DNA Metabarcoding Analysis of Arthropod Diversity in Dust from the Natural History Museum, Vienna
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
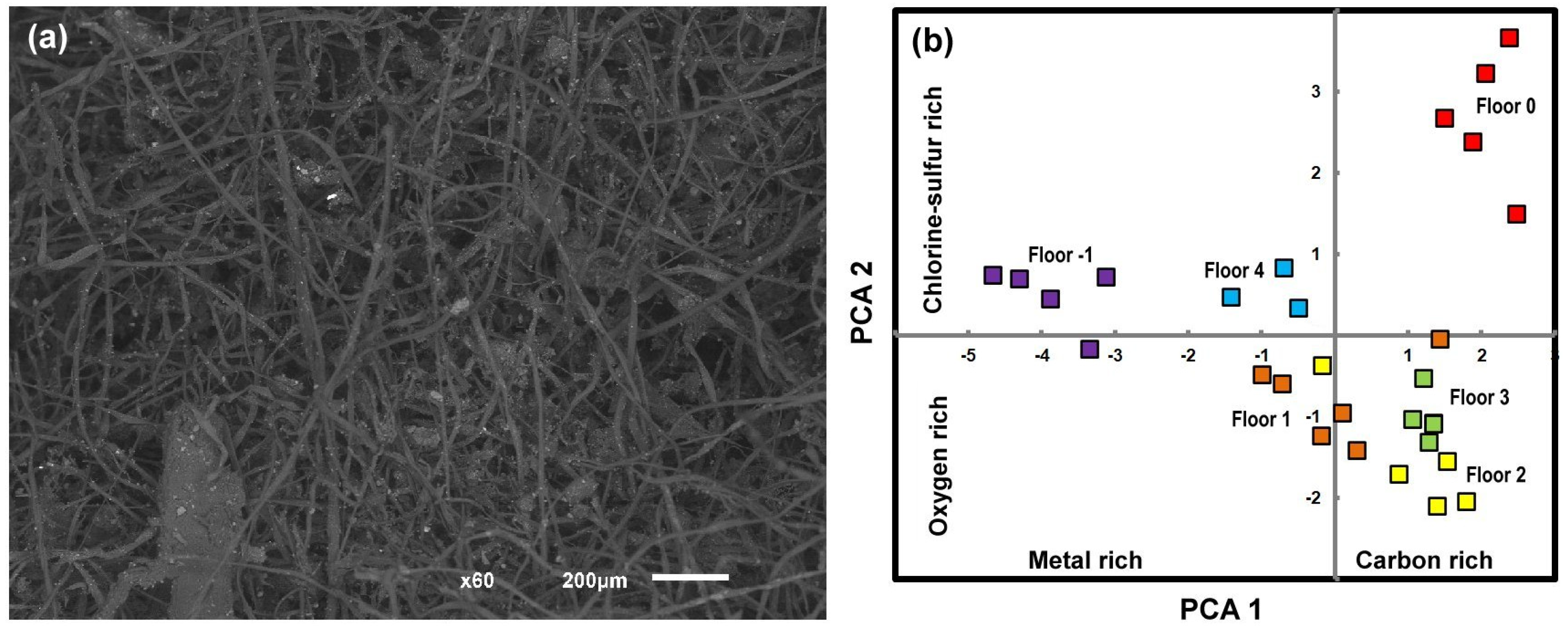
2.2. Dust Sampling to Collect DNA Within
2.3. Insect Trapping to Collect DNA Within
2.4. DNA Analysis
2.5. Species Reduction
3. Results
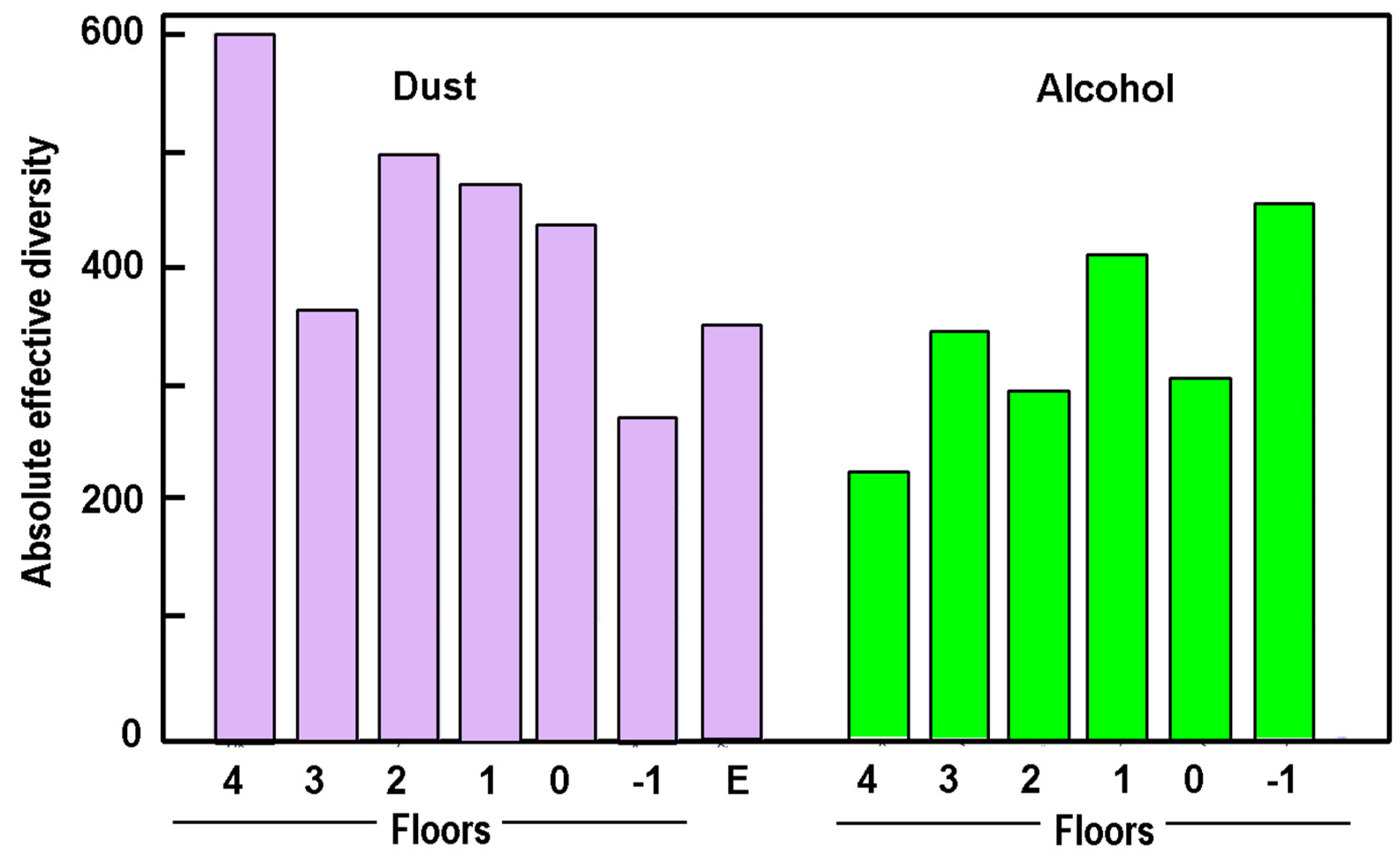
3.1. Total Species Richness
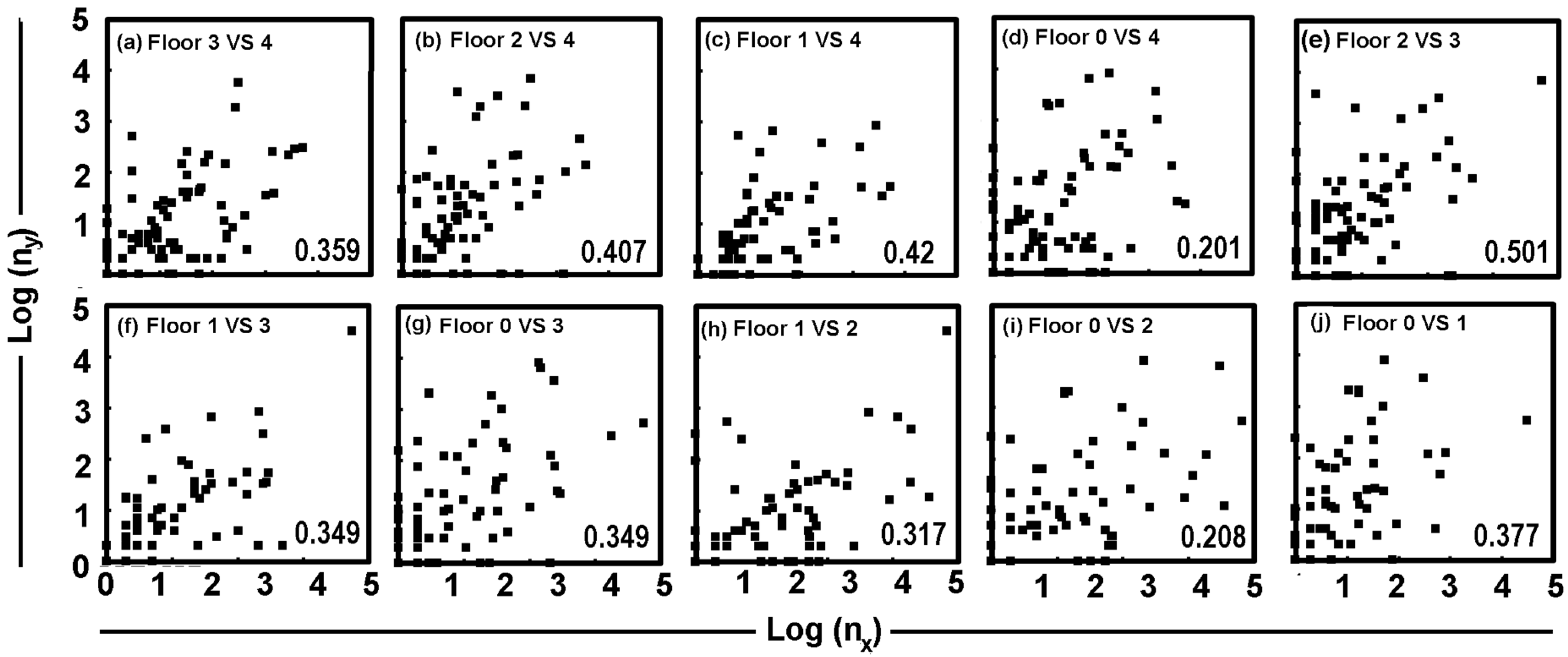
3.2. Species Richness in the Dust Samples and Trap Extracts
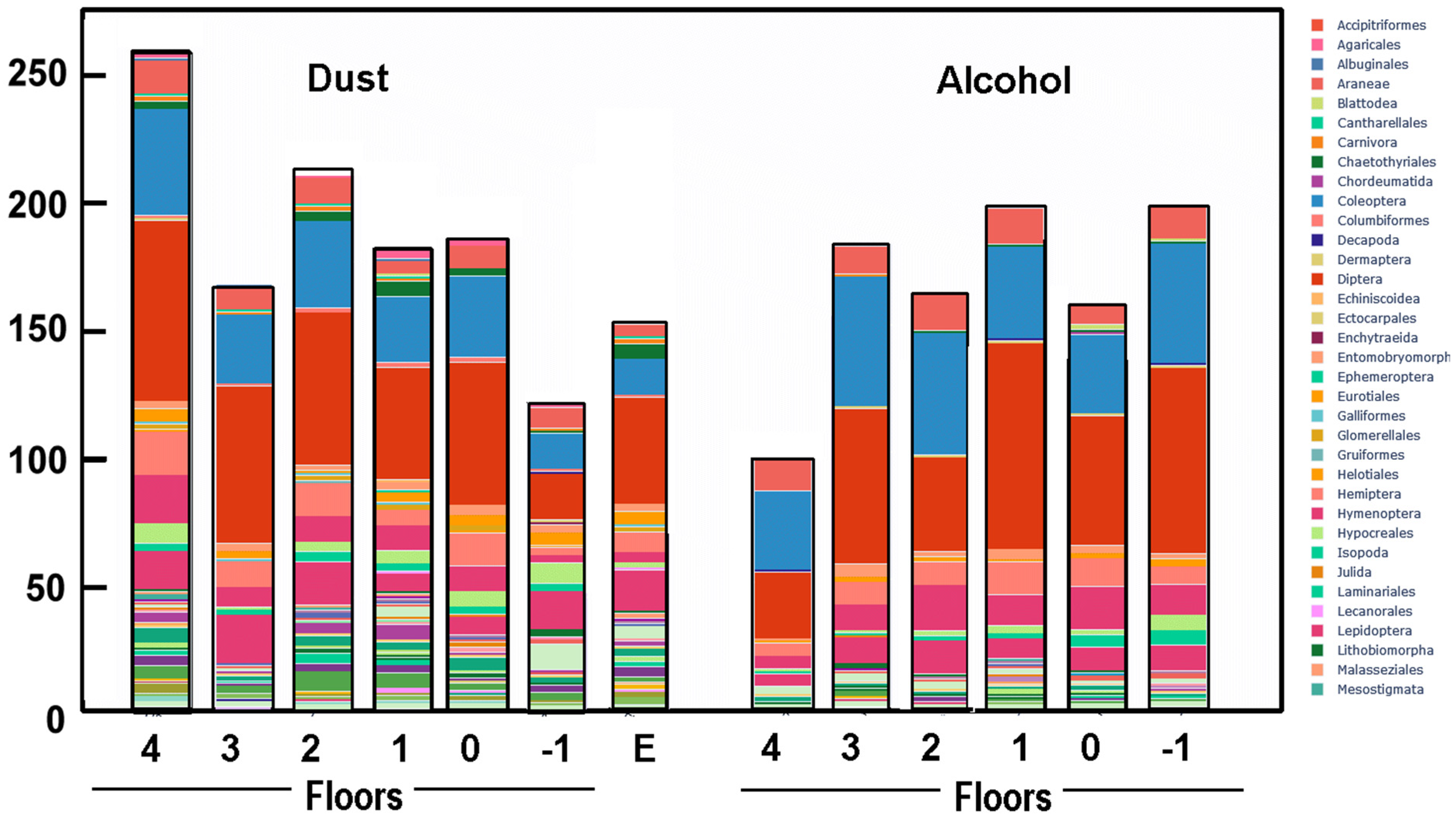
3.3. Arthropod Species Assemblage
3.4. Museum Pests in the Dust Samples
3.5. Species Richness of Pests across the Floors
3.6. Comparison of Pest DNA and Insect Monitoring
3.7. Non Arthropods Species Found in the DNA Samples
4. Discussion
4.1. Species in DNA Dust and Trapped Samples
4.2. Usefulness of DNA Samples
- Advantages of DNA analysis of dust samples
- Cheap sampling method and requires just a few samples
- Simple to collect, no specific training needed to take the samples
- Not only pest species are identified to the species level but also a broad list of arthropods
- Disadvantages of DNA analysis of dust samples:
- Not sure if the animals found were alive or already long dead
- Not sure how many individuals were collected
- The number of reads gives only a rough estimate on species abundance
- Get a long list of species, but many of them are not relevant to IPM
