Abstract
The effect of invertebrates like termites and beetle larvae on dead wood could be time-dependent due to changes in wood traits and invertebrate species composition over time. This study assessed changes in the impact of termites and beetle larvae on dead wood decomposition in two tree species, Pinus densiflora and Quercus acutissima, in South Korea over a 6-year period (2016–2022). Wood samples were prepared, with half of them encased in a stainless-steel mesh to prevent access by invertebrates larger than 0.26 mm. These samples were placed in three regions representative of different environments in South Korea (southern, eastern, and western). Significant variations in the mass loss of dead wood were observed based on the tree species, region, and time (p < 0.05). The mean standardized invertebrate effect, assessed with Hedges’ d and a 95% confidence interval, was 0.83 ± 2.19 for P. densiflora and 1.08 ± 2.26 for Q. acutissima. Termites were found in the southern and western regions, with the highest invertebrate effect after two years. Our results indicate that the influence of invertebrates, especially termites, on dead wood decomposition could be most significant during the initial decomposition stages, as noted in the southern region of this research.
1. Introduction
Termites and wood-boring beetles are dominant invertebrates contributing to dead wood decomposition in temperate forests [1]. Termite preference for wood largely depends on tree species traits, which are categorized based on wood type (softwood/hardwood) [2,3] or clade (angiosperm/gymnosperm) [1,4,5], owing to their distinctive wood traits. By contrast, many wood-boring beetles are considered generalists in terms of tree species and tend to exhibit high diversity in areas with high tree diversity [6,7,8]. Notably, native phloem-feeding beetles primarily colonize dead wood in the early stages of decomposition [9]. Where termites and beetles co-exist, termites generally play a more important role in wood consumption [1].
Two species of subterranean termite (Reticulitermes speratus and R. kanmonensis) and one invasive drywood termite (Glyptotermes nakajima) inhabit South Korea and utilize dead wood, with R. speratus being particularly prevalent throughout the country [10]. As the geographic distribution and activity period of subterranean termites and beetles could change due to increasing temperatures resulting from climate change, the effects of termites and beetles on dead wood could change in the future [11,12].
Termites and wood-boring beetles can directly consume and fragment dead wood [1]. Likewise, the activities of invertebrates and microorganisms can alter dead wood density, C, and nutrient concentrations (e.g., Nitrogen; N) during decomposition processes [2]. Additionally, tunnels and feces created by invertebrates can facilitate the dispersal and access of other organisms to dead wood [13]. Wood-decomposing fungi are also known as one of the dominant decomposers of dead wood and tend to accumulate N, resulting in an increase in N concentration [14]. The main termite predators such as ants can also reduce dead wood decomposition through termite predation and inhibition of fungal activities [15].
Previous studies in the field have estimated the influence of invertebrates on dead wood [2,3,5,15,16]. While the decomposition rate of dead wood needs to be considered based on wood traits, the effects of these traits tend to diminish in the later stages of decomposition, accompanied by changes in the abundance and composition of invertebrates within the dead wood [17,18]. However, many studies on the contribution of invertebrates to dead wood decomposition have been limited to the early stages of wood decomposition, typically the first 2 or 3 years. This limitation could lead to a misunderstanding that dead wood decomposition progresses at a constant rate. Conducting a long-term experiment to assess the effects of invertebrates on dead wood is necessary to comprehensively recognize their impact on the decomposition process.
Two dominant tree species found in South Korea, Pinus densiflora Sieb. et Zucc. (softwood and gymnosperm) and Quercus acutissima Carruth (hardwood and angiosperm), were selected to quantify the effects of wood-decaying invertebrates on the decomposition of dead wood over a six-year period. We hypothesized that the effects of termites and beetle larvae on dead wood are likely to vary due to potential shifts in the dominant decomposers across different decomposition stages. Additionally, we expected that dead wood decomposition varies by region, by tree species influenced by variations in microclimatic conditions, and by the feeding preferences of termites and beetle larvae for specific tree species.
2. Materials and Methods
2.1. Experimental Design
To investigate the decomposition rate of dead wood in South Korea, seven study sites were selected across the southern, western, and eastern regions of the Korean Peninsula (Figure 1). Each region is categorized as having a temperate climate; however, the microclimate at each site differs (Table 1). For example, the southern region has relatively warmer temperatures at lower altitudes. The eastern region is characterized by a colder and moister climate with N-rich soils, while the western region experiences a moderate microclimate compared to the other regions and has acidic soils [19]. Pinus densiflora (Korean red pine) and Quercus spp. (oak) were the dominant tree species at the study sites, with varying stand densities.
