The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Parasites
2.2. Morphological Study
2.3. Genetic Study
3. Results
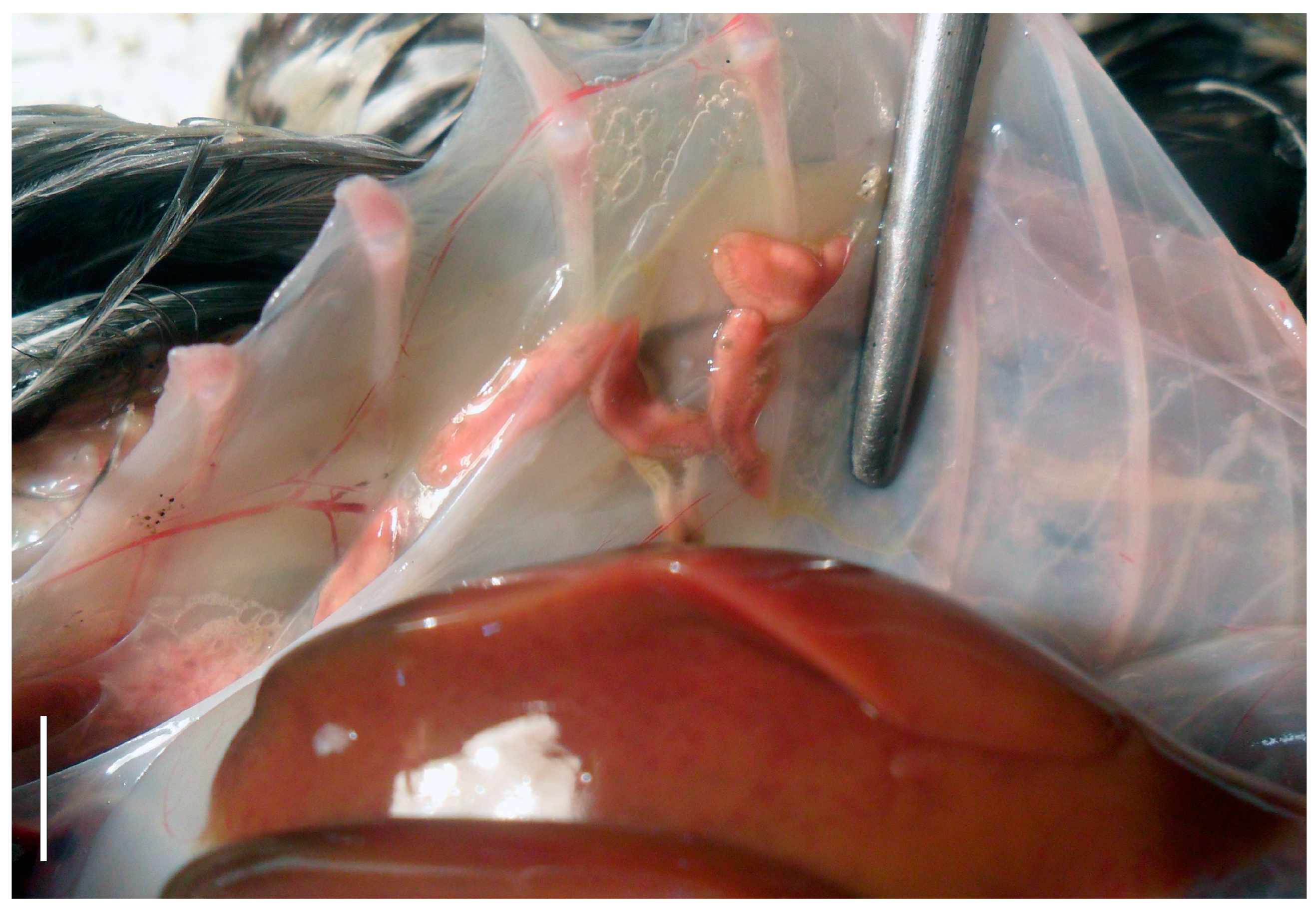
3.1. Parasitological Data
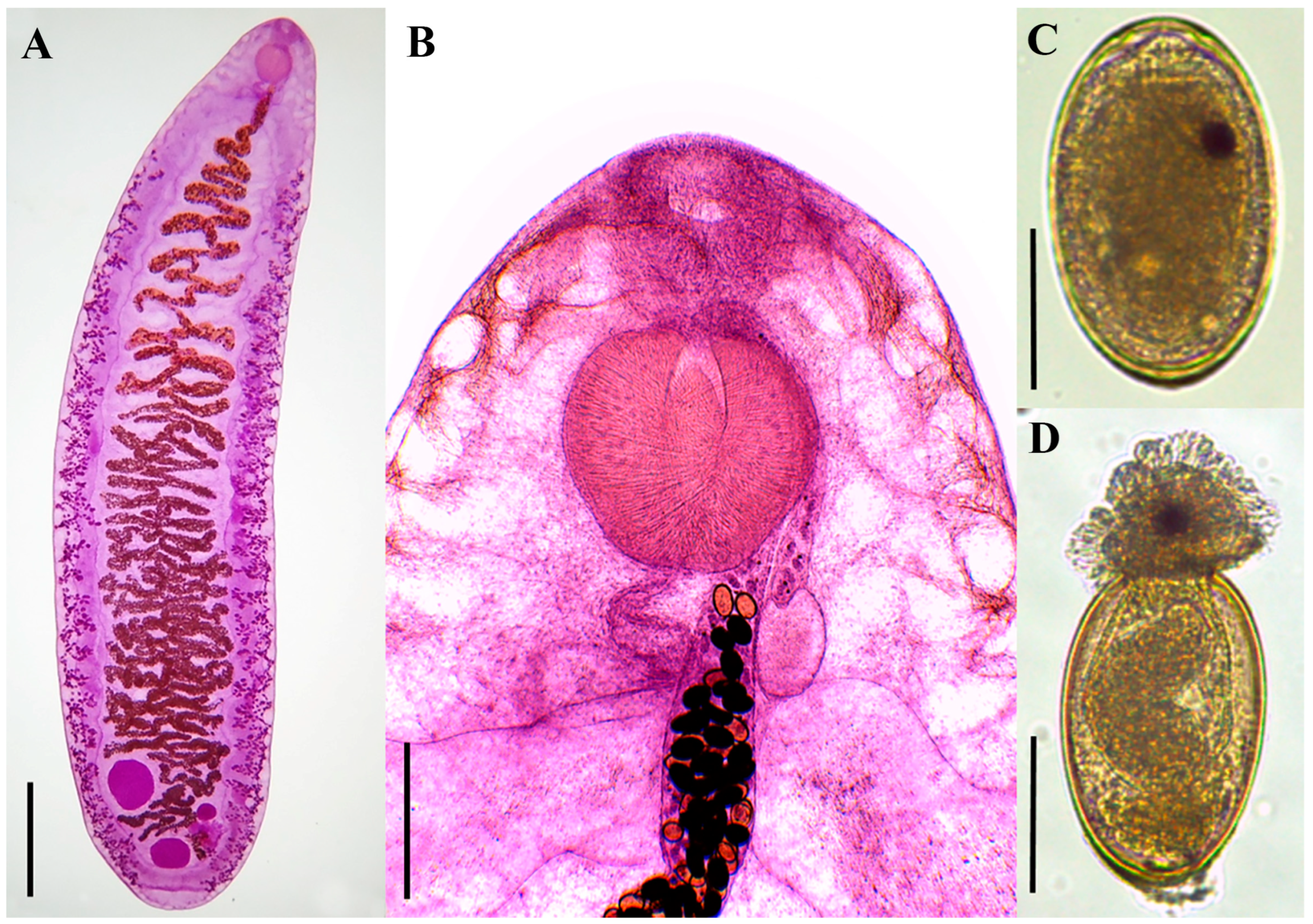
3.2. Morphological Characterization
Remarks
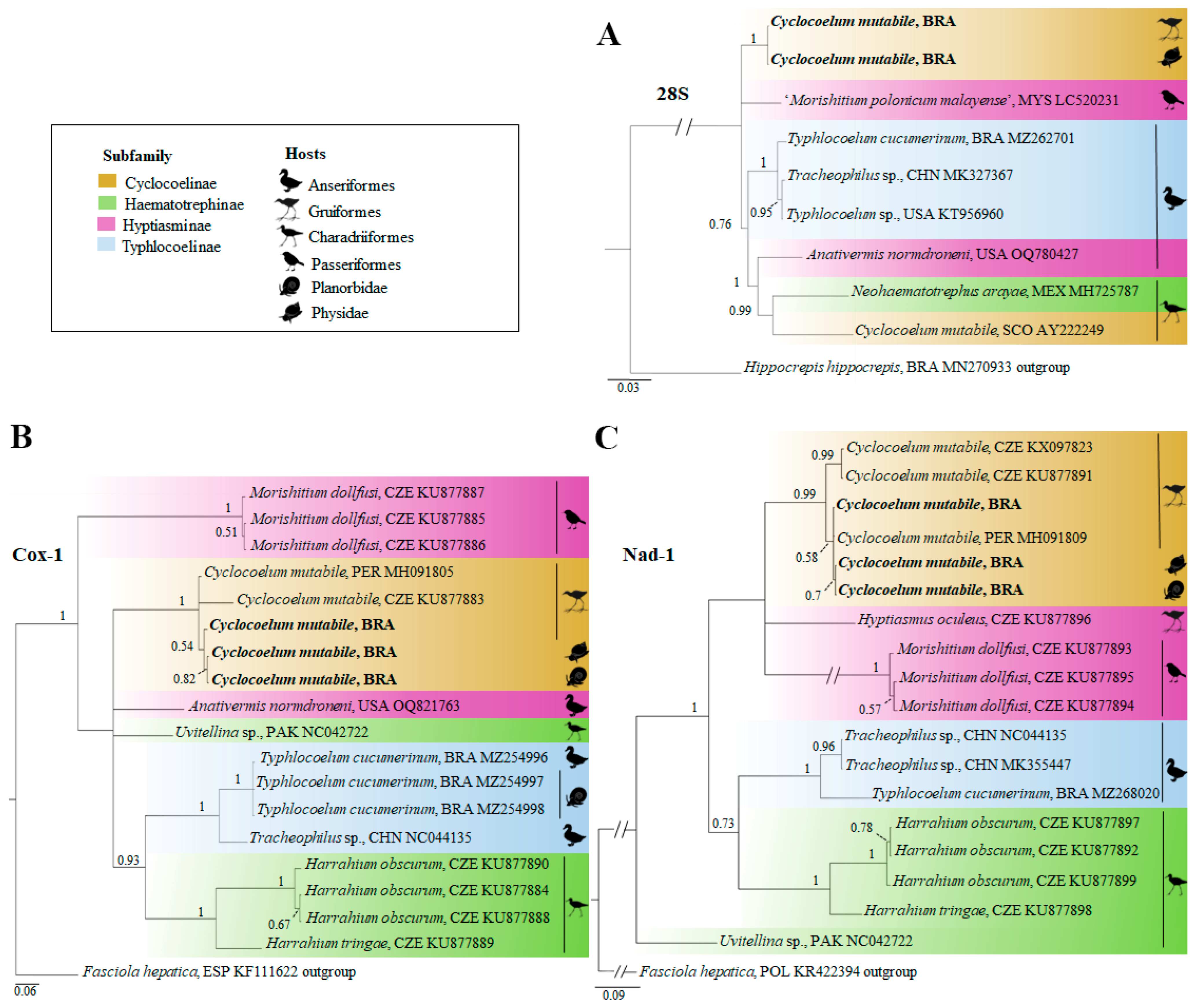
3.3. Genetic Characterization
3.4. Taxonomic Summary
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubois, G. Revision des Cyclocoelidae Kossack 1911. Rev. Suisse Zool. 1959, 66, 67–147. [Google Scholar] [CrossRef]
- Kanev, I.; Radev, V.; Fried, B. family Cyclocoelidae Stossich, 1902. In Keys to the Trematoda; Gibson, D.I., Jones, A., Bray, R.A., Eds.; CAB International and The Natural History Museum: London, UK, 2002; Volume 1, pp. 131–145. [Google Scholar]
- Dronen, N.O.; Blend, C.K. Updated keys to the genera in the subfamilies of Cyclocoelidae Stossich, 1902, including a reconsideration of species assignments, species keys and the proposal of a new genus in Szidatitreminae Dronen, 2007. Zootaxa. 2015, 4053, 1–100. [Google Scholar] [CrossRef][Green Version]
- Dutton, H.R.; Bullard, S.A.; Kelly, A.M. New genus and species of Cyclocoelidae Stossich, 1902 (Platyhelminthes: Digenea) infecting the nasopharyngeal cavity of Canada Goose, Branta canadensis (Anseriformes: Anatidae) from Western Alabama. J. Parasitol. 2023, 190, 349–356. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.D. The migratory route of Cyclocoelum mutabile (Zeder) (Trematoda: Cyclocoelidae) in the American coot, Fulica americana (Gm.). Can. J. Zool. 1977, 55, 274–279. [Google Scholar] [CrossRef]
- Branton, S.L.; Deaton, J.W.; Gerlach, H.; Ruff, M.D. Cyclocoelum mutabile infection and aortic rupture in an American coot (Fulica americana). Avian Dis. 1985, 29, 246–249. [Google Scholar] [CrossRef]
- Galosi, L.; Heneberg, P.; Rossi, G.; Sitko, J.; Magi, G.E.; Perrucci, S. Air sac trematodes: Morishitium polonicum as a newly identified cause of death in the common blackbird (Turdus merula). Int. J. Parasitol. Parasites Wildl. 2019, 9, 74–79. [Google Scholar] [CrossRef]
