Abstract
A synopsis of current knowledge of the diversity of the New Zealand landhopper fauna is provided. A combination of morphological and molecular analysis was employed on material from across New Zealand. Thirteen new endemic genera soon to be formally described have been discovered, including four belonging to the widespread families Talitridae and Arcitalitridae. These are families that had not been previously reported from New Zealand. We document the existence of at least 48 new provisional native species. This number far exceeds the 28 species currently described. Some described species are now shown to be species complexes, and a few of these are very diverse with numerous cryptic species. Six changes to the existing taxonomy are proposed. Dallwitzia simularis (Hurley, 1957) is transferred from Makawidae Myers & Lowry, 2020 to Talitridae Rafinesque, 1815; Kellyduncania hauturu (Duncan, 1994) is reinstated as a member of Dana Lowry, 2011; Kellyduncania (Lowry & Myers, 2019) is relegated to a synonym of Dana Lowry, 2011; Kanikania Duncan, 1994 is transferred from Makawidae Myers & Lowry, 2020 to Arcitalitridae Myers & Lowry, 2020; Parorchestia longicornis is transferred to Kanikania Duncan, 1994; Waematau kaitaia (Duncan, 1994) is transferred to Kohuroa Lowry, Myers & Nakano, 2019; and Waematau unuwhao (Duncan, 1994) is transferred to Omaiorchestia Lowry & Myers, 2019. This reduces the number of described New Zealand genera from 17 to 16.
1. Introduction
Currently, the amphipod superfamily Talitroidea contains seven families: Arcitalitridae Myers & Lowry, 2020, Protorchestiidae Myers & Lowry, 2020, Uhlorchestiidae Myers & Lowry, 2020, Brevitalitridae Myers & Lowry, 2020, Curiotalitridae Myers & Lowry, 2020, Makawidae Myers & Lowry, 2020 and Talitridae Rafinesque, 1815. Landhoppers are found in all families except Uhlorchestiidae, and four of the families (Arcitalitridae, Brevitalitridae, Curiotalitridae and Makawidae) are exclusively landhoppers.
All the known native species of landhopper in New Zealand until now have been attributable to the family Makawidae, which is restricted to Zealandia and Tasmania [1]. Most beachhoppers and sandhoppers included in Talitridae are not traditionally regarded as ‘landhoppers’ by amphipod workers [2,3,4,5] in New Zealand despite some species penetrating deeply into terrestrial environments above the supralittoral zone [6].
The described landhopper fauna of New Zealand currently stands at 28 species in 17 endemic genera and two introduced Arcitalitrus species. Although there is a long history of taxonomic study of landhoppers in New Zealand [2,7,8,9,10,11], progress has been sporadic and limited in extent. Progress in understanding their ecology is even more incomplete.
Here, we provide a synopsis of current knowledge of New Zealand’s native landhoppers using morphological and molecular analytical methods. We resolve some discrepancies in the current classification of New Zealand’s native landhoppers and assess the extent of the knowledge deficit that exists for this group. We also provide direction for some of the key taxonomic challenges that lie ahead and critically examine the role of molecular methods in helping to delineate species and elucidate evolutionary relationships. While this study focuses on native species, information on exotic species is also presented.
2. Materials and Methods
2.1. Morphological Examination
Landhoppers from a variety of sources and sites from across New Zealand and its offshore islands were examined during these studies. Material was collected or obtained from the Three Kings Islands, Northland (including Te Paki Ecological District and Poor Knights Islands), Auckland, Coromandel (Little Barrier Island), Waikato, Taranaki, Whanganui, Wairarapa, Nelson, Mid Canterbury, Dunedin, Southland, Fiordland, Stewart Island, Chatham Islands, Snares Islands, Antipodes Islands and Bounty Islands (area location names are based on the boundary definitions of Crosby et al. [12] shown in Figure 1). Most of this material is currently held in a private collection of one of the authors (OB) but will be deposited in museum collections on completion of formal species descriptions. All landhoppers were stored in 80% ethanol. Additional material, including type specimens, from the amphipod collections at Canterbury Museum (CNMNZ), Museum of New Zealand Te Papa (MNZ) and Auckland Museum (AMNZ), was also inspected. Landhoppers were examined in 80% ethanol using a dissecting microscope at 6.3–60 times magnification. Landhoppers were identified to species level where possible or were designated as distinct Operational Taxonomic Units (OTUs) based on differences in their morphology. Some OTUs were obviously affiliated with existing species or genera, whereas others were not.
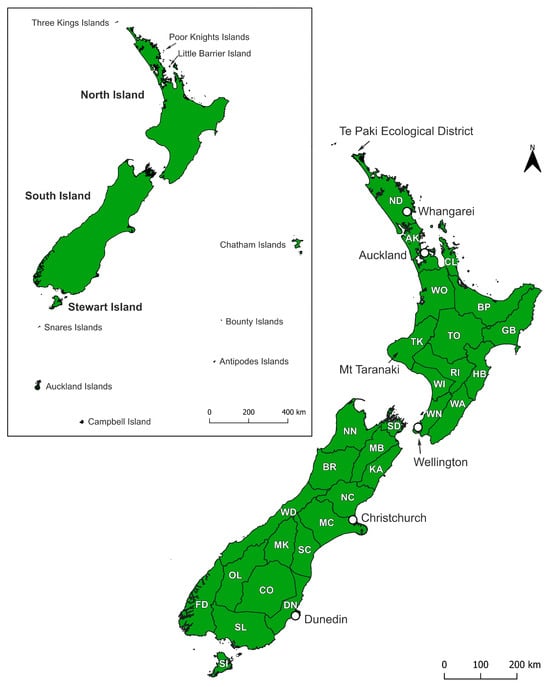
Figure 1.
Map of New Zealand’s main islands showing area codes used to describe landhopper distribution records [12]. Inset map shows the New Zealand subregion including nearshore and offshore islands. Crosby area codes for the North Island: AK = Auckland; BP = Bay of Plenty; CL = Coromandel; GB = Gisborne; HB = Hawke’s Bay; ND = Northland; RI = Rangitikei; TK = Taranaki; TO = Taupo; WA = Wairarapa; WI = Whanganui; WN = Wellington; WO = Waikato. Crosby area codes for the South Island and Stewart Island: BR = Buller; CO = Central Otago; DN = Dunedin; FD = Fiordland; KA = Kaikoura; MB = Marlborough; MC = Mid Canterbury; MK = Mackenzie; NC = North Canterbury; NN = Nelson; OL = Otago Lakes; SC = South Canterbury; SD = Marlborough Sounds; SI = Stewart Island; SL = Southland; WD = Westland.
Six described species of New Zealand landhopper, Dendrorchestia parva (Chilton, 1909), Dracorchestia insularis (Chilton, 1909), Dracorchestia maynei (Chilton, 1909), Kanikania improvisa (Chilton, 1909), Leslieorchestia lesliensis (Hurley, 1957) and Makawe waihekensis Duncan, 1994, were not subject to morphological or molecular investigation in this study as fresh material suitable for morphological examination and genetic analysis was not available.
2.2. DNA Isolation, PCR Amplification and Sequencing
Selected landhoppers including those showing varying degrees of morphological deviation from described species and genera were subjected to DNA analysis. Where sufficient material was available (i.e., in the vast majority of cases), sequences from multiple individuals (up to 17) of each species or OTU were generated. Individuals affiliated with 22 described species in 13 of the 17 described New Zealand genera, as well as many individuals apparently not affiliated with described species or genera, were analysed. Three coastal talitrids, Subantarctorchestia aucklandiae (Spence Bate, 1862), Bellorchestia quoyana (Milne-Edwards, 1840) and Tatahipeke tumida (Stebbing, 1887), were also included in our investigations to assist with elucidating evolutionary associations at deeper levels. DNA extraction, 16S amplification and sequencing of much of the material followed Ball et al. [11] and used the primers Y16F (5′-GGTAATTTGACCGTGCTAAG-3′) and Y16F (5′-CCGRTTTGAACTCARATCATGT-3′) [13]. For more recent molecular investigations (after 2017), the same methods were used except that PCR was performed in 12 μL volumes containing 1× MyTaq mix (Bioline, Australia), 0.3 M of bovine serum albumin (BSA) and 0.5 μM of each primer, and thermocycling conditions involved an initial denaturation of 98 °C for 1 min and then 35 cycles of 98 °C for 20 s, 50 °C for 20 s and 72 °C for 30 s, followed by a final extension of 72 °C for 5 min.
2.3. Phylogenetic Analyses
Newly generated sequences were edited in Sequencer 5.4.6 (Gene Codes Corporation, Ann Arbor, MI, USA) and have been deposited in GenBank (Supplementary Table S1). We also downloaded available 16S sequences of other New Zealand amphipods from GenBank to determine whether any additional species had been sequenced. Sequences that represented species not included in our study were added to our analyses.
The alignment of DNA sequences and the removal of regions of low homology followed Ball et al. [11], as did phylogenetic analyses with Bayesian analysis and maximum likelihood. The 16S sequences were tested for substitution saturation by plotting transitions and transversions against F84 genetic distance in DAMBE 7.3.32 Xia [14].
Landhoppers were designated as distinct Molecular Operational Taxonomic Units (MOTUs) based on a sequence threshold difference of 16% or greater, with uncorrected pairwise genetic distances calculated in Geneious Prime 2022.2.2 (Biomatters). The 16% threshold was chosen as we have found that morphological distinctions within most genera are difficult to identify at lower thresholds. This was also the threshold suggested by Lefébure et al. [15] for delineating new or uncertain species of Crustacea using the COI locus. Although Lefébure et al. [15] concluded that 16S evolved more slowly than COI, our results with New Zealand Talitroidea are less conclusive in this regard. The diagnosis of the New Zealand landhopper fauna was determined through a reciprocal recognition of distinctive taxa as described species/OTUs and MOTUs. Where there were discrepancies between described species/OTUs and MOTUs, priority was given to described species and OTUs rather than MOTUs, unless the molecular data were unequivocal. This is because detailed and formal morphological investigations would be required to change the status of any described species, and because we consider it more likely that significant morphological differences are more informative and precautionary about species boundaries than molecular differences at a single DNA locus. Putative genera were recognised by morphological synapomorphies. To improve clarity, a single individual of each described species, OTU and MOTU was selected to generate a 16S phylogenetic tree. The example chosen for each taxon was selected based on criteria such as geographic proximity to the type locality (for described species) and likely future type locality (for OTUs and MOTUs). However, in some cases, selection of the individual was random. The phylogenetic tree was rooted using a species of Hyalidae, considered to be the closest family to the Talitroidea [6].
Although differences in gross morphology of most described species and OTUs are outlined in the discussion, detailed morphological descriptions are not included. Formal descriptions will be provided in upcoming publications.
3. Results
3.1. Molecular and Morphological Results
DNA sequences from the 16S locus were obtained for at least one individual of most described and undescribed taxa subjected to morphological examination and molecular analysis. The exceptions were Genus M (which did not yield any genetic results) and Genus A sp. nov. 6 (the material for which was too old to sequence). Also, we have conservatively assumed that individuals of Kanikania longicornis, Kanikania motuensis, Omaiorchestia rubroannulata, Parorchestia ihurawao, Puhuruhuru aotearoa, Sinbadorchestia sinbadensis and Snaresorchestia patersoni true to the definition of each of these species have been sequenced for this study. However, these may be incorrect assumptions as fresh material from the type localities for these species has not been examined or subjected to molecular analysis. There is a particular reservation about P. aotearoa as there is doubt that the male holotype and female allotype belong to the same species (see later discussion on this species).
The final 16S alignment was 456 bp in length, and 270 characters were parsimony-informative. The plot of the transitions and transversions at the 16S locus against genetic distance (Figure 2) showed that transversions exceeded transitions for much of the plot. This result indicates considerable site saturation at this locus, except for very recent divergences, and that multiple substitutions are likely to mislead phylogenetic reconstruction.
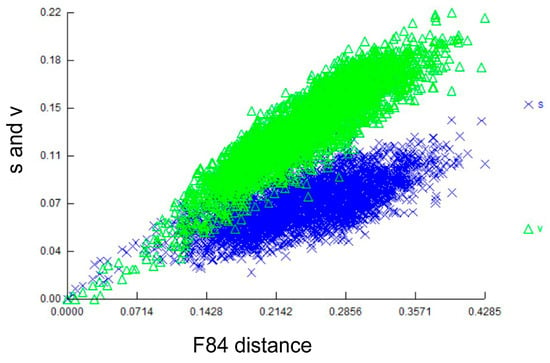
Figure 2.
