Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption
Abstract
1. Introduction
| Functional Group (1) | Functional/ Ecosystemic Role | Major Faunal Group | Effects over Forest (2) | Main Groups and Species Involved | |
|---|---|---|---|---|---|
| ACEP | IWP | ||||
| Biogeochemistry mediators | C, N, P, Fe, and S cycle mediators | Micro-organisms | Effect over ecological processes that cycle nutrients and metals C cycling/N fixation, ammonification, nitrification, denitrification/sulphate reduction Changes in C:N:P ratio of organic matter > changes digestibility and biodegradability of OM Changes trace (essential and non-essential) elements’ availability to plants > regulation plant growth/Hg methylation Control redox conditions > induces iron plaque formation > regulate nutrient uptake by roots > protect trees from toxic substances | Bacteria; Archaea; Cyanobacteria; Fungi (Mycorrhizae); Algae (Diatomacea); Protists; N fixing, ammonifying, nitrifying, and denitrifying bacteria; Phosphate-solubilizing bacteria; Sulphate-reducing bacteria. | Bacteria; Archaea; Cyanobacteria; Fungi (Mycorrhizae); Algae (Diatomacea); Protists; N fixing, ammonifying, nitrifying, and denitrifying bacteria; Phosphate-solubilizing bacteria; Sulphate-reducing bacteria. |
| Bioturbators/burrowers | Ecosystem engineering by sediment disturbance (burrowing, scraping, feeding), sediment oxygenation, particle reworking, sediment architecture, and creation of new habitats | Decapod Crustaceans: Ocypodoidea Xanthoidea Grapsoidea Portunidae Stomatopoda Alpheidae Thalassinidae | Oxygenation > increases redox potential > increase OM mineralisation > dissociate sulphides Decrease sediment OM content > accelerate nutrient and pollutant cycling > maintain productivity Sediment architecture > creation of new habitats > increases micro- and macro-biota diversity Burial/topple of small propagules > biased recruitment > forest structure > AGB/BGB content > carbon content and cycling | Semi-terrestrial Decapods: Fiddler crabs and Ucides cordatus, U. occidentalis (*) (Ocypodoidea) (3) Sesarma rectum, S. curacaoense, S. aequatoriale (*), S. rhizophorae (*), Armases angustipes, A. occidentale (*) (Sesarmidae, (3) Grapsoidea) + other crabs inhabiting ACBS (4) Xanthoidea Callinectes spp. (Portunidae) Pistol shrimp Alpheus spp. (Alpheidae) | Semi-terrestrial Decapods: Fiddler crabs (Ocypodidae) Episesarma versicolor Neosarmatium spp. Perisesarma dussumieri (Sesarmidae) (3) Mud crab Scylla spp. (Portunidae) Xanthoidea Mantis shrimp Squilla spp. (Stomatopoda) Pistol shrimp Alpheus spp.(Caridea) Mud lobters Thalassina spp. (Thalassinidae) |
| Fishes | Burrows making/occupancy > sediment disturbance > oxidation > biota diversification | Cyprinodonts, Gobiids, Fundulids, Rivulins, Poeciliids, Eleotrids | Cyprinodonts, Eleotrids, Gobiids: Periophthalmodon spp., Periophthalmus spp., Scartelaos spp., Boleophthalmus spp. (Mudskippers) | ||
| Herbivores (including Omnivore crabs) | Propagules consumption | Brachyuran and Sesarmid crabs | Propagule consumption > biased recruitment > forest structure/architecture > AGB/BGB content > carbon content and cycling | Goniopsis cruentata, G. pelii (A), G. pulchra (*) (Grapsidae) Ucides cordatus, U. occidentalis (*) (Ucididae) | Neosarmatium smithii, N. meinerti, N. africanum, Metopograpsus latifrons (Sesarmidae) |
| Folivory | Sesarmid crabs | Selective canopy consumption > forest productivity and biomass? | Aratus pisonii, A. pacificus (*) (Sesarmidae, Grapsoidea) | Neosarmatium spp., Perisesarma spp., Parasesarma spp. Episesarma versicolor (Sesarmidae) | |
| Insects | Leaf consumption, defoliation, sap feeding >affect leaf and bud areas > canopy and forest architecture/structure >reproductive and vegetative growth > reproductive output > tree species recruitment | Lepidopterans, Dipterans, Homopterans, Hemipterans, Orthopterans, Coleopterans. | Coleopterans, Lepidopterans, Dipterans, Homopterans, Hemipterans, Orthopterans. | ||
| Litter/detritus consumption | Crabs and gastropod molluscs (5) | Alter soil carbon content and cycling Increase export of carbon and nutrients | Goniopsis cruentata, G. pelii (A), G. pulchra (*) (Grapsidae) Ucidescordatus, U. occidentalis (*) (Ucididae) Sesarmidae (Grapsoidea) Littoraria spp. Thais spp. (*) Cerithidea spp. (*) (Gastropoda) | Parasesarma eumolpe, P. onychophorum, Perisesarma spp. (Sesarmidae) Fiddler crabs (Ocypodidae) Telescopium telescopium, Terebralia palustris Potamiidae Littoraria spp. Cerithidea spp. (Gastropoda) | |
| leaves, flowers, and fruits | Primates | Seed dispersal? | Sapajus libidinosus, S. xanthosternos, S. apella, Alouatta palliata, A. pigra Cebus capucinus (*) | Langurs (Presbytis), proboscis monkey (Nasalis larvatus), Macaca fascicularis, Trachypithecus auratus, T. cristatus, Piliocolobus badius | |
| Wood-borers/ chewers (Xylovores) | Wood-boring Cellulose-lignin processers | Crustaceans: Brachyuran crabs, Isopoda | Consumption of aerial roots meristems > forest architecture > changes in live and dead biomass > carbon stock Promotion of root sprouting (in Rhizophora mangle) > increase in structural complexity of roots > increase in root biomass Effects on structural support and nutrient supply > changes in epifauna and epifloral communities > community-level impacts | Sphaeroma terebrans, S. peruvianum (*) Limnoria lignorum (*) (Isopoda, Crustacea) G. cruentata (Grapsidae) | Sphaeroma terebrans Limnoria spp. (Isopoda) |
| Teredinid and Pholadidae molluscs | Biodegradation > increase in OM lability > accelerate nutrient cycling and outwelling of POM from the tunnelling of deadwood Significant tunnelling of deadwood enhances the benthic structural and niche complexity > provide habitat for many taxa and enhances environmental buffering within deadwood | Teredo spp. (Teredinidae) | Bactronophorus thoracites, Dicyathifer mannii, Lyrodus massa, Spathoteredo obtusa, Teredo spp., Bankia spp. (Teredinidae) | ||
| Insects | Wood consumption > increasing OM biodegradability Effects on canopy architecture, tree growth and reproduction, and internal nutrient cycling | Coccotrypes rhizophorae, Euplatypus sp. (*) (Scolytidae, Coleoptera) Termites (Blattodeans) | Dendroctonus spp. Phaenops spp. (Coleoptera) Cenoloba spp. Zeuzera conferta (Lepidoptera) Termites (Blattodeans) | ||
| Pollinators | Pollination | Insects | Enable tree reproduction > forest structure/architecture > AGB/BGB content > carbon content and cycling | Hymenopteran, Lepidopteran, -Dipteran | Hymenopteran, Lepidopteran, Dipteran |
| Bats | Phyllostomidae | Pteropodidae: Macroglossus minimus, Eonycteris spelaea | |||
