Current and Future Distribution of the Cataglyphis nodus (Brullé, 1833) in the Middle East and North Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Species Occurrence
2.2. Environmental Data and Processing
2.3. Bioclimatic Data and Processing
2.4. Variables Selection
2.5. Model Simulation
2.6. Model Performance
3. Result and Discussion
3.1. Variables Contribution and Model Performance
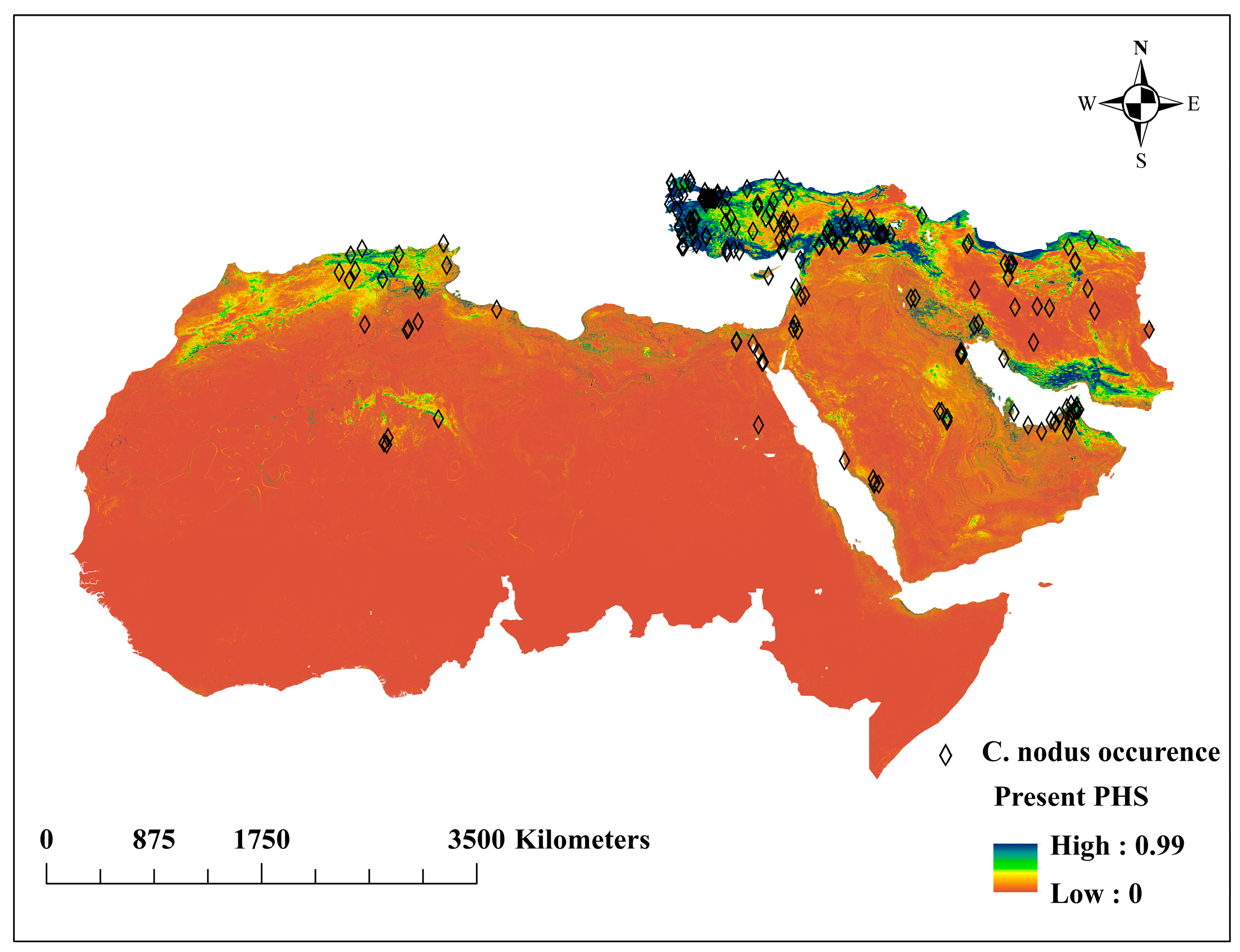
3.2. Present Potential Habitat Suitability (PHS)
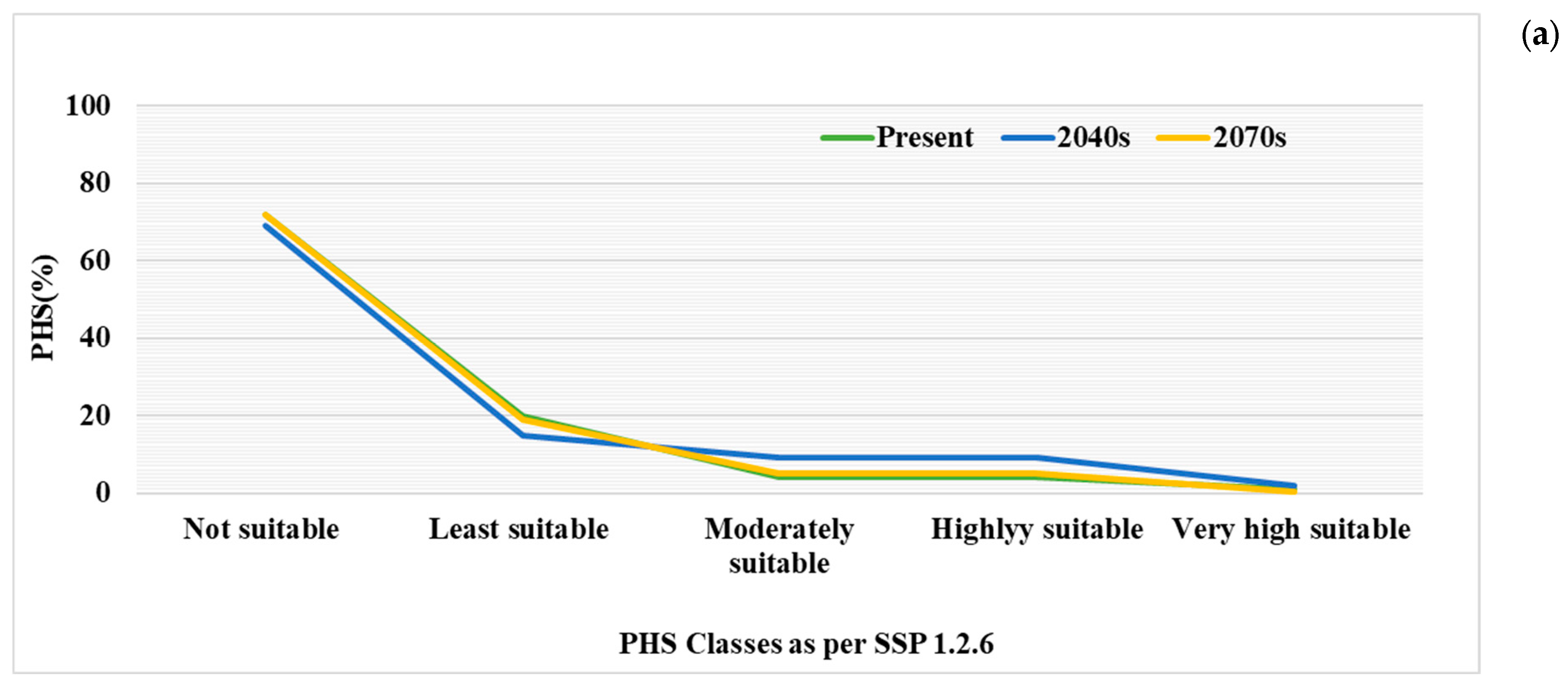
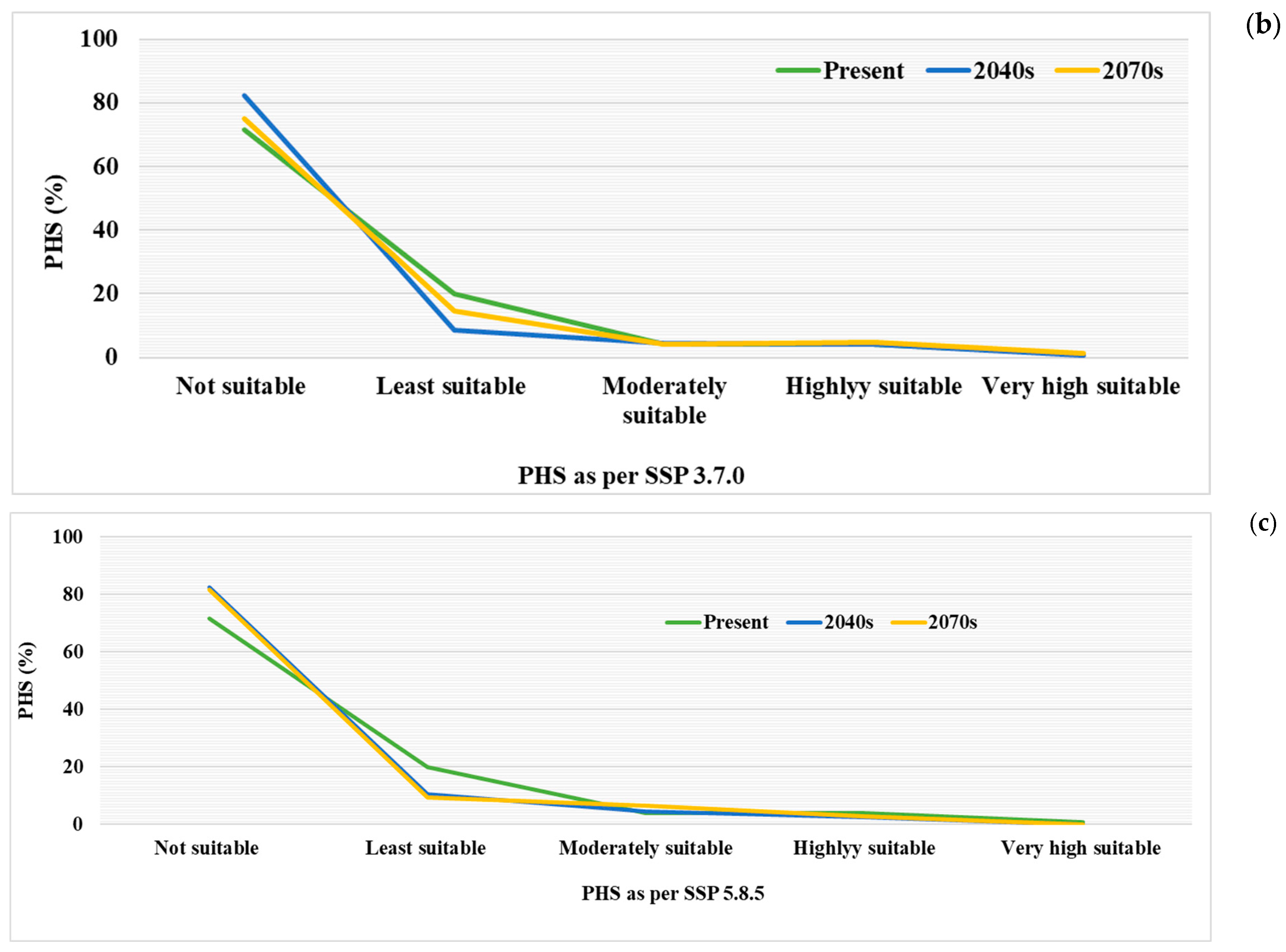
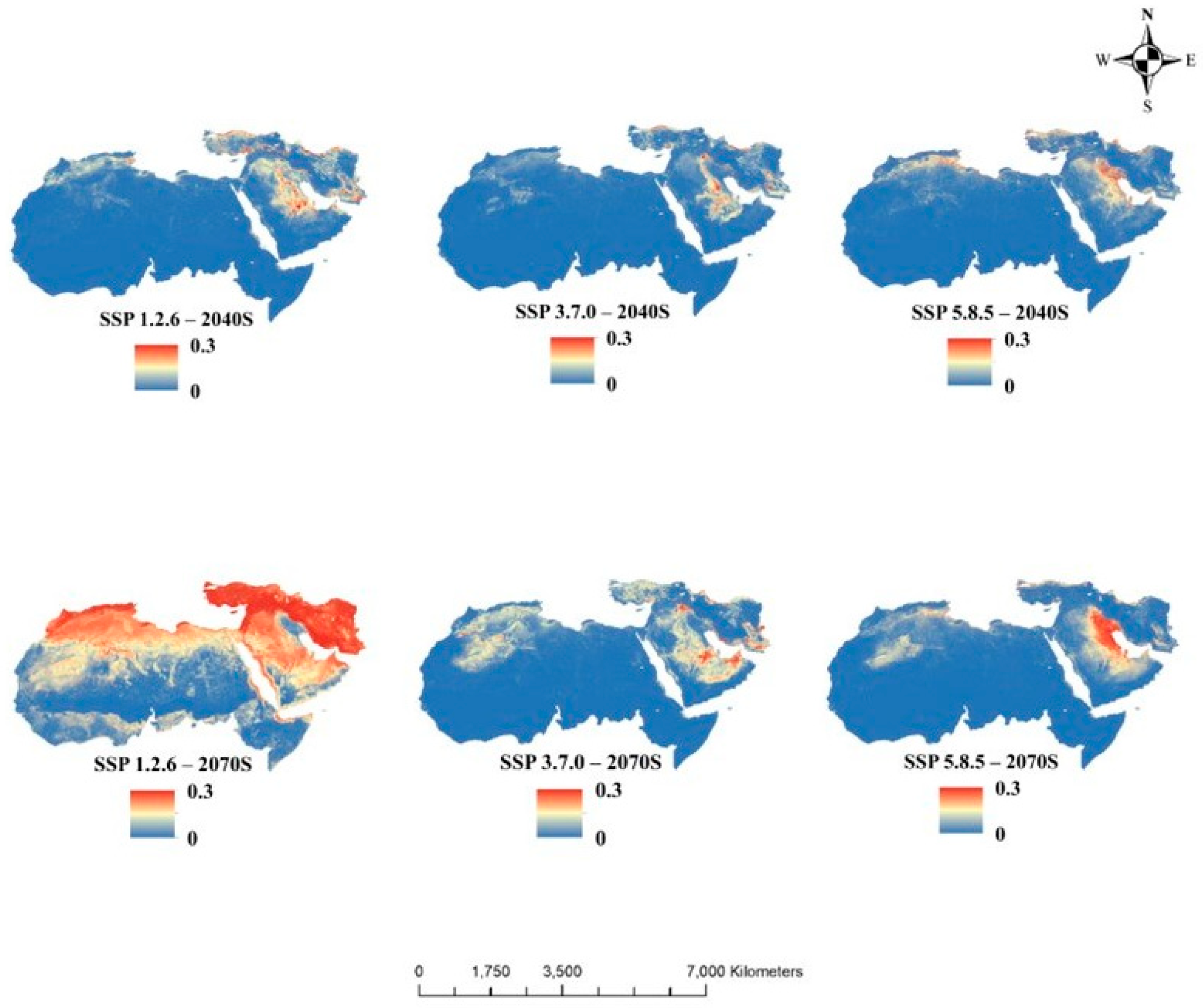
3.3. Future Potential Habitat Suitability
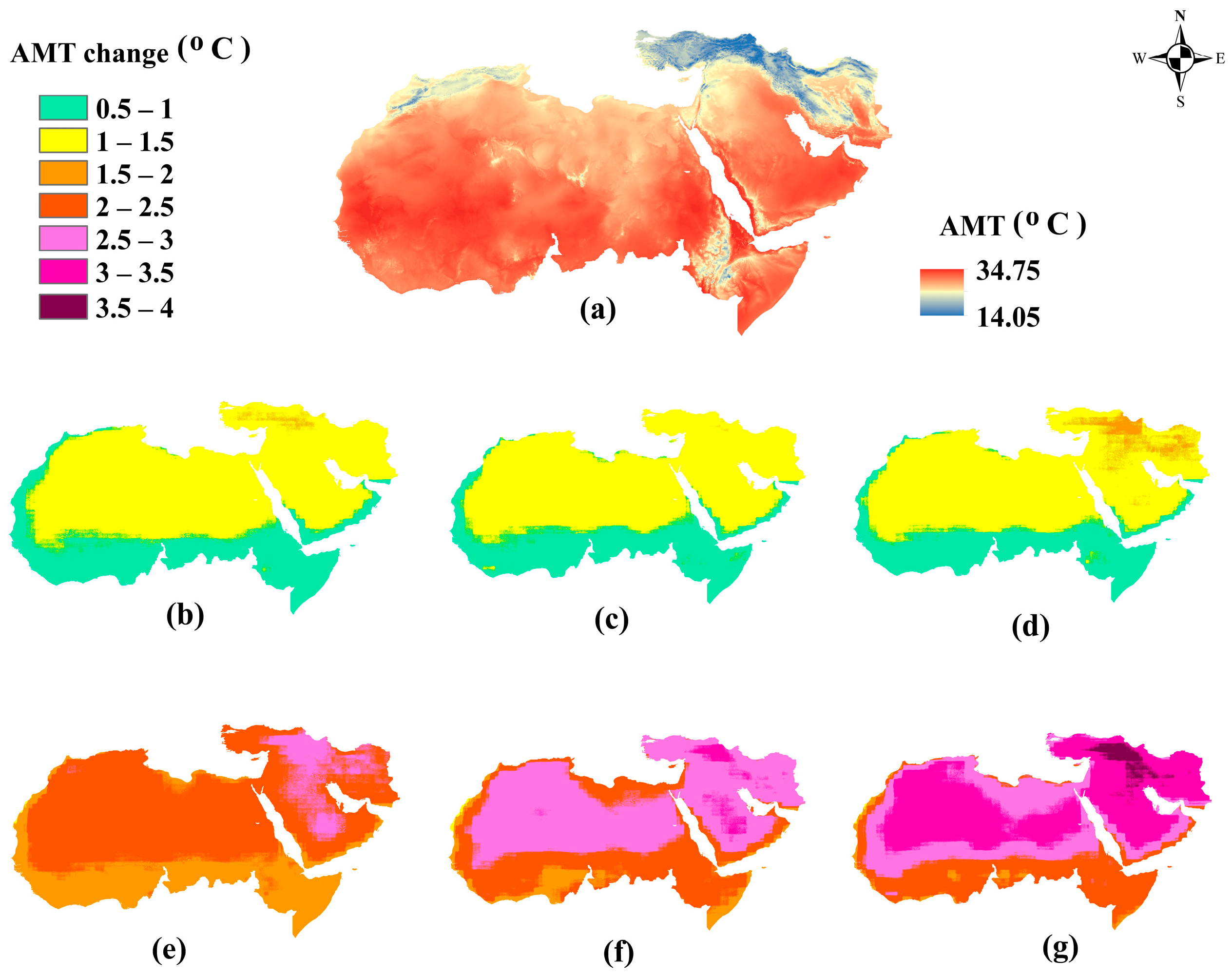
3.4. Uncertainty in Future Bioclimatic Conditions
4. Conclusions
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Burrows, M.T.; Schoeman, D.S.; Richardson, A.J.; García, J. Climate velocity and geographical limits to shifts in species’ distributions. Nature 2014, 507, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Rosenweig, M.L. Species Diversity in Space and Time; Cambridge University Press: New York, NY, USA, 1995. [Google Scholar]
- Dornelas, M.; Magurran, A.E.; Buckland, S.T.; Chao, A.; Chazdon, R.L.; Colwell, R.K.; Curtis, T.; Gaston, K.J.; Gotelli, N.J.; Kosnik, M.A.; et al. Quantifying temporal change in biodiversity: Challenges and opportunities. Proc. R. Soc. B Biol. Sci. 2013, 280, 20121931. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, Q.W.; Fu, S.; Wu, X.F.; Xue, F.S. Effect of photoperiod and temperature on the intensity of pupal diapause in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Bull. Entomol. Res. 2014, 104, 12–18. [Google Scholar] [CrossRef]
- Macfadyen, S.; McDonald, G.; Hill, M.P. From species distributions to climate change adaptation: Knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agric. Ecosyst. Environ. 2018, 253, 208–219. [Google Scholar] [CrossRef]
- Schultheiss, P.; Nooten, S.S.; Wang, R.; Wong, M.K.; Brassard, F.; Guénard, B. The abundance, biomass, and distribution of ants on Earth. Proc. Natl. Acad. Sci. USA 2022, 119, 2201550119. [Google Scholar] [CrossRef]
- Brian, M.V. (Ed.) Production Ecology of Ants and Termites; Cambridge University Press: Cambridge, UK, 1978; Volume 13. [Google Scholar]
