Dispersal Ecology of Golden Eagles (Aquila chrysaetos) in Northern Greece: Onset, Ranging, Temporary and Territorial Settlement
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species
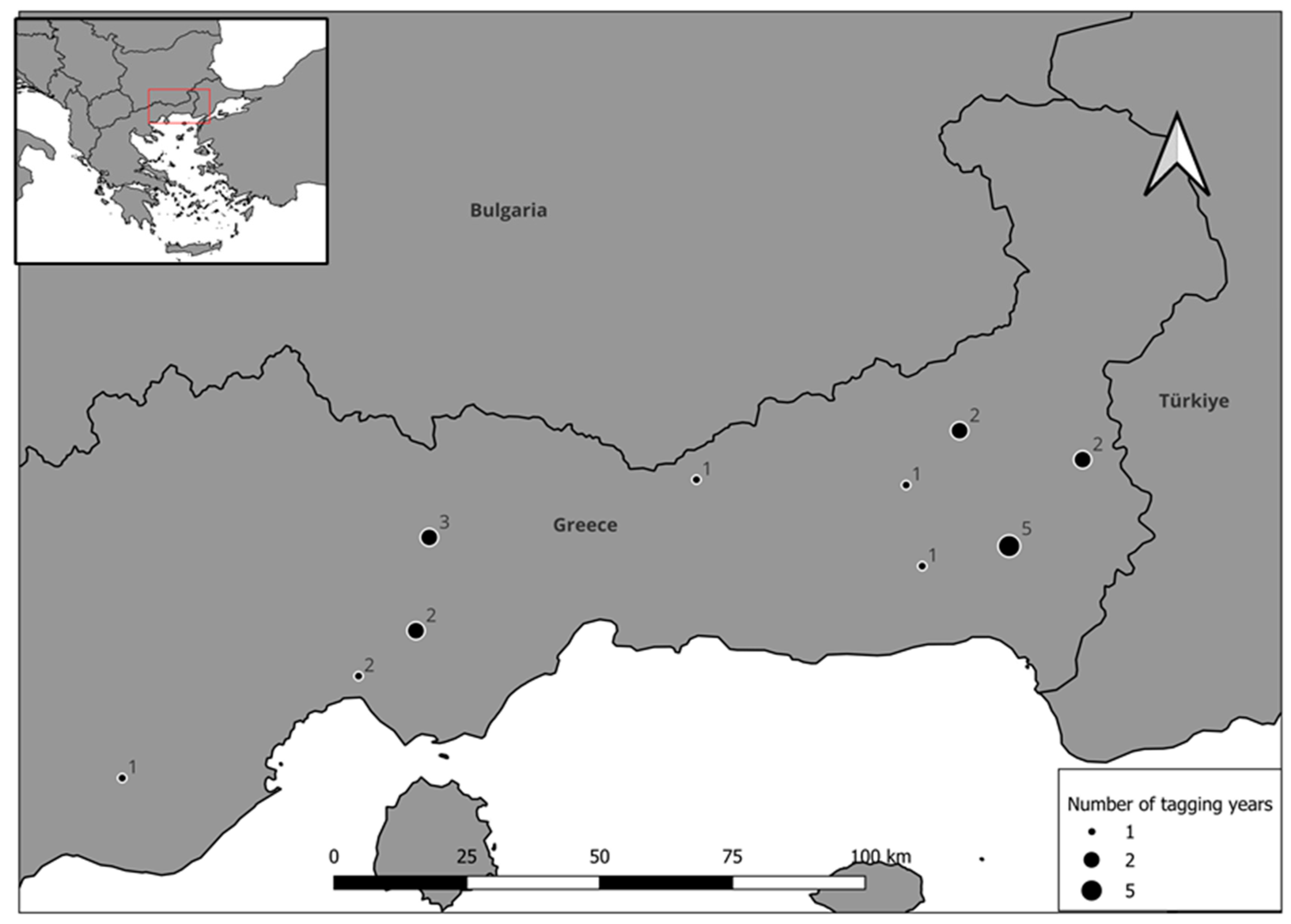
2.2. Satellite Tagging, Data Collection and Determination of Dispersal Onset
2.3. Dispersal Area Use
2.4. Temporary Settlement Areas
2.5. Territorial Settlement
2.6. Habitat Analysis
2.6.1. Habitat Suitability for Dispersing Golden Eagles
2.6.2. Temporary Settlement Area Selection and Use
3. Results
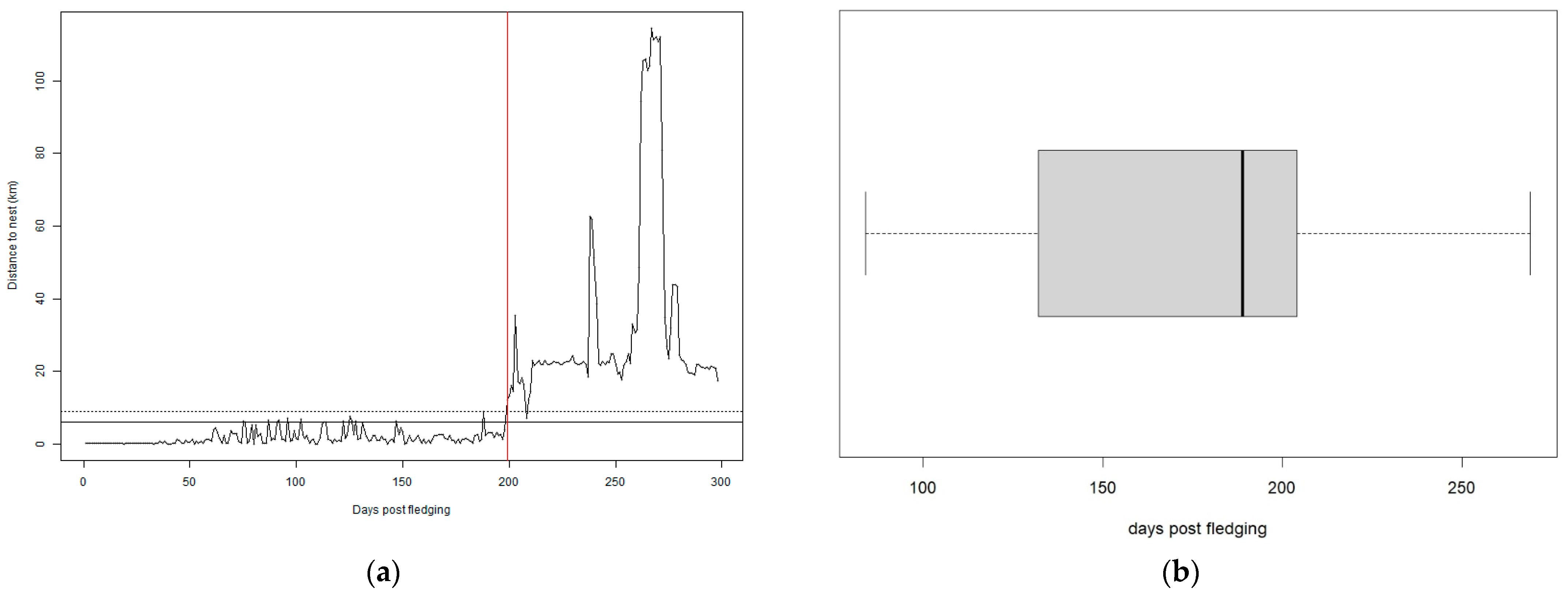
3.1. Dispersal Onset
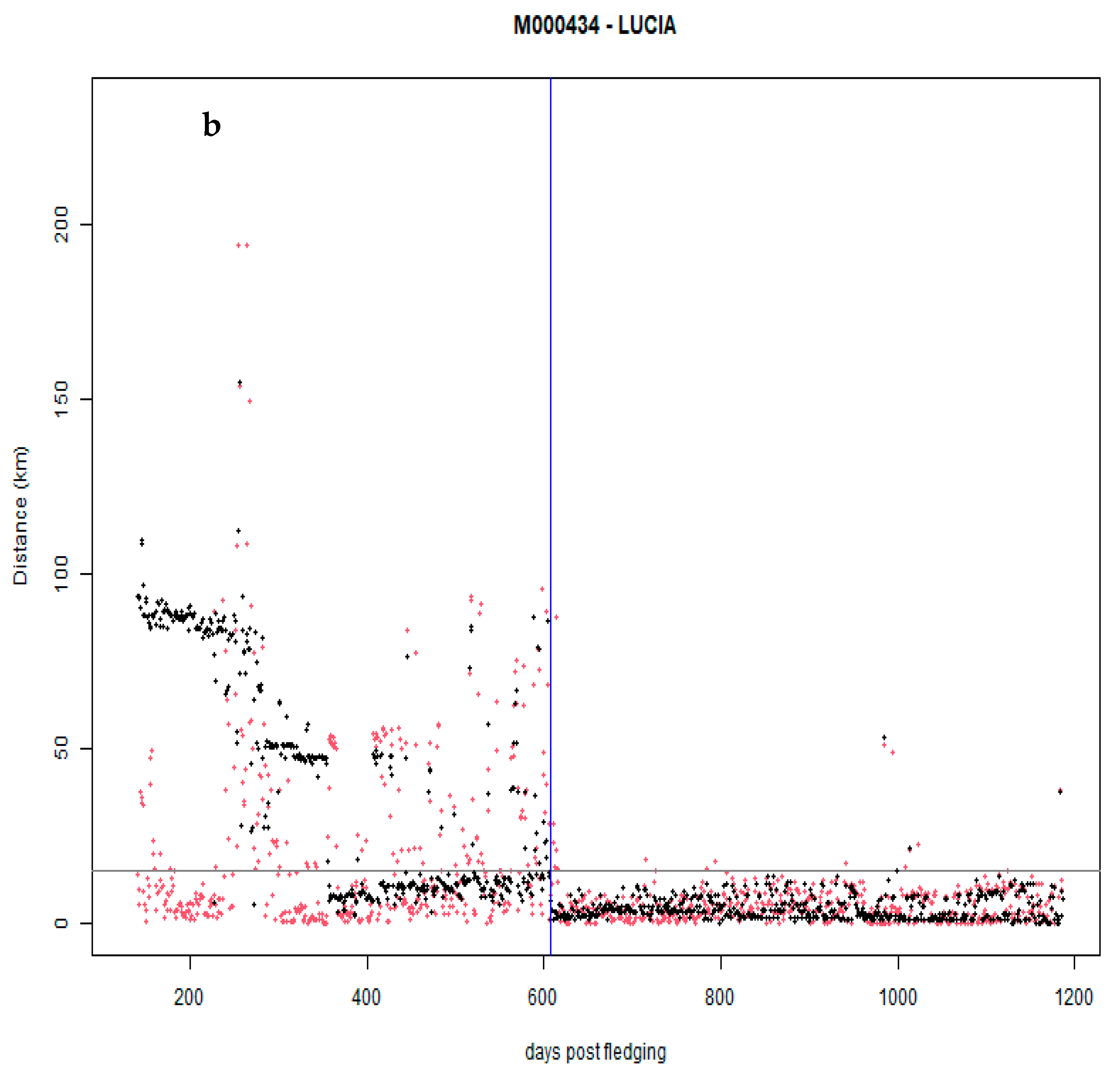
3.2. Distancing from the Natal Territory and Dispersal Area Ranging
3.3. Temporary Settlement Behavior
3.4. Territorial Settlement
3.5. Habitat Preferences of Dispersing Golden Eagles
3.5.1. Habitat Suitability during the NDP
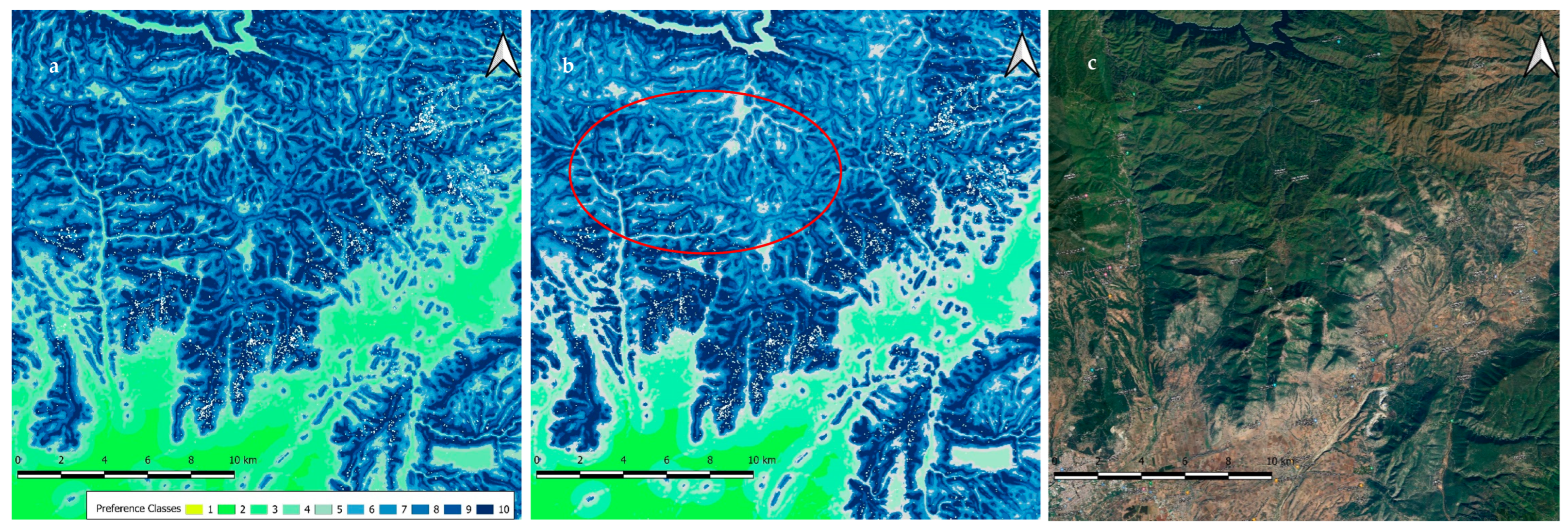
3.5.2. Preferences within Temporary Settlement Areas
4. Discussion
4.1. Dispersal Onset and Ranging
4.2. Temporary Settlement Behavior
4.3. Territorial Settlement
4.4. Habitat Preferences
5. Conservation Implications
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Individual (Ring Number) | Sex | Date Tagged | Last Day of Dispersal Tracking | Tag Model | Tag Attachment | n of Raw Fixes Retrieved | Fix Intervals (Seconds) | Analyses Where Data Used | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dispersal Onset | Temporary Settlement | Territorial Settlement | Habitat Use | ||||||||
| M000413—HEAG01 | F 1 | 7 July 2014 | 16 May 2015 | ECOTONE KITE | BP | 3016 | 3600 | + | |||
| M000414—WEE_BUG | M | 20 June 2018 | 5 June 2019 | ECOTONE KITE | BP | 1325 | 3600 | + | |||
| M000422—ARCHONTAS | M 1 | 15 June 2019 | 6 February 2022 | Ornitela OT50 | BP | 129,999 | 300 | + | + | + | + |
| M000426—ROY | M 1 | 24 June 2019 | 16 August 2019 | Ornitela OT50 | BP | 2521 | 64 | ||||
| M000434—LUCIA | F 1 | 5 July 2020 | 15 March 2022 | Ornitela OT50 | BP | 122,567 | 300 | + | + | + | + |
| M000429—AIMO | M 1 | 29 June 2019 | 4 December 2020 | Ornitela OT50 | BP | 83,684 | 300 | + | + | + | + |
| M000423—LADI | M 1 | 20 June 2019 | 19 September 2021 | Ornitela OT50 | BP | 135,188 | 300 | + | + | + | + |
| M000424—DOBRI | M 1 | 20 June 2019 | 25 September 2019 | Ornitela OT50 | BP | 8103 | 181 | ||||
| M000433—ANASIA | F 1 | 27 June 2020 | 5 March 2023 | Ornitela OT50 | BP | 198,745 | 299 | + | + | + | + |
| M000425—AKRITAS | M 1 | 22 June 2019 | 2 September 2020 | Ornitela OT50 | BP | 99,809 | 180 | + | + | + | + |
| M000427—APOSTOLIA | F 1 | 28 June 2019 | 4 July 2022 | Ornitela OT50 | PH | 199,471 | 299 | + | + | + | + |
| M000428—PANAGIOTAM | M 1 | 27 June 2019 | 20 January 2021 | Ornitela OT30 | PH | 55,000 | 300 | + | + | + | + |
| M000435—PATRICK | M 2 | 8 July 2021 | 23 June 2022 | Ornitela OT50 | BP | 40,805 | 300 | + | + | + | + |
| M000436—LORCA | F 1 | 19 June 2022 | 18 December 2023 | Ornitela OT50 | PH | 9625 | 300 | + | + | + | + |
| M000437—RASPUTIN | M | 22 January 2022 | 21 December 2022 | Ornitela OT50 | BP | 33,897 | 300 | + | + | + | + |
| M000440—FREEDOM | M | 6 July 2022 | 15 March 2024 | Ornitela OT50 | BP | 13,901 | 300 | + | + | + | + |
| M900551—LOLA | F 1 | 29 June 2023 | 29 March 2024 | Ornitela OT50 | PH | 28,588 | 300 | + | |||
| M900552—ALAN | M 1 | 30 June 2023 | 15 March 2024 | Eobs Solar 55 | PH | 32,698 | 300 | + | + | ||
| M900553—MOONCHILD | M 1 | 1 July 2024 | 28 January 2024 | Ornitela OT50 | PH | 17,896 | 300 | + | + | + | |
| M900554—VALIENTE | M 1 | 7 January 2024 | 15 March 2024 | Ornitela OT50 | PH | 22,169 | 300 | + | + | + | |
| MCP vs. 95% MKDE | 95% vs. Core Area MKDE | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Friedman’s H | p | Mean Difference | Wilcoxon’s Matched Pairs | p | Mean Difference | Wilcoxon’s Matched Pairs | p |
| % Extensive agriculture 1 | 63.25 | 0.0000 | 6.79 | 1261 | 0 | 2.12 | 990 | 0 |
| % Woods and scrub 2 | 12.49 | 0.0019 | −3.75 | 410 | 0.007 | −1.45 | 618 | 0.39 |
| % Open and transitional habitats 3 | 3.66 | 0.1604 | −3.39 | 488 | 0.044 | −0.37 | 655 | 0.595 |
| % Natural vegetation 4 | 52.89 | 0.0000 | −7.14 | 109 | 0 | −1.82 | 296 | 0.001 |
| Tree cover density 5 | 5.55 | 0.0624 | 0.46 | 682 | 0.77 | 1.58 | 1091 | 0.001 |
| % Area with <50% cover 6 | 5.02 | 0.0813 | −1.22 | 676 | 0.73 | −2.75 | 308 | 0 |
| % Ridge pixels 7 | 65.25 | 0.0000 | −6.41 | 35 | 0 | −5.7 | 187 | 0 |
| Northness 8 | 5.25 | 0.0726 | 0.03 | 973 | 0.023 | −0.01 | 558 | 0.165 |
| Eastness 8 | 0.15 | 0.9273 | −0.01 | 671 | 0.697 | 0 | 683 | 0.777 |
| Slope 9 | 51.66 | 0.0000 | −1.7 | 117 | 0 | −1.13 | 132 | 0 |
| Altitude 9 | 9.51 | 0.0086 | −8.03 | 467 | 0.028 | 1.81 | 625 | 0.426 |
| Road density (km/km2) 10 | 8.19 | 0.0167 | 0.51 | 985 | 0.017 | −0.41 | 457 | 0.022 |
| Turbines/km2 11 | 16.00 | 0.0003 | 0 | 88 | 0.931 | 0.03 | 36 | 0.014 |
| Division 12 | 87.96 | 0.0000 | −22 | 30 | 0 | −8 | 114 | 0 |
| Model | AICc | Concordance | LR | LR P | χ2 | p |
|---|---|---|---|---|---|---|
| Slope | 48.26 | 0.84 | 31.5 | <0.0001 | ||
| % Open | 80.45 | 0.66 | 10.33 | 0.001 | ||
| Division | 59.89 | 0.81 | 30.89 | <0.0001 | ||
| Slope + % Open | 40.51 | 0.86 | 52.41 | <0.0001 | 9.89 | <0.002 |
| Slope + Division | 43.35 | 0.87 | 49.56 | <0.0001 | 7.04 | <0.01 |
| Slope + Open + Division | 37.89 | 0.91 | 57.23 | <0.0001 | 4.82 | <0.03 |