2. Functional Groups, Their Specific Roles, and Effects over Mangroves
2.1. Biogeochemistry Mediators
2.2. Bioturbators/Burrowers
2.2.1. Crabs and Thalassinids
2.2.2. Fish
2.3. Herbivores
2.3.1. Crabs
2.3.2. Insects
2.3.3. Primates
2.4. Wood Borers
2.4.1. Crustaceans and Molluscs
2.4.2. Insects
2.5. Pollinators
2.5.1. Insects
2.5.2. Bats
3. Specific Responses of Functional Groups to Climate Change and Effects over Mangroves
3.1. Sea Level Rise (SLR)
3.2. Global and Regional Increases in Air and Soil Temperature
3.3. Altered Precipitation Regimes (Floods and Extended Drought Events)
3.4. Increase in Extreme Weather Events (Storms, Hurricanes)
3.5. Water Acidification
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bunting, P.; Rosenqvist, A.; Hilarides, L.; Lucas, R.M.; Thomas, N.; Tadono, T.; Worthington, T.A.; Spalding, M.; Murray, N.J.; Rebelo, L.-M. Global Mangrove Extent Change 1996–2020: Global Mangrove Watch Version 3.0. Remote Sens. 2022, 14, 3657. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Latham, R.E. Global patterns of diversity in mangrove floras. In Species Diversity in Ecological Communities: Historical and Geographical Perspectives; Ricklefs, R.E., Schluter, D., Eds.; University of Chicago Press: Chicago, IL, USA, 1993; pp. 215–229. [Google Scholar]
- Krauss, K.W.; Lovelock, C.E.; McKee, K.L.; López-Hofman, L.; Ewe, S.M.L.; Sousa, W.P. Environmental drivers in mangrove establishment and early development: A review. Aquat. Bot. 2008, 89, 105–127. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Ferreira, A.C.; Borges, R.; Ward, R. Mangroves of Brazil. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 521–563. [Google Scholar] [CrossRef]
- Cannicci, S.; Burrows, B.; Fratini, S.; Smith, T.J., III; Ofenberg, J.; Dahdouh-Guebas, F. Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 2008, 89, 186–200. [Google Scholar] [CrossRef]
- Kristensen, E. Mangrove crabs as ecosystem engineers; with emphasis on sediment processes. J. Sea Res. 2008, 59, 30–43. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ganade, G.; Attayde, J.L. Restoration versus natural regeneration in a neotropical mangrove: Effects on plant biomass and crab communities. Ocean Coast. Manag. 2015, 110, 38–45. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Bezerra, L.E.A.; Mathews-Cascon, H. Aboveground stock in a restored Neotropical mangrove: Influence of management and brachyuran crab assemblage. Wetlands Ecol. Manag. 2019, 27, 223–242. [Google Scholar] [CrossRef]
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; ITTO-ISME-FAO: Okinawa, Japan, 2011. [Google Scholar] [CrossRef]
- Hogarth, P.J. The Biology of Mangroves; Oxford University Press: London, UK, 1999. [Google Scholar]
- Lee, S.Y. Mangrove macrobenthos: Assemblages, services, and linkages. J. Sea Res. 2008, 59, 16–29. [Google Scholar] [CrossRef]
- Lee, S.Y. Ecological role of grapsid crabs in mangrove ecosystems: A review. Mar. Freshw. Res. 1998, 49, 335–343. [Google Scholar] [CrossRef]
- Cannicci, S.; Lee, S.Y.; Bravo, H.; Cantera-Kintz, J.R.; Dahdouh-Guebas, F.; Fratini, S.; Fusi, M.; Jimenez, P.J.; Nordhaus, I.; Porri, F.; et al. A functional analysis reveals extremely low redundancy in global mangrove invertebrate fauna. Proc. Natl. Acad. Sci. USA 2021, 118, e2016913118. [Google Scholar] [CrossRef]
- Duke, N.C. Mangrove Floristics and Biogeography Revisited: Further Deductions from Biodiversity Hot Spots, Ancestral Discontinuities, and Common Evolutionary Processes. In Mangrove Ecosystems: A Global Biogeographic Perspective; Rivera-Monroy, V.H., Lee, S.Y., Kristensen, E., Teilley, R.R., Eds.; Springer: Cham, Switzerland, 2017; pp. 17–54. [Google Scholar]
- Hendy, I.W.; Shipway, J.R.; Tupper, M.; Etxabe, A.G.; Ward, R.D.; Cragg, S.M. Biodegraders of large woody debris across a tidal gradient in an Indonesian mangrove ecosystem. Front. Glob. Chang. 2022, 5, 852217. [Google Scholar] [CrossRef]
- Ashton, E.C. Threats to Mangroves and Conservation Strategies. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 217–230. [Google Scholar] [CrossRef]
- Alongi, D.M. Climate Change and Mangroves. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 175–198. [Google Scholar] [CrossRef]
- Gilman, E.; Ellison, J.; Duke, N.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Moomaw, W.R.; Chmura, G.L.; Davies, G.T.; Finlayson, C.M.; Middleton, B.A.; Natali, S.M.; Perry, J.E.; Roulet, N.; Sutton-Grier, A.E. Wetlands in a changing climate: Science, policy and management. Wetlands 2018, 38, 183–205. [Google Scholar] [CrossRef]
- Ward, R.; Friess, D.; Day, R.; Mackenzie, R. Impacts of climate change on global mangrove ecosystems: A region by region overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef]
- I.P.C.C. Climate Change 2013: The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- da Rocha Júnior, R.L.; dos Santos Silva, F.D.; Pinto, D.D.C.; Costa, R.L.; Gomes, H.B.; Herdies, D.L.; Guidson Farias de Freitas, I.; Silva Vila Nova, T. Analysis of temperature extremes in the South of Brazil. Rev. Bras. Climatol. 2022, 30, 445–460. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Meireles, A.J.A.; Lacerda, L.D. Mangrove response to land use change in estuaries along the semiarid coast of Ceará, Brazil. J. Coast. Res. 2018, 34, 524–533. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Borges, R.; Ferreira, A.C. Neotropical mangroves: Conservation and sustainable use in a scenario of global climate change. Aquatic Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1347–1364. [Google Scholar] [CrossRef]
- Godoy, M.D.P.; Lacerda, L.D. Mangroves response to climate change: A review of recent findings on mangrove extension and distribution. An. Acad. Bras. Cien. 2015, 87, 651–667. [Google Scholar] [CrossRef]
- Ward, R.D.; Lacerda, L.D.; Cerqueira, A.S.; Silva, V.H.M.C.; Hernandez, O.C. Vertical accretion rates of mangroves in northeast Brazil: Implications for future responses and management. Estuar. Coast. Shelf. Sci. 2023, 289, 108382. [Google Scholar] [CrossRef]
- Mafi-Gholami, D.; Zenner, E.K.; Jaafari, A.; Ward, R.D. Modeling multi-decadal mangrove leaf area index in response to drought along the semi-arid southern coasts of Iran. Sci. Tot. Env. 2019, 656, 1326–1336. [Google Scholar] [CrossRef]
- Ward, R.; Lacerda, L.D. Responses of mangrove ecosystems to sea level change. In Dynamic Sedimentary Environment of Mangrove Coasts; Friess, D., Sidik, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 235–253. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Lacerda, L.D.; Rodrigues, J.V.M.; Bezerra, L.E.A. New contributions to mangrove rehabilitation/restoration protocols and practices. Wetlands Ecol. Manag. 2023, 31, 89–114. [Google Scholar] [CrossRef]