- Reference database is incomplete; this is also true for many pest species.
- Active infestations of objects mean visual inspection remains necessary
- Hard to compare results over time (months, years)
- Problem with contamination of non-target DNA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sattler, T.; Obrist, M.K.; Duelli, P.; Moretti, M. Urban arthropod communities: Added value or just a blend of surrounding biodiversity? Landsc. Urban Plan. 2011, 103, 347–361. [Google Scholar] [CrossRef]
- Urban, M.C.; Alberti, M.; De Meester, L.; Zhou, Y.; Verrelli, B.C.; Szulkin, M.; Schmidt, C.; Savage, A.M.; Roberts, P.; Rivkin, L.R.; et al. Interactions between climate change and urbanization will shape the future of biodiversity. Nat. Clim. Change 2024, 14, 436–447. [Google Scholar] [CrossRef]
- Turo, K.J.; Gardiner, M.M. The balancing act of urban conservation. Nat. Commun. 2020, 11, 3773. [Google Scholar] [CrossRef]
- McKinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosyst. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Marzluff, J.M. Worldwide urbanization and its effects on birds. In Avian Ecology and Conservation in an Urbanizing World; Springer: Boston, MA, USA, 2001; pp. 19–47. [Google Scholar]
- Grimm, N.B.; Faeth, S.H.; Golubiewski, N.E.; Redman, C.L.; Wu, J.; Bai, X.; Briggs, J.M. Global Change and the Ecology of Cities. Science 2008, 319, 756–760. [Google Scholar] [CrossRef]