| Slope + Open + Division + Eastness + Eastness: Season | 36.4 | 0.92 | 63.35 | <0.0001 | 6.1 | <0.05 |
| Model | Variable | Coefficient | Odds | Lower CI | Upper CI | Z | p |
|---|---|---|---|---|---|---|---|
| Slope + Open + Division [Season n = 64] | Slope | 1.50 | 4.5 | 1.77 | 11.42 | 3.15 | 0.002 |
| % Open | 0.17 | 1.18 | 1.03 | 1.35 | 2.43 | 0.015 | |
| Division | 0.10 | 1.11 | 0.98 | 1.25 | 1.64 | 0.1 | |
| Slope + Open + Division + Eastness + Eastness: Season | Slope | 2.08 | 8.06 | 2.01 | 32.55 | 2.93 | 0.003 |
| % Open | 0.21 | 1.23 | 1.06 | 1.43 | 2.77 | 0.006 | |
| Division | 0.24 | 1.27 | 1.02 | 1.57 | 2.14 | 0.03 | |
| Eastness | −0.23 | 0.79 | 0.65 | 0.97 | −2.21 | 0.03 | |
| Eastness: Season | 0.26 | 1.30 | 1.01 | 1.67 | 2.08 | 0.04 |

Appendix B
Appendix B.1. GET and GETc Model Supplementary Notes
Appendix B.2. Stepwise Model Selection of Conditional Logistic Regression Modeling
References
- Serrano, D. Dispersal in Raptors. In Birds of Prey Conservation in the XXI Century; Sarasola, J.H., Grande, J.M., Negro, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 95–121. ISBN 978-3-319-73745-4. [Google Scholar]
- Wright, S. Evolution in Mendelian Populations. Bull. Math. Biol. 1931, 52, 241–295. [Google Scholar] [CrossRef] [PubMed]
- Penteriani, V.; Delgado, M.M. Birthplace-Dependent Dispersal: Are Directions of Natal Dispersal Determined a Priori? Ecography 2011, 34, 729–737. [Google Scholar] [CrossRef]
- Poessel, S.A.; Woodbridge, B.; Smith, B.W.; Murphy, R.K.; Bedrosian, B.E.; Bell, D.A.; Bittner, D.; Bloom, P.H.; Crandall, R.H.; Domenech, R.; et al. Interpreting Long-Distance Movements of Non-Migratory Golden Eagles: Prospecting and Nomadism? Ecosphere 2022, 13, e4072. [Google Scholar] [CrossRef]
- Stamps, J.A. Habitat Selection by Dispersers: Integrating Proximate and Ultimate Approaches. In Dispersal; Clobert, J., Danchin, E., Dhondt, A.A., Nichols, J.D., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 230–242. ISBN 978-0-19-850660-7. [Google Scholar]
- Watson, J. The Golden Eagle; Poyser: London, UK, 2010. [Google Scholar]
- Morrison, J.L.; Wood, P.B. Broadening Our Approach to Studying Dispersal in Raptors. J. Raptor Res. 2009, 43, 81–89. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Anderson, D.; Benn, S.; Reid, R.; Tingay, R.; Weston, E.D. Seasonal Variation in First Territory Settlement of Dispersing Golden Eagles: An Innate Behaviour? Diversity 2024, 16, 82. [Google Scholar] [CrossRef]
- Soutullo, A.; Urios, V.; Ferrer, M.; Peñarrubia, S.G. Post-Fledging Behaviour in Golden Eagles Aquila Chrysaetos: Onset of Juvenile Dispersal and Progressive Distancing from the Nest. Ibis 2006, 148, 307–312. [Google Scholar] [CrossRef]
- Urios, V.; Soutullo, A.; López-López, P.; Cadahía, L.; Limiñana, R.; Ferrer, M. The First Case of Successful Breeding of a Golden Eagle Aquila Chrysaetos Tracked from Birth by Satellite Telemetry The First Case of Successful Breeding of a Golden Eagle Aquila Chrysaetos Tracked from Birth by Satellite Telemetry. Acta Ornithol. 2007, 42, 205–209. [Google Scholar] [CrossRef]
- The Golden Eagle around the World; Bautista, J., Ellis, D.H., Eds.; Hancock House Publishers: Blaine, WA, USA; Surrey, BC, Canada, 2024. [Google Scholar]
- Cruz-Romo, L.J.; Sanchez-Vilchis, M.; Sanchez-Cordero, V.; Murphy, R.K.; Cruz-Molina, I.; Vargas-Velasco, J.J.; Valdes-Alargon, M.; Millsap, B.A. First Satellite Telemetry Study of Movement Behavior of Juvenile Golden Eagles from Mexico. J. Raptor Res. 2022, 56, 28–39. [Google Scholar] [CrossRef]
- Cadahía, L.; López-lópez, P.; Urios, V.; Negro, J.J. Estimating the Onset of Dispersal in Endangered Bonelli ’ s Eagles Hieraaetus Fasciatus Tracked by Satellite Telemetry: A Comparison between Methods. Ibis 2008, 150, 416–420. [Google Scholar] [CrossRef]
- Weston, E.D.; Whitfield, D.P.; Travis, J.M.J.; Lambin, X. When Do Young Birds Disperse? Tests from Studies of Golden Eagles in Scotland. BMC Ecol. 2013, 13, 42. [Google Scholar] [CrossRef]
- Delgado, M.M.; Penteriani, V. Behavioral States Help Translate Dispersal Movements into Spatial Distribution Patterns of Floaters. Am. Nat. 2008, 172, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Fielding, A.H.; Anderson, D.; Benn, S.; Reid, R.; Tingay, R.; Weston, E.D.; Whitfield, D.P. Substantial Variation in Prospecting Behaviour of Young Golden Eagles Aquila Chrysaetos Defies Expectations from Potential Predictors. Diversity 2023, 15, 506. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Mcleod, D.R.A.; Haworth, P.F. Modelling the Effects of Persecution on Population Dynamics of Golden Eagles in Scotland. Biol. Conserv. 2004, 119, 319–333. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Mcleod, D.R.A.; Haworth, P.F. A Conservation Framework for Golden Eagles: Implications for Their Conservation and Management in Scotland. Scott. Nat. Herit. Comm. Rep. 2008, 193, 151. [Google Scholar]
- Hernández-Matías, A.; Real, J.; Parés, F.; Pradel, R. Electrocution Threatens the Viability of Populations of the Endangered Bonelli’s Eagle (Aquila Fasciata) in Southern Europe. Biol. Conserv. 2015, 191, 110–116. [Google Scholar] [CrossRef]
- Newton, I.A.N.; Mcgrady, M.J.; Oli, M.K. A Review of Survival Estimates for Raptors and Owls. Ibis 2016, 158, 227–248. [Google Scholar] [CrossRef]
