Abstract
Every year, several hundred million birds cross the Mediterranean on their migration from Eurasia to their wintering quarters in Africa. As many migrants travel at night or at high altitudes, direct observations of bird migration are difficult and thus our information about migrating species, numbers and timing is incomplete. An indirect way to assess autumn migration is the analysis of prey remains of Eleonora’s Falcons (Falco eleonorae). These falcons breed in large colonies on islands in the Mediterranean and on the Canary Islands. Many migrants have to pass these islands on their flight to their African wintering quarters. Eleonora’s Falcons appear to be adapted to the autumn bird migration and raise their young between August and October, when migrating birds are abundant. When nestlings have to be fed, falcons exclusively hunt small birds of 10 to 150 g body mass, whereas they prey mostly on aerial invertebrates (Coleoptera, Hymenoptera, Diptera, Orthoptera, Hemiptera, Odonata, Lepidoptera) from November to July. We studied Eleonora’s Falcons from 1965 to 2001 on a rocky islet, north of Crete, which harboured a colony of about 200 breeding pairs. In 1969, 1971, 1977, and 1988 we systematically monitored and collected the pluckings and cached food items in 22 to 36 nest sites each year. Pluckings were systematically analysed later in Germany using a reference collection of bird feathers for identification. In total, we determined more than 111 prey species (mostly Passerines) comprising more than 13,450 individuals. The top 12 prey species were: Willow Warbler (27.8% of all prey items), Red-backed Shrike (10.7%), Spotted Flycatcher (9.9%), Whinchat (8.8%), Common Whitethroat (5.1%), Wood Warbler (3.8), Tree Pipit (2.9%), Icterine Warbler (2.5%), Greater Short-toed Lark (2.5%), Northern Wheatear (1.8%), Common Nightingale (1.6%), and European Pied Flycatcher (1.5%). Eleonora’s Falcons are selective hunters to some degree; thus, the phenology and abundance data derived from the plucking analyses are biased towards slow-flying species or smaller birds (only up to a body mass of 150 g). When the young falcons develop and grow, food demand increases concomitantly. Comparing the total weight of prey over time indicates a correlation with food demand and in consequence with the number of prey items brought to the nest sites by the falcons.
1. Introduction
This paper forms part 30 of a series on the biology of Eleonora’s Falcon.
Although several millions of Eurasian birds migrate each autumn from their breeding grounds to their wintering quarters in Sub-Saharan Africa [1,2], the elucidation of bird migration has been an ongoing challenge for centuries. Migration can be assessed by direct and systematic observations, by radar analysis, standardized mist-netting and ringing, but also by new technologies including radio tracking, geolocators, GPS and satellite telemetry (overview in Bairlein [2]).
It has been assumed that additional information about the seasonal changes and abundance of migrating birds can be revealed from a prey analysis of Eleonora’s Falcons (Falco eleonorae) which relies on migrating birds to raise its young [3,4,5,6,7,8,9,10]. As can be seen from our studies and those from other colonies, Eleonora’s falcons are selective hunters, so that prey lists are biased to some degree. Nevertheless, for the main prey species, these plucking analyses provide interesting additional details.
Eleonora’s Falcon belongs to a small group of Eurasian falcons that are long-distance migrants [11,12]. According to DNA analyses [13,14] this group consists of Eleonora’s Falcon, Sooty Falcon (Falco concolor), and Hobby (F. subbuteo). Eleonora’s Falcons breed colonially on islands in the Mediterranean and Atlantic (from Cyprus in the east to the Canary Islands in the west) and winter in Africa, especially on Madagascar [15,16].
In contrast to most other falcon species, Eleonora’s Falcons breed in small to large colonies of up to more than 300 breeding pairs on small rocky islands or on rocky coasts. Though, being a colonial raptor, it defends individual territories [17], neighbouring pairs often breed only 10 metres apart (Figure 1). About 80% of Eleonora’s Falcons breed in Greece on small Aegean islands. The total number of breeding falcons was estimated to be between 32,400 and 33,300 adult individuals distributed across a range of <20,000 km2 [18,19,20]. Although there are local declines in breeding populations, overall, Eleonora’s Falcons have exhibited a population increase during the last 30 years as a result of public awareness campaigns to reduce human persecution [20].
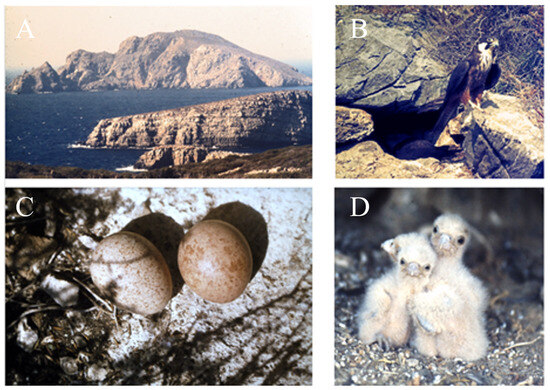
Figure 1.
Eleonora’s Falcons: (A) Study site (the islet of Paximada in the background) and Eleonora’s Falcons: (B) Adult male at its nest site, (C) clutch with two eggs, (D) three young falcons in September (Photos: M. Wink, D. Ristow).
Since these falcons breed colonially, they are accessible for detailed research projects with regards to their ecology, evolution, and breeding biology. After 1965, several ornithologists became interested in this species and many studies, several of them long term, have been carried out on Cyprus, the Aegean Islands, Sardinia, Sicily, Balearic Islands, Morocco, Algeria, and the Canary Islands. Studies include topics such as breeding biology and ecology [3,4,6,17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], hunting and prey studies [4,5,8,10,16,22,34,38,39,40,41,42,43], morph polymorphism [44,45,46,47,48], pesticide contamination of eggs [49,50,51,52], and population genetics [27,53,54,55,56]. Movements of migratory Eleonora’s Falcons have been tracked with GPS and satellite telemetry which have elucidated the routes from the breeding sites in the Mediterranean and Canary Islands across the Sahara to east Africa and Madagascar [15,34,57,58,59,60,61,62,63,64,65,66,67].
Similar to the closely related Sooty Falcon, Eleonora’s Falcons appear to be adapted to autumn bird migration [4,68]. The adaptation can be seen in the timing of breeding, the choice of breeding sites and its hunting behaviour. Eleonora’s Falcons raise their young in late summer and autumn, when most other Eurasian birds have terminated their breeding seasons, because food in the form of migrants becomes abundant as several hundred million birds cross the Mediterranean on their way to Sub-Saharan Africa [1,2,4,66,67,69]. In addition, most breeding colonies are located at strategically optimal sites, such as islands or coastal rocks over which migrants pass in substantial numbers. Whereas most raptors hunt individually, Eleonora’s Falcons exhibit communal hunting and catch prey in the air and never on the ground [21,38,39,40,41]. Typically, many falcons circle over the islands and wait for approaching migrants. When migrants are detected, gangs of falcons chase the prey together. These group hunts end mostly with aerial capture of the migrant. Most of the hunting occurs above the open sea [4,21].
