; Balsamo, M.; Fregni, E.; Tongiorgi, P. Inventario dei gastrotrichi marini italiani: Una checklist annotata. Atti Della Soc. Toscana Sci. Nat. Mem. Ser. B 2001, 107, 75–137. [Google Scholar]
- Remane, A. Organisation und systematische stellung der aberranten Gastrotrichen. Verhandlungen Dtsch. Zool. Ges. 1925, 30, 121–128. [Google Scholar]
- Rao, G.C.; Clausen, C. Planodasys marginalis gen. et sp. nov. and Planodasyidae fam. nov. (Gastotricha Magrodasyoidea). Sarsia 1970, 42, 73–82. [Google Scholar] [CrossRef]
- Hummon, W.D.; Todaro, M.A. Analytic taxonomy and notes on marine, brackish-water and estuarine Gastrotricha. Zootaxa 2010, 2392, 1–32. [Google Scholar] [CrossRef]
- Gagne, G.D. Dolichodasys elongatus n.g., n.sp., a new macrodasyid gastrotrich from New England. Trans. Am. Microsc. Soc. 1977, 96, 19. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rossaro, S.; Dal Zotto, M.; Giere, O. Marine gastrotrichs from west-Mediterranean calcareous sediments. In Proceedings of the 12th International Meiofauna Conference, Ravenna, Italy, 11–16 July 2004. [Google Scholar]
- Wilke, U. Mediterrane Gastrotrichen. Zoologische Jahrbücher Abteilung für Systematik. Geogr. Biol. Tiere 1954, 82, 497–550. [Google Scholar]
- Todaro, M.A. (Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy). Personal communication, 2024.
- Strand, E. Zoological and Palaeontological Nomenclatorical Notes; Arbeiten aus dem Systematisch-Zoologischen Institut der Lettländischen Universität: Riga, Latvia, 1929. [Google Scholar]
- Todaro, M.A.; Hummon, W. An overview and a dichotomous key to genera of the phylum Gastrotricha. Meiofauna Mar 2008, 16, 3–20. [Google Scholar]
- Todaro, M.A. (Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy). Unpublished work. 2024.
- Remane, A. Neue aberrante Gastrotrichen. I. Macrodasys buddenbrocki nov. gen. nov. spec. Zool. Anz. 1924, 61, 289–297. [Google Scholar]
- Remane, A. Morphologie und verwandtschaftsbeziehungen der aberranten Gastrotrichen I. Z. Morphol. Okol. Tiere 1926, 5, 625–754. [Google Scholar] [CrossRef]
- Schoepfer-Sterrer, C. Five new species of Urodasys and remarks on the terminology of the genital organs in Macrodasyidae (Gastrotricha). Cah. Biol. Mar. 1974, 15, 229–259. [Google Scholar]
- Hochberg, R.; Atherton, S.; Kieneke, A. Marine Gastrotricha of Little Cayman island with the description of one new species and an initial assessment of meiofaunal diversity. Mar. Biodivers. 2014, 44, 89–113. [Google Scholar] [CrossRef]
- Cesaretti, A.; Kosakyan, A.; Saponi, F.; Todaro, M.A. Gaining and losing on the way: The evolutionary scenario of reproductive diversification in genus Urodasys (Macrodasyida, Gastrotricha) inferred by multi-gene phylogeny. Zool. J. Linn. Soc. 2024, 202, zlae148. [Google Scholar] [CrossRef]