- Griffiths, H.M.; Ashton, L.A.; Walker, A.E.; Hasan, F.; Evans, T.A.; Eggleton, P.; Parr, C.L. Ants are the major agents of resource removal from tropical rainforests. J. Anim. Ecol. 2018, 87, 293–300. [Google Scholar] [CrossRef]
- Frouz, J.; Jilková, V. The effect of ants on soil properties and processes (Hymenoptera: Formicidae). Myrmecol. News 2008, 11, 191–199. [Google Scholar]
- Grodsky, S.M.; Roeder, K.A.; Campbell, J.W. Effects of solar energy development on ants in the Mojave Desert. Ecosphere 2023, 14, 4668. [Google Scholar] [CrossRef]
- Hoffmann, B.D. Using ants for rangeland monitoring: Global patterns in the responses of ant communities to grazing. Ecol. Indic. 2010, 10, 105–111. [Google Scholar] [CrossRef]
- Schultz, T.R. In search of ant ancestors. Proc. Natl. Acad. Sci. USA 2000, 97, 14028–14029. [Google Scholar] [CrossRef] [PubMed]
- Andersen, A.N. The use of and communities to evaluate change in Australian terrestrial ecosystems: A review and a recipe. Proc. Ecol. Soc. Aust. 1990, 16, 347–357. [Google Scholar]
- Hansen, R.R.; Kristiansen, S.M.; Damgaard, C.F.; Offenberg, J. Effects of ant mounts (Formica exsecta) on subsoil properties, in a heathland. Eur. J. Soil Sci. 2024, 120, 103597. [Google Scholar] [CrossRef]
- Folgarait, P.J.; Perelman, S.; Gorosito, N.; Pizzio, R.; Fernández, J. Effects of Camponotus punctulatus ants on plant community composition and soil properties across land-use histories. Plant Ecol. 2002, 163, 1–13. [Google Scholar] [CrossRef]
- Folgarait, P.J. Ant biodiversity and its relationship to ecosystem functioning: A review. Biodivers. Conserv. 1998, 7, 1221–1244. [Google Scholar] [CrossRef]
- Dantas, A.; Fonseca, C.R. Global biogeographical patterns of ants and their abiotic determinants. Perspect. Ecol. Conserv. 2023, 21, 237–246. [Google Scholar] [CrossRef]
- Economo, E.P.; Narula, N.; Friedman, N.R.; Weiser, M.D.; Guénard, B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 2018, 9, 1778. [Google Scholar] [CrossRef]
- Samson, D.A.; Rickart, E.A.; Gonzales, P.C. Ant Diversity and Abundance along an Elevational Gradient in the Philippines. Biotropica 1997, 29, 349–363. [Google Scholar] [CrossRef]
