Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. Laboratory Protocol
2.3. Phylogenetic Analyses
2.4. Species Delimitation Analyses
2.5. Morphological Analyses
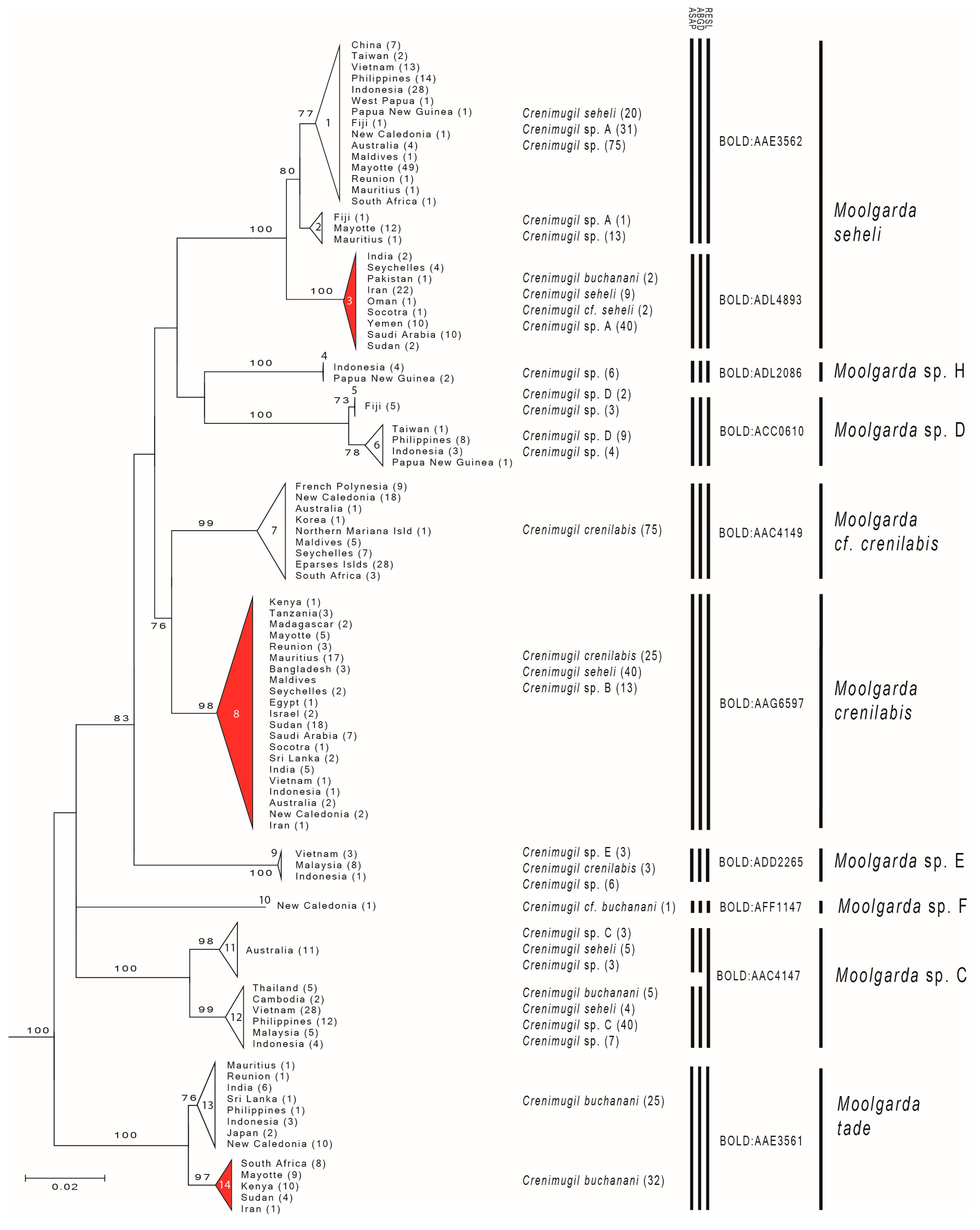
3. Results
3.1. Reviving the Genus Moolgarda
3.2. Genetic Analyses
3.3. Morphological Analyses
4. Key to the Red Sea species of Moolgarda
- 1a. Second dorsal fin 1.4–1.8 times higher than first dorsal fin, second dorsal and anal fins falcate, subequal in height; scales in longitudinal series 33–37……………………………M. tade
- 1b. Second dorsal fin subequal in height to first dorsal fin and slightly lower than anal fin; scales in longitudinal series 36–40…………………………………………………………………2
- 2a. Upper lip very thick anteriorly, its lower part with several rows of small papillae; lower lip with fringed anterior edge…………………………………………………….M. crenilabis
- 2b. Upper lip thin, its lower part not papillated; anterior edge of lower lip not fringed………………………………………………………… …………………………………………M. seheli
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durand, J.D.; Chen, W.J.; Shen, K.N.; Fu, C.; Borsa, P. Genus-level taxonomic changes implied by the mitochondrial phylogeny of grey mullets (Teleostei: Mugilidae). Comptes Rendus Biol. 2012, 335, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.D.; Shen, K.N.; Chen, W.J.; Jamandre, B.W.; Blel, H.; Diop, K.; Nirchio, M.; Garcia de León, F.J.; Whitfield, A.K.; Chang, C.W.; et al. Systematics of the grey mullets (Teleostei: Mugiliformes: Mugilidae): Molecular phylogenetic evidence challenges two centuries of morphology-based taxonomy. Mol. Phylogenet. Evol. 2012, 64, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Durand, J.D.; Borsa, P. Mitochondrial phylogeny of grey mullets (Acanthopterygii: Mugilidae) suggests high proportion of cryptic species. Comptes Rendus Biol. 2015, 338, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Durand, J.D.; Fu, C. Multilocus resolution of Mugilidae phylogeny (Teleostei: Mugiliformes): Implications for the family’s taxonomy. Mol. Phylogenet. Evol. 2016, 96, 161–177. [Google Scholar] [CrossRef]
- Thieme, P.; Bogorodsky, S.V.; Alpermann, T.J.; Whitfield, A.K.; Freitas, R.; Durand, J.D. Contributions to the taxonomy of the mugilid genus Chelon Artedi (Teleostei: Mugilidae), with a major review of the status of C. persicus Senou, Randall & Okiyama, 1995. Zootaxa 2022, 5188, 1–42. [Google Scholar] [CrossRef]