- Diaz, E.A.; Donoso, G.; Mosquera, J.D.; Ramírez-Villacís, D.X.; Gonzáles, G.; Zapata, S.; Cisneros-Heredia, D.F. Death by massive air sac fluke (Trematoda: Bothriogaster variolaris) infection in a free-ranging snail kite (Rostrhamus sociabilis). Int. J. Parasitol. 2022, 19, 155–160. [Google Scholar] [CrossRef]
- Sitko, J.; Bizos, J.; Heneberg, P. Central European parasitic flatworms of the Cyclocoelidae Stossich, 1902 (Trematoda: Plagiorchiida): Molecular and comparative morphological analysis suggests the reclassification of Cyclocoelum obscurum (Leidy, 1887) into the Harrahium Witenberg, 1926. Parasitology 2017, 144, 368–383. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, A.; García-Varela, M.; Hernández-Orts, J.S. Review of five species of cyclocoelids (Digenea: Cyclocoelidae) from aquatic birds in Mexico with notes on their interspecific variation. Syst. Parasitol. 2018, 95, 921–942. [Google Scholar] [CrossRef]
- Gomez-Puerta, L.A.; Salas, M.Y.; Lopez-Urbina, M.T.; Gonzalez, A.E. Diagnóstico morfológico y molecular de Cyclocoelum mutabile (Trematoda: Cyclocoelidae) en el Perú. Rev. Peru. Biol. 2018, 25, 315–320. [Google Scholar] [CrossRef][Green Version]
- Ginetsinskaya, T.A. Life cycle and biology of the developmental stages of Cyclocoelum microstomum (Trematoda). Uchenye Zap. Leningr. Univ. Ser. Biol. 1954, 172, 90–112. (In Russian) [Google Scholar]
- Yamaguti, S. A Synoptical Review of Life Histories of Digenetic Trematodes of Vertebrates; Keigaku Publishing: Tokyo, Japan, 1975. [Google Scholar]
- McLaughlin, J.D. Experimental studies on the life cycle of Cyclocoelum mutabile (Zeder) (Trematoda: Cyclocoelidae). Can. J. Zool. 1976, 54, 48–54. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McLaughlin, J.D. The biology of Cyclocoelum mutabile (Trematoda) infections in American coots. Proc. Helminthol. Soc. Wash. 1986, 53, 177–181. [Google Scholar]
