Abstract
Records from online biodiversity databases (including citizen science data) can play a crucial role in enhancing scientific knowledge about the abundance, distribution, and population trends of poorly studied species which are usually not properly monitored. This study aims to demonstrate the utility of data hosted in GBIF in detecting the likely decline of species common and widely distributed in the past, but whose conservation status is now uncertain, such as the weasel (Mustela nivalis) in Spain. To address this, we analyzed data on its presence in Spain from 2008 to 2022 available on GBIF.org, and compared it with the distribution data from the Atlas of Mammals of Spain published in 2007. The results indicate that: (i) data from GBIF.org reveal a moderate decline (negative trend) in the weasel population in Spain during the study period; (ii) the species has been recorded in a limited number of 10 × 10 km UTM-squares (Universal Transverse Mercator) within its distribution range as defined by the 2007 atlas; and (iii) there are large areas of Spain in which the species has not been detected in recent years. These findings highlight the concerning conservation status of this carnivorous species and underscore the value of data from open access platforms such as GBIF in identifying potential silent extinctions.
1. Introduction
The population abundance and distribution of wild species is a basic component in conservation programs. Accurate population trends are essential for identifying species of concern, targeting the necessary conservation measures to prioritize species and sites, and assessing their effectiveness [1,2]. However, to achieve these goals, monitoring species at a large scale can be very difficult to implement by scientists and public administrators for certain reasons: technological and financial constraints, great fieldwork demands, or logistical support [3,4]. Fortunately, the recent development of open-access database platforms has made it possible to collect, store, and manage biodiversity data, enhancing our understanding of the distribution of numerous species [5]. The Global Biodiversity Information Facility (GBIF: https://www.gbif.org/, accessed on 15 July 2024) is likely the most comprehensive open-access biodiversity database integrating records from other data repositories [6]. It is an international network and data infrastructure funded by the world’s governments and aimed at providing anyone, anywhere, with open access to data about all types of life on Earth [7]. As of July 2024, 2.9 billion species occurrence records have been published on GBIF from over 100,000 databases by more than 2000 publishing institutions, which have been used in more than 10,000 peer-reviewed papers.
More specifically, these databases have greatly increased in volume thanks to the significant rise of citizen science in recent years [8,9]. Opportunistic data from citizen science integrated in these platforms can, therefore, be complementary to monitoring species on a broad scale and over the long-term with little resource investment [10,11,12].
However, there is significant taxonomic bias in these database platforms [13], since not all species are equally represented, with some species being overrepresented with large volumes of data, while others are underrepresented with very few records [14,15]. In the case of mammals, some animal taxa are underrepresented for different reasons: smaller body size, elusive behavior, nocturnal activity, a habitat with poor visibility, rare species, difficulty to identify, or smaller distribution range [16,17]. For some of these species, the low number of records could also suggest the scarcity of the species and a plausible population decline. Recently, some scientists and conservationists (e.g., [18,19]) have coined the term ‘silent extinction’ for those species whose populations trends have not been adequately assessed and whose number are decreasing or may even be locally extinct. This could be the case for weasels (Carnivora: Mustelidae: Mustela), the world’s smallest carnivores [20] and probably one of the most difficult species to detect [20] and later register in these open-access platforms. For these reasons, some new methods have been developed in recent years to monitor weasels, such as non-invasive genetic surveys, detection dogs, camera-traps adapted to detect weasels, or the use of citizen science (see the review [21]).
Despite the least weasel Mustela nivalis (Linnaeus, 1758) being widely distributed in Europe, basic information about its ecology, distribution, and population trends remains significantly undisclosed [22,23], probably, among other reasons, because of the difficulty of detecting it and the lack of long-term and large-scale data. The decline of weasel populations has been documented recently in the USA, [24], United Kingdom [25], and Tunisia [26]. However, this information gap is particularly noteworthy in Spain, where very few studies have been focused on this species [27]. Almost the only study on the population trends of weasels in Spain suggested a strong decrease in Catalonia (NE Spain) from the first sampling period (1995–1998) to the second period (2005–2008), since in the latter they only recorded one weasel with a sampling effort greater than 20.000 trap nights [28].
