Lake Champlain Zooplankton Community Dynamics Following an Extreme Flood Event
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Field and Laboratory Methods
2.3. Data Analysis
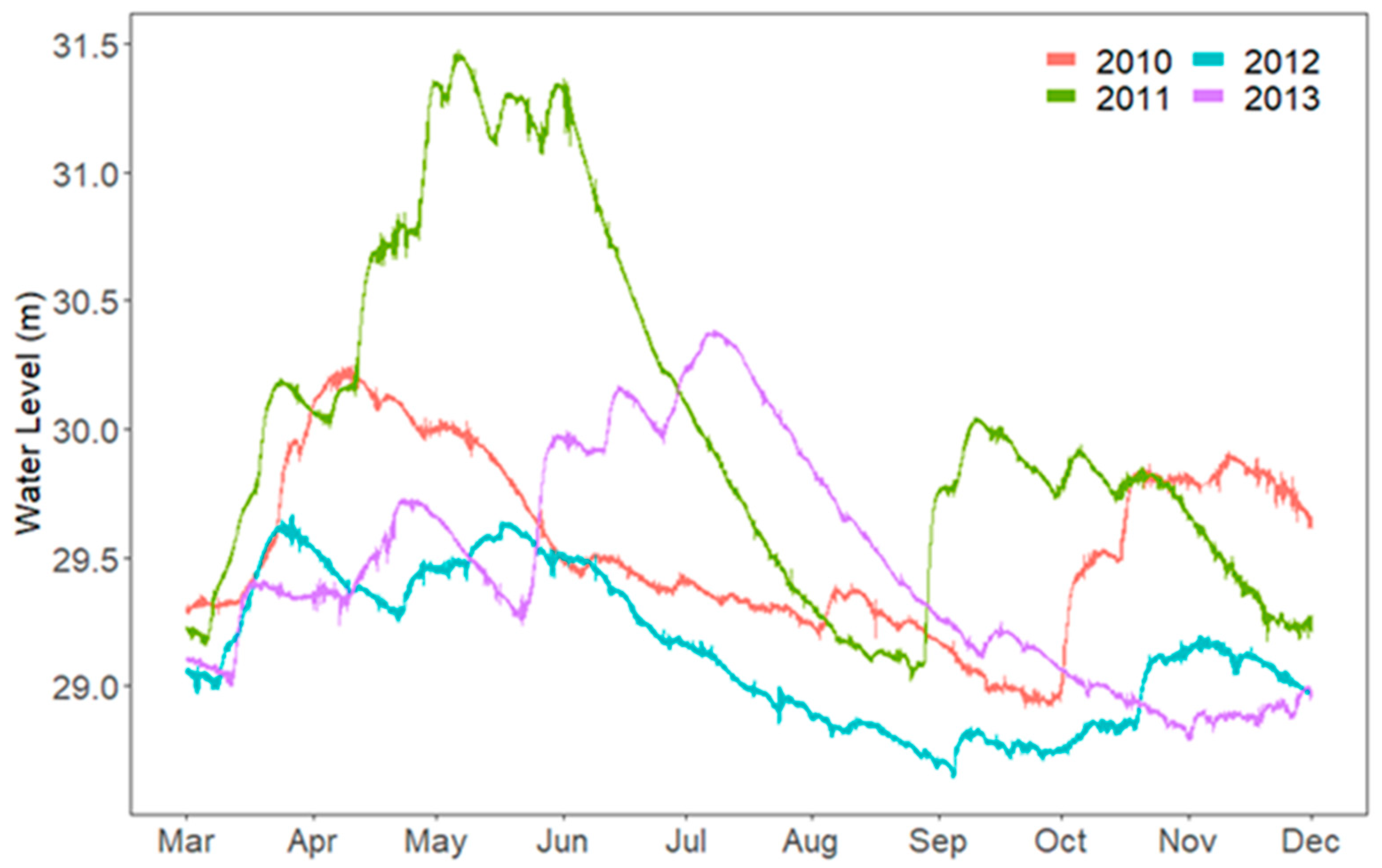
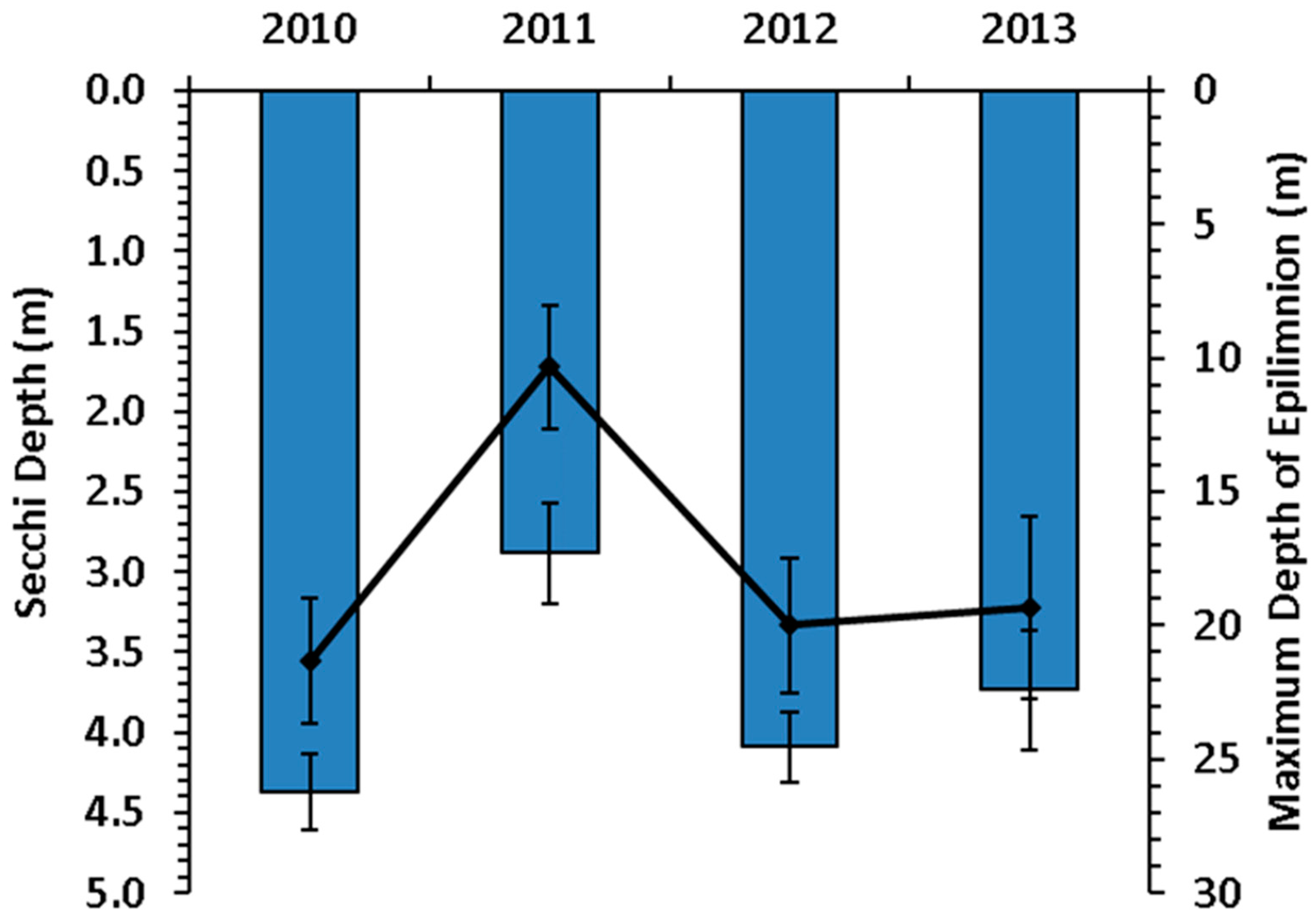
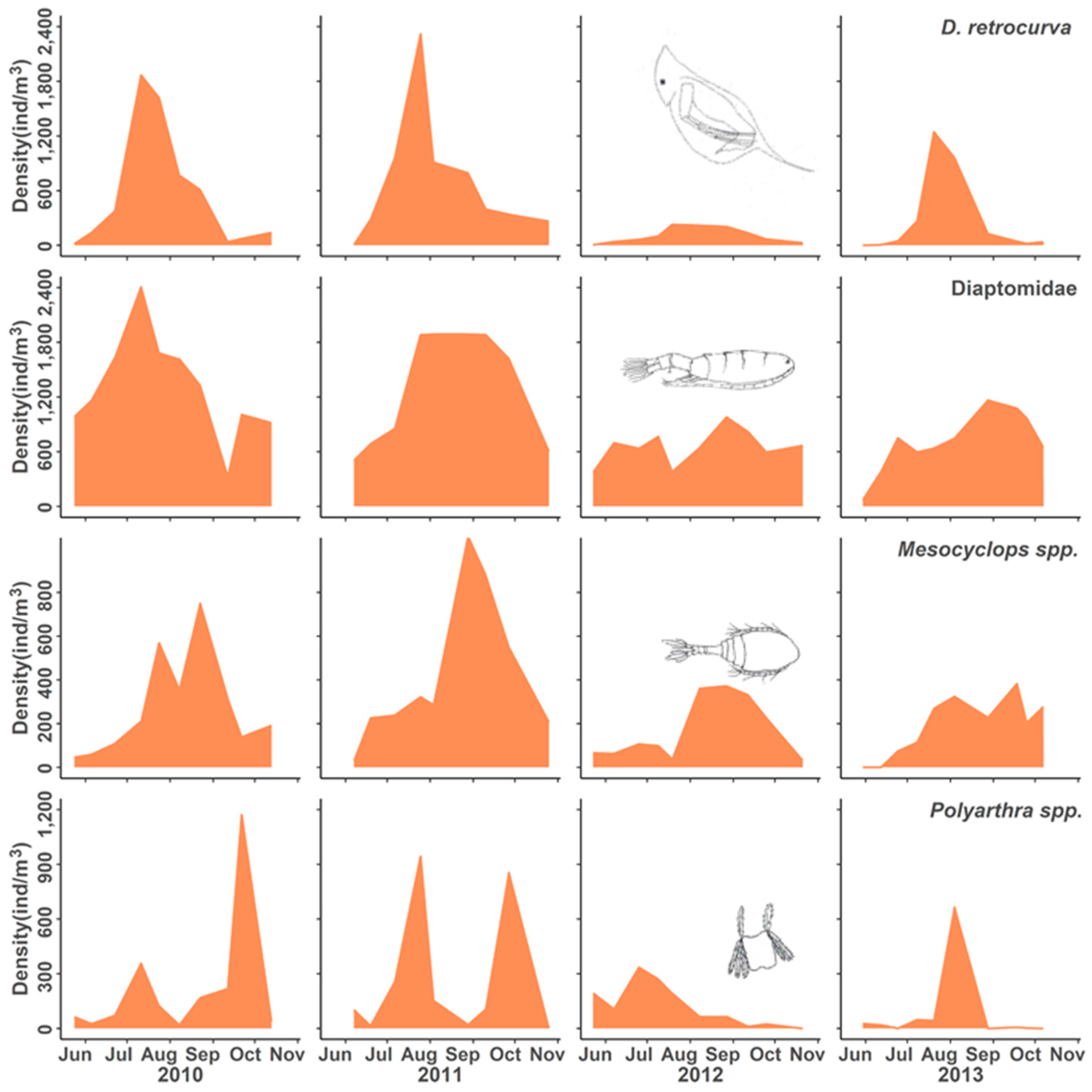
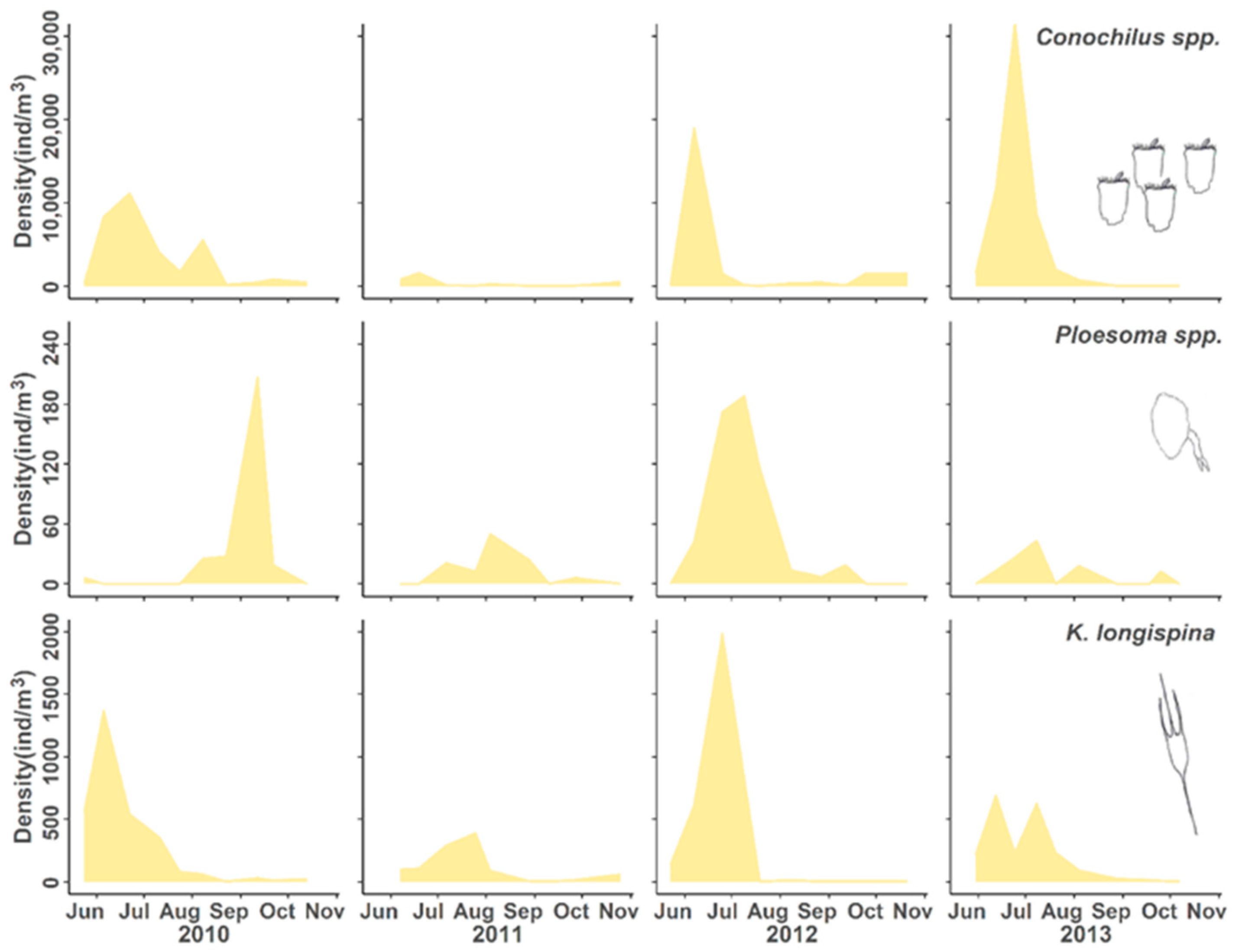
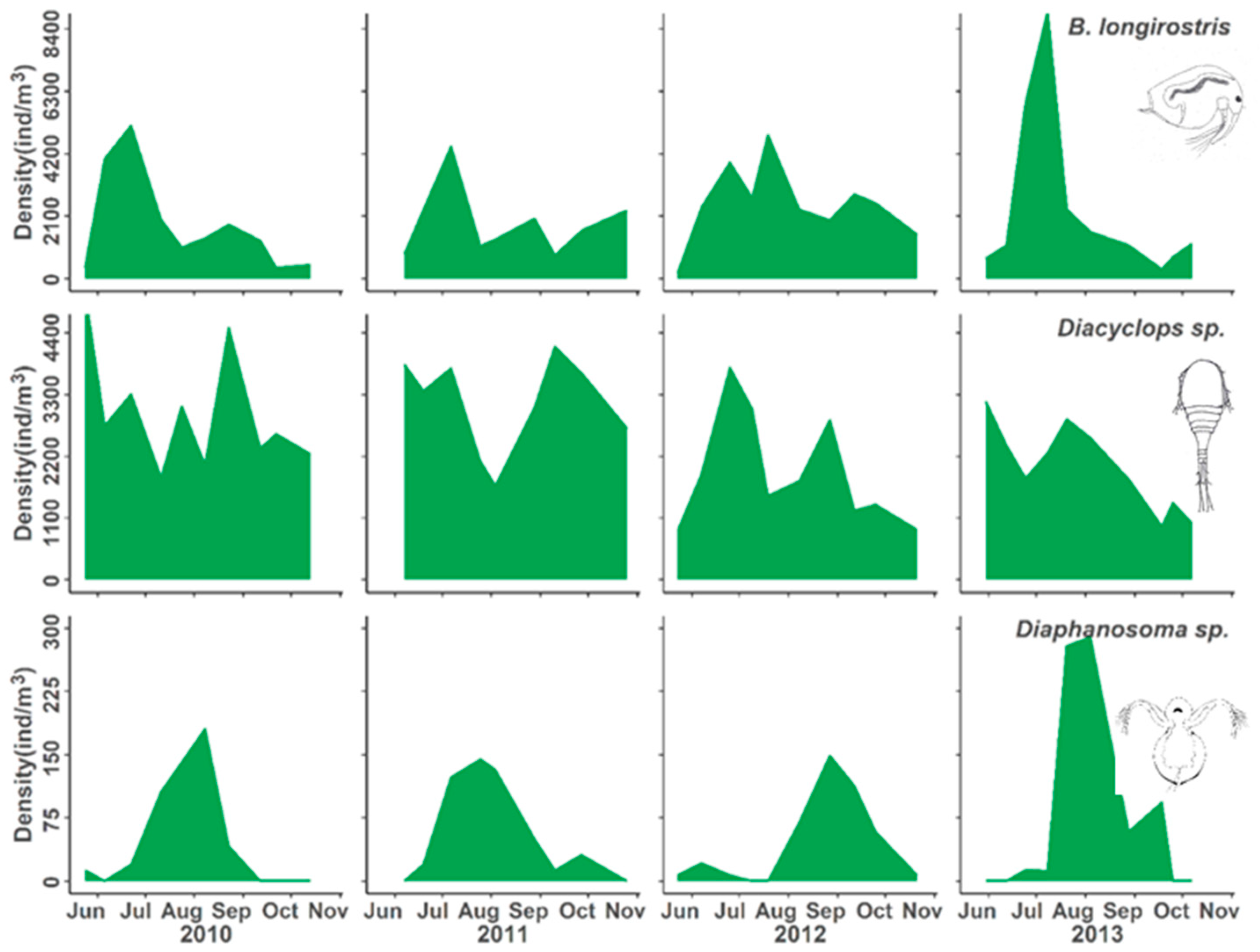
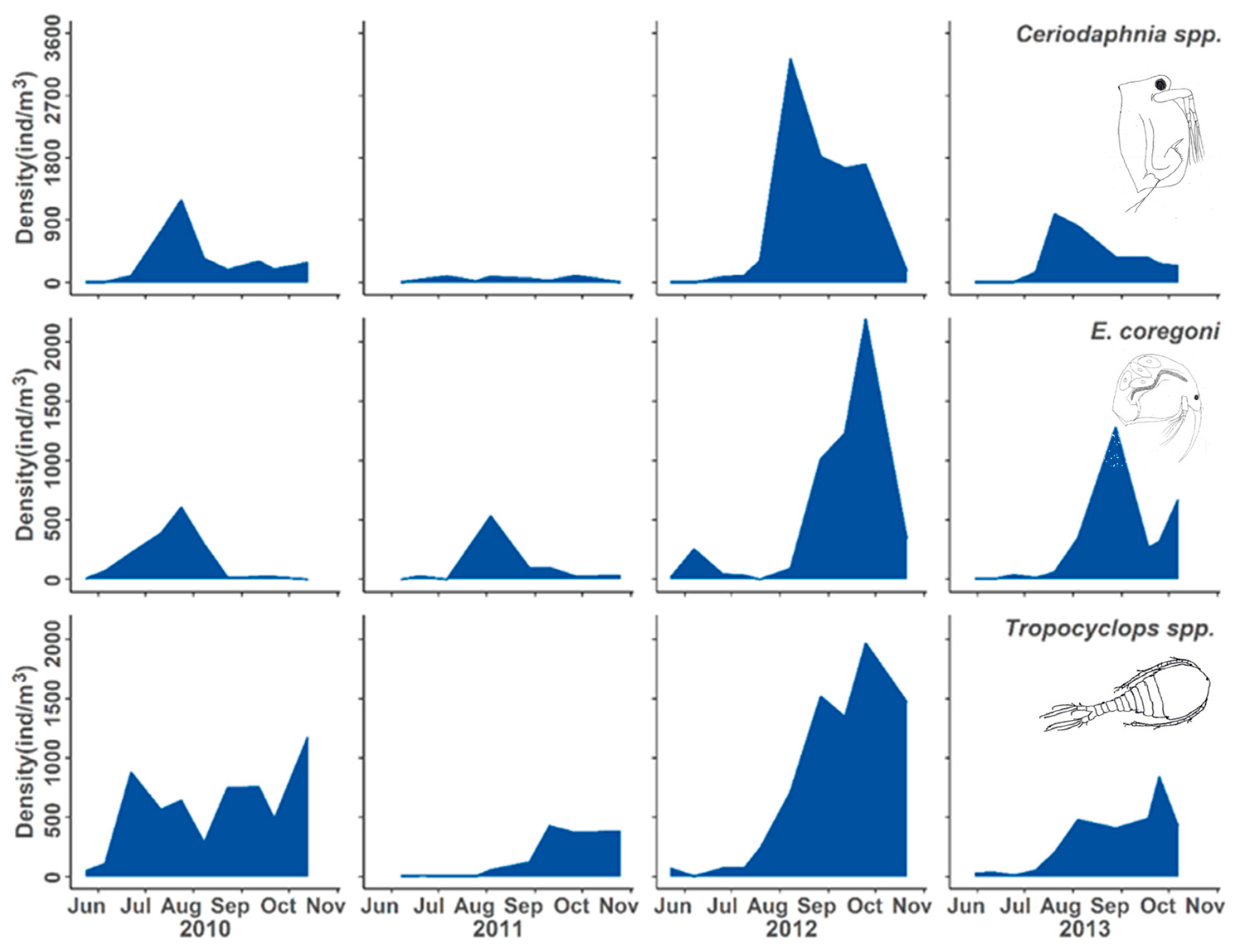
3. Results
3.1. Secchi and Epilimnion Depth
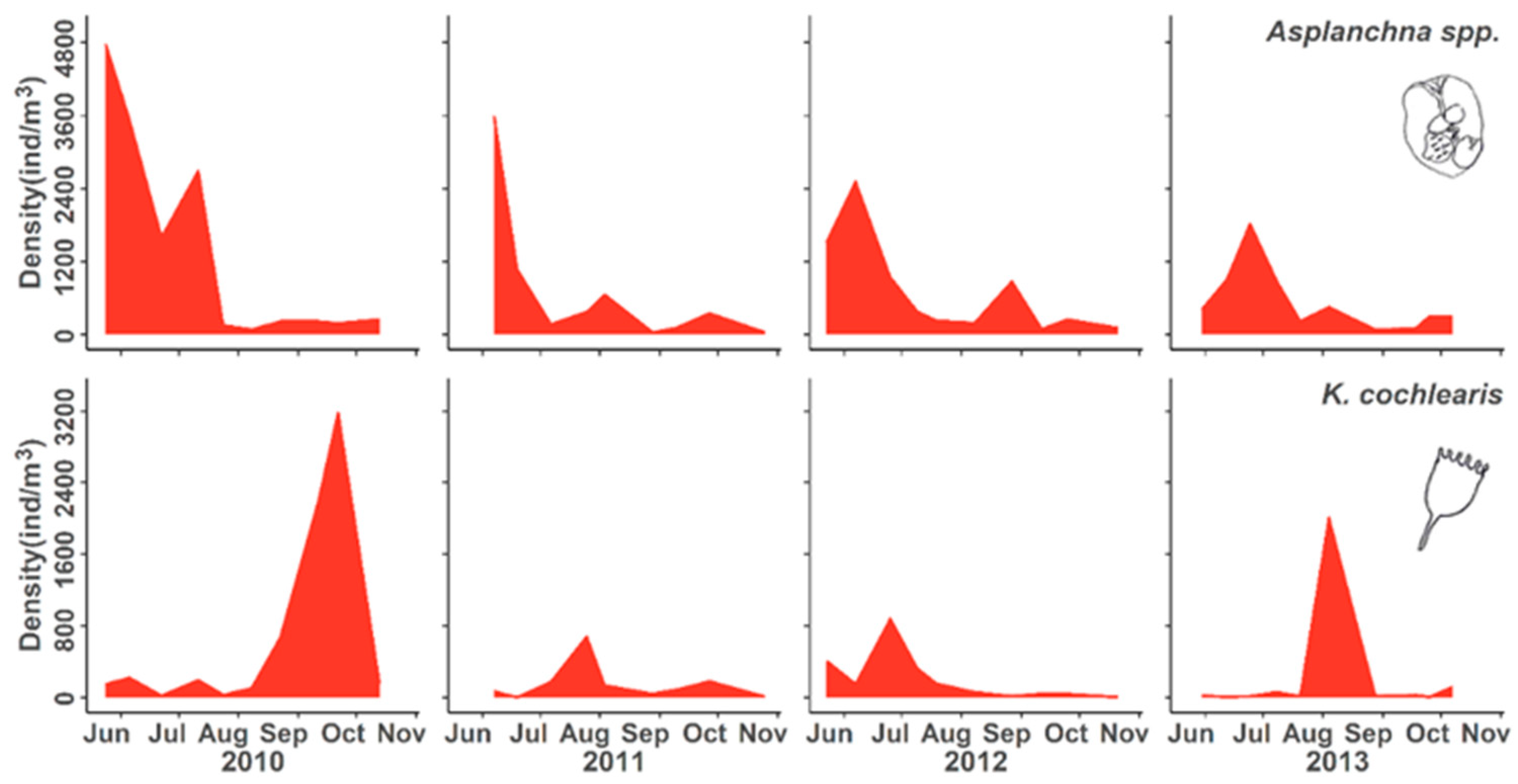
3.2. Population Responses to Flooding
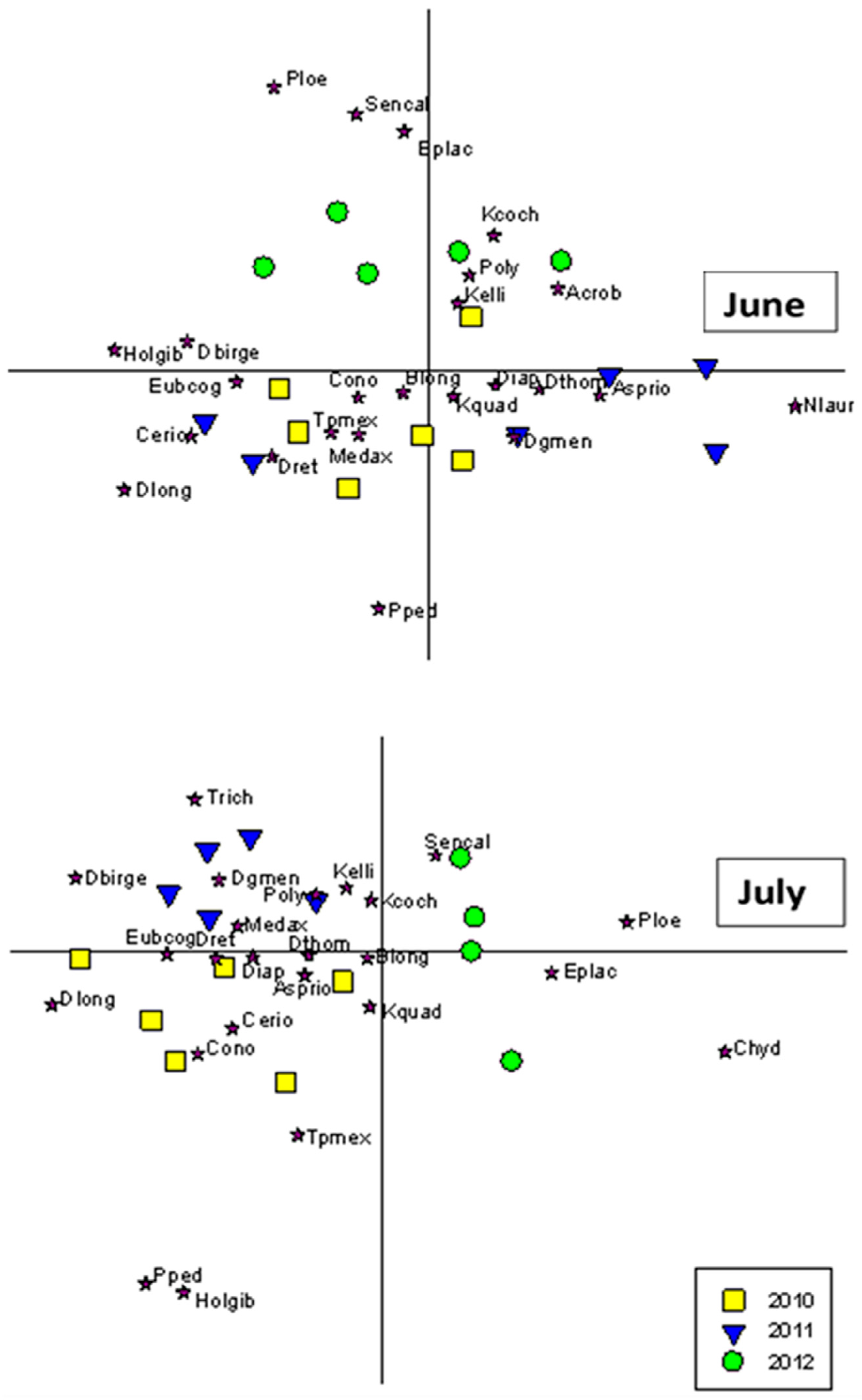
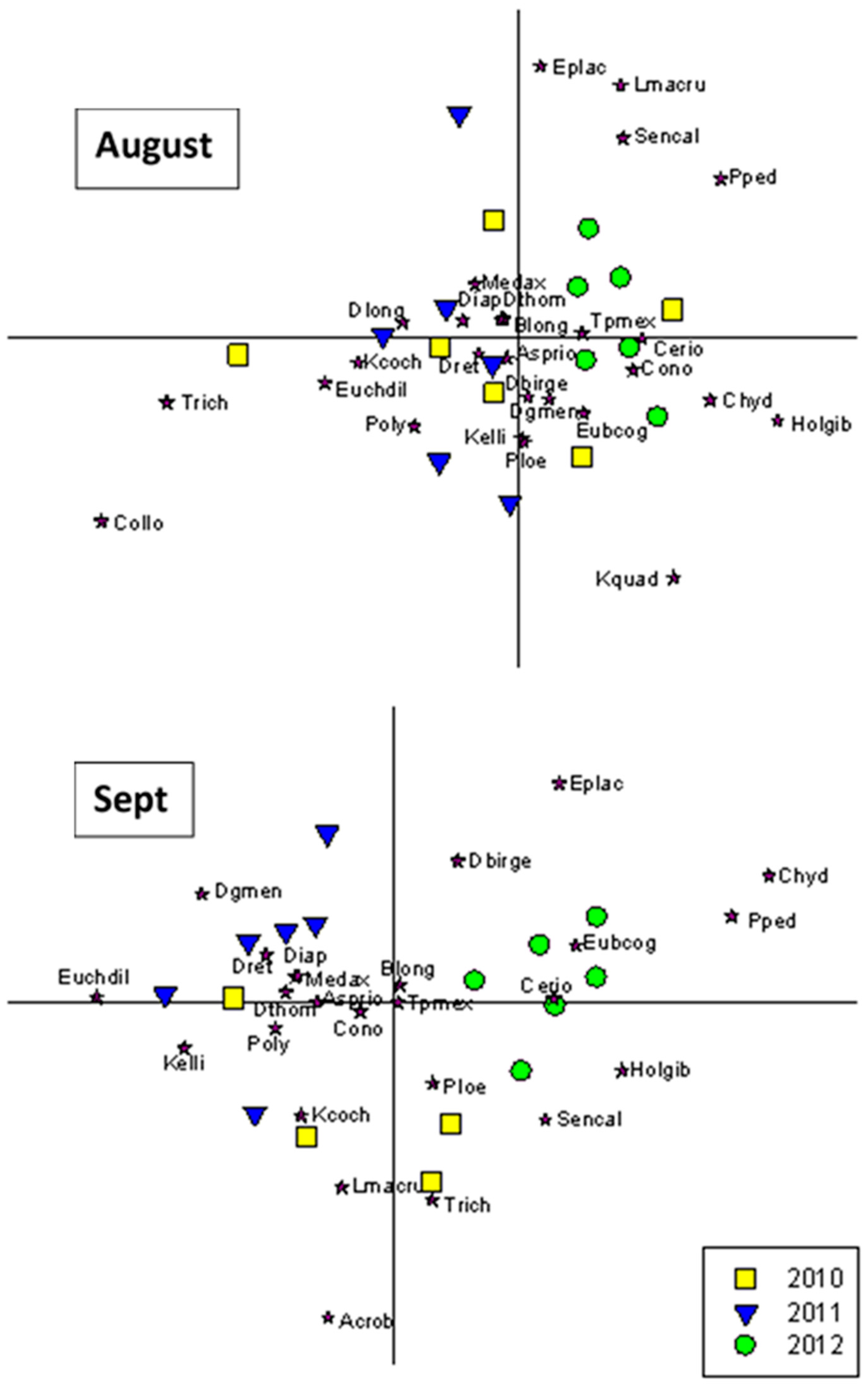
3.3. Community Composition Ordinations
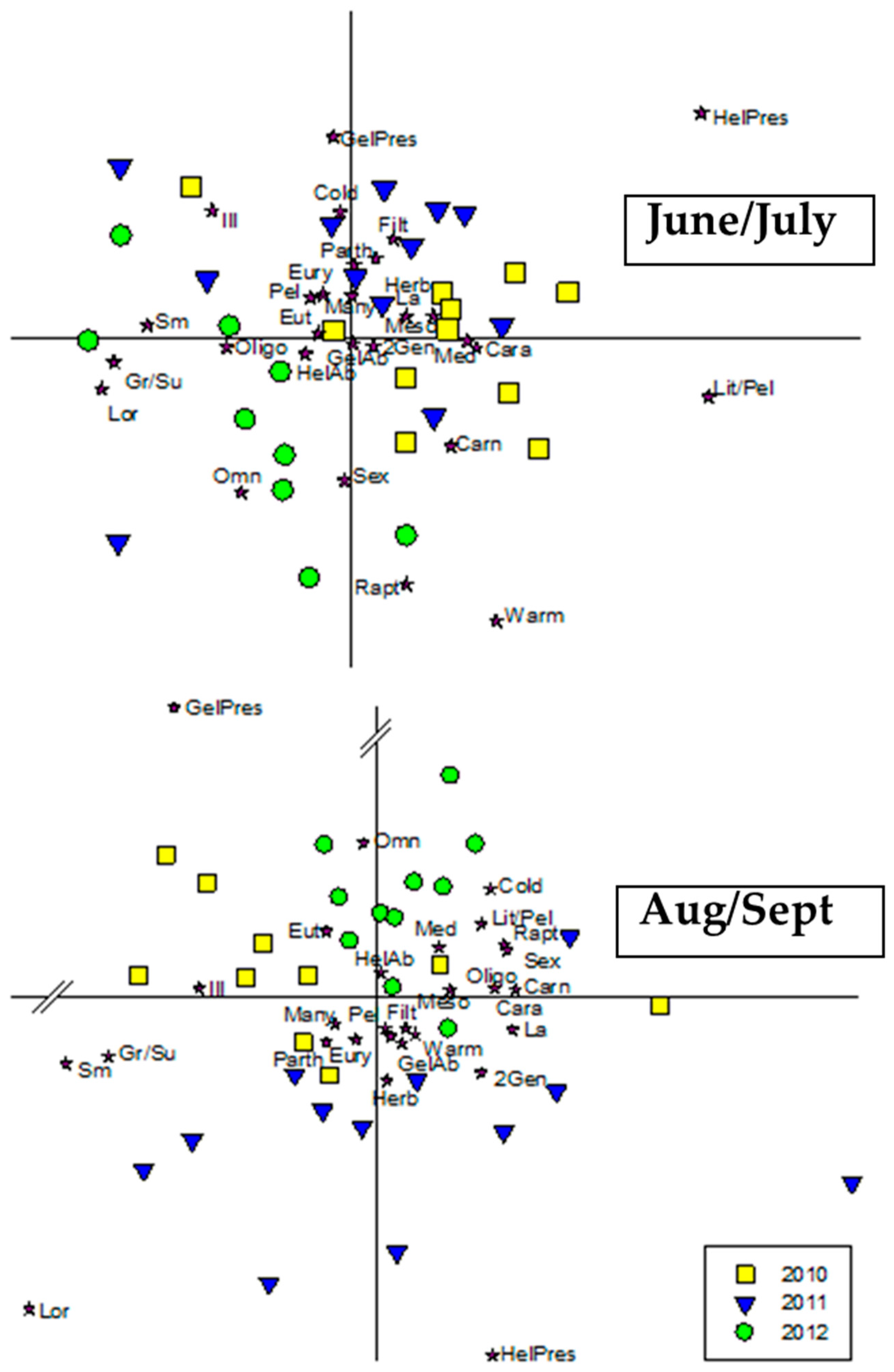
3.4. Ecological Traits
4. Discussion
4.1. Population Dynamics
4.2. Negative Responses to Flood Event
4.3. Positive Responses to the Flood Event
4.4. Neutral Responses to the Flood Event
4.5. Shifts in Community Composition
4.6. Ecological Traits
4.7. Ecological Comparison with Other Lakes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Tables of Abbreviations
| Abbreviation | Meaning |
|---|---|
| Acrob | Acanthocyclops robustus |
| Asc | Ascomorpha spp. |
| Asprio | Asplanchna spp. |
| Bcaly | Brachionus calyciflorus |
| Blong | Bosmina longirostris |
| Cerio | Ceriodaphnia reticulata |
| Chyd | Chydorus sphaericus |
| Collo | Collotheca spp. |
| Cono | Conochilus spp. unicornis |
| Dbirge | Diaphanosoma birgei |
| Dgmen | Daphnia galeata mendotae |
| Diap | Diaptomidae |
| Dlong | Daphnia longiremis |
| Dret | Daphnia retrocurva |
| Dthom | Diacyclops thomasi |
| Eplac | Epischura lacustris |
| Erg | Ergasilus spp. |
| Eubcog | Eubsomina coregoni |
| Euchdil | Euchlanis dilatata |
| Fil | Filinia terminalis |
| Holgib | Holopedium gibberum |
| Kcoch | Keratella cochlearis |
| Kelli | Kellicottia longispina |
| Kquad | Keratella quadrata |
| Lec | Lecane spp. |
| Lmacru | Limnocalanus macrurus |
| Medax | Mesocyclops edax |
| Mon | Monostyla spp. |
| Nlaur | Notholca laurentiae |
| Ploe | Ploesoma spp. |
| Poly | Polyarthra spp. |