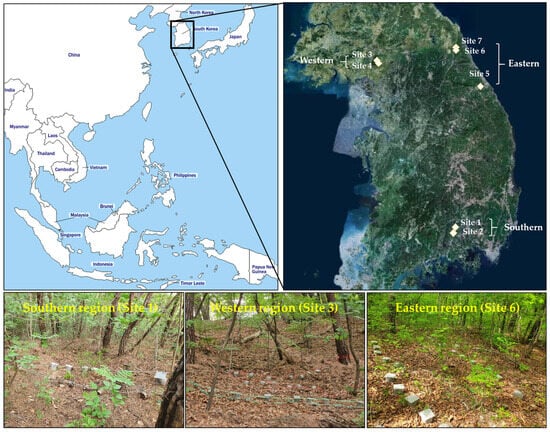
Figure 1.
The locations and pictures of the study sites across the southern, western, and eastern regions of the Korean Peninsula.

Table 1.
The coordinates, microclimate (annual mean temperature, precipitation, and humidity; mean ± SE), altitude, tree density, and dominant tree species of the seven study sites.
In March 2016, Pinus densiflora and Quercus acutissima of the same age (20 years old) and with a diameter at breast height (DBH) of 11 cm were collected after thinning in Bonghwa, South Korea. To minimize differences in initial traits among the dead wood samples, the trees were freshly harvested and cut into similar cylindrical shapes, each with a length of 10 cm and a diameter of 11 cm. Half of the dead wood samples were wrapped with a stainless-steel mesh, featuring holes with a diameter of 0.26 mm to physically prevent access to wood-decaying invertebrates larger than 0.26 mm, such as termites and beetles (Figure 2a). The mean density of dead wood samples was 0.4 ± 0.0 g cm−3 for P. densiflora and 0.6 ± 0.0 g cm−3 for Q. acutissima. The mean C and N concentrations of the dead wood samples were 48.9 ± 0.3% and 0.05 ± 0.01% for P. densiflora and 48.4 ± 0.2% and 0.11 ± 0.00% for Q. acutissima, respectively (the initial wood trait data came from Kim et al. [19]).

Figure 2.
(a) Control dead wood sample, (b) dead wood samples wrapped with stainless-steel mesh for invertebrate exclusion (treatment), and (c) transects linking dead wood samples.
In May 2016, 1200 dead wood samples (100 wood samples × two tree species × two exclusion treatments × three regions) were produced. At each site, 133–200 dead wood samples were allocated based on geographical conditions and placed on the forest ground at distances of 5–10 m. Ten transects linking the dead wood samples were established, and the samples were positioned on the forest ground to mimic downed dead wood (Figure 2b). The dead wood samples were secured with strings, and any humus and litter on the spot was removed to maintain the dead wood samples at the designated locations (Figure 2c).
In May of 2018, 2020, and 2022, the fresh weight of all the dead wood samples was measured in the field. Additionally, 6–11 samples from each region were randomly collected to measure the traits of the collected samples (fresh-to-dry ratio, C and N concentrations, and C:N ratio) in 2020 and 2022. After drying at 85 °C, the C and N concentrations were determined using an elemental analyzer (Vario Macro CHN, Elementar Analysen system GmbH, Langenselbold, Germany). The dry weight of the dead wood samples was calculated using a fresh-to-dry weight ratio of the collected samples by exclusion treatment and control samples among the regions. In the field, the presence of termites, beetle larvae, and ants around the dead wood samples was also checked. As this study did not employ destructive sampling, the presence of beetle larvae in all samples was not comprehensively examined. Beetle larvae seasonally occupy the deepest part of dead wood samples, and it is challenging to confirm their presence without destructive sampling.
2.2. Data Analysis
Throughout the study period, the number of dead wood samples varied because of samples removed for measuring wood traits and samples lost due to harsh weather conditions, such as heavy rain. Consequently, we monitored the weights of the remaining samples as thoroughly as possible until 2022 (Table 2). Any dead wood samples with broken or opened stainless-steel mesh were excluded from the data analyses. A total of 740 dead wood samples were collected and analyzed for mass loss tracking, whereas 224 dead wood samples were collected and analyzed to measure wood traits.

Table 2.
The number of Pinus densiflora and Quercus acutissima dead wood samples collected and analyzed for mass loss and C and N concentrations.
Mass loss and decomposition constant k were calculated using a single exponential decay model.
where Y0 is the initial dry weight, Yt is the dry weight at time t, and t is the year. The invertebrate effect was estimated based on mass loss, using a standardized equation from Kim et al. [19], employing Hedges’ d, and a 95% confidence interval as follows:
where MLcontrol and MLtreatment are the mass loss in the control and invertebrate exclusion treatment samples, ncontrol and ntreatment are the number of study sites, and SDcontrol and SDtreatment are the standard deviations of the mass loss of control and invertebrate exclusion treatment samples, respectively.