Recent concerns about possible declines in weasel populations in other countries highlight the need to critically evaluate available data on abundance and distribution to ascertain whether there is also a decline in Spain. For this purpose, we have verified whether the data available on GBIF corroborate the negative population trend of this species over recent years. Accordingly, we aim to highlight the importance of biodiversity databases in analyzing the population trends of species whose population decline is going unnoticed by science.
2. Materials and Methods
2.1. Data Collection and Management
The records of M. nivalis in Spain from 2008 to 2022 were obtained from the Global Biodiversity Information Facility (GBIF.org, accessed on 16 July 2024), using the GBIF plugin implemented in QGIS (Figure 1). We used this timeframe because it aligns with the development of biodiversity data collection on this platform and because the Atlas of Mammals of Spain, the most comprehensive data about mammal distributions in Spain, was published in 2007 [29]. In total, 644 records of M. nivalis were obtained for this period [30], of which 639 were provided via human observations, 4 via preserved specimens, and 1 via machine observation. As GBIF integrates data from different sources, the data of M. nivalis in Spain are formed by different datasets, including citizen science platforms (iNaturalist.org, Observation.org, or MammalNet), and data collected by research institutions and public organizations.
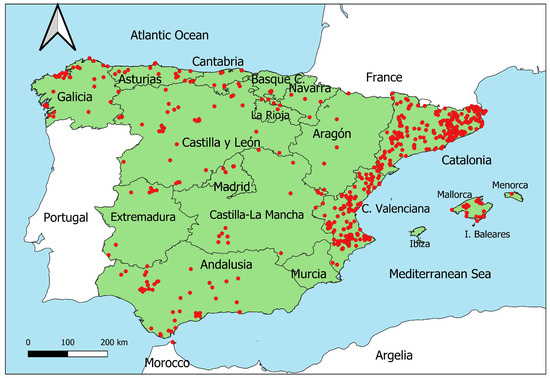
Figure 1.
Spatial location of the records (red spots) of Mustela nivalis in Spain from 2008 to 2022 on GBIF.org. The black lines represent the limits of the autonomous communities (ACs) in Spain.
Since there can be significant year-to-year variations in sampling efforts and/or data quantities (e.g., due to the COVID-19 lockdown in 2020 [31]), for each year the number of records of M. nivalis was standardized for every 1000 records of all carnivores (Carnivora; relative abundance 1; Formula (3)) and all mustelids (Mustelidae; relative abundance 2; Formula (2)), following the methodology suggesting by Santos et al. [32].
The yearly number of records was also calculated for each autonomous community (hereafter AC), which also made it possible to partly control the differences in sampling efforts, and the spatial distribution. The Region of Murcia and the Canary Islands were excluded from the analysis because M. nivalis is missing in these ACs.
The records of the presence of the M. nivalis in Spain from 2008 to 2022 were compared with the distribution of this species according to the Atlas of Mammals of Spain [29] in order to verify the percentage of UTM 10 × 10 cells where the species is assumed to be present in 2007 and where it was detected afterward (occurrence frequency; Formula (3)).
2.2. Statistical Analysis
The population trends from 2008 to 2022 were estimated with TRIM software version 3.54 (trends and indices for monitoring data), which uses a log-linear Poisson regression model to estimate temporal population trends [33]. In the analysis, the experimental unit was the standardized number of records of weasels in each year in each AC (n = 225), and the AC (n = 15) was included in the analysis as the site. Two different analyses were performed, using the relative abundance of M. nivalis standardized by the records of Mustelidae (RA1) and Carnivora (RA2). Since the ACs of Catalonia and Valencia have their own systems to collect biodiversity data (Ornitho.cat and Banco de Datos de la Biodiversidad de la Comunidad Valenciana, respectively) and make these local data available on the GBIF website, representing a high proportion of the data (see results), we performed different models separately for Valencia and Catalonia and the rest of Spain excluding these ACs.
Based on the slopes of the regression model and standard errors, TRIM classifies trends into six categories: Increase, Moderate increase, Stable, Moderate decline, Decline, and Uncertain trends [33]. The population change estimated as the % of change from the first to the last year (Formula (4)) [32].