| Pped | Polyphemus pediculus |
| Sencal | Senecella calanoides |
| Synch | Synchaeta spp. |
| Tpmex | Tropocyclops prasinus mexicanus |
| Trich | Trichocerca spp. |
| Abbreviation | Meaning |
|---|---|
| Herb | Primary consumer (herbivore, bacteriovore) |
| Omn | Omnivore |
| Carn | Carnivore |
| Filt | Filter feeding |
| GrSu | Grasping/sucking |
| Rapt | Raptorial feeding |
| Lit | Littoral |
| LitPel | Littoral or pelagic |
| Pel | Pelagic |
| Cold | Cold |
| Eury | Eurytherm |
| Warm | Warm |
| Oligo | Oligotrophic |
| Meso | Mesotrophic |
| Eut | Eutrophic |
| 1Gen | 1 Generation per year |
| 2Gen | 2 Generations per year |
| Many | Multiple generations per year |
| Sex | Sexual reproduction (exclusive) |
| Parth | Parthenogenetic reproduction |
| Ill | Illoricate |
| Lor | Loricate |
| Car | With carapace |
| GelAb | Gelatinous sheath absent |
| GelPres | Gelatinous sheath present |
| Sm | Small (<0.5 mm) |
| Med | Medium (0.5–1.0 mm) |
| La | Large (>1.0 mm) |
| HelAb | Helmet absent |
| HelPres | Helmet present |
Appendix B. Species Traits Summary Tables
| Species | Trophic Group | Feeding Mode | Habitat | Thermal | Lake Productivity | Generations Per Year | Reproduction | Armor | Gelatinous Sheath | Size Bin | Helmet |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bosmina longirostris | herbivore [18] | filtration [18] | pelagic [18] | cold-adapted eurytherm [13] | meso-eutrophic [18] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | 0.5–1 mm | no [18] |
| Eubosmina coregoni | herbivore [18] | filtration [18] | pelagic [18] | warm-adapted eurytherm [13] | mesotrophic [13] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | 0.5–1 mm | no [18] |
| Ceriodaphnia reticulata | herbivore [18] | filtration [18] | both [50] | warm-adapted eurytherm [13] | meso-eutrophic [55] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | 0.5–1 mm | no [18] |
| Daphnia longiremis | herbivore [13] | filtration [18] | both [13] | cold stenotherm [13,47,48] | oligo-mesotrophic [13,54] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | 0.5–1 mm | yes [18] |