Eleonora’s Falcons return from their wintering quarters on Madagascar to the Mediterranean breeding grounds in April and May, and immediately occupy territories, although they do not immediately start breeding (Figure 2). Between arrival and egg laying the falcons may roost on the islands or may be hundreds of kilometres away for several days in order to hunt aerial insects and to a lesser extent on birds on the mainland [70]. Breeding (egg laying, incubation and hatching) is strongly synchronized in a colony and takes place at the same time each year [6,23,26] (Figure 2). Egg laying in an Aegean colony starts around July 18 with a variation of 5 days between years and is completed in the first days of August [6,23,26,54]. Eggs are incubated for 28 days and young falcons hatch between 14 August and 1 September [6,23,26]. Young falcons need 35 to 40 days to fledge [71]. In October, both young and adult falcons leave the breeding sites [6,23,26,71] and migrate to Africa, often crossing the Sahara [15,34,57,58,59,60,61,62,63,64,65,66,67].
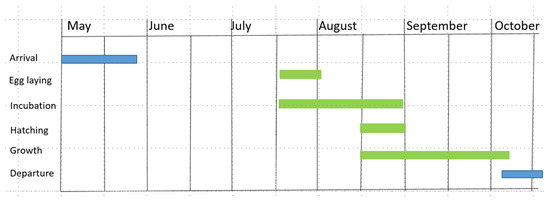
Figure 2.
Breeding phenology of Eleonora’s Falcon in an Aegean colony (from [6,21]. Blue bars indicate non-breeding, green bars breeding season.
The diet of Eleonora’s Falcons undergoes a dramatic change between breeding and non-breeding periods. During spring and early summer months, when bird migrants are scarce at the breeding cliffs, the main prey for the falcons are aerial invertebrates, such as Coleoptera (e.g., Buprestidae), Hymenoptera (e.g., ants including Camponotus, Pheidole, and Tetramorium), Diptera, Orthoptera, Hemiptera, Odonata, and Lepidoptera [5,8,9,21,72].
When bird prey becomes available, as during autumn migration, Eleonora’s Falcons prefer birds over invertebrates. Eleonora’s Falcons sample a wide range of migrant birds, and more than 111 prey species have been observed. Systematic analyses, all of them much smaller than our present study, have been reported from an island off of eastern Crete [3,5,22], the Aegean Sea [9], Italy [73], the Balearic Islands [72,74], the Columbretes [75], Morocco [3,76], Algeria [8,10], Tunisia [77] and the Canary Islands [7].
Bird prey varies in size from Goldcrests to Little Bittern (e.g., roughly between 10 and 150 g body mass). Falcons bring their catch to their nest sites and pluck them there. Typically, nest sites are sheltered from wind between rocks so that the feathers remain there for a couple of days. When food is abundant (mostly on days with northerly winds, which are frequent in autumn), Eleonora’s Falcons continue to catch as many birds as they can and cache the intact dead birds in the shade of rocks or in a bush close to the nest site [5,6,23,25]. The cached birds are eaten later, when food becomes scarce in the afternoon or on windless days. Most of the hunting is done by the male falcons from dawn to midday, and not at night; they supply food for the incubating female and the young falcons [5,6,21,23].
Our own fieldwork was carried out between 1969 and 2001 on the Cretan islet Paximada (where H. Walter and D. Ristow started the studies in 1965) with the aim to investigate the breeding biology and ecology of Eleonora’s Falcons. On this island, a breeding colony of Eleonora’s Falcons of about 200 pairs exists.
As most migrant birds pass the Mediterranean at night, our knowledge of bird migration in the eastern Mediterranean is limited [69,78]. The aim and scope of this work is to document the diet composition of Eleonora’s Falcon in the eastern Mediterranean during the breeding season and report migration profiles of more than 60 migrants, which we obtained from systematic and non-systematic monitoring. In 1969, 1971, 1978 and 1988 we collected pluckings and other prey remains systematically from 20 to 36 nest sites each year. In all of the years, we also recorded cached prey items and made visual observations of migrating birds. During the four years (1969, 1971, 1977, and 1988) we have covered the whole breeding cycle of this species. The present analysis is comprehensive and widens the database of previous analyses [5,6,21] which were based only on prey items sampled in 1977. Our present study provides new data on Mediterranean bird migration for over 110 species derived from the prey analyses of Eleonora’s Falcons.
2. Materials and Methods
We studied Eleonora’s Falcons on a group of small rocky islands, the Dionysades off the coast of eastern Crete and north of Sitia, which includes the islands Gianysada, Dragonada, Paximada, and Paximadaki. Our main study site was the uninhabited islet of Paximada, a rocky islet of triangular size 430 × 1300 metres (Figure 1). We have visited these islands 26 times—1965, 1969, 1975 and then almost yearly until 2001. Overall, we spent 745 field days on the islands. Table S1 provides an overview of these activities. Ideally, a prey analysis as presented in this publication should be carried out over the complete breeding season in a single year. Because fieldwork was very exhausting on the hot, bare limestone islands (which had no settlements, housing facilities nor fresh water), this proved to be impossible. We were not able to stay longer than three to five weeks on the islands without a break or we worked in shifts. Thus, our present study is a compromise and represents a summary of fieldwork from several years.
In a breeding colony, a crew of two to four persons can monitor falcon pluckings systematically in up to 36 nest sites in a season if they spend half a day at the beginning and up to a full day towards the end of the season for daily feather collecting. Later, the collected prey items have to be examined carefully, in order to identify the species and the number of individuals from a particular nest site [5,22].
In the years 1969, 1971, 1977, and 1988 (Table S1) we carried out a systematic monitoring of prey remains at 22 to 36 sheltered nest sites so that feathers would not be blown away by the wind. Due to the difficult study conditions, we could neither visit each nest site on a single day nor during a complete breeding season. Instead, we completely collected all feathers in three-day intervals at each study nest site in several years, but over the years (1969, 1971, 1977 and 1988) covered the complete breeding season. In 1977 and 1988 we sampled prey items through the main breeding period, whereas samples from 1969 and 1971 came from the end of the breeding period (Table S1). In Table S3 we also noted the number of prey items recorded in each of these four years. Although we did not do a systematic plucking collection during the last decade of our field study, the impression was that the frequency of prey numbers was reduced for Calandrella brachydactyla, Phoenicurus phoenicurus, and Passer hispaniolensis in particular. Additionally, cached food items were recorded; as these concerned bird species known to us from central Europe, we could identify them directly at the collection site. We placed plucked feathers into individual envelopes for each nesting site and sprayed them with an insecticide to avoid degradation during later storage. In addition, pluckings and cached food were monitored non-systematically in all study years and sight observations of all bird species seen on the island or flying past it were recorded. In this analysis, we have not included prey remains from insects, which were mostly observed between May and August. These data have been published elsewhere [70].
A major effort was required to identify the species and the quantity of individuals in a plucking sample. For identification, one of us (DR) had built up a comprehensive feather collection of about 300 European bird species (now archived in the Zoologische Staatssammlung in Munich). In addition, monographs of feather identification were regularly consulted [79,80]. These references helped to identify most species. In case of ambiguous identifications, other feather specialists were consulted and the museum collections of bird skins at the Zoologisches Forschungsmuseum Alexander Koenig in Bonn and the Zoologische Staatssammlung in Munich used for direct comparison. As a consequence, we were able to identify more than 99% of the samples, with some of the ambiguous identifications mentioned in Tables 2 and 3. Apparent difficulties in separating prey species (in case of incomplete plucking) included the following species which all had a low abundance in the prey list: (a) Regulus spec, (b) Bonelli’s Warbler Phylloscopus bonelli, Hippolais spec, (c) Red-Breasted Flycatcher Ficedula parva, (d) reed warblers, and (e) pipits.