- Brener, A.G.F.; Ruggiero, A. Leaf-cutting ants (Atta and Acromyrmex) inhabiting Argentina: Patterns in species richness and geographical range sizes. J. Biogeogr. 1994, 21, 391–399. [Google Scholar] [CrossRef]
- Gerlach, J.; Samways, M.; Pryke, J. Terrestrial invertebrates as bioindicators: An overview of available taxonomic groups. J. Insect Conserv. 2013, 17, 831–850. [Google Scholar] [CrossRef]
- Schmidt, F.A.; Ribas, C.R.; Schoereder, J.H. How predictable is the response of ant assemblages to natural forest recovery? Implications for their use as bioindicators. Ecol. Indic. 2013, 24, 158–166. [Google Scholar] [CrossRef]
- Nascimento, G.; Câmara, T.; Arnan, X. Critical thermal limits in ants and their implications under climate change. Biol. Rev. 2022, 97, 1287–1305. [Google Scholar] [CrossRef] [PubMed]
- Parr, C.L.; Bishop, T.R. The response of ants to climate change. Glob. Change Biol. 2022, 28, 3188–3205. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.; Aron, S. Adaptations to thermal stress in social insects: Recent advances and future directions. Biol. Rev. 2020, 95, 1535–1553. [Google Scholar] [CrossRef] [PubMed]
- Boulay, R.; Aron, S.; Cerdá, X.; Doums, C.; Graham, P.; Hefetz, A.; Monnin, T. Social life in arid environments: The case study of Cataglyphis ants. Annu. Rev. Entomol. 2017, 62, 305–321. [Google Scholar] [CrossRef]
- Cerda, X.; Arnan, X.; Retana, J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecol. News 2013, 18, 131–147. [Google Scholar]
- Boulay, R.; Arnan, X.; Cerdá, X.; Retana, J. The ecological benefits of larger colony size may promote polygyny in ants. J. Evol. Biol. 2014, 27, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Agosti, D. Review and reclassification of Cataglyphis (Hymenoptera, Formicidae). J. Nat. Hist. 1990, 24, 1457–1505. [Google Scholar] [CrossRef]
- Lenoir, A.; Aron, S.; Cerda, X.; Hefetz, A. Cataglyphis desert ants: A good model for evolutionary biology in Darwin’s anniversary year—A review. Isr. J. Entomol. 2009, 39, 1–32. [Google Scholar]
- Borghesi, S.; Ticci, E. Climate change in the MENA region: Environmental risks, socioeconomic effects and policy challenges for the future. In IEMed Mediterranean Yearbook 2019; IEMed: Barcelona, Spain, 2019; pp. 289–292. Available online: http://hdl.handle.net/11365/1121314 (accessed on 16 January 2023).