- Hasan, M.E.; Hasan, A.; Béarez, P.; Shen, K.N.; Chang, C.W.; Tran, T.T.V.; Golani, D.; Al-Saboonchi, A.; Siddiqui, P.J.A.; Durand, J.D. Planiliza lauvergnii (Eydoux & Souleyet, 1850), a senior synonym of Planiliza affinis (Günther, 1861) with a re-evaluation of keeled back mullets (Mugiliformes: Mugilidae). Zootaxa 2022, 5194, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Fricke, R.; Eschmeyer, W.N.; Van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 5 October 2023).
- Golani, D.; Fricke, R. Checklist of the Red Sea Fishes with delineation of the Gulf of Suez, Gulf of Aqaba, endemism and Lessepsian migrants. Zootaxa 2018, 4509, 1–215. [Google Scholar] [CrossRef]
- Thomson, J.M. The Mugilidae of the World. Mem. Qld. Mus. 1997, 41, 457–562. [Google Scholar]
- Whitley, G.P. New sharks and fishes from Western Australia. Part 2. Aust. Zool. 1945, 11, 1–42. [Google Scholar]
- Kottelat, M. The fishes of the inland waters of southeast Asia: A catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull. Zool. Suppl. 2013, 27, 1–663. [Google Scholar]
- Durand, J.D.; Hubert, N.; Shen, K.N.; Borsa, P. DNA barcoding grey mullets. Rev. Fish. Biol. Fish. 2017, 27, 233–243. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R. Soc. B-Biol. Sci. 2005, 360, 1847–1857. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.H. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Elsevier: Amsterdam, The Netherlands, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Thieme, P.; Moritz, T. The osteology of the golden grey mullet Liza aurata (Teleostei: Mugiliformes: Mugilidae) including interactive three-dimensional reconstructions. J. Fish Biol. 2020, 96, 1320–1340. [Google Scholar] [CrossRef] [PubMed]
- Schultz, L.P. A revision of the genera of mullets, fishes of the family Mugilidae, with descriptions of three new genera. In Proceedings of the United States National Museum; Smithsonian Institution Press: Washington, DC, USA, 1946; Volume 96, pp. 377–395. [Google Scholar]
- Nakabo, T. Fishes of Japan with Pictorial Keys to the Species; Tokai University Press: Tokyo, Japan, 2002; p. 1749. [Google Scholar]
- Harrison, I.J.; Senou, H. Mugilidae. In Species identification guide for fisheries purposes. In The Living Marine Resources of the Western Central Pacific. Bony Fishes Part 2 (Mugilidae to Carangidae); FAO: Rome, Italy, 1999; Volume 4. [Google Scholar]
- Smith, J.L.B. A generic revision of the mugilid fishes of South Africa. Ann. Mag. Nat. Hist. 1948, 14, 833–843. [Google Scholar] [CrossRef]
- Ghasemzadeh, J. Mugilidae. In Coastal Fishes of the Western Indian Ocean; Heemstra, P.C., Heemstra, E., Ebert, D.A., Holleman, W., Randall, J.E., Eds.; South African Institute for Aquatic Biodiversity: Makhanda, South Africa, 2022; Volume 2, pp. 308–326. [Google Scholar]