In a first step of the analysis, feathers were sorted by species. Then, within the same species, we focussed on counting the outer primaries, which were found to be more useful than the tail feathers used by H. Walter [3] to determine from how many individuals the feathers had derived from. Emarginations of the primaries were used to determine the number of individuals represented in the envelope’s sample. Sometimes, a few feathers had been overlooked on the first visit but were found on the consecutive one. In order to avoid counting such feathers twice, we decided that up to five feathers from an incomplete plucking in the second visit would belong to the previous sample. The analysis effort back home roughly equalled the amount of man hours spent for plucking collection in the field.
3. Results and Discussion
3.1. Diet of Eleonora’s Falcons
We systematically collected pluckings from individual nest sites of Eleonora’s Falcons, which were monitored every two to three days in 1969, 1971, 1978 and 1988 (→systematic data). In addition, we monitored cached prey in food stores, which were in close vicinity of a particular nest site. In other years (Table S1), we investigated the breeding biology of the falcons, regularly visiting all parts of the colony, and any apparent prey remains or pluckings were recorded (see non-systematic data; Table 2 and Table S3).
Excluding insects, we collected more than 13,450 prey items (Table 1). The main avian prey species were Willow Warbler (27.8% of all prey items), Red-Backed Shrike (10.7%), Spotted Flycatcher (9.9%), Whinchat (8.8%), Common Whitethroat (5.1%), Wood Warbler (3.8%), Tree Pipit (2.9%), Icterine Warbler (2.5%), Greater Short-toed Lark (2.5%), Northern Wheatear (1.8%), Common Nightingale (1.6%), and European Pied Flycatcher (1.5%). Xirouchakis et al. [9] reported a similar prey composition on other Aegean islands. Table 1 provides an overview of all 111 avian prey species, which we had recorded mostly on the island of Paximada. Some records from neighbouring islands, which we had visited in some years, were also included. Our list reveals new prey species that are not mentioned in the literature. From otherwise published data, plus our data, a total of 125 bird prey species have been recorded as avian prey of Eleonora’s Falcon. If we take the body mass of the prey into consideration (Table 1 and Table S2), the top 9 species which contributed more than 10 kg to the overall diet of the colony include Lanius collurio (43.1 kg), Phylloscopus trochilus (33.6 kg), Saxicola rubetra (19 kg), Muscicapa striata (18.6 kg), Streptopelia turtur (13.2 kg), Oriolus oriolus (13.1 kg), Cuculus canorus (12.7 kg), Upupa epops (10.5 kg), and Sylvia communis (10.3 kg). Prey with a higher body mass (shrike, Turtle Dove, Oriole, Cuckoo and Hoopoe) contributed substantially to the diet. From the aspect of energetics, these species appear to be more profitable prey than smaller migrants, such as warblers.

Table 1.
Summary of prey items of Eleonora’s Falcons from Paximada and Dragonada and their contribution to the diet (species are listed in relation to their abundance in the diet).
Table 2 provides a more detailed phenology of avian prey species from which we had 50 or more records. A complete list (Table S3) of our systematic and non-systematic prey monitoring includes sight observations of migrating or resting birds on the island. For the more abundant migrant species, Figure 3 documents their phenology and quantitative changes during autumn migration. For a few species, we could also obtain direct observations of migrants resting on the island (such as Anthus trivialis, Oenanthe oenanthe and Phylloscopus trochilus). As can be seen in Figure 3, their abundance agrees with the number of birds in the prey list.

Table 2.
Diet of Falco eleonorae (number of prey items) and direct observations of migrating birds (total >50 samples/individuals); a complete list can be found in Table S3. Species are listed in a systematic order: 5-day periods (pentads): 1–6 = July; 7–12 = August; 13–18 = September; 19–21 = October.
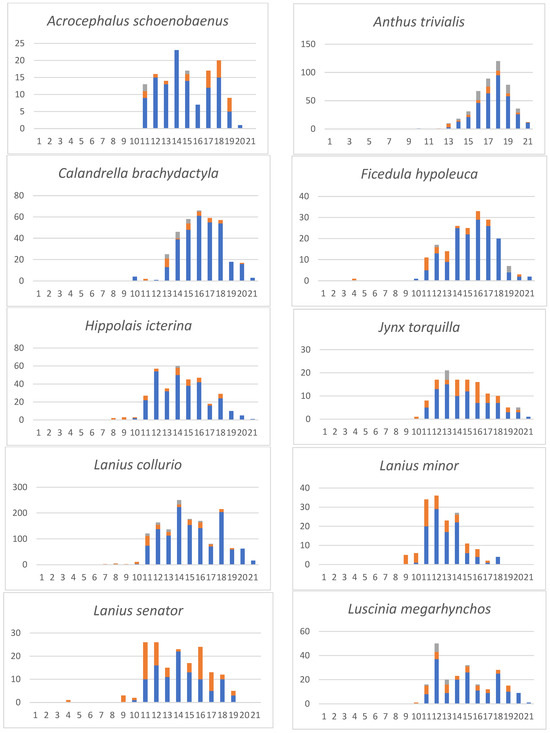
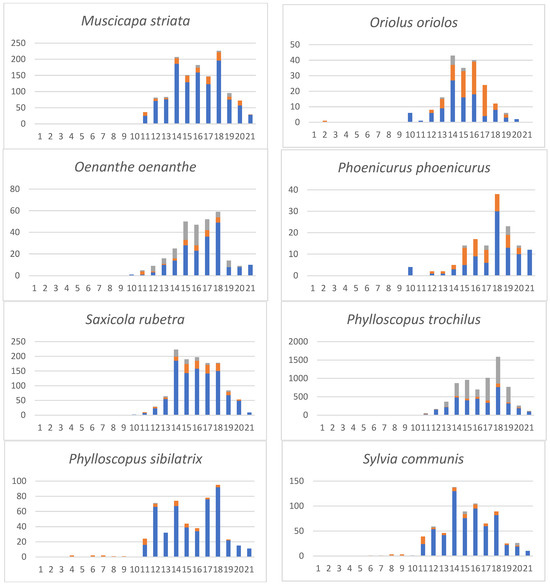
Figure 3.
Phenology of the more abundant migrants on the Dionysades Islands. X axis: 5-day periods (pentads): 1–6 = July; 7–12 = August; 13–18 = September; 19–21 = October. Y axis: number of individuals. Columns: Blue: systematic sampling of pluckings and cached prey; orange: non-systematic sampling of pluckings and cached prey; grey: sightings.
No substantial differences can be seen in the abundances and phenology of the prey species during the assessment period 1969–1988, although it covers a wide time span (Table S3). As can be seen from Figure 3 and Table 2, species-specific differences exist. Early migrants in July/August include Hoopoes, Cuckoos, Bee Eaters and Shrikes. The main migration occurs in the second half of August and especially in September. Late migrants in October were Meadow Pipits and sparrows.
Eleonora’s Falcons catch any bird from small warblers (>10 g) up to the size of Little Bitterns or Corncrakes (>150 g) (Table 2, Tables S2 and S3), which they encounter migrating above the breeding colony and the sea next to it. Birds which fly slowly are apparently easier to catch than fast-flying species. Birds which fly slowly are caught after a few stoops. However, prey species of median agility are repeatedly stooped at and driven to lower altitudes while avoiding capture. Meanwhile more falcons join the pursuit in hot competition so that a group of more than twenty falcons stoop at the prospective prey in short intervals until one falcon succeeds or else the prey reaches solid ground on the island.