- Blondel, J. Guilds or functional groups: Does it matter? Oikos 2003, 100, 223–231. [Google Scholar] [CrossRef]
- Holguín, G.; Vazquez, P.; Bashan, Y. The role of sediment microorganisms in the productivity, conservation, and rehabilitation of mangrove ecosystems: An overview. Biol. Fertil. Soils 2001, 33, 265–278. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; Sham, A.; Elbadawi, A.A.; Hassan, A.H.; Alhosani, B.K.K.; El-Esawi, M.A.; AlKhajeh, A.S.; AbuQamar, S.F. A Consortium of Rhizosphere-Competent Actinobacteria Exhibiting Multiple Plant Growth-Promoting Traits Improves the Growth of Avicennia marina in the United Arab Emirates. Front. Mar. Sci. 2021, 8, 715123. [Google Scholar] [CrossRef]
- Farrer, E.C.; Van Bael, S.A.; Clay, K.; Smith, M.K.H. Plant microbial symbioses in coastal systems: Their ecological importance and role in coastal restoration. Estuar. Coasts 2022, 45, 1805–1822. [Google Scholar] [CrossRef]
- Barletta, M.; Saint-Paul, U.; Barletta-Bergan, A.; Ekau, W.; Schories, D. Spatial and temporal distribution of Myrophis punctatus (Ophichtidae) and associated fish fauna, in a north Brazilian intertidal mangrove forest. Hydrobiologia 2000, 426, 65–74. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Alencar, C.E.R.D.; Bezerra, L.E.A. Interrelationships among ecological factors of brachyuran crabs, trees and soil in mangrove community assemblage in Northeast Brazil. Community Ecol. 2019, 20, 277–290. [Google Scholar] [CrossRef]
- Lira, M.G.S.; Berbel-Filho, W.M.; Espírito-Santo, H.M.V.; Tatarenkov, A.; Avise, J.C.; Leaniz, C.G.; Consuegra, S.; Lima, S.M.Q. Filling the gaps: Phylogeography of the self-fertilizing Kryptolebias species (Cyprinodontiformes: Rivulidae) along South American mangroves. J. Fish Biol. 2021, 99, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.D.; Carvalho, C.E.V.; Tanizaki, K.F.; Ovalle, A.R.; Rezende, C.E. The biogeochemistry and trace metals distribution of mangrove rhizospheres. Biotropica 1993, 25, 252–257. [Google Scholar] [CrossRef]
- Smith, T.J., III; Chan, H.T.; McIvor, C.C.; Robblee, M.B. Comparisons of seed predation in tropical tidal forests from three continents. Ecology 1989, 70, 146–151. [Google Scholar] [CrossRef]
- McKee, K.L. Mangrove species distribution and propagule predation in Belize: An exception to the dominance-predation hypothesis. Biotropica 1995, 27, 334–345. [Google Scholar] [CrossRef]
- Bosire, J.O.; Kairo, J.G.; Kazungu, J.; Koedam, N.; Dahdouh-Guebas, F. Predation on propagules regulates regeneration in a high-density reforested mangrove plantation. Mar. Ecol. Prog. Ser. 2005, 299, 149–155. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ganade, G.; Freire, F.A.M.; Attayde, J.L. Propagule predation in a Neotropical mangrove: The role of the Grapsid crab Goniopsis cruentata. Hydrobiologia 2013, 707, 135–146. [Google Scholar] [CrossRef]
- Lima-Gomes, R.C.; Cobo, V.J.; Fransozo, A. Feeding behaviour and ecosystem role of the red mangrove crab Goniopsis cruentata (Latreille, 1803) (Decapoda, Grapsoidea) in a subtropical estuary on the brazilian coast. Crustaceana 2011, 84, 735–747. [Google Scholar] [CrossRef]
- Cutrim, A.S.T.; Sousa, L.K.S.; Ribeiro, R.P.; Oliveira, V.M.; Almeida, Z.S. Structure of a Polychaete community in a mangrove in the northern coast of Brazil. Acta Biol. Col. 2018, 23, 286–294. [Google Scholar] [CrossRef]
- Murugesan, P.; Sarathy, P.P.; Muthuvelu, S.; Mahadevan, G. Diversity and distribution of Polychaetes in Mangroves of East Coast of India. In Mangrove Ecosystem Ecology and Function; Sharma, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Spedicato, A.; Zeppilli, D.; Thouzeau, G.; Michaud, E. Nematode diversity patterns in mangroves: A review of environmental drivers at different spatial scales. Biodivers. Conserv. 2023, 32, 1451–1471. [Google Scholar] [CrossRef]
- Chew, L.L.; Chong, V.C. Copepod community structure and abundance in a tropical mangrove estuary, with comparisons to coastal waters. Hydrobiologia 2011, 666, 127–143. [Google Scholar] [CrossRef]
- Jaime, R.; Cantera, K.; Thomassin, B.A.; Arnaud, P.M. Faunal zonation and assemblages in the Pacific Colombian mangroves. Hydrobiologia 1999, 413, 17–33. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Blaber, S.J.M.; Bouillon, S.; Green, P.; Haywood, M.; Kirton, L.G.; Meynecke, J.-O.; Pawlik, J.; Penrose, H.M.; Sasekumar, A.; et al. The habitat function of mangroves for terrestrial and marine fauna: A review. Aquat. Bot. 2008, 89, 155–185. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, Q.; Li, J.; Jian, S.; Ren, H. Mangrove succession enriches the sediment microbial community in South China. Sci. Rep. 2016, 6, 27468. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguín, G. Plant growth-promoting bacteria: A potential tool for arid mangrove reforestation. Trees 2002, 16, 159–166. [Google Scholar] [CrossRef]
- Alongi, D.M. Macro- and Micronutrient Cycling and Crucial Linkages to Geochemical Processes in Mangrove Ecosystems. J. Mar. Sci. Eng. 2021, 9, 456. [Google Scholar] [CrossRef]
- Alongi, D.M. Bacterial productivity and microbial biomass in tropical mangrove sediments. Micro. Ecol. 1988, 15, 59–79. [Google Scholar] [CrossRef] [PubMed]
- Bano, N.; Nisa, M.; Khan, N.; Saleem, M.; Harrison, P.J.; Ahmed, S.I.; Azam, F. Significance of bacteria in the flux of organic matter in the tidal creeks of the mangrove ecosystem of the Indus River delta, Pakistan. Mar. Ecol. Prog. Ser. 1997, 157, 1–12. Available online: https://www.int-res.com/articles/meps/157/m157p001.pdf (accessed on 1 May 2024). [CrossRef]
- Brown, D.R.; Marotta, H.; Peixoto, R.B.; Enrich-Prast, A.; Barroso, G.C.; Soares, M.L.G.; Machado, W.; Pérez, A.; Smoak, J.M.; Sanders, L.M.; et al. Hypersaline tidal flats as important “blue carbon” systems: A case study from three ecosystems. Biogeosciences 2021, 18, 2527–2538. [Google Scholar] [CrossRef]
- Lacerda, L.D.; Ward, R.D.; Borges, R.; Ferreira, A.C. Mangrove trace-metal Biogeochemistry response to global climate change. Front. For. Glob. Chang. 2022, 5, 817992. [Google Scholar] [CrossRef]
- Zuberer, D.A.; Silver, W.S. Biological dinitrogen fixation (Acetylene reduction) associated with Florida mangroves. Appl. Environ. Microbiol. 1978, 35, 567–575. [Google Scholar] [CrossRef]
- Abhijith, R.; Vennila, A.; Purushothaman, C.S.; Padua, S.; Shilta, M.T.; Mahesh, V. Influence of sediment chemistry on mangrove-phosphobacterial relationship. Int. J. Chem. Stud. 2018, 6, 1677–1686. Available online: https://core.ac.uk/download/pdf/158619563.pdf (accessed on 1 May 2024).