3. Results
During the study period (2008–2022), 644 records of M. nivalis were available on GBIF.org, with an annual mean of 43.5 (±26.5 S.D.) records, which represent only the 1.21% and 2.3% of all records of Carnivora (54,401) and Mustelidae (28,797) during this time period, respectively. The records calculated in every AC showed that the mean yearly value was 2.59 (±7.8), being 0 for the median value, since in many years for many AC there were no records of M. nivalis (of the 225 combinations of 15 years × 15 AC, in 118 there were no records of M. nivalis). The spatial distribution was uneven, with 65.5% of the presences located in the Catalonia and Valencia ACs, which means that only 229 records were obtained in the rest of the Spain in 15 years.
The two TRIM analyses of the data for Spain, excluding Valencia and Catalonia, show a significant negative trend (Table 1; Figure 2), with an estimated population change of −50.6 and −59.19%, respectively, being classified as a moderate decline in both cases, which means a significant decline but not significantly more than 5% per year. The TRIM analysis of the data from Catalonia and Valencia shows a negative non-significant trend, classified as uncertain, which means no significant decline but without certainty that the trends are less than 5% per year.

Table 1.
Results of the TRIM models for the relative abundance (RA) of M. nivalis standardized to the records of Mustelidae (RA1) and Carnivora (RA2), for the data from Valencia and Catalonia together and for the rest of Spain. S.E. = standard errors of means.
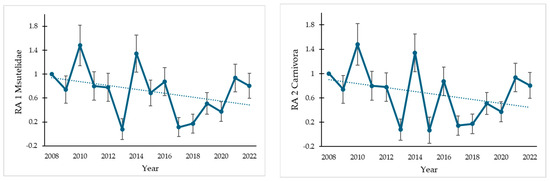
Figure 2.
Trends in the relative abundance (RA) of Mustela nivalis in Spain standardized to the number of records of Mustelidae (RA1) and Carnivora (RA2), using the data of 2008 as a reference value (RA in 2008 = 1). The vertical error bars show the standard error.
The presence of M. nivalis was recorded in 274 UTM 10 × 10 km cells out of the 2336 cells in which the species was present according to the 2007 atlas (Figure 3), which means that the species was registered from 2008 to 2022 in 11.73% of the cells with a presence in 2007. However, the records of M. nivalis during this period were spatially uneven (Figure 3). Of the 274 UTM cells with a presence recorded between 2008 and 2022, 180 (65.7%) are located in Catalonia and Valencia, and 94 (34.3%) are located in the rest of the country. That is, in Catalonia and Valencia this species was recorded in the 32.8% of the UTM cells with a presence in 2007, but in the rest of the country the species was registered in only 5% of the cells.
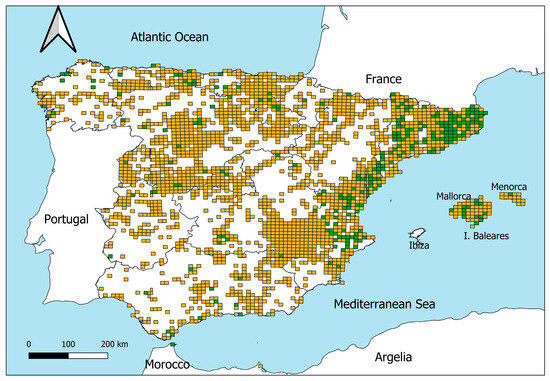
Figure 3.
Potential distribution (orange squares) of M. nivalis in 2007 according to the Atlas of Mammals of Spain [30]. The green squares show those 10 × 10 km UTM cells in which the species were registered from 2008 to 2022.
4. Discussion
The data stored on GBIF.org suggest that the weasel is a scarce species in Spain, and although these data should be interpreted with caution (see below), this approach can be useful in detecting the rarity of some species considered common and widely distributed the past but that are not being adequately monitored, thus leading to uncertainty about its conservation status. Given that funds for biodiversity conservation are limited, monitoring programs are usually focused on some species, particularly those with a known worrisome conservation status, higher ecological and economic importance, or that are flagship species [34,35]. Under this scenario, the silent extinction of some species might be occurring, and therefore checking their population trends using the data on GBIF and other open-access databases can provide the initial information about the conservation status of a species.