- Todaro, M.A.; Rocha, C.E.F. Further data on marine Gastrotrichs from the state of São Paulo and the first records from the state of Rio de Janeiro (Brazil). Meiofauna Mar. 2005, 14, 27–31. [Google Scholar]
- Todaro, M.A.; Balsamo, M.; Tongiorgi, P. Marine gastrotrichs from the Tuscan Archipelago (Tyrrhenian Sea): I. Macrodasyida, with description of three new species. Boll. Zool. 1992, 59, 471–485. [Google Scholar] [CrossRef]
- Balsamo, M.; Fregni, E.; Tongiorgi, P. Marine Gastrotricha from the coasts of Sardinia (Italy). Bolletino Zool. 1995, 62, 273–286. [Google Scholar] [CrossRef]
- Fregni, E.; Faienza, M.G.; De Zio Grimaldi, S.; Tongiorgi, P.; Balsamo, M. Marine gastrotrichs from the Tremiti Archipelago in the Southern Adriatic Sea, with the description of two new species of Urodasys. Ital. J. Zool. 1999, 66, 183–194. [Google Scholar] [CrossRef]
- Schmidt, P. Interstitielle Fauna von Galapagos, IV.Gastrotricha. Mikrofauna Meeresbod. 1974, 26, 1–76. [Google Scholar]
- Boaden, P.J.S. Three new thiobiotic Gastrotricha. Cah. Biol. Mar. 1974, 15, 267–378. [Google Scholar]
- Kisielewski, J. New records of marine Gastrotricha from the French coasts of Manche and Atlantic I. Macrodasyida, with descriptions of seven new species. Bull. Muséum Natl. D’histoire Nat. 1987, 9, 837–877. [Google Scholar] [CrossRef]
- Remane, A. Neue Gastrotricha Macrodasyoidea. Zool. Jahrbuecher Abt. Syst. Oekologie Geogr. Tiere 1927, 54, 203–242. [Google Scholar]
- Ruppert, E.E. The reproductive system of gastrotrichs. III. Genital organs of Thaumastodermatinae subfam. n. and Diplodasyinae subfam. n. with discussion of reproduction in Macrodasyida. Zool. Scr. 1978, 7, 93–114. [Google Scholar] [CrossRef]
- Swedmark, B. Etude de la microfaune des sables marins de la région de Marseille. Arch. Zool. Expérimentale Générale 1956, 2, 70–95. [Google Scholar]
- Todaro, M.A.; Balsamo, M.; Tongiorgi, P. Marine gastrotrich fauna in Corsica (France), with a description of a new species of the genus Tetranchyroderma (Macrodasyida, Thaumastodermatidae). Sarsia 2002, 87, 248–257. [Google Scholar] [CrossRef]
- Todaro, M.A.; Balsamo, M.; Tongiorgi, P. Gastrotricha. In Check list della flora e della fauna dei mari Italiani:(Parte I) (ed. G. Relini). Biol. Mar. Mediterr. 2009, (Suppl. S15), 160–169. [Google Scholar]
- Gerlach, S.A. Gastrotrichen aus dem Küstengrundwasser des Mittelmeeres. Zool. Anz. 1953, 150, 203–211. [Google Scholar]
- Thane-Fenchel, A. Interstitial gastrotrichs in some south Florida Beaches. Ophelia 1970, 7, 113–137. [Google Scholar] [CrossRef]
- Hummon, W.D. Tetranchyroderma parapapii n. sp.(Gastrotricha, Thaumastodermatidae), a North American analog to the european T. papii, with a redescription of the latter. Meiofauna Mar. 2009, 17, 121–132. [Google Scholar]
- Todaro, M.A. (Department of Life Sciences, University of Modena and Reggio Emilia, Modena, Italy). Unpublished work. 2020.