- Warren, J.H.; Underwood, A.J. Effects of burrowing crabs on the topography of mangrove swamps in New South Wales. J. Exp. Mar. Biol. Ecol. 1986, 102, 223–235. [Google Scholar] [CrossRef]
- Barbanera, A.; Markesteijn, L.; Kairo, J.; Juma, J.A.; Karythis, S.; Skov, M.W. Functional responses of mangrove fauna to forest degradation. Mar. Freshw. Res. 2022, 73, 762–773. [Google Scholar] [CrossRef]
- Nozarpour, R.; Shojaei, M.G.; Naderloo, R.; Nasi, F. Crustaceans functional diversity in mangroves and adjacent mudflats of the Persian Gulf and Gulf of Oman. Marine Environ. Res. 2023, 186, 105919. [Google Scholar] [CrossRef]
- Macintosh, D.J. Ecology and productivity of Malaysian mangrove crab populations (Decapoda: Brachyura). In Proceedings of the Asian Symposium on Mangrove Environment: Research and Management, Kuala Lumpur, Malaysia, 25–29 August 1980; Soepadmo, E., Rao, A.N., Macintosh, D.J., Eds.; University of Malaya Press: Kuala Lumpur, Malaysia, 1984; pp. 354–377. [Google Scholar]
- Smith, T.J., III; Boto, K.G.; Frusher, D.D.; Giddins, R.L. Keystone species and mangrove forest dynamics: The influence of burrowing by crabs on soil nutrient status and forest productivity. Estuar. Coast. Shelf. Sci. 1991, 33, 19–32. [Google Scholar] [CrossRef]
- Havanond, S. Effects of mud lobster (Thalassina anomala Herbst) on plant succession in mangrove forests. Bull. Mar. Sci. 1987, 41, 635–636. [Google Scholar]
- Hossain, M.S.; Bujang, J.S.; Kamal, A.H.M.; Zakaria, M.H.; Muslim, A.M.; Nadzri, M.I. Effects of burrowing mud lobsters (Thalassina anomala Herbst 1804) on soil macro- and micronutrients in a Malaysian mangrove. Estuar. Coast. Shelf Sci. 2019, 228, 106358. [Google Scholar] [CrossRef]
- Felder, D.L. Diversity and ecological significance of deep-burrowing macrocrustaceans in coastal tropical waters of the Americas (Decapoda: Thalassinidea). Interciencia 2001, 26, 440–449. [Google Scholar]
- Arceo-Carranza, D.; Gamboa, E.; Teutli-Hernández, C.; Badillo-Alemán, M.; Herrera-Silveira, J.A. Fish as an indicator of ecological restoration of mangroves on the north coast of Yucatán. Rev. Mex. Biodivers. 2016, 87, 489–496. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5548498 (accessed on 1 May 2024). [CrossRef]
- Lewis, R.R.; Gilmore, R.G. Important considerations to achieve successful mangrove forest restoration with optimum fish habitat. Bull. Mar. Sci. 2007, 80, 823–837. [Google Scholar]
- Hernández-Mendoza, L.C.; Escalera-Vázquez, L.; Arceo-Carranza, D. Estuarine fish feeding changes as indicator to mangrove restoration success in seasonal karstic wetlands. Front. Glob. Chang. 2022, 4, 743232. [Google Scholar] [CrossRef]
- Hendy, I.; Michie, L.; Taylor, B.W. Habitat creation and biodiversity maintenance in mangrove forests: Teredinid bivalves as ecosystem engineers. PeerJ 2014, 2, e591. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, A.; Khoo, K.H.; Takita, T. Deposition of air in burrows of tropical mudskippers as an adaptation to the hypoxic mudflat environment. Science 1998, 81, 289–297. Available online: https://www.jstor.org/stable/i40135492 (accessed on 1 May 2024).
- Lacerda, L.D.; José, D.V.; Rezende, C.E.; Francisco, M.C.F.; Wasserman, J.C.; Martins, J.C. Leaf chemical characteristics affecting herbivory in a New World mangrove forest. Biotropica 1986, 18, 350–355. [Google Scholar] [CrossRef]
- Ong, J.E.; Gong, W.K.; Wong, C.H. Allometry and partitioning of the mangrove, Rhizophora apiculata. For. Ecol. Manag. 2004, 188, 395–408. [Google Scholar] [CrossRef]
- Ananda, K.; Sridhar, K.R. Diversity of filamentous fungi on decomposing leaf and woody litter of mangrove forests in the southwest coast of India. Curr. Sci. 2004, 87, 1431–1437. Available online: https://www.jstor.org/stable/24109484 (accessed on 1 May 2024).