In the case of M. nivalis in Spain, a widely distributed species in the past (Figure 3), it has recently been recorded in only few locations within its potential distribution area (Figure 3), its moderate population decline (Figure 2), and the few existing studies [27] suggesting a possible silent extinction. Except in the eastern Iberian Peninsula (see below), the number of records is very low, and the species has not been detected in vast areas of the country (Figure 1 and Figure 3). Indeed, in 8 of the 15 ACs, there are fewer than 20 records in 15 years. Moreover, some comparisons can be useful to contextualize the amount of data on weasels. For instance, if we compare, during the same period, the amount of data on M. nivalis with two mustelids such as the stone marten (Martes foina) and the European polecat (Mustela putorius), these species have 3432 and 2930 presences in GBIF, respectively, which represents 5 and 4.5 times more data. On the other hand, our research group has performed many camera-trap sampling campaigns in the Cordoba province (Andalusia, southern Spain) in the last four years (see [36,37] for more details; and unpublished data), with an accumulated sampling effort greater than 4500 trap nights, but we have obtained only one record of the least weasel (Figure 4), which could suggest the current low abundance of the species.

Figure 4.
The only photo of Mustela nivalis that the research team has been able to obtain through photo-trapping for four years (2020–2024).
The decline and scarcity of the weasel is likely due to several factors: land-use changes; loss of optimal habitat as a result of the natural afforestation of Mediterranean areas due to rural abandonment [28,38]; road kill [39]; exposure to anticoagulant rodenticides [40]; a decrease in prey abundance [28]; top–down regulation by larger predators, particularly in poor habitat with low refuge availability [28]; climate change effects [41]; or even some diseases with an unknown impact. Moreover, these factors can act synergistically and, for instance, climate change can decrease habitat favorability due to negative effects on prey [28,41,42], or vulnerability to by top-predators can increase because of land-use changes. More studies are therefore necessary to determine the main harmful factors, which is an indispensable requirement for designing appropriate conservation measures. The decline of weasel populations could disrupt predator–prey relationships and ecosystem dynamics, since this species is an important predator for rodents but also prey for some species.
Our approach has two limitations (particularly common in data from citizen science; [10,13]) which should be considered: (1) the difficulty of detecting small animals such as weasels and their possible underrepresentation; (2) the spatial and temporal sampling bias in data collection. Indeed, the AC of Catalonia and Valencia have their own systems for collecting biodiversity data (Ornitho.cat and Banco de Datos de la Biodiversidad de la Comunidad Valenciana, respectively) and make these local data available on the GBIF website, including data from citizen science but also from regional monitoring programs. Data from the 2008–2022 Catalonia and Valencia dataset represented 65.5% of the data of M. nivalis in Spain (see Results), making necessary the separate interpretation of the spatial data for the rest of the country. In these two ACs with greater data availability, the population trends of M. nivalis are uncertain. Despite these limitations, data from GBIF are widely used in the scientific literature, and most criticisms are usually aimed at using occurrence data to reconstruct and model species’ distribution ranges, but they can be useful in answering a simple question, as in our case: has the weasel been recorded anywhere during a given period [43]? In the particular case of citizen science, with a proper design, training, and validation protocols, and, later, proper interpretation, these data can play a crucial role in monitoring and understanding population trends in weasels, being a suitable complementary tool together with data collected by scientists and public administrations. The combination of different methodologies (e.g., modified camera traps to detect small mammals, non-invasive genetics, or live captures [21]) could increase the number of records and thus help us to better understand the conservation status of the species.