- Swedmark, B. Description de Paraturbanella teissieri, n.sp. (Gastrotriche Macrodasyoide). Bull. Sociéte Zool. Fr. 1954, 79, 46–49. [Google Scholar]
- Todaro, M.A. Paraturbanella solitaria a new psammic species (Gastrotricha: Macrodasyida), from the coast of California. Proc. Biol. Soc. Wash. 1995, 108, 553–559. [Google Scholar]
- Tongiorgi, P. Two interesting Macrodasyoidea (Gastrotricha) from the coast of Tuscany. Boll. Zool. 1975, 42, 275–278. [Google Scholar] [CrossRef]
- Balsamo, M.; Ferraguti, M.; Guidi, L.; Todaro, A.M.; Tongiorgi, P. Reproductive system and spermatozoa of Paraturbanella teissieri (Gastrotricha, Macrodasyida): Implications for sperm transfer modality in Turbanellidae. Zoomorphology 2002, 121, 235–241. [Google Scholar] [CrossRef]
- Schultze, M. Über Chaetonotus und Ichthydium (Ehrb.) und eine ueue verwandte gattung Turbanella. Müller’s Arch. Anat. Physiol. Wiss. Med. 1853, 6, 241–254. [Google Scholar]
- Kaplan, G. Premières observations sur les Gastrotriches psammophiles des côtes du Calvados. Arch. Zool. Exp. Gén. 1958, 1, 27–37. [Google Scholar]
- Hummon, W.D. Gastrotricha of the North Atlantic Ocean: 1. Meiofauna Mar. 2008, 16, 117–174. [Google Scholar]
- Dal Zotto, M.; Leasi, F.; Todaro, M.A. A new species of Turbanellidae (Gastrotricha, Macrodasyida) from Jamaica, with a key to species of Paraturbanella. ZooKeys 2018, 734, 105–119. [Google Scholar] [CrossRef] [PubMed]
- d’Hondt, J.L. Gastrotricha. Oceanogr. Mar. Biol. Annu. Rev. 1971, 9, 141–192. [Google Scholar]
- Gosse, P.H. The natural history of the hairy-backed animalcules (Chaetonotidae). Intellect. Obs. 1864, 5, 387–406. [Google Scholar]
- Kisielewski, J. Inland-water Gastrotricha from Brazil. Ann. Zool. 1991, 43, 1–168. [Google Scholar]
- Voigt, M. Eine neue Gastrotrichenspecies (Chaetonotus arquatus) aus dem Schlossparkteiche Zu Plön. Plöner Forschungsberichte 1903, 10, 90–93. [Google Scholar]
- Remane, A. Gastrotricha. In Die Tierwelt der Nord-und Ostsee; Grimpe, G., Ed.; Akademische Verlagsgesellschaft: Leipzig, Germany, 1927. [Google Scholar]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Contribution à la connaissance des gastrotriches des côtes de Toscane. Cah. Biol. Mar. 1971, 12, 433–455. [Google Scholar]
- Balsamo, M.; Todaro, M.A.; Tongiorgi, P. Marine gastrotrichs from the Tuscan Achipelago (Tyrrhenian Sea): II. Chaetonotida, with description of three new species. Boll. Zool. 1992, 59, 487–498. [Google Scholar] [CrossRef]
- Hummon, W.D. Gastrotricha from Beaufort, North Carolina, USA. Cah. Biol. Mar. 1974, 15, 85–92. [Google Scholar]
- Ehrenberg, C.G. Organisation, Systematik, Und Geographisches Verhältnis Der Infusionstierchen. In Druckerei dei Koningliche Akademie der Wissenschaften.t. I–VIII; F. Dümmler: Berlin, Germany, 1830; 108p. [Google Scholar]
- Hummon, W.D. Marine Gastrotricha of the Caribbean sea: A review and new descriptions. Bull. Mar. Sci. 2010, 86, 661–708. [Google Scholar]