- Murray, M.H.; Sánchez, C.A.; Becker, D.J.; Byers, K.A.; Worsley-Tonks, K.E.L.; Craft, M.E. City sicker? A meta-analysis of wildlife health and urbanization. Front. Ecol. Environ. 2019, 17, 575–583. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Lepczyk, C.A.; Aronson, M.F.J.; Evans, K.L.; Goddard, M.A.; Lerman, S.B.; MacIvor, J.S. Biodiversity in the City: Fundamental Questions for Understanding the Ecology of Urban Green Spaces for Biodiversity Conservation. BioScience 2017, 67, 799–807. [Google Scholar] [CrossRef]
- Niemelä, J. Ecology and urban planning. Biodivers. Conserv. 1999, 8, 119–131. [Google Scholar] [CrossRef]
- Dunn, R. Never Home Alone—From Microbes to Millipedes, Camel Crickets, and Honeybees, the Natural History of Where We Live; Basic Books; Hachette Book Group: New York, NY, USA, 2018. [Google Scholar]
- Pernstich, A.; Krenn, H. (Eds.) Die Tierwelt des Botanischen Gartens der Universität Wien eine Oase Inmitten der Großstadt anläßlich des 250-jährigen Bestehens des Botanischen Gartens der Universität Wien (1754–2004); Institut für Angewandte Biologie und Umweltbildung Wien: Vienna, Austria, 2004. [Google Scholar]
- Dunn, R.R.; Burger, J.R.; Carlen, E.J.; Koltz, A.M.; Light, J.E.; Martin, R.A.; Munshi-South, J.; Nichols, L.M.; Vargo, E.L.; Yitbarek, S.; et al. A Theory of City Biogeography and the Origin of Urban Species. Front. Cons. Sci. 2022, 3, 761449. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Urban_ecology (accessed on 8 June 2024).
