A Molecular-Informed Species Inventory of the Order Ceramiales (Rhodophyta) in the Narragansett Bay Area (Rhode Island and Massachusetts), USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection
2.2. Morphological Examination
2.3. DNA Extraction, Amplification and Sequencing
| Marker | Name | Sequence (5′-3′) | Source |
|---|---|---|---|
| UPA | p23SrV_f1 | GGA CAG AAA GAC CCT ATG AA | [25] |
| p23SrV_r1 | TCA GCC TGT TAT CCC TAG AG | [25] | |
| rbcL-3P | F753 | GGA AGA TAT GTA TGA AAG AGC | [37] |
| rbcLrevNEW | ACA TTT GCT GTT GGA GTY TC | [32] | |
| R1442 | AAA CAT TAG CTG TTG GAG TTT CTA C | [38] |
2.4. Molecular Analysis
2.5. Species Determinations
3. Results
3.1. Sequencing
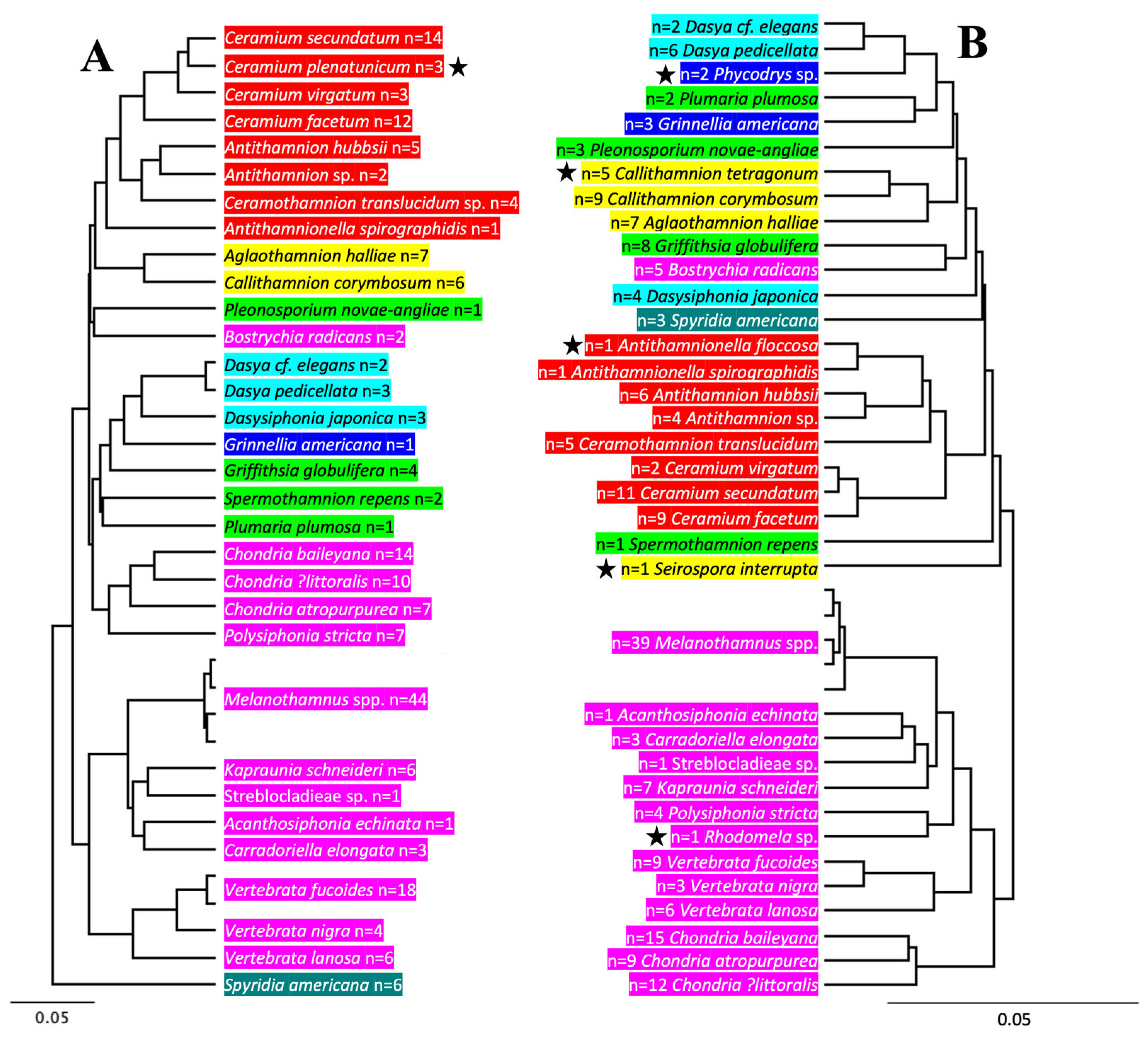
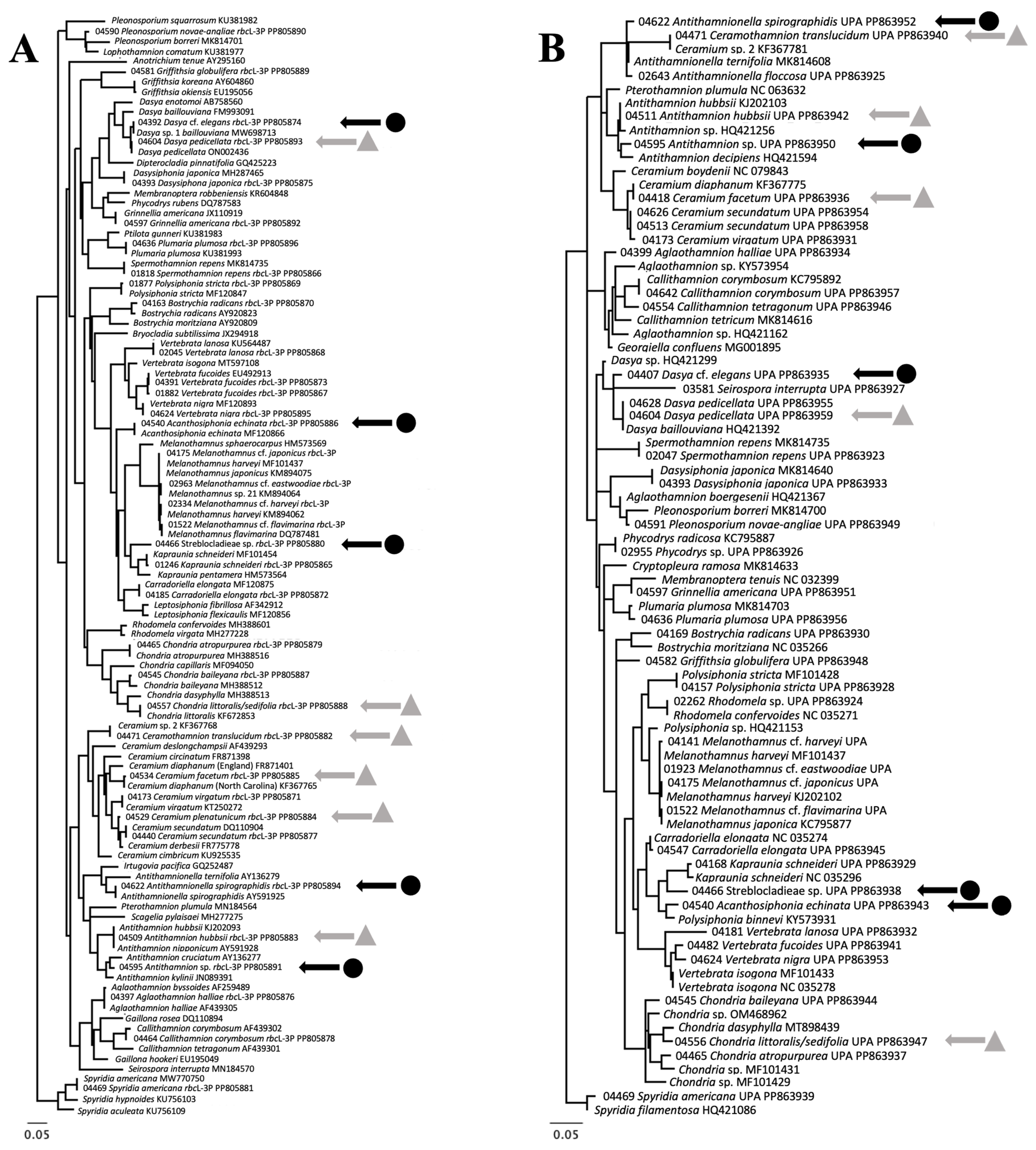
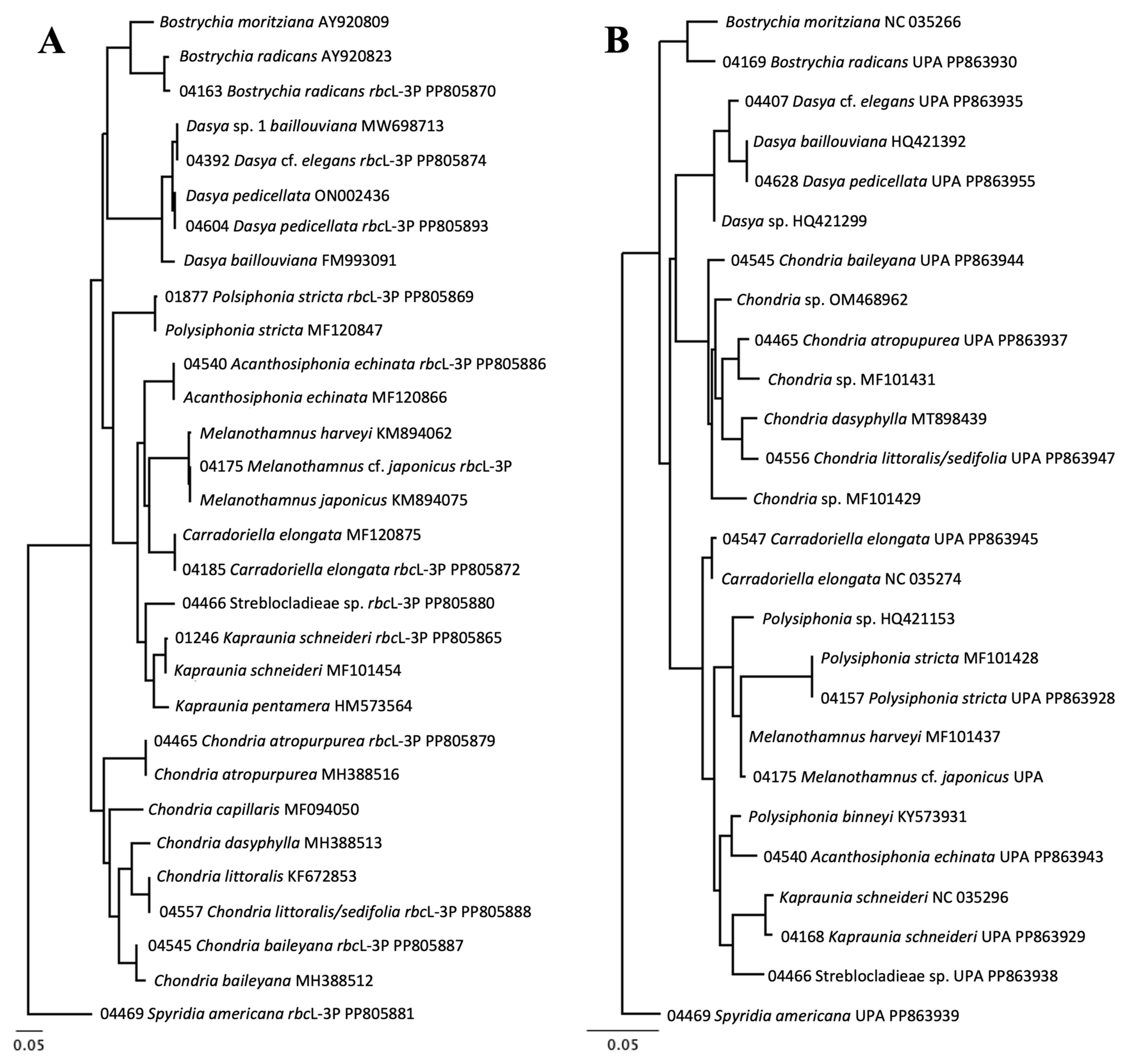
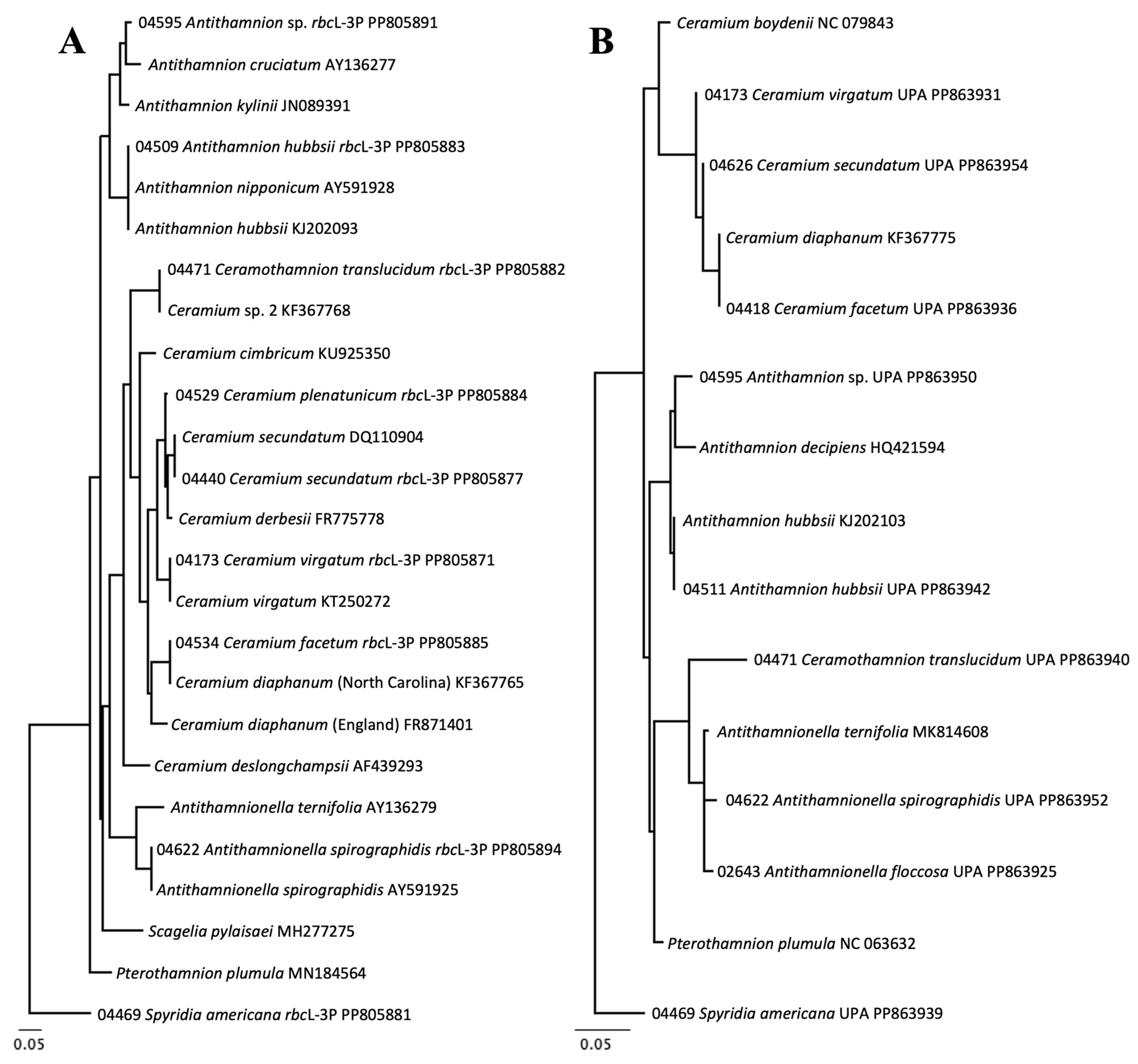
3.2. Molecular Biodiversity of Ceramiales