A few fast-flying species are apparently more difficult to catch. Although we saw them migrating over the islands (Table 2 and Table S3), they are under-represented in the prey list. These species include swifts (Apus apus, Tachymarptis melba), Turtle Dove, waders, swallows, and wagtails (Motacilla flava, M. alba). Thus, Eleonora’s Falcons are selective hunters, and our data are therefore somewhat biased. This bias can be seen in our data (Table 1 and Table S2), which reflects the way in which most of the birds which contribute to the diet are slow-flying species. Eleonora’s Falcons do not feed on caught birds in the air or at sites outside the breeding area. Thus, we assume that the plucking lists are representative for the hunting success of the falcons.
Among the pluckings of migrants, we found several bird species that are hardly seen by birders (Table 1). These include rails (Gallinula chloropus, Porzana porzana, Zapornia parva, Zapornia pusilla, Crex crex), Ixobrychus minutus and the Great Spotted Cuckoo Clamator glandarius. Waders apparently migrate close to the island; however, because mud flats do not exist there, the numbers are very small. A comparison with the pluckings of Peregrines (Falco peregrinus) from the same study plot [81] indicates that Peregrines, which breed earlier, are more successful hunters of waders and gulls/terns. Species to be expected as prey but not yet encountered in our study include Water Rail (Rallus aquaticus), Woodcock Scolopax rusticola, Masked Shrike Lanius nubicus, Dunnock Prunella modularis, Grey Wagtail Motacilla cinerea, and Reed Bunting Emberiza schoeniclus. Apparently, Chiffchaffs (Phylloscopus collybita) (a short-distance migrant) are not part of the diet, despite earlier claims by Walter [3]. Walter had apparently misidentified the feathers. Additionally, when mist-netting on Dragonada, Phillips and Round [78] only caught Willow Warblers but no Chiffchaffs. In our own analyses of over 3700 warblers (Table 2 and Table S3), we did not obtain any evidence for P. collybita.
Eleonora’s Falcons also hunt on the mainland of Crete (about 20 km away), where they catch insects and occasionally resident birds. We assume that the following prey species (Table 1, Table 2 and Table S3) were caught on the mainland: Wood Lark Lulla arborea, Crested Lark Galerida cristata, Cetti’s Warbler Cettia cetti, Stonechat Saxicola torquata, Greenfinch Chloris chloris, Goldfinch Carduelis carduelis, Linnet Linaria cannabina, and Corn Bunting Emberiza calandra. In addition to migrants, some birds breeding or foraging at the Dionysades Islands turn up in the prey list (Table 1, Table 2 and Table S2) such as Storm Petrel Hydrobates pelagicus, Rock Dove Columba livia, Swifts Apus apus, A. pallidus, Tachymarptis melba, Sardinian Warbler Sylvia melanocephala, and Blue Rock Thrush Monticola solitarius.
It is possible to distinguish adult and juvenile (first year) birds in the pluckings from quite a few prey species by shape of rectrices tip, by bleached and worn feather tips, position of growth bars, or colour differences [80]. We did not carry out a comprehensive study on adult to juvenile ratios, so Table 3 only represents trends for a few species. In most passerine species, first-year juveniles are more abundant than adult birds during autumn migration, because a single pair of passerine birds can produce more than two offspring. Thus, juveniles become more abundant than adults. This trend can also be seen in mist-net captures during autumn [2].

Table 3.
Differentiation between adult and immature birds in species which were found more than 20 times as prey items (species are listed in systematic order).
Bird migration is active on windy days, but almost non-existent on windless days [21]. As already mentioned, on windy days the falcons catch more than they and their nestlings can eat; the surplus food is cached in food stores and is consumed, when food becomes scarce. When windless and hot days last longer than three days, young falcons begin to starve. In this situation, the siblings sometimes kill the weakest young in a brood for food [6,21,71].
The diet of Eleonora’s Falcons can differ between colonies, depending on the composition and abundance of migrants across the Mediterranean. On the island Kel Amor in Algeria, the main prey species (in 513 dietary samples) were Sylvia borin, Phoenicurus phoenicurus, Oenanthe oenanthe, Apus apus, Saxicola rubetra, Jynx torquilla, and Erithacus rubecula [10]. Whereas in accordance with our analysis, studies of prey remains from the Aegean region provided similar results [9].
Are molluscs or lizards part of the diet of Eleonora’s Falcons? Xirouchakis et al. [9] include snails and lizards as part of the diet of Eleonora’s Falcons, but this appears to be unlikely from our own experience. Eleonora’s Falcon is an aerial hunter and, per our own observations between 1969 and 2001, does not target any prey on the ground Walter [4]. However, digestive stones and fragments of molluscs are sometimes found in pellets of falcons, and Eleonora’s Falcon is no exception [4]. We, however, are not aware of a study investigating whether small calcium-rich stones or seashells are preferentially swallowed or taken up by chance. What about lizards? Small lizards (such as Podarcis erhardii) live on many of the Aegean islands, often in the neighbourhood of Eleonora’s Falcon nests, where they are tolerated. When observing nests from close distance, we have several additional records that show that lizards sometimes try to bite off bits of flesh from fresh prey, delivered to the nest by the male falcon, while the female stands on it to tear it apart and feed the nestlings. We presented a film at the WWGBP 1982 conference in Thessaloniki/Greece with two scenes, one that shows a lizard resting on the rump of an incubating female falcon and signalling territorial behaviour, and one that shows two lizards walking from behind and underneath a feeding female falcon to bite off bits of flesh from the prey under the talons. The female briefly snapped at them two times, but the lizards escaped the bite attempts and returned to continue their piracy. If a lizard does not pay attention, it may be killed by a bite. However, we once observed that a female falcon killed a lizard, but left the dead animal untouched. As the dead lizard is not removed from the nest, its dry carcass lies at the nest for days. When inspecting nests, an observer could wrongly assume that the lizard would be part of the prey list. On the Algerian island Kef Amor, the remains of a wall gecko was discovered in a falcon eyrie [10]. Again, we would argue, as in the case of Podarcis erhardii, that the gecko was accidentally killed. For these reasons, the inclusion of molluscs and reptiles in prey lists of Eleonora’s Falcon may be misleading [9,17]. Bakour and Moulai [8] mention that they found some gastropods as prey items; however, from our own observations, we know that rats, which are abundant on falcon islands, may transport the gastropods to the eyrie and store them in corners sheltered from the wind and that such corners are preferred by the falcons for egg laying later on. Thus, we assume that these gastropods were not killed by the falcons and are not part of their diet [8].
3.2. Prey Abundance in Relation to Food Demand of Young Falcons
Breeding of Eleonora’s Falcons is highly synchronized within a breeding colony (Figure 2) [6,23,24,26,55,71]. In Figure 4 we summarize our findings on the synchrony of egg laying, hatching and the development of young falcons. Egg laying data derive from 1975, while hatching and growth data derive from 1977. For the number of prey items, we used the data from our systematic monitoring in 1969, 1971, 1977 and 1988 (Table 1 and Table S3). For July and early August, we used the data from 1975, when we monitored the island on a 3-day basis to find nests and to record egg laying. We used body mass data of prey species from Limbrunner et al. [82] and calculated the overall body mass of all prey items per 5-day period. As can be seen from Figure 4, egg laying and hatching is a synchronized process in the colony. It coincides with the main migration of migrants from the middle of August to middle October. The number of prey items is small during egg laying and incubation. As soon as the young falcons start to grow and fledge, the number of prey items increases concomitantly with the growing demand.