- Pain, D.J.; Donald, P.F. Outside the Reserve: Pandemic Threaths to Bird Biodiversity. In Conserving Birds Biodiversity General Principles and their Application; Norris, K., Pain, D.J., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 157–179. [Google Scholar]
- Orgeret, F.; Grüebler, M.U.; Scherler, P.; Bergen, V.S.V.; Kormann, U.G. Shift in Habitat Selection during Natal Dispersal in a Long-Lived Raptor Species. Ecography 2023, 2023, e06729. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; McLeod, D.R.A.; Haworth, P.F.; Watson, J. A Conservation Framework for the Golden Eagle in Scotland: Refining Condition Targets and Assessment of Constraint Influences. Biol. Conserv. 2006, 130, 465–480. [Google Scholar] [CrossRef]
- Balbontín, J. Identifying Suitable Habitat for Dispersal in Bonelli’s Eagle: An Important Issue in Halting Its Decline in Europe. Biol. Conserv. 2005, 126, 74–83. [Google Scholar] [CrossRef]
- Bautista, J.; Gómez, G.J.; Otero, M.; Garrido, J.R. Golden Eagles in Southern Spain Recolonize Human-Dominated Landscapes (1998–2021). In The Golden Eagle Around the World; Bautista, J., Ellis, D.H., Eds.; Hancock House Publishers: Blaine, WA, USA; Surrey, BC, Canada, 2024; pp. 220–253. [Google Scholar]
- Bautista, J.; Otero, M.; Gómez, G.J.; Garrido, J.R. Golden Eagles and Humans: Expansion into Human Dominated Landscapes; Wilder Sur: Granada, Spain, 2022. [Google Scholar]
- Cadahía, L.; López-López, P.; Urios, V.; Negro, J.J. Satellite Telemetry Reveals Individual Variation in Juvenile Bonelli’s Eagle Dispersal Areas. Eur. J. Wildl. Res. 2010, 56, 923–930. [Google Scholar] [CrossRef]
- Ferrer, M. Juvenile Dispersal Behaviour and Natal Philopatry of a Long-lived Raptor, the Spanish Imperial Eagle Aquila Adalberti. Ibis 1993, 135, 132–138. [Google Scholar] [CrossRef]
- Ferrer, M. Reduction in Hunting Success and Settlement Strategies in Young Spanish Imperial Eagles. Anim. Behav. 1993, 45, 406–408. [Google Scholar] [CrossRef][Green Version]
- Hunt, G. A Pilot Golden Eagle Population Study in the Altamont Pass Wind Resource Area in California; Predatory Bird Research Group: Santa Cruz, CA, USA, 1995; p. 219. [Google Scholar]
- Penteriani, V.; Otalora, F.; Sergio, F.; Ferrer, M. Environmental Stochasticity in Dispersal Areas Can Explain the ‘Mysterious’ Disappearance of Breeding Populations. Proc. R. Soc. B Biol. Sci. 2005, 272, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Penteriani, V.; Otalora, F.; Ferrer, M. Floater Survival Affects Population Persistence. The Role of Prey Availability and Environmental Stochasticity. Oikos 2005, 108, 523–534. [Google Scholar] [CrossRef]
- Real, J.; Bosch, R.; Tinro, A.; Hernandez-matias, A. Identifying Key Habitats for the Conservation of Bonelli ’s Eagle Aquila Fasciata Using Radio- Tracking. Ibis 2016, 158, 556–568. [Google Scholar] [CrossRef]
- Real, J.; Mañosa, S. Demography and Conservation of Western European Bonelli’s Eagle Hieraaetus Fasciatus Populations. Biol. Conserv. 1997, 79, 59–66. [Google Scholar] [CrossRef]
- Braham, M.; Miller, T.; Duerr, A.E.; Lanzone, M.; Fesnock, A.; LaPre, L.; Driscoll, D.; Katzner, T. Home in the Heat: Dramatic Seasonal Variation in Home Range of Desert Golden Eagles Informs Management for Renewable Energy Development. Biol. Conserv. 2015, 186, 225–232. [Google Scholar] [CrossRef]
- Dennhardt, A.J.; Duerr, A.E.; Brandes, D.; Katzner, T.E. Modeling Autumn Migration of a Rare Soaring Raptor Identifies New Movement Corridors in Central Appalachia. Ecol. Model. 2015, 303, 19–29. [Google Scholar] [CrossRef]
- Fielding, A.H.; Haworth, P.F.; Anderson, D.; Benn, S.; Dennis, R.; Weston, E.; Whitfield, D.P. A Simple Topographical Model to Predict Golden Eagle Aquila Chrysaetos Space Use during Dispersal. Ibis 2019, 162, 400–415. [Google Scholar] [CrossRef]
- McLeod, D.R.A.; Whitfield, D.P.; Fielding, A.H.; Haworth, P.F.; Mcgrady, M.J. Predicting Home Range Use by Golden Eagles Aquila Chrysaetos in Western Scotland. Avian Sci. 2002, 2, 183–198. [Google Scholar]
- Singh, N.J.; Moss, E.; Hipkiss, T.; Ecke, F.; Dettki, H.; Sandström, P.; Bloom, P.; Kidd, J.; Thomas, S.; Hörnfeldt, B. Habitat Selection by Adult Golden Eagles Aquila Chrysaetos during the Breeding Season and Implications for Wind Farm Establishment. Bird Study 2016, 63, 233–240. [Google Scholar] [CrossRef]
- Pedrini, P.; Sergio, F. Golden Eagle Aquila Chrysaetos Density and Productivity in Relation to Land Abandonment and Forest Expansion in the Alps. Bird Study 2001, 48, 194–199. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Gregory, M.J.P.; Gordon, A.G.; McLeod, D.R.A.; Haworth, P.F. Complex Effects of Habitat Loss on Golden Eagles Aquila Chrysaetos. Ibis 2006, 149, 26–36. [Google Scholar] [CrossRef]
- Sandgren, C.; Hipkiss, T.; Dettki, H.; Ecke, F.; Hörnfeldt, B. Habitat Use and Ranging Behaviour of Juvenile Golden Eagles Aquila Chrysaetos within Natal Home Ranges in Boreal Sweden. Bird Study 2014, 61, 9–16. [Google Scholar] [CrossRef]
- Bounas, A.; Sidiropoulos, L. Aquila Chrysaetos. The Greek Red List of Threatened Species. Available online: https://redlist.necca.gov.gr/en/assessment/?id=224415705 (accessed on 8 June 2024).
- Sidiropoulos, L.; Whitfield, D.P.; Astaras, C.; Vasilakis, D.; Alivizatos, H.; Kati, V. Pronounced Seasonal Diet Diversity Expansion of Golden Eagles (Aquila Chrysaetos) in Northern Greece during the Non-Breeding Season: The Role of Tortoises. Diversity 2022, 14, 135. [Google Scholar] [CrossRef]