3.3. Molecular-Assisted Identification of Ceramialean Species in the NBA
3.4. Species Treatments
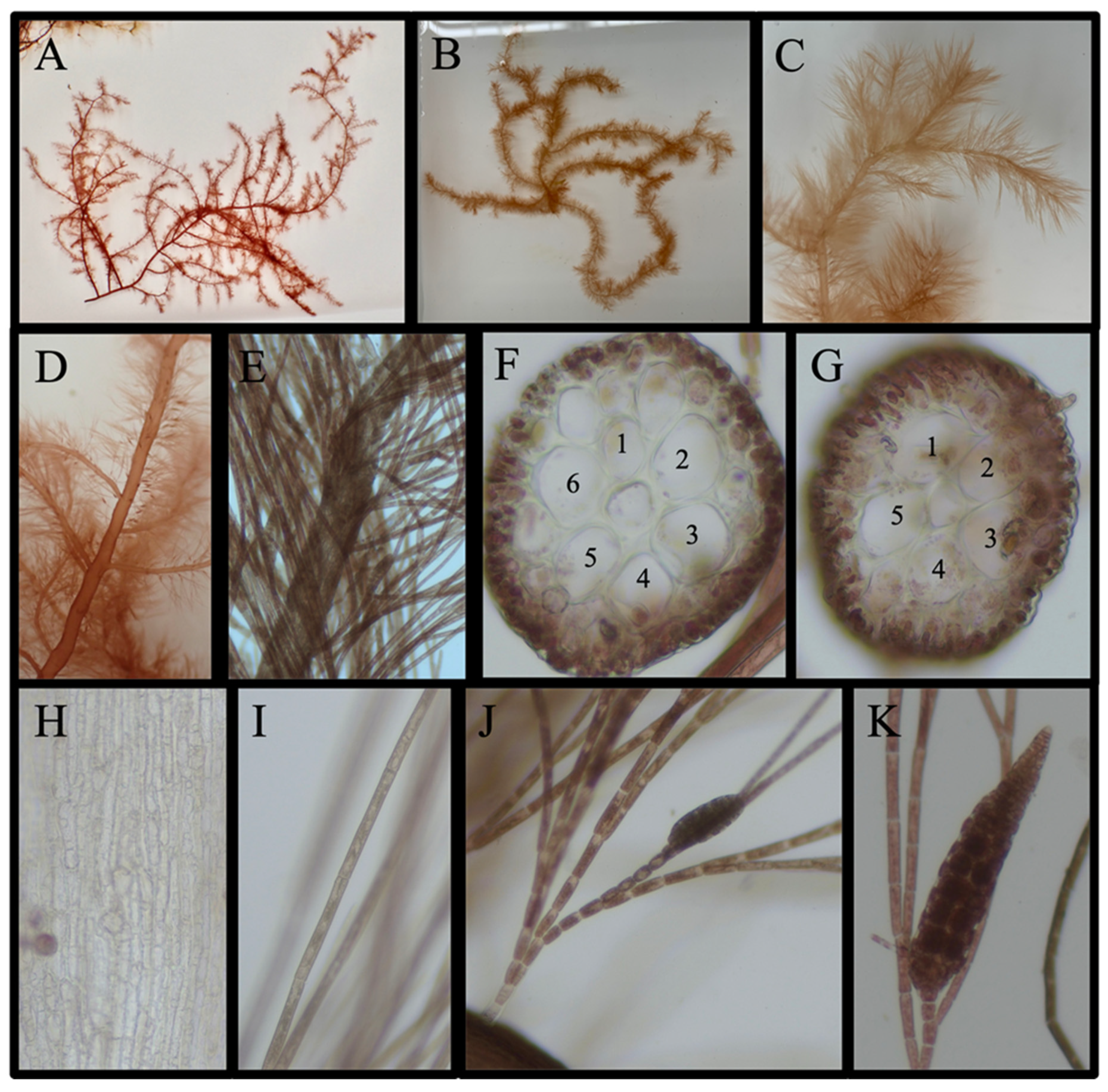
3.4.1. New Reports for the Narragansett Bay Area (5 Species)
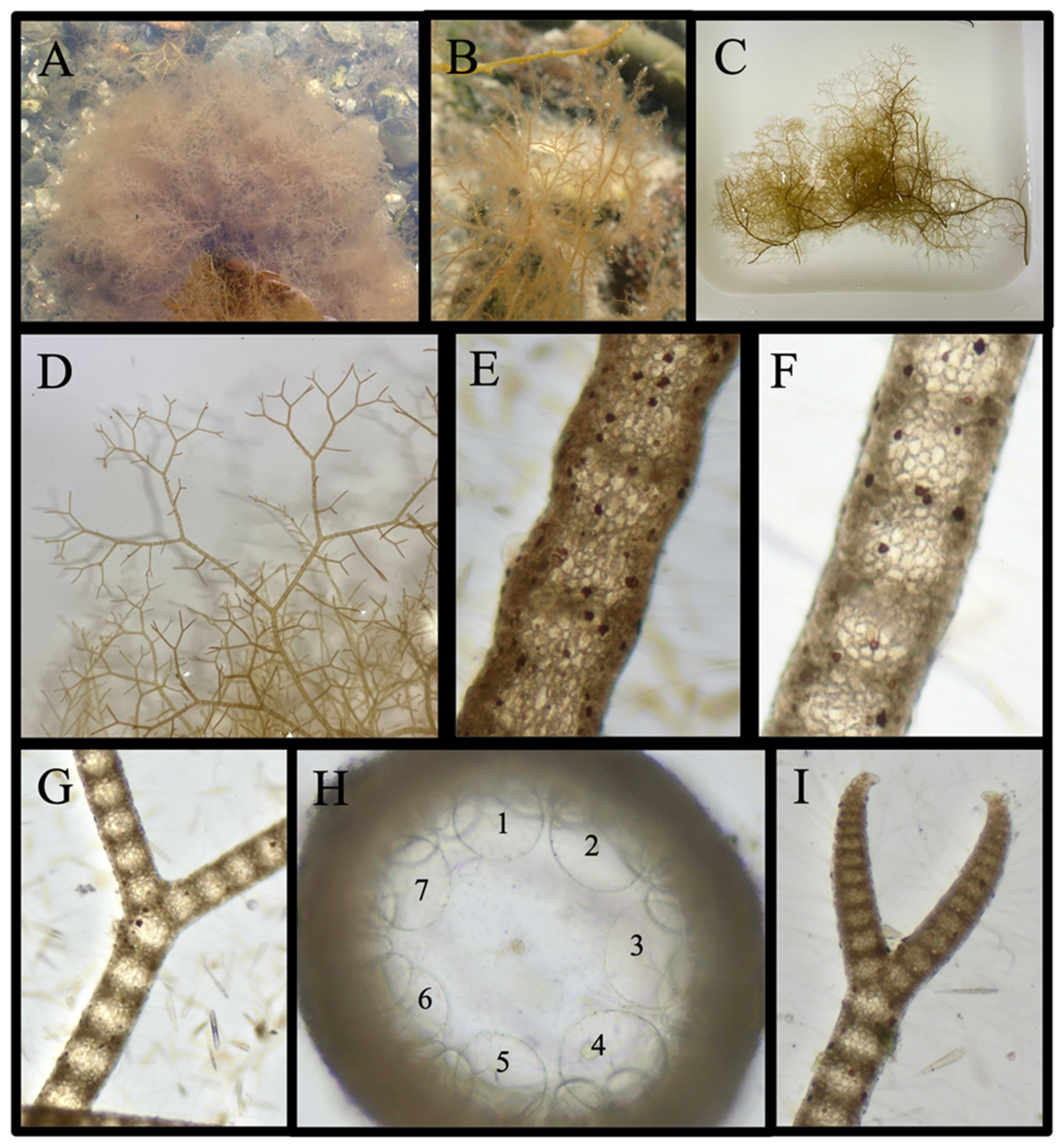
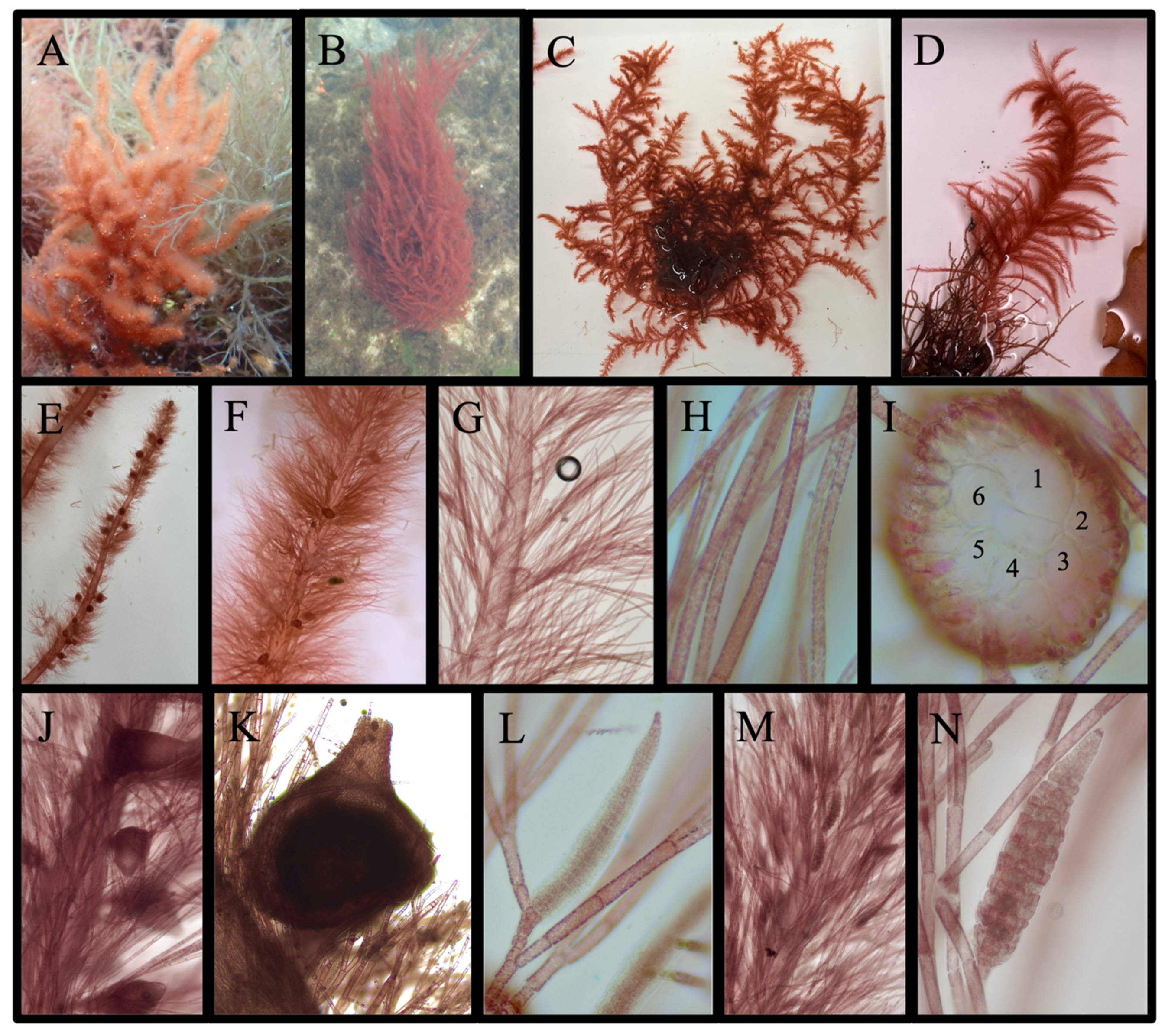
Acanthosiphonia echinata (Harvey) Savoie & G. W. Saunders
Antithamnion sp.
Antithamnionella spirographidis (Schiffner) E. M. Wollaston, 1968
Dasya elegans (G. Martens) C. Agardh
Streblocladieae sp.