Repeated measures analysis was conducted using a general linear mixed model (GLM) to assess the effects of time, tree species, region, and their interactions on the mass loss of dead wood samples. The Scheffé test was used to compare the means. Regression lines were used to describe the relationship between temperature and mass loss. To analyze changes in C and N concentrations due to mass loss, a GLM was performed, and regression lines were utilized. Outlier variables were checked and excluded from all statistical tests. In addition, the z-score normalization method was applied to the C and N values to ensure normality. Statistical analyses were performed using SAS software (version 9.4; SAS System, Cary, NC, USA).
3. Results
In 2018, beetle larvae were found at all study sites. At that time, the termite R. speratus was detected on P. densiflora in the southern (Sites 1 and 2) and western (Site 3) regions, whereas it was absent in the eastern region (Table 3). Conversely, dead wood samples of Q. acutissima in the southern region (Site 1) exclusively contained termites. Subsequently, tunnels and traces of termites and beetle larvae P. densiflora and Q. acutissima were identified and checked from 2018 to 2022 (Figure 3a,b). In 2022, ant colonies were observed within the tunnels of dead wood samples across all the regions (Sites 1, 3, 5, and 6; Figure 3c).

Table 3.
The number of dead wood samples in which invertebrates were found during 2018–2022.
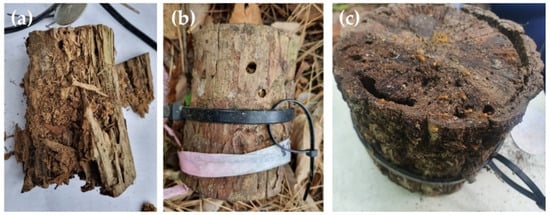
Figure 3.
(a,b) Dead wood samples with invertebrate traces and (c) dead wood samples with traces of ants in 2022.
During the study period, the mean difference in mass loss of dead wood samples with and without invertebrates was 8.85 ± 2.82% for P. densiflora and 6.90 ± 2.45% for Q. acutissima. The mean difference was highest in 2018, reaching 21.74% (range 8.42–45.77%) for P. densiflora and 11.88% (range 9.20–15.70%) for Q. acutissima, after which, by 2022, it had decreased to 7.33% (range 5.00–9.77%) for P. densiflora and 5.99% (range 0.00–12.26%) for Q. acutissima.
On average, the standardized invertebrate effect, using Hedges’ d and a 95% confidence interval, was 1.04 ± 2.12 for P. densiflora and 1.30 ± 2.22 for Q. acutissima during the study period. The greatest invertebrate effect was observed in P. densiflora in the southern region in 2018 (Figure 4). For Q. acutissima, the invertebrate effect was greatest in 2018 during the study period. Additionally, for Q. acutissima, the invertebrate effect was greatest in the southern region, followed by the eastern and western regions in 2018 (southern > eastern > western). Notably, P. densiflora tended to have a greater invertebrate effect than Q. acutissima in the southern and western regions, whereas the effect was reversed in the eastern region in 2018.
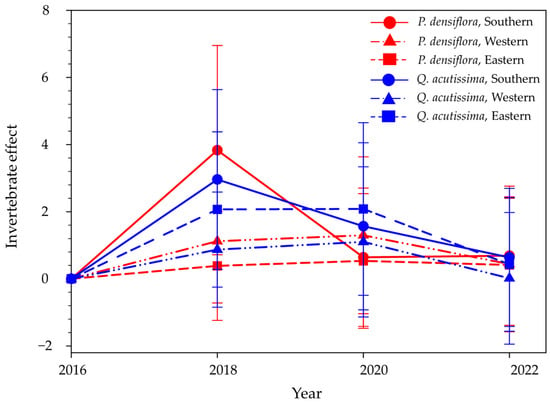
Figure 4.
Invertebrate effect of Pinus densiflora and Quercus acutissima in the southern, western, and eastern regions of South Korea from 2016 to 2022. The effect size was calculated using Hedges’ d ± 95% confidence interval. Vertical bars indicate the 95% confidence interval.
The overall mean mass loss was 84.24 ± 6.93% (range 79.10–88.41%; k = 0.33 ± 0.10 year−1) for P. densiflora and 74.25 ± 7.74% (range 65.35–81.82%; k = 0.23 ± 0.07 year−1) for Q. acutissima during the 6 years of study. The highest mass loss was found in P. densiflora installed in the southern region, which was 88.41 ± 6.46% (k = 0.46 ± 0.13 year−1) after 6 years (Figure 5). At the time, Q. acutissima in the eastern region showed the lowest mass loss of 65.35 ± 8.12% (k = 0.18 ± 0.04 year−1). Mass loss in P. densiflora and Q. acutissima showed significant differences among the regions during the study period (southern > western ≥ eastern).