- Waha, K.; Krummenauer, L.; Adams, S.; Aich, V.; Baarsch, F.; Coumou, D.; Fader, M.; Hoff, H.; Jobbins, G.; Marcus, R.; et al. Climate change impacts in the Middle East and Northern Africa (MENA) region and their implications for vulnerable population groups. Reg. Environ. Chang. 2017, 17, 1623–1638. [Google Scholar] [CrossRef]
- Giorgi, F.; Lionello, P. Climate change projections for the Mediterranean region. Glob. Planet. Chang. 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Essa, Y.H.; Hirschi, M.; Thiery, W.; El-Kenawy, A.M.; Yang, C. Drought characteristics in Mediterranean under future climate change. NPJ Clim. Atmos. Sci. 2023, 6, 133. [Google Scholar] [CrossRef]
- Abdulwahab, U.A.; Hammill, E.; Hawkins, C.P. Choice of climate data affects the performance and interpretation of species distribution models. Ecol. Model. 2022, 471, 110042. [Google Scholar] [CrossRef]
- Almarinez, B.J.M.; Fadri, M.J.A.; Lasina, R.; Tavera, M.A.A.; Carvajal, T.M.; Watanabe, K.; Legaspi, J.C.; Amalin, D.M. A bioclimate-based maximum entropy model for Comperiella calauanica Barrion, Almarinez and Amalin (Hymenoptera: Encyrtidae) in the Philippines. Insects 2021, 12, 26. [Google Scholar] [CrossRef]
- Khatri-Chhetri, P.; Hendryx, S.M.; Hartfield, K.A.; Crimmins, M.A.; Leeuwen, W.J.V.; Kane, V.R. Assessing vegetation response to multi-scalar drought across the Mojave, Sonoran, Chihuahuan deserts and Apache highlands in the Southwest United States. Remote Sens. 2021, 13, 1103. [Google Scholar] [CrossRef]
- Soliman, M.M.; Al-Khalaf, A.A.; El-Hawagry, M.S. Effects of Climatic Change on Potential Distribution of Spogostylum ocyale (Diptera: Bombyliidae) in the Middle East Using Maxent Modelling. Insects 2023, 14, 120. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. J. Syst. Evol. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Brambilla, M.; Bazzi, G.; Ilahiane, L. The effectiveness of species distribution models in predicting local abundance depends on model grain size. Ecology 2024, 105, 4224. [Google Scholar] [CrossRef]