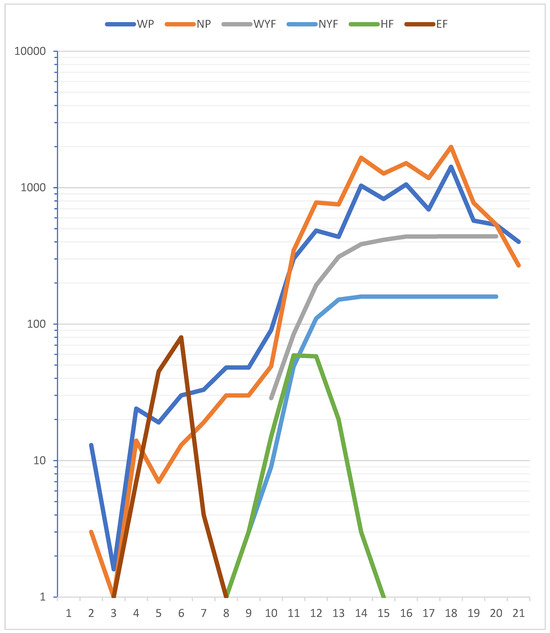
Figure 4.
Phenology of breeding in Eleonora’s falcons in relation to the number of prey items and their weight. X axis: 5-day periods (pentads): 1–6 = July; 7–12 = August; 13–18 = September; 19–21 = October; logarithmic Y axis: number of individuals (eggs, falcons) or total mass of prey items (in grams). EF (dark brown line) = numbers of eggs laid, HF (green) = number of hatching falcons, NYF (light blue) = number of young falcons, WYF (grey) = mass of young falcons (in grams), WP (dark blue) = total mass of prey items (in grams), NP (orange) = total number of prey items.
3.3. Direct Observations of Migrating Birds That Are Not Hunted by Eleonora’s Falcons
In addition to the birds which are part of the diet of Eleonora’s Falcons, we were able to record a number of migrating herons and raptors, which flew over the island. As these data are relevant for bird migration in the eastern Mediterranean we have recorded and documented these observations in Table 4. In addition, while working on the islands, we also regularly observed a number of resident species on Paximada and the neighbouring islands where they breed and many observations were also obtained from non-breeding birds in late summer and autumn. These bird species include Shag Phalacrocorax aristotelis (n = 90), Audouin’s Gull Ichthyaetus audounii (n = 111), Yellow-Legged Gull Larus michahellis (n = 350), Yelkouan Shearwater Puffinus yelkouan (n = 34), Scopoli’s Shearwater Calonectris diomedea, and Peregrine Falcon Falco peregrinus. Scopoli’s Shearwater Puffinus diomedea and Yellow-Legged Gulls breed on the island Paximada in large numbers. Regular guests were Kestrel Falco tinnunculus (n = 27), two pairs of which breed on the neighbouring island, and Raven Corvus corax (n = 16) from mainland Crete.

Table 4.
Direct observation of migrating herons and raptors on Paximada. Time intervals: 5-day periods (pentads): 1–6 = July; 7–12 = August; 13–18 = September; 19–21 = October.
3.4. Potential Impact of Eleonora’s Falcons on Rare Migrants
Many trans-Saharan migrants are arthropod feeding species. As food is becoming less abundant in the breeding and wintering areas because of extensive industrial agriculture, many bird species have seen substantial population decline. Further threats for many migrants are extensive mist-netting along the north African coast, illegal hunting, and more importantly loss of suitable habitats for breeding and wintering [83].
For several endangered migrants (such as Wryneck, shrikes, Cuckoo, Hoopoe, Nightjar, and Oriole), Eleonora’s Falcons may have some additional impact on their population, which can be considered as moderate in comparison with other threats for these species.
How many migrants are caught every year by Eleonora’s Falcons in the eastern Mediterranean? Our analysis is representative of the number of prey items caught by Eleonora’s Falcons during the autumn migration in the Aegean Sea. From Table 1 and Table 2, we can derive an estimate of how many birds are caught by 100 or more pairs of breeding falcons during a breeding season (Table S3). Depending on the number of breeding falcons in the Aegean and on Cyprus, we can make a first assumption on the impact of Eleonora’s Falcons on migrating birds, though it is rather speculative.
4. Conclusions
We have systematically collected plucked feathers and cached food systematically on the islet Paximada, northeast of Crete over four years (1969, 1971, 1978 and 1988). We recorded more than 13,450 prey items and from more than 110 prey species. The falcons are selective hunters, preferring smaller and slow-flying birds (up to 150 g body mass); fast-flying species, such as swifts and swallows, can be seen migrating over the islands, but they are underrepresented in the prey list. The diet of Eleonora’s Falcons is not identical across the range of the Mediterranean region and can differ between colonies, depending on the local composition and abundance of migrants [3,8,9,10,73,76].
It would be interesting, if our prey analyses could be repeated, as such an analysis could reveal changes in the abundance of endangered Eurasian migrants or climate-dependent phenology shifts.
Our prey analysis of Eleonora’s Falcons, which is the most comprehensive investigation so far, describes the timing of bird migration across the Mediterranean Sea for more than 20 migrant species, of which we had obtained more than 100 records. Although our data are biased and selective, this method can supplement other studies on bird migration that are based on direct observations, mist-netting, ringing and satellite or GPS telemetry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16090538/s1, Table S1: Summary of monitoring days on the Dionysades Islands; Table S2: Top prey items—sorted by body mass; Table S3: Complete data set of prey items from Falco eleonorae and of direct observations of migrating birds on Paximada; Table S4: Estimated numbers of prey items caught by Eleonora’s Falcons within a single breeding season in the Aegean Sea.
Author Contributions
Both authors collected samples in the field. D.R. identified the collected feathers. Both authors compiled the data and interpreted them. M.W. drafted a first version of the manuscript, which was corrected by D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Fieldwork on Crete was carried out under the permit number 97403/2613 from the Ministry of Agriculture in Athens (Greece). The storage of feathers and their investigation occurred under permit no. 830-8642-6/83 from the Regierung Oberbayern Obere Naturschutzbehörde München (Germany).
Data Availability Statement
The original contributions presented in the study are included in the article and Supplementary Material, further inquiries can be directed to the corresponding author.
Acknowledgments
Several friends and colleagues participated in the fieldwork on Paximada and Dragonada between 1969 and 2001: We wish to especially thank Coralie Wink, Winfried Scharlau (died in 2020), H. Friemann, Falko Feldmann, Ludger Witte (died in 2001 while doing fieldwork on Paximada), and Ingrid Swatschek. We thank two reviewers for their helpful recommendations.
Conflicts of Interest
Michael Wink is editor-in-chief of DIVERSITY.