| Daphnia galeata mendotae | herbivore [18] | filtration [18] | pelagic [18] | warm-adapted eurytherm [13] | oligo-mesotrophic [13] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | >1 mm | yes [18] |
| Daphnia retrocurva | herbivore [18] | filtration [18] | pelagic [18] | warm-adapted eurytherm [13] | mesotrophic [13] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | >1 mm | yes [18] |
| Chydorus sphaericus | herbivore [18] | filtration [18] | littoral [18] | Warm | eutrophic [54] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | <0.5 mm | no [18] |
| Diaphanosoma birgei | herbivore [18] | filtration [18] | pelagic [13] | warm-adapted eurytherm [13] | mesotrophic [13] | multi [13] | parthenogenetic [13] | carapace [13] | absent [13] | >1 mm | no [18] |
| Holopedium gibberum | omnivore [13] | filtration [18] | pelagic [18] | cold [13] | oligo-mesotrophic [13] | multi [13] | parthenogenetic [13] | carapace [13] | present [13] | >1 mm | no [18] |
| Polyphemus pediculus | carnivore [18] | raptorial [18] | both [13,50] | warm-adapted eurytherm [13] | mesotrophic | two [13] | parthenogenetic [13] | reduced carapace [13] | absent [13] | >1 mm | no [18] |
| Species | Trophic Group | Feeding | Habitat | Thermal | Lake Productivity | Generations Per Year | Reproduction | Armor | Gelatinous Sheath | Size | Helmet |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diacyclops thomasi Cyclopoid | Omnivore-carnivore [18] | raptorial [18] | pelagic [13] | eurytherm [13] | mesotrophic [13] | two [13] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Mesocyclops edax | Omnivore-carnivore [18] | raptorial [18] | both [13] | eury-warm [13] | mesotrophic | two [13] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Tropocyclops prasinus mexicanus | Omnivore-herbivore [18] | raptorial [18] | pelagic [49] | warm-adapted eurytherm [13,38] | mesotrophic | multi [13] | sexual [13] | carapace [13] | absent [13] | 0.5–1 mm | no [13] |