- Eastman, J.R. Idrisi Selva Tutorial; Idrisi Production, Clark Labs-Clark University: Worcester, MA, USA, 2012; Volume 45, pp. 51–63. [Google Scholar]
- Hosseini, N.; Ghorbanpour, M.; Mostafavi, H. Habitat potential modelling and the effect of climate change on the current and future distribution of three Thymus species in Iran using MaxEnt. Sci. Rep. 2024, 14, 3641. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E. Predictive habitat distribution models in ecology. Ecol. Model. 2000, 135, 147–186. [Google Scholar] [CrossRef]
- Kalarikkal, R.K.; Kim, Y.; Ksiksi, T. Incorporating satellite remote sensing for improving potential habitat simulation of Prosopis cineraria (L.) Druce in United Arab Emirates. Glob. Ecol. Conserv. 2022, 37, e02167. [Google Scholar] [CrossRef]
- Sallam, M.F.; Al Ahmed, A.M.; Abdel-Dayem, M.S.; Abdullah, M.A. Ecological niche modeling and land cover risk areas for rift valley fever vector, Culex tritaeniorhynchus Giles in Jazan, Saudi Arabia. PLoS ONE 2013, 8, 65786. [Google Scholar] [CrossRef]
- Macedo, F.L.; Ragonezi, C.; Reis, F.; de Freitas, J.G.; Lopes, D.H.; Aguiar, A.M.F.; Cravo, D.; Carvalho, M.A.P.D. Prediction of the Potential Distribution of Drosophila suzukii on Madeira Island Using the Maximum Entropy Modeling. Agriculture 2023, 13, 1764. [Google Scholar] [CrossRef]
- Holuša, J.; Kaláb, O. The habitat-suitability models of the European mole cricket (Gryllotalpa gryllotalpa) as information tool for conservation and pest management. Heliyon 2023, 9, e14826. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Song, J.W.; Yoon, S.H.; Jung, J.M. Spatial evaluation of machine learning-based species distribution models for prediction of invasive ant species distribution. Appl. Sci. 2022, 12, 10260. [Google Scholar] [CrossRef]
- Istifanus, A.P.; Abdelmutalab, A.G.; Pirk, C.W.; Yusuf, A.A. Predicting the Habitat Suitability and Distribution of Two Species of Mound-Building Termites in Nigeria Using Bioclimatic and Vegetation Variables. Diversity 2023, 15, 157. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Kim, S.H.; Lee, E.J. Identifying high-priority conservation areas for endangered waterbirds using a flagship species in the Korean DMZ. Ecol. Eng. 2021, 159, 106080. [Google Scholar] [CrossRef]
