Taxonomic Exploration of Rare Amphipods: A New Genus and Two New Species (Amphipoda, Iphimedioidea, Laphystiopsidae) Described from Seamounts in the Western Pacific †
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Preservation
2.2. DNA Extraction, Sequencing, and Phylogenetic Analyses
3. Results
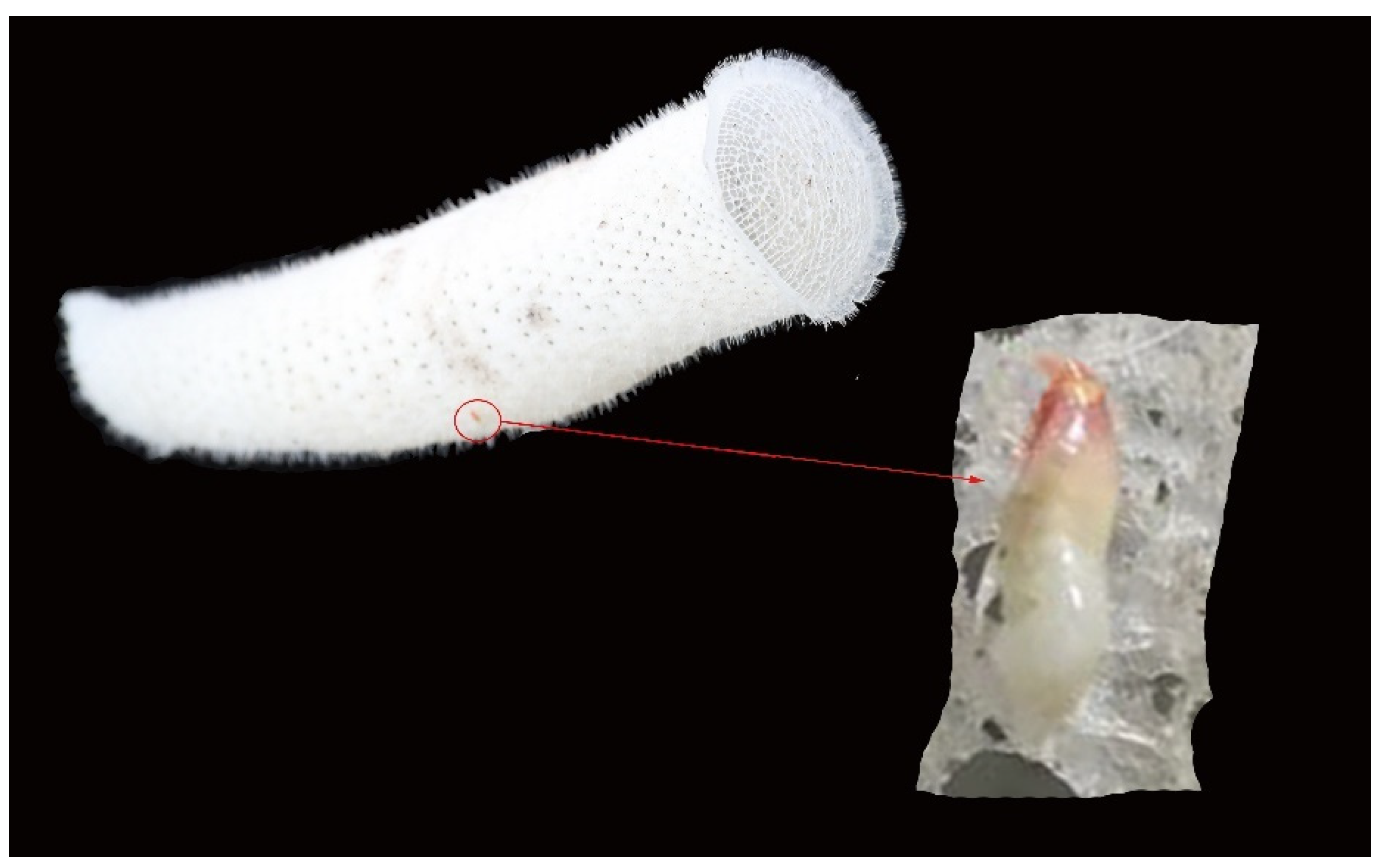
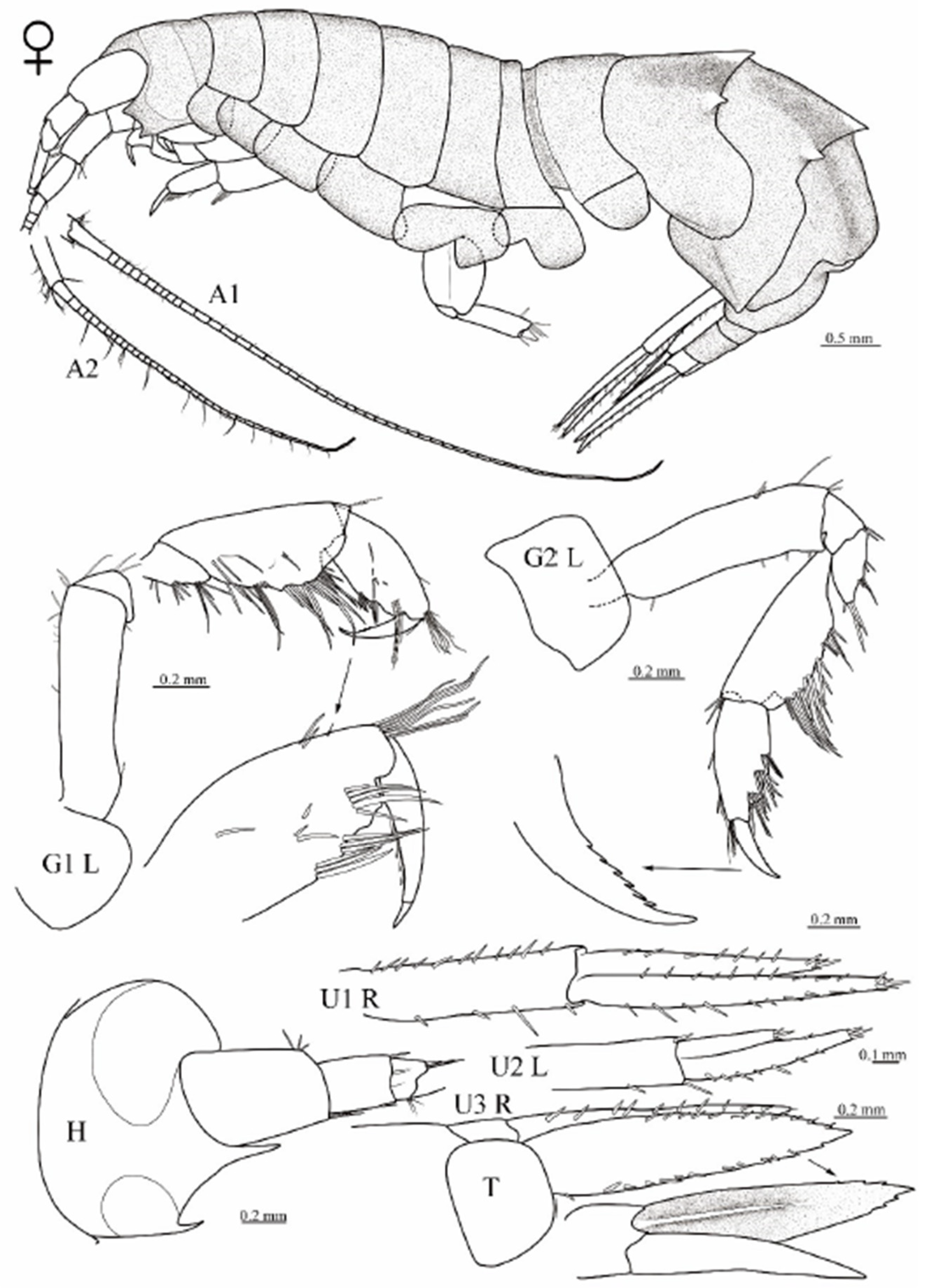
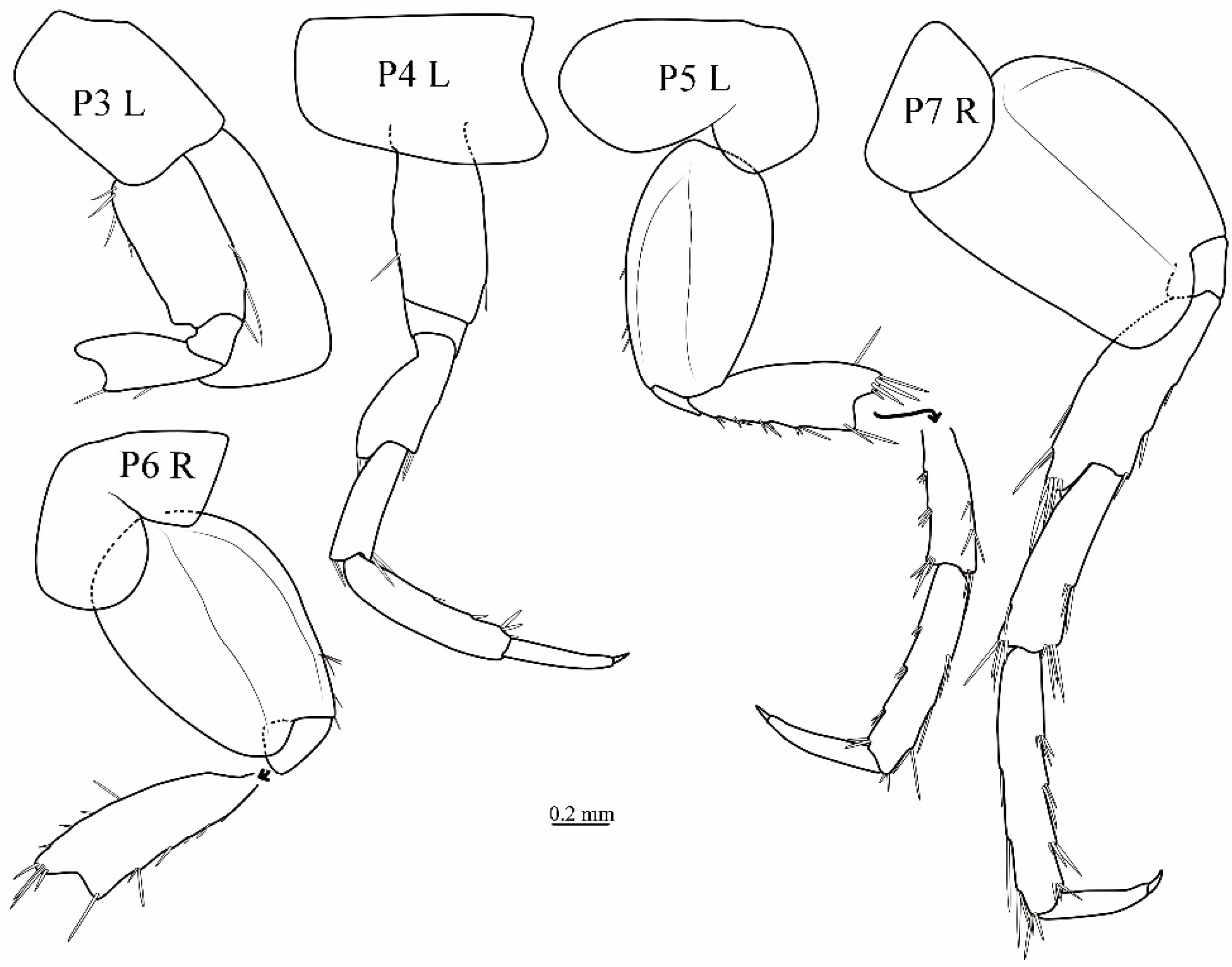
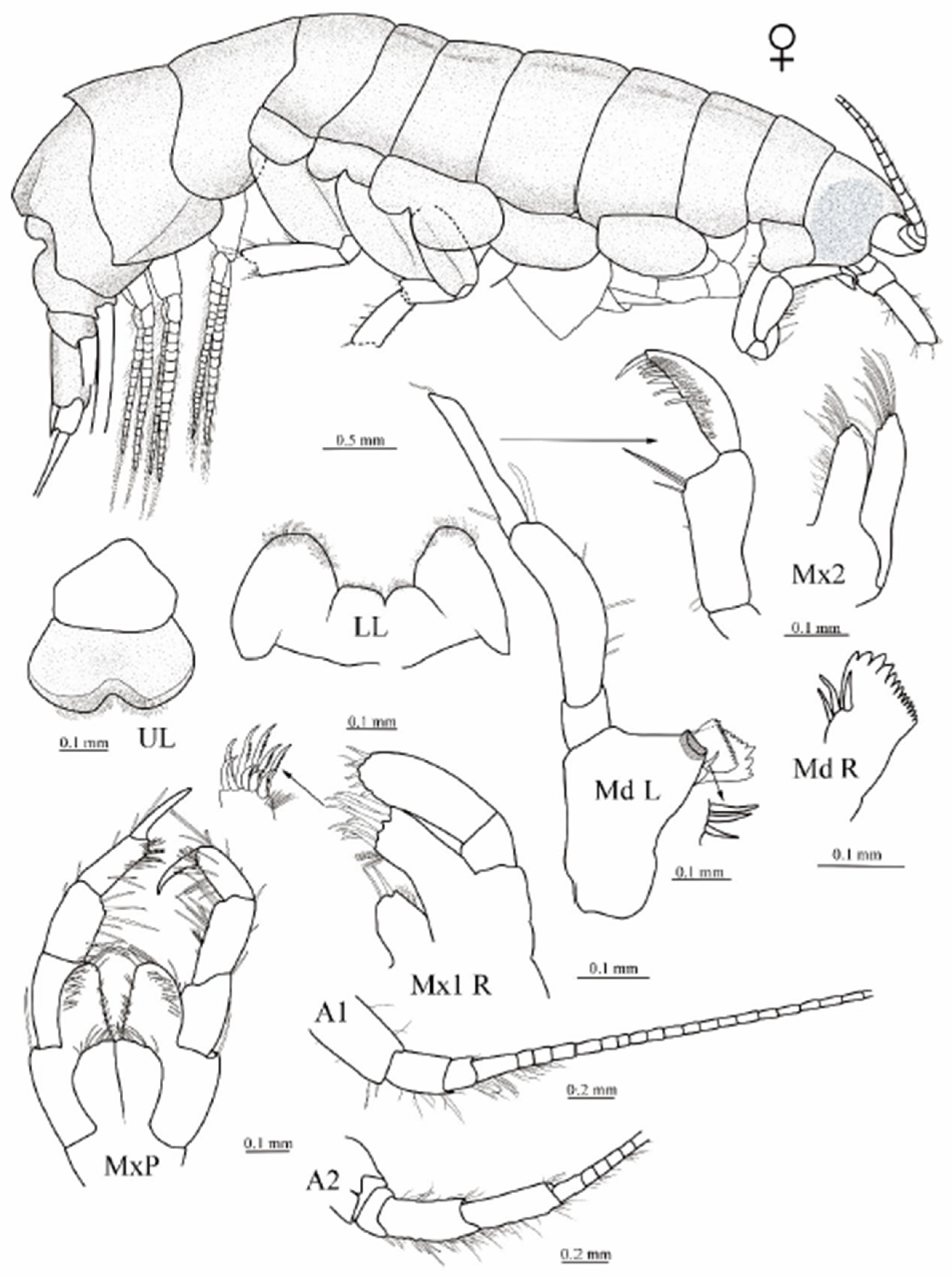
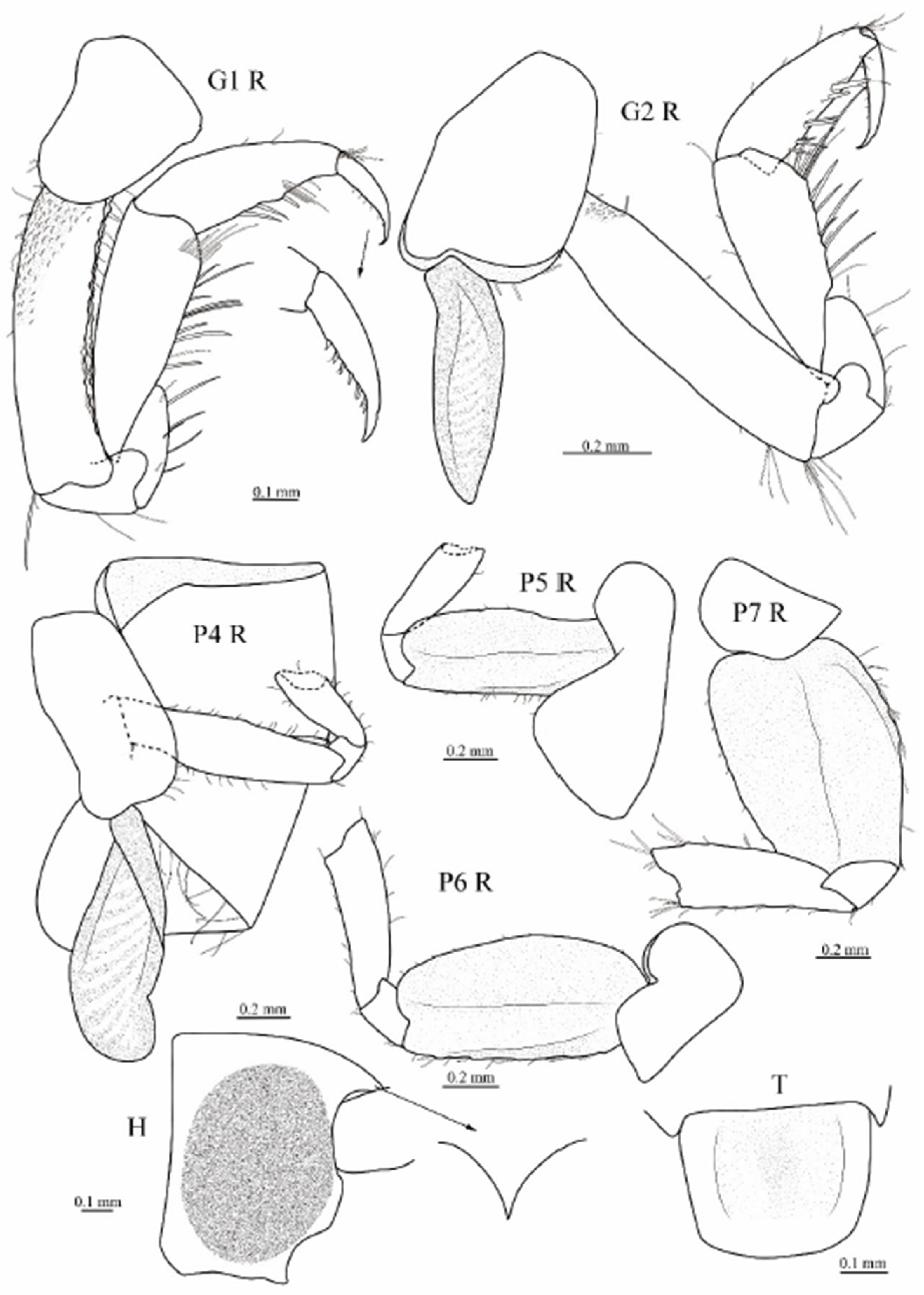
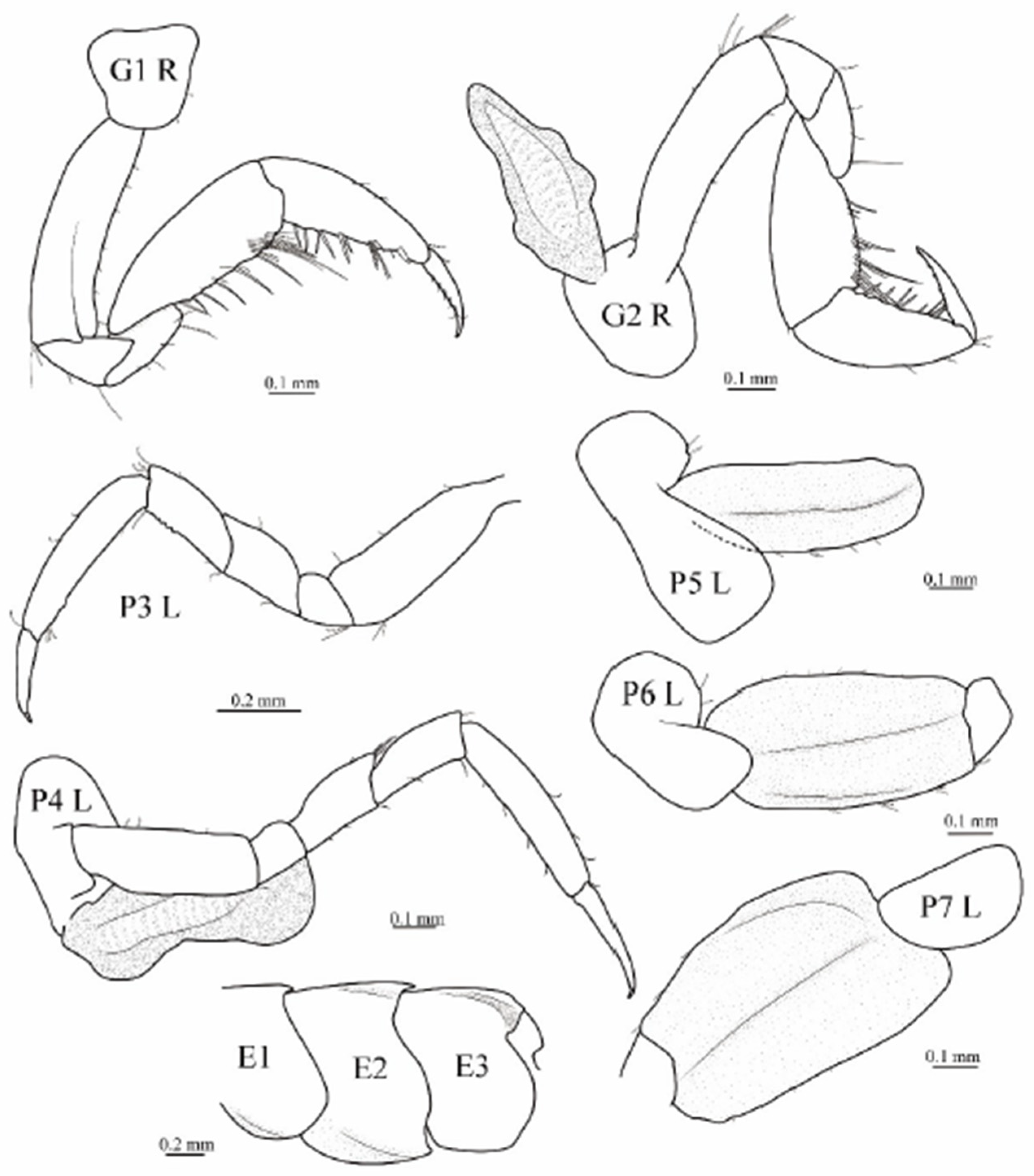
3.1. Taxonomy