| Acanthocyclops robustus | omnivore [18] | raptorial [18] | both [13,49] | warm-adapted eurytherm [13] | meso-eutrophic [13] | multi [3] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Ergasilus spp. | ectoparasite [42,49] | grasping | pelagic [42,51] | warm-adapted eurytherm [53] | mesotrophic [42] | one [42] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Epischura lacustris | omnivore [39] | raptorial [39] | littoral [13] | eury [13] | oligotrophic [13] | one [13] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Diaptomidae | herbivore [39] | filtration [13] | pelagic [13] | cold-adapted eurytherm [1] | oligo-mesotrophic [54] | two [13] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Senecella calanoides | omnivore [39] | filtration | pelagic [13] | cold stenotherm [13,54] | oligotrophic [13] | one [38] | sexual [13] | carapace [1] | absent [13] | >1 mm | no [13] |
| Limnocalanus macrurus | Omnivore-carnivore [39,52] | raptorial [39] | pelagic [13] | cold stenotherm [13,48,54] | oligotrophic [13] | one [38] | sexual [13] | carapace [13] | absent [13] | >1 mm | no [13] |
| Species | Trophic Group | Feeding | Habitat | Thermal | Lake Productivity | Generations Per Year | Reproduction | Armor | Gelatinous | Size (µm) | Trophi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ascomorpha ecaudis | herbivore [45] | sucking [45] | littoral [14] | eurythermal [44] | mesotrophic [40] | multi [45] | parthenogenetic [14] | illoricate [14] | absent [14] | 130–200 | virgate [14] |
| Ascomorpha ovalis | herbivore [45] | sucking [45] | littoral [14] | eurythermal [44] | mesotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 95–150 | virgate [14] |
| Ascomorpha saltans | herbivore [45] | sucking [45] | littoral [14] | eurythermal [44] | mesotrophic [40] | multi [45] | parthenogenetic [14] | thin lorica [14] | absent [14,45] | 100–200 | virgate [14] |
| Asplanchna spp. | omnivore [14] | grasping [45] | pelagic [46] | eurythermal [41] | eu-mesotrophic [40] | multi [45] | parthenogenetic [14] | illoricate [14] | absent [14] | 420–1500 | incudate [14] |