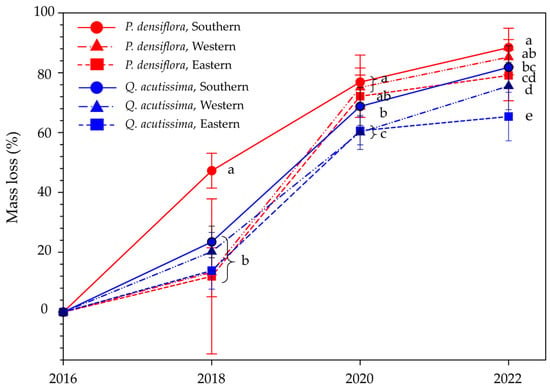
Figure 5.
Mass loss (%) of Pinus densiflora and Quercus acutissima in the southern, western, and eastern regions of South Korea from 2016 to 2022. Vertical bars indicate standard errors. Different letters within the same year indicate significant differences in the Scheffé test (significant differences at p < 0.05).
The mass loss of dead wood samples from invertebrates was statistically different based on the tree species, exclusion treatment, region, and time (Table 4). The interaction effect between the exclusion treatment and region was also significant (p < 0.001). Mass loss increased with the annual mean temperature (°C) across the sites for all tree species (Figure 6).

Table 4.
The results of the general linear model representing the mass loss of control dead wood samples by tree species (S), invertebrate exclusion (E), region (R), time (T), and their interactions (NS: not significant; * p < 0.05; *** p < 0.0001).
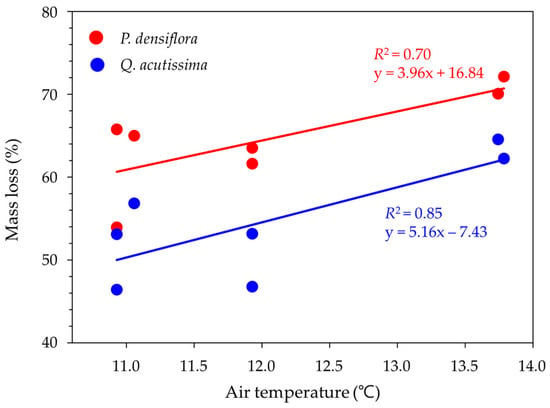
Figure 6.
Relationship between mass loss (%) and annual mean temperature (°C) for Pinus densiflora and Quercus acutissima in the study sites (n = 7). The solid red and blue lines denote the linear regressions for both tree species, respectively.
In 2020, the mean N concentration of P. densiflora was 0.80 ± 0.14% for dead wood samples with invertebrates (control), and it was 0.79 ± 0.18% for dead wood samples without invertebrates (treatment). In 2022, the mean N concentration in P. densiflora was 0.65 ± 0.14% in the control group, whereas it measured 0.54 ± 0.23% in the treatment group. For Q. acutissima, the N concentration was 1.05 ± 0.06% and 0.94 ± 0.09% for the control group and the treatment group, respectively, in 2020. the N concentration was 0.95 ± 0.15% in the control group and 0.84 ± 0.19% in the treatment group in 2022. The C concentration in P. densiflora was 47.67 ± 5.02% in the control group and 48.44 ± 3.92% in the treatment group. Similarly, for Q. acutissima, the C concentration was 46.97 ± 3.20% in the control group and 47.35 ± 2.95% in the treatment group. The C:N ratio for P. densiflora was 76.75 ± 21.60 in the control group and 111.86 ± 61.32 in the treatment group. For Q. acutissima, the C:N ratio was 50.99 ± 10.71 in the control group and 60.37 ± 18.16 in the treatment group.
The N concentration differed by the exclusion treatment (p < 0.001) and was positively correlated with mass loss (p < 0.05; Figure 7a,b), except in the control dead wood samples of Q. acutissima (p > 0.05; Figure 7b). By contrast, the C concentration was not significantly different by species, exclusion treatment, and regions (p > 0.05). A significant difference in the C:N ratio was found in the exclusion treatment (p < 0.001), and the C:N ratio decreased with mass loss in P. densiflora (control: n = 23, p < 0.001; R2 = 0.48; treatment: n = 23, p < 0.001; R2 = 0.48).