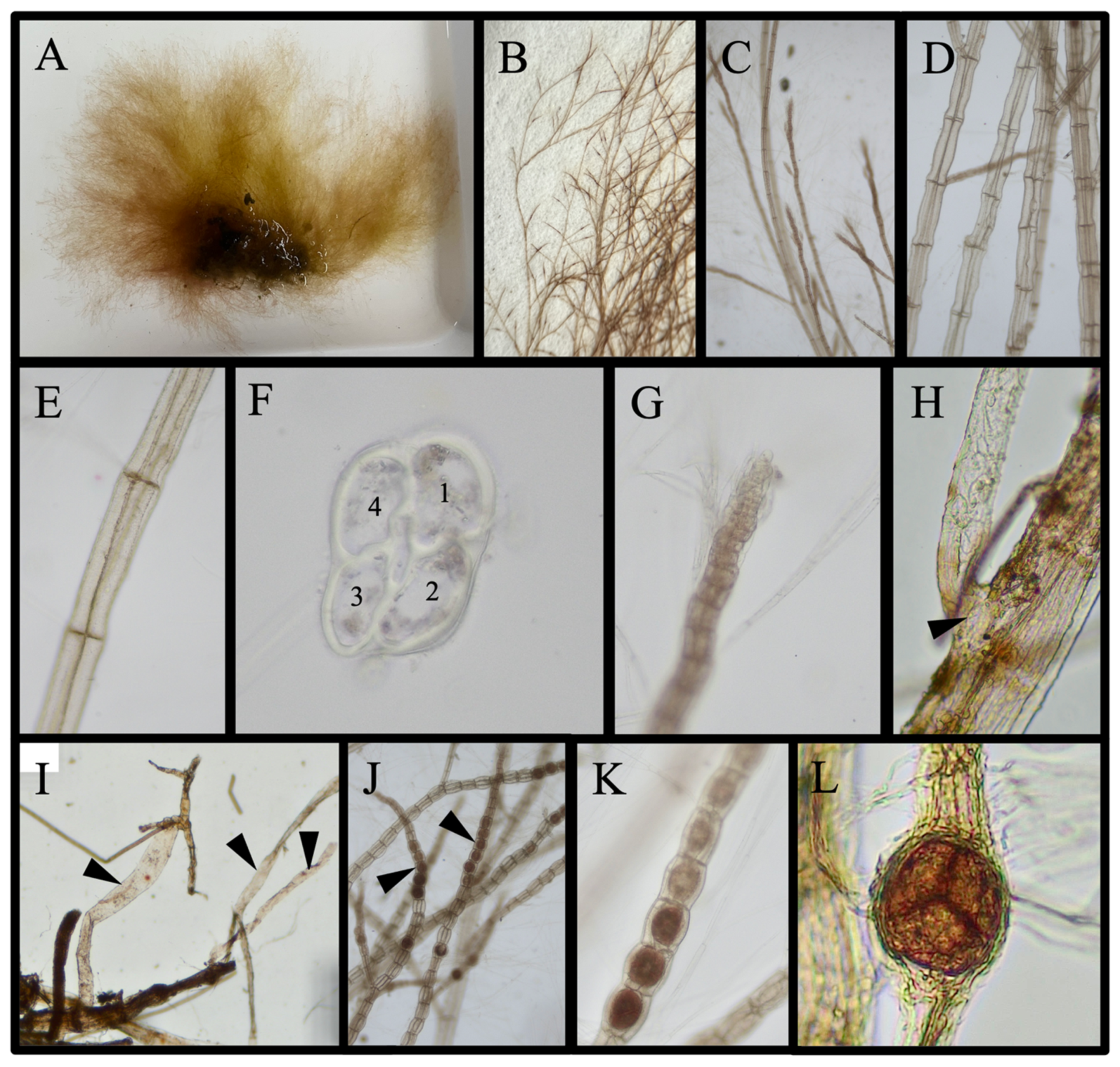
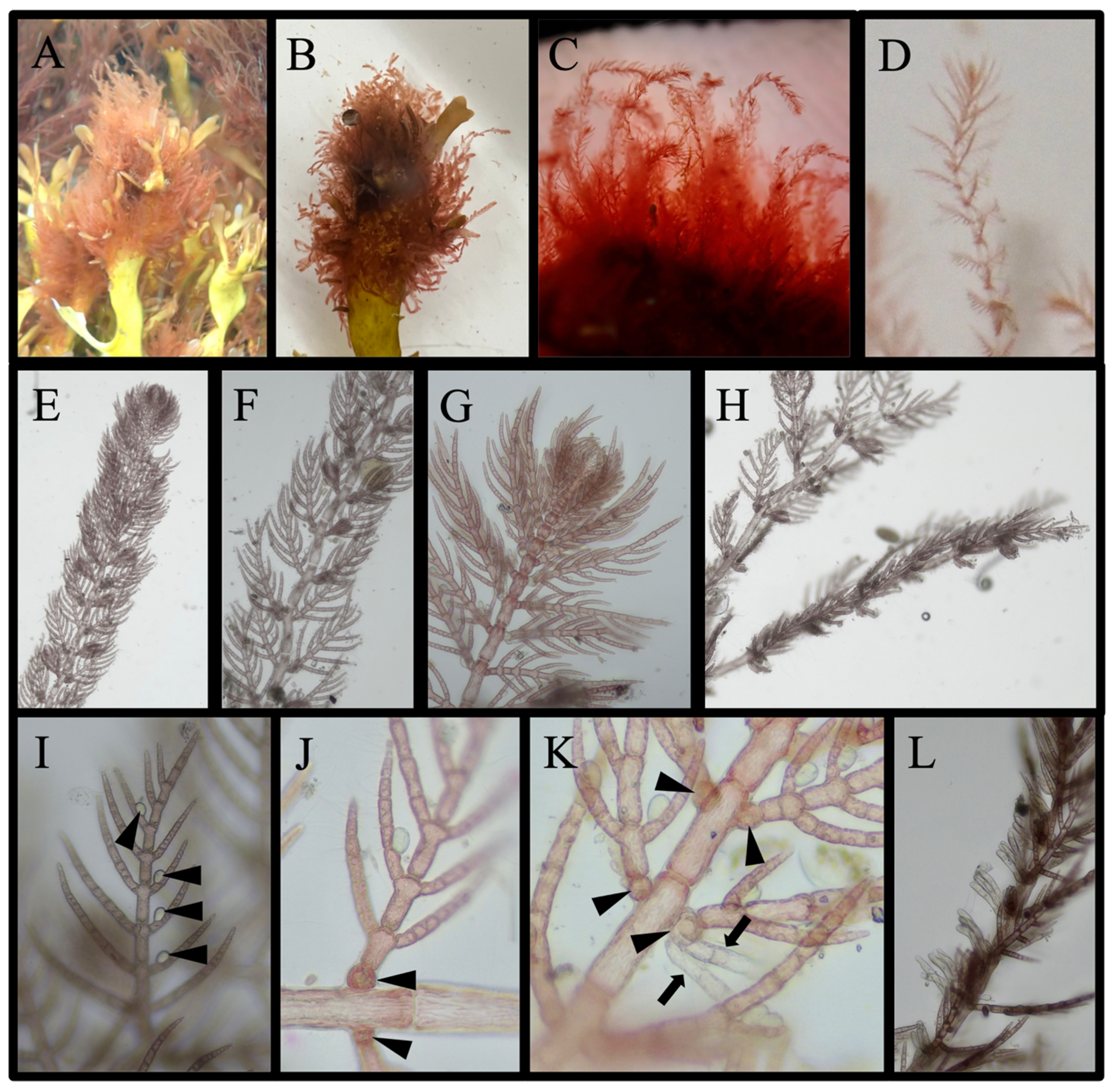
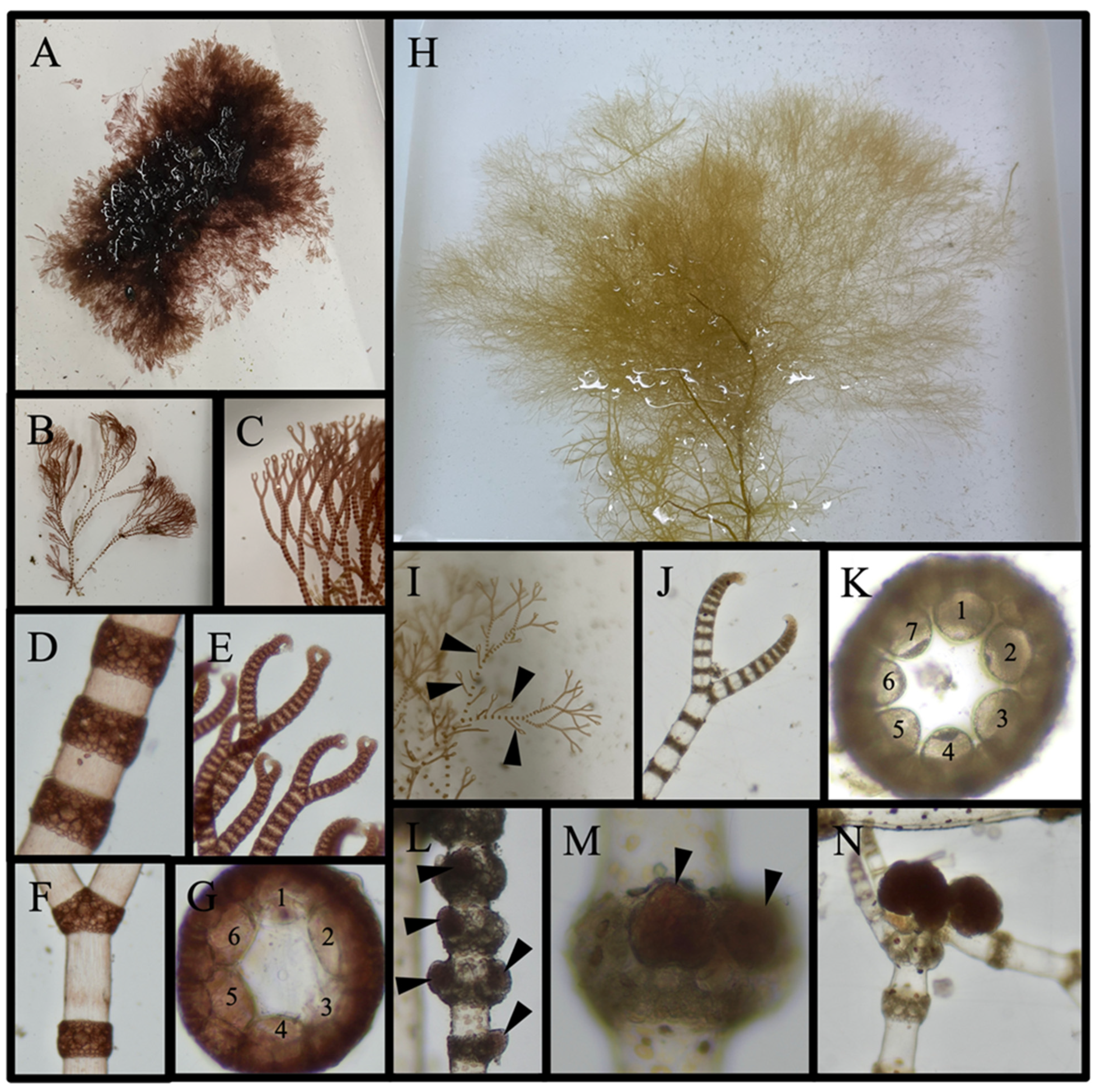
3.4.2. Species with Taxonomic Ambiguities That Are Not New Reports: (6 Species)
Antithamnion hubbsii E. Y. Dawson
Ceramium facetum G.W. Saunders & C. W. Schneider, 2024
Ceramium plenatunicum G. W. Saunders & C. W. Schneider, 2024
Ceramothamnion translucidum G. W. Saunders & C. W. Schneider, 2024
Chondria littoralis Harvey 1853/Chondria sedifolia Harvey 1853
Dasya pedicellata (C. Agardh) C. Agardh, 1824
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streftaris, N.; Zenetos, A. Alien marine species in the Mediterranean-the 100 ‘Worst Invasives’ and their impact. Med. Mar. Sci. 2006, 7, 87–118. [Google Scholar] [CrossRef]
- Lorenti, M.; Gambi, M.C.; Guglielmo, R.; Patti, F.P.; Scipione, M.B.; Zupo, V.; Buia, M.C. Soft-bottom macrofaunal assemblages in the Gulf of Salerno, Tyrrhenian Sea, Italy, an area affected by the invasion of the seaweed Caulerpa racemosa var. cylindracea. Mar. Ecol. 2011, 32, 320–334. [Google Scholar] [CrossRef]
- Cohen, A.N.; Carlton, J.T. Accelerating invasion rate in a highly invaded estuary. Science 1998, 279, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, L.; Cinelli, F. Evaluation of benthic macroalgal invasion in a harbour area of the western Mediterranean Sea. Eur. J. Phycol. 2003, 38, 223–231. [Google Scholar] [CrossRef]
- Palumbi, S.R.; Sandifer, P.A.; Allan, J.D.; Beck, M.W.; Fautin, D.G.; Fogarty, M.J.; Halpern, B.S.; Incze, L.S.; Leong, J.-A.; Norse, E.; et al. Managing for ocean biodiversity to sustain marine ecosystem services. Front. Ecol. Environ. 2009, 7, 204–211. [Google Scholar] [CrossRef]
- Deudero, S.; Blanco, A.; Box, A.; Mateu-Vicens, G.; Cabanellas-Reboredo, M.; Sureda, A. Interaction between the invasive macroalga Lophocladia lallemandii and the bryozoan Reteporella grimaldii at seagrass meadows: Density and physiological responses. Biol. Invasions 2010, 12, 41–52. [Google Scholar] [CrossRef]
- Gravez, V.; Ruitton, S.; Boudouresque, C.F.; Meinesz, A.; Scabbia, G.; Verlaque, M. (Eds.) Fourth International Workshop on Caulerpa taxifolia; GIS Posidonie Publ.: Marseille, France, 2001; p. 406. [Google Scholar]
- Salvaterra, T.; Green, D.S.; Crowe, T.P.; O’Gorman, E.J. Impacts of the invasive alga Sargassum muticum on ecosystem functioning and food web structure. Biol. Invasions 2013, 15, 2563–2576. [Google Scholar] [CrossRef]
- Guy-Haim, T.; Lyons, D.A.; Kotta, J.; Ojaveer, H.; Queirós, A.M.; Chatzinikolaou, E.; Arvanitidis, C.; Como, S.; Magni, P.; Blight, A.J.; et al. Diverse effects of invasive ecosystem engineers on marine biodiversity and ecosystem functions: A global review and meta-analysis. Glob. Chang. Biol. 2018, 24, 906–924. [Google Scholar] [CrossRef]
- Choi, K.H.; Kimmerer, W.; Smith, G.; Ruiz, G.M.; Lion, K. Post-exchange zooplankton in ballast water of ships entering the San Francisco Estuary. J. Plankton Res. 2005, 27, 707–714. [Google Scholar] [CrossRef]
- Godwin, L.S. Hull fouling of maritime vessels as a pathway for marine species invasions to the Hawaiian Islands. Biofouling 2003, 19, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [PubMed]
- Smale, D.A.; Teagle, H.; Hawkins, S.J.; Jenkins, H.L.; Frontier, N.; Wilding, C.; King, N.; Jackson-Bué, M.; Moore, P.J. Climate-driven substitution of foundation species causes breakdown of a facilitation cascade with potential implications for higher trophic levels. J. Ecol. 2022, 110, 2132–2144. [Google Scholar] [CrossRef]
- Williams, S.; Smith, J. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase; World-Wide Electronic Publication, National University of Ireland: Galway, Ireland, 2024; Available online: https://www.algaebase.org (accessed on 24 March 2023).