- Bertone, M.A.; Leong, M.; Bayless, K.M.; Malow, T.L.; Dunn, R.R.; Trautwein, M.D. Arthropods of the great indoors: Characterizing diversity inside urban and suburban homes. PeerJ 2016, 4, e1582. [Google Scholar] [CrossRef] [PubMed]
- Madden, A.A.; Barberán, A.; Bertone, M.A.; Menninger, H.L.; Dunn, R.R.; Fierer, N. The diversity of arthropods in homes across the United States as determined by environmental DNA analyses. Mol. Ecol. 2016, 25, 6214–6224. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.; Bertone, M.A.; Bayless, K.M.; Dunn, R.R.; Trautwein, M.D. Exoskeletons and economics: Indoor arthropod diversity increases in affluent neighbourhoods. Biol. Lett. 2016, 12, 20160322. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.; Bertone, M.A.; Savage, A.M.; Bayless, K.M.; Dunn, R.R.; Trautwein, M.D. The habitats humans provide: Factors affecting the diversity and composition of arthropods in houses. Sci. Rep. 2017, 7, 15347. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H. Urban Insects and Arachnids—A Handbook of Urban Entomology; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar] [CrossRef]
- Schoelitsz, B.; Meerburg, B.G.; Takken, W. Influence of the public’s perception, attitudes, and knowledge on the implementation of integrated pest management for household insect pests. Entomol. Exp. Appl. 2019, 167, 14–26. [Google Scholar] [CrossRef]
- Cranshaw, W. A review of nuisance invader household pests of the United States. Am. Entomol. 2011, 57, 165–169. [Google Scholar] [CrossRef][Green Version]
- Rust, M.K.; Su, N.Y. Managing social insects of urban importance. Annu. Rev. Entomol. 2012, 57, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Brokerhof, A.W.; Zanen, W.B.; Watering, K.; Porck, H. Buggy Biz, Integrated Pest Management in Collections; Netherlands Institute for Cultural Heritage (ICN) and IADA: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Pinniger, D. Integrated Pest Management in Cultural Heritage; Archetype Publications: London, UK, 2015. [Google Scholar]
- Pinniger, D.; Lauder, D. Pests in Houses Great and Small: Identification, Prevention and Eradication; English Heritage: Swindon, UK, 2018. [Google Scholar]
- Querner, P. Insect pests and integrated pest management in museums, libraries and historic buildings. Insects 2015, 6, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Trematerra, P.; Pinniger, D. Museum pests–cultural heritage pests. In Recent Advances in Stored Product Protection; Springer: Berlin/Heidelberg, Germany, 2018; pp. 229–260. [Google Scholar]
- Strang, T.; Jacobs, J.; Kigawa, R. Integrated pest management for museum collections. In Preventive Conservation: Collection Storage; Society for the Preservation of Natural History Collections: New York, NY, USA, 2019; pp. 375–406. [Google Scholar]
- Brimblecombe, P.; Querner, P. Investigating insect catch metrics from a large Austrian museum. J. Cult. Herit. 2024, 66, 375–383. [Google Scholar] [CrossRef]
- Brimblecombe, P. Predicting the changing insect threat in the UK heritage environment. J. Inst. Conserv. 2024, 47, 133–148. [Google Scholar] [CrossRef]
- Available online: www.wheateatingyourcollection (accessed on 8 June 2024).
- Querner, P. Linking webbing clothes moths to infested object or other food source in museums. Stud. Conserv. 2016, 61, 111–117. [Google Scholar] [CrossRef]
- Beltrame, T.N. A Matter of Dust, Powdery Fragments, and Insects. Object Temporalities Grounded in Social and Material Museum Life. Centaurus 2023, 65, 365–385. [Google Scholar] [CrossRef]
- Marcotte, S.; Estel, L.; Minchin, S.; Leboucher, S.; Le Meur, S. Monitoring of lead, arsenic and mercury in the indoor air and settled dust in the Natural History Museum of Rouen (France). Atmos. Pollut. Res. 2017, 8, 483–489. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Brimblecombe, P. Contribution of dust at floor level to particle deposit within the Sainsbury Centre for Visual Arts. Stud. Conserv. 2000, 45, 127. [Google Scholar] [CrossRef]
- Butte, W.; Heinzow, B. Pollutants in house dust as indicators of indoor contamination. Rev. Environ. Contam. Toxicol. 2002, 175, 46. [Google Scholar]
- Craine, J.M.; Barberán, A.; Lynch, R.C.; Menninger, H.L.; Dunn, R.R.; Fierer, N. Molecular analysis of environmental plant DNA in house dust across the United States. Aerobiologia 2017, 33, 71–86. [Google Scholar] [CrossRef]
- Lennartz, C.; Kurucar, J.; Coppola, S.; Crager, J.; Bobrow, J.; Bortolin, L.; Comolli, J. Geographic source estimation using airborne plant environmental DNA in dust. Sci. Rep. 2021, 11, 16238. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.G.; Smith, D. Biodiversity and museum pest management. In Insect Biodiversity: Science and Society; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 221–243. [Google Scholar]
- Querner, P.; Morelli, M. Nachweis von Museumsschädlingen in Schmutz. Restauro 2009, 2, 85. [Google Scholar]
- Arbes, S.J., Jr.; Cohn, R.D.; Yin, M.; Muilenberg, M.L.; Burge, H.A.; Friedman, W.; Zeldin, D.C. House dust mite allergen in US beds: Results from the First National Survey of Lead and Allergens in Housing. J. Allergy Clin. Immunol. 2003, 111, 408–414. [Google Scholar] [CrossRef]
- Gilbert, M.T. Documenting DNA in the dust. Mol. Ecol. 2017, 26, 969–971. [Google Scholar] [CrossRef][Green Version]
- Barberán, A.; Dunn, R.R.; Reich, B.J.; Pacifici, K.; Laber, E.B.; Menninger, H.L.; Morton, J.M.; Henley, J.B.; Leff, J.W.; Miller, S.L.; et al. The ecology of microscopic life in household dust. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151139. [Google Scholar] [CrossRef] [PubMed]