- Lonsdale, D.; Pautasso, M.; Holdenrieder, O. Wood-decaying fungi in the forest: Conservation needs and management options. Eur. J. For. Res. 2008, 127, 1–22. [Google Scholar] [CrossRef]
- Lee, S.Y. Herbivory as an ecological process in a Kandelia candel (Rhizophoraceae) mangal in Hong Kong. J. Trop. Ecol. 1997, 7, 337–348. Available online: https://www.jstor.org/stable/2559631 (accessed on 1 May 2024). [CrossRef]
- Camilleri, J. Leaf choice by crustaceans in a mangrove forest in Queensland. Mar. Biol. 1989, 102, 453–459. [Google Scholar] [CrossRef]
- Thongtham, N.; Kristensen, E.; Purangprasan, S.Y. Leaf removal by sesarmid crabs in Bangrong Mangrove forest, Phuket, Thailand: With emphasis on the feeding ecology of Neoepisesarma versicolor. Estuar. Coast. Shelf. Sci. 2008, 80, 573–580. [Google Scholar] [CrossRef]
- Medina-Contreras, D.; Arenas-González, F.; Cantera-Kintz, J.; Sánchez-González, A.; Giraldo, A. Food web structure and isotopic niche in a fringe macro-tidal mangrove system, Tropical Eastern Pacific. Hydrobiologia 2020, 847, 3185–3199. [Google Scholar] [CrossRef]
- Erickson, A.A.; Saltis, M.; Bell, S.S.; Dawes, C.J. Herbivore feeding preferences as measured by leaf damage and stomatal ingestion: A mangrove crab example. J. Exp. Mar. Biol. Ecol. 2003, 289, 123–138. [Google Scholar] [CrossRef]
- Rani, V.; Sreelakshmi, C.; Nandan, S.B.; Santu, K.S.; Preethy, C.M. Feeding ecology of Parasesarma plicatum and its relation to carbon structuring in mangrove ecosystem. Hydrobiologia 2023, 850, 911–927. [Google Scholar] [CrossRef]
- Feller, I.C. The role of herbivory by wood-boring insects in mangrove ecosystems in Belize. Oikos 2002, 97, 153–176. [Google Scholar] [CrossRef]
- Alongi, D.M.; Christoffersen, P. Benthic infauna and organism-sediment relations in a shallow, tropical coastal area—Influence of outwelled mangrove detritus and physical disturbance. Mar. Ecol. Prog. Ser. 1992, 81, 229–245. Available online: https://www.jstor.org/stable/24827336 (accessed on 1 May 2024). [CrossRef]
- Calderon, D.G.; Echeverri, B.R. Obtaining Rhizophora mangle seedlings by stimulation of adventitious roots using an air-layering technique. In Mangrove Ecosystem Studies in Latin America and Africa; Kjerfve, B., Ed.; UNESCO: Paris, France, 1997; pp. 98–107. [Google Scholar]
- Schories, D.; Barletta-Bergan, A.; Barletta, M.; Krumme, U.; Mehlig, U.; Rademaker, V. The keystone role of leaf-removing crabs in mangrove forests of North Brazil. Wetlands Ecol. Manag. 2003, 11, 243–255. [Google Scholar] [CrossRef]
- Ashton, E.C. Mangrove sesarmid crab feeding experiments in Peninsular Malaysia. J. Exp. Mar. Biol. Ecol. 2002, 273, 97–119. [Google Scholar] [CrossRef]
- Burggren, W.; McMahon, B. Biology of the Land Crabs; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar] [CrossRef]
- Yeo, D.; Srivathsan, A.; Puniamoorthy, J.; Maosheng, F.; Grootaert, P.; Chan, L.; Guènard, B.; Damken, C.; Wahab, R.A.; Yuchen, A.; et al. Mangroves are an overlooked hotspot of insect diversity despite low plant diversity. BMC Biol. 2021, 19, 202. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.H. The natural history of insect herbivory on mangrove trees in and near Singapore. Raffles Bull. Zool. 1990, 38, 119–203. [Google Scholar]
- Feller, I.C.; Mathis, W.N. Primary herbivory by wood-boring insects along an architectural Gradient of Rhizophora mangle. Biotropica 1997, 29, 440–451. [Google Scholar] [CrossRef]
- Robertson, A.I.; Duke, N.C. Insect herbivory on mangrove leaves in North Queensland. Aust. J. Ecol. 1987, 12, 1–7. [Google Scholar] [CrossRef]
- Farnsworth, E.J.; Ellison, A.M. Patterns of Herbivory in Belizean Mangrove Swamps. Biotropica 1991, 23, 555–567. [Google Scholar] [CrossRef]
- Veenakumari, K.; Mohanraj, P.; Bandyopadbyay, A.K. Insect herbivores and their natural enemies in the mangals of the Andaman and Nicobar Islands. J. Nat. Hist. 1997, 31, 1105–1126. [Google Scholar] [CrossRef]
- Whitten, A.J.; Damanik, S.J. Mass defoliation of mangroves in Sumatra, Indonesia. Biotropica 1986, 18, 176. [Google Scholar] [CrossRef]
- Lu, W.; Xiao, J.; Cui, X.; Xu, F.; Lin, G.; Lin, G. Insect outbreaks have transient effects on carbon fluxes and vegetative growth but longer-term impacts on reproductive growth in a mangrove forest. Agric. For. Meteorol. 2019, 279, 107747. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Freire, F.A.M.; Rodrigues, J.V.M.; Bezerra, L.E.A. Mangrove recovery in semiarid coast shows increase in ecological processes from biotic and abiotic drivers in response to hydrological restoration. Wetlands 2022, 42, 80. [Google Scholar] [CrossRef]
- Onuf, C.P.; Teal, J.M.; Valiela, I. Interactions of nutrients, plant growth and herbivory in a mangrove ecosystem. Ecology 1977, 58, 514–526. [Google Scholar] [CrossRef]
- Feller, I.C. The effects of nutrient enrichment on growth and herbivory in dwarf red mangrove (Rhizophora mangle L.). Ecol. Monogr. 1995, 65, 477–505. [Google Scholar] [CrossRef]
- Maldonado-López, Y.; Vaca-Sánchez, M.S.; Canché-Delgado, A.; García-Jaín, S.E.; González-Rodríguez, A.; Cornelissen, T.; Cuevas-Reyes, P. Leaf herbivory and fluctuating asymmetry as indicators of mangrove stress. Wetlands Ecol. Manag. 2019, 27, 571–580. [Google Scholar] [CrossRef]