In summary, the moderate decline of the weasel in Spain, the lack of records for many regions and many years, and its detection in few areas where the species was present in the past, suggests that the species could be scarcer than expected. We deem that our approach of using data from GBIF could be useful for detecting the scarcity and likely recent decline of some species which are not usually well monitored (known as silent extinctions), as a first step to warn about their possibly concerning conservation status. Therefore, long-term and large-scale monitoring programs should be implemented to effectively assess the conservation status of M. nivalis in Spain, and whether the lack of data in recent years in GBIF is a consequence of population decline. This is a necessary step before the implementation of conservation actions to boost species populations.
Author Contributions
Conceptualization, J.G.-C. and F.S.T.; methodology, A.B.L., F.S.T. and J.G.-C.; formal analysis, A.B.L. and J.G.-C.; investigation, A.B.L. and J.G.-C.; data curation, A.B.L. and J.G.-C.; writing—original draft preparation, A.B.L.; writing—review and editing, A.B.L., F.S.T. and J.G.-C.; visualization, J.G.-C.; supervision, F.S.T. and J.G.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used in this article are freely available at https://www.gbif.org/ (accessed on 15 July 2024).
Acknowledgments
We are grateful to all people who collect data on biodiversity and share them on citizen science platforms. We are also particularly grateful to all of the landowners, volunteers, and students who have helped us to deploy the camera traps.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kleiman, D.G.; Reading, R.P.; Miller, B.J.; Clark, T.W.; Scott, J.M.; Robinson, J.; Wallace, R.L.; Cabin, R.J.; Felleman, F. Essays Improving the Evaluation of Conservation Programs. Conserv. Biol. 2000, 14, 356–365. [Google Scholar] [CrossRef]
- Moussy, C.; Burfield, I.J.; Stephenson, P.J.; Newton, A.F.E.; Butchart, S.H.M.; Sutherland, W.J.; Gregory, R.D.; McRae, L.; Bubb, P.; Roesler, I.; et al. A quantitative global review of species population monitoring. Conserv. Biol. 2022, 36, e13721. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, P.J. Technological advances in biodiversity monitoring: Applicability, opportunities and challenges. Curr. Opin. Environ. Susta. 2020, 45, 36–41. [Google Scholar] [CrossRef]
- Kerry, R.G.; Montalbo, F.J.P.; Das, R.; Patra, S.; Mahapatra, G.P.; Maurya, G.K.; Nayak, V.; Jena, A.B.; Ukhurebor, K.E.; Jena, R.C.; et al. An overview of remote monitoring methods in biodiversity conservation. Environ. Sci. Pollut. Res. 2022, 29, 80179–80221. [Google Scholar] [CrossRef] [PubMed]
- Troia, M.J.; McManamay, R.A. Filling in the GAPS: Evaluating completeness and coverage of open-access biodiversity databases in the United States. Ecol. Evol. 2016, 6, 4654–4669. [Google Scholar] [CrossRef] [PubMed]
- Saran, S.; Kumar Chaudhary, S.; Singh, P.; Tiwari, A.; Kumar, V. A comprehensive review on biodiversity information portals. Biodivers. Conserv. 2022, 31, 1445–1468. [Google Scholar] [CrossRef]
- GBIF Secretariat. What is GBIF? 2024. Available online: https://www.gbif.org/what-is-gbif (accessed on 15 July 2024).