Plot of transitions (s) and transversions (v) at the 16S locus versus F84 genetic distance. The 16S locus is saturated with transversions exceeding transitions, indicating that some nucleotides are likely to have experienced multiple substitutions.
The molecular analyses yielded a phylogenetic tree where many of the deeper relationships were poorly resolved with weak support (Figure 3), which is likely a consequence of sequence saturation. However, many of the recovered clades are associated with described genera. Several clades are not closely associated with described genera, and when considered alongside the morphological data, these represent provisional (undescribed) genera.
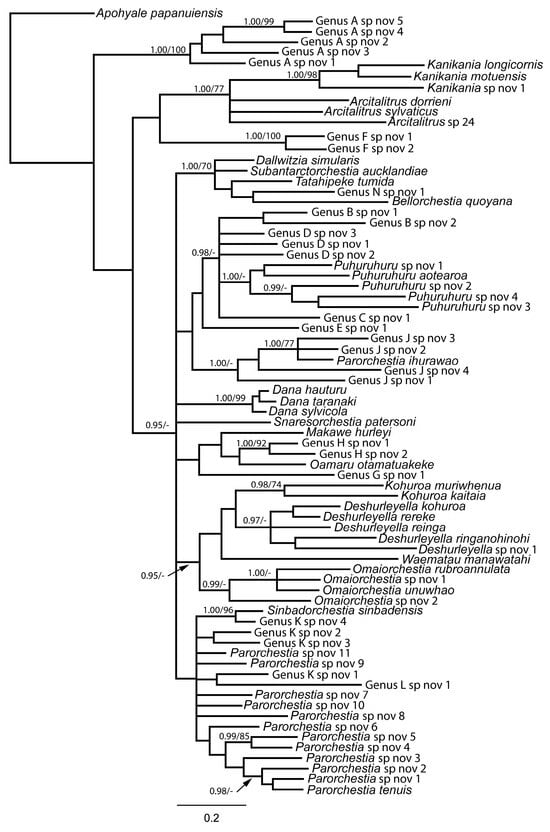
Figure 3.
Phylogeny of Makawidae, Arcitalitridae and Talitridae present in New Zealand based on mitochondrial 16S DNA sequences. The phylogeny is rooted with Apohyale papanuiensis (Hyalidae). Support values are shown in the order Bayesian posterior probability (PP)/maximum likelihood bootstrap support (BS). Only nodes with >0.95 PP or 70% BS are shown.
Sixty-six native landhopper MOTUs and three exotic landhopper MOTUs (one of which was downloaded from GenBank) were identified from the 16S sequences. Three coastal talitrid MOTUs were also identified. Based on morphology, 69 described native landhopper species and OTUs (i.e., excluding the six species not subject to morphological investigation in this study and the three described coastal talitrid species) were identified in three families, Makawidae, Arcitalitridae and Talitridae. From the morphology alone, two exotic described species in the family Arcitalitridae were also detected.
There were several instances where OTUs and MOTUs were not congruent. In two genera, differences in 16S sequences revealed more MOTUs than OTUs. Omaiorchestia sp. nov. 1 and Omaiorchestia sp. nov. 2 were morphologically almost indistinguishable from O. rubroannulata and O. unuwhao, respectively, and the two provisional species in Genus F were also morphologically very similar to each other. As genetic distances were considered sufficient in these cases, the decision was made to delineate each of the undescribed taxa as separate species based on 16S differences alone. Conversely, in Sinbadorchestia and Dallwitzia, differences in morphology revealed more OTUs than MOTUs within each genus. Sinbadorchestia and Dallwitzia were each thought to comprise two morphologically distinct species, but the molecular distances between congeners suggested that each genus constituted a single species, and that morphological variation was intraspecific. In the case of Genus K sp. nov. 4 and S. sinbadensis, the genetic distance between them was only 8%, but clear morphological differences between them were given precedence over the molecular data and they were not considered monospecific. The genetic distances between Dana hauturu, D. taranaki and D. sylvicola were only 5–6%, and morphological differences between them were also small. Nevertheless, despite molecular distances being well below our set threshold, for the moment we treat these as different species because more in-depth morphological investigation would be necessary to change their current status as described species. There were two instances where two taxa comprising one MOTU surpassed the 16% threshold for sequence differences. In Genus J sp. nov. 2 and Puhuruhuru sp. nov. 3, sequence differences between the two least similar individuals in the taxon spanned 17%. However, individuals at the extreme limits of each of these two clades were considered conspecific due to the presence of a gradient of intermediate genotypes.
There was generally very good agreement between described species/OTUs and MOTUs, which greatly assisted in determining species boundaries. Our interpretation of the morphological and molecular data indicates the existence of at least 48 provisional (undescribed) native species. Three exotic species were also detected. A checklist of all of these described and provisional species, both native and exotic, now known from New Zealand is provided in Table 1.

Table 1.
Species checklist of New Zealand terrestrial Amphipoda (includes synonyms for described species).
3.2. Taxonomic Changes
Based on the results, six taxonomic changes are proposed for the described fauna. Details of the rationale for these changes are discussed in Section 4.
- Dallwitzia simularis (Hurley, 1957) is transferred from Makawidae Myers & Lowry, 2020 to Talitridae Rafinesque, 1815;
- Kellyduncania hauturu (Duncan, 1994) is reinstated as a member of Dana Lowry, 2011, and Kellyduncania Lowry & Myers, 2019 is relegated to a synonym of Dana Lowry, 2011;
- Kanikania Duncan, 1994 is transferred from Makawidae Lowry & Myers, 2020 to Arcitalitridae Lowry & Myers, 2020;
- Parorchestia longicornis (Stephensen, 1938) is transferred to Kanikania Duncan, 1994;
- Waematau kaitaia (Duncan, 1994) is transferred to Kohuroa Lowry, Myers & Nakano, 2019;
- Waematau unuwhao (Duncan, 1994) is transferred to Omaiorchestia Lowry & Myers, 2019.
4. Taxonomic Changes
4.1. Transfer of Dallwitzia simularis (Hurley, 1957) from Makawidae Myers & Lowry, 2020 to Talitridae Rafinesque, 1815
Lowry and Myers [16] speculated that D. simularis may have been independently derived from a New Zealand beachhopper (i.e., a coastal talitrid). Morphological examination of specimens from different populations of this species from Fiordland to the Antipodes and Bounty Islands supports this view. Dallwitzia simularis shows characters associated with many species in the Talitridae (e.g., a pronounced distomedial lobe on article 2 of the maxilliped palp, obvious dactylar cusps, and sexual dimorphism in the structure of pereopod 7). The morphological evidence is supported by the molecular data (including sequences from the Snares Islands, the type locality), which group D. simularis with the three coastal talitrids and another new terrestrial talitrid, Genus N from the Three Kings Islands, with moderate to high support (1.00 Bayesian posterior probability (PP)/70% maximum likelihood bootstrap support (BS); Figure 3). We therefore transfer Dallwitzia simularis from Makawidae to Talitridae.
Until now, D. simularis was thought to be a semi-mascupod landhopper. Inspection of sexually mature males for this study indicates the species is fully mascupod, supporting Hurley’s hypothesis [2] that an immature male was used in the original description. Furthermore, D. simularis was considered to be restricted to the Snares Islands [2,3], whereas our investigations show that the species is also present in Fiordland (Seal Islands, Dusky Sound), Antipodes Island and the Bounty Islands (Proclamation Island). Genetic testing of material from the Snares Islands, Antipodes Island and Bounty Islands supports the contention that these geographically widespread populations are monospecific (OB and LS, unpublished data). However, this interpretation of D. simularis should be treated with a degree of caution. Dallwitzia simularis shares obvious morphological characters with species in the genus Subantarctorchestia, and the close association between the two is supported by the genetic data (Figure 3). From the material available to us, we have discovered considerable morphological variation in D. simularis, which genetic testing suggests is intraspecific (likely, at least in part, due to differences in maturity). The relationship between D. simularis and Subantarctorchestia bollonsi (Chilton, 1909), which have largely overlapping distributions, therefore requires investigation.
4.2. Dana hauturu (Duncan, 1994)
In his revision of the New Zealand landhopper fauna, Duncan [3] erected the new genus Tara Duncan, 1994 in which he placed three species, T. hauturu, T. sylvicola and T. taranaki. However, as Tara was found to be preoccupied, all three species were transferred to Dana Lowry, 2011 [17]. Duncan [3] described Dana sylvicola and D. taranaki as mascupod species, and D. hauturu as a femipod (for a definition of these terms, see Lowry and Myers, [16]). Based on this, Lowry and Myers [16] transferred D. hauturu to a new monotypic femipod genus, Kellyduncania Lowry & Myers, 2019. Recently, large mascupod males of a species otherwise fitting the description of K. hauturu have been found on Little Barrier Island. It is therefore evident that the original description of the male was from an immature individual still bearing the femipod gnathopod 2. Genetic analysis strongly supports the view that D. sylvicola, D. taranaki and K. hauturu are closely related and form a well-supported monophyletic clade in the 16S phylogeny (1.00PP/99% BS; Figure 3). For this reason, K. hauturu is here reinstated as a member of Dana Lowry, 2011. Kellyduncania is relegated to a synonym of Dana.
4.3. Transfer of Kanikania Duncan, 1994 from Makawidae Myers & Lowry, 2020 to Arcitalitridae Myers & Lowry, 2020
The 16S phylogeny indicates that species of Kanikania have closer affinities with introduced species of Arcitalitrus in the family Arcitalitridae than they do with members of the Makawidae (1.00PP/77% BS; Figure 3). This conclusion has not been falsified by the morphological data. Examination of the pereopods shows that species in Kanikania, like all Arcitalitridae, lack dactylar cusps and are therefore simplidactylate. They possess other morphological characters often associated with the Arcitalitridae (e.g., they are femipods, and the exopods of uropods 1 and 2 have no robust setae), and compared with all other landhoppers in New Zealand, they have uniquely structured pleopods. The males of some species of Kanikania also have an enlarged and characteristically shaped gnathopod 1 propodus. Similarly structured pleopods and/or the enlarged gnathopod 1 propodus can be seen in several Australian Arcitalitridae, most notably in Mysticotalitrus tasmaniae (Ruffo, 1949). Therefore, it is evident that Kanikania should no longer be included in the Makawidae, so we herein transfer it to Arcitalitridae.
4.4. Kanikania longicornis (Stephensen, 1938) comb. nov.
Lowry and Myers [16] considered Parorchestia longicornis to be incertae sedis because insufficient detail was available to identify which genus the species belonged to. Our study has produced strong molecular evidence indicating that P. longicornis does not belong in Parorchestia but rather is a congener of Kanikania motuensis. Phylogenetic analyses of 16S sequences show that P. longicornis forms a well-supported monophyletic clade with K. motuensis and an undescribed species (1.00PP/98% BS; Figure 3). The morphological evidence linking them is also overwhelming. As well as generally comparable body shapes, all three species of Kanikania have very similar and distinctive pleopods. The shape of the gnathopod 1 propodus, in addition to the exceptionally long second antennae, is also characteristic of this group. Consequently, we transfer P. longicornis to Kanikania.
4.5. Kohuroa kaitaia (Duncan, 1994) comb. nov.
Following their revision, Lowry and Myers [16] and Lowry et al. [18] retained Kohuroa kaitaia in Waematau but transferred K. muriwhenua from Waematau to the newly erected genus Kohuroa due to perceived differences in cuspidactylation. Microscopic examination has now confirmed that cuspidactylation in K. kaitaia and K. muriwhenua is very similar (both are bicuspidactylate). Genetic analysis and further morphological studies have also shown that the two species are very similar and undoubtedly closely related sister species [11] in a moderate to well-supported monophyletic clade (0.98PP/74% BS; Figure 3). We therefore conclude that K. kaitaia is a congener of K. muriwhenua and transfer it from Waematau to Kohuroa.
4.6. Omaiorchestia unuwhao (Duncan, 1994) comb. nov.
Following Duncan’s [3] original description of Waematau unuwhao, Ball et al. [11] clarified various morphological characters of this species, but retained it in Waematau, with the qualification that a review of the genus needed to occur. In their review, Lowry and Myers [16] also retained the species in Waematau. However, it is now evident that Waematau has become a heterogeneous collection of disparate species, and that W. unuwhao should no longer be included in that genus. Waematau unuwhao is transferred here to the genus Omaiorchestia.