- Salter, R.E.; Mackenzie, N.A.; Nightingale, N.; Aken, K.M.; Chai, P.P.K. Habitat use, ranging behaviour and food habits of the Proboscis monkey, Nasalis larvatus (van Wurmb), in Sarawak. Primates 1985, 26, 436–451. [Google Scholar] [CrossRef]
- Yeager, C.P. Feeding ecology of the Proboscis monkey (Nasalis larvatus). Int. J. Primatol. 1989, 10, 497–530. [Google Scholar] [CrossRef]
- Dierenfeld, E.S.; Koontz, E.W.; Goldstein, R.S. Feed intake, digestion and passage of the Proboscis monkey (Nasalis larvatus) in captivity. Primates 1992, 33, 399–405. [Google Scholar] [CrossRef]
- Tangah, J.; Ashton, E.C.; Chan, H.T.; Baba, S. Mangroves of Malaysia. In Mangroves: Biodiversity, Livelihoods and Conservation; Das, S.C., Thammineni, P., Ashton, E.C., Eds.; Springer: Singapore, 2022; pp. 373–395. [Google Scholar] [CrossRef]
- Snarr, K.A. Seismic activity response as observed in mantled howlers (Alouatta palliata), Cuero y Salado Wildlife Refuge, Honduras. Primates 2005, 46, 281–285. [Google Scholar] [CrossRef]
- Luecke-Bridgeman, L. Diet of the black howler monkey (Alouatta pigra) in mangrove forests and the phytochemistry of mangrove plants. Am. J. Phys. Anthropol. 2012, 147, 197. [Google Scholar] [CrossRef]
- Mendez-Carvajal, P. Population Study of Coiba Howler Monkeys (Alouatta coibensis coibensis) and Coiba Capuchin Monkeys (Cebus capucinus imitator), Coiba Island National Park, Republic of Panama. J. Primatol. 2012, 1, 104. [Google Scholar] [CrossRef]
- Santos, R.R.; Bridgeman, L.L. Mangrove-living primates in the Neotropics: An ecological review. In Primates in Flooded Habitats: Ecology and Conservation; Nowak, K., Barnett, A.A., Matsuda, I., Eds.; Cambridge University Press: Cambridge, UK, 2019; pp. 54–58. [Google Scholar] [CrossRef]
- Cuddington, K.; Byers, J.E.; Wilson, W.G.; Hastings, A. Ecosystem Engineers: Plants to Protists; Academic Press: Cambridge, MA, USA, 2007; ISBN 978-0123738578. [Google Scholar]
- Davidson, T.; de Rivera, C.E.; Hsieh, H.-L. Damage and alteration of mangroves inhabited by a marine wood-borer. Mar. Ecol. Prog. Ser. 2014, 516, 177–185. [Google Scholar] [CrossRef]
- Perry, D.M.; Brusca, R.C. Effect of the root-boring isopod Sphaeroma peruvianum on red mangrove forests. Mar. Ecol. Prog. Ser. 1989, 57, 287–292. [Google Scholar] [CrossRef]
- Svavarsson, J.; Melckzedeck, K.W.; Osore, E.O. Does the wood-borer Sphaeroma terebrans (Crustacea) shape the distribution of the mangrove Rhizophora mucronata? Ambio 2002, 31, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Simberloff, D.; Brown, B.J.; Lowrie, S. Isopod and insect root borers may benefit Florida mangroves. Science 1978, 201, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Ribi, G. Does the wood boring isopod Sphaeroma terebrans benefit red mangroves (Rhizophora mangle)? Bull. Mar. Sci. 1981, 31, 925–928. [Google Scholar]
- Olafsson, E. Are wood-boring isopods a real threat to the well-being of mangrove forests? Ambio 1998, 27, 760–761. [Google Scholar]
- Brooks, R.A. Discovery of Sphaeroma terebrans, a wood-boring isopod, in the red mangrove, Rhizophora mangle, habitat of Northern Florida Bay. Ambio 2004, 33, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.B.; Angelini, C. Wood traits and tidal exposure mediate shipworm infestation and biofouling in southeastern U.S. estuaries. Ecol. Eng. 2019, 132, 1–12. [Google Scholar] [CrossRef]
- Nadia, T.C.L. Fenologia, Ecologia da Polinização e Reprodução de Espécies de Manguezal, no Município de Goiana—PE. Ph.D. Dissertation, Universidade Federal de Pernambuco, Recife, Brazil, 2009. [Google Scholar]
- Diniz, U.M.; Nadia, T.L.; Mello, M.A.R.; Machado, I.C. Few plants and one dominant fly shape a unique pollination network in a neotropical mangrove. Aquat. Bot. 2022, 180, 103526. [Google Scholar] [CrossRef]
- Landry, C.L. Pollinator-mediated competition between two coflowering Neotropical mangrovespecies, Avicennia germinans (Avicenniaceae) and Laguncularia racemosa (Combretaceae). Ann. Bot. 2013, 111, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Nadia, T.L.; Machado, I.C. Wind pollination and propagule formation in Rhizophora mangle L. (Rhizophoraceae): Resource or pollination limitation? An. Acad. Bras. Cien. 2014, 86, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Núnez, D.A.; Mancera-Pineda, J.E. Pollination and fruit set in the main neotropical mangrove species from the Southwestern Caribbean. Aquat. Bot. 2012, 103, 60–65. [Google Scholar] [CrossRef]
- Fleming, T.H.; Geiselman, C.; Kress, W.J. The evolution of bat pollination: A phylogenetic perspective. Ann. Bot. 2009, 104, 1117–1143. [Google Scholar] [CrossRef] [PubMed]
- Villamayor, B.M.R.; Rollon, R.N.; Samson, M.S.; Albano, G.M.G.; Primavera, J.H. Impact of Haiyan on Philippine mangroves: Implications to the fate of the widespread monospecifc Rhizophora plantations against strong typhoons. Ocean Coast. Manag. 2016, 132, 1–14. [Google Scholar] [CrossRef]
- Castellanos-Galindo, G.A.; Cantera, J.; Valencia, N.; Giraldo, S.; Peña, E.; Kluger, L.C.; Wolff, M. Modeling trophic flows in the wettest mangroves of the world: The case of Bahía Málaga in the Colombian Pacific coast. Hydrobiologia 2017, 803, 13–27. [Google Scholar] [CrossRef]