| Brachionus calyciflorus | herbivore [14] | filtration [45] | littoral [14] | eurythermal [41] | eutrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 180–570 | malleate [14] |
| Collotheca mutabilis | omnivore [14] | trapping [45] | pelagic [37] | eurythermal [41] | mesotrophic [41] | multi [45] | parthenogenetic [14] | illoricate [14] | present [14,45] | 178–315 | uncinate [14] |
| Conochilus spp. unicornis | herbivore [37] | filtration [45] | pelagic [37] | eurythermal [44] | eu-mesotrophic [41] | multi [45] | parthenogenetic [14] | illoricate [14] | present [14,45] | 200–450 | malleoramate [14] |
| Euchlanis dilatata | herbivore [37] | filtration [45] | littoral [37] | warm [44] | eutrophic [14] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 200–270 | malleate [14] |
| Filinia terminalis | herbivore [14] | filtration [45] | pelagic [45] | cold [41] | mesotrophic [41] | multi [45] | parthenogenetic [14] | illoricate [14] | absent [14] | malleoramate [14] | |
| Kellicottia longispina | herbivore [37] | filtration [45] | pelagic [37] | cold-adapted eurytherm [14] | oligotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 400–860 | malleate [14] |
| Keratella cochlearis | herbivore [37] | filtration [45] | pelagic [37] | eury [43,44] | mesotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 115–200 | malleate [14] |
| Keratella quadrata | herbivore [37] | filtration [45] | pelagic [37] | eury [14] | mesotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 224–385 | malleate [14] |
| Monostyla spp. | herbivore [37] | filtration [37] | littoral [37] | warm [14] | eutrophic [14] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 78–142 | malleate [14] |
| Notholca laurentiae | herbivore [45] | filtration [45] | pelagic [14] | cold [14,37,41] | oligotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 192–250 | malleate [14] |
| Ploesoma spp. | omnivore [37] | grasping [45] | pelagic [37] | warm [41] | oligotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 150–600 | virgate [14] |