- Korol, E.; Stenko, P. Larvae of trematodes of the family Cyclocoelidae (Trematoda, Digenea) in Crimean Mollusks. Vestn. Zool. 2005, 18, 192–194. (In Ukrainian) [Google Scholar]
- Galaktionov, K.V.; Dobrovolskij, A.A. The Biology and Evolution of Trematodes; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2003. [Google Scholar]
- Ginetsinskaya, T.A.; Saakova, E.O. Migration of trematodes of the family Cyclocoelidae Koss in the body of the final host. Dokl. Akad. Nauk. SSSR. 1952, 85, 1432–1436. (In Russian) [Google Scholar]
- Ẑdárská, Z. Larvální stadia motolic z vodních plů na území ČSSR. Česk. Parasitol. 1963, 10, 207262. [Google Scholar]
- Urabe, M.; Hashim, N.E.N.; Uni, S.; Takachi, I.; Halim, M.R.A.; Marzuki, M.E.; Udin, A.S.M.; Zainuri, N.A.; Omar, H.; Agatsuma, T.; et al. Description and molecular characteristics of Morishitium polonicum malayense Urabe, Nor Hashim & Uni, n. subsp. (Trematoda: Cyclocoelidae) from the Asian glossy starling, Aplonis panayensis strigata (Passeriformes: Sturnidae) in Peninsular Malaysia. Parasitol. Int. 2020, 76, 102074. [Google Scholar] [CrossRef]
- Assis, J.C.A.; López-Hernández, D.; Favoretto, S.; Medeiros, L.B.; Melo, A.L.; Martins, N.R.S.; Pinto, H.A. Identification of the avian tracheal trematode Typhlocoelum cucumerinum (Trematoda: Cyclocoelidae) in a host-parasite-environment system: Diagnosis, life cycle and molecular phylogeny. Parasitology 2021, 148, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Paraense, W.L. Planorbídeos hospedeiros intermediários do Schistosoma mansoni. In Esquistossomose Mansoni; Cunha, A.S., Ed.; Ed USP: São Paulo, Brasil, 1970; pp. 13–30. [Google Scholar]
- Paraense, W.L.; Pointier, J.P. Physa acuta Draparnaud, 1805 (Gastropoda: Physidae): A study of topotypic specimens. Mem Inst Oswaldo Cruz. 2003, 98, 513–517. [Google Scholar] [CrossRef]
- Fernandes, B.M.M. Sobre as espécies brasileiras da família Cyclocoelidae Kossak, 1911 (Trematoda, Cyclocoelidae). Mem. Inst. Oswaldo Cruz. 1976, 74, 289–294. [Google Scholar] [CrossRef][Green Version]