- Sidiropoulos, L.; Xirouchakis, S.; Alivizatos, H.; Boukas, N.; Bounas, A.; Bourdakis, E.; Christopoulos, A.; Damianakis, K.; Evangelidis, A.; Kret, E.; et al. The Golden Eagle in Greece. In The Golden Eagle Around the World; Ellis, D.H., Bautista, J., Ellis, C.H., Eds.; Hancock House Publishers: Blaine, WA, USA; Surrey, BC, Canada, 2024; pp. 397–415. [Google Scholar]
- Sidiropoulos, L. The Golden Eagle (Aquila Chrysaetos L.) in the Rhodope Mts: Modelling Densities, Distribution and Population Viability to Inform Conservation. Masters Thesis, Division of Biology. Imperial College London, London, UK, 2012. [Google Scholar]
- Bloom, P.H.; Kidd, J.W.; Hipkiss, S.E.T.; Kuehn, H.J. Trapping Success Using Carrion with Bow Nets to Capture Adult Golden Eagles in Sweden. J. Raptor Res. 2015, 49, 92–97. [Google Scholar] [CrossRef]
- Anderson, D.; Arkumarev, V.; Bildstein, K.; Botha, A.; Bowden, C.G.R.; Davies, M.; Duriez, O.; Forbes, N.A.; Godino, A.; Green, R.E.; et al. A Practical Guide to Methods for Attaching Research Devices to Vultures and Condors. Vulture News 2020, 78. [Google Scholar] [CrossRef]
- Fridolfsson, A.-K.; Ellegren, H. A Simple and Universal Method for Molecular Sexing of Non-Ratite Birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Peshev, H.; Grozdanov, A.; Kmetova–Biro, E.; Ivanov, I.; Stoyanov, G.; Tsiakiris, R.; Marin, S.; Marinković, S.; Sušić, G.; Lisichanets, E.; et al. New Insight into Spatial Ecology of Griffon Vulture (Gyps Fulvus) on the Balkans Provides Opportunity for Focusing Conservation Actions for a Threatened Social Scavenger. Biodivers. Data J. 2021, 9, e71100. [Google Scholar] [CrossRef]
- Kranstauber, B.; Cameron, A.; Weinzerl, R.; Fountain, T.; Tilak, S.; Wikelski, M.; Kays, R. The Movebank Data Model for Animal Tracking. Environ. Model. Softw. 2011, 26, 834–835. [Google Scholar] [CrossRef]
- Benhamou, S. Dynamic Approach to Space and Habitat Use Based on Biased Random Bridges. PLoS ONE 2011, 6, e14592. [Google Scholar] [CrossRef]
- Calenge, C. Home Range Estimation in R: The adehabitatHR Package; Office National de la Classe et la Faune Sauvage: Auffargis, France, 2015. [Google Scholar]
- R-Core_Team. R: A Language and Environment for Statistical Computing. v 4.2.3; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Rymešová, D.; Raab, R.; Machálková, V.; Horal, D.; Dorňáková, D.; Rozsypalová, L.; Spakovszky, P.; Literák, I. First-Year Dispersal in White-Tailed Eagles Haliaeetus Albicilla. Eur. J. Wildl. Res. 2021, 67, 44. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Anderson, D.; Benn, S.; Dennis, R.; Grant, J.; Weston, E.D. Age of First Territory Settlement of Golden Eagles Aquila Chrysaetos in a Variable Competitive Landscape. Front. Ecol. Evol. 2022, 10, 743598. [Google Scholar] [CrossRef]
- Powell, R.A. Animal Home Ranges and Territories and Home Range Estimatiors. In Research Techniques in Animal Ecology. 441pp; Boitani, L., Fuller, T.K., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 65–103. [Google Scholar]
- Thieurmel, B.; Elmarhraoui, A. Package “Suncalc”: Compute Sun Position, Sunlight Phases, Moon Position and Lunar Phase. Available online: https://cran.r-project.org/web/packages/suncalc/ (accessed on 10 December 2023).
- Hardey, J.; Crick, H.; Wernham, C.; Riley, H.; Etheridge, B.; Thompson, D. Raptors: A Field Guide to Survey and Monitoring; The Stationary Office: Edinburgh, UK, 2006. [Google Scholar]
- European Union/Copernicus Land Monitoring Service. CORINE Land Cover Dataset; European Environment Agency: København, Danmark, 2018. [Google Scholar]
- European Commission/Copernicus Land Monitoring Service. European Digital Elevation Model; European Environment Agency: København, Danmark, 2016. [Google Scholar]
- European Union/Copernicus Land Monitoring Service. High Resolution Tree Cover Density; European Environment Agency: København, Danmark, 2018; ISBN 2-01-220152-0. [Google Scholar]
- Hesselbarth, M.H.K.; Sciaini, M.; With, K.A.; Wiegand, K.; Nowosad, J. Landscapemetrics: An Open-source R Tool to Calculate Landscape Metrics. Ecography 2019, 42, 1648–1657. [Google Scholar] [CrossRef]
- McGarigal, K.; Cushman, S.A.; Ene, E. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer Software Program Produced by the Authors at the University of Massachusetts, Amherst. Available online: http://www.umass.edu/landeco/research/fragstats/fragstats.html (accessed on 10 May 2024).
- Crandall, R.H.; Bedrosian, B.E.; Craighead, D. Habitat Selection and Factors Influencing Nest Survival of Golden Eagles in South-Central Montana. J. Raptor Res. 2015, 49, 413–428. [Google Scholar] [CrossRef]
- Blondel, J.; Arronson, J. Biology and Wildlife of the Mediterranean Region; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Grove, A.T.; Rackham, O. The Nature of Mediterranean Europe—An Ecological History; Yale University Press: New Heaven, CT, USA, 2001. [Google Scholar]
- Vander Wal, E.; Rodgers, A.R. An Individual-Based Quantitative Approach for Delineating Core Areas of Animal Space Use. Ecol. Model. 2012, 224, 48–53. [Google Scholar] [CrossRef]
- QGIS Development Team QGIS Geographic Information System v3.34. Available online: http://qgis.osgeo.org (accessed on 16 January 2024).
- Therneau, T.M. Survival: A Package for Survival Analysis in R. Available online: https://cran.r-project.org/web/packages/survival/vignettes/survival.pdf (accessed on 15 June 2024).