- Remane, A. Gastrotricha und Kinorhyncha. In Klassen und Ordnungen des Tierreichs; Akademische Verlagsgesellschaft: Leipzig, Germany, 1935–1936; Band IV, Abt. 2, Buch 1,Teil 2, Lieferung 1: pp. 1–160, 2: pp. 161–242 + Appendix, pp. 373–385. [Google Scholar]
- ICZN. International Code of Zoological Nomenclature, 4th ed.; The Natural History Museum, The International Trust for Zoological Nomenclature: London, UK, 1999. [Google Scholar]
- ICZN. Declaration 45—Addition of recommendations to article 73 and of the term “specimen, preserved” to the glossary. Bull. Zool. Nomencl. 2017, 73, 96–97. [Google Scholar] [CrossRef]
- Evans, W.A. Five new species of marine Gastrotricha from the Atlantic coast of Florida. Bull. Mar. Sci. 1992, 51, 315–328. [Google Scholar]
- Hummon, W.D.; Balsamo, M.; Todaro, M.A. Italian marine Gastrotricha: I. Six new and one redescribed species of Chaetonotida. Boll. Zool. 1992, 59, 499–516. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.D.; Bownes, S.J.; Perissinotto, R. First records of Gastrotricha from South Africa, with description of a new species of Halichaetonotus (Chaetonotida, Chaetonotidae). ZooKeys 2011, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mock, H. Chaetonotoidea (Gastrotricha) der Nordseeinsel Sylt. Mikrofauna Meeresbod. 1979, 78, 1–107. [Google Scholar]
- Todaro, M.A.; Balsamo, M. Marine gastrotrichs from Sicily (Italy). Biol. Gallo-Hell. 1995, 22, 291–292. [Google Scholar]
- Schrom, H. Nordadriatische Gastrotrichen. Helgol. Wiss. Meeresunters. 1972, 23, 286–351. [Google Scholar] [CrossRef]
- Balsamo, M.; Fregni, E.; Tongiorgi, P. Marine Gastrotricha from Sicily with the description of a new species of Chaetonotus. Ital. J. Zool. 1996, 63, 173–183. [Google Scholar] [CrossRef]
- Remane, A. Xenotrichula velox nov. gen. nov. spec., ein Chaetonotoides Gastrotrich mit Männlichen Geschlechtsorganen. Zool. Anz. 1927, 71, 289–294. [Google Scholar]
- Ruppert, E. Morphology and systematics of the Xenotrichulidae. Mikrofauna Meeresbod. 1979, 76, 5–56. [Google Scholar]
- Hummon, W.D. Some taxonomic revisions and nomenclatural notes concerning marine and brackish-water Gastrotricha. Trans. Am. Microsc. Soc. 1974, 93, 194. [Google Scholar] [CrossRef]
- Renaud-Mornant, J. Présence du Genre Polymerurus en Milieu Marin, Description de Deux Espèces Nouvelles (Gastrotricha, Chaetonotoidae); Pubblicazioni della Stazione Zoologica di Napoli: Naples, Italy, 1968. [Google Scholar]
- Luporini, P.; Magagnini, G.; Tongiorgi, P. Chaetonotoid gastrotrichs of the Tuscan Coast. Boll. Zool. 1973, 40, 31–40. [Google Scholar] [CrossRef]
- Araújo, T.Q.; Wieloch, A.H.; Hochberg, R.; Garraffoni, A.R.S. Description of Xenotrichula tropicalis sp. nov. (Gastrotricha: Chaetonotida) and new records of Xenotrichulidae species from Brazil and USA. Ann. Zool. 2022, 72, 167–185. [Google Scholar] [CrossRef]
- Todaro, M.A. Gastrotricha World Portal: Marine Species. Available online: http://www.gastrotricha.unimore.it/marine.htm (accessed on 13 November 2024).