- Mathieson, A.C.; Dawes, C.J. Seaweeds of the Northwest Atlantic; University of Massachusetts: Amherst, MA, USA, 2017; p. 798. [Google Scholar]
- Piñeiro-Corbeira, C.; Verbruggen, H.; Díaz-Tapia, P. Molecular survey of the red algal family Rhodomelaceae (Ceramiales, Rhodophyta) in Australia reveals new introduced species. J. Appl. Phycol. 2020, 32, 2535–2547. [Google Scholar] [CrossRef]
- Serio, D.; Furnari, G.; Moro, I.; Sciuto, K. Molecular and morphological characterisation of Melanothamnus testudinis sp. nov. (Rhodophyta, Rhodomelaceae) and its distinction from Polysiphonia carettia. Phycologia 2020, 59, 281–291. [Google Scholar]
- Cassano, V.; Santos, G.D.N.; Pestana, E.M.D.S.; Nunes, J.M.D.C.; Oliveira, M.C.; Fujii, M.T. Laurencia longiramea sp. nov. for Brazil and an emendation of the generic delineation of Corynecladia (Ceramiales, Rhodophyta). Phycologia 2019, 58, 115–127. [Google Scholar]
- Robba, L.; Russell, S.J.; Barker, G.L.; Brodie, J. Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). Am. J. Bot. 2006, 93, 1101–1108. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 1879–1888. [Google Scholar]
- Cianciola, E.N.; Popolizio, T.R.; Schneider, C.W.; Lane, C.E. Using molecular-assisted alpha taxonomy to better understand red algal biodiversity in Bermuda. Diversity 2010, 2, 946–958. [Google Scholar] [CrossRef]
- Wolf, M.A.; Sciuto, K.; Betto, V.M.; Moro, I.; Maggs, C.A.; Sfriso, A. Updating Ceramium (Rhodophyta, Ceramiales) biodiversity in the North Adriatic Sea (Mediterranean): Ceramium rothianum sp. nov. and rediscovery of three forgotten species. Eur. J. Phycol. 2019, 54, 571–584. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Kurihara, A.; Conklin, K.Y.; Sauvage, T.; Presting, G.G. The Hawaiian Rhodophyta Biodiversity Survey (2006–2010): A summary of principal findings. BMC Plant Biol. 2010, 10, 258. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Idol, J.N.; Parham, S.L.; Fernández-García, C.; León, N.; Gabrielson, P.W.; Wysor, B. Molecular assisted identification reveals hidden red algae diversity from the Burica Peninsula, Pacific Panama. Diversity 2017, 9, 19. [Google Scholar] [CrossRef]
- Gabriel, D.; Ferreira, A.I.; Micael, J.; Fredericq, S. Non-Native Marine Macroalgae of the Azores: An Updated Inventory. Diversity 2023, 15, 1089. [Google Scholar] [CrossRef]
- Bast, F.; Bhushan, S.; Ahmad John, A. Brown barcoded as red but reality is green! How epiphytic green algae confuse phycologists? Webbia 2015, 70, 59–63. [Google Scholar] [CrossRef]
- Zangaro, F.; Saccomanno, B.; Tzafesta, E.; Bozzeda, F.; Specchia, V.; Pinna, M. Current limitations and future prospects of detection and biomonitoring of NIS in the Mediterranean Sea through environmental DNA. NeoBiota 2021, 70, 151–165. [Google Scholar] [CrossRef]
- Sherwood, A.R.; Presting, G.G. Universal primers amplify a 23S rDNA plastid marker in eukaryotic algae and cyanobacteria. J. Phycol. 2007, 43, 605–608. [Google Scholar] [CrossRef]
- Freshwater, D.W.; Tudor, K.; O’shaughnessy, K.; Wysor, B. DNA barcoding in the red algal order Gelidiales: Comparison of COI with rbcL and verification of the “barcoding gap”. Cryptogam. Algol. 2010, 3, 435–449. [Google Scholar]
- Saunders, G.W.; Moore, T.E. Refinements for the amplification and sequencing of red algal DNA barcode and RedToL phylogenetic markers: A summary of current primers, profiles and strategies. Algae 2013, 28, 31–43. [Google Scholar] [CrossRef]
- Davidson, A.D.; Campbell, M.L.; Hewitt, C.L.; Schaffelke, B. Assessing the impacts of nonindigenous marine macroalgae: An update of current knowledge. Bot. Mar. 2015, 58, 55–79. [Google Scholar] [CrossRef]
- Sears, J.R. NEAS Keys to Benthic Marine Algae of the Northeastern Coast of North America from Long Island Sound to the Strait of Belle Isle; Northeast Algal Society Contr. No. 2; Univ. Mass. Dartmouth Campus Book Store: Dartmouth, MA, USA, 2002; pp. 1–161. [Google Scholar]
- Villalard-Bohnsack, M. Illustrated Key to the Seaweeds of New England; Rhode Island Natural History Survey: South Kingstown, RI, USA, 2003; pp. 1–149. [Google Scholar]
- Saunders, G.W. NEAS Keys to the Benthic Marine Algae of the Northwest Atlantic and Canadian Arctic from Long Island Sound to Cambridge Bay, 3rd ed.; With the Description of Eight New Species; Northeast Algal Society Contr. No. 3; Northeast Algal Society: Bronx, NY, USA, 2024; pp. 1–87. [Google Scholar]
- Hommersand, M.H.; Fredericq, S.; Freshwater, D.W. Phylogenetic systematics and biogeography of the Gigartinaceae (Gigartinales, Rhodophyta) based on sequence analysis of rbcL. Bot. Mar. 1994, 37, 193–203. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, S.Y.; Nelson, W. Symphyocladia lithophila sp. nov. (Rhodomelaceae, Ceramiales), a new Korean red algal species based on morphology and rbcL sequences. Bot. Mar. 2010, 53, 233–241. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Molec. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.R.; Saunders, G.W. A molecular-assisted investigation of diversity, biogeography and phylogenetic relationships for species of Neoptilota and Ptilota (Wrangeliaceae, Rhodophyta) reported along Canadian coasts. Phycologia 2017, 56, 36–53. [Google Scholar] [CrossRef]
- Savoie, A.M.; Saunders, G.W. Evidence for the introduction of the Asian red alga Neosiphonia japonica and its introgression with Neosiphonia harveyi (Ceramiales, Rhodophyta) in the Northwest Atlantic. Mol. Ecol. 2015, 24, 5927–5937. [Google Scholar] [CrossRef]
- Schneider, C.W.; Wynne, M.J.; Saunders, G.W. On the nomenclatural reinstatement and lectotypification of Spyridia americana Durant (1850). Bot. Mar. 2021, 64, 221–225. [Google Scholar] [CrossRef]
- Schneider, C.W.; Saunders, G.W. Correcting an historical oversight: Chondria atropurpurea Harvey (Rhodomelaceae, Rhodophyta) is present in the northeastern North American flora. Not. Algarum 2023, 279, 1–5. [Google Scholar]
- Schneider, C.W.; Saunders, G.W. Australasian Lophothamnion J. Agardh aligns genetically with Pleonosporium Nägeli (Wrangeliaceae, Spongoclonieae): New species from the western Atlantic. Cryptogam. Algol. 2024, 45, 1–10. [Google Scholar]
- Vis, M.L.; Necchi Jr, O.; Chiasson, W.B.; Entwisle, T.J. Molecular phylogeny of the genus Kumanoa (Batrachospermales, Rhodophyta). J. Phycol. 2012, 48, 750–758. [Google Scholar] [CrossRef]
- Lyra Gde, M.; Costa Eda, S.; de Jesus, P.B.; de Matos, J.C.; Caires, T.A.; Oliveira, M.C.; Oliveira, E.C.; Xi, Z.; Nunes, J.M.; Davis, C.C. Phylogeny of Gracilariaceae (Rhodophyta): Evidence from plastid and mitochondrial nucleotide sequences. J Phycol. 2015, 51, 356–366. [Google Scholar] [CrossRef]
- Savoie, A.M.; Saunders, G.W. A molecular assessment of species diversity and generic boundaries in the red algal tribes Polysiphonieae and Streblocladieae (Rhodomelaceae, Rhodophyta) in Canada. Eur. J. Phycol. 2019, 54, 1–25. [Google Scholar]
- Wolf, M.A.; Buosi, A.; Sfriso, A. First record of Acanthosiphonia echinata (Rhodomelaceae, Rhodophyta) in the Mediterranean Sea, molecular and morphological characterization. Bot. Mar. 2020, 63, 241–245. [Google Scholar] [CrossRef]
- Bustamante, D.E.; Won, B.Y.; Cho, T.O. First record of Neosiphonia echinata (Rhodomelaceae, Rhodophyta) in the South Pacific: An introduced species in Southeast Asia. Bot. Mar. 2015, 58, 345–354. [Google Scholar] [CrossRef]
- Mamoozadeh, N.R.; Freshwater, D.W. Taxonomic notes on Caribbean Neosiphonia and Polysiphonia (Ceramiales, Florideophyceae): Five species from Florida, USA and Mexico. Bot. Mar. 2011, 54, 269–292. [Google Scholar] [CrossRef]
- Cho, T.O.; Yeon Won, B.; Fredericq, S. Antithamnion nipponicum (Ceramiaceae, Rhodophyta), incorrectly known as A. pectinatum in western Europe, is a recent introduction along the North Carolina and Pacific coasts of North America. Eur. J. Phycol. 2005, 40, 323–335. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G.; Serio, D. Flora marina bentonica del Mediterraneo: Rhodophyta—Rhodymeniophycidae III: Ceramiales I (Rhodomelaceae escluse). Bull. Gioenia Acad. Nat. Sci. Catania 2023, 56, FP81–FP615. [Google Scholar] [CrossRef]
- Maggs, C.A.; Stegenga, H. Red algal exotics on North Sea coasts. Helgoländer Meeresunters. 1998, 52, 243–258. [Google Scholar]
- Stegenga, H.; Prud’homme Van Reine, W.F. Changes in the seaweed flora of the Netherlands. Changes Mar. Flora North Sea 1998, 7, 7–8. [Google Scholar]
- Verlaque, M.; Ruitton, S.; Mineur, F.; Boudouresque, C.F. CIESM atlas of exotic species in the Mediterranean: 4. Macrophytes 2009. Available online: https://www.ciesm.org/atlas/appendix4.html (accessed on 25 August 2024).