- Duchesne, T.; Fortin, D.; Courbin, N. Mixed Conditional Logistic Regression for Habitat Selection Studies. J. Anim. Ecol. 2010, 79, 548–555. [Google Scholar] [CrossRef]
- Iverson, A.R.; Humple, D.L.; Cormier, R.L.; Hull, J. Land Cover and NDVI Are Important Predictors in Habitat Selection along Migration for the Golden-Crowned Sparrow, a Temperate-Zone Migrating Songbird. Mov. Ecol. 2023, 11, 2. [Google Scholar] [CrossRef]
- Murphy, R.K.; Dunk, J.R.; Woodbridge, B.; Stahlecker, D.W.; LaPlante, D.W.; Millsap, B.A.; Jacobson, K.V. First-Year Dispersal of Golden Eagles from Natal Areas in the Southwestern United States and Implications for Second-Year Settling. J. Raptor Res. 2017, 51, 216–233. [Google Scholar] [CrossRef]
- Hemery, A.; Mugnier-Lavorel, L.; Itty, C.; Duriez, O.; Besnard, A. Timing of Departure from Natal Areas by Golden Eagles Is Not Constrained by Acquisition of Flight Skills. J. Avian Biol. 2023, 2023, e03111. [Google Scholar] [CrossRef]
- Weston, E.D.; Whitfield, D.P.; Travis, J.M.J.; Lambin, X. The Contribution of Flight Capability to the Post-Fledging Dependence Period of Golden Eagles. J. Avian Biol. 2018, 49, 1–11. [Google Scholar] [CrossRef]
- Kitowski, I. Play Behaviour and Active Training of Montagu’s Harrier (Circus Pygargus) Offspring in the Post-Fledging Period. J. Ethol. 2005, 23, 3–8. [Google Scholar] [CrossRef]
- Kitowski, I. Social Learning of Hunting Skills in Juvenile Marsh Harriers Circus Aeruginosus. J. Ethol. 2009, 27, 327–332. [Google Scholar] [CrossRef]
- Bloom, P.H.; Michael Scott, J.; Papp, J.M.; Thomas, S.E.; Kidd, J.W. Vagrant Western Red-Shouldered Hawks: Origins, Natal Dispersal Patterns, and Survival. The Condor 2011, 113, 538–546. [Google Scholar] [CrossRef]
- Morandini, V.; González, E.; Bildstein, K.; Ferrer, M. Juvenile Dispersal in an Uninhabited Continent: Young Spanish Imperial Eagles in Africa. J. Ornithol. 2020, 161, 373–380. [Google Scholar] [CrossRef]
- Soutullo, A.; López-López, P.; Cortés, G.D.; Urios, V.; Ferrer, M. Exploring Juvenile Golden Eagles’ Dispersal Movements at Two Different Temporal Scales. Ethol. Ecol. Evol. 2013, 25, 117–128. [Google Scholar] [CrossRef]
- Watson, J. The Golden Eagle and Pastoralism across Europe. In Proceedings of the Birds and Pastoral Agriculture in Europe; Curtis, D.J., Bignal, E.M., Curtis, M.A., Eds.; Joint Nature Conservation Commitee-Scottish Chough Study Group: Port Erin, Isle of Man, 1991; pp. 56–57. [Google Scholar]
- Sanchez-Zapata, J.A.; Eguia, S.; Blazquez, M.; Moleon, M.; Botella, F. Unexpected Role of Ungulate Carcasses in the Diet of Golden Eagles Aquila Chrysaetos in Mediterranean Mountains. Bird Study 2010, 57, 352–360. [Google Scholar] [CrossRef]
- Arkumarev, V.; Dobrev, D.; Stamenov, A.; Terziev, N.; Delchev, A.; Stoychev, S. Seasonal Dynamics in the Exploitation of Natural Carcasses and Supplementary Feeding Stations by a Top Avian Scavenger. J. Ornithol. 2021, 162, 723–735. [Google Scholar] [CrossRef]
- Murphy, R.; Stahlecker, D.; Millsap, B.; Jacobson, K.; Johnson, A.; Smith, C.; Tator, K.; Kruse, K. Natal Dispersal Distance of Golden Eagles in the Southwestern United States. J. Fish Wildl. Manag. 2018, 10, 213–218. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Fielding, A.H.; Anderson, D.; Benn, S.; Reid, R.; Tingay, R.; Weston, E. Sex Difference in Natal Dispersal Distances of Golden Eagles Aquila Chrysaetos in Scotland. Ibis 2024, 166, 146–155. [Google Scholar] [CrossRef]
- Pirotta, E.; Katzner, T.; Miller, T.A.; Duerr, A.E.; Braham, M.A.; New, L. State-Space Modelling of the Flight Behaviour of a Soaring Bird Provides New Insights to Migratory Strategies. Funct. Ecol. 2018, 32, 2205–2215. [Google Scholar] [CrossRef]
- Sur, M.; Duerr, A.E.; Bell, D.A.; Fisher, R.N.; Tracey, J.A.; Bloom, P.H.; Miller, T.A.; Katzner, T.E. Relevance of Individual and Environmental Drivers of Movement of Golden Eagles. Ibis 2020, 162, 381–399. [Google Scholar] [CrossRef]
- Bogel, R.; Eberhardt, R. A GIS Model for Predicting Flight Conditions for Soaring Raptors, Evaluated with Data on Eurasian Griffon Vultures (Gyps Fulvus) and Golden Eagles (Aquila Chrysaetos). In Spatial Analysis in Raptor Ecology and Conservation; Rodriguez-Estrella, R., Tapia, L.A., Eds.; CIB: Mexico City, Mexico, 2004; pp. 39–55. [Google Scholar]
- Whitfield, D.P.; Mcleod, D.R.A.; Fielding, A.H.; Broad, R.A.; Evans, R.J.; Haworth, P.F. The Effects of Forestry on Golden Eagles on the Island of Mull, Western Scotland. J. Appl. Ecol. 2001, 38, 1208–1220. [Google Scholar] [CrossRef]
- Miller, T.A.; Brooks, R.P.; Lanzone, M.J.; Cooper, J.; O’Malley, K.; Brandes, D.; Duerr, A.; Katzner, T.E. Summer and Winter Space Use and Home Range Characteristics of Golden Eagles ( Aquila Chrysaetos ) in Eastern North America. The Condor 2017, 119, 697–719. [Google Scholar] [CrossRef]
- Moss, E.H.; Hipkiss, T.; Ecke, F.; Dettki, H.; Standstrom, P.; Bloom, P.H.; Kidd, J.W.; Thomas, S.E.; Hornfeldt, B. Home-Range Size and Examples of Post-Nesting Movements for Adult Golden Eagles (Aquila Chrysaetos) in Boreal Sweden. J. Raptor Res. 2014, 48, 93–105. [Google Scholar] [CrossRef]
- Caro, J.; Ontiveros, D.; Pizarro, M.; Pleguezuelos, J.M. Habitat Features of Settlement Areas Used by Floaters of Bonellis and Golden Eagles. Bird Conserv. Int. 2011, 21, 59–71. [Google Scholar] [CrossRef]
- Stojadinović, D.M.; Milošević, D.D.; Sretić, K.S.; Cvetković, M.P.; Jovanović, T.R.; Jovanović, B.L.; Crnobrnja-Isailović, J.M. Activity Patterns and Habitat Preference of Eastern Hermann’s Tortoise (Testudo Hermanni Boettgeri) in Serbia. Turk. J. Zool. 2017, 41, 1036–1044. [Google Scholar] [CrossRef]
- Nikolíc, M.; Cvetkovíc, J.; Stojadinovíc, D.; Crnobrnja-Isailovíc, J. Macro- and Microhabitat Preferences of Eastern Hermann’s Tortoise (Testudo Hermanni Boettgeri). Amphib. Reptil. 2020, 41, 313–322. [Google Scholar] [CrossRef]
- Fielding, A.H.; Anderson, D.; Benn, S.; Dennis, R.; Geary, M.; Weston, E.; Whitfield, D.P. Non-Territorial GPS-Tagged Golden Eagles Aquila Chrysaetos at Two Scottish Wind Farms: Avoidance Influenced by Preferred Habitat Distribution, Wind Speed and Blade Motion Status. PLoS ONE 2021, 16, e0254159. [Google Scholar] [CrossRef]
- Triantakonstantis, D.P.; Kollias, V.J.; Kalivas, D.P. Forest Re-Growth since 1945 in the Dadia Forest Nature Reserve in Northern Greece. New For. 2006, 32, 51–69. [Google Scholar] [CrossRef]
- Zakkak, S.; Radovic, A.; Nikolov, S.C.; Shumka, S.; Kakalis, L.; Kati, V. Assessing the Effect of Agricultural Land Abandonment on Bird Communities in Southern-Eastern Europe. J. Environ. Manage. 2015, 164, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Poirazidis, K.; Bontzorlos, V.; Xofis, P.; Zakkak, S.; Xirouchakis, S.; Grigoriadou, E.; Kechagioglou, S.; Gasteratos, I.; Alivizatos, H.; Panagiotopoulou, M. Bioclimatic and Environmental Suitability Models for Capercaillie (Tetrao Urogallus) Conservation: Identification of Optimal and Marginal Areas in Rodopi Mountain-Range National Park (Northern Greece). Glob. Ecol. Conserv. 2019, 17, e00526. [Google Scholar] [CrossRef]
- Kati, V.; Foufopoulos, J.; Ioannidis, Y.; Papaioannou, H.; Poirazidis, K.; Lebrun, P. Diversity, Ecological Structure and Conservation of Herpetofauna in a Mediterranean Area (Dadia National Park, Greece). Amphib. Reptil. 2007, 28, 517–529. [Google Scholar] [CrossRef]
- National Observatory of Forest Fires (NOFFi). Available online: http://fmrsvm.for.auth.gr/ (accessed on 10 June 2024).