- Todaro, M.A.; Fleeger, J.W.; Hu, Y.P.; Hrincevich, A.W.; Foltz, D.W. Are meiofaunal species cosmopolitan? Morphological and molecular analysis of Xenotrichula intermedia (Gastrotricha: Chaetonotida). Mar. Biol. 1996, 125, 735–742. [Google Scholar] [CrossRef]
- Todaro, M.A.; Dal Zotto, M.; Segura-Bermúdez, O.A.; Cambronero-Bolaños, R.; Vargas, J.A.; Sibaja-Cordero, J.A. Biodiversity and distribution of marine Gastrotricha along the Pacific coast of Costa Rica. Estuar. Coast. Shelf Sci. 2025, 313, 109097. [Google Scholar] [CrossRef]
- Dice, L.R. Measures of the amount of ecologic association between Species. Ecology 1954, 26, 297–302. [Google Scholar] [CrossRef]
- Sørensen, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Kong. Dan. Vidensk. Selsk. Biol. Skr. 1948, 5, 1–34. [Google Scholar]



























| Location | Station | Latitude | Longitude | Salinity (‰) | Temperature (°C) | Date of Sampling |
|---|---|---|---|---|---|---|
| Cap Angela | St1 | 37°19′53″ N | 09°46′10″ E | 40 | 25 | 16 September 2023 |
| Les Grottes | St2 | 37°19′60″ N | 09°50′38″ E | 40 | 25 | 16 September 2023 |
| Rimel Beach | St3 | 37°15′34″ N | 09°54′12″ E | 40 | 25 | 16 September 2023 |
| Rimel Epave | St4 | 37°15′11″ N | 09°56′35″ E | 40 | 25 | 16 September 2023 |
| Bouficha | a | 36°16′46″ N | 10°29′37″ E | 38.5 | 15 | 13 March 2008 |
| Mahdia | b | 35°31′17″ N | 11°02′37″ E | 38.5 | 15 | 13 March 2008 |
| Station | Mean Grain Size (phi) | Size Class | Sorting | Sorting Class | Kurtosis | Skewness |
|---|---|---|---|---|---|---|
| St1 | 1.96 | Medium sand | 0.61 | Moderately well-sorted | 2.29 | −0.17 |
| St2 | 1.94 | Medium sand | 0.62 | Moderately well-sorted | 2.40 | −0.17 |
| St3 | 2.05 | Fine sand | 0.63 | Moderately well-sorted | 2.68 | −0.54 |
| St4 | 1.71 | Medium sand | 0.77 | Moderately sorted | 2.58 | −0.24 |
| a | 1.37 | Medium sand | 0.72 | Moderately sorted | 3.08 | −0.21 |
| b | 2.33 | Fine sand | 0.83 | Moderately sorted | 6.50 | −1.68 |
| Taxon | St1 | St2 | St3 | St4 |
|---|---|---|---|---|
| MACRODASYIDA | ||||
| Cephalodasyidae | ||||
| Dolychodasys sp1 | - | - | - | + |
| Dactylopodolidae | ||||
| Dactylopodola typhle | - | - | + | + |
| Macrodasyidae | ||||
| Urodasys viviparus | - | - | - | + |
| Planodasyidae | ||||
| Megadasys sp1 | - | - | - | + |
| Thaumastodermatidae | ||||
| Acanthodasys aculeatus | + | - | + | - |
| Diplodasys sanctimariae | - | + | - | - |
| Pseudostomella etrusca | - | - | + | - |
| Tetranchyroderma heterotubulatum | - | + | + | - |
| Tetranchyroderma papii | + | - | - | - |
| Turbanellidae | ||||
| Paraturbanella teissieri | + | - | + | - |
| Turbanella bocqueti | - | + | - | + |
| Number of macrodasyidan species by locations | 3 | 3 | 5 | 5 |
| CHAETONOTIDA | ||||
| Chaetonotidae | ||||
| Aspidiophorus mediterraneus | + | - | - | - |
| Aspidiophorus paramediterraneus | + | - | - | + |
| Chaetonotus dispar | + | - | - | + |
| Halichaetonotus bizertae sp. nov. | + | + | - | - |
| Halichaetonotus thalassopais | + | + | - | - |
| Halichaetonotus euromarinus | + | - | - | - |
| Heterolepidoderma loricatum | + | - | + | - |