Omaiorchestia rubroannulata and O. unuwhao are distinctive species in the New Zealand fauna, and their resemblance to one another is striking. Females of the two species are morphologically almost identical. As well as having very similar and distinctive gills, both species have vestigial second and third pleopods, and their urosomes are all but indistinguishable. Also, freshly preserved (in ethanol) individuals of the two species have a similar and quite unique colour pattern for New Zealand taxa (vivid red hoops running dorsolaterally (Figure 4 shows the colour pattern of an individual O. rubroannulata preserved in ethanol)). These morphological similarities are consistent with the molecular data which show that the two species are sister taxa, which is well supported in the Bayesian analysis (1.00 PP; Figure 3). However, mature males of the two species show two distinctive differences. Omaiorchestia rubroannulata males have a well-developed strongly subchelate gnathopod 1 propodus (an apparent adaptation to digging in sandy soils [3]) and are femipod, whereas O. unuwhao males have a normally developed (though also subchelate) gnathopod 1 propodus and are mascupod. As Bousfield and Howarth [19] and Lowry and Myers [16] have argued, it is likely that genera would have evolved exclusively as either femipods or mascupods, not both. A similar argument could be used for the enlarged gnathopod 1 propodus in O. rubroannulata. We agree that the femipod and mascupod categories are normally indicative of independent evolutionary processes. However, we consider that in this case, feminisation (through neoteny) has occurred independently in the Omaiorchestia line, which would be unique for the Makawidae. The enlarged gnathopod 1 propodus, also seen in Kanikania, appears to be a homoplasy.
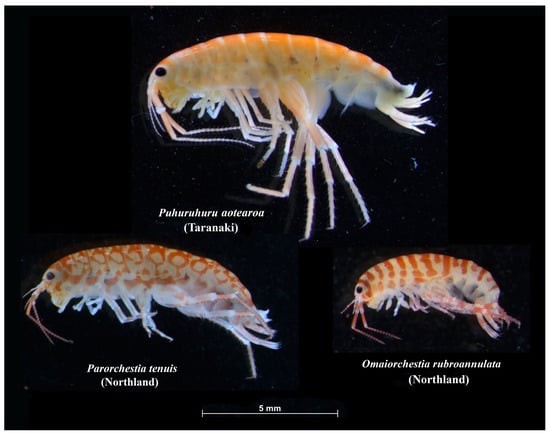
Figure 4.
Examples of colour patterns in New Zealand landhoppers following preservation in ethanol. The landhoppers are illustrated to scale. (Note: colour patterns generally fade after several weeks or months in ethanol.)
5. Diversity of New Zealand’s Landhopper Fauna
With the relegation of Kellyduncania to a synonym of Dana, 28 endemic species in 16 endemic genera are currently described from New Zealand (Table 1). However, this study has documented the existence of at least 48 provisional native species and 13 provisional native genera. The New Zealand landhopper fauna is therefore considerably more speciose than currently described. Including the six species not subject to morphological or molecular investigation in this study, the total number of described and provisional native species amounts to 76. These are distributed among 29 described and provisional genera. The New Zealand landhopper fauna has therefore undergone substantial radiation along multiple evolutionary lines.
The increase in our knowledge of the diversity of New Zealand landhoppers is a result of the following:
- The recognition of morphologically and genetically distinct new species that have clear affiliations to described taxa;
- The realisation that some described species are complexes of genetically distinct but morphologically very similar species;
- The discovery of morphologically and genetically distinct species of Makawidae that have no clear affiliations with described genera;
- The recognition that some newly recognised species, as well as previously described species, belong to families other than Makawidae.
5.1. New Species with Close Affiliations to Described Taxa
5.1.1. Deshurleyella sp. nov. 1
Deshurleyella sp. nov. 1 (Table 1, Figure 3) is, like all Deshurleyella, a femipod species that lacks lobes covered in palmate setae on the merus, carpus and propodus of the male gnathopod 1. It also has the distinctive antenna 1 apex of the genus where the penultimate article is slightly swollen and the distal-most article is attached in such a way as to give it a slightly lopsided appearance. It is morphologically most similar to D. ringanohinohi but differs fundamentally from it as it has a vestigial pleopod 3 (biramous in D. ringanohinohi). This small (approx. 8–10 mm) and almost completely white species is relatively widespread and has been found in native forests in several populations from Northland and Auckland.
5.1.2. Genus G sp. nov. 1; Genus H sp. nov. 1; Genus H sp. nov. 2
From the morphological data available, these three species are closely related to landhoppers in Makawe and Oamaru. The molecular data also recovered this relationship, although the support for this clade was poor (Table 1, Figure 3).
Genus G sp. nov. 1 has been collected in Aorangi Forest in southern Wairarapa. It differs from other clade members in having more elongate pleopod peduncles and a distinctly reticulate body pigmentation (body pigmentation of the other species in the clade ranges from diffuse orange-red bands to semi-reticulate patterning). DNA sequence data and morphological evidence indicate that Genus G sp. nov. 1 is the most distinct species in the clade. It will be given a monospecific genus.
Genus H sp. nov. 1 has been found at sites around Dunedin (see [20] for reference to this taxon) and is sympatric with O. otamatuakeke at most sites. It possesses an overall body plan similar to O. otamatuakeke, but differs in having very broad and stout pleopod 1 and 2 peduncles, a much reduced third pleopod with tiny rami and no obvious peduncular setae, one row (rather than two) of robust setae on the endopods of uropods 1 and 2, and a single (rather than two), long, robust seta apically on the telson. Its body pigmentation is also most similar to that in O. otamatuakeke, with freshly preserved (in ethanol) individuals of both species possessing faint orange bands and semi-reticulate areas. However, subtle differences in the colouration of preserved individuals can still be discerned between the two species. Morphological characters indicate closer affinities of Genus H sp. nov. 1 to O. otamatuakeke than M. hurleyi, and DNA sequence data provide only limited support for this view (Figure 3). A further new genus in the group will therefore be erected to accommodate Genus H sp. nov. 1.
Molecular and morphological data indicate that Genus H sp. nov. 2 is most similar to Genus H sp. nov. 1, so these will be congeneric when formally described. Genus H sp. nov. 2 has been detected only in Fiordland. Pleopod 3 in this species is reduced to a long peduncular stump, and in this, it differs significantly from all other known species in the clade.
5.2. Species or Genus Complexes Affiliated with Described Taxa
This is the most significant contributor to the increase in the number of known undescribed species. Some are cryptic species and difficult to distinguish without the assistance of molecular techniques [21]. Our investigations have revealed that at least five described species in four of the current genera comprise species or genus complexes, with complexes ranging in size from 2 to 16 species.
5.2.1. Kanikania Species Complex: K. improvisa (Chilton, 1909); K. longicornis (Stephensen, 1938); K. motuensis Duncan 1994; Kanikania sp. nov. 1
As well as showing that Kanikania longicornis is a congener of K. improvisa and K. motuensis (see earlier discussion in Section 4.4), our investigations suggest the existence of at least one more species in this complex (Figure 3). The new species appears more closely aligned with K. longicornis as males of two species in the clade lack the enlarged gnathopod 1 propodus, the character most associated with this genus. However, differences in 16S sequences suggest that a slightly closer, albeit weakly supported, genetic association exists between the two species differing in the enlargement of the gnathopod 1 propodus (K. motuensis and K. longicornis from Figure 3). Variants can be sympatric, increasing our confidence that the taxa constitute distinct species. As well as differences in the enlargement of the gnathopod 1 propodus, subtle morphological variation is observable in several characters, including the shape of the epimera, the presence of accessory setae on the endopodite of uropod 2, the number of setae on the uropod 3 ramus and the shape and setation of the telson at the apex. However, females of all three species so far identified from Stewart Island are so similar that they can easily be confused. This may also be the case for females of K. improvisa from the Snares Islands, a species previously regarded as conspecific with K. motuensis [2,8]. It should also be noted that we are assuming one of our study taxa belongs to K. motuensis sensu stricto and one belongs to K. longicornis sensu stricto. However, material from the type localities should be examined to confirm our hypotheses.
5.2.2. Omaiorchestia Species Complex: O. rubroannulata (Hurley, 1957); O. unuwhao (Duncan, 1994); Omaiorchestia sp. nov. 1; Omaiorchestia sp. nov. 2
The analysis of 16S sequences suggests that Omaiorchestia consists of at least four, rather than two, species (Figure 3), two femipods (O. rubroannulata and Omaiorchestia sp. nov. 1) and two mascupods (O. unuwhao and Omaiorchestia sp. nov. 2).
As individuals of O. rubroannulata from the type locality have not been examined or genetically tested, we have tentatively assumed that the material from Whangārei (Northland) used in this investigation belongs to O. rubroannulata sensu stricto. Although morphologically very similar, the genetically distinct femipod Omaiorchestia sp. nov. 1 occurs on the Three Kings Islands. Considering the widespread distribution of O. rubroannulata around New Zealand, further sampling may reveal more femipod species in the complex. Similarly, the mascupod Omaiorchestia unuwhao is considered to be endemic to Te Paki Ecological District at the northern extremity of New Zealand’s North Island. However, mascupod specimens morphologically very similar to O. unuwhao have been collected from the Poor Knights Islands, Little Barrier Island and Whangārei in Northland. Molecular analyses indicate that these populations comprise a second new species in the Omaiorchestia complex (Omaiorchestia sp. nov. 2).
5.2.3. Genus J Species Complex: Parorchestia ihurawao Duncan, 1994; Genus J sp. nov. 1; Genus J sp. nov. 2; Genus J sp. nov. 3; Genus J sp. nov. 4
The Genus J species complex is based around Parorchestia ihurawao. When Duncan [3] described P. ihurawao, he included it in the genus Parorchestia as it had no long setae on the pleopod peduncles and no dorsal robust setae on the exopods of uropods 1 and 2. It also has a distinct resemblance to P. tenuis (the type species for the genus), the only other species currently included in Parorchestia, and females or small colourless individuals of the two ‘species’ can easily be confused if not inspected carefully. In their review of New Zealand landhoppers, Lowry and Myers [16] considered P. ihurawao to be incertae sedis, due in part to difficulties in interpreting some of the characters. Our investigations show that P. ihurawao is genetically quite different from P. tenuis (Figure 3). Despite their superficial similarities, this genetic difference is supported by differences at the morphological level, and it is evident that P. ihurawao should be accommodated in a new genus. Until a new genus is formally described, we continue to treat P. ihurawao as incertae sedis.
Duncan [3] described P. ihurawao as a small but abundant species with a widespread distribution from Kaikoura down the east coast all the way to Dunedin and Fiordland in the South Island. We have also found representatives on Stewart Island during our investigations, which extends the range of this taxon. However, Duncan [3] noticed some geographic variation in several characters including body size, body pigmentation and the relative length of antenna 1. Though he concluded that this was probably no more than subspecific variation, he also acknowledged that P. ihurawao could be a species complex. Morphological and molecular investigations into some members of this clade confirm that it is a monophyletic species complex (Figure 3). To date, investigations have been focussed on the Dunedin, Southland and Stewart Island regions, and thus far, five taxa in the Genus J species complex have emerged. These are based on 16S sequence data and subtle differences in morphology, specifically around the colouration of preserved specimens, body length and form, length of pleopod 3, shape of the epimera, general form of uropods 1, 2 and 3 (long and slender or short and stout), length of the distolateral robust seta, and number of robust setae on uropod 3 and the telson. At some sites, we have found two of the provisional species in sympatry, supporting the contention that these are distinct. We are conservatively assuming that one of the five taxa belongs to P. ihurawao sensu stricto and four are new and undescribed species in this clade. However, material from the type locality (Peel Forest in South Canterbury) should be examined to confirm whether this interpretation is correct.
5.2.4. Parorchestia–Genus K Complex: Parorchestia tenuis (Dana, 1852); Parorchestia sp. nov. 1; Parorchestia sp. nov. 2; Parorchestia sp. nov. 3; Parorchestia sp. nov. 4; Parorchestia sp. nov. 5; Parorchestia sp. nov. 6; Parorchestia sp. nov. 7; Parorchestia sp. nov. 8; Parorchestia sp. nov. 9; Parorchestia sp. nov. 10; Parorchestia sp. nov. 11; Genus K sp. nov. 1; Genus K sp. nov. 2; Genus K sp. nov. 3; Genus K sp. nov. 4
Parorchestia tenuis is the most confused of all the New Zealand terrestrial taxa and awaits detailed study. It was one of the first landhoppers described from New Zealand [7]. Duncan [3] regarded it as one of the most common New Zealand landhoppers. Landhoppers that are diagnosed as (or close to) P. tenuis as currently defined are distributed from Northland to Southland to Stewart Island. All species possess most or all of the key diagnostic characters associated with this species such as a reticulate body pigmentation pattern for individuals freshly preserved in ethanol (Figure 4), subchelate gnathopod 1 propodus, mascupod gnathopod 2, fully developed and biramous pleopods with narrow peduncles that lack setae, and dorsal robust setae on uropod 1 and 2 endopods but not on the exopods.