- Hailey, A. The Effects of Fire and Mechanical Habitat Destruction on Survival of the Tortoise Testudo Hermanni in Northern Greece. Biol. Conserv. 2000, 92, 321–333. [Google Scholar] [CrossRef]
- Kati, V.; Poirazidis, K.; Dufrêne, M.; Halley, J.M.; Korakis, G.; Schindler, S.; Dimopoulos, P. Towards the Use of Ecological Heterogeneity to Design Reserve Networks: A Case Study from Dadia National Park, Greece. Biodivers. Conserv. 2010, 19, 1585–1597. [Google Scholar] [CrossRef]
- Sanchez-zapata, J.A.; Calvo, J.F. Raptor Distribution in Relation to Landscape Composition in Semi-Arid Mediterranean Habitats. J Appl. Ecol. 1999, 36, 254–262. [Google Scholar] [CrossRef]
- Soutullo, A.; López-López, P.; Urios, V. Incorporating Spatial Structure and Stochasticity in Endangered Bonelli’s Eagle’s Population Models: Implications for Conservation and Management. Biol. Conserv. 2008, 141, 1013–1020. [Google Scholar] [CrossRef]
- Angelov, I.D. Exceptionally Low Proportion of Adult Pairs and Male-Skewed Adult Sex Ratio in a Declining Population of the Golden Eagle Aquila Chrysaetos (L., 1758) (Accipitriformes: Accipitridae). Acta Zool. Bulg. 2024, 76, 241. [Google Scholar]
- Ntemiri, K.; Saravia, V.; Angelidis, C.; Baxevani, K.; Probonas, M.; Kret, E.; Mertzanis, Y.; Iliopoulos, Y.; Georgiadis, L.; Skartsi, D.; et al. Animal Mortality and Illegal Poison Bait Use in Greece. Environ. Monit. Assess. 2018, 190, 488. [Google Scholar] [CrossRef]
- Tintó, A.; Real, J.; Mañosa, S. Predicting and Correcting Electrocution of Birds in Mediterranean Areas. J. Wildl. Manag. 2010, 74, 1852–1862. [Google Scholar] [CrossRef]
- Vasilakis, D.P.; Whitfield, D.P.; Kati, V. A Balanced Solution to the Cumulative Threat of Industrialized Wind Farm Development on Cinereous Vultures (Aegypius Monachus) in South-Eastern Europe. PLoS ONE 2017, 12, e0172685. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.; Otero, M.; Gómez, G.J.; Madero, A. [Golden Eagles “Sentinels” Identify Biodiversity Threats.] in Spanish. Rev. Quercus. 2024, pp. 35–37. Available online: https://www.revistaquercus.es/noticia/8666/nacional/aguilas-reales-centinelas-identifican-las-amenazas-para-la-biodiversidad.html (accessed on 10 May 2024).