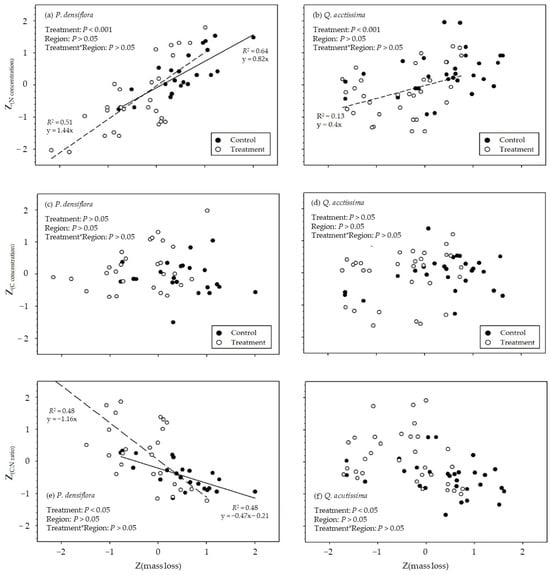
Figure 7.
Relationships of N concentration of (a) Pinus densiflora control and treatment and (b) Quercus acutissima control and treatment; C concentration of (c) P. densiflora control and treatment and (d) Q. acutissima control and treatment; and C:N ratio of (e) P. densiflora control and treatment and (f) Q. acutissima control and treatment with mass loss of dead wood samples. Control: dead wood samples with invertebrates (Supplementary Table S1); treatment: dead wood samples wrapped with stainless-steel mesh. Z-score transformation was applied for all variables; the solid line indicates the linear regressions of control dead wood samples, and dashed lines represent the linear regression of treatment dead wood samples (p < 0.05). Only significant regression lines are presented on each graph.
4. Discussion
The average difference in mass loss of dead wood samples with and without invertebrates during the six years was in between the results of previous studies (11.5% of mass loss in Warren and Bradford 2012 [15]; 13.7% of specific gravity loss in Ulyshen [16]; 6.3% of mass loss in Seibold et al. [5]). However, the difference in mass loss between the control and treated dead wood samples in 2018 was greater than that reported in other studies estimating termite effects on dead wood decomposition in temperate forests. By contrast, the difference in mass loss between the control and treated dead wood samples in 2022 was similar to the result reported by Seibold et al. [5] and lower than that in previous studies. These results indicate variations in the effects of invertebrates on dead wood and highlight the challenges in identifying their contribution to dead wood decomposition, especially where termites exist.
In the southern region, the invertebrate effect peaked in the first two years and then decreased significantly. The diminished invertebrate effect on dead wood could have been influenced by ants, which are known predators of termites [20,21]. When termites encounter ants, termites could forage for new dead wood [22] or abandon their nesting areas [20]. Despite the absence of termites in dead wood, fungi within the dead wood may lead the mass loss process of dead wood thereafter [23,24]. In the eastern region, where termites were not found, succession in the beetle larvae species might affect the mass loss of dead wood decomposition [18].
The mass loss of dead wood samples with invertebrates could have occurred due to both wood-decomposing invertebrates and fungi. In this study, the highest mass loss observed in the southern region aligns with the findings of Kim and Chung [10], who measured the greatest termite damage to the Korean wood cultural heritage in the southern region. Additionally, our results align with previous studies indicating that the distribution and foraging activities of termites are restricted by low temperatures [25,26]. Laboratory studies have shown that the feeding activities of the termite R. speratus peak around 30 °C [25]. Thus, the higher mass loss of dead wood found in the southern region may be caused by activated invertebrate feeding activity, especially that of termites, at higher temperatures [27]. Indeed, temperature emerged as the most significant factor controlling the mass loss in this study (Figure 6).
The results of the overall mass loss by tree species analysis coincided with previous studies by Takamura [2] and Peralta et al. [3], who examined the feeding preferences of subterranean termites and suggested a preference for softwood over hardwood. By contrast, Weedon et al. [4] reported that angiosperm decompose faster than gymnosperm due to their lower density, higher N concentrations, and different xylem architecture. However, the wood traits of the tree species did not fit into the categories identified by Weedon et al. [4], as Q. acutissima (an angiosperm) exhibited higher density and N concentration than P. densiflora (a gymnosperm). The faster decomposition rate of Quercus spp. compared to P. densiflora was also reported by Noh et al. [14] in a study from the western region of South Korea over two years. Specifically, in this study, the decomposition rate of Q. acutissima in the western region surpassed that of P. densiflora two years after the experiment commenced, particularly during the early decomposition stage.