| 1. Rostrum acute, not spatulate; maxilla 1 with inner plate bearing 2 apical setae, outer plate with 10–11 spines; telson rectangular | |
| Phoxirostus gen. nov. | |
| -. Rostrum spatulate, or absent; maxilla 1 with inner plate bearing 0–1 apical seta, outer plate with 5–7 spines; telson oval | |
| 2 | |
| 2. Rostrum absent; coxa 4 almost as long as broad, deeply excavate posteriorly | |
| Prolaphystius KH Barnard, 1930 | |
| -. Rostrum present; coxa 4 small, much wider than long, not excavate posteriorly | |
| 3 | |
| 3. Peduncular article 1 of antenna 1 having apical projection; rostrum apically constricted, margin rounded; eye lobes strongly bulging | |
| Prolaphystiopsis Schellenberg, 1931 | |
| -. Peduncular article 1 of antenna 1 lacking apical projection; rostrum not constricted, truncate; eye lobes not bulging | |
| Laphystiopsis GO Sars, 1893 | |
| 1. Eyes rounded; pleonite 1 without posterodorsal acute protrusion and posterior margin of pleonites 1 and 2 only with one acute protrusion medially | |
| P. yapensis sp. nov. | |
| -. Eyes reniform; pleonite 1 with posterodorsal acute protrusions and posterior margin of pleonites 1 and 2 with three acute protrusions | |
| P. longicarpus sp. nov. | |