References
- Cresswell, W. Migratory connectivity of Palaearctic-African migratory birds and their responses to environmental change: The serial residency hypothesis. Ibis 2014, 156, 493–510. [Google Scholar] [CrossRef]
- Bairlein, F. Das große Buch vom Vogelzug: Eine umfassende Gesamtdarstellung; Aula: Wiebelsheim, Germany, 2022. [Google Scholar]
- Walter, H. Zur Abhängigkeit des Eleonorenfalken (Falco eleonorae) vom mediterranen Vogelzug. J. Ornithol. 1968, 109, 323–365. [Google Scholar] [CrossRef]
- Walter, H. Eleonora’s Falcon: Adaptations to Prey and Habitat in a Social Raptor; The University of Chicago Press: Chicago, IL, USA, 1979. [Google Scholar]
- Ristow, D.; Wink, C.; Wink, M. Assessment of Mediterranean autumn migration by prey analysis of Eleonora’s Falcon. In Proceedings of the First Conference on Birds Wintering in the Mediterranean Region, Alla, Italy, 23–25 February 1984; Farina, A., Ed.; Supplemento alle Ricerche di Biologia della Selvaggina, Istituto Superiore per la Protezione e la Ricerca Ambientale: Ozzano dell’Emilia, Italy, 1986; Volume X, pp. 285–295. [Google Scholar]
- Wink, M.; Biebach, H.; Feldmann, F.; Scharlau, W.; Swatschek, I.; Wink, C.; Ristow, D. breeding biology of Eleonora’s falcon (Falco eleonorae). In Biology of Small Falcons; Nicholls, M.K., Clarke, R., Eds; The Hawk & Owl Trust: London, UK, 1993; pp. 59–72.eleonorae). In Biology of Small Falcons; Nicholls, M.K., Clarke, R., Eds.; The Hawk & Owl Trust: London, UK, 1993; pp. 59–72. [Google Scholar]
- De León, L.; Rodríguez, B.; Aurelio Martín, A.; Nogales, M.; Alonso, J.; Izquierdo, C. Status, distribution, and diet of Eleonora’s Falcon (Falco eleonorae) in the Canary Islands. J. Raptor Res. 2007, 41, 331–336. [Google Scholar] [CrossRef]
- Bakour, S.; Moulai, R. Dietary analysis across breeding seasons of Eleonora’s Falcon Falco eleonorae on the western coast of Algeria. Ostrich 2019, 90, 63–72. [Google Scholar] [CrossRef]
- Xirouchakis, S.M.; Alivizatos, H.; Georgopoulou, E.; Dimalexis, A.; Latsoudis, P.; Portolou, D.; Karris, G.; Georgiakis, P.; Fric, J.; Saravia, V.; et al. The diet of the Eleonora’s Falcon (Falco eleonorae) in the Aegean archipelago (Greece). J. Nat. Hist. 2019, 53, 1767–1785. [Google Scholar] [CrossRef]
- Samraoui, B.; Kayser, Y.; Touati, L.; Samraoui, F.; Boucheker, A.; El-Serehy, H.A.; Samraoui, K.R. Diet of breeding Eleonora’s Falcon Falco eleonorae in Algeria: Insights for the autumn trans-Mediterranean avian migration. Ecol. Evol. 2022, 12, e9065. [Google Scholar] [CrossRef] [PubMed]
- Cramp, S. (Ed.) Hawks to Bustards. In Handbook of the Birds of Europe, the Middle East and North Africa; Oxford University Press: Oxford, UK, 1980; Volume 2. [Google Scholar]
- Del Hoyo, J.; Ellitt, J.; Sargatal, J. (Eds.) New World Vultures to Guineafowl. In Handbook of the Birds of the World; Lynx Edicions: Barcelona, Spain, 1994; Volume 2. [Google Scholar]
- Wink, M. Phylogeny of Falconidae and phylogeography of Peregrine Falcons. Ornis Hung. 2018, 26, 27–37. [Google Scholar] [CrossRef][Green Version]
- Wilcox, J.J.; Boissinot, S.; Idaghdour, Y. Falcon genomics in the context of conservation, speciation, and human culture. Ecol. Evol. 2019, 9, 14523–14537. [Google Scholar] [CrossRef]
- Gschweng, M.; Kalko, E.; Querner, U.; Fiedler, W.; Berthold, P.; Fahr, J. Multi-temporal distribution modelling with satellite tracking data: Predicting responses of a long-distance migrant to changing environmental conditions. J. Appl. Ecol. 2012, 49, 803–813. [Google Scholar] [CrossRef]
- Hadjikyriakou, T.G.; Kassinis, N.; Skarlatos, D.; Charilaou, P.; Kirschel, A.N. Breeding success of Eleonora’s Falcon in Cyprus revisited using survey techniques for cliff-nesting species. Condor 2020, 122, 1–13. [Google Scholar] [CrossRef]
- Ristow, D.; Wink, C.; Wink, M. Biology of Eleonora`s Falcon. 1. Individual and social defense behavior. Raptor Res. 1982, 16, 65–70. [Google Scholar]
- Dimalexis, A.; Xirouchakis, S.M.; Portolou, D.; Latsoudis, P.; Karris, G.; Fric, J.; Georgiakakis, P.; Barboutis, C.; Bourdakis, S.; Ivovič, M.; et al. The status of Eleonora’s Falcon (Falco eleonorae) in Greece. J. Ornithol. 2007, 149, 23–30. [Google Scholar] [CrossRef]
- Kassara, C.; Fric, J.; Sfenthourakis, S. Factors influencing the occurrence of Eleonora’s Falcon Falco eleonorae breeding colonies on Greek islands. Wildl. Biol. 2013, 19, 202–209. [Google Scholar] [CrossRef] [PubMed]
- BirdLife International. Species Factsheet: Falco eleonorae. Available online: http://datazone.birdlife.org/species/factsheet/eleonoras-falcon-falco-eleonorae. (accessed on 23 January 2024).
- Ristow, D.; Wink, C.; Wink, M. Biologie des Eleonorenfalken 12. Die Anpassung des Jagdverhaltens an die vom Wind abhängigen Zugvogelhäufigkeiten. Vogelwarte 1983, 32, 7–13. [Google Scholar]
- Ristow, D.; Wink, C.; Wink, M.; Friemann, H. Biologie des Eleonorenfalken. 14. Das Brutreifealter der Weibchen. J. Ornithol. 1983, 124, 291–293. [Google Scholar] [CrossRef]
- Wink, M.; Wink, C.; Ristow, D. Biologie des Eleonorenfalken: 8. Die Gelegegröße in Relation zum Nahrungsangebot, Jagderfolg und Gewicht der Altfalken. J. Ornithol. 1980, 121, 387–390. [Google Scholar] [CrossRef]
- Wink, M.; Wink, C.; Ristow, D. Biologie des Eleonorenfalken. 9. Eitemperaturen und Körpertemperaturen juveniler und adulter Falken. Vogelwarte 1980, 30, 320–325. [Google Scholar]
- Wink, M.; Wink, C.; Ristow, D. Biologie des Eleonorenfalken: 10. Einfluß der Horstlage auf den Bruterfolg. J. Ornithol. 1982, 123, 401–408. [Google Scholar] [CrossRef]
- Wink, M.; Wink, C.; Ristow, D. Biology of Eleonora`s Falcon. 7. Variability of clutch size, egg dimensions and egg colouring. Raptor Res. 1985, 19, 8–14. [Google Scholar]
- Swatschek, I.; Ristow, D.; Scharlau, W.; Wink, C.; Wink, M. Populationsgenetik und Vaterschaftsanalyse beim Eleonorenfalken (Falco eleonorae). J. Ornithol. 1993, 134, 137–143. [Google Scholar] [CrossRef]