DNA sequence data and slight differences in body colouration and in character states involving gills, epimera, oostegites, pleopods, uropods and the telson indicate that most do not belong to P. tenuis sensu stricto. Conservatively, there are at least 16 species (including P. tenuis sensu stricto) in two genera, Parorchestia and Genus K gen. nov. masquerading under the name P. tenuis, making the Parorchestia–Genus K genus complex the largest detected thus far (Table 1; Figure 3). There are probably more cryptic species yet to be discovered in this complex, particularly in geographic regions not yet sampled. Some of these provisional species may be sympatric, supporting the view that they are separate species.
Individuals from Northland (where the type locality is situated), Auckland, Little Barrier Island (Coromandel), Taranaki and Nelson form a single, monospecific clade, which we tentatively regard as P. tenuis sensu stricto (OB and LS, unpublished data). Although intraspecific genetic distances between the populations in Nelson and the North Island were high (up to 14%), we conservatively regard the Nelson population as belonging to this species for now. As noted by Duncan [3], P. tenuis seems to be relatively widespread, but this is probably only the case in the North Island and northwest of the South Island. All but one of the provisional species in the P. tenuis complex have been found in the South Island.
5.2.5. Puhuruhuru Species Complex: P. aotearoa (Duncan, 1994); Puhuruhuru sp. nov. 1; Puhuruhuru sp. nov. 2; Puhuruhuru sp. nov. 3; Puhuruhuru sp. nov. 4
Duncan [3] noted that P. aotearoa was very widespread across New Zealand, occurring on the Three Kings Islands and throughout much of the North Island, South Island and Stewart Island. He considered it to be one of the most abundant native terrestrial animals in the country. Duncan [3] based his description on a male holotype and female allotype collected from ‘Castle Hill’ near Alfredton in the Wairarapa, though he also examined many other specimens from across New Zealand. Duncan [3] noted that the male possessed a parachelate or subchelate gnathopod 1 propodus (i.e., it had a distinct palm) and dorsal robust setae on the exopod of uropod 1, whereas the female possessed a simple gnathopod 1 propodus and lacked dorsal robust setae on the exopod of uropod 1. These differences are more likely to be associated with interspecific rather than intraspecific differences. We consider that either the male holotype was atypical or two closely related but different species in sympatry were used as the types. Males and females encountered in the North Island from Mt Taranaki (Taranaki) northwards do not show the sexual differences that Duncan [3] observed in his types (though individuals sometimes possess a weakly parachelate gnathopod 1 propodus). This northern North Island taxon is also very similar to the female allotype. More variants have since emerged, including at least four from the northern part of the South Island, each exhibiting slight differences in characters such as the form of the gnathopod 1 propodus, the configuration of dorsal robust setae on the endopods and exopods of uropods 1 and 2, and the shape and setal configuration of the telson. These morphological differences are supported by differences in 16S sequence data (Figure 3). We interpret all four of the South Island variants as representing different species. It is likely that the size of this complex will grow further when other regions of New Zealand are properly surveyed.
5.2.6. Other Potential Species Complexes
Leslieorchestia lesliensis (Hurley, 1957): This species has a very widespread distribution in the South Island, with records from Nelson, Mid Canterbury, South Canterbury, Central Otago, and Southland. Hurley [2] also noted its presence in the North Island (Blue Mountains, Silverstream, Wellington), though Duncan [3] was not able to confirm its presence there despite extensive searching. As the type specimens from Leslie Valley (Nelson) had been lost, Duncan [3] extended the description of this species using paratypes from Banks Peninsula (Mid Canterbury), as well as material from various unspecified localities. He did not document any variation in morphology in the material he examined. However, considering how widespread this species is, an investigation into its monospecific status is warranted.
Snaresorchestia patersoni (Stephensen, 1938): This species is known to occur in the southern South Island (Dunedin and Southland), Stewart Island and the Snares Islands [3]. A small, perhaps introduced population is also known from sub-Antarctic Macquarie Island [22]. The type specimens of S. patersoni collected from “Paterson Bay” on Stewart Island have unfortunately been lost. Hurley [2] established topotypes and hypotypes as replacements for the lost types, but the information around these is incomplete. Little locality information is provided for the topotypes, though these are presumably from habitats beside what is now called Paterson Inlet. The hypotypes are from the Snares Islands and Bench Island off the coast of Stewart Island’s Paterson Inlet. In his redescription of S. patersoni, Hurley [2] does not discriminate between these widely separated populations. However, Duncan [3] noted that specimens from the Snares Islands were smaller, and males had a simple rather than subchelate gnathopod 1 propodus as well as a less enlarged gnathopod 2 propodus than those from Stewart Island and the South Island. He hypothesised the existence of a north-to-south cline and recognised two subspecies, P. patersoni patersoni in the north and the more neotenous P. patersoni snarensis from the Snares Islands. Our investigations of the species throughout the Dunedin area reveal the presence of males with either a fully enlarged, normally shaped propodus (mascupod) or a smaller partially enlarged propodus (semi-mascupod) at the same sites. Genetic analysis confirms they are the same species, indicating that the morphological variation is due to differences in the maturity of the specimens. Populations throughout the Dunedin area therefore appear to constitute a single widespread species (Figure 3).
A recent study by Parvizi et al. [23] into the origins of the Macquarie Island population has helped in understanding this taxon further. Parvizi et al. [23] generated mtDNA COI data for specimens of S. patersoni sampled from across its known range (including from Bench Island off Stewart Island), as well as from a new population detected on the Auckland Islands, and concluded that the taxon was most likely a complex of four species. Surprisingly, three of these were present on Bench Island alone, which adds considerably to the challenge of defining the type species.
Sinbadorchestia sinbadensis (Hurley, 1957): Duncan [3] noted variation in the setal configuration of the uropods in specimens of Sinbadorchestia sinbadensis from different populations and postulated that this variation could have been due to differences in the maturity of the specimens. Recent examination of specimens from two sites in Fiordland, Homer Cirque and Lake Roe, that are approximately 130 km apart show significant differences in the setal configuration of the uropods. However, the morphological variation between the populations is only supported by low to moderate differences at the molecular level, suggesting they may still be monospecific. Morphological differences (in such characters as body size, length of antenna 2, structure of the male gnathopod 2 propodus and setal configuration of uropods 1 and 2) between these populations and the species as currently described from the type locality in Sinbad Gully, Milford Sound in Fiordland are also evident. Only when fresh material from the type locality is studied will it be possible to consolidate the description of S. sinbadensis sensu stricto, confirm its distribution patterns and determine whether it is a species complex or is monospecific.
5.3. Provisional Species of Makawidae with No Close Affiliations to Described Genera
In Section 5.1 and Section 5.2, we have identified at least 30 provisional native species belonging to described or undescribed genera with clear affiliations to described species or genera. In this section, we identify at least a further nine provisional native species of Makawidae belonging to five provisional genera which show no close morphological and/or molecular (where molecular data are available) affiliations with described genera. These genera are Genus B, Genus C, Genus D, Genus E and Genus F.
Genus B comprises at least two species (Table 1; Figure 3) with populations in Nelson and Taranaki (Mt Taranaki) and is morphologically remarkably similar to P. tenuis. However, contrary to P. tenuis, their pleopod peduncles have long setae, and the configuration of robust setae on the rami of uropods 1 and 2 is different.
One provisional species from Nelson has been identified as belonging to Genus C (Table 1; Figure 3). Superficially, Genus C bears some resemblance to Genus B and Genus D, but a simple gnathopod 1 propodus, very sparsely setose pleopod peduncles and the presence of non-apical marginal setae on the telson make this a distinctive taxon.
Three provisional species are associated with Genus D (Table 1; Figure 3), with populations in Nelson and Taranaki (Mt Taranaki). Genus D has morphological similarities to Genus B and Genus C, but a combination of differences in femipod/mascupod status, as well as the structure of the male gnathopod 1, gills, and configuration of robust setae on the urosome, shows that species in Genus D are distinct.
One provisional species occurs within Genus E. Genus E sp. nov. 1 (Table 1; Figure 3) is morphologically most similar to Genus D, though it has obvious differences in the configuration of robust setae on the uropods. Genus E sp. nov. 1 is also superficially very similar in appearance to most species in the P. tenuis species complex apart from the presence of setose pleopod peduncles. Genus E has only been found at one South Island location, Secretary Island in Fiordland.
The clade consisting of Genus F from Stewart Island is morphologically similar to M. hurleyi, the most obvious difference being that the pleopod peduncles are not setose in Genus F. The 16S tree also indicates that Genus F comprises two similar species, differing slightly in the shape of the epimera.
5.4. Provisional and Described Species That Belong to Families Other than Makawidae
Until now, all endemic landhoppers known to occur in New Zealand were attributed to a single family, the Makawidae. Our investigations have shown that endemic landhoppers in this country are distributed across three families—Makawidae, Arcitalitridae and Talitridae—rather than one. This discovery represents a significant development in the history of New Zealand landhopper taxonomy.
Prior to this study, at least two species of Arcitalitridae were already known from New Zealand, but these are recent introductions from Australia. Arcitalitridae are naturally found in Australia, South Africa and Asia and have been introduced to several countries around the world. The four species of Kanikania (discussed earlier), as well as Genus A, Genus L and Genus M, all appear to belong to the family Arcitalitridae as they are simplidactylate (i.e., lack cusps on the pereopod dactyls) and possess other morphological characters most associated with this family (e.g., the exopods of uropods 1 and 2 have no robust setae).
Genus A is a species complex consisting of six provisional species (Table 1; Figure 3). Representatives of Genus A are distributed from the Three Kings Islands, Northland, Poor Knights Islands and Auckland with a seemingly disjunct population at Bushy Park in Whanganui. Genus L sp. nov. 1 (Table 1; Figure 3), which has a population in the northern South Island, bears a close resemblance to Genus A, though it differs fundamentally in the structure of the pleopods and maxilliped. Little is known about Genus M sp. nov. 1 (Table 1) as so few individuals have been found, and at only one site on Stewart Island. Also, it has not been possible to obtain DNA sequences from this species, making it more difficult to place taxonomically.
Recently, two terrestrial native species in the family Talitridae have been detected in New Zealand, D. simularis and Genus N (Table 1; Figure 3). Dallwitzia simularis was thought to belong to the Makawidae, and the transfer of this species to Talitridae has already been discussed. Genus N sp. nov. 1 was found in forest leaf litter on the Three Kings Islands.
6. New Zealand Landhopper Groups
Based on phylogenetic analysis of the 16S sequences, many major groups and smaller subgroups can be recognised in the New Zealand landhopper fauna that has been subjected to analysis, some with weak support (Figure 3). Although the congruence between 16S and morphology is generally very high, it is likely that a small number of relationships depicted in the tree are phylogenetically misleading given the sequence saturation of 16S. Careful consideration of both morphological and genetic information is needed. Here, we discuss the composition of the major clades from evolutionary, genetic, morphological, distributional and biogeographical perspectives and identify where discrepancies between the molecular and morphological lines of evidence occur.
6.1. Genus A
The species comprising Genus A are members of the family Arcitalitridae. The arcitalitrids belong to the Protorchestoidae, which are considered ancestral to the Talitroidae (which includes the makawids and talitrids), so their position as the basally diverging lineage in the phylogeny (Figure 3) supports this interpretation. Five of the six species comprising Genus A appear to be restricted to Northland and several of its offshore islands and show strong evidence of repeated bouts of geographic isolation during the Pliocene and Pleistocene epochs [24,25]. The distribution of the sixth species (Genus A sp. nov. 5) is biogeographically unusual, with genetically and morphologically almost identical and disjunct populations being found in Auckland and Bushy Park (Whanganui), around 450 km apart. Further surveys are needed to confirm whether these populations are truly disjunct. It is also possible that the current distribution of the species arose anthropogenically (e.g., through the movement of soil and vegetation).