| Polyarthra spp. | herbivore [37] | grasping [45] | pelagic [37] | eury [41] | mesotrophic [14] | multi [45] | parthenogenetic [14] | illoricate [14] | absent [14] | 72–200 | virgate [14] |
| Synchaeta spp. | herbivore [37] | grasping [45] | pelagic [37] | eury [41] | mesotrophic [40] | multi [45] | parthenogenetic [14] | loricate [14] | absent [14] | 90–600 | virgate [14] |
| Trichocerca spp. | omnivore [14] | sucking [45] | littoral [14] | eury [41] | eu-mesotrophic [14] | multi [45] | parthenogenetic [14] | ill/lor [14] | absent [14] | 85–310 | virgate [14] |
References
- Milly, P.C.D.; Wetherald, R.T.; Cunne, K.A.; Delworth, T.L. Increasing risk of great floods in a changing climate. Nature 2002, 415, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Arruda, J.A.; Marzolf, G.R.; Faulk, R.T. The role of suspended sediments in the nutrition of zooplankton in turbid reservoirs. Ecology 1983, 64, 1225–1235. [Google Scholar] [CrossRef]
- Threlkeld, S.T. Life table responses and population dynamics of four cladoceran zooplankton during a reservoir flood. J. Plankton Res. 1986, 8, 639–647. [Google Scholar] [CrossRef]
- Godlewska, M.; Mazurkiewicz-Boron, G.; Pociecha, A.; Wilk-Wozniak, E.; Jelonek, M. Effects of flood on the functioning of the Dobczyce reservoir ecosystem. Hydrobiologia 2003, 504, 305–313. [Google Scholar] [CrossRef]
- Muylaert, K.; Vyverman, W. Impact of a flood event on the planktonic food web of the Schelde estuary (Belgium) in spring 1998. Hydrobiologia 2006, 559, 385–394. [Google Scholar] [CrossRef]
- Paidere, J.; Gruberts, D.; Škute, A.; Druvietis, I. Impact of two different flood pulses on planktonic communities of the largest floodplain lakes of the Daugava River (Latvia). Hydrobiologia 2007, 592, 303–314. [Google Scholar] [CrossRef]
- USGS. 2012. USGS 04294500 Lake Champlain at Burlington, VT. Available online: http://waterdata.usgs.gov/nwis/nwisman/?site_no=04294500&agency_cd=USGS (accessed on 20 April 2012).
- Howland, W.G. The Floods of 2011—A Quick View. Lake Champlain Basin Program. 2011. Available online: http://www.lcbp.org/2011Flood.htm (accessed on 14 March 2012).