- Urios, G.; Martínez-Abraín, A. The study of nest-site preferences in Eleonora’s Falcon Falco eleonorae through digital terrain models on a western Mediterranean island. J. Ornithol. 2005, 147, 13–23. [Google Scholar] [CrossRef]
- Kassara, C.; Dimalexis, A.; Fric, J.; Karris, G.; Barboutis, C.; Sfenthourakis, S. Nest-site preferences of Eleonora’s Falcon (Falco eleonorae) on uninhabited islets of the Aegean Sea using GIS and species distribution models. J. Ornithol. 2012, 153, 663–675. [Google Scholar] [CrossRef]
- Gangoso, L.; López-López, P.; Grande, J.M.; Mellone, U.; Limiñana, R.; Figuerola, J.; Alonso-Alvarez, C. Ecological specialization to fluctuating resources prevents long-distance migratory raptors from becoming sedentary on islands. PLoS ONE 2013, 8, e61615. [Google Scholar] [CrossRef]
- Gutiérrez-López, R.; Gangoso, L.; Martínez-de la Puente, J.; Fric, J.; López-López, P.; Mailleux, M.; Muñoz, J.; Touati, L.; Samraoui, B.; Figuerola, J. Low prevalence of blood parasites in a long-distance migratory raptor: The importance of host habitat. Parasites Vectors 2015, 8, 189. [Google Scholar] [CrossRef]
- Steen, R.; Miliou, A.; Tsimpidis, T.; Selås, V.; Sonerud, G.A. Nonparental infanticide in colonial Eleonora’s Falcons (Falco eleonorae). J. Raptor Res. 2016, 50, 217–220. [Google Scholar] [CrossRef]
- Touati, L.; Nedjah, R.; Samraoui, F.; Alfarhan, A.H.; Gangoso, L.; Figuerola, J.; Samraoui, B. On the brink: Status and breeding ecology of Eleonora’s Falcon Falco eleonorae in Algeria. Bird Conserv. Int. 2017, 27, 594–606. [Google Scholar] [CrossRef]
- Hadjikyriakou, T.G.; Nwankwo, E.C.; Virani, M.Z.; Kirschel, A.N.G. Habitat availability influences migration speed, refueling patterns and seasonal flyways of a fly-and-forage migrant. Mov. Ecol. 2020, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Jemaa, H.B.; Nefla, A.; Bouragaoui, Z.; Nouira, S. Breeding biology of the Eleonora’s Falcon, Falco eleonorae within the Galite archipelago. Biologia 2021, 76, 1491–1499. [Google Scholar]
- Xirouchakis, S.M.; Botsidou, P.; Baxevani, K.; Andreou, G.; Tsaparis, D. Brood sex ratio variation in a colonial raptor, the Eleonora’s Falcon, Falco eleonorae. Anim. Behav. 2023, 195, 93–106. [Google Scholar] [CrossRef]
- Xirouchakis, S.M.; Fric, B.J.; Kassara, B.C.; Portolou, D.; Dimalexis, B.A.; Karris, B.G.; Barboutis, C.; Latsoudis, B.P.; Bourdakis, B.S.; Kakalis, E.; et al. Variation in breeding parameters of Eleonora’s Falcon (Falco eleonorae) and factors affecting its reproductive performance. Ecol. Res. 2012, 27, 407–416. [Google Scholar] [CrossRef]
- Rosén, M.; Hedenström, A.; Badami, A.; Spina, F.; Åkesson, S. Hunting flight behaviour of the Eleonora’s Falcon Falco eleonorae. J. Avian Biol. 1999, 30, 342–350. [Google Scholar] [CrossRef]
- Hedenström, A.; Rosen, M. Predator versus prey: On aerial hunting and escape strategies in birds. Behav. Ecol. 2001, 12, 150–156. [Google Scholar] [CrossRef]
- Hedenström, A.; Rosén, M.; Åkesson, S.; Spina, F. Flight performance during hunting excursions in Eleonora’s Falcon Falco eleonorae. J. Exp. Biol. 1999, 202, 2029–2039. [Google Scholar] [CrossRef]
- Rosén, M.; Hedenström, A. Soaring Flight in the Eleonora’s Falcon (Falco eleonorae). Auk 2002, 119, 835–840. [Google Scholar] [CrossRef]
- Buij, R.; Gschweng, M. Nocturnal hunting by Eleonora’s Falcons Falco eleonorae on their breeding and non-breeding grounds. Acta Ornithol. 2017, 52, 35–49. [Google Scholar] [CrossRef]
- Xirouchakis, S.M.; Panuccio, M. Hunting altitude of Eleonora’s Falcon (Falco eleonorae) over a breeding colony. J. Raptor Res. 2019, 53, 56–65. [Google Scholar] [CrossRef]
- Wink, M.; Wink, C.; Ristow, D. 1978: Biologie des Eleonorenfalken (Falco eleonorae). 2. Zur Vererbung der Gefiederphasen (hell- dunkel). J. Ornithol. 1978, 119, 421–428. [Google Scholar] [CrossRef]
- Ristow, D.; Wink, C.; Wink, M.; Scharlau, W. Colour polymorphism in Eleonora’s Falcon, Falco eleonorae. Sandgrouse 1998, 20, 56–64. [Google Scholar]
- Ristow, D.; Witte, L.; Wink, M. A characterization of the homozygous dark morph of Eleonora’s Falcon. Sandgrouse 2000, 22, 118–121. [Google Scholar]
- Gangoso, L.; Afán, I.; Grande, J.M.; Figuerola, J. Sociospatial structuration of alternative breeding strategies in a color polymorphic raptor. Behav. Ecol. 2015, 26, 1119–1130. [Google Scholar] [CrossRef]
- Gangoso, L.; Grande, J.M.; Ducrest, A.L.; Figuerola, J.; Bortolotti, G.R.; Andrés, J.A.; Roulin, A. MC1R-dependent, melanin-based colour polymorphism is associated with cell-mediated response in the Eleonora’s Falcon. J. Evol. Biol. 2011, 24, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Ristow, D.; Conrad, B.; Wink, C.; Wink, M. Pesticide residues of failed eggs of Eleonoras Falcon, Falco eleonorae, from an Aegean colony. Ibis 1980, 122, 74–76. [Google Scholar] [CrossRef]
- Bianchi, N.P.; Ancora, S.; di Fazio, N.; Leonzio, C. Cadmium, lead, and mercury levels in feathers of small passerine birds: Noninvasive sampling strategy. Environ. Toxicol. Chem. 2008, 27, 2064–2070. [Google Scholar] [CrossRef] [PubMed]
- Tsarpali, V.; Kassara, C.; Barboutis, C.; Papadimitraki, M.; Kloukinioti, M.; Giokas, S.; Dailianis, S. Assessing the seasonal and intrinsic variability of neurotoxic and cyto-genotoxic biomarkers in blood of free-living Eleonoras’ Falcons. Sci. Total Environ. 2020, 711, 135101. [Google Scholar] [CrossRef] [PubMed]
- Rial-Berriel, C.; Acosta-Dacal, A.; Cabrera Pérez, M.Á.; Suárez-Pérez, A.; Melián, A.; Zumbado, M.; Henríquez Hernández, L.A.; Ruiz-Suárez, N.; Rodriguez Hernández, Á.; Boada, L.D.; et al. Intensive livestock farming as a major determinant of the exposure to anticoagulant rodenticides in raptors of the Canary Islands (Spain). Sci. Total. Environ. 2021, 768, 144386. [Google Scholar] [CrossRef]
- Ristow, D.; Scharlau, W.; Wink, M. Population structure and mortality of Eleonora’s Falcon Falco eleonorae. In Raptors in the Modern World; Meyburg, B.-U., Chancellor, R.D., Eds.; WWGBP: Berlin, Germany; London, UK; Paris, France, 1989; pp. 321–326. [Google Scholar]
- Ristow, D.; Wink, M. Seasonal variation in sex ratio of fledging Eleonora’s Falcon, Falco eleonorae. Raptor Res. 2004, 38, 320–325. [Google Scholar]