- Foster, N.R.; Martin, B.; Hoogewerff, J.; Aberle, M.G.; de Caritat, P.; Roffey, P.; Edwards, R.; Malik, A.; Thwaites, P.; Waycott, M.; et al. The utility of dust for forensic intelligence: Exploring collection methods and detection limits for environmental DNA, elemental and mineralogical analyses of dust samples. Forensic Sci. Int. 2023, 344, 111599. [Google Scholar] [CrossRef] [PubMed]
- Meiklejohn, K.A.; Scheible, M.K.R.; Boggs, L.M.; Dunn, R.R.; Ricke, D.O. Using Fast ID to analyze complex SNP mixtures from indoor dust. J. Forensic Sci. 2023, 68, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Mol. Ecol. Resour. 2007, 7, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, A.M.; Gan, H.; Wickings, K.; Fierer, N. A DNA metabarcoding approach to characterize soil arthropod communities. Soil Biol. Biochem. 2018, 125, 37–43. [Google Scholar] [CrossRef]
- Banerjee, P.; Dey, G.; Antognazza, C.M.; Kumar Sharma, R.; Maity, J.P.; Chan, M.W.Y.; Huang, Y.-H.; Lin, P.-Y.; Chao, H.-C.; Lu, C.-M.; et al. Reinforcement of Environmental DNA Based Methods (Sensu Stricto) in Biodiversity Monitoring and Conservation: A Review. Biology 2021, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- Roger, F.; Ghanavi, H.R.; Danielsson, N.; Wahlberg, N.; Löndahl, J.; Pettersson, L.B.; Andersson, G.K.S.; Olén, N.B.; Clough, Y. Airborne environmental DNA metabarcoding for the monitoring of terrestrial insects—A proof of concept from the field. Environ. DNA 2022, 4, 790–807. [Google Scholar] [CrossRef]
- Elbrecht, V.; Braukmann, T.W.A.; Ivanova, N.V.; Prosser, S.W.J.; Hajibabaei, M.; Wright, M.; Zakharov, E.V.; Hebert, P.D.N.; Steinke, D. Validation of COI metabarcoding primers for terrestrial arthropods. PeerJ 2019, 7, e7745. [Google Scholar] [CrossRef]
- Elbrecht, V.; Bourlat, S.J.; Hörren, T.; Lindner, A.; Mordente, A.; Noll, N.W.; Schäffler, L.; Sorg, M.; Zizka, V.M.A. Pooling size sorted Malaise trap fractions to maximize taxon recovery with metabarcoding. PeerJ 2021, 9, e12177. [Google Scholar] [CrossRef]
- Hospodsky, D.; Qian, J.; Nazaroff, W.W.; Yamamoto, N.; Bibby, K.; Rismani-Yazdi, H.; Peccia, J. Human occupancy as a source of indoor airborne bacteria. PLoS ONE 2014, 9, e103425. [Google Scholar] [CrossRef]
- Adams, R.I.; Bateman, A.C.; Bik, H.M.; Meadow, J.F. Microbiota of the indoor environment: A meta-analysis. Microbiome 2015, 3, 49. [Google Scholar] [CrossRef]
- Barberán, A.; Ladau, J.; Leff, J.W.; Pollard, K.S.; Menninger, H.L.; Dunn, R.R.; Fierer, N. Continental-scale distributions of dust-associated bacteria and fungi. Proc. Nat. Acad. Sci. USA 2015, 112, 5756–5761. [Google Scholar] [CrossRef]
- Linard, B.; CramptonPlatt, A.; Gillett, C.P.D.T.; Timmermans, M.J.T.N.; Vogler, A.P. Metagenome skimming of insect specimen pools: Potential for comparative genomics. Genome Biol. Evol. 2015, 7, 1474–1489. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Liu, S.; Yang, Q.; Su, X.U.; Zhou, L.; Tang, M.; Fu, R.; Li, J.; Huang, Q. Ultra-deep sequencing enables high-fidelity recovery of biodiversity for bulk arthropod samples without PCR amplification. GigaScience 2013, 2, 4. [Google Scholar] [CrossRef]
- Elbrecht, V.; Lindner, A.; Manerus, L.; Steinke, D. A bright idea-metabarcoding arthropods from light fixtures. PeerJ 2021, 9, e11841. [Google Scholar] [CrossRef]
- Ritter, C.D.; Häggqvist, S.; Karlsson, D.; Sääksjärvi, I.E.; Muasya, A.M.; Nilsson, R.H.; Antonelli, A. Biodiversity assessments in the 21st century: The potential of insect traps to complement environmental samples for estimating eukaryotic and prokaryotic diversity using high-throughput DNA metabarcoding. Genome 2019, 62, 147–159. [Google Scholar] [CrossRef]
- Elbrecht, V.; Leese, F. Can DNA-based ecosystem assessments quantify species abundance? Testing primer bias and biomass—sequence relationships with an innovative metabarcoding protocol. PLoS ONE 2015, 10, e0130324. [Google Scholar] [CrossRef]
- Lynggard, C.; Nielsen, M.; Santos-Bay, L.; Gastauer, M.; Oliveira, G.; Bohmann, K. Vertebrate diversity revealed by metabarcoding of bulk arthropod samples from tropical forests. Environ. DNA 2019, 1, 329–341. [Google Scholar] [CrossRef]
- Sickel, W.Z.; Vera, M.A.; Scherges, A.; Bourlat, S.; Dieker, P. Abundance estimation with DNA metabarcoding—Recent advancements for terrestrial arthropods. Metabarcoding Metagenomics 2023, 7, e112290. [Google Scholar] [CrossRef]
- Clarke, L.J.; Soubrier, J.; Weyrich, L.S.; Cooper, A. Environmental metabarcodes for insects: In silico PCR reveals potential for taxonomic bias. Mol. Ecol. Resour. 2014, 14, 1160–1170. [Google Scholar] [CrossRef]
- Creedy, T.J.; Ng, W.S.; Vogler, A.P. Toward accurate species-level metabarcoding of arthropod communities from the tropical forest canopy. Ecol. Evol. 2019, 9, 3105–3116. [Google Scholar] [CrossRef] [PubMed]
- Krehenwinkel, H.; Fong, M.; Kennedy, S.; Huang, E.G.; Noriyuki, S.; Cayetano, L.; Gillespie, R. The effect of DNA degradation bias in passive sampling devices on metabarcoding studies of arthropod communities and their associated microbiota. PLoS ONE 2018, 13, e0189188. [Google Scholar] [CrossRef] [PubMed]
- Prosser, S.W.J.; de Waard, J.R.; Miller, S.E.; Hebert, P.D.N. DNA barcodes from century-old type specimens using next-generation sequencing. Mol. Ecol. Resour. 2016, 16, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, K.M.; Kline, R.J.; Rahman, M.S. Past, present, and future perspectives of environmental DNA (eDNA) metabarcoding: A systematic review in methods, monitoring, and applications of global eDNA. Glob. Ecol. Conserv. 2019, 17, e00547. [Google Scholar] [CrossRef]