6.2. Arcitalitrus Hurley, 1975/Kanikania Duncan, 1994/Genus F
Arcitalitrus spp. and Kanikania spp. comprise species belonging to the family Arcitalitridae. These form an early diverging lineage in the phylogeny (Figure 3), further supporting the ancestral credentials of Arcitalitridae. Arcitalitrus spp. and Kanikania spp. share several character states with Genus A. Critically for Arcitalitridae, they are all simplidactylate. Also, they are all femipods and all have robust setae on the endopods but not exopods of uropods 1 and 2. However, contrary to expectations, Arcitalitrus and Kanikania do not form a clade with Genus A in the tree. The situation is exacerbated by the presence of a fourth arcitalitrid genus in New Zealand, Genus L, which falls in the Parorchestia clade, albeit with weak support. Arcitalitrus, Kanikania, Genus A and Genus L differ from each other in several key morphological characters. Arcitalitrus and Genus A possess distally arcuate maxilliped exopodites, whereas Kanikania and Genus L do not. Also, Arcitalitrus, Genus A and Genus L possess a simple or parachelate gnathopod 1 propodus and similar-shaped urosomes, but Kanikania have a subchelate gnathopod 1 propodus and a differently shaped urosome. The form of the pleopods differs significantly between all four genera with respect to characters such as their vestigiality, the presence of peduncular setae, articulation of the rami and width of the peduncles. These results may be a consequence of the limitations of 16S to portray phylogenetic relationships accurately at deeper levels (see discussion in Section 7).
Kanikania is the most distinct of the four arcitalitrid genera in New Zealand. As a genus, Kanikania has a southern New Zealand distribution, with species being found in the southern South Island (Dunedin and Southland), Stewart Island and the Snares Islands.
Genus F unexpectedly grouped with Arcitalitrus and Kanikania in the phylogeny, and this poorly supported relationship is likely to be incorrect. Genus F is not simplidactylate and shares few characters with Arcitalitrus and Kanikania. The two provisional species in Genus F are morphologically far more similar to Makawe hurleyi than to any other species of New Zealand landhopper, but the molecular tree does not indicate a close link between these two genera, suggesting convergent evolution. The two provisional species in Genus F are only known from Stewart Island.
6.3. The Talitridae
The five species in this group all belong to the family Talitridae. Three of the species, Subantarctorchestia aucklandiae, Bellorchestia quoyana, and Tatahipeke tumida, are coastal talitrids and are beachhoppers and sandhoppers rather than landhoppers. They are included here to show the close genetic and evolutionary relationships between these talitrids (Figure 3). The transfer of Dallwitzia simularis to Talitridae has already been discussed, so its presence in this clade was expected. Dallwitzia simularis has been considered a landhopper, though it probably could just as well be regarded as a beachhopper, often occupying very similar habitats to Subantarctorchestia species. We have identified the species of Subantarctorchestia from Antipodes Island that was used to generate the 16S sequence as S. aucklandiae, as corrugations were present on the pereon and a large distal sinus was present on the male gnathopod 2 propodus [5]. Subantarctorchestia aucklandiae is known to be present on the Auckland Islands, Campbell Island, the Snares Islands, Stewart Island and Otago Peninsula (Dunedin), but there are no records of S. aucklandiae or any Subantarctorchestia on Antipodes Island. This therefore represents a new locality record for the species and genus.
Genus N has only been found on the Three Kings Islands and is the only known species of Talitridae that appears to have invaded the terrestrial environment on any part of the North Island or its immediate offshore islands.
6.4. Puhuruhuru Duncan, 1994/Genus B/Genus C/Genus D/Genus E/Genus J/Parorchestia ihurawao Duncan, 1994
This group containing the Puhuruhuru species complex as well as Genus B, Genus C, Genus D and Genus E, in addition to the Genus J species complex (Figure 3), is large and morphologically diverse. Excluding the Genus J complex, there are several morphological similarities between the other genera in this group indicating it may comprise a true evolutionary clade. For example, all species and genera possess setose (or partially/sparsely setose) pleopod peduncles and a distolateral robust seta, and all have robust setae on the endopod of uropod 1 and the endopod and exopod of uropod 2. In the context of the New Zealand fauna, these similarities, particularly the presence of long setae on the pleopod peduncles, are likely indicators of a common ancestry. However, the cohesiveness of Puhuruhuru, Genus B, Genus C, Genus D and Genus E from a morphological standpoint is otherwise poorly defined. Pigmentation patterns of individuals preserved in ethanol vary from diffuse bands in P. aotearoa (Figure 4) to fully reticulate patterns in Genus B, Genus C and Genus D, and the structure of the gnathopod 1 propodus ranges from simple in Genus C to simple–parachelate in the P. aotearoa complex to fully subchelate in Genus B, Genus D and Genus E. Other differences include whether lobes covered in palmate setae are present or not on the merus, carpus and propodus of the male gnathopod 1 (they are present in Genus B but not in Genus C and Genus D and are indeterminate in P. aotearoa and Genus E), whether robust setae are present on the uropod 1 exopod, and whether genera are mascupod (Genus B) or femipod (all other genera except Genus E which is unknown). Variation in gill structure and telson structure is also evident. Differences in the structure of the gills are particularly revealing. Gills 3 and 4 are lobed in the P. aotearoa species complex and in provisional species from Genus B, but they are not lobed in provisional species from Genus C, Genus D and Genus E. These morphological differences are closely matched by sequence differences at the 16S locus (Figure 3). This scenario with subgroups possessing either lobed or unlobed gills may be similar to the situation in the Parorchestia–Genus K genus complex and may be related to the genetically regulated convergent evolution of convoluted gills associated with montane transitions revealed by Liu et al. [26] in other talitroid groups.
Diversity in the subgroup consisting of Puhuruhuru, Genus B, Genus C, Genus D and Genus E appears to be largely centred in Nelson in the South Island, where at least ten provisional species are located (Table 1). Apart from one record of Genus E from Secretary Island in Fiordland, these provisional genera have not been detected elsewhere in the South Island or on Stewart Island. There are historical records of P. aotearoa throughout the South Island [3], though these are unlikely to be P. aotearoa sensu stricto. In the North Island, three species in this subgroup have been found so far. Puhuruhuru aotearoa (see earlier discussion on the taxonomic uncertainties of this species complex) appears to be widespread across the North Island. Two provisional species, Genus B sp. nov. 1 and Genus D sp. nov. 2, have only been recorded from Taranaki (Mt Taranaki), but these are likely conspecific with populations in Nelson. This biogeographic link between now widely separated species provides more strong evidence of land connections between the Nelson region and south Taranaki at the time of the glacial periods (including the last glacial maximum) during the Pleistocene epoch [24,27,28,29].
The subgroup containing the Genus J species complex (which also includes P. ihurawao) is morphologically and genetically distinct from the other genera in this clade. Morphological comparisons would suggest a closer association with the Parorchestia–Genus K complex than the other genera in the Puhuruhuru group, but homoplasy is an ever-present concern. Genus J sp. nov. 1 and Genus J sp. nov. 2 are both widely distributed species, and are found, often in sympatry, in Dunedin and Southland. A third provisional species, Genus J sp. nov. 4, has been recorded from a single site on the outskirts of Dunedin city, where it is sympatric with Genus J sp. nov. 1 and Genus J sp. nov. 2. Genus J sp. nov. 3 has only been recorded from one area of Stewart Island.
6.5. Dana Lowry, 2011
The Dana group is a monogeneric clade (Figure 3) containing three species, D. hauturu, D. sylvicola and D. taranaki. With no more than a 6% divergence at the 16S locus, genetic distances between the three species are small. These genetic affinities are closely paralleled by their striking morphological homogeneities. All three species are mascupod, and all possess very similar gills, three pairs of pleopods lacking peduncular setae, the same configuration of robust setae on uropods 1, 2 and 3, and very similarly shaped telsons. Also, D. sylvicola and D. taranaki are known to have a reticulate body pigmentation pattern [3], although no descriptions of the body patterning are available for D. hauturu. As molecular differences between all three species are well below the 16% threshold set, detailed morphological studies are needed to determine whether they are distinct species or monospecific. Until such studies are conducted, they continue to be treated as different species.
Dana hauturu and D. taranaki are restricted to Little Barrier Island (Coromandel) and Mt Taranaki (Taranaki), respectively [3]. Dana sylvicola was thought to be restricted to Northland [3], though recent surveys that have included the use of DNA sequencing have also detected the species in Auckland and the Waikato (OB and LS, unpublished data), showing it is more widely distributed than previously thought.
6.6. Snaresorchestia Lowry & Myers, 2019
Snaresorchestia patersoni is not closely related to any other sampled taxon (though see discussion on the likely existence of a species complex) (Figure 3). This should come as no surprise as Snaresorchestia patersoni is unique in the New Zealand fauna as it is the only taxon where all three pleopods are reduced to vestigial stumps. It is therefore likely that S. patersoni is an evolutionarily distinctive species in in the New Zealand fauna. Snaresorchestia patersoni has a similar geographic distribution to Kanikania, naturally occurring in Dunedin, Southland, Stewart Island and the Snares Islands, though it is also present on the Auckland Islands.
6.7. Makawe Duncan, 1994/Oamaru Lowry & Myers, 2019/Genus G/Genus H
Although the group consisting of Makawe and Oamaru species and the three provisional species in Genus G and Genus H received poor support in the 16S phylogeny, there are morphological features uniting them. All of the constituent species are femipods, and all possess similarly structured propodi of gnathopods 1 and 2, setose pleopod peduncles, a variously reduced pleopod 3 and similar-shaped telsons. Despite marked differences in the colouration of preserved specimens (ranging from semi-reticulate to semi-vivid hoops to more diffuse patterning) and differences in the configuration of robust setae on the rami of uropods 1 and 2, the degree of integrity of this clade (Figure 3) is high, indicating divergence times may be more recent than in some other major clades.
Most of the described and provisional species in this clade are found in the South Island, and mostly to the east of the Southern Alps divide from North Canterbury to Southland and Fiordland. However, Genus G sp. nov. 1 has been collected from southern Wairarapa in the North Island, and the only known population of Makawe waihekensis is recorded from Waiheke Island, a small island in the Hauraki Gulf in Auckland, which is a considerable distance from where the rest of the clade occur. There is also a record of M. hurleyi from the Chatham Islands, though no further details are provided [3,10]. Individuals from the Chatham Islands need to be examined to determine whether this population is a recent (i.e., human-assisted) arrival, or whether it has more ancient origins.
6.8. Deshurleyella Lowry, Myers & Nakano, 2019/Kohuroa Lowry, Myers & Nakano, 2019/Waematau Duncan, 1994/Omaiorchestia Lowry & Myers, 2019
Ostensibly, it would appear that the group comprising Deshurleyella, Kohuroa, Waematau and Omaiorchestia, which received moderate support in the 16S phylogeny (0.95 PP; Figure 3), is a random assortment of genera that are morphologically distinct and not closely related. The colour patterns of preserved individuals in this group range from vividly hooped (Figure 4) to more diffuse patterns to partly reticulate to almost colourless, and there is significant variation in many other key character states such as the shape of antenna 1, the form of the gnathopod 1 propodus, whether there are palmate lobes on the merus, carpus and propodus of the male gnathopod 1 (all genera bar Deshurleyella), whether genera are femipods (Deshurleyella), mascupod (Kohuroa and Waematau) or both (Omaiorchestia), the shape of the gills, the presence/absence of the distolateral robust seta and the shapes of uropod 3 and the telson. However, they also share some key character states. For example, there are no setae on the pleopod peduncles in any genus, and the pleopod structure (where these are fully developed) is similar across all genera. Also, all have robust setae on the uropod 1 and 2 rami except for the uropod 1 exopod. Furthermore, it is notable that with the exception of the O. rubroannulata species complex (which extends down the east coast of the North Island), all genera and species are otherwise restricted to the Auckland and Northland areas. We consider it probable that this northern group is reflective of evolutionary histories, and that the extent of the morphological differences indicates that divergence times are more ancient relative to several other clades.
6.9. Parorchestia Stebbing, 1899/Genus K/Sinbadorchestia Lowry & Myers, 2019
Parorchestia tenuis sensu stricto and all of the provisional species within the P. tenuis–Genus K complex, Sinbadorchestia sinbadensis and Genus L sp. nov. 1 form a large, albeit poorly supported clade in the 16S phylogeny (Figure 3). The obvious morphological outlier in this group is Genus L, which will be discussed later in this section. All taxa (other than Genus L) in this clade share many common characters. All possess a gnathopod 1 that is subchelate with lobes (in males) covered in palmate setae on the merus, carpus and propodus, three pairs of moderately developed and biramous pleopods with relatively narrow peduncles lacking setae, a distolateral robust seta on uropod 1, and a similar overall morphology of the epimera and urosome. Moreover, all representatives of this clade are mascupod and have a reticulate-type pigmentation pattern.