- Pena-Martín, C.; Gómez-Garreta, A.; Crespo, M.B. Proposals to conserve or reject names. Taxon 2016, 65, 882–883. [Google Scholar] [CrossRef]
- Lam, D.W.; García-Fernández, M.E.; Aboal, M.; Vis, M.L. Polysiphonia subtilissima (Ceramiales, Rhodophyta) from freshwater habitats in North America and Europe is confirmed as conspecific with marine collections. Phycologia 2013, 52, 156–160. [Google Scholar] [CrossRef]
- Curiel, D.; Marzocchi, M.; Bellemo, G. First report of fertile Antithamnion pectinatum (Ceramiales, Rhodophyceae) in the North Adriatic Sea (Lagoon of Venice, Italy). Bot. Mar. 1996, 39, 19–22. [Google Scholar] [CrossRef]
- Verlaque, M. Checklist of the macroalgae of Thau Lagoon (Hérault, France), a hot spot of marine species introduction in Europe. Oceanol. Acta 2001, 24, 29–49. [Google Scholar] [CrossRef]
- Athanasiadis, A. Typification of Antithamnion nipponicum Yamada et Inagaki (Antithamnieae, Ceramioideae, Ceramiaceae, Ceramiales, Rhodophyta). Bot. Mar. 2009, 52, 256–261. [Google Scholar] [CrossRef]
- Verlaque, M.; Riouall, R. Introduction de Polysiphonia nigrescens et d’Antithamnion nipponicum Rhodophyta, Ceramiales sur le littoral méditerranéen français. Cryptogamie. Algol. 1989, 10, 313–323. [Google Scholar]
- Foertch, J.R.; Swenarton, J.; Keser, M. Introduction of a new Antithamnion (cf. nipponicum) to Long Island Sound. In Proceedings of the 48th Northeast Algal Symposium, Amherst, MA, USA, 17–19 April 2009; University of Massachusetts: Amherst, MA, USA, 1991; p. 21. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Saunders, G.W.; Schneider, C.W. Taxonomic addendum. In NEAS Keys to the Benthic Marine Algae of the Northwest Atlantic and Canadian Arctic from Long Island Sound to Cambridge Bay, 3rd ed.; With the Description of Eight New Species; Northeast Algal Society Contr. No. 3; Saunders, G.W., Ed.; Northeast Algal Society: Bronx, NY, USA, 2024; pp. 63–69. [Google Scholar]
- Barros-Barreto, M.B.; Jaramillo, M.A.; Hommersand, M.H.; Ferreira, P.C.G.; Maggs, C.A. Phylogenetic analysis of the red algal tribe Ceramieae reveals multiple morphological homoplasies but defines new genera. Cryptogam. Algol. 2023, 44, 13–58. [Google Scholar] [CrossRef]
- Schneider, C.W.; Suyemoto, M.M.; Yarish, C. An annotated checklist of Connecticut seaweeds. Bull. Connecticut State Geol. Nat. Hist. Survey 1979, 108, 1–20. [Google Scholar]
- Dawes, C.J.; Mathieson, A.C. The Seaweeds of Florida; University Press of Florida: Gainesville, FL, USA, 2008; 592p. [Google Scholar]
- Agardh, C.A. Systema Algarum; Lundae: Lund, Sweden; Literis Berlingianis: Berlin, Germany, 1824; pp. 1–312. [Google Scholar]
- Carlton, J.T. Transoceanic and interoceanic dispersal of coastal marine organisms: The biology of ballast water. Oceanogr. Mar. Biol. Ann. Rev. 1985, 23, 313–371. [Google Scholar]
- Carlton, J.T. Man’s role in changing the face of the ocean: Biological invasions and implications for conservation of near-shore environments. Cons. Biol. 1989, 3, 265–273. [Google Scholar] [CrossRef]
- Ellegaard, M.; Ribeiro, S. The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’. Biol. Rev. Camb Philos. Soc. 2018, 93, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Gollasch, S.; MacDonald, E.; Belson, S.; Botnen, H.; Christensen, J.T.; Hamer, J.P.; Houvvenaghel, G.; Jelmert, A.; Luca, I.; Masson, D.; et al. Life in Ballast Tanks. In Invasive Aquatic Species of Europe, Distribution, Impact and Management; Leppakoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 217–231. [Google Scholar]
- Hallegraeff, G.M.; Bolch, C.J. Transport of toxic dinoflagellate cysts in ship’s ballast water. Mar. Pollut. Bull. 1991, 22, 27–30. [Google Scholar] [CrossRef]
- McCarthy, H.; Crowder, L. An overlooked scale of global transport: Phytoplankton species richness in ship’s ballast water. Biol. Invas. 2000, 2, 321–322. [Google Scholar] [CrossRef]
- Smith, L.D.; Wonham, M.J.; McCann, L.D.; Ruize, G.M.; Hines, A.H.; Carlton, J.T. Invasion pressure to a ballast-flooded estuary and an assessment of inoculant survival. Biol. Invas. 1999, 1, 67–87. [Google Scholar] [CrossRef]
- Ware, C.; Berge, J.; Sundet, J.H.; Kirkpatrick, J.B.; Coutts, A.D.M.; Jelmert, A.; Olsen, S.M.; Floerl, O.; Wisz, M.S.; Alsos, I.G.; et al. Climate change, non-indigenous species and shipping: Assessing the risk of species introduction to a high-Arctic archipelago. Divers. Distrib. 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Flagella, M.M.; Verlaque, M.; Soria, A.; Buia, M.C. Macroalgal survival in ballast water tanks. Mar. Pollut. Bull. 2007, 54, 1395–1401. [Google Scholar] [CrossRef]
- Flagella, M.M.; Andreakis, N.; Hiraoka, M.; Verlaque, M.; Buia, M.C. Identification of cryptic Ulva species (Chlorophyta, Ulvales) transported by ballast water. J. Biol. Res. Thessalon. 2010, 13, 47–57. [Google Scholar]
- Zaiko, A.; Martinez, J.L.; Schmidt-Petersen, J.; Ribicic, D.; Samuiloviene, A.; Garcia-Vazquez, E. Metabarcoding approach for the ballast water surveillance—An advantageous solution or an awkward challenge? Mar. Pollut. Bull. 2015, 92, 25–34. [Google Scholar] [CrossRef] [PubMed]







| Reagent | Reaction Volume (mL) | Final PCR Concentration |
|---|---|---|
| BioLine 2× MyTaq Red HS Mix | 10 | 1× |
| 10 mM Forward Primer | 1 | 0.5 mM |
| 10 mM Reverse Primer | 1 | 0.5 mM |
| 5M Betaine | 4 | 1 M |
| PCR Water | 3 | -- |
| DNA Template | 1 | -- |
| PCR Stage | Temperature (°C) | Time (min) | # Cycles | |
|---|---|---|---|---|
| Initial Denature | 95 | 5 | 1 | |
| Cycle Start | Denature | 95 | 0.5 | 35 |
| Annealing | 50 | 0.5 | 35 | |
| Cycle End | Extension | 72 | 1.5 | 35 |
| Final Extension | 72 | 5 | 1 |
| Primer Pair | PCR | Sequencing | #Sequences |
|---|---|---|---|
| UPA-F/UPA-R | 185/198 (93.4%) | 136/172 (79.1%) | 133 |
| F753/R1442 | 204/294 (69.4%) | 101/169 (59.8%) | 91 |
| F753/rbcLrevNEW | 48/61 (78.7%) | 17/31 (54.8%) | 17 |
| Family | Species | # of rbcL-3P | # of UPA | Overlap | rbcL-3P Acc. # | rbcL-3P MARI # | UPA Acc. # | UPA MARI # |
|---|---|---|---|---|---|---|---|---|
| Callithamniaceae—4 spp. | 13 | 22 | 13 | |||||
| Aglaothamnion halliae | 7 | 7 * | 7 | PP805876 | MARI-04397 | PP862934 | MARI-04399 | |
| Callithamnion corymbosum | 6 | 9 | 6 | PP805878 | MARI-04464 | PP862957 | MARI-04642 | |
| Callithamnion tetragonum | 0 | 5 * | 0 | --- | --- | PP862946 | MARI-04554 | |
| Seirospora interrupta | 0 | 1 * | 0 | --- | --- | PP862927 | MARI-03581 | |
| Ceramiaceae—9 spp. | 44 | 39 | 29 | |||||
| Antithamnion hubbsii | 5 | 6 | 4 | PP805883 | MARI-04509 | PP862942 | MARI-04511 | |
| Antithamnion sp. | 2 * | 3 * | 2 | PP805891 | MARI-04595 | PP862950 | MARI-04595 | |
| Antithamnionella floccosa | 0 | 1 * | 0 | --- | --- | PP862925 | MARI-02643 | |
| Antithamnionella spirographidis | 1 * | 1 * | 1 | PP805894 | MARI-04622 | PP862952 | MARI-04622 | |
| Ceramium facetum | 12 | 9 | 8 | PP805885 | MARI-04534 | PP862936 | MARI-04418 | |
| Ceramium plenatunicum | 3 | 0 | 0 | PP805884 | MARI-04529 | --- | --- | |
| Ceramium secundatum | 14 | 11 * | 9 | PP805877 | MARI-04440 | PP862954, PP862958 | MARI-04626, MARI-04513 | |
| Ceramium virgatum | 3 | 2 * | 2 | PP805871 | MARI-04173 | PP862931 | MARI-04173 | |