The mass loss was highest in the southern region, followed by the western and eastern regions (southern > western > eastern). However, the invertebrate effect on dead wood varied across regions and did not precisely align with the regional mass loss pattern (southern > eastern > western). Furthermore, the initial invertebrate effect was higher for P. densiflora than for Q. acutissima in the southern region. By contrast, the invertebrates preferred Q. acutissima over P. densiflora in the eastern and western regions. Given the regional differences in the presence of termites and beetles, as well as the lack of clear identification of invertebrate feeding preferences for specific tree species in this study, we suggest that the observed results may be attributed to differences in the composition of termites and beetles and their feeding preferences. Since many wood-boring beetles are recognized as non-specialist species [8], the well-known feeding preference of termites for certain tree species may be attenuated in regions with relatively low termite density. In this study, termites were not detected in dead wood samples from the eastern region throughout the study period. Considering that the contribution of beetles to the mass loss of dead wood may be smaller than that of termites [1], the observed regional trends in mass loss and invertebrate effects likely reflect the significance of local-scale variations in dominant dead wood decomposers [28].
We anticipated that termites and beetle larvae would induce more dynamic C and N processes in dead wood decomposition by mixing mineral and organic soils around the dead wood [1]. Our results, which indicate an increase in N concentration due to mass loss, may signify processes involving N immobilization and accumulation in dead wood [29,30,31]. Additionally, considering the alterations in N concentration and the C:N ratio of the two tree species with mass loss, the influence of termites and beetle larvae on dead wood traits may vary between tree species.
In this study, although soils and other materials (e.g., mushrooms) were removed during the experiments, soils and feces introduced by termites could remain inside of the dead wood samples in the field. Thus, the estimated decomposition rates of dead woods were likely to be underestimated. We acknowledge that our study’s 6-year duration may not have been sufficient to capture the full diversity of invertebrate fauna associated with different stages of wood decomposition. According to Parisi et al. [18], there are four stages of wood decomposition, each associated with various invertebrate families. Future research should extend the monitoring period and employ enhanced sampling methods of invertebrates to fully understand the abundance, temporal dynamics, and succession of invertebrate communities in decomposing wood. Moreover, our study primarily focused on the role of termites and beetles, as well as other invertebrates larger than 0.26 mm, in the decomposition process. This approach does not encompass the contributions of smaller and abundant invertebrates such as springtails, mites, nematodes and proturans, and fungi, which are also significant decomposers. In addition, although we suggested changes in the invertebrate effects and different feeding preferences of termites and beetles for dead wood, the predominant beetle communities affecting dead wood at the sites and decomposition stages could not be clearly identified. Since the sampling of dead wood for C and N concentrations was conducted randomly, control dead wood samples without traces of termites and beetle larvae were included in the analysis of wood traits. Furthermore, since dead wood sampling was conducted only in 2020 and 2022, when the invertebrate effect had already decreased, the estimation of the impact of the invertebrates on dead wood traits may have been underestimated. Likewise, the effects of microorganisms on dead woods needed to be considered to explain the changes in overall mass loss and N processes in the dead woods.
As the distribution of termites is expected to expand in South Korea due to climate change, the decomposition rate of dead wood could increase in forests. Our findings may provide valuable insights in estimating the potential damage to woody materials caused by termites and changes in the N cycle in forest ecosystems. This study also emphasized the challenges associated with observing the fluctuating impact of invertebrates on dead wood decomposition and identifying the dominant invertebrate species. These challenges need to be taken into account in future studies.
5. Conclusions
This study investigated the effects of termites, beetle larvae, and ants on dead wood decomposition, focusing on two dominant tree species (P. densiflora and Q. acutissima) in South Korea. To conduct this study, we implemented two key measures: (1) an invertebrate (larger than 0.26 mm) exclusion treatment, where half of the dead wood samples of P. densiflora and Q. acutissima were wrapped in a stainless-steel mesh to physically prevent the invertebrates, and (2) the installation of dead wood samples in forests located in three regions across South Korea. Experimental measurements were conducted over a 6-year period to assess changes in mass loss and wood traits, such as C and N concentrations. The southern region exhibited higher mass loss, possibly due to increased termite activity caused by higher temperatures. While distinct trends in the effects of termites and beetle larvae on dead wood decomposition according to tree species were not evident, an interaction of tree species and exclusion treatment on the mass loss of dead wood was significantly different. Thus, it remains crucial to account for potential differences in feeding preferences among termites and beetles. Additionally, the effect of invertebrates larger than 0.26 mm on dead wood decomposition may be maximized in the early stages (as observed in year 2 of this study). Our results highlight the intricate interplay of various microclimatic and biological factors in dead wood decomposition. As dead wood in forests can be influenced not only by various organisms but also by the survival and activities of these organisms, multiple factors and their interactions need to be considered and managed.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16080452/s1, Table S1: Decomposition rate (%), nitrogen (N) and carbon (C) concentrations (%) and C/N ratio of Pinus densiflora and Quercus acutissima samples collected during 2016-2022. Control: dead wood samples without stainless-steel mesh; Exclusion: dead wood samples with stainless-steel mesh.