The most obvious morphological character that separates Parorchestia species from Genus K species is their fundamentally different gill structure. Gills 3 and 4 in Parorchestia species are unlobed, whereas gills 3 and 4 in Genus K species are lobed. This divide between closely related genera that possess lobed or unlobed gills was also observed in the Puhuruhuru group, so it may be a second example of genetically regulated convergent evolution of convoluted gills documented by Liu et al. [26]. Sinbadorchestia sinbadensis also possesses lobed gills, so it is morphologically closer to Genus K than it is to Parorchestia. However, S. sinbadensis differs from Genus K (and all species of Parorchestia) as it possesses dorsal robust setae on the exopods of uropods 1 and 2. We have found that the presence or absence of dorsal robust setae on uropods 1 and 2 can be a variable character state within some New Zealand genera.
It is evident that radiation in this group has been extensive but almost entirely confined to the South Island. Only P. tenuis sensu stricto and Genus K sp. nov. 3 have been found in the North Island. Parorchestia tenuis sensu stricto is widespread across the North Island and the top of the South Island, whereas Genus K sp. nov. 3 has only been found on Mt Taranaki (Taranaki) (Table 1). The other 15 described and provisional species we have documented from this clade are all found in the South Island and Stewart Island and are likely restricted to there. Seven provisional species have been identified from Fiordland alone, and a further five (excluding P. tenuis sensu stricto) from Nelson, indicating that radiation in these areas has been particularly prolific. Only one of the provisional species from the South Island (Parorchestia sp. nov. 11) appears to be fairly widespread, with most others being recorded thus far from small geographic areas or single locations (Table 1). Morphological and genetic differences between many of the provisional species are comparatively small, perhaps suggesting divergence times more recent than in most other genera.
In the case of Genus L, we surmise that the inability of 16S to accurately and consistently depict phylogenetic relationships, particularly at deeper levels, is responsible for the inclusion of this genus in this clade. Being a member of the Arcitalitridae, Genus L is fundamentally different in morphology from all other members of the Parorchestia group, as its long branch in the phylogeny (Figure 3) also suggests. Currently, Genus L contains one species, Genus L sp. nov. 1, and although it has strong morphological affiliations with the other three arcitalitrid genera present in New Zealand, it is also quite distinct from them (see earlier discussions on Genus L in Section 6.2). Thus far, Genus L has only been found in Nelson.
7. Molecular Interpretations
Traditionally, species identifications and taxonomic descriptions have relied exclusively on the interpretation of morphological characters. However, this process often takes many years, and despite calls to increase research capacity in taxonomy, investment in this area remains low [30]. One way to potentially accelerate the critical taxonomic process is to follow a system of provisional nomenclature [31]. Many amphipod studies have shown how valuable the incorporation of genetic data can be to reveal, for example, the presence of cryptic species and instances of morphological convergences and simply increase confidence around species delimitation [26,32,33,34,35,36,37,38,39,40,41]. There is a risk of species discovery using molecular methods not being followed up by formal species descriptions, which could exacerbate the problems associated with a backlog of undescribed species [33]. However, in the absence of longer-term solutions, the categorisation of species as Molecular Operational Taxonomic Units (MOTUs) provides a convenient interim solution. Therefore, in combination with the usual morphological criteria, this study used genetic data to more rapidly advance our understanding of the taxonomy of New Zealand landhoppers than would have otherwise been possible.
Many studies on amphipod taxonomy incorporating a genetic component use multiple molecular markers, both mitochondrial and nuclear, to assist in the decision-making process [26,35,36,37,40,41]. Initially, we used two mitochondrial (16S and COI) and two nuclear (histone H3 and 28S) markers. The 16S locus was successfully amplified from most specimens, with the nuclear markers being least likely to be amplified. We found that gene trees constructed from different loci showed some differences, but these were generally in poorly supported deeper nodes in the phylogenies. There was higher sequence variation in the mitochondrial markers compared with the nuclear markers, which therefore resulted in better-resolved mitochondrial trees. A cautious approach in the use of mitochondrial markers for species delimitation therefore needs to be taken in order to avoid overestimating species richness [42]. Such patterns in molecular data are commonly reported in amphipod studies elsewhere [36,37,40,43], and discrepancies between different genetic markers and putative species boundaries are commonly attributed to hybridisation and the random sorting of ancestral variation into descendant lineages (incomplete lineage sorting) [37].
We found that variation in 16S was significantly more consistent with the morphological information than COI or the two nuclear markers. In a similar result, Waller et al. [40] concluded that 12S was more reliable at resolving relationships than COI for the genus Hyalella. However, the reasons for this appear to be clearer as they also found that 12S was more conserved and less saturated, and therefore more accurate, than COI. A concatenated or consensus tree incorporating all markers is often produced [26,36,37,40]. However, combining markers in this study led to a noticeable decline in congruence between morphological and molecular data. Thus, a single gene tree using the 16S marker was preferred, even though some discrepancies between the morphological information and genetic phylogenies remained.
Apparently anomalous results were cross-checked where possible across multiple loci to reduce uncertainties around delimiting species or clades. For example, the association of Kanikania with Arcitalitrus in the 16S tree was unexpected given their distinct morphologies, but sequences from COI, histone H3 and 28S showed similar results (OB and LS, unpublished data). This approach using evidence from multiple molecular markers (both mitochondrial and nuclear) will be used for producing descriptions of New Zealand landhoppers in the future.
For K. longicornis, K. motuensis, O. rubroannulata, P. ihurawao, P. aotearoa, S. sinbadensis and S. patersoni, it was not possible to confirm whether any of our material would be viewed as belonging to the respective species sensu stricto. In some cases, the quality of the type material was poor, and the original descriptions were either not detailed or not consistent enough for us to be certain about the identity of our own material. But in all of these cases, we did not have access to fresh material from the type localities. Most of these taxa have been shown to be complexes where differences between species are difficult to detect. Descriptions for each species in such complexes therefore need to be particularly detailed with key differences between each species clearly highlighted. Because of this, we used a precautionary approach and presumed, correctly or not, that one of the taxa in our possession represented the species. Only when each species complex is subjected to rigorous morphological and molecular investigation will a more accurate interpretation emerge.
We now consider the need to integrate genetic and morphological data for the delimitation of species is crucial for talitroids (and no doubt for many other groups), and we hope that the molecular insights obtained as a result of this study might be used to inform future research. However, in order to provide more certainty around the deeper phylogenetic relationships in landhoppers (at the family and distantly related genera levels), multiple, independently evolving loci evolving at a suitable rate are required.
8. Landhopper Distributions
Landhoppers are distributed widely over the three main islands of New Zealand (North, South and Stewart Islands) and many of its nearshore and offshore islands, including the sub-Antarctic islands. However, the vast majority (84%) of described and provisional species appear to be endemic to only one major island or offshore island group (Table 1). For most species, oceans or sea channels represent barriers that are practically insurmountable. A notable exception is D. simularis, which has a distribution encompassing Antipodes Island, Bounty Island, the Snares Islands and Fiordland in the South Island. Furthermore, 55% of species appear to have occupancy areas that are restricted to a single ecological district or less, leaving 45% of species with more widespread distributions, either across multiple ecological districts or regions within a main island or between islands. These distributional patterns correlate well with their generally poor vagility [44]. Although they can sometimes be found under rocks in areas with little leaf litter, most New Zealand landhoppers are found in the leaf litter layer of habitats dominated by native terrestrial trees and shrubs. Some native landhopper species appear quite tolerant of disturbance and of the presence of exotic plant species (e.g., K. kaitaia, M. hurleyi, O. otamatuakeke, O. unuwhao and S. patersoni), but others (e.g., D. kohuroa and most species in Genus A) do not. As they are small and disperse poorly, even small fragments of woody vegetation in a matrix of production land can represent valuable refugia for these creatures.
Native landhoppers belonging to the Makawidae and now Arcitalitridae are found on all three main islands of New Zealand as well as some offshore islands (e.g., Three Kings and Poor Knights Islands). Except for specific (often disturbed) locations where exotic Arcitalitrus species occur in large numbers, the fauna is usually dominated by the makawids.
Representatives of the Talitridae appear notably absent from most terrestrial habitats on New Zealand’s three main islands. Only the supralittoral zones on these islands appear to be occupied by sandhoppers and beachhoppers [2,5]. However, a small number of Talitridae on some smaller islands may have penetrated slightly further inland. Dallwitzia simularis has been collected from leaf litter on the Snares Islands [2], and its presence in soil and leaf litter associated with terrestrial plants 500 m inland at an elevation of 135 m has now been confirmed on Antipodes Island (Robin Long, pers. comm.). In fact, D. simularis can thrive in a variety of habitats, spanning environments occupied by beachhoppers and landhoppers. Another member of the Talitridae, the provisional representative of Genus N, occurs in coastal (pohutukawa) leaf litter on the Three Kings Islands.
The family Talitridae is generally associated with the marine littoral habitat, occurring above high tides amongst various types of litter–vegetation, including mangroves, and in marshes close to the sea. In several parts of the world, talitrids have moved inland, even to the tops of high mountains, e.g., Canariorchestia Lowry & Myers, 2019 in the Canary Islands, Laniporchestia Lowry & Myers, 2019 in Hawaii and Opunorchestia Lowry & Myers, 2019 in Tahiti. However, although D. simularis and Genus N may be able to move beyond the supralittoral zone, they are apparently restricted to coastal areas and are therefore still influenced by coastal conditions. The apparent absence of talitrids in inland habitats on New Zealand’s three main islands is puzzling. It is possible that talitrids have struggled to conquer areas already inhabited by competitively superior makawids and arcitalitrids.
9. Areas of Endemism
Landhopper diversity is not distributed evenly across New Zealand. Including the provisional taxa identified in this study, species richness is currently around 18% higher in the South Island compared with the North Island (Table 1). However, within each island, there are also centres of richness and endemicity. In the South Island, Nelson appears to be the most species-rich area with 18 described and provisional species, 14 (78%) of which have not been detected elsewhere. Although it is still too early to conclusively affirm whether these species are all regionally endemic, high levels of endemism and species diversity in invertebrates have been noted for the Nelson area previously [45,46]. Fiordland could be considered another centre of endemism in the South Island, with 8 of the 13 described and provisional species (or 62%) apparently restricted to the area. The centre of species richness and endemicity in the North Island appears to be Northland, with 16 described and provisional species, eight of which (50%) are endemic (this excludes the Poor Knights and Three Kings Islands). Moreover, within Northland, nine described and provisional species are known from Te Paki Ecological District at the northern tip of the North Island, six of which are only found in this district, resulting in an endemism rate of 67%. This indicates that relative to its size, Te Paki Ecological District is a disproportionately important centre of endemism for landhoppers. Both Northland and Te Paki specifically have been identified as centres of endemism for various taxa, including invertebrates [46,47,48,49]. The high rates of endemism at Te Paki Ecological District (and on Northland’s offshore islands) are most likely caused by prolonged and repeated periods of isolation of high-elevation points within the district from the mainland during the Pliocene and Pleistocene epochs [24,25].
10. Introduced Species
Two introduced landhoppers, both in the family Arcitalitridae, are known historically to occur in New Zealand. These are Arcitalitrus sylvaticus (Haswell, 1879) and A. dorrieni (Hunt, 1925) (Table 1; Figure 3). Fenwick and Webber [4] report a third introduced species, Talitroides topitotum (Burt, 1934), a member of the Brevitalitridae, but place a question mark around its distribution, which they list as “northern New Zealand?”. We have regularly encountered A. sylvaticus and A. dorrieni during surveys and in collections, but have not encountered T. topitotum. It is possible that this species is present in New Zealand and that it has been missed in field surveys and in museum collections. However, in his earlier investigations, Duncan [50] appeared to misidentify species of Arcitalitrus for T. topitotum. Talitroides topitotum was first described from Sri Lanka but is almost cosmopolitan, so its region of origin is unknown. The existence of T. topitotum in New Zealand requires confirmation.
Recently, the study by Liu et al. [26] included sequences of an arcitalitrid (“Arcitalitrus sp 24”) collected from Mission Bay in Auckland that are different from A. sylvaticus and A. dorrieni (Figure 3). Material from this locality has not been subjected to morphological examination, but it now appears that three species of Arcitalitrus are present in New Zealand.