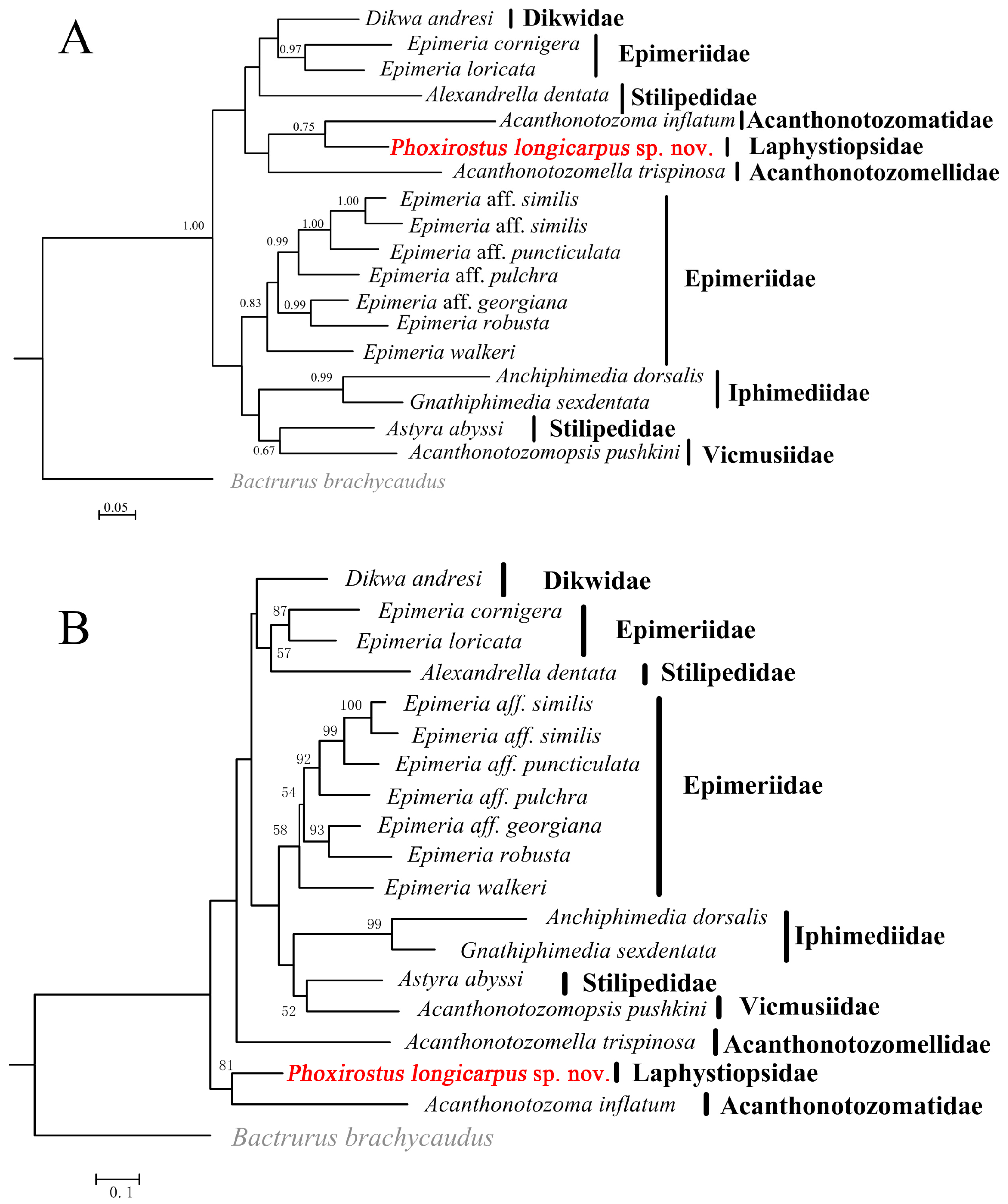
3.2. Phylogenetic Analyses
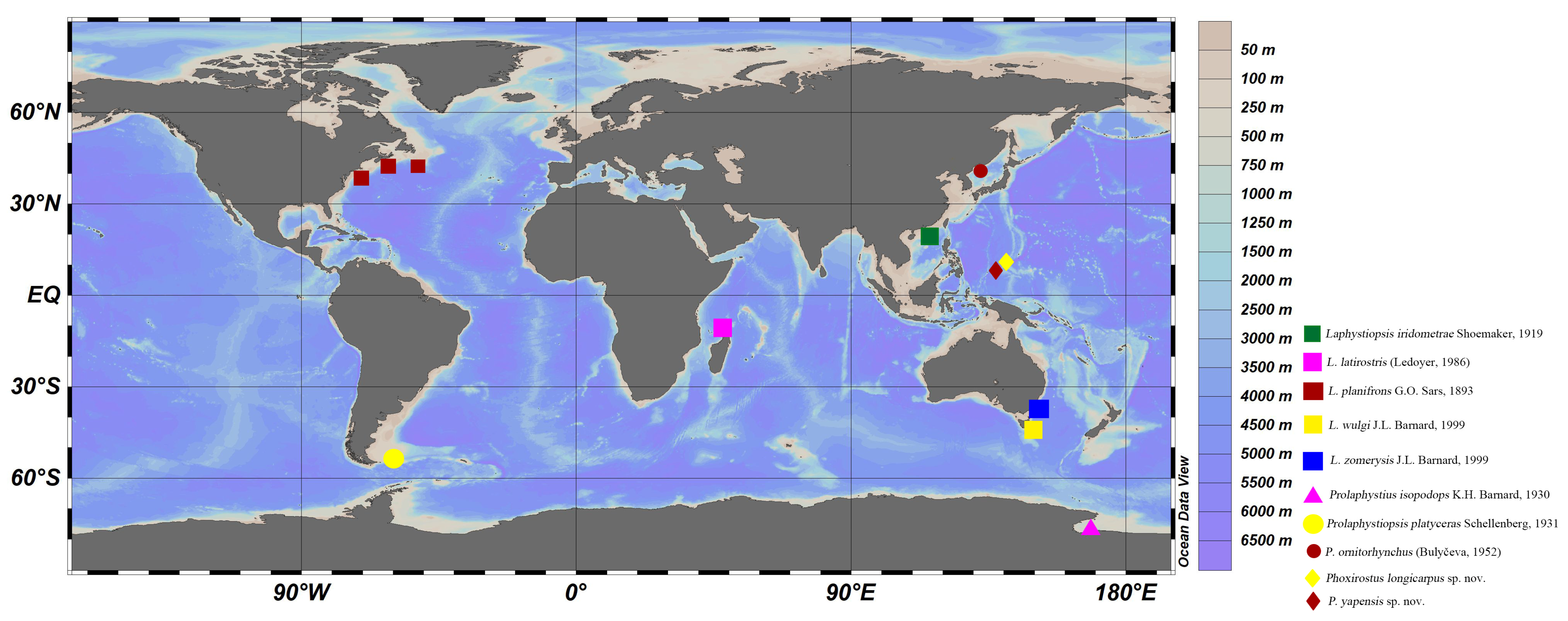
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sars, G.O. Amphipoda. Part XVII. Epimeridae (concluded), Syrrhoidae (part). In An Account of the Crustacea of Norway, with Short Descriptions and Figures of All the Species; Cammermeyer: Oslo, Norway, 1893; Volume I, pp. 365–388. [Google Scholar]
- Barnard, K.H. Crustacea. Part XI—Amphipoda. British Antarctic (“Terra Nova”) Expedition, 1910. Natural History Report. Zoology 1930, 8, 307–454. [Google Scholar]