- Steinke, D.; Braukmann, T.W.; Manerus, L.; Woodhouse, A.; Elbrecht, V. Effects of Malaise trap spacing on species richness and composition of terrestrial arthropod bulk samples. Metabarcoding Metagenomics 2021, 5, 43–50. [Google Scholar] [CrossRef]
- Steinke, D.; DeWaard, S.L.; Sones, J.E.; Ivanova, N.; Prosser, S.W.J.; Perez, K.; Braukmann, T.W.A.; Milton, M.; Zakharov, E.; DeWaard, J.R.; et al. Message in a Bottle-Metabarcoding enables biodiversity comparisons across ecoregions. GigaScience 2022, 11, giac040. [Google Scholar] [CrossRef]
- Meusnier, I.; Singer, G.A.C.; Landry, J.F.; Hickey, D.A.; Hebert, P.D.; Hajibabaei, M. A universal DNA mini-barcode for biodiversity analysis. BMC Genom. 2008, 9, 214. [Google Scholar] [CrossRef]
- Yu, D.W.; Ji, Y.; Emerson, B.C.; Wang, X.; Ye, C.; Yang, C.; Ding, Z. Biodiversity soup: Metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring: Biodiversity soup. Methods Ecol. Evol. 2012, 3, 613–623. [Google Scholar] [CrossRef]
- Wang, C.; Abbar, S.; Pan, X.; Ranabhat, S.; Cooper, R. Diversity and prevalence of nuisance arthropods detected by sticky traps in apartments in New Jersey. J. Econ. Entomol. 2023, 4, 1317–1320. [Google Scholar] [CrossRef]
- Chua, P.Y.S.; Bourlat, S.J.; Ferguson, C.; Korlevic, P.; Zhao, L.; Ekrem, T.; Meier, R.; Lawniczak, M.K.N. Future of DNA-based insect monitoring. Trends Genet. 2023, 39, 531–544. [Google Scholar] [CrossRef]
- Cheolwoon, W.; Mohammad Imtiaj, U.B.; Donghyun, K.; Priyanka, K.; Seung-Kyung, L.; Ji, Y.P.; Ke, D.; Kiyoung, L.; Naomichi, Y. DNA metabarcoding-based study on bacteria and fungi associated with house dust mites (Dermatophagoides spp.) in settled house dust. Exp. Appl. Acarol. 2022, 88, 329–347. [Google Scholar]
- Sepúlveda, C.N.; Biebl, S.; Pöllath, N.; Seifert, S.; Weiss, M.; Weibulat, T.; Triebel, D. GBIF-Compliant Data Pipeline for the Management and Publication of a Global Taxonomic Reference List of Pests in Natural History Collections. Biodivers. Inf. Sci. Stand. 2023, 7, e112391. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Querner, P. Webbing clothes moth catch and the management of heritage environments. Int. Biodeterior. Biodegrad. 2014, 96, 50–57. [Google Scholar] [CrossRef]
- Cox, P.; Pinniger, D. Biology, behaviour and environmentally sustainable control of Tineola bisselliella (Hummel) (Lepidoptera: Tineidae). J. Stored Prod. Res. 2007, 43, 2–32. [Google Scholar] [CrossRef]
- Querner, P.; Simon, S.; Morelli, M.; Fürenkranz, S. Insect pest management programs and results from their application in two large museum collections in Berlin and Vienna. Int. Biodeterior. Biodegrad. 2013, 84, 275–280. [Google Scholar] [CrossRef]
- Querner, P.; Sterflinger, K.; Piombino-Mascali, D.; Morrow, J.J.; Pospischil, R.; Piñar, G. Insect pests and Integrated Pest Management in the Capuchin Catacombs of Palermo, Italy. Int. Biodeterior. Biodegrad. 2018, 131, 107–114. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Lankester, P. Long-term changes in climate and insect damage in historic houses. Stud. Conserv. 2013, 58, 13–22. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Brimblecombe, C.T.; Thickett, D.; Lauder, D. Statistics of insect catch within historic properties. Herit. Sci. 2013, 1, 34. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Brimblecombe, C.T. Trends in insect catch at historic properties. J. Cult. Herit. 2015, 16, 127–133. [Google Scholar] [CrossRef]
- Shimoda, M.; Honda, K. Insect reactions to light and its applications to pest management. Appl. Entomol. Zool. 2013, 48, 413–421. [Google Scholar] [CrossRef]
- Doxon, E.D.; Davis, C.A.; Fuhlendorf, S.D. Comparison of two methods for sampling invertebrates: Vacuum and sweep-net sampling. J. Field Ornithol. 2011, 82, 60–67. [Google Scholar] [CrossRef]
- Available online: https://biblio.naturalsciences.be/rbins-publications/abc-txa/abc-taxa-08/chapter-15.pdf (accessed on 8 June 2024).
- Hava, J. Beetles of the Family Dermestidae of the Czech and Slovak Republics; Zoological Keys; Academia: Prague, Czech Republic, 2021. [Google Scholar]
- Weidner, H.; Sellenschlo, U. Vorratsschädlinge und Hausungeziefer: Bestimmungstabellen für Mitteleuropa; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010. [Google Scholar]
- Leray, M.; Yang, J.Y.; Meyer, C.P.; Mills, S.C.; Agudelo, N.; Ranwez, V.; Boehm, J.T.; Machida, R.J. A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: Application for characterizing coral reef fish gut contents. Front Zool 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Morinière, J.; de Araujo, B.C.; Lam, A.W.; Hausmann, A.; Balke, M.; Schmidt, S.; Hendrich, L.; Doczkal, D.; Fartmann, B.; Arvidsson, S.; et al. Species Identification in Malaise Trap Samples by DNA Barcoding Based on NGS Technologies and a Scoring Matrix. PLoS ONE 2016, 11, e0155497. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ftp.ncbi.nlm.nih.gov/blast/ (accessed on 8 June 2024).
- Available online: www.boldsystems.org (accessed on 8 June 2024).
- Porter, T.M.; Hajibabaei, M. Scaling up: A guide to high-throughput genomic approaches for biodiversity analysis. Mol. Ecol. 2018, 27, 313–338. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ftp.ncbi.nlm.nih.gov/pub/taxonomy/ (accessed on 8 June 2024).
- Uhler, J.; Redlich, S.; Zhang, J.; Hothorn, T.; Tobisch, C.; Ewald, J.; Thorn, S.; Seibold, S.; Mitesser, O.; Morinière, J.; et al. Relationship of insect biomass and richness with land use along a climate gradient. Nat. Commun. 2021, 12, 5946. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://en.wikipedia.org/wiki/Berlese_funnel (accessed on 8 June 2024).