- Di Lieto, G.; Esposito, C. [Recolonisation after 30 Years of a Reproductive Site in the Lepini Mountains Area (Latium, C Italy) by Golden Eagle Aquila Chrysaetos. Riv. Ital. Ornithol. 2012, 80, 114–116. [Google Scholar] [CrossRef][Green Version]
- Morollón, S.; López-López, P.; Urios, V. A New View of Territoriality in Large Eagles: The Territory Pre-Exists Regardless of Their Occupants. J. Zool. 2024, 323, 177–186. [Google Scholar] [CrossRef]
- Kati, V.; Petridou, M.; Tzortzakaki, O.; Papantoniou, E.; Galani, A.; Psaralexi, M.; Gotsis, D.; Papaioannou, H.; Kassara, C. How Much Wilderness Is Left? A Roadless Approach under the Global and the European Biodiversity Strategy Focusing on Greece. Biol. Conserv. 2023, 281, 110015. [Google Scholar] [CrossRef]
- REGULATORY AUTHORITY FOR ENERGY Geospatial Map for Energy Units and Requests. Available online: https://geo.rae.gr/?tab=viewport_maptab (accessed on 13 December 2022).
- Bartoń, K. Package MuMIn. Multimodel Inference. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 10 May 2024).
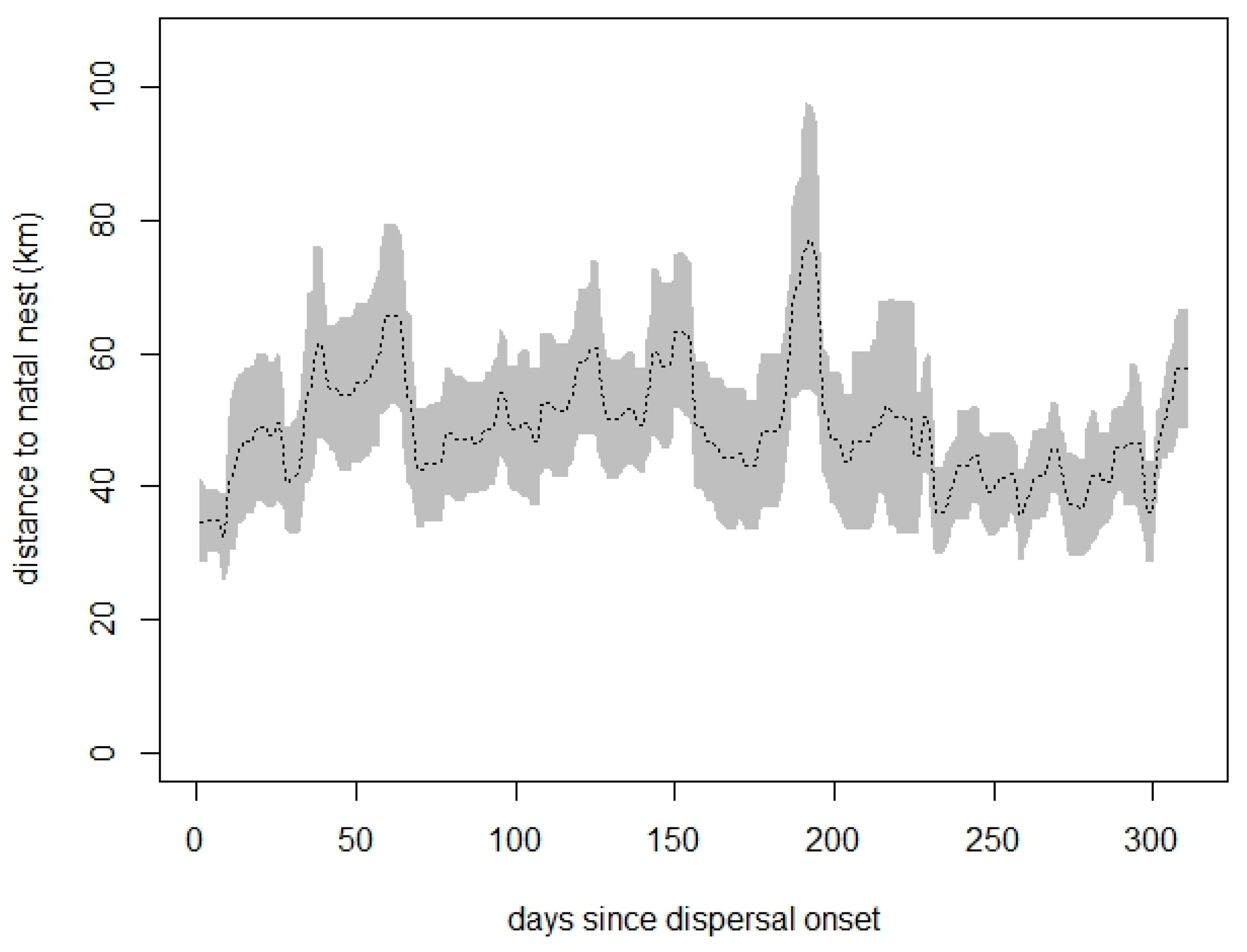
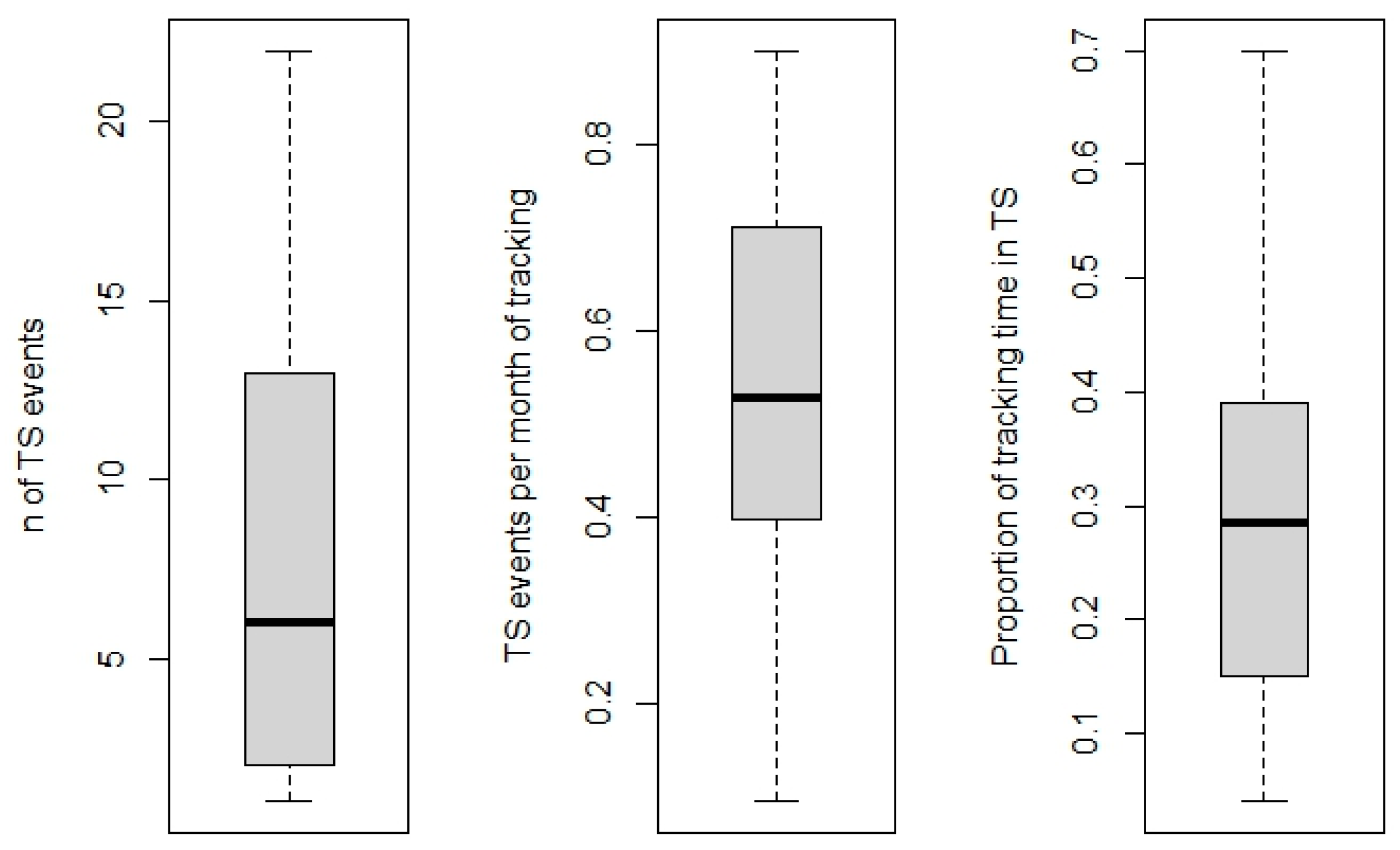
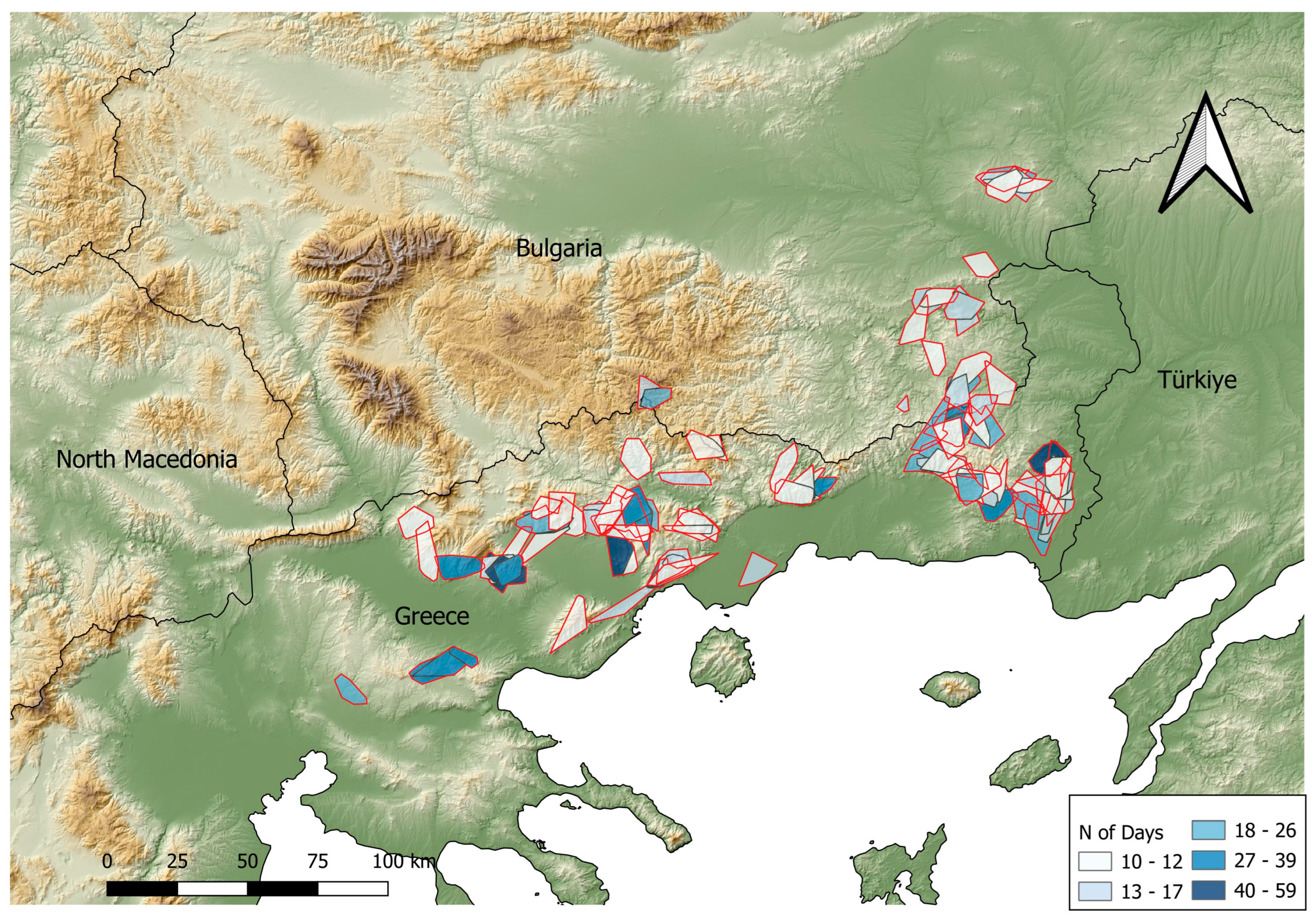
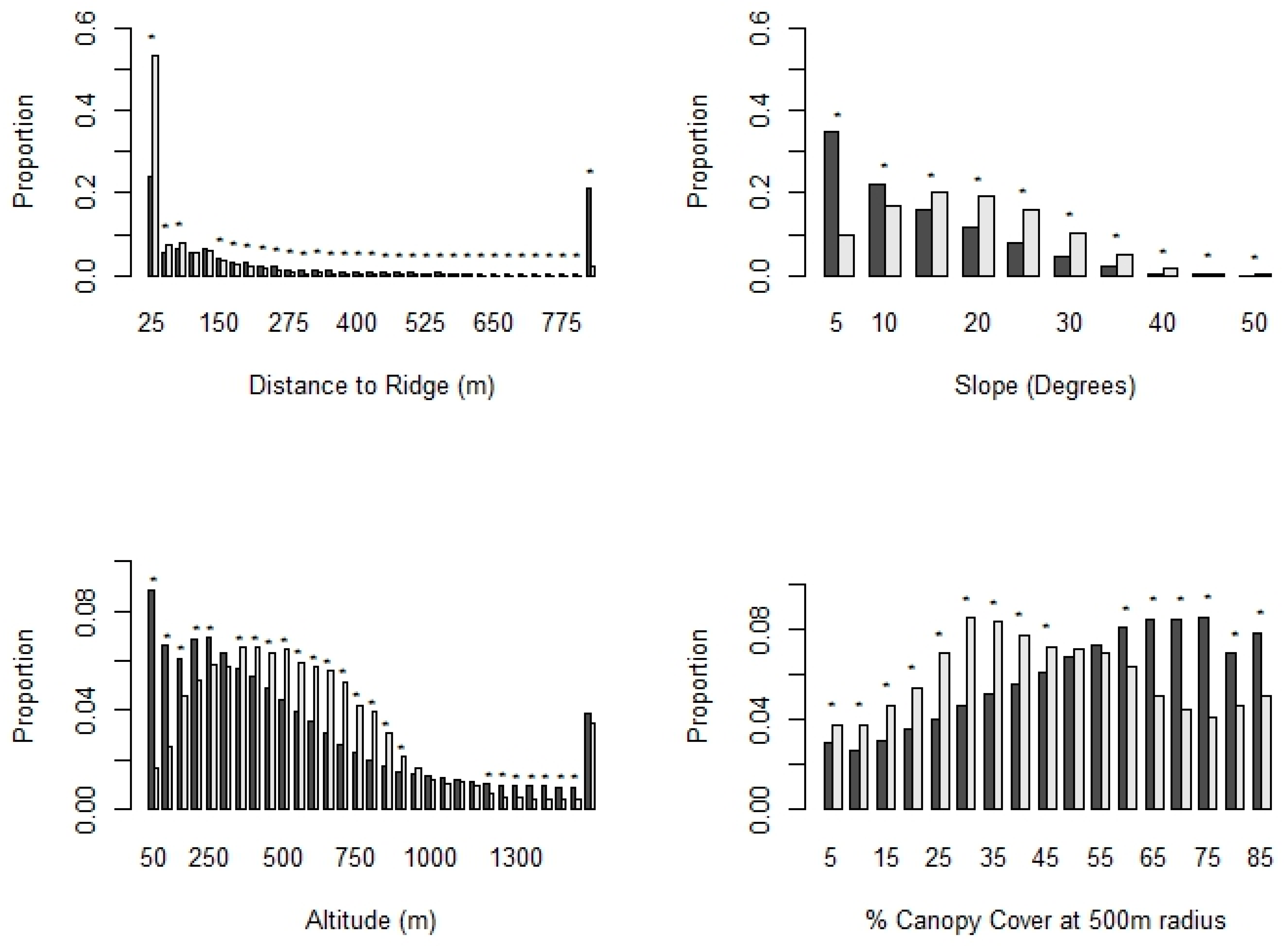
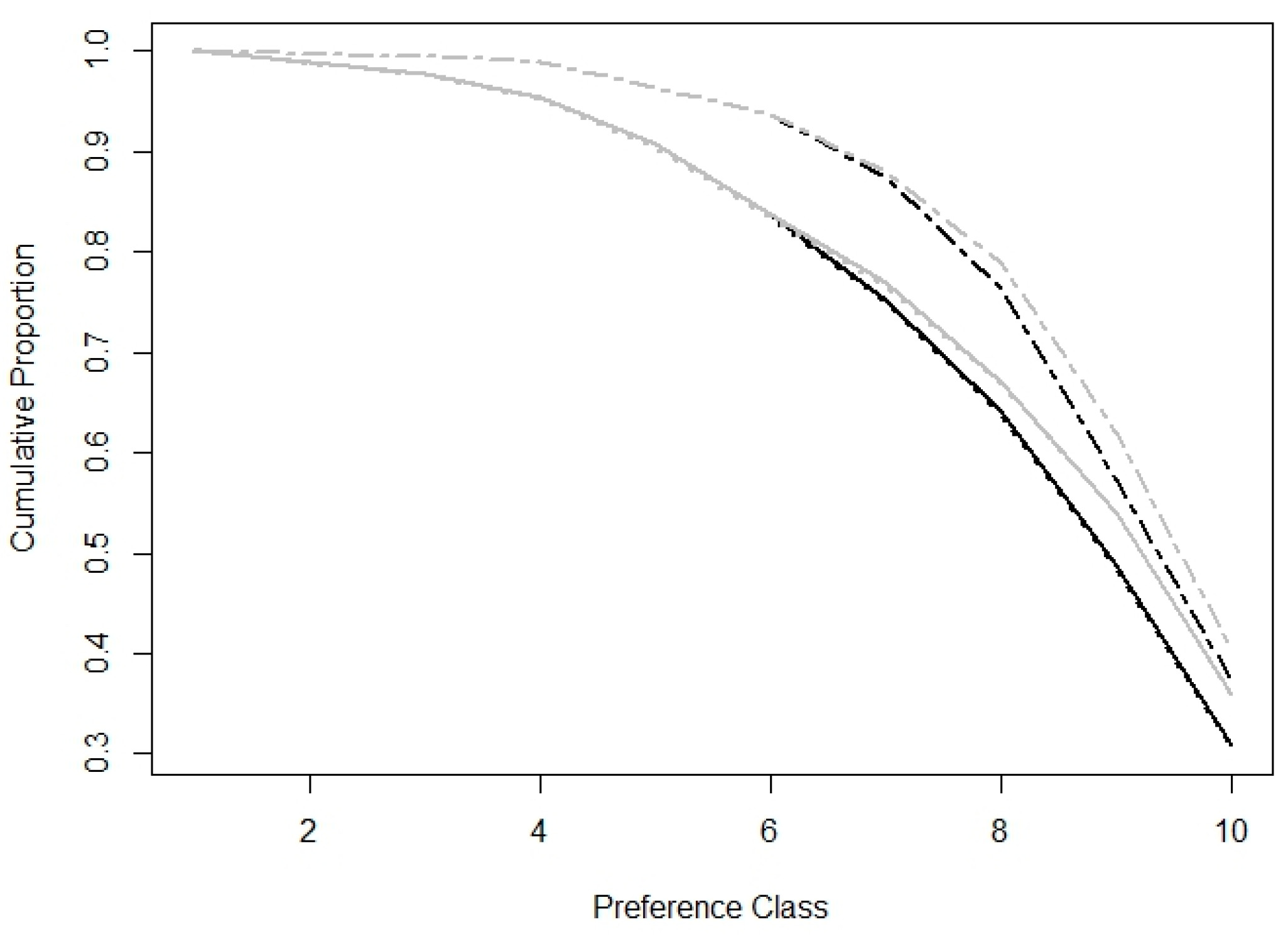

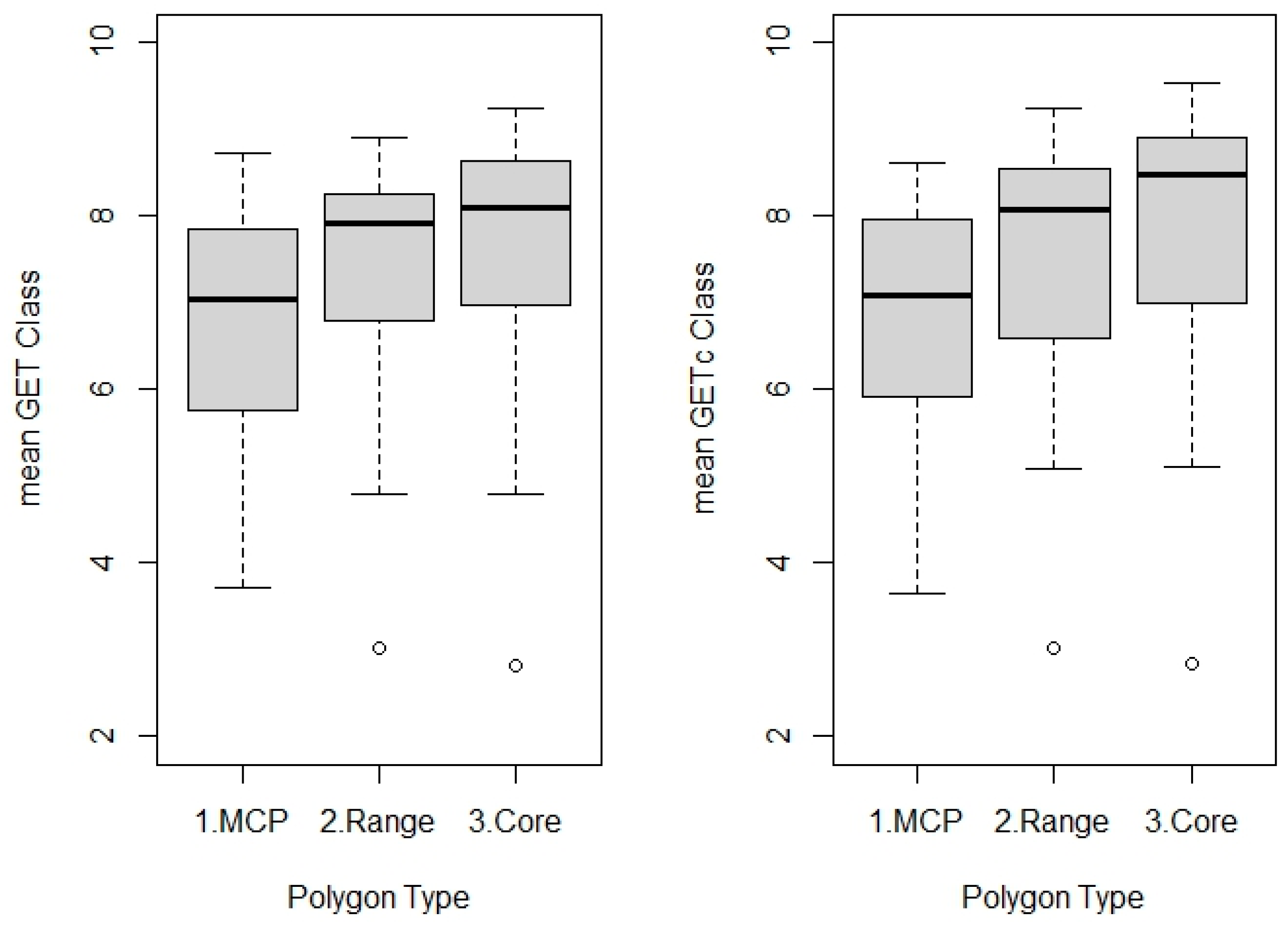
| TS Polygon Type | Season | Mean Area (km2) |
|---|---|---|
| 95% MCP | Winter | 84.89 ± 23.58 |
| Summer | 82.65 ± 23.41 | |
| 95% MKDE | Winter | 24.62 ± 13.62 |
| Summer | 18.28 ± 11.53 | |
| Core Area MKDE | Winter | 6.44 ± 10.19 |
| Summer | 5.29 ± 8.82 |
| GET Model | GETc Model | |||
|---|---|---|---|---|
| Class | Percentage | Area (km2) | Percentage | Area (km2) |
| 1 | 5.6 | 8659 | 5.6 | 8659 |
| 2 | 14.3 | 22,065 | 14.3 | 22,065 |
| 3 | 18.3 | 28,084 | 18.3 | 28,084 |
| 4 | 13.0 | 20,060 | 13.0 | 20,060 |
| 5 | 9.8 | 15,015 | 9.9 | 15,159 |
| 6 | 8.8 | 13,479 | 8.7 | 13,420 |
| 7 | 8.4 | 12,900 | 8.4 | 12,857 |
| 8 | 7.8 | 12,049 | 7.6 | 11,632 |
| 9 | 7.2 | 11,115 | 7.6 | 11,720 |
| 10 | 6.8 | 10,422 | 6.6 | 10,193 |
| Variables | AICc | Concordance | Z | p | χ2 | χ2 p |
|---|---|---|---|---|---|---|
| Slope | 43.01 | 0.85 | 32.5 | <0.003 | ||