- Kosmala, M.; Wiggins, A.; Swanson, A.; Simmons, B. Assessing data quality in citizen science. Front. Ecol. Environ. 2016, 14, 551–560. [Google Scholar] [CrossRef]
- Fischer, H.; Gerber, L.R.; Wentz, E.A. Evaluating the Fitness for Use of Citizen Science Data for Wildlife Monitoring. Front. Ecol. Evol. 2021, 9, 620850. [Google Scholar] [CrossRef]
- Horns, J.J.; Adler, F.R.; Şekercioğlu, Ç.H. Using opportunistic citizen science data to estimate avian population trends. Biol. Conserv. 2018, 221, 151–159. [Google Scholar] [CrossRef]
- Neate-Clegg, M.H.C.; Horns, J.J.; Adler, F.R.; Çisel, M.; Aytekin, K.; Şekercioğlu, Ç.H. Monitoring the world’s bird populations with community science data. Biol. Conserv. 2020, 248, 108653. [Google Scholar] [CrossRef]
- Arenas-Castro, S.; Regos, A.; Martins, I.; Honrado, J.; Alonso, J. Effects of input data sources on species distribution model predictions across species with different distributional ranges. J. Biogeogr. 2022, 49, 1299–1312. [Google Scholar] [CrossRef]
- Troudet, J.; Grandcolas, P.; Blin, A.; Vignes-Lebbe, R.; Legendre, F. Taxonomic bias in biodiversity data and societal preferences. Sci. Rep. 2017, 7, 9132. [Google Scholar] [CrossRef]
- Binley, A.D.; Bennett, J.R. The data double standard. Methods Ecol. Evol. 2023, 14, 1389–1397. [Google Scholar] [CrossRef]
- Ward, D.F. Understanding sampling and taxonomic biases recorded by citizen scientists. J. Insect Conserv. 2014, 18, 753–756. [Google Scholar] [CrossRef]
- Kays, R.; Lasky, M.; Parsons, A.W.; Pease, B.; Pacifici, K. Evaluation of the Spatial Biases and Sample Size of a Statewide Citizen Science Project. Citiz. Sci. Theory Pract. 2021, 6, 1–12. [Google Scholar] [CrossRef]
- Steger, C.; Butt, B.; Hooten, M.B. Safari Science: Assessing the reliability of citizen science data for wildlife surveys. J. Appl. Ecol. 2017, 54, 2053–2062. [Google Scholar] [CrossRef]
- Howard, S.D.; Bickford, D.P. Amphibians over the edge: Silent extinction risk of Data Deficient species. Divers. Distrib. 2014, 20, 837–846. [Google Scholar] [CrossRef]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.T. The Silent Extinction of Species and Taxonomists—An Appeal to Science Policymakers and Legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- King, C.M.; Powell, R.A. The Natural History of Weasels and Stoats: Ecology, Behavior, and Management, 2nd ed.; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Jachowski, D.S.; Bergeson, S.M.; Cotey, S.R.; Croose, E.; Hofmeester, T.R.; MacPherson, J.; Wright, P.; Calderón-Acevedo, C.A.; Carter, S.P.; Dürst, A.C.; et al. Non-invasive methods for monitoring weasels: Emerging technologies and priorities for future research. Mammal Rev. 2024, 54, 243–260. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Díaz-Ruiz, F.; Viñuela, J.; de Diego, N.; Illanas, S.; Olea, P.P.; Santamaría, A.E.; Oñate, J.J.; Herránz, J.; Aceves, P.; et al. New distribution data of the least weasel Mustela nivalis in Castilla y León, Spain. Galemys Span. J. Mammal. 2018, 30, 66–70. [Google Scholar] [CrossRef]
- Palazón, S. Comadreja—Mustela nivalis. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Barja, I., Eds.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2017; Available online: http://www.vertebradosibericos.org/ (accessed on 10 June 2024).
- Jachowski, D.; Kays, R.; Butler, A.; Hoylman, A.M.; Gompper, M.E. Tracking the decline of weasels in North America. PLoS ONE 2021, 16, e0254387. [Google Scholar] [CrossRef]
- Sainsbury, K.A.; Shore, R.F.; Schofield, H.; Croose, E.; Campbell, R.D.; McDonald, R.A. Recent history, current status, conservation and management of native mammalian carnivore species in Great Britain. Mammal Rev. 2019, 49, 171–188. [Google Scholar] [CrossRef]
- Hayder, F.; Madikiza, Z.J.K.; San, E.D.L. Updated Distribution and Current Population Status of the Least Weasel (Mustela nivalis) in Tunisia: A Countrywide Interview Survey. Afr. J. Wildl. Res. 2023, 53, 11–18. [Google Scholar] [CrossRef]
- Rosalino, L.M.; Matias, G.; Fernandes, C.; Palomares, F. Three decades of research on Iberian wild Carnivora: Trends, highlights, and future directions. Mammal Rev. 2023, 53, 254–270. [Google Scholar] [CrossRef]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. Weasel (Mustela nivalis) decline in NE Spain: Prey or land use change? Mammal Res. 2018, 63, 501–505. [Google Scholar] [CrossRef]
- Palomo, L.J.; Gisbert, J.; Blanco, J.C. Atlas y Libro Rojo de los Mamíferos Terrestres de España; Dirección General para la Biodiversidad-SECEM-SECEMU: Madrid, Spain, 2007; 588p. [Google Scholar]
- GBIF.org. GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.yzrgns (accessed on 16 July 2024).