- Hardy, A.C.; Milne, P.S. Studies in the distribution of insects by aerial currents. J. Anim. Ecol. 1938, 7, 199–229. [Google Scholar] [CrossRef]
- Glick, P.A. The Distribution of Insects, Spiders and Mites in the Air; Technical Bulletin; U.S. Department of Agriculture: Washington DC, USA, 1939; Volume 673. [Google Scholar]
- Johnson, C.G. The distribution of insects in the air and the empirical relation of density to height. J. Anim. Ecol. 1957, 26, 479–494. [Google Scholar] [CrossRef]
- Bell, J.R.; Bohan, D.A.; Shaw, E.M.; Weyman, G.S. Ballooning dispersal using silk: World fauna, phylogenies, genetics and models. Bull. Entomol. Res. 2005, 95, 69–114. [Google Scholar] [CrossRef]
- Komposch, C. Rote Liste der Weberknechte (Opiliones) Österreichs. In Rote Listen Gefährdeter Tiere Österreichs. Checklisten, Gefährdungsanalysen, Handlungsbedarf; Zulka, P., Ed.; Grüne The Distribution of Insects, Spiders and Mites in the Air; Technical Bulletin Reihe des Lebensministeriums: Vienna, Austria, 2009; Volume 14/3, pp. 397–483. [Google Scholar]
- Martens, J. Die Tierwelt Deutschlands 64. Teil, Weberknechte, Opiliones; VEB Gustav Fischer Verlag: Jena, Germany, 1978; Volume 464. [Google Scholar]
- Martens, J. Vier Dekaden Weberknechtforschung. Arachnol. Mitteilungen 2021, 62, 35–60. [Google Scholar] [CrossRef]
- Pinol, J.; San Andres, V.; Clare, E.L.; Mir, G.; Symondson, W.O.C. A pragmatic approach to the analysis of diets of generalist predators: The use of next-generation sequencing with no blocking primers. Mol. Ecol. Resour. 2014, 14, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Molero-Baltanás, R.; Mitchell, A.; Gaju-Ricart, M.; Robla, J. Worldwide revision of synanthropic silverfish (Insecta: Zygentoma: Lepismatidae) combining morphological and molecular data. J. Insect Sci. 2024, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Aak, A.; Hage, M.; Magerøy, Ø.; Byrkjeland, R.; Lindstedt, H.H.; Ottesen, P.; Rukke, B.A. Introduction, dispersal, establishment and societal impact of the long-tailed silverfish Ctenolepisma longicaudata (Escherich, 1905) in Norway. BioInvasions Rec. 2021, 10, 483–498. [Google Scholar] [CrossRef]
- Aak, A.; Rukke, B.A.; Ottesen, P.S. Long-Tailed Silverfish (Ctenolepisma longicadaudata)—Biology and Control; Norwegian Institute of Public Health Report; Norwegian Institute of Public Health: Oslo, Norway, 2019. [Google Scholar]
- Kulma, M.; Vrabec, V.; Patoka, J.; Rettich, F. The first established population of the invasive silverfish Ctenolepisma longicaudata (Escherich) in the Czech Republic. BioInvasions Rec. 2018, 7, 329–333. [Google Scholar] [CrossRef]
- Kulma, M.; Bubová, T.; Davies, M.P.; Boiocchi, F.; Patoka, J. Ctenolepisma longicaudatum Escherich (1905) Became a Common Pest in Europe: Case Studies from Czechia and the United Kingdom. Insects 2021, 12, 810. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Molero-Baltanás, R.; Petrtýl, M.; Patoka, J. Invasion of synanthropic silverfish continues: First established populations of Ctenolepisma calvum (Ritter, 1910) revealed in the Czech Republic. BioInvasions Rec. 2022, 11, 110–123. [Google Scholar] [CrossRef]
- Querner, P.; Szucsich, N.; Landsberger, B.; Erlacher, S.; Trebicki, L.; Grabowski, M.; Brimblecombe, P. Identification and spread of the Ghost Silverfish (Ctenolepisma calvum) among museums and homes in Europe. Insects 2022, 13, 855. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Pachler, M.C.; Querner, P. Effect of Indoor Climate and Habitat Change on Museum Insects during COVID-19 Closures. Heritage 2021, 4, 3497–3506. [Google Scholar] [CrossRef]
- Brimblecombe, P.; Querner, P. Silverfish (Zygentoma) in Austrian Museums before and during COVID-19 lockdown. Int. Biodeterior. Biodegrad. 2021, 164, 105296. [Google Scholar] [CrossRef]
- Edgar, R.C.; Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 2015, 31, 3476–3482. [Google Scholar] [CrossRef]
- Available online: https://quelestcetanimal-lagalerie.com/wp-content/uploads/2012/11/Family-Dermestidae-illustrated-key-to-British-species.pdf (accessed on 8 June 2024).