| Ceramothamnion translucidum | 4 | 5 | 3 | PP805882 | MARI-04471 | PP862940 | MARI-04471 | |
| Dasyaceae—3 spp. | 8 | 14 | 8 | |||||
| Dasya cf. elegans | 2 | 2 | 2 | PP805874 | MARI-04392 | PP862935 | MARI-04407 | |
| Dasya pedicellata | 3 | 6 | 3 | PP805893 | MARI-04604 | PP862955, PP862959 | MARI-04628, MARI-04604 | |
| Dasysiphonia japonica | 3 | 4 | 3 | PP805875 | MARI-04393 | PP862933 | MARI-04393 | |
| Delesseriaceae—2 spp. | 1 | 5 | 1 | |||||
| Grinnellia americana | 1 | 3 * | 1 | PP805892 | MARI-04597 | PP862951 | MARI-04597 | |
| Phycodrys sp. | 0 | 2 * | 0 | --- | --- | PP862926 | MARI-02955 | |
| Rhodomelaceae – 14 spp. | 123 | 115 | 81 | |||||
| Acanthosiphonia echinata | 1 | 1 * | 1 | PP805886 | MARI-04540 | PP862943 | MARI-04540 | |
| Bostrychia radicans | 2 | 5 * | 2 | PP805870 | MARI-04163 | PP862930 | MARI-04169 | |
| Carradoriella elongata | 3 | 3 | 2 | PP805872 | MARI-04185 | PP862945 | MARI-04547 | |
| Chondria atropurpurea | 7 | 9 * | 5 | PP805879 | MARI-04465 | PP862937 | MARI-04465 | |
| Chondria baileyana | 14 | 15 * | 10 | PP805887 | MARI-04545 | PP862944 | MARI-04545 | |
| Chondria littoralis/sedifolia | 10 | 12 * | 10 | PP805888 | MARI-04557 | PP862947 | MARI-04556 | |
| Kapraunia schneideri | 6 | 7 | 5 | PP805865 | MARI-01246 | PP862929 | MARI-04168 | |
| Melanothamnus spp. | 44 | 39 | 29 | --- | --- | --- | --- | |
| Polysiphonia stricta | 7 | 4 | 1 | PP805869 | MARI-02324 | PP862928 | MARI-04157 | |
| Rhodomela sp. | 0 | 1 | 0 | --- | --- | PP862924 | MARI-02262 | |
| Streblocladieae sp. | 1 * | 1 * | 1 | PP805880 | MARI-04466 | PP862938 | MARI-04466 | |
| Vertebrata fucoides | 18 | 9 * | 7 | PP805867, PP805873 | MARI-01882, MARI04391 | PP862941 | MARI-04482 | |
| Vertebrata lanosa | 6 | 6 | 5 | PP805868 | MARI-02045 | PP862932 | MARI-04181 | |
| Vertebrata nigra | 4 | 3 * | 3 | PP805895 | MARI-04624 | PP862953 | MARI-04624 | |
| Spyridiaceae—1 sp. | 6 | 3 | 3 | |||||
| Spyridia americana | 6 | 3 * | 3 | PP805881 | MARI-04469 | PP862939 | MARI-04469 | |
| Wrangeliaceae—4 spp. | 8 | 14 | 7 | |||||
| Griffithsia globulifera | 4 * | 8 * | 4 | PP805889 | MARI-4581 | PP862948 | MARI-04582 | |
| Pleonosporium novae-angliae | 1 | 3 * | 1 | PP805890 | MARI-04590 | PP862949 | MARI-04591 | |
| Plumaria plumosa | 1 | 2 | 1 | PP805896 | MARI-04636 | PP862956 | MARI-04636 | |
| Spermothamnion repens | 2 | 1 | 1 | PP805866 | MARI-01818 | PP862923 | MARI-02047 | |
| Family Species | rbcL-3P (% ID) | Nearest BLAST | UPA (% ID) | Nearest BLAST | rbcL-3P Intra. Div. | UPA Intra. Div |
|---|---|---|---|---|---|---|
| Callithamniaceae—4 spp. | ||||||
| Aglaothamnion halliae | 100% | Aglaothamnion halliae AF439305 | 98.10% | Aglaothamnion sp. KY573954 | 0% | 0% |
| Callithamnion corymbosum | 99.40% | Callithamnion corymbosum DQ110896 | 100% | Callithamnion corymbosum KC795892 | 0–0.30% | 0–0.28% |
| Callithamnion tetragonum | --- | --- | 97.83% | Callithamnion tetragonum MK814616 | --- | 0% |
| Seirospora interrupta | --- | --- | 94.86% | Cryptopleura ramosa MK814633 | --- | --- |
| Ceramiaceae—9 spp. | ||||||
| Antithamnion hubbsii | 100% | Antithamnion hubbsii KJ202093 | 100% | Antithamnion hubbsii KJ202103 | 0% | 0% |
| Antithamnion sp. | 96.40% | Antithamnion kylinii JN089393 | 98.37% | Antithamnion hubbsii KJ202103 | 0% | 0% |
| Antithamnionella floccosa | --- | --- | 99.19% | Antithamnionella ternifolia MK814608 | --- | --- |
| Antithamnionella spirographidis | 100% | Antithamnionella spirographidis DQ022810 | 99.19% | Antithamnionella ternifolia MK814608 | --- | --- |
| Ceramium facetum | 99.85% | Ceramium diaphanum KF367765 | 100% | Ceramium diaphanum KF367765 | 0–0.07% | 0–0.28% |
| Ceramium plenatunicum | 98.80% | Ceramium derbesii FR775779 | --- | --- | 0% | --- |
| Ceramium secundatum | 100% | Ceramium secundatum DQ110904 | 98.92% | Ceramium diaphanum KF367775 | 0% | 0% |
| Ceramium virgatum | 100% | Ceramium virgatum KT250272 | 98.38% | Ceramium diaphanum KF367775 | 0.07–0.22% | 0% |
| Ceramothamnion translucidum | 100% | “Ceramium sp. 2” KF367768 | 100% | “Ceramium sp. 2” KF367781 | 0–0.15% | 0% |
| Dasyaceae—3 spp. | ||||||
| Dasya cf. elegans | 100% | “Dasya sp. 1 baillouviana” MW698713 | 98.64% | Dasya sp. HQ421299 | 0% | 0% |
| Dasya pedicellata | 100% | Dasya pedicellata ON002436 | 100% | Dasya baillouviana HQ421392 | 0% | 0% |
| Dasysiphonia japonica | 100% | Dasysiphonia japonica MH287465 | 100% | Dasysiphonia japonica MK814640 | 0% | 0% |
| Delesseriaceae—2 spp. | ||||||
| Grinnellia americana | 100% | Grinnellia americana AF254184 | 97.83% | Membranoptera tenuis NC_032399 | --- | 0% |
| Phycodrys sp. | --- | --- | 100% | Phycodrys radicosa KC795887 | --- | 0% |
| Rhodomelaceae—14 spp. | ||||||
| Acanthosiphonia echinata | 100% | Acanthosiphonia echinate MF120866 | 97.57% | Polysiphonia binneyi KY573931 | --- | --- |
| Bostrychia radicans | 100% | Bostrychia radicans AY920882 | 96.48% | Bostrychia moritziana NC_035266 | 0.30% | 0% |
| Carradoriella elongata | 100% | Carradoriella elongata MF120875 | 99.73% | Carradoriella elongata NC_035274 | 0% | 0% |
| Chondria atropurpurea | 100% | Chondria atropurpurea MH388516 | 98.10% | Chondria sp. MF101431 | 0–0.08% | 0% |
| Chondria baileyana | 100% | Chondria baileyana KU564500 | 97.83% | Chondria sp. OM468962 | 0–0.22% | 0–0.28% |
| Chondria littoralis/sedifolia | 100% | Chondria littoralis KF672853 | 97.02% | Chondria sp. MF101429 | 0–0.15% | 0–0.27% |
| Kapraunia schneideri | 100% | Kapraunia schneideri MT597079 | 99.19% | Kapraunia schneideri NC_035296 | 0–0.29% | 0% |
| Melanothamnus spp. | --- | --- | --- | --- | 0–1.94% | 0–0.73% |
| Polysiphonia stricta | 99.85% | Polysiphonia stricta EU492916 | 100% | Polysiphonia stricta MF101428 | 0% | 0–0.31% |
| Rhodomela sp. | --- | --- | 100% | Rhodomela confervoides NC_035271 | --- | --- |
| Streblocladieae sp. | 93.30% | Kapraunia pentamera HM573564 | 97.83% | Polysiphonia sp. HQ421052 | --- | --- |
| Vertebrata fucoides | 100% | Vertebrata fucoides EU492913 | 98.37% | Vertebrata isogona NC_035278 | 0–0.30% | 0% |
| Vertebrata lanosa | 100% | Vertebrata lanosa KU564487 | 100% | Vertebrata lanosa KP208097 | 0% | 0% |
| Vertebrata nigra | 100% | Vertebrata nigra MF120893 | 98.37% | Vertebrata isogona NC_035278 | 0% | 0% |
| Spyridiaceae—1 sp. | ||||||
| Spyridia americana | 100% | Spyridia americana MW770750 | 99.19% | Spyridia filamentosa HQ421086 | 0–0.15% | 0% |
| Wrangeliaceae—4 spp. | ||||||
| Griffithsia globulifera | 89.66% | Griffithsia okiensis EU195056 | 95.93% | Plumaria plumosa MK814703 | 0–0.16% | 0% |
| Pleonosporium novae-angliae | 96.20% | Lophothamnion comatum KU381977 | 99.18% | Aglaothamnion boergesenii HQ421367 | --- | 0% |
| Plumaria plumosa | 100% | Plumaria plumosa KU381993 | 99.19% | Plumaria plumosa MK814703 | --- | 0.27% |
| Spermothamnion repens | 100% | Spermothamnion repens MK814735 | 100% | Spermothamnion repens MK814735 | 0.15% | --- |
| Families and Species | Dates Collected | Substratum | Habitat | Localities |
|---|---|---|---|---|
| Callithamniaceae—4 spp. | ||||
| Aglaothamnion halliae | JUN | Epiphytic on Gracilaria, Spartina, and shells | Low intertidal to shallow subtidal, estuarine, common in June | RWU |