- Fernandes, B.M.M.; Justo, M.C.N.; Cárdenas, M.Q.; Cohen, S. South American Trematodes Parasites of Birds and Mammals; Fundação Oswaldo Cruz: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Tkach, V.V.; Littlewood, T.J.; Olson, P.D.; Kinsella, M.; Swiderski, Z. Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Syst. Parasitol. 2003, 56, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miura, O.; Kuris, A.M.; Torchin, M.E.; Hechinger, R.F.; Dunham, E.J.; Chiba, S. Molecular-genetic analyses reveal cryptic species of trematodes in the intertidal gastropod, Batillaria cumingi (Crosse). Int. J. Parasitol. 2005, 35, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.A.T.; Blair, D. Relative merits of nuclear ribosomal internal transcribed spacers and mitochondrial CO1 and ND1 genes for distinguishing among Echinostoma species (Trematoda). Parasitology 1998, 116, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Van Steenkiste, N.; Locke, S.A.; Castelin, M.; Marcogliese, D.J.; Abbott, C.L. New primers for DNA barcoding of digeneans and cestodes (Platyhelminthes). Mol. Ecol. Resour. 2015, 15, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stunkard, H.W. The life history of Typhlocoelum cymbium (Diesing, 1850) Kossack. 1911 (Trematoda, Cyclocoelidae). A contribution to the phylogeny of the monostomes. Bull. Soc. Zool. Fr. 1934, 59, 447–466. [Google Scholar]
- Schafranski, N.L.; Freitas, M.G.; Costa, J.O. Ciclo biológico de Typhlocoelum cucumerinum (Rudolphi, 1809) (Trematoda: Cyclocoelidae). Rev. Bras. Biol. 1975, 35, 519–526. [Google Scholar]
- Taft, S.J.; Heard, R.W. Aspects of the larval development of Ophthalmophagus sp. (Trematoda: Cyclocoelidae). J. Parasitol. 1978, 64, 597–600. [Google Scholar] [CrossRef]