- Qiao, H.; Orr, M.; Yang, Q.; Zhan, X.; Lei, F.; Hughes, A.C. Global birdwatching data reveal uneven consequences of the COVID-19 pandemic. Biol. Conserv. 2023, 288, 110351. [Google Scholar] [CrossRef]
- Santos, X.; Pleguezuelos, J.M.; Chergui, B.; Geniez, P.; Cheylan, M. Citizen-science data shows long-term decline of snakes in southwestern Europe. Biodivers. Conserv. 2022, 31, 1609–1625. [Google Scholar] [CrossRef]
- Pannekoek, J.; Van Strien, A. TRIM 3 Manual (Trends and Indices for Monitoring Data); Statistics Netherlands: Voorburg, The Netherlands, 2005. [Google Scholar]
- Ruiz-Gutierrez, V.; Hooten, M.B.; Campbell Grant, E.H. Uncertainty in biological monitoring: A framework for data collection and analysis to account for multiple sources of sampling bias. Methods Ecol. Evol. 2016, 7, 900–909. [Google Scholar] [CrossRef]
- Mair, L.; Ruete, A. Explaining Spatial Variation in the Recording Effort of Citizen Science Data across Multiple Taxa. PLoS ONE 2016, 11, e0147796. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Carpio, A.J.; Mendoza-Lozano, A.; Tortosa, F.S. Detección de mesocarnívoros en zonas agrícolas del sur de España mediante trampeo fotográfico en puntos de agua. Galemys Span. J. Mammal. 2022, 34, 28–32. [Google Scholar] [CrossRef]
- Mendoza-Lozano, A.; Llorca, A.B.; Ponte-González, M.; Mármol-Melendo, M.; Guerrero-Casado, J. Nuevos registros de mamíferos silvestres en la provincia de Córdoba (España) mediante fototrampeo: ¿especies en expansión o poco muestreadas? Trianoi 2022, 7, 69–79. [Google Scholar]
- McDonald, R.A.; Abramov, A.V.; Stubbe, M.; Herrero, J.; Maran, T.; Tikhonov, A.; Cavallini, P.; Kranz, A.; Giannatos, G.; Kryštufek, B.; et al. Mustela nivalis (amended version of 2016 assessment). IUCN Red List. Threat. Species 2019, E.T70207409A147993366. [Google Scholar] [CrossRef]
- Červinka, J.; Riegert, J.; Grill, S.; Šálek, M. Large-scale evaluation of carnivore road mortality: The effect of landscape and local scale characteristics. Mammal Res. 2015, 60, 233–243. [Google Scholar] [CrossRef]
- Fernandez-de-Simon, J.; Coeurdassier, M.; Couval, G.; Fourel, I.; Giraudoux, P. Do bromadiolone treatments to control grassland water voles (Arvicola scherman) affect small mustelid abundance? Pest Manag. Sci. 2019, 75, 900–907. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guilhaumon, F.; Rodrigues-Neto, D.; Pozo-Ortego, I.; Gómez Calmaestra, R. Impactos, Vulnerabilidad y Adaptación al Cambio Climático de la Biodiversidad Española. 2. Fauna de Vertebrados; Dirección General de Medio Natural y Política Forestal; Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011; 640p. [Google Scholar]
- Gisbert, J.; Santos-Reis, M. Mustela nivalis Linnaeus, 1766. In Atlas y Libro Rojo de los Mamíferos Terrestres de España; Palomo, L.J., Gisbert, J., Blanco, J.C., Eds.; Dirección General de Conservación de la Naturaleza-SECEM-SECEMU: Madrid, Spain, 2007; pp. 283–286. [Google Scholar]
- Zattara, E.E.; Aizen, M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).