- Arakaki, J.; Grande, F.R.; Arvigo, A.L.; Pardo, J.C.F.; Fogo, B.R.; Sanches, F.H.C.; Miyai, C.A.; Marochi, M.Z.; Costa, T.M. Battle of the borders: Is a range-extending fiddler crab affecting the spatial niche of a congener species? J. Exp. Mar. Biol. Ecol. 2020, 532, 151445. [Google Scholar] [CrossRef]
- Jimenez, P.J.; Vorsatz, L.D.; Costa, T.M.; Cannicci, S. Temperature Extremes and Sex-Related Physiology, Not Environmental Variability, Are Key in Explaining Thermal Sensitivity of Bimodal-Breathing Intertidal Crabs. Front. Mar. Sci. 2022, 9, 858280. [Google Scholar] [CrossRef]
- Booth, J.M.; Fusi, M.; Marasco, R.; Mbobo, T.; Daffonchio, D. Fiddler crab bioturbation determines consistent changes in bacterial communities across contrasting environmental conditions. Sci. Rep. 2019, 9, 3749. [Google Scholar] [CrossRef]
- Fusi, M.; Booth, J.M.; Marasco, R.; Merlino, G.; Garcias-Bonet, N.; Barozzi, A.; Garuglieri, E.; Mbobo, T.; Diele, K.; Duarte, C.M.; et al. Bioturbation Intensity Modifies the Sediment Microbiome and Biochemistry and Supports Plant Growth in an Arid Mangrove System. Microbiol. Spectr. 2022, 10, e0111722. [Google Scholar] [CrossRef]
- Otero, X.L.; Araújo Jr, J.M.C.; Barcellos, D.; Queiroz, H.M.; Romero, D.J.; Nóbrega, G.N.; Siqueira Neto, M.; Ferreira, T.O. Crab Bioturbation and Seasonality Control Nitrous Oxide Emissions in Semiarid Mangrove Forests (Ceará, Brazil). App. Sci. 2020, 10, 2215. [Google Scholar] [CrossRef]
- Thirukanthan, C.S.; Azra, M.N.; Seman, N.J.A.; Agos, S.M.; Arifin, H.; Aouissi, H.A.; Lananan, F.; Gao, H. A scientometric review of climate change and research on crabs. J. Sea Res. 2023, 193, 102386. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest insects and climate change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Kumar, A.; Ramanathan, A. Speciation of selected trace metals (Fe, Mn, Cu and Zn) with depth in the sediments of Sundarban mangroves: India and Bangladesh. J. Soils Sediments 2015, 15, 2476–2486. [Google Scholar] [CrossRef]
- Nurdiani, R.; Zeng, C. Effects of temperature and salinity on the survival and development of mud crab, Scylla serrata (Forsskål), larvae. Aquacult. Res. 2007, 38, 1529–1538. [Google Scholar] [CrossRef]
- Dahdouh-Guebas, F.; Jayatissa, L.P.; Di Nitto, D.; Bosire, J.O.; Seen, D.L.; Koedam, N. How effective were mangroves as a defence against the recent tsunami? Curr. Biol. 2005, 15, R443–R447. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Rajendran, N. Coastal mangrove forests mitigated tsunami. Estuar. Coast. Shelf Sci. 2005, 65, 601–606. [Google Scholar] [CrossRef]
- Baldwin, A.; Egnotovich, M.; Ford, M.; Platt, W. Regeneration in Fringe Mangrove Forests Damaged by Hurricane Andrew. Plant Ecol. 2001, 157, 151–164. [Google Scholar] [CrossRef]
- Cai, W.; Ng, B.; Geng, T.; Jia, F.; Wu, L.; Wang, G.; Liu, Y.; Gan, B.; Yang, K.; Santoso, A.; et al. Anthropogenic impacts on twentieth-century ENSO variability changes. Nat. Rev. Earth Environ. 2023, 4, 407–418. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove dieback during fluctuating sea levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- Servino, R.N.; Gomes, L.E.O.; Bernardino, A.F. Extreme weather impacts on tropical mangrove forests in the Eastern Brazil Marine Ecoregion. Sci. Total Environ. 2018, 628–629, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.O.; Otero, X.L.; Nóbrega, G.N.; Queiroz, H.M.; Barcellos, D.; Vidal-Torrado, P. Mangroves Along the Brazilian Coast. In The Soils of Brazil; Schaefer, C.E.G.R., Ed.; World Soils Book Series; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Diele, K.; Tran Ngoc, D.M.; Geist, S.J.; Meyer, F.W.; Pham, Q.H.; Saint-Paul, U.; Tran, T.; Berger, U. Impact of typhoon disturbance on the diversity of key ecosystem engineers in a monoculture mangrove forest plantation, Can Gio Biosphere Reserve, Vietnam. Global Planet. Chang. 2013, 110, 236–248. [Google Scholar] [CrossRef]
- Muzaki, F.K.; Giffari, A.; Saptarini, D. Community structure of fish larvae in mangroves with different root types in Labuhan coastal area, Sepulu–Madura. AIP Conf. Proc. 2017, 1854, 020025. [Google Scholar] [CrossRef]
- Landry, C.L. Changes in pollinator assemblages following hurricanes affect the mating system of Laguncularia racemosa (Combretaceae) in Florida, USA. J. Trop. Ecol. 2013, 29, 209–216. [Google Scholar] [CrossRef]
- Lovelock, C.E. Soil Respiration and Belowground Carbon Allocation in Mangrove Forests. Ecosystems 2008, 11, 342–354. [Google Scholar] [CrossRef]
- Saavedra-Hortua, D.A.; Friess, D.A.; Zimmer, M.; Gillis, L.G. Sources of particulate organic matter across mangrove forests and adjacent ecosystems in different geomorphic settings. Wetlands 2020, 40, 1047–1059. [Google Scholar] [CrossRef]
- Ashton, E.C.; Macintosh, D.J.; Hogarth, P.J. A baseline study of the diversity and community ecology of crab and molluscan macrofauna in the Sematan mangrove forest, Sarawak, Malaysia. J. Trop. Ecol. 2003, 19, 127–142. [Google Scholar] [CrossRef]
- McNaughton, S.J.; Wolf, L.L. Dominance and the Niche in Ecological Systems. Science 1970, 167, 131–139. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Carscadden, K.; Mirotchnick, N. Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 2011, 48, 1079–1087. [Google Scholar] [CrossRef]