Morphologically, A. dorrieni and A. sylvaticus are very similar. We have found that the most obvious and useful character separating the two species is the shape of gill 6. In A. dorrieni, gill 6 has smooth margins and is deeply and narrowly cleft posterodistally, giving it a chelate appearance. In contrast, the margins of gill 6 in A. sylvaticus are convoluted, and it is very widely cleft lower down the posterior margin, giving an almost anseriform appearance. The shape of epimeron 2 is a second useful character for distinguishing between the two species. In A. sylvaticus, epimeron 2 is very asymmetrical, with the deepest point situated anteriorly and the ventral margin being relatively straight. In contrast, in A. dorrieni, epimeron 2 has a convex ventral margin and is nearly symmetrical, the deepest point lying centrally or slightly posteriorly. Sequencing of material from various parts of New Zealand has shown significant sequence variation in A. sylvaticus. This is accompanied by small though consistent differences in gill 6 morphology. It is therefore likely that introductions of A. sylvaticus in the past have originated from different sources and probably therefore on more than one occasion.
11. Discussion and Conclusions
This paper has reviewed the current state of knowledge of New Zealand landhoppers and explored their patterns of radiation. It has provided valuable insight into the undescribed portion of the New Zealand fauna. Twenty-eight native species in 16 genera are currently described, all endemic to New Zealand. We have transferred four species to pre-existing genera to resolve taxonomic inconsistencies, and one species (Parorchestia ihurawao) is currently considered incertae sedis and awaiting transfer to a new genus. Until now, all described native species in New Zealand were thought to belong to the family Makawidae. However, species from two genera were found to belong to Arcitalitridae and Talitridae to which they have been transferred. These are the first records of fully terrestrial landhoppers belonging to families other than Makawidae from New Zealand. Moreover, we have documented the existence of at least 48 provisional (undescribed) native species, greatly expanding our knowledge of the diversity of the New Zealand fauna. At 28 species, our described fauna is therefore already dwarfed by the now-known undescribed species, and more species and genera will doubtless emerge as studies continue.
The most significant contributor to the increase in the number of known undescribed species is the existence of several species and genus complexes with affiliations to described species or genera. Evidence for such complexes has accumulated for many invertebrate groups, including Amphipoda [33,51,52,53]. Our investigations have revealed that species in at least four of the 16 existing genera comprise species complexes or genus complexes. Currently, the number of additional (provisional) species within each complex ranges from one to 15. The Parorchestia–Genus K complex is particularly large and complicated. It is evident that interspecific and intergeneric differences have been underestimated in the past and that subtle differences in just one or two characters denote species or genus status.
Also contributing to the increase in the number of provisional species are those that show no close morphological and/or molecular affiliations with described genera. New genera will need to be erected to accommodate these unaffiliated species. Most (nine) of these provisional genera belong to the Makawidae, but three belong to the Arcitalitridae and one to the Talitridae, further expanding the known diversity of New Zealand landhoppers. Many are small, which could explain why they have eluded taxonomic authorities until now. However, some are relatively large animals, which indicates a historic lack of research investment in this group.
Combining the described with the provisional species and genera, this study has shown that the New Zealand landhopper fauna comprises a total of 76 native species distributed among 29 genera in three families (Table 1), and more are sure to be identified in future studies. Thus, the New Zealand landhopper fauna is far richer and much more diverse than currently described.
The distributions of landhopper species are often more limited than previously thought. Species such as P. tenuis and P. aotearoa were thought to have nationwide distributions, and others such as P. ihurawao and K. longicornis were also considered widespread species with distributions spanning several islands, provinces or ecological regions. However, the discovery that these and many others are actually complexes of species and even complexes of genera has significantly reduced the recorded ranges of their constituent species. Though a few species (e.g., P. tenuis sensu stricto, Puhuruhuru aotearoa (the taxon present in the northern North Island) and M. hurleyi) are still relatively widespread, these appear to be the exceptions, and the distributions of most species seldom span more than one to two provinces or island groups, often less. As area of occupancy in relation to historical habitat loss is a key metric for assessing the conservation status of species in New Zealand [54], the generally small species distributions, often in the context of recent and extensive habitat destruction, have potentially serious consequences for the conservation of landhoppers. There is little doubt that New Zealand has already lost species of landhopper due to habitat destruction in high-clearance areas such as Waikato, Gisborne, Hawke’s Bay, Wairarapa, the Canterbury Plains (North, Mid and South Canterbury) and Southland.
Diagnoses of the New Zealand landhoppers were determined through a reciprocal recognition of taxa as morphological units (OTUs—Operational Taxonomic Units) and molecular units (MOTUs—Molecular Operational Taxonomic Units). However, some associations identified using molecular methods were questioned or rejected due to incongruence with morphology.
As with all amphipod taxonomy, it can sometimes be a challenge to distinguish between synapomorphies and homoplasies when establishing phylogenetic relationships based on morphological characters alone. In this regard, the added support provided by the molecular data has been invaluable. For example, the presence or absence of marginal robust setae on the rami of uropods 1 and 2 has been used in generic diagnoses of Makawidae. While these character states can indeed be important in distinguishing genera and even larger groups, they may not always be diagnostic, since the loss of setae (naked ramus) may have evolved more than once in independent lineages. Some characters such as the form of the gnathopod 1 propodus (from simple to subchelate) and reduction in pleopod size appear to evolve quite rapidly within certain genera and some of the major groups identified in this study. One character state that appears to be very conserved within lineages and therefore informative is the presence of long plumose setae on the pleopod peduncles. Even when species undergo dramatic changes in multiple other characters, the presence or absence of plumose setae on the pleopod peduncles tends to remain relatively constant. Another useful character that has emerged is the presence or absence of lobed gills. This appears to be an example of genetically regulated convergent evolution [26]. It has appeared independently in two of the major groups identified in this study and can be a useful diagnostic character. A rarely acknowledged [3,10] character that we have found useful in delineating species is body pigmentation and patterning in material freshly preserved in ethanol. This can vary significantly between species (even within a species complex) and genera. Unfortunately, the colouring fades post-mortem irrespective of the method used for preservation.
Although results are provisional and more fieldwork needs to be undertaken, centres of species richness and endemism in both the North and South Islands are beginning to be recognised. In the South Island, Nelson appears to be the most species-rich area, and most species found there are yet to be detected elsewhere. Fiordland appears to be another centre of endemism in the South Island. In the North Island, Northland, and more specifically Te Paki Ecological District, appears to be a centre of endemism for landhoppers.
The process of describing species by their morphological characters is a time-consuming activity, requiring detailed dissections and illustrations. Though not a silver bullet [37,42,43], the advent of molecular techniques has greatly facilitated the identification of new amphipod species [23,33,52]. Together with an initial examination of the critical morphological characters, our study affirms the value of using molecular data as a preliminary step in detecting and delineating potential new species within complexes of closely related and morphologically similar species. Phylogenetic relationships that are inferred by analyses based on the 16S gene alone agree more consistently with morphological analyses than do relationships inferred by COI, histone H3 and 28S, even when analyses are based on concatenated or consensus trees using all available markers.
Although we have used an integrative taxonomic approach that combines the use of molecular data with morphological observations to further our understanding of the New Zealand landhopper fauna, our conclusions remain tentative. Detailed morphological investigations leading to formal descriptions are required to validate our findings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16100632/s1: Supplementary Table S1. Specimens used for genetic analyses, their collection locality and GenBank accession number.
Author Contributions
Conceptualisation, A.A.M., O.J.-P.B., S.R.P. and L.D.S.; design and funding, O.J.-P.B. and S.R.P.; fieldwork, O.J.-P.B. and S.R.P.; examination of material including type specimens, O.J.-P.B. and A.A.M.; genetic and statistical analyses, L.D.S.; writing of manuscript, O.J.-P.B., A.A.M., L.D.S. and S.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the Department of Conservation’s Threatened Species Research Workstream. DNA sequencing was funded by Te Papa. NorthTec provided further funding and support for the project.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Sequence data are deposited in GenBank under https://www.ncbi.nlm.nih.gov/genbank/ (accessed on 8 August 2024); see Table S1 for accession numbers.
Acknowledgments
We would like to acknowledge the support from the Department of Conservation, without which this paper could not have been produced. We would particularly like to acknowledge Tara Murray, who has supported the project over recent years. We also acknowledge the long-term support of Te Papa Museum, Wellington, and the research committee of NorthTec, Whangārei. We also thank (in alphabetical order) the following people and organisations for allowing access to sites, access to material held in collections, passing on collected material, assistance in the field and/or supporting the research: Robyn Abernethy (Dunedin City Council), Barbara Barratt (AgResearch), Aaron Bertoia (University of Otago), Bernie Buhler (Friends of Matakohe-Limestone Island), Victoria Campbell (Ngai Tahu), Geoff Churchill (Nelson City Council), Phil Clements (P.F. Olsen), Bridget Coughlan (Te Rūnanga o Ōtākou), Max Crowe (Waitaki District Council), Francesca Cunninghame (Forest and Bird), Mike Dickison (Whanganui Regional Museum), Tom Drinan (Department of Conservation), John Early (Auckland Museum), Eric Edwards (Department of Conservation), David Glenn (Dunedin City Council), Nick Goldwater (Wildland Consultants Ltd.), Owen Graham (Dunedin City Council), Chris Green (Department of Conservation), Grace Hall (Manaaki Whenua-Landcare Research), Danilo Hegg (Wētā Conservation Charitable Trust), Lindsay Hyde (Waitaki District Council), Erin Jeffery (Craigmore Sustainables), Julia Kasper (Te Papa Tongarewa-Museum of New Zealand), Matthew Lanyon (Waitaki District Council), Robin Long (Tawaki Project), Alan Matchett (Dunedin City Council), Jane Matchett (Waitaki District Council), Cathy and Pete Mitchell (Bream Head Conservation Trust), Muriwhenua Incorporation, Pineaha Murray (Ngāti Kuri), Tom Myers (Dunedin City Council), Eric Odell (Dunedin City Council), Joyce Palmer (Department of Conservation), Vicky Rawnsley (Department of Conservation), Tōrea Scott-Fyfe (University of Otago), Dave Seldon (University of Auckland), Matthew Shaw (Canterbury Museum), Brian Smith (NIWA, Hamilton), John and Andrea Sturgess (Lucklaw Farm), Craig Treloar (P.F. Olsen), Cor Vink (Canterbury Museum), Malcom Walker (Runaka Puketeraki), Rick Webber (Te Papa Tongarewa-Museum of New Zealand), Patrick Whaley (Department of Conservation), Gavin White (Forest and Bird) and Jane Williams (Department of Conservation).