- Morales, N.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Lee, D.S.; Kwon, T.S.; Athar, M.; Park, Y.S. Predicting the global distribution of Solenopsis geminata (Hymenoptera: Formicidae) under climate change using the MaxEnt model. Insects 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Brodetzki, R.T.; Inbar, S.; Cohen, P.; Aron, S.; Privman, E.; Hefetz, A. The Interplay between Incipient Species and Social Polymorphism in the Desert Ant Cataglyphis. Sci. Rep. 2019, 9, 9495. [Google Scholar] [CrossRef]
- Knaden, M.; Tinaut, A.; Stoekl, J.; Cerda, X.; Wehner, R. Molecular phylogeny of the desert ant genus Cataglyphis (Hymenoptera: Formicidae), Myrmecol. News 2012, 16, 123–132. [Google Scholar]
- Aron, S.; Mardulyn, P.; Leniaud, L. Evolution of reproductive traits in Cataglyphis desert ants: Mating frequency, queen number, and thelytoky. Behav. Ecol. Sociobiol. 2016, 70, 1367–1379. [Google Scholar] [CrossRef]
- Ionescu, A.; Eyer, P.A. Notes on Cataglyphis Foerster, 1850 of the bicolor species-group in Israel, with description of a new species (Hymenoptera: Formicidae). Isr. J. Entomol. 2016, 46, 109–131. [Google Scholar]
- Guénard, B.; Weiser, M.D.; Gomez, K.; Narula, N.; Economo, E.P. The Global Ant Biodiversity Informatics (GABI) Database: Synthesizing Data on the Geographic Distribution of Ant Species (Hymenoptera: Formicidae). Ph.D. Thesis, Okinawa Institute of Science and Technology Graduate University, Okinawa, Japan, 2017. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Reichle, R.; De Lannoy, G.; Koster, R.D.; Crow, W.T.; Kimball, J.S.; Liu, Q. SMAP L4 Global 3-Hourly 9 km EASE-Grid Surface and Root Zone Soil Moisture Analysis Update, Version 6. [Indicate Subset Used]; NASA National Snow and Ice Data Center Distributed Active Archive Center: Boulder, CO, USA, 2021. [Google Scholar] [CrossRef]
- AppEEARS Team. Application for Extracting and Exploring Analysis Ready Samples (AppEEARS); NASA EOSDIS Land Processes Distributed Active Archive Center (LP DAAC), USGS/Earth Resources Observation and Science (EROS) Center: Sioux Falls, SD, USA, 2023. Available online: https://appeears.earthdatacloud.nasa.gov/ (accessed on 16 January 2023).