- Lake, P.S. Disturbance, patchiness, and diversity in streams. J. N. Am. Benthol. Soc. 2000, 19, 573–592. [Google Scholar] [CrossRef]
- Myer, G.E.; Gruendling, G.K. Limnology of Lake Champlain; Lake Champlain Basin Study: Boston, MA, USA, 1979; 407p. [Google Scholar]
- Mihuc, T.B.; Dunlap, F.; Binggeli, C.; Myers, L.; Pershyn, C.; Groves, A.; Waring, A. Long-term patterns in Lake Champlain’s zooplankton: 1992–2010. J. Great Lakes Res. 2012, 38, 49–57. [Google Scholar] [CrossRef]
- Smeltzer, E.; Shambaugh, A.D.; Stangel, P. Environmental change in Lake Champlain revealed by long-term monitoring. J. Great Lakes Res. 2012, 38, 6–18. [Google Scholar] [CrossRef]
- Balcer, M.; Korda, N.; Dodson, S.A. Guide to the Identification and Ecology of the Common Crustacean Species; The University of Wisconsin Press: Madison, WI, USA, 1984; pp. 74–101. [Google Scholar]
- Stemberger, R.S. A Guide to the Rotifers of the Laurentian Great Lakes; US EPA: Cincinnati, OH, USA, 1979; 200p. [Google Scholar]
- Grothe, D.W.; Grothe, D.R. An Illustrated Key to the Planktonic Rotifers of the Laurentian Great Lakes; US EPA: Chicago, IL, USA, 1977; 91p. [Google Scholar]
- Doledec, S.; Chessel, D.; Gimaret-Carpentier, G. Niche separation in community analysis: A new method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Weithoff, G.; Beisner, B.E. Measures and Approaches in Trait-Based Phytoplankton Community Ecology—From Freshwater to Marine Ecosystems. Front. Mar. Sci. 2019, 6, 40. [Google Scholar] [CrossRef]
- Barnett, A.J.; Finlay, F.; Beisner, B.E. Functional diversity of crustacean zooplankton communities: Towards a trait-based classification. Freshw. Biol. 2007, 52, 796–813. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS for Windows, Version 14.0; SPSS Inc.: Chicago, IL, USA, 2006. [Google Scholar]
- Clarke, K.R.; Ainsworth, M. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 1993, 92, 205–219. [Google Scholar] [CrossRef]
- Oksanen, J.F.; Blanchet, G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. vegan: Community Ecology Package. R Package Version 2.0-5. 2012. Available online: http://CRAN.R-project.org/package-vegan (accessed on 1 November 2012).
- Gannon, J.E.; Bricker, K.S.; Bricker, F.J. Zooplankton Community Composition in Green Bay, Lake Michigan; US EPA: Chicago, IL, USA, 1982; 93p. [Google Scholar]
- Lamouroux, N.; Dolédec, S.; Gayraud, S. Biological traits of stream macroinvertebrate communities: Effects of microhabitat, reach and basin filters. J. North Am. Benthol. Soc. 2004, 23, 449–466. [Google Scholar] [CrossRef]
- Gilbert, J.J. Competition between rotifers and Daphnia. Ecology 1985, 66, 1943–1950. [Google Scholar] [CrossRef]
- Kang, H.K. Effects of suspended sediments on reproductive responses of Paracalanus sp. (Copepoda: Calanoida) in the laboratory. J. Plankton Res. 2012, 34, 626–635. [Google Scholar] [CrossRef]
- Dejen, E.; Vijverberg, J.; Nagelkerke, L.A.J.; Sibbing, F.A. Temporal and spatial distribution of microcrustacean zooplankton in relation to turbidity and other environmental factors in a large tropical lake (L. Tana, Ethiopia). Hydrobiologia 2004, 513, 39–49. [Google Scholar] [CrossRef]
- Hart, R.C. Zooplankton abundance, community structure and dynamics in relation to inorganic turbidity, and their implications for a potential fisher in subtropical Lake le Roux, South Africa. Freshw. Biol. 1986, 16, 351–371. [Google Scholar] [CrossRef]
- Lynch, M. Predation, competition, and zooplankton community structure: An experimental study. Limnol. Oceanogr. 1979, 24, 253–272. [Google Scholar] [CrossRef]
- Brooks, J.L.; Dodson, S.I. Predation, Body Size, and Composition of Plankton: The effect of a marine planktivore on lake plankton illustrates theory of size, competition, and predation. Science 1965, 150, 28–35. [Google Scholar] [CrossRef]
- Muenscher, W.G. Plankton studies in the Lake Champlain watershed. In A Biological Survey of the Lake Champlain Watershed; Supplement to the 19th Annual Report; NY State Conservation Dept.: New York, NY, USA, 1929. [Google Scholar]
- DeMott, W.R.; Kerfoot, W.C. Competition among cladocerans: Nature of the interaction between Bosmina and Daphnia. Ecology 1982, 63, 1949–1966. [Google Scholar] [CrossRef]
- Kirk, K.L.; Gilbert, J.J. Suspended clay and the population dynamics of planktonic rotifers and cladocerans. Ecology 1990, 71, 1741–1755. [Google Scholar] [CrossRef]
- Okogwu, O.I.; Nwani, C.D.; Ugwumba, A.O. Seasonal variations in the abundance and biomass of microcrustaceans in relation to environmental variables in two shallow tropical lakes within the cross river floodplain, Nigeria. Acta Zool. Litu. 2009, 19, 205–215. [Google Scholar] [CrossRef]
- Napiórkowski, P.; Napiórkowska, T. The impact of catastrophic flooding on zooplankton. Pol. J. Environ. Stud. 2014, 23, 409–417. [Google Scholar]
- Dube, T.; DeNecker, L.; Van Vuren, J.H.; Wepene, V.; Smit, N.J.; Brendonck, L. Spatial and temporal variation of invertebrate community structure in flood-controlled tropical floodplain wetlands. J. Freshw. Ecol. 2017, 32, 1–15. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Z.; Chen, W. Effects of Floods on Zooplankton Community Structure in the Huayanghe Lake. Diversity 2023, 15, 250. [Google Scholar] [CrossRef]
- Pedruski, M.T. The Effects of Habitat Connectivity and Regional Heterogeneity on Artificial Pond Metacommunities. Master’s Thesis, Queens University, Kingston, ON, Canada, 2008. [Google Scholar]
- Carter, J.C.H. Life cycles of Limnocalanus macrurus and Senecella calanoides, and seasonal abundance and vertical distributions of various planktonic copepods, in Parry Sound, Georgian Bay. J. Fish. Res. Board Can. 1969, 26, 2543–2560. [Google Scholar] [CrossRef]
- Wong, C.K. A study of the relationships between the mouthparts and food habits in several species of freshwater calanoid copepods. Can. J. Zool. 1984, 62, 1588–1595. [Google Scholar] [CrossRef]
- Sladecek, V. Rotifers as indicators of water quality. Hydrobiologia 1983, 100, 169–201. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Bolortsetseg, E. Mongolian rotifers (Rotifera, Monogononta)—A checklist with annotations on global distribution and autecology. Proc. Acad. Nat. Sci. Phila. 2010, 159, 119–168. [Google Scholar] [CrossRef]