| Division | 43.32 | 0.85 | 32.2 | <0.02 | ||
| % Open | 61.4 | 0.66 | 14.11 | <0.001 | ||
| Slope + Open | 35.6 | 0.86 | 42.09 | <0.0001 | 9.6 | <0.002 |
| Slope + Division | 35.1 | 0.91 | 42.6 | <0.0001 | 10.1 | <0.002 |
| Slope + Open + Division | 32.5 | 0.91 | 47.5 | <0.0001 | 4.8 | <0.03 |
| Model | Model Term | Coefficient | Odds | Lower CI | Upper CI | Z | p |
|---|---|---|---|---|---|---|---|
| Slope + Open | Slope | 1.61 | 4.99 | 2.02 | 12.35 | 3.48 | <0.0001 |
| Open | 0.23 | 1.26 | 1.06 | 1.50 | 2.59 | <0.001 | |
| Slope + Division | Slope | 1.61 | 4.99 | 1.29 | 6.90 | 2.57 | 0.01 |
| Division | 0.19 | 1.21 | 0.999 | 1.47 | 1.95 | 0.051 | |
| Slope + Open + Division | Slope | 1.24 | 3.24 | 1.34 | 9.07 | 2.56 | 0.01 |
| Open | 0.18 | 1.19 | 0.999 | 1.4 | 1.95 | 0.051 | |
| Division | 0.13 | 1.14 | 0.97 | 1.33 | 1.56 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidiropoulos, L.; Whitfield, D.P.; Poirazidis, K.; Navarrete, E.; Vasilakis, D.P.; Bounas, A.; Kret, E.; Kati, V. Dispersal Ecology of Golden Eagles (Aquila chrysaetos) in Northern Greece: Onset, Ranging, Temporary and Territorial Settlement. Diversity 2024, 16, 580. https://doi.org/10.3390/d16090580
Sidiropoulos L, Whitfield DP, Poirazidis K, Navarrete E, Vasilakis DP, Bounas A, Kret E, Kati V. Dispersal Ecology of Golden Eagles (Aquila chrysaetos) in Northern Greece: Onset, Ranging, Temporary and Territorial Settlement. Diversity. 2024; 16(9):580. https://doi.org/10.3390/d16090580
Chicago/Turabian StyleSidiropoulos, Lavrentis, D. Philip Whitfield, Konstantinos Poirazidis, Elisabeth Navarrete, Dimitris P. Vasilakis, Anastasios Bounas, Elzbieta Kret, and Vassiliki Kati. 2024. "Dispersal Ecology of Golden Eagles (Aquila chrysaetos) in Northern Greece: Onset, Ranging, Temporary and Territorial Settlement" Diversity 16, no. 9: 580. https://doi.org/10.3390/d16090580
APA StyleSidiropoulos, L., Whitfield, D. P., Poirazidis, K., Navarrete, E., Vasilakis, D. P., Bounas, A., Kret, E., & Kati, V. (2024). Dispersal Ecology of Golden Eagles (Aquila chrysaetos) in Northern Greece: Onset, Ranging, Temporary and Territorial Settlement. Diversity, 16(9), 580. https://doi.org/10.3390/d16090580