- Woodward, F.I. Climate and Plant Distribution; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Davis, M.B.; Shaw, R.G. Range shifts and adaptive responses to Quaternary climate change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Karger, D.N.; Conrad, O.; Böhner, J.; Kawohl, T.; Kreft, H.; Soria-Auza, R.W.; Zimmermann, N.E.; Linder, H.P.; Kessler, M. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 2017, 4, 170122. [Google Scholar] [CrossRef]
- Brun, P.; Zimmermann, N.E.; Hari, C.; Pellissier, L.; Karger, D.N. CHELSA-BIOCLIM+ A Novel Set of Global Climate-Related Predictors at Kilometre-Resolution; EnviDat, 2022. [Google Scholar] [CrossRef]
- Lange, S. Trend-preserving bias adjustment and statistical downscaling with ISIMIP3BASD (v1. 0). Geosci. Model Dev. 2019, 12, 3055–3070. [Google Scholar] [CrossRef]
- Dunne, J.P.; John, J.G.; Adcroft, A.J.; Griffies, S.M.; Hallberg, R.W.; Shevliakova, E.; Stouffer, R.J.; Cooke, W.; Dunne, K.A.; Harrison, M.J.; et al. GFDL’s ESM2 global coupled climate–carbon earth system models. Part I: Physical formulation and baseline simulation characteristics. J. Clim. 2012, 25, 6646–6665. [Google Scholar] [CrossRef]
- Boucher, O.; Servonnat, J.; Albright, A.L.; Aumont, O.; Balkanski, Y.; Bastrikov, V.; Bekki, S.; Bonnet, R.; Bony, S.; Bopp, L.; et al. Presentation and evaluation of the IPSL-CM6A-LR climate model. J. Adv. Model. Earth Syst. 2020, 12, e2019MS002010. [Google Scholar] [CrossRef]
- Gutjahr, O.; Putrasahan, D.; Lohmann, K.; Jungclaus, J.H.; von Storch, J.-S.; Brüggemann, N.; Haak, H.; Stössel, A. Max Planck Institute Earth System Model (MPI-ESM1.2) for the High-Resolution Model Intercomparison Project (HighResMIP). Geosci. Model Dev. 2019, 12, 3241–3281. [Google Scholar] [CrossRef]
- Yukimoto, S.; Kawai, H.; Koshiro, T.; Oshima, N.; Yoshida, K.; Urakawa, S.; Tsujino, H.; Deushi, M.; Tanaka, T.; Hosaka, M.; et al. The Meteorological Research Institute Earth System Model version 2.0, MRI-ESM2.0: Description and basic evaluation of the physical component. J. Meteorol. Soc. Jpn. Ser. II 2019, 97, 931–965. [Google Scholar] [CrossRef]
- Sellar, A.A.; Jones, C.G.; Mulcahy, J.P.; Tang, Y.; Yool, A.; Wiltshire, A.; O’connor, F.M.; Stringer, M.; Hill, R.; Palmieri, J.; et al. UKESM1: Description and evaluation of the UK Earth System Model. J. Adv. Model. Earth Syst. 2019, 11, 4513–4558. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Ghehsareh Ardestani, E.; Ebrahimi, A.; Borhani, M. Climate change impacts on optimal habitat of Stachys inflata medicinal plant in central Iran. Sci. Rep. 2023, 13, 6580. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.K.M.; Stevens, M.H.H. Temperature, topography, soil characteristics, and NDVI drive habitat preferences of a shade-tolerant invasive grass. Ecol. Evol. 2020, 10, 10785–10797. [Google Scholar] [CrossRef] [PubMed]
- Jarnevich, C.S.; Reynolds, L.V. Challenges of predicting the potential distribution of a slow-spreading invader: A habitat suitability map for an invasive riparian tree. Biol. Invasions 2011, 13, 153–163. [Google Scholar] [CrossRef]
- Wei, B.O.; Wang, R.; Hou, K.; Wang, X.; Wu, W. Predicting the current and future cultivation regions of Carthamus tinctorius L. using MaxEnt model under climate change in China. Glob. Ecol. Conserv. 2018, 16, e00477. [Google Scholar] [CrossRef]
- Tesfamariam, B.G.; Gessesse, B.; Melgani, F. MaxEnt-based modeling of suitable habitat for rehabilitation of Podocarpus forest at landscape-scale. Environ. Syst. Res. 2022, 11, 4. [Google Scholar] [CrossRef]
- Mahato, R.; Abujam, S.; Bushi, D.; Dai Nimasow, O.; Nimasow, G.; Das, D.N. Distribution modelling of Tor putitora (Hamilton, 1822), an endangered cyprinid in the Himalayan river system using MaxEnt. Acta Ecol. Sin. 2023, 43, 343–351. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, C.; Liu, Z. Optimized maxent model predictions of climate change impacts on the suitable distribution of Cunninghamia lanceolata in China. Forests 2020, 11, 302. [Google Scholar] [CrossRef]
- Gebrewahid, Y.; Abrehe, S.; Meresa, E.; Eyasu, G.; Abay, K.; Gebreab, G.; Kidanemariam, K.; Adissu, G.; Abreha, G.; Darcha, G. Current and future predicting potential areas of Oxytenanthera abyssinica (A. Richard) using MaxEnt model under climate change in Northern Ethiopia. Ecol. Process. 2020, 9, 6. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. 2017. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 12 December 2023).