| Callithamnion corymbosum | MAY–OCT, NOV *, DEC * | Most often lithophytic, occasionally epiphytic on coarse algae | Low intertidal to subtidal, estuarine, common | BH, CSP *, HHP, NP, PLC *, SPG |
| Callithamnion tetragonum | MAR, JUN–OCT | Epiphytic on Codium, Chondrus and other coarse algae | Lower intertidal, subtidal, tidepools, open coast, more abundant JUL—OCT | BSP, BTP, FW, KB, SCB * |
| Seirospora interrupta | AUG | (Drift) | Estuarine | GB |
| Ceramiaceae—9 spp. | ||||
| Antithamnion hubbsii | JUN–OCT | Epiphytic on Phyllophora, Chondrus, and other coarse algae | Subtidal, open coast with high wave action, common annuals with new growth appearing in June and larger growth in fall | BP, CRN, FW, KB, SCB * |
| Antithamnion sp. | AUG–SEP | Epiphytic on coarse algae and shells | Subtidal, open coast to unprotected estuarine waters, uncommon | FBS, KB |
| Antithamnionella floccosa | APR | Epiphytic on jetty | “Common, exposed area 2 feet above datum” | PB |
| Antithamnionella spirographidis | OCT | (Drift) | Open coast | LHB |
| Ceramium facetum | MAY–SEP | Mostly lithophytic, occasionally epiphytic on Gracilaria | Upper intertidal, shallow subtidal, estuarine and open coast, common | BH, BP, FBN, FW, PLC, RWU |
| Ceramium plenatunicum | AUG | Epiphytic on Gracilaria | Low intertidal to shallow subtidal, protected estuarine, abundant at this site | FBN |
| Ceramium secundatum | MAY–NOV | Epiphytic on coarse algae or lithophytic | Subtidal, mostly open coast but occasionally in estuarine drift, common | BP, BSP, FW, HNB, KB, LHB, RWU |
| Ceramium virgatum | MAY–AUG | Epiphytic on coarse algae or lithophytic | Subtidal, open coast, uncommon | FW, SCB |
| Ceramothamnion translucidum | JUN–SEP | Epiphytic on Fucus and other coarse and mixed with soft algae | On algae growing on floating docks or attached to drift algae, estuarine and open coast, inconspicuous but common | HNB, NP, SP |
| Dasyaceae—3 spp. | ||||
| Dasya cf. elegans | MAY–JUN | Lithophytic | Low intertidal to subtidal, estuarine | RWU |
| Dasya pedicellata | JUL, SEP–NOV | Mostly lithophytic, but younger thalli epiphytic on coarse algae and Zostera | Subtidal, open coast, common | BM, HNB, KB, LHB |
| Dasysiphonia japonica | JUN, JUL, AUG *–MAY * | Lithophytic or epiphytic on coarse algae | Low intertidal to subtidal, estuarine and open coast, common and widespread | BH *, BM *, CSP *, FW *, HNB *, KB, NP, RWU, SCB * |
| Delesseriaceae—2 spp. | ||||
| Grinnellia americana | JUN, JUL *, AUG *, OCT, NOV * | Lithophytic, occasionally on shells, once collected on raphyrus mass of Cliona celata | Subtidal, estuarine and open coast, common and widespread | CSP *, FBS, FW, PLC *, RWU |
| Phycodrys sp. | OCT, NOV | (Drift) | Subtidal, open coast | BSP, NMS |
| Rhodomelaceae—14 spp. | ||||
| Acanthosiphonia echinata | JUL | Epiphytic on Chorda in drift | Probably subtidal, open coast | HNB |
| Bostrychia radicans | JUN, JUL, NOV * | Mostly epiphytic on live Geukensia, occasionally on Spartina or spreading over rocks | High intertidal, estuarine, inconspicuous but common at these sites | PLC, RWU |
| Carradoriella elongata | APR *, JUL, AUG *–OCT *, NOV | (Drift) | Probably subtidal, open coast, new growth in April and older growth growth JUL–OCT, common | BM *, CWB *, HNB, KB *, SCB |
| Chondria atropurpurea | JUN–OCT | Strictly lithophytic | Shallow subtidal, open coast and estuarine, widespread and common | BM *, FBN, FBS, HNB, KB, NP, RWU, SCB |
| Chondria baileyana | JUN–OCT | Lithophytic or epiphytic on coarse algae and Zostera | Low intertidal to shallow subtidal and in tidepools, estuarine and open coast, widespread and common, new growth appearing in June, older growth in the fall | BH, BP, EAS, FW, HNB, KB, PLC, RWU |
| Chondria littoralis/sedifolia | JUN–OCT | Mostly lithophytic, but younger thalli epiphytic on coarse algae and Zostera | Subtidal, open coast, new growth appearing in June, older growth in the fall, common | BM *, FW, HNB, KB, SCB |
| Kapraunia schneideri | JUN–AUG | Lithophytic or on pilings or floating docks | Subtidal, estuarine or open coast, widespread and common | BH, BP *, PLC, RWU |
| Melanothamnus spp. | APR–OCT, DEC *–MAR * | Lithophytic or epiphytic on coarse algae or floating docks | Mid intertidal to shallow subtidal or in tidepools, estuarine and open coast, widespread and common | BH, BSP, BTP, CRN, CSP, EAS, GOO, FW *, KB, RWU, SCB, SPG |
| Polysiphonia stricta | DEC *, FEB *, MAR–JUN | Lithophytic | Mid intertidal to shallow subtidal, new growth appearing in December, older growth present in June. | BSP, CSP, FW, KB, RWU |
| Rhodomela sp. | APR | Lithophyte | In tidepool, open coast | BSP |
| Streblocladieae sp. | JUN | Lithophytic | Shallow subtidal, estuarine | NP |
| Vertebrata fucoides | FEB *, MAY, JUN, AUG *, OCT | Lithophytic | Subtidal, estuarine and open coast, widespread, common, very old growth present in August | BSP, COR, CSP, GOO, FW, KB, RWU, SCB, SPG |
| Vertebrata lanosa | JUN, JUL *, AUG *, SEP–NOV | Growing on Ascophyllum nodosum | Mid intertidal to low intertidal, open coast, common | BP, GOO, FW, KB, SCB |
| Vertebrata nigra | APR *, JUN, OCT | Lithophytic | Subtidal, open coast, uncommon | CWB *, GOO, FW *, LHB |
| Spyridiaceae—1 sp. | ||||
| Spyridia americana | JUN, JUL, AUG *–OCT * | Mostly lithophytic, occasionally in tangled masses with other algae | Subtidal, open coast and estuarine, widespread, common in certain localities, older growth present in October | KB *, NP, RWU |
| Wrangeliaceae—4 spp. | ||||
| Griffithsia globulifera | JUN, JUL, SEP | Mostly lithophytic, occasionally epiphytic on shells | Shallow subtidal, estuarine, common in certain localities | FBS, KR, PLC, RWU * |
| Pleonosporium novae-angliae | AUG, SEP | Epiphytic on coarse algae | Subtidal, open coast, uncommon, new growth appearing in August | KB |
| Plumaria plumosa | OCT | (Drift) | Open coast | NTB |
| Spermothamnion repens | JUN *–SEP * | Lithophytic or spreading onto basal axes of coarse algae | Subtidal, open coast, common | BP *, FW *, KB *, SCB * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irvine, T.; Wysor, B.; Beauvais, A. A Molecular-Informed Species Inventory of the Order Ceramiales (Rhodophyta) in the Narragansett Bay Area (Rhode Island and Massachusetts), USA. Diversity 2024, 16, 554. https://doi.org/10.3390/d16090554
Irvine T, Wysor B, Beauvais A. A Molecular-Informed Species Inventory of the Order Ceramiales (Rhodophyta) in the Narragansett Bay Area (Rhode Island and Massachusetts), USA. Diversity. 2024; 16(9):554. https://doi.org/10.3390/d16090554
Chicago/Turabian StyleIrvine, Thomas, Brian Wysor, and Alicia Beauvais. 2024. "A Molecular-Informed Species Inventory of the Order Ceramiales (Rhodophyta) in the Narragansett Bay Area (Rhode Island and Massachusetts), USA" Diversity 16, no. 9: 554. https://doi.org/10.3390/d16090554
APA StyleIrvine, T., Wysor, B., & Beauvais, A. (2024). A Molecular-Informed Species Inventory of the Order Ceramiales (Rhodophyta) in the Narragansett Bay Area (Rhode Island and Massachusetts), USA. Diversity, 16(9), 554. https://doi.org/10.3390/d16090554