- Bricker, K.S.; Gannon, J.E.; Roberts, L.S.; Torke, B.G. Observations on the ecology and distribution of free-living Ergasilidae (Copepoda, Cyclopoida). Crustaceana 1987, 35, 313–317. [Google Scholar] [CrossRef]
- May, L. Rotifer occurrence in relation to water temperature in Loch Leven, Scotland. Hydrobiologia 1983, 104, 311–315. [Google Scholar] [CrossRef]
- Berzins, B.; Pejler, B. Rotifer occurrence in relation to water temperature. Hydrobiologia 1989, 175, 223–231. [Google Scholar] [CrossRef]
- Ruttner-Kolisko, A. Plankton Rotifers: Biology and Taxonomy; Kolisko, G., Translator; E. Schweizerbart’sche Verlagsbuchhandlung: Stuttgart, Germany, 1974; 145p. [Google Scholar]
- Preißler, K. Do rotifers show “avoidance of the shore”? Oecologia 1977, 27, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Berzins, B.; Bertilsson, J. Occurrence of limnic micro-custaceans in relation to pH and humic content in Swedish water bodies. Hydrobiologia 1990, 199, 65–71. [Google Scholar] [CrossRef]
- Bertilsson, J.; Berzins, B.; Pejler, B. Occurrence of limnic micro-crustaceans in relation to temperature and oxygen. Hydrobiologia 1995, 299, 163–167. [Google Scholar] [CrossRef]
- Smith, K.; Fernando, C.H. A Guide to the Freshwater Calanoid and Cyclopoid Copepod Crustacean of Ontario; Department of Biology, University of Waterloo: Waterloo, ON, Canada, 1978. [Google Scholar]
- Brandlova, J.; Brandl, Z.; Fernando, C.H. The Cladocera of Ontario with remarks on some species and distribution. Can. J. Zool. 1972, 50, 1373–1403. [Google Scholar] [CrossRef]
- Hogue, C.C.; Sutherland, D.R.; Christensen, B.M. Ecology of metazoan parasites infecting Catostomus spp. (Catostomidae) from southwestern Lake Superior. Can. J. Zool. 1993, 71, 1646–1652. [Google Scholar] [CrossRef]
- Bowers, J.A.; Warren, G.J. Predaceous feeding by Limnocalanus macrurus upon Diaptomus ashlandi. J. Great Lakes Res. 1977, 3, 234–236. [Google Scholar] [CrossRef]
- Cloutman, D.G.; Becker, D.A. Some ecological aspects of Ergasilus centarchidarum Wright (Crustacea: Copepoda) on largemouth and spotted bass in Lake Fort Smith, Arkansas. J. Parasitol. 1977, 63, 372–376. [Google Scholar] [CrossRef]
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 97, 16–35. [Google Scholar] [CrossRef]
- Weber, C.I. Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms; US EPA: Cincinnati, OH, USA, 1993; 253p. [Google Scholar]
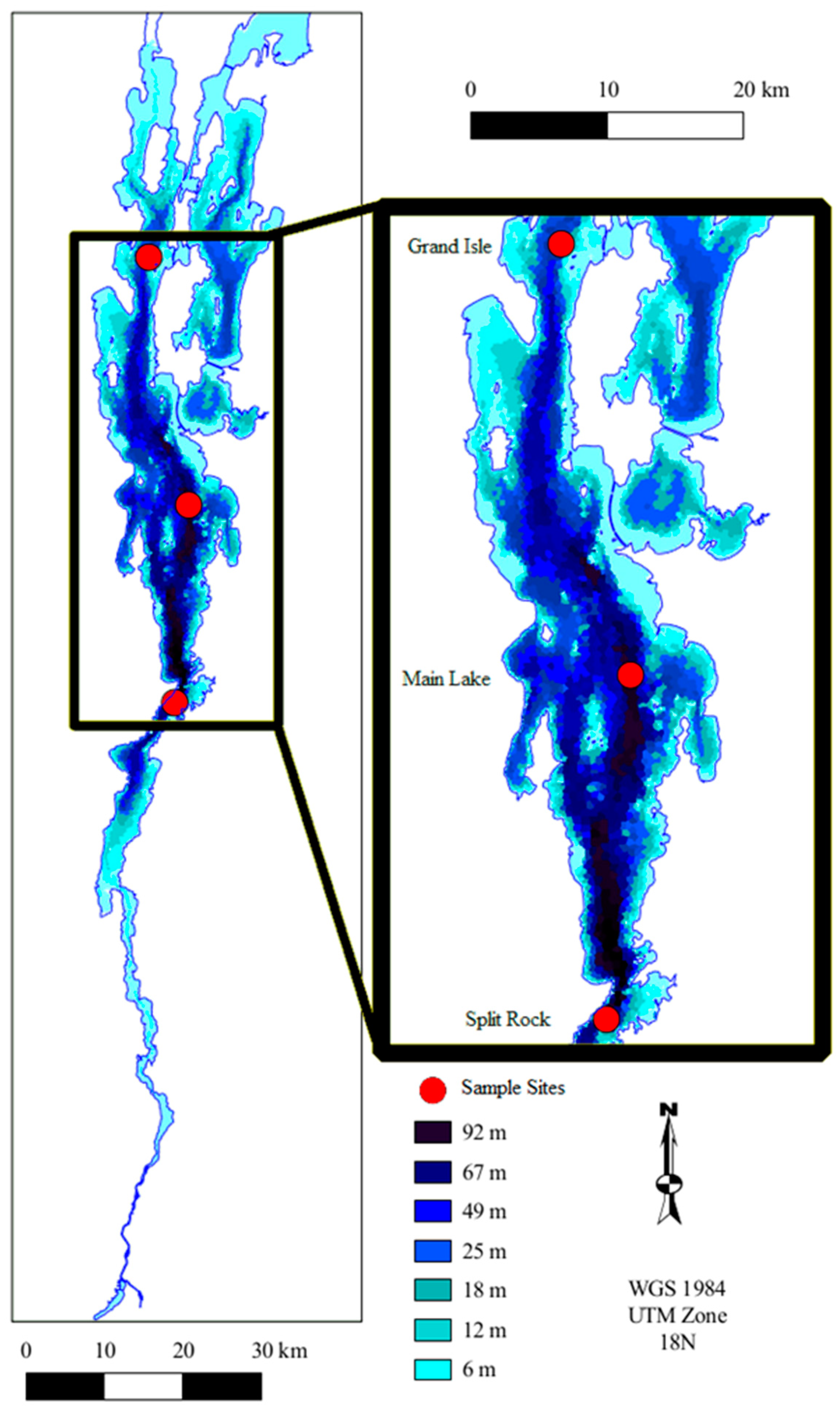
| Station # | Station Name | Latitude | Longitude | Depth (m) |
|---|---|---|---|---|
| 9 | Split Rock | 44°14.53′ | 73°24.77′ | 87 |
| 19 | Main Lake | 44°28.26′ | 73°17.95′ | 100 |
| 36 | Grand Isle | 44°45.37′ | 73°21.30′ | 50 |
| Category | Taxon | Response | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|---|
| Asplanchnid Rotifer | Asplanchna spp. | Decrease | ^ | v | v | v |
| Brachionid rotifer | Keratella cochlearis | Decrease | ^ | v | v | v |
| Daphnidae | Daphnia retrocurva | Delayed decrease | ^ | ^ | v | v |
| Calanoid copepod | Diaptomidae | Delayed decrease | ^ | ^ | v | v |
| Cyclopoid copepod | Mesocyclops edax | Delayed decrease | > | ^ | v | v |
| Synchaetid rotifer | Polyarthra spp. | Delayed decrease | ^ | > | v | v |
| Conochilid rotifer | Conochilus spp. | Decrease, recovery | ^ | v | ^ | ^ |
| Synchaetid rotifer | Ploesoma spp. | Decrease, recovery | ^ | v | ^ | ^ |
| Brachionid rotifer | Kellicottia longispina | Decrease, recovery | > | v | ^ | > |
| Bosminidae | Bosmina longirostris | Neutral | > | > | > | > |
| Cyclopoid copepod | Diacyclops thomasi | Neutral | > | > | > | > |
| Sididae | Diaphanosoma birgei | Neutral | > | > | > | > |
| Daphnidae | Ceriodaphnia reticulata | Delayed increase | v | v | ^ | > |
| Bosminidae | Eubosmina coregoni | Delayed increase | v | v | ^ | > |
| Cyclopoid copepod | Tropocyclops prasinus mexicanus | Delayed increase | > | v | ^ | > |
| Trait | Trait States |
|---|---|
| Trophic | herbivore (primary consumer), omnivore, carnivore |
| Feeding mode | filtration, grasping/sucking, raptorial |
| Habitat | littoral, littoral/pelagic, pelagic |
| Thermal | cold, eurytherm, warm |
| Lake productivity | oligotrophic, mesotrophic, eutrophic |
| Generations per year | one, two, many |
| Reproduction | sexual, parthenogenetic |
| Armor | illoricate, loricate, carapace |
| Gelatinous sheath | absent, present |
| Size class | <0.5 mm, 0.5–1 mm, >1 mm |
| Helmet | absent, present |
| Category | Taxon | Flood Adaptation |
|---|---|---|
| Asplanchnid rotifer | Asplanchna spp. | Flood intolerant |
| Brachionid rotifer | Keratella cochlearis | |
| Daphnid cladoceran | Daphnia retrocurva | Delayed Flood intolerant |
| Calanoid copepod | Diaptomidae | |
| Cyclopoid copepod Synchaetid rotifer | Mesocyclops edax Polyarthra spp. | |
| Conochilid rotifer | Conochilus spp. unicornis | Flood intolerant Fast recovery |
| Synchaetid rotifer Brachionid rotifer | Ploesoma spp. Kellicottia longispina | |
| Bosminid cladoceran | Bosmina longirostris | Flood tolerant |
| Cyclopoid copepod | Diacyclops thomasi | |
| Sidid cladoceran | Diaphanosoma birgei | |
| Daphnid cladoceran | Ceriodaphnia reticulata | Flood adapted |
| Bosminid cladoceran | Eubosmina coregoni | |
| Cyclopoid copepod | Tropocyclops prasinus mexicanus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihuc, T.; Hayes-Pontius, E.; Arnwine, M.; Cutter, Z.; Myers, L. Lake Champlain Zooplankton Community Dynamics Following an Extreme Flood Event. Diversity 2024, 16, 451. https://doi.org/10.3390/d16080451
Mihuc T, Hayes-Pontius E, Arnwine M, Cutter Z, Myers L. Lake Champlain Zooplankton Community Dynamics Following an Extreme Flood Event. Diversity. 2024; 16(8):451. https://doi.org/10.3390/d16080451
Chicago/Turabian StyleMihuc, Timothy, Erin Hayes-Pontius, Marshall Arnwine, Zachary Cutter, and Luke Myers. 2024. "Lake Champlain Zooplankton Community Dynamics Following an Extreme Flood Event" Diversity 16, no. 8: 451. https://doi.org/10.3390/d16080451
APA StyleMihuc, T., Hayes-Pontius, E., Arnwine, M., Cutter, Z., & Myers, L. (2024). Lake Champlain Zooplankton Community Dynamics Following an Extreme Flood Event. Diversity, 16(8), 451. https://doi.org/10.3390/d16080451