- Háva, J. Dermestidae. In Catalogue of Palaearctic Coleoptera, Volume 4. Elateroidea, Derodontoidea, Bostrichoidea, Lymexyloidea, Cleroidea and Cucujoidea; Löbl, I., Smetana, A., Eds.; Stenstrup Apollo Books: Stenstrup, Denmark, 2007; pp. 299–320. [Google Scholar]
- Tsvetanov, T.; Herrmann, A. First record of Attagenus smirnovi (Zhantiev, 1973) in Bulgaria (Insecta: Coleoptera: Dermestidae). ZooNotes 2022, 211, 1–4. [Google Scholar]
- Holloway, G.; Pinniger, D. Anthrenus species (Coleoptera; Dermestidae) found in UK museums with special reference to A. museorum Linnaeus, 1761, the museum beetle. J. Nat. Sci. Collect. 2020, 7, 68–71. [Google Scholar]
- Holloway, G.; Bakaloudis, D. Anthrenus flavipes LeConte, 1854 (Coleoptera; Dermestidae); a destructive pest of natural history specimens. J. Nat. Hist. Collect. 2021, 8, 39–43. [Google Scholar]
- Holloway, G. A review of the species of Anthrenus Geoffroy, 1762, (Coleoptera: Dermestidae) on the British list. Entomol. Mon. Mag. 2020, 156, 11–18. [Google Scholar] [CrossRef]
- Adams, R.G. Anthrenus olgae Kalik, new to Britain (Coleoptera: Dermestidae) with notes of separation from A. caucasicus Reitter. Entomol. Gaz. 1988, 39, 207–210. [Google Scholar]
- Querner, P. Thylodrias contractus Motschulsky, 1839, ein neuer Material- und Museumsschädling in Wien und Österreich. Beiträge Entomofaunist. 2018, 19, 127–132. [Google Scholar]
- Robinson, J.; Jackson, J.C.; Whiffin, A.L. Battling booklice in Scottish galleries, libraries, archives and museums. In Integrated Pest Management for Collections: Pest Odyssey 2021-The Next Generation; Ryder, S., Crossman, A., Eds.; Archetype Books: London, UK, 2022; pp. 210–213. [Google Scholar]
- New, T.R. Psocids: Psocoptera (Booklice and Barklice). In Handbooks for the Identification of British Insects; Royal Entomological Society: London, UK, 2005; pp. 68–69. [Google Scholar]



| Floor | Description | Collection Type and Room Use | Dust Sample in g | Perimeter in m | Floor Area in m2 | Flooring and Climate Control | Sticky Blunder Traps | Pheromone Traps | Museum Visitors | Open Windows |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | Attic, modern | Archive, library, botany | 37 | 311 | 1461 | Linoleum/ HVAC | 97 | 16 | No | No |
| 3 | historic | Mammal, library, botany | 118 | 376 | 2197 | Wood only heating | 152 | 26 | No | Partly |
| 2 | historic | Bird, library, exhibition | 132 | 439 | 2695 | Wood/ only heating | 135 | 58 | Yes | Yes |
| 1 | historic | Exhibition, offices | 179 | 510 | 3943 | Wood/ only heating | 36 | 35 | Yes | Yes |
| 0 | Ground floor, historic | Library, taxidermy studio, offices | 86 | 230 | 592 | Wood/ only heating | 36 | 17 | No | No |
| −1 | basement, historic | Technical rooms, storage, hallways | 103 | 200 | 2367 | Concrete/ no control | 35 | 35 | No | No |
| 4 + 3 | modern and historic | Entomology | 43 | 209 | 865 | Linoleum/wood only heating | 126 | 13 | No | No |
| Floor | SR in Dust (DNA) | Entropy- Dust | SR in Trap (DNA) | Entropy- Traps | Species Overlap | % of Human DNA in Dust |
|---|---|---|---|---|---|---|
| 4 | 166 | 2.73 | 101 | 2.58 | 65 | 20 |
| 3 | 128 | 2.15 | 148 | 2.44 | 81 | 42 |
| 2 | 129 | 2.52 | 132 | 2.72 | 61 | 49 |
| 1 | 109 | 0.72 | 162 | 2.26 | 55 | 39 |
| 0 | 124 | 2.54 | 133 | 2.21 | 69 | 5 |
| −1 | 58 | 1.74 | 192 | 2.57 | 35 | 1 |
| Entomology | 61 | 2.30 | - | - | - | 62 |
| Floor | Pest SR in Dust DNA | Pests SR in Trap DNA | Species Overlap | Pest SR in Monitoring |
|---|---|---|---|---|
| 4 | 17 | 15 | 12 | 13 |
| 3 | 12 | 14 | 10 | 13 |
| 2 | 12 | 15 | 9 | 12 |
| 1 | 11 | 14 | 8 | 8 |
| 0 | 13 | 15 | 12 | 12 |
| −1 | 4 | 17 | 4 | 10 |
| entomology | 9 | - | - | 11 |
| Floor | −1 | 0 | 1 | 2 | 3 | 4 | Entomology |
|---|---|---|---|---|---|---|---|
| Tineola bisselliella | 50 | 263 | 42 | 27 | 146 | 3 | 11 |
| Monopis crocicapitella | 7 | - | - | - | - | - | - |
| Plodia interpunctalla | 4 | 3 | 3 | - | - | - | 3 |
| Anthrenus verbasci | - | 9 | 1 | - | 7 | 4 | 5 |
| Anthrenus olgae/caucasicus | 1 | 1 | - | 13 | 41 | 3 | 14 |
| Anthrenus larvae | 2 | 6 | 5 | 3 | 14 | 21 | 25 |
| Attagenus smirnovi | 1 | 4 | 39 | 32 | 99 | - | 54 |
| Attagenus unicolor/brunneus | - | 4 | - | 11 | 4 | - | 25 |
| Attagenus larvae | 1 | - | 7 | 15 | 91 | 2 | 58 |
| Thylodrias contractus | 1 | 1 | 10 | 117 | 4 | 2 | - |
| Reesa vespulae | 2 | 2 | - | 12 | 2 | 9 | 18 |
| Dermestes maculatus | - | 1 | - | 1 | - | - | |
| Stegobium paniceum | - | - | 2 | 7 | 7 | 15 | 10 |
| Lasioderma serricorne | - | - | - | - | - | 1 | 1 |
| Ptinus cf. sexpunctatus | - | - | - | 1 | 1 | 2 | - |
| Lepisma saccharinum | 20 | 12 | 4 | 5 | 2 | 2 | - |
| Ctenolepisma longicaudatum | 74 | 1 | - | - | 1 | 2 | - |
| C. calvum | 10 | 71 | - | 4 | 7 | 5 | 19 |
| C. lineatum | - | - | 4 | 16 | 13 | 3 | 3 |
| Total pest SR per floor: | 12 | 13 | 10 | 14 | 15 | 14 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Querner, P.; Szucsich, N.; Landsberger, B.; Brimblecombe, P. DNA Metabarcoding Analysis of Arthropod Diversity in Dust from the Natural History Museum, Vienna. Diversity 2024, 16, 476. https://doi.org/10.3390/d16080476
Querner P, Szucsich N, Landsberger B, Brimblecombe P. DNA Metabarcoding Analysis of Arthropod Diversity in Dust from the Natural History Museum, Vienna. Diversity. 2024; 16(8):476. https://doi.org/10.3390/d16080476
Chicago/Turabian StyleQuerner, Pascal, Nikola Szucsich, Bill Landsberger, and Peter Brimblecombe. 2024. "DNA Metabarcoding Analysis of Arthropod Diversity in Dust from the Natural History Museum, Vienna" Diversity 16, no. 8: 476. https://doi.org/10.3390/d16080476
APA StyleQuerner, P., Szucsich, N., Landsberger, B., & Brimblecombe, P. (2024). DNA Metabarcoding Analysis of Arthropod Diversity in Dust from the Natural History Museum, Vienna. Diversity, 16(8), 476. https://doi.org/10.3390/d16080476