Conflicts of Interest
Author Olivier J.-P. Ball was employed by the company Wildland Consultants Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Myers, A.A.; Lowry, J.K. A phylogeny and classification of the Talitroidea (Amphipoda, Senticaudata) based on interpretation of morphological synapomorphies and homoplasies. Zootaxa 2020, 4778, 281–310. [Google Scholar] [CrossRef]
- Hurley, D.E. Terrestrial and littoral amphipods of the genus Orchestia, Family Talitridae. Trans. R. Soc. N. Z. 1957, 85, 149–199. [Google Scholar]
- Duncan, K.W. Terrestrial Talitridae (Crustacea: Amphipoda). In Fauna of New Zealand; Manaaki Whenua Press: Lincoln, New Zealand, 1994; Volume 31, p. 128. [Google Scholar] [CrossRef]
- Fenwick, G.; Webber, R. Identification of New Zealand’s terrestrial amphipods (Crustacea: Amphipoda: Talitridae). Tuhinga 2008, 19, 29–56. [Google Scholar]
- Hughes, L.E.; Lowry, J.K. Review of New Zealand coastal talitroids with description of three new genera (Crustacea, Amphipoda, Senticaudata). Zootaxa 2023, 5268, 001–081. [Google Scholar] [CrossRef]
- Myers, A.A. The conquest of land by the Amphipoda (Senticaudata, Talitroidea) explained by global tectonics and vicariance. Zootaxa 2022, 5214, 224–234. [Google Scholar] [CrossRef]
- Dana, J.D. Conspectus Crustaceorum quae in Orbis Terrarum circumnavigatione, Carola Wilkes e Classe Reipublicae Foederatae Duce, lexit et descripsit Jacobus D. Dana. Pars III. Proc. Am. Acad. Arts Sci. 1852, 2, 201–220. [Google Scholar]
- Chilton, C. The Crustacea of the Subantarctic Islands of New Zealand. In The Subantarctic Islands of New Zealand; Chilton, C., Ed.; Philosophical Institute of Canterbury: Christchurch, New Zealand, 1909; Volume 2, pp. 601–671. [Google Scholar]
- Stephensen, K. Amphipoda, Tanaidacea und Pycnogonida. Senckenbergiana 1938, 20, 236–264. [Google Scholar]
- Duncan, K.W. A description of a new species of terrestrial amphipod (Fam. Talitridae) from New Zealand. Trans. R. Soc. N. Z. Zool. 1968, 10, 205–210. [Google Scholar]
- Ball, O.J.-P.; Webber, W.R.; Shepherd, L.D. New species and phylogeny of landhoppers in the genus Waematau Duncan, 1994 (Crustacea: Amphipoda: Talitridae) from northern New Zealand. Zootaxa 2017, 4306, 151–207. [Google Scholar] [CrossRef]
- Crosby, T.K.; Dugdale, J.S.; Watt, J.C. Area codes for recording specimen localities in the New Zealand subregion. N. Z. J. Zool. 1998, 25, 175–183. [Google Scholar] [CrossRef]
- Yang, L.; Hou, Z.; Li, S. Marine incursion into East Asia: A forgotten driving force of biodiversity. Proc. R. Soc. B 2013, 280, 20122892. [Google Scholar] [CrossRef]
- Xia, X. DAMBE7: New and improved tools for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2018, 35, 1550–1552. [Google Scholar] [CrossRef]
- Lefébure, T.; Douady, C.J.; Gouy, M.; Gibert, J. Relationship between morphological taxonomy and molecular divergence within Crustacea: Proposal of a molecular threshold to help species delimitation. Mol. Phylogenet. Evol. 2006, 40, 435–447. [Google Scholar] [CrossRef]
- Lowry, J.K.; Myers, A.A. New genera of Talitridae in the revised Superfamily Talitroidea Bulycheva, 1957 (Crustacea, Amphipoda, Senticaudata). Zootaxa 2019, 4553, 1–100. [Google Scholar] [CrossRef]
- Lowry, J.K. Dana, replacement name for the New Zealand talitrid amphipod genus Tara Duncan, 1994 (Crustacea, Amphipoda, Talitridae), preoccupied by Tara Peckham & Peckham, 1886 (Araneae, Salticidae, Amycinae, Astiinae). Zootaxa 2011, 2912, 68. [Google Scholar]
- Lowry, J.K.; Myers, A.A.; Nakano, T. Replacement names for four preoccupied talitrid genus-group names proposed by Lowry and Myers 2019 (Crustacea: Amphipoda: Senticaudata). Zootaxa 2019, 4615, 395–396. [Google Scholar] [CrossRef]
- Bousfield, E.L.; Howarth, F.G. The cavernicolous fauna of Hawaiian lava tubes. 8, Terrestrial Amphipoda (Talitridae), including a new genus and species with notes on its biology. Pac. Insects 1976, 17, 144–154. [Google Scholar]
- Barratt, B.I.P.; Wing, J.M.; Ball, O.J.-P.; Johnstone, P.D.; Dickinson, K.J.M. The effect of fire on terrestrial amphipods (Crustacea: Amphipoda) in a natural grassland community. Pedobiologia 2019, 77, 150590. [Google Scholar] [CrossRef]
- Lawley, J.W.; Gamero-Mora, E.; Maronna, M.M.; Chiaverano, L.M.; Stampar, S.N.; Hopcroft, R.R.; Collins, A.G.; Morandini, A.C. Morphology is not always useful for diagnosis, and that’s ok: Species hypotheses should not be bound to a class of data. Reply to Brown and Gibbons (South African Journal of Science 2022;118(9/10), Art. #12590). S. Afr. J. Sci. 2022, 118, 9–10. [Google Scholar] [CrossRef]
- Richardson, A.M.M.; Jackson, J.E. The first record of a terrestrial landhopper (Crustacea: Amphipoda: Talitridae) from Macquarie Island. Polar Biol. 1995, 15, 419–422. [Google Scholar] [CrossRef]
- Parvizi, E.; McGaughran, A.; Stevens, M.I. Tracking the origins of the introduced terrestrial amphipod, Puhuruhuru patersoni, on sub-Antarctic Macquarie Island. N. Z. J. Zool. 2023, 51, 77–87. [Google Scholar] [CrossRef]
- Fleming, C.A. The Geological History of New Zealand and Its Life; Auckland University Press: Auckland, New Zealand, 1979; p. 141. [Google Scholar]
- Balance, P.F.; Williams, P.W. The geomorphology of Auckland and Northland. In Landforms of New Zealand; Soons, J.M., Selby, M.J., Eds.; Longman Paul: Auckland, New Zealand, 1982; pp. 127–146. [Google Scholar]
- Liu, H.; Zheng, Y.; Zhu, B.; Tong, Y.; Xin, W.; Yang, H.; Jin, P.; Hu, Y.; Huang, M.; Chang, W.; et al. Marine-montane transitions coupled with gill and genetic convergence in extant crustacean. Sci. Adv. 2023, 9, eadg4011. [Google Scholar] [CrossRef]
- Lewis, K.B.; Carter, L.; Davey, F.J. The opening of Cook Strait: Interglacial tidal scour and aligning basins at a subduction to transform plate edge. Mar. Geol. 1994, 116, 293–312. [Google Scholar] [CrossRef]
- Balance, P.F. New Zealand geology: An illustrated guide. Geosci. Soc. N. Z. Misc. Publ. 2009, 148, 128. [Google Scholar]
- Trewick, S.A.; Bland, K.J. Fire and slice: Palaeogeography for biogeography at New Zealand’s North Island/South Island juncture. J. R. Soc. N. Z. 2012, 42, 153–183. [Google Scholar] [CrossRef]
- Nelson, W.; Breitwieser, I.; Fordyce, E.; Bradford-Grieve, J.; Penman, D.; Roskruge, N.; Trnski, T.; Waugh, S.; Webb, C. National Taxonomic Collections in New Zealand; The Royal Society of New Zealand: Wellington, New Zealand, 2015; p. 63. [Google Scholar]
- Schindel, D.E.; Miller, S.E. Provisional nomenclature: The on-ramp to taxonomic names. In Systema Naturae 250: The Linnaean ark; Polaszek, A., Ed.; Taylor & Francis Group: London, UK; New York, NY, USA; CRC Press: Boca Raton, FL, USA, 2010; pp. 109–115. [Google Scholar]
- Grabowski, M.; Mamos, T.; Bącela-Spychalska, K.; Rewicz, T.; Wattier, R.A. Neogene paleogeography provides context for understanding the origin and spatial distribution of cryptic diversity in a widespread Balkan freshwater amphipod. Peer J. 2017, 5, e3016. [Google Scholar] [CrossRef]
- Grabowski, M.; Wysocka, A.; Mamos, T. Molecular species delimitation methods provide new insight into taxonomy of the endemic gammarid species flock from the ancient Lake Ohrid. Zool. J. Linn. Soc. 2017, 181, 272–285. [Google Scholar] [CrossRef]
- Adamowicz, S.J.; Marinone, M.C.; Menu-Marque, S.; Martin, J.W.; Allen, D.C.; Pyle, M.N.; De los Ríos, P.; Sobel, C.N.; Ibañez, C.; Pinto, J.; et al. The Hyalella (Crustacea: Amphipoda) species cloud of the ancient Lake Titicaca originated from multiple colonizations. Mol. Phylogenet. Evol. 2018, 125, 232–242. [Google Scholar] [CrossRef]
- Liu, H.; Li, S.; Ugolini, A.; Momtazi, F. Tethyan closure drove tropical marine biodiversity: Vicariant diversification of intertidal crustaceans. J. Biogeogr. 2018, 45, 941–951. [Google Scholar] [CrossRef]
- Rendoš, M.; Delić, T.; Copilaș-Ciocianu, D.; Fišer, C. First insight into cryptic diversity of a Caucasian subterranean amphipod of the genus Niphargus (Crustacea: Amphipoda: Niphargidae). Zool. Anz. 2021, 290, 1–11. [Google Scholar] [CrossRef]
- Mamos, T.; Jażdżewski, K.; Čiamporová-Zatovičová, Z.; Čiampor, F., Jr.; Grabowski, M. Fuzzy species borders of glacial survivalists in the Carpathian biodiversity hotspot revealed using a multimarker approach. Sci. Rep. 2021, 11, 21629. [Google Scholar] [CrossRef]
- Zapelloni, F.; Pons, J.; Jurado-Rivera, J.A.; Jaume, D.; Juan, C. Phylogenomics of the Hyalella amphipod species-flock of the Andean Altiplano. Sci. Rep. 2021, 11, 366. [Google Scholar] [CrossRef]
- Tong, Y.; Hao, J.; Liu, H.; Li, S.; Hou, Z. Floresorchestia xueli, a new terrestrial crustacean (Amphipoda, Talitridae) from Yunnan, China. Zootaxa 2021, 4991, 318–330. [Google Scholar] [CrossRef]
- Waller, A.; Gonzáles, E.R.; Verdi, A.; Tomasco, I.H. Genus Hyalella (Amphipoda: Hyalellidae) in Humid Pampas: Molecular diversity and a provisional new species. Arthropod Syst. Phylogeny 2022, 80, 261–278. [Google Scholar] [CrossRef]
- Park, E. Understanding the diversity and phylogenetic placements of New Zealand amphipods within a global context. N. Z. J. Mar. Freshw. Res. 2024, 58, 60–72. [Google Scholar] [CrossRef]
- Hupało, K.; Copilaș-Ciocianu, D.; Leese, F.; Weiss, M. Morphology, nuclear SNPs and mate selection reveal that COI barcoding overestimates species diversity in a Mediterranean freshwater amphipod by an order of magnitude. Cladistics 2023, 39, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Poulin, R. Extremely divergent COI sequences within an amphipod species complex: A possible role for endosymbionts? Ecol. Evol. 2022, 12, e9448. [Google Scholar] [CrossRef]
- Hurley, D.E. Transition from water to land in amphipod crustaceans. Am. Zool. 1968, 8, 327–353. [Google Scholar] [CrossRef]
- Buckley, T.R.; Krotsch, M.; Leschen, R.A.B. Evolution of New Zealand insects: Summary and prospectus for future research. Austral Entomol. 2015, 54, 1–27. [Google Scholar] [CrossRef]
- Taylor-Smith, B.; Morgan-Richards, M.; Trewick, S.A. Patterns of regional endemism among New Zealand invertebrates. N. Z. J. Zool. 2020, 47, 1–19. [Google Scholar] [CrossRef]
- Larochelle, A.; Larivière, M.-C. Harpalini (Insecta: Coleoptera: Carabidae: Harpalinae). In Fauna of New Zealand; Manaaki Whenua Press: Lincoln, New Zealand, 2005; Volume 53, p. 160. [Google Scholar]
- Marshall, B.A.; Barker, G.M. A revision of New Zealand landsnails of the genus Cytora Kobelt & Möllendorff, 1897 (Mollusca: Gastropoda: Pupinidae). Tuhinga 2007, 18, 49–113. [Google Scholar]
- Ball, O.J.-P.; Fitzgerald, B.M.; Pohe, S.R.; Whaley, P.T. Effect of plant composition on epigeal spider communities in northern New Zealand forest remnants. N. Z. J. Ecol. 2022, 46, 3480. [Google Scholar] [CrossRef]
- Duncan, K.W. The Systematics, Ecology and Physiology of New Zealand Landhoppers (Crustacea: Amphipoda: Talitridae). Part I: Systematics and Phylogeny. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 1984; p. 343. [Google Scholar]
- Cheng, Y.T.; Nakazono, K.; Lin, Y.K.; Chan, B.K.K. Cryptic diversity of the semi-terrestrial amphipod Platorchestia japonica (Tattersall, 1922) (Amphipoda: Talitrida: Talitridae) in Japan and Taiwan, with description of a new species. Zootaxa 2011, 2787, 1–18. [Google Scholar] [CrossRef]
- Katouzian, A.R.; Sari, A.; Macher, J.N.; Weiss, M.; Saboori, A.; Leese, F.; Weigand, A.M. Drastic underestimation of amphipod biodiversity in the endangered Irano-Anatolian and Caucasus biodiversity hotspots. Sci. Rep. 2016, 6, 22507. [Google Scholar] [CrossRef][Green Version]
- Desiderato, A.; Costa, F.O.; Serejo, C.S.; Abbiati, M.; Queiroga, H.; Vieira, P.E. Macaronesian islands as promoters of diversification in amphipods: The remarkable case of the family Hyalidae (Crustacea, Amphipoda). Zool. Scr. 2019, 48, 359–375. [Google Scholar] [CrossRef]
- Townsend, A.J.; de Lange, P.J.; Duffy, C.A.J.; Miskelly, C.M.; Molloy, J.; Norton, D.A. New Zealand Threat Classification Manual; Science and Technical Publishing Department of Conservation: Wellington, New Zealand, 2008; p. 35. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).