- Timon-David, T. Cycle évolutif d’un Trématode Cyclocoeidé: Pseudhyptiasmus dollfusi Timon-David 1950. Recherches expérimentales. Ann. Parasitol. Hum. Comp. 1955, 30, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.M. Contribuição ao estudo de formas larvárias de trematódeos brasileiros. 3—Fauna de Belo Horizonte e Jaboticatubas, Estado de Minas Gerais. Mem. Inst. Butantan. 1952, 24, 45–62. [Google Scholar]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 735–755. [Google Scholar] [CrossRef]
- Suleman, M.S.K.; Heneberg, P.; Zhou, C.Y.; Muhammad, N.; Zhu, X.Q.; Ma, J. Characterization of the complete mitochondrial genome of Uvitellina sp., representative of the family Cyclocoelidae and phylogenetic implications. Parasitol Res. 2019, 118, 2203–2211. [Google Scholar] [CrossRef] [PubMed]
- McKindsey, C.W.; McLaughlin, J.D. Field studies on the transmission and survival of Cyclocoelum mutabile (Digenea) infections in natural snail populations in Southern Manitoba, Canada. J. Parasitol. 1995, 81, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Ohlweiler, F.P.; Eduardo, J.M.; Takahashi, F.Y.; Crein, G.A.; Luca, L.R.; Oliveira, R.C. Larvas de trematódeos associadas a moluscos de água doce em municípios da Região Metropolitana de São Paulo, Estado de São Paulo, Brasil. Rev. Pan-Amaz Saúde 2013, 4, 37–48. [Google Scholar] [CrossRef]
- Feizullaev, A. On specific characters and distinct status of Cyclocoelum mutabile (Zeder, 1800) and Cyclocoelum microstomum (Creplin, 1829) (Trematoda, Cyclocoelidae). Parazitologiya 1970, 4, 39–42. (In Russian) [Google Scholar]
- Tkach, V.V.; Kudlai, O.; Kostadinova, A. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int. J. Parasitol. 2016, 46, 171–185. [Google Scholar] [CrossRef]

| Host | Biomphalaria glabrata | Physella acuta | Mollusk species 1 | Mollusk species 2 | Mollusk species 3 | |||
| Locality | Brazil | Brazil | Russia | Canada | Crimea | |||
| Reference | Present study | Present study | Ginetsinskaia [12] | McLaughlin [14] | Korol and Stenko [16] | |||
| Condition | Alive | Hot killed and Fixed | Alive | Fixed | Alive | Alive | Hot killed | |