- Lohbeck, M.; Bonger, F.; Martinez-Ramos, M.; Poorter, L. The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 2016, 97, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Delfan, N.; Shojaei, M.J.; Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Est. Coast. Shelf. Sci. 2021, 252, 107288. [Google Scholar] [CrossRef]
- Henderson, C.J.; Gilby, B.L.; Schlacher, T.A.; Connolly, R.M.; Sheaves, M.; Maxwell, P.S.; Flint, N.; Borland, H.P.; Martin, T.S.H.; Olds, A.D. Low redundancy and complementarity shape ecosystem functioning in a low-diversity ecosystem. J. Anim. Ecol. 2020, 89, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Walker, B. Conserving biological diversity through ecosystem resilience. Conserv. Biol. 1995, 9, 747–752. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge and needs for future research. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Sacco, A.D.; Hardwick, K.A.; Blakesley, D.; Brancalion, P.H.S.; Breman, E.; Rebola, L.C.; Chomba, S.; Dixon, K.; Elliott, S.; Ruyonga, G.; et al. Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Chang. Biol. 2021, 27, 328–1348. [Google Scholar] [CrossRef]

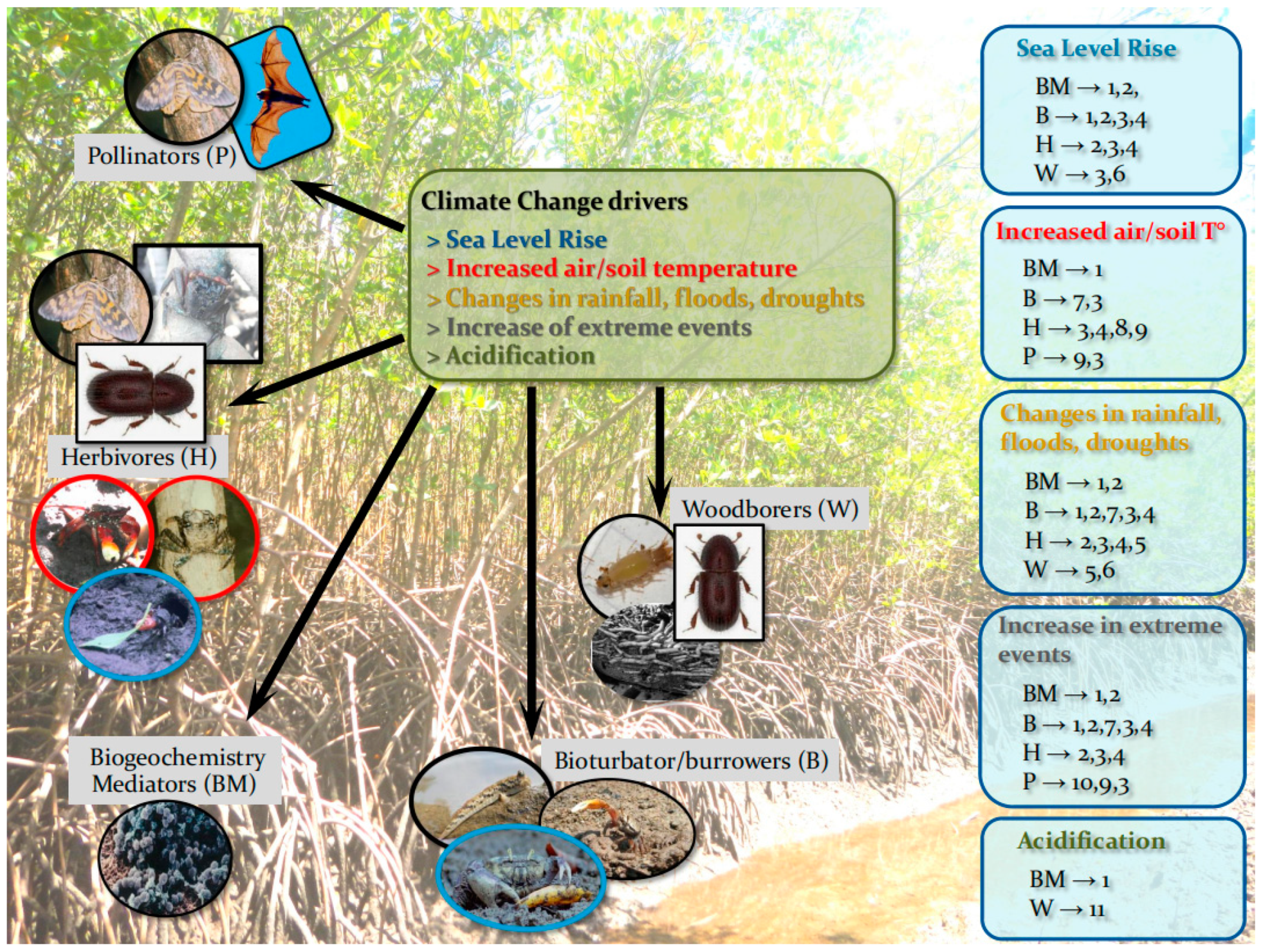
| Climate Change Driver | Impact of Climate Driver on the Ecosystem | FG Reached/ Affected | Impacts of Climate Driver over FG | Direct and Indirect Impacts of FGs on Forest Structure | Impact in ACEP | Impact in IWP |
|---|---|---|---|---|---|---|
| Sea level rise | Erosion of fringe forests Increase flooded period and redox Prolonged periods of anoxic conditions Poleward and inland migration Increase nutrient and pollutant availability and toxicity Augmenting the export of nutrients and pollutants from forest to adjacent coastal areas | Biogeochemistry mediators Burrowers/ bioturbators Herbivores Wood-borers | Negative impacts on soil metabolism due to increased anoxia periods Changes in bio-geochemical processes mediated by microbiota–soil relationships Disappearing of major intertidal burrowers (crabs and Thalassinids), most of them herbivores Decrease in activity of basidiomycetes, coleopterans, and termites Increase Isopod/ teredinid drilling |
| MAJOR | MAJOR Extreme in low-lying areas |
| Global and regional increase in air and soil temperature | Changing distribution patterns of functional groups components Increase in abundance of specific groups (ex. insects) | Biogeochemistry mediators Burrowers/ bioturbators Herbivores Pollinators | Changes in biogeochemical processes mediated by microbiota–soil relationships Effects on crab distribution (e.g., fiddler crabs) due to their limited range of temperature > Some burrowers can increase their burrowing to protect from thermal stress, while others can be excluded by it Changes in crab assemblages (Thalassinids?) Disruption in reproductive periods Insect outbreaks Decrease quality of plant material available to herbivores, e.g., increasing ash content Migration |
| MINOR MAJOR in semiarid coasts | MINOR MAJOR in semiarid coasts |
| Changes in rainfall, floods, and extended drought events | Increase erosion/sedimentation rates Change flooding periods | Biogeochemistry mediators Burrowers/ Bioturbators Herbivores Wood-borers | Changes in biogeochemical processes mediated by microbiota–soil relationships Mortality due to burrows collapse Mortality by osmotic limitations Change in crab assemblages and Thalassinid species (to species more resistant to higher inundation or resistant to thermal stress) Floods increase Isopod/ teredinid boring |
| MAJOR in semiarid coasts | MAJOR in semiarid coasts |
| Increase frequencies of extreme events (storms, cyclones, and hurricanes) | Accumulation of large debris Breaking of branches and trunks Mass defoliation | Biogeochemistry mediators Burrowers/ Bioturbators Herbivores Pollinators | Changes in biogeochemical processes mediated by microbiota–soil relationships Soil disruption Change in crab assemblages and Thalassinid species Burrow collapse Mortality due to trunks and debris smashing Disruption of populations of mangrove habitat dependent insects and fishes Local extinction of specific pollinators |
| MAJOR in Greater Caribbean South-eastern Atlantic | MAJOR in Monsoon regions |
| Acidification | Biogeochemistry mediators Wood-borers | Changes in biogeochemical processes mediated by microbiota–soil relationships Shell hardness and structure |
| MINOR | MAJOR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, A.C.; Ashton, E.C.; Ward, R.D.; Hendy, I.; Lacerda, L.D. Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption. Diversity 2024, 16, 423. https://doi.org/10.3390/d16070423
Ferreira AC, Ashton EC, Ward RD, Hendy I, Lacerda LD. Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption. Diversity. 2024; 16(7):423. https://doi.org/10.3390/d16070423
Chicago/Turabian StyleFerreira, Alexander C., Elizabeth C. Ashton, Raymond D. Ward, Ian Hendy, and Luiz D. Lacerda. 2024. "Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption" Diversity 16, no. 7: 423. https://doi.org/10.3390/d16070423
APA StyleFerreira, A. C., Ashton, E. C., Ward, R. D., Hendy, I., & Lacerda, L. D. (2024). Mangrove Biodiversity and Conservation: Setting Key Functional Groups and Risks of Climate-Induced Functional Disruption. Diversity, 16(7), 423. https://doi.org/10.3390/d16070423