- Dahlsjö, C.A.; Valladares Romero, C.S.; Espinosa Iñiguez, C.I. Termite diversity in Ecuador: A comparison of two primary forest national parks. J. Insect Sci. 2020, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Vezza, P.; Muñoz-Mas, R.; Martinez-Capel, F.; Mouton, A. Random forests to evaluate biotic interactions in fish distribution models. Environ. Model. Softw. 2015, 67, 173–183. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Majdi, F.; Hosseini, S.A.; Karbalaee, A.; Kaseri, M.; Marjanian, S. Future projection of precipitation and temperature changes in the Middle East and North Africa (MENA) region based on CMIP6. Theor. Appl. Climatol. 2022, 147, 1249–1262. [Google Scholar] [CrossRef]
- Cerdá, X.; Retana, J. Alternative strategies by thermophilic ants to cope with extreme heat: Individual versus colony level traits. Oikos 2000, 89, 155–163. [Google Scholar] [CrossRef]
- Perez, R.; Benbachir, M.; Decroo, C.; Mascolo, C.; Wattiez, R.; Aron, S. Cataglyphis desert ants use distinct behavioral and physiological adaptations to cope with extreme thermal conditions. J. Therm. Biol. 2023, 111, 103397. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Synthesis. Am. Mus. Nat. Hist. 2007, 50, 54–89. [Google Scholar]
- Sharaf, M.R.; Collingwood, C.A.; Aldawood, A.S. Notes on the ant genus Cataglyphis Foerster, 1850 (Hymenoptera, Formicidae) in the Arabian Peninsula with description of a new species and a key to species of the C. pallida-group. ZooKeys 2015, 545, 101. [Google Scholar] [CrossRef]
- Paknia, O.; Pfeiffer, M. Steppe versus desert: Multi-scale spatial patterns in diversity of ant communities in Iran. Insect Conserv. Divers. 2011, 4, 297–306. [Google Scholar] [CrossRef]
- Mao, M.; Chen, S.; Ke, Z.; Qian, Z.; Xu, Y. Using MaxEnt to predict the potential distribution of the little fire ant (Wasmannia auropunctata) in China. Insects 2022, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Saha, A.; Rahman, S.; Alam, S. Modeling current and future potential distributions of desert locust Schistocerca gregaria (Forskål) under climate change scenarios using MaxEnt. J. Asia-Pac. Biodivers. 2021, 14, 399–409. [Google Scholar] [CrossRef]
- Rato, C.; Silva-Rocha, I.; Sillero, N. What does the future hold for a thermophilic and widely introduced gecko, Tarentola mauritanica (Squamata: Phyllodactylidae)? Biol. Invasions 2024, 26, 1061–1074. [Google Scholar] [CrossRef]
- Gull, E.; Fareen, A.; Mahmood, T.; Bodlah, I.; Rashid, A.; Khalid, A.; Mahmood, S. Modeling potential distribution of newly recorded ant, Brachyponera nigrita using Maxent under climate change in Pothwar region, Pakistan. PLoS ONE 2022, 17, 0262451. [Google Scholar]
- Vale, C.G.; Brito, J.C. Desert-adapted species are vulnerable to climate change: Insights from the warmest region on Earth. Glob. Ecol. Conserv. 2015, 4, 369–379. [Google Scholar] [CrossRef]


| SI No. | Model Name | Developers |
|---|---|---|
| 1 | GFDL | Geophysical Fluid Dynamics Laboratory Earth System Model version 4.1 [70] |
| 2 | IPSLCM6 | Institute Pierre-Simon Laplace Climate Model [71] |
| 3 | MPIESM12-HR | Max Planck Institute for Meteorology Earth System Models [72] |
| 4 | MRIESM | Meteorological Research Institute-Earth System Model Version 2 [73] |
| 5 | UKESM | UK Earth System Model [74] |
| SI No. | Final Variables Used | Abbreviations | Contribution (%) |
|---|---|---|---|
| 1 | Elevation | ELEV | 37.8 |
| 2 | Mean monthly precipitation amount of the coldest quarter | Bio19 | 26.8 |
| 3 | Temperature seasonality | Bio4 | 17.6 |
| 4 | Precipitation amount of the driest month | Bio14 | 7.7 |
| 5 | Mean diurnal air temperature range | Bio2 | 4 |
| 6 | Nighttime land surface temperature | LSTN | 2.7 |
| 7 | Mean daily mean air temperatures of the driest quarter | Bio9 | 1 |
| 8 | Root-zone soil moisture | SM | 1 |
| 9 | Mean daily mean air temperatures of the warmest quarter | Bio10 | 0.5 |
| 10 | Mean daily maximum air temperature of the warmest month | Bio5 | 0.4 |
| 11 | Enhanced vegetation index | EVI | 0.3 |
| 12 | Precipitation seasonality | Bio15 | 0.2 |
| 13 | Mean monthly precipitation amount of the warmest quarter | Bio18 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalarikkal, R.K.; Park, H.; Georgiadis, C.; Guénard, B.; Economo, E.P.; Kim, Y. Current and Future Distribution of the Cataglyphis nodus (Brullé, 1833) in the Middle East and North Africa. Diversity 2024, 16, 563. https://doi.org/10.3390/d16090563
Kalarikkal RK, Park H, Georgiadis C, Guénard B, Economo EP, Kim Y. Current and Future Distribution of the Cataglyphis nodus (Brullé, 1833) in the Middle East and North Africa. Diversity. 2024; 16(9):563. https://doi.org/10.3390/d16090563
Chicago/Turabian StyleKalarikkal, Remya Kottarathu, Hotaek Park, Christos Georgiadis, Benoit Guénard, Evan P. Economo, and Youngwook Kim. 2024. "Current and Future Distribution of the Cataglyphis nodus (Brullé, 1833) in the Middle East and North Africa" Diversity 16, no. 9: 563. https://doi.org/10.3390/d16090563
APA StyleKalarikkal, R. K., Park, H., Georgiadis, C., Guénard, B., Economo, E. P., & Kim, Y. (2024). Current and Future Distribution of the Cataglyphis nodus (Brullé, 1833) in the Middle East and North Africa. Diversity, 16(9), 563. https://doi.org/10.3390/d16090563