| Mature rediae | N | 3 | - | - | - | - | - | - |
| Body | L | 4649 ± 1105 | - | - | 2000–3000 | 3500–4000 | 700–2030 | - |
| (3578–6171) | ||||||||
| W | 1079 ± 183) | - | - | - | 620 | - | - | |
| (906–1000) | ||||||||
| Pharynx | L | 138 ± 60 (86–221) | - | - | 160 | - | - | - |
| W | 117 ± 29 (88–157) | - | - | 180 | - | - | - | |
| Cercariae | N | 15 | 15 | 10 | - | - | - | - |
| Body | L | 685 ± 122 | 661 ± 96 | 669 ± 229 | 340–370 | 625–764 | 520–550 | 318 |
| (490–936) | (504–794) | (509–922) | ||||||
| W | 271 ± 49 (170–367) | 198 ± 54 (144–378) | 307 ± 30 (238–344) | 90–100 | 208–222 | 166–180 | 143 | |
| Anterior | L | 100 ± 22 (70–159) | 85 ± 7 (73–94) | 96 ± 14 (80–109) | 65 | 83–87 | 98–117 | 55 |
| organ | W | 85 ± 16 (66–119) | 72 ± 8 (65–89) | 89 ± 9 (82–98) | 65 | 83–87 | 57 | |
| Pharynx | L | 38 ± 6 (30–48) | 27 ± 0.6 (27–28) | 40 ± 3 (36–44) | - | 41–49 | 41–46 | 18 |
| W | 41 ± 8 (30–53) | 26 ± 3 (23–31) | 45 ± 2 (43–47) | - | 41–49 | 39–42 | 18 | |
| Ventral | L | 75 ± 7 (67–97) | 69 ± 9 (58–89) | 76 ± 7 (69–838) | 34 | 75–83 | 68–83 | 47 |
| sucker | W | 74 ± 6 (67–89) | 68 ± 9 (60–92) | 78 ± 7 (71–85) | 37 | 75–83 | 68–83 | 42 |
| Metacercariae | N | 10 | 10 | 10 | - | - | - | - |
| D | 317 ± 35 (241–358) | 384 ± 11 (359–400) | 338 ± 25 (287–368) | 180–230 | 300 | 250–270 | - | |
| Hosts | Galinulla galeata | G. galeata | Fulica atra | G. galeata | Tringa semipalmata | |
| Locality | Brazil | Brazil | Czech Republic | Peru | Mexico | |
| Reference | Present study | Fernandes | Sitko | Gomez-Puerta | López-Jiménez | |
| [24] | et al. [9] | et al. [11] | et al. [10] | |||
| N | 10 | 7 | 30 | 4 | 7 | |
| Body (mm) | L | 15.2 ± 5.6 (13–18.5) | 13.6–18.9 | 19.3 ± 3.1 (15–28.3) | 12.2 ± 2.7 (10.2–13.2) | 17.5–20.5 |
| W | 4.5 ± 0.6 (3.5–5.0) | 4–6 | 5.2 ± 0.9 (3.8–7.4) | 4.5 ± 0.9 (3.8–4.8) | 3.2–4.4 | |
| Oral opening | L | 328 ± 34 (283–354) | - | 615 ± 209 (373–1286) | 614 ± 13 (580–642) | - |