Author Contributions
Conceptualization and investigation, D.R., S.K., H.-S.K., S.H.H. and G.K.; supervision, Y.S.; Analysis and writing, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of the Republic of Korea (NRF-2022R1A2C1011309), ‘R&D Program for Forest Science Technology’ provided by Korea Forest Service (Korea Forestry Promotion Institute) (RS-2022-KF002024), and Korea Environment Industry & Technology Institute (KEITI) through Climate Change R&D Project for New Climate Regime, funded by the Korea Ministry of Environment (MOE) (RS-2022-KE002294).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are available on request from the first author.
Acknowledgments
The authors gratefully acknowledge the assistance of all the students in the Ecosystem Ecology Laboratory at Korea University during wood sample preparation and field measurements. Choonsig Kim of Gyeongnam National University of Science and Technology helped to select and establish the sites in the southern region.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cornwell, W.K.; Cornelissen, J.H.; Allison, S.D.; Bauhus, J.; Eggleton, P.; Preston, C.M.; Scarff, F.; Weedon, J.T.; Wirth, C.; Zanne, A.E. Plant traits and wood fates across the globe: Rotted, burned, or consumed? Glob. Chang. Biol. 2009, 15, 2431–2449. [Google Scholar] [CrossRef]
- Takamura, K. Effects of termite exclusion on decay of heavy and light hardwood in a tropical rain forest of Peninsular Malaysia. J. Trop. Ecol. 2001, 17, 541–548. [Google Scholar] [CrossRef]
- Peralta, R.C.G.; Menezes, E.B.; Carvalho, A.G.; Aguiar-Menezes, E.D.L. Wood consumption rates of forest species by subterranean termites (Isoptera) under field conditions. Rev. Árvore 2004, 28, 283–289. [Google Scholar] [CrossRef]
- Weedon, J.T.; Cornwell, W.K.; Cornelissen, J.H.; Zanne, A.E.; Wirth, C.; Coomes, D.A. Global meta-analysis of wood decomposition rates: A role for trait variation among tree species? Ecol. Lett. 2009, 12, 45–56. [Google Scholar] [CrossRef]
- Seibold, S.; Rammer, W.; Hothorn, T.; Seidl, R.; Ulyshen, M.D.; Lorz, J.; Cadotte, M.W.; Lindenmayer, D.B.; Adhikari, Y.P.; Aragón, R.; et al. The contribution of insects to global forest deadwood decomposition. Nature 2021, 597, 77–81. [Google Scholar] [CrossRef]
- Gossner, M.M.; Wende, B.; Levick, S.; Schall, P.; Floren, A.; Linsenmair, K.E.; Steffan-Dewenter, I.; Schulze, E.D.; Weisser, W.W. Deadwood enrichment in European forests–Which tree species should be used to promote saproxylic beetle diversity? Biol. Conserv. 2016, 201, 92–102. [Google Scholar] [CrossRef]
- Horák, J. Niche partitioning among dead wood-dependent beetles. Sci. Rep. 2021, 11, 15178. [Google Scholar] [CrossRef]
- Milberg, P.; Bergman, K.O.; Johansson, H.; Jansson, N. Low host-tree preferences among saproxylic beetles: A comparison of four deciduous species. Insect Conserv. Diver. 2014, 7, 508–522. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Wagner, T.L. Quantifying arthropod contributions to wood decay. Methods Ecol. Evol. 2013, 4, 345–352. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, Y.J. Analysis of factors affecting termite damage to wooden architectural heritage buildings in Korea. Forests 2022, 13, 465. [Google Scholar] [CrossRef]
- Lee, H.B.; Seo, M.S.; Lee, S.B.; Lee, W. New distribution of Reticulitermes speratus speratus (Blattodea: Rhinotermitidae) in Korea. J. Econ. Entomol. 2023, 116, 2027–2034. [Google Scholar] [CrossRef]
- Kwon, T.S.; Lee, C.M.; Kim, S.S. Prediction of abundance of beetles according to climate warming in South Korea. J. Asia-Pac. Biodivers. 2015, 8, 7–30. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Wood decomposition as influenced by invertebrates. Biol. Rev. 2016, 91, 70–85. [Google Scholar] [CrossRef]
- Noh, N.J.; Yoon, T.K.; Kim, R.H.; Bolton, N.W.; Kim, C.; Son, Y. Carbon and nitrogen accumulation and decomposition from coarse woody debris in a naturally regenerated Korean red pine (Pinus densiflora S. et Z.) forest. Forests 2017, 8, 214. [Google Scholar] [CrossRef]