- Wink, M.; Ristow, D. Biology and molecular genetics of Eleonora’s Falcon (Falco eleonorae), a colonial raptor of Mediterranean islands. In Raptors at Risk; Chancellor, R.D., Meyburg, B.-U., Eds.; WWGBP/Hancock House: Surrey, BC, Canada, 2000; pp. 653–668. [Google Scholar]
- Wink, M.; Wink, C.; Scharlau, W.; Ristow, D. Ortstreue und Genfluß bei Inselvogelarten: Eleonorenfalke (Falco eleonorae) und Gelbschnabelsturmtaucher (Calonectris diomedea). J. Ornithol. 1987, 128, 485–488. [Google Scholar] [CrossRef]
- Gschweng, M.; Kalko, E.; Querner, U.; Fiedler, W.; Berthold, P. All across Africa: Highly individual migration routes of Eleonora’s Falcon. Proc. R. Soc. B. 2008, 275, 2887–2896. [Google Scholar] [CrossRef]
- López-López, P.; Limiñana, R.; Urios, V. Autumn migration of Eleonora’s Falcon Falco eleonorae tracked by satellite telemetry. Zool. Stud. 2009, 48, 485–491. [Google Scholar]
- Mellone, U.; López-López, P.; Limiñana, R.; Urios, V. Summer pre-breeding movements of Eleonora’s Falcon Falco eleonorae revealed by satellite telemetry: Implications for conservation. Bird Conserv. Int. 2013, 23, 487–494. [Google Scholar] [CrossRef]
- Mellone, U.; López-López, P.; Limiñana, R.; Urios, V. Weather conditions promote route flexibility during open ocean crossing in a long-distance migratory raptor. Int. J. Biometeorol. 2011, 55, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Kassara, C.; Evangelidis, A.; Tsiopelas, N.; Barboutis, C.; Giokas, S. Seasonal and daily activity patterns by Eleonora’s Falcon Falco eleonorae based on GPS telemetry: A contribution to the species’ movement ecology at its breeding grounds. Bird Conserv. Int. 2021, 32, 154–171. [Google Scholar] [CrossRef]
- Kassara, C.; Fric, J.; Gschweng, M.; Sfenthourakis, S. Complementing the puzzle of Eleonora’s Falcon (Falco eleonorae) migration: New evidence from an eastern colony in the Aegean Sea. J. Ornithol. 2012, 153, 839–848. [Google Scholar] [CrossRef]
- Kassara, C.; Fric, J.; Sfenthourakis, S. Distribution modelling of Eleonora’s Falcon Falco eleonorae Géné, 1839 occurrence in its wintering grounds: A niche-based approach with satellite telemetry data. Bird Conserv. Int. 2014, 24, 100–113. [Google Scholar] [CrossRef]
- Kassara, C.; Gangoso, L.; Mellone, U.; Piasevoli, G.; Hadjikyriakou, T.G.; Tsiopelas, N.; Giokas, S.; López-López, P.; Urios, V.; Figuerola, J.; et al. Current and future suitability of wintering grounds for a long-distance migratory raptor. Sci. Rep. 2017, 7, 8798. [Google Scholar] [CrossRef] [PubMed]
- Hadjikyriakou, T.G.; Kassara, C.; de Roland, L.-A.R.; Giokas, S.; Tsiopelas, N.; Evangelidis, A.; Thorstrom, R.; Kirschel, A.N.G. Phenology, variation in habitat use, and daily activity patterns of Eleonora’s Falcon overwintering in Madagascar. Landsc. Ecol. 2020, 35, 159–172. [Google Scholar] [CrossRef]
- Vansteelant, W.M.; Gangoso, L.; Viana, D.S.; Shamoun-Baranes, J.Z.; Figuerola, J. A trans-African migrant shows repeatable route choice in males and repeatable timing in females. J. Avian Biol. 2023, e03050. [Google Scholar] [CrossRef]
- Vansteelant, W.; Gangoso, L.; Bouten, W.; Viana, D.S.; Figuerola, J. Adaptive drift and barrier-avoidance by a fly-forage migrant along a climate-driven flyway. Mov. Ecol. 2021, 9, 37. [Google Scholar] [CrossRef]
- Ferguson-Lees, J.; Christie, D.A. Raptors of the World; Christopher Helm: London, UK, 2001. [Google Scholar]
- Bateson, P.P.G.; Nisbet, J.C.T. Autumn migration in Greece. Ibis 1961, 1003, 503–514. [Google Scholar] [CrossRef]
- Ristow, D. On the insect diet of Eleonora’s Falcon Falco eleonorae and its importance for coloniality. In Raptors Worldwide; Chancellor, R.D., Meyburg, B.-U., Eds.; WWGP/MME: Budapest, Hungary, 2004; pp. 705–712. [Google Scholar]
- Wink, M.; Wink, C.; Ristow, D. Biologie des Eleonorenfalken. 11. Biometrie des Sexualdimorphismus adulter und flügger Falken. Vogelwelt 1982, 103, 225–229. [Google Scholar]
- Araujo, J.; Munoz-Cobo, J.; Purroy, F.J. Las rapaces y aves marinas del archipelago de Cabrera. Nat. Hisp. 1977, 12, 1–94. [Google Scholar]
- Spina, F.; Scappi, A.; Berthemy, B.; Pinna, G. The diet of Eleonora’s Falcon (Falco eleonorae) in a colony of the western coast of Sardinia with some remarks on the migration of small passerines through the Mediterranean. Suppl. Ric. Biol. Selvag. 1982, 12, 235–252. [Google Scholar]
- Mayol, J. Estudios sobre el halcon de Eleonor, Falco eleonorae, en las islas Baleares. Ardeola 1976, 23, 104–136. [Google Scholar]
- Belenguer, R.; Tena, V.; Ménez, J. Halcón de Eleonora: El pirata de Columbretes. Quercus 2004, 24, 9–16. [Google Scholar]
- Clark, A.L. Ecology of Eleonora’s Falcon in Morocco. Ph.D. Thesis, Cornell University, Ithaca, NY, USA, 1981. [Google Scholar]
- Azafzaz, H. Numbers of Falco Eleonorae Breeding in Tunisia in 2004; Manuscript 2004; GTO/AAO: Ariana, Tunisia, 2004. [Google Scholar]
- Phillips, N.J.; Round, P.D. Crete Ringing Group 1975. Report 1973–1975.
- Hansen, W.; Oelke, H. Bestimmungsbuch für Rupfungen und Mauserfedern; Beiträge zur Naturkunde Niedersachsens 1973–1983; Niedersächsisches Landesmuseum Hannover: Hannover, Germany, 1973; pp. 26–36. [Google Scholar]
- Blasco-Zumeta, J.; Heinze, G.-M. Identification Atlas of the Continental Birds of Southwestern Europe; Tundra Ediciones: Castellón, Spain, 2023. [Google Scholar]
- Pieper, H.; Ristow, D. Prey of a Peregrine Falco peregrinus pair off Crete. Il-Merill 2002, 30, 29–31. [Google Scholar]
- Limbrunner, A.; Bezzel, E.; Richarz, K.; Singer, D. Enzyklopädie der Brutvögel Europas; Kosmos-Verlag: Stuttgart, Germany, 2013. [Google Scholar]
- Brochet, A.-L.; Bossche, W.V.D.; Jones, V.R.; Arnardottir, H.; Damoc, D.; Demko, M.; Driessens, G.; Flensted, K.; Gerber, M.; Ghasabyan, M.; et al. Illegal killing and taking of birds in Europe outside the Mediterranean: Assessing the scope and scale of a complex issue. Bird Conserv. Int. 2019, 29, 10–40. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).