| W | 470 ± 67 (425–567) | - | 789 ± 255 (289–1286) | 723 ± 49 (601–822) | - | |
| Prepharynx | L | 342 ± 77 (284–447) | 430–510 | - | - | 192 |
| Pharynx | L | 695 ± 68 (528–764) | 790–820 | 855 ± 130 (596–1118) | 741 ± 15 (710–771) | 234–323 |
| W | 783 ± 100 (586–878) | 1090–1240 | 935 ± 153 (596–1237) | 759 ± 20 (724–805) | 237–326 | |
| Cirrus sac | L | 1170 ± 80 (1100–1300) | 900–940 | - | 901 ± 6 (886–912) | 818–1035 |
| Anterior testes | L | 675 ± 154 (425–1009) | 580–970 | 1095 ± 366 (328–1857) | 831 ± 40 (745–935) | 1078–2082 |
| W | 770 ± 154 (531–1027) | 580–970 | 1098 ± 395 (373–1857) | 889 ± 50 (779–1008) | 1169–1974 | |
| Posterior testes | L | 653 ± 130 (443–835) | 460–640 | 940 ± 357 (373–1771) | 803 ± 41 (685–873) | 962–1668 |
| W | 755 ± 140 (514–992) | 780–1160 | 940 ± 263 (447–1571) | 837 ± 79 (670–986) | 1165–1983 | |
| Ovary | L | 379 ± 54 (286–457) | 300–490 | 557 ± 76 (429–771) | 416 ± 10 (402–445) | 374–465 |
| W | 400 ± 60 (286–514) | 310–450 | 527 ± 87 (328–771) | 485 ± 36 (430–589) | 408–532 | |
| Egg (N = 50) | L | 109 ± 4 (104–114) | 75–102 | 111 ± 5 (104–116) | 116 ± 1 (112–118) | 130–146 |
| W | 64 ± 2 (60–67) | 47–75 | 60 ± 4 (52–64) | 57 ± 1 (54–60) | 77–88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, J.C.A.d.; Pinto, H.A. The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil. Diversity 2024, 16, 422. https://doi.org/10.3390/d16070422
Assis JCAd, Pinto HA. The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil. Diversity. 2024; 16(7):422. https://doi.org/10.3390/d16070422
Chicago/Turabian StyleAssis, Jordana Costa Alves de, and Hudson Alves Pinto. 2024. "The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil" Diversity 16, no. 7: 422. https://doi.org/10.3390/d16070422
APA StyleAssis, J. C. A. d., & Pinto, H. A. (2024). The Natural Infection of Freshwater Snails with the Avian Air Sac Fluke, Cyclocoelum mutabile (Trematoda: Cyclocoelidae), in Brazil. Diversity, 16(7), 422. https://doi.org/10.3390/d16070422