- Schellenberg, A. Gammariden und Caprelliden des Magellangebietes, Sudgeorgiens und der Westantarktis. In Further Zoological Results of the Swedish Antarctic Expedition 1901–1903; Stockholm Norstedt: Stockholm, Sweden, 1931; Volume 2, pp. 1–290. [Google Scholar]
- Ledoyer, M. Crustacés Amphipodes Gammariens. Familles des Haustoriidae à Vitjazianidae. Faune Madag. 1986, 59, 599–1112. [Google Scholar]
- Barnard, J.L. Revision of Laphystiopsidae (Crustacea: Amphipoda): New and old Species from South China Sea, Southeastern Australia, Falkland Islands and Western Atlantic Ocean. Mem. Mus. Vic. 1999, 57, 287–310. [Google Scholar] [CrossRef][Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, G.; Lohman, D.J.; Meier, R. Sequencematrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogeny. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; Von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Ledoyer, M. Amphipodes Gammariens de la frondaison des herbiers d’Enhalus de la région de Nosy-Bé (Madagascar) (Systématique et écologie). Comparaison avec la faune des herbiers de Tuléar (Cymodocea, Thalassia etc). Tethys Suppl. 1973, 5, 25–36. [Google Scholar]
- Barnard, J.L.; Karaman, G.S. The Families and Genera of Marine Gammaridean Amphipoda (except Marine Gammaroids), Part 1; Australian Museum: Darlinghurst, Australia, 1991; Volume 13, pp. 1–417. [Google Scholar]
- Woolley, S.N.C.; Tittensor, D.P.; Dunstan, P.K.; Guillera-Arroita, G.; Lahoz-Monfort, J.J.; Wintle, B.A.; Worm, B.; O’Hara, T.D. Deep-sea diversity patterns are shaped by energy availability. Nature 2016, 533, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Lowry JK, Myers AA A Phylogeny and Classification of the Amphipoda With the Establishment of the New Order Ingolfiellida (Crustacea: Peracarida). Zootaxa 2017, 4265, 1–89.