- Warren, R.J.; Bradford, M.A. Ant colonization and coarse woody debris decomposition in temperate forests. Insectes Soc. 2012, 59, 215–221. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Interacting effects of insects and flooding on wood decomposition. PLoS ONE 2014, 9, e101867. [Google Scholar] [CrossRef]
- Zuo, J.; Fonck, M.; van Hal, J.; Cornelissen, J.H.C.; Berg, M.P. Diversity of macro-detritivores in dead wood is influenced by tree species, decay stage and environment. Soil Biol. Biochem. 2014, 78, 288–297. [Google Scholar] [CrossRef]
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests—A review. iForest 2018, 11, 423–436. [Google Scholar] [CrossRef]
- Kim, S.; Han, S.H.; Li, G.; Roh, Y.; Kim, H.J.; Son, Y. The initial effects of microclimate and invertebrate exclusion on multi-site variation in the mass loss of temperate pine and oak deadwoods. Sci. Rep. 2021, 11, 14840. [Google Scholar] [CrossRef]
- Tuma, J.; Eggleton, P.; Fayle, T.M. Ant-termite interactions: An important but under-explored ecological linkage. Biol. Rev. 2020, 95, 555–572. [Google Scholar] [CrossRef]
- Wong, N.; Lee, C.-Y. Effects of disturbance and the presence of termite and other invertebrate carcasses at feeding sites on the behavior of the subterranean termite Microceratermes crassus (Blattodea: Termitidae). Sociobiology 2010, 55, 353–367. [Google Scholar]
- Viana-Junior, A.B.; Côrtes, M.O.; Cornelissen, T.G.; Neves, F.D.S. Interactions between wood-inhabiting fungi and termites: A meta-analytical review. Arth.-Plant Int. 2018, 12, 229–235. [Google Scholar] [CrossRef]
- Bani, A.; Pioli, S.; Ventura, M.; Panzacchi, P.; Borruso, L.; Tognetti, R.; Tonon, G.; Brusetti, L. The role of microbial community in the decomposition of leaf litter and deadwood. Appl. Soil Ecol. 2018, 126, 75–84. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Kauserud, H.; Sverdrup-Thygeson, A.; Bjorbækmo, M.M.; Birkemoe, T. Wood-inhabiting insects can function as targeted vectors for decomposer fungi. Fungal Ecol. 2017, 29, 76–84. [Google Scholar] [CrossRef]
- Gautam, B.K.; Henderson, G. Wood consumption by Formosan subterranean termites (Isoptera: Rhinotermitidae) as affected by wood moisture content and temperature. Ann. Entomol. Soc. Am. 2011, 104, 459–464. [Google Scholar] [CrossRef]
- Cao, R.; Su, N.Y. Temperature preferences of four subterranean termite species (Isoptera: Rhinotermitidae) and temperature-dependent survivorship and wood-consumption rate. Ann. Entomol. Soc. Am. 2016, 109, 64–71. [Google Scholar] [CrossRef]
- Zanne, A.E.; Flores-Moreno, H.; Powell, J.R.; Cornwell, W.K.; Dalling, J.W.; Austin, A.T.; Classen, A.T.; Eggleton, P.; Okada, K.I.; Parr, C.L.; et al. Termite sensitivity to temperature affects global wood decay rates. Science 2022, 377, 1440–1444. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.A.; Warren II, R.J.; Baldrian, P.; Crowther, T.W.; Maynard, D.S.; Oldfield, E.E.; Wieder, W.R.; Wood, S.A.; King, J.R. Climate fails to predict wood decomposition at regional scales. Nat. Clim. Chang. 2014, 4, 625–630. [Google Scholar] [CrossRef]
- Palviainen, M.; Laiho, R.; Mäkinen, H.; Finer, L. Do decomposing Scots pine, Norway spruce, and silver birch stems retain nitrogen? Can. J. For. Res. 2008, 38, 3047–3055. [Google Scholar] [CrossRef]
- Köster, K.; Metslaid, M.; Engelhart, J.; Köster, E. Dead wood basic density, and the concentration of carbon and nitrogen for main tree species in managed hemiboreal forests. For. Ecol. Manag. 2015, 354, 35–42. [Google Scholar] [CrossRef]
- Purahong, W.; Wubet, T.; Lentendu, G.; Hoppe, B.; Jariyavidyanont, K.; Arnstadt, T.; Baber, K.; Otto, P.; Kellner, H.; Hofrichter, M.; et al. Determinants of deadwood-inhabiting fungal communities in temperate forests: Molecular evidence from a large scale deadwood decomposition experiment. Front. Microbiol. 2018, 9, 2120. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).