| Xenotrichulidae | ||||
| Draculiciteria tesselata | - | - | + | - |
| Heteroxenotrichula sp1 | + | - | - | - |
| Heteroxenotrichula sp2 | - | - | + | - |
| Number of chaetonotidan species by locations | 8 | 2 | 3 | 2 |
| Total gastrotrich species by locations | 11 | 5 | 8 | 7 |
| Trait | Specimen | Mean ± SD | ||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
| Total length | 129 | 128 | 124 | 127.0 ± 2.2 |
| Pharynx length | 30 | 30 | 29 | 29.9 ± 0.5 |
| Furca length | 27 | 26 | 23 | 25.3 ± 1.7 |
| Adhesive tubes length | 15 | 15 | 12 | 13.9 ± 1.4 |
| Head width | 25 | 23 | 19 | 22.5 ± 2.4 |
| Neck width | 15 | 15 | 14 | 14.8 ± 0.7 |
| Trunk width | 30 | 25 | 23 | 26.1 ± 2.6 |
| Furcal base width | 13 | 14 | 14 | 13.7 ± 0.3 |
| Anterior pharynx width | 11 | 10 | 10 | 10.1 ± 0.3 |
| Mid pharynx width | 6 | 6 | 6 | 6.1 ± 0.3 |
| Posterior pharynx width | 9 | 9 | 9 | 9.0 ± 0.2 |
| Head scale length | 5 | 5 | 5 | 4.7 ± 0.0 |
| Head scale width | 3 | 4 | 4 | 3.6 ± 0.3 |
| Head spine length | 5 | - | - | 5.2 ± 0.0 |
| Neck scale length | 3 | 3 | 4 | 3.5 ± 0.6 |
| Neck scale width | 3 | 3 | 4 | 3.4 ± 0.4 |
| Neck spine length | - | 3 | - | 3.0 ± 0.0 |
| Trunk scale length | 8 | 7 | - | 7.2 ± 0.4 |
| Trunk scale width | 5 | 4 | - | 4.6 ± 0.2 |
| Trunk spine length | 10 | 8 | - | 9.1 ± 0.0 |
| Terminal interciliary scale length | 7 | 7 | 7 | 7.0 ± 0.2 |
| Terminal interciliary scale width | 4 | 4 | 4 | 3.6 ± 0.1 |
| Spine length | 8 | 8 | 8 | 8.0 ± 0.3 |
| Cephalion length | 1 | 1 | 2 | 1.3 ± 0.2 |
| Cephalion width | 10 | 8 | 8 | 8.5 ± 0.8 |
| Mouth diameter | 7 | 6 | 6 | 6.4 ± 0.2 |
| Bristle scale length | 4 | 5 | 4 | 4.3 ± 0.5 |
| Bristle scale width | 3 | 4 | 3 | 3.3 ± 0.5 |
| Egg length | - | 42 | - | 41.5 ± 0.0 |
| Egg width | - | 21 | - | 21.0 ± 0.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souid, A.; Gammoudi, M.; Saponi, F.; El Cafsi, M.; Todaro, M.A. First Investigation of the Marine Gastrotrich Fauna from the Waters of North Tunisia, with the Description of a New Species of Halichaetonotus (Gastrotricha, Chaetonotida). Diversity 2025, 17, 17. https://doi.org/10.3390/d17010017
Souid A, Gammoudi M, Saponi F, El Cafsi M, Todaro MA. First Investigation of the Marine Gastrotrich Fauna from the Waters of North Tunisia, with the Description of a New Species of Halichaetonotus (Gastrotricha, Chaetonotida). Diversity. 2025; 17(1):17. https://doi.org/10.3390/d17010017
Chicago/Turabian StyleSouid, Aicha, Mehrez Gammoudi, Francesco Saponi, M’hamed El Cafsi, and M. Antonio Todaro. 2025. "First Investigation of the Marine Gastrotrich Fauna from the Waters of North Tunisia, with the Description of a New Species of Halichaetonotus (Gastrotricha, Chaetonotida)" Diversity 17, no. 1: 17. https://doi.org/10.3390/d17010017
APA StyleSouid, A., Gammoudi, M., Saponi, F., El Cafsi, M., & Todaro, M. A. (2025). First Investigation of the Marine Gastrotrich Fauna from the Waters of North Tunisia, with the Description of a New Species of Halichaetonotus (Gastrotricha, Chaetonotida). Diversity, 17(1), 17. https://doi.org/10.3390/d17010017