- Copilaş-Ciocianu, D.; Borko, Š.; Fišer, C. The late blooming amphipods: Global change promoted post-Jurassic ecological radiation despite Palaeozoic origin. Mol. Phylogenet. Evol. 2020, 143, 106664. [Google Scholar] [CrossRef] [PubMed]
- Verheye, M.L.; Backeljau, T.; d’Udekem d’Acoz, C. Locked in the icehouse: Evolution of an endemic Epimeria (Amphipoda, Crustacea) species flock on the Antarctic shelf. Mol. Phylogenet. Evol. 2017, 114, 14–33. [Google Scholar] [CrossRef] [PubMed]


| Taxon | COI | H3 |
|---|---|---|
| Acanthonotozomatidae Stebbing, 1906 | ||
| Acanthonotozoma inflatum (Krøyer, 1842) | N/A | KJ530648 |
| Acanthonotozomellidae Coleman and J.L. Barnard, 1991 | ||
| Acanthonotozomella trispinosa (Bellan-Santini, 1972) | KY907618 | KY825836 |
| Dikwidae Coleman and J.L. Barnard, 1991 | ||
| Dikwa andresi Lörz and Coleman, 2003 | KY907614 | KY825833 |
| Epimeriidae Boeck, 1871 | ||
| Epimeria aff. similis Chevreux, 1912 | KU870895 | KY825955 |
| Epimeria aff. similis Chevreux, 1912 | KU870865 | KY825885 |
| Epimeria aff. georgiana Schellenberg, 1931 | KU870894 | KY825952 |
| Epimeria aff. puncticulata K.H. Barnard, 1930 | KU870888 | KY825933 |
| Epimeria aff. pulchra Coleman, 1990 | KU870881 | KY825925 |
| Epimeria cornigera (Fabricius, 1779) | KY907659 | KY825950 |
| Epimeria loricata G.O. Sars, 1879 | KY907626 | KY825847 |
| Epimeria robusta K.H. Barnard, 1930 | KU870854 | KY825866 |
| Epimeria walkeri (K.H. Barnard, 1930) | KU870819 | N/A |
| Iphimediidae Boeck, 1871 | ||
| Anchiphimedia dorsalis K.H. Barnard, 1930 | KY907612 | KY825816 |
| Gnathiphimedia sexdentata (Schellenberg, 1926) | KU870835 | KY825841 |
| Stilipedidae Holmes, 1908 | ||
| Alexandrella dentata Chevreux, 1912 | KY907619 | KY825837 |
| Astyra abyssi Boeck, 1871 | KY907617 | KY825835 |
| Vicmusiidae Just, 1990 | ||
| Acanthonotozomopsis pushkini (Bushueva, 1978) | KY907620 | KY825838 |
| Crangonyctidae Bousfield, 1973 | ||
| Bactrurus brachycaudus Hubricht and Mackin, 1940 | MN175619 | KF484707 |
| Laphystiopsidae | ||
| Phoxirostus longicarpus gen. et ap. nov. | PQ193948 | PQ268529 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Sha, Z.; Ren, X. Taxonomic Exploration of Rare Amphipods: A New Genus and Two New Species (Amphipoda, Iphimedioidea, Laphystiopsidae) Described from Seamounts in the Western Pacific. Diversity 2024, 16, 564. https://doi.org/10.3390/d16090564
Wang Y, Sha Z, Ren X. Taxonomic Exploration of Rare Amphipods: A New Genus and Two New Species (Amphipoda, Iphimedioidea, Laphystiopsidae) Described from Seamounts in the Western Pacific. Diversity. 2024; 16(9):564. https://doi.org/10.3390/d16090564
Chicago/Turabian StyleWang, Yanrong, Zhongli Sha, and Xianqiu Ren. 2024. "Taxonomic Exploration of Rare Amphipods: A New Genus and Two New Species (Amphipoda, Iphimedioidea, Laphystiopsidae) Described from Seamounts in the Western Pacific" Diversity 16, no. 9: 564. https://doi.org/10.3390/d16090564
APA StyleWang, Y., Sha, Z., & Ren, X. (2024). Taxonomic Exploration of Rare Amphipods: A New Genus and Two New Species (Amphipoda, Iphimedioidea, Laphystiopsidae) Described from Seamounts in the Western Pacific. Diversity, 16(9), 564. https://doi.org/10.3390/d16090564