- Niebuhr, C. Descriptiones Animalium Avium, Amphibiorum, Piscium, Insectorum, Vermium; Quae in Itinere Orientali Observavit… Post Mortem Auctoris Edidit Carsten Niebuhr; ex Officina Mölleri, Hauniae: Copenhagen, Denmark, 1775. [Google Scholar]
- Rüppell, W.P.E.S. Atlas zu der Reise im Nördlichen Afrika. Fische des Rothen Meers; Heinrich Ludwig Brönner: Frankfurt am Main, Germany, 1828–1830; pp. 1–141+143. [Google Scholar]
- Cuvier, G.; Valenciennes, A. Histoire Naturelle des Poissons; Tome onzième. Livre treizième. De la famille des Mugiloïdes. Livre quatorzième. De la famille des Gobioïdes; F.G. Levrault: Paris, France, 1836; pp. xx + 506 + 2, 307–343. [Google Scholar]
- Klunzinger, C.B. Synopsis der Fische des Rothen Meeres. I. Theil. Percoiden-Mugiloiden; Verhandlungen der Kaiserlich-Königlichen Zoologisch-Botanischen Gesellschaft: Vienna, Austria, 1870; Volume 20. [Google Scholar]
- Klunzinger, C.B. Die Fische des Rothen Meeres. Eine kritische Revision mit Bestimmungstabellen. I. Teil. Acanthopteri veri Owen; E. Schweizerbart’sche Verlagshandlung (E. Koch): Stuttgart, Germany, 1884. [Google Scholar]
- Fowler, H.W. The fishes obtained by the De Schauensee South African Expedition: 1930. Proc. Acad. Nat. Sci. Phila. 1931, 83, 233–249. [Google Scholar]
- Roux-Estève, R.; Fourmanoir, P. Poissons capturés par la mission de la Calypso en Mer Rouge. Ann. L’institut Oceanogr. Monaco New Ser. 1955, 30, 195–203. [Google Scholar]
- Roux-Estève, R. Résultats scientifiques des campagnes de la Calypso. 10. Poissons. Ann. L’institut Oceanogr. Monaco New Ser. 1956, 32, 61–115. [Google Scholar]
- Marshall, N.B. The “Manihine” expedition to the Gulf of Aqaba 1948–1949. IX. Fishes. Bull. Br. Mus. Zool. 1952, 1, 221–252. [Google Scholar] [CrossRef]
- Klausewitz, W. Die physiographische Zonierang der Saumriffe von Sarso. In Meteor Forschungsergebnisse: Reihe D, Biologie; Meteor: Stuttgart, Germany, 1967; pp. 44–68. Available online: https://eurekamag.com/research/022/420/022420179.php (accessed on 5 October 2023).
- Ben-Tuvia, A. Mugilid fishes of the Red Sea with a key to the Mediterranean and Red Sea species. Bamidgeh 1975, 27, 14–20. [Google Scholar] [CrossRef]
- Dor, M. CLOFRES: Checklist of the Fishes of the Red Sea; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1984; pp. i–xxii + 1–437. [Google Scholar]
- Thomson, J.M.; Luther, G. Mugilidae; FAO: Rome, Italy, 1984. [Google Scholar]
- Goren, M.; Dor, M. An Updated Checklist of the Fishes of the Red Sea–CLOFRES II; Israel Academy of Sciences and Humanities: Jerusalem, Israel, 1994; p. 120. [Google Scholar]
- Khalaf, M.A.; Disi, A.M. Fishes of the Gulf of Aqaba; Marine Science Station Aqaba: Aqaba, Jordan, 1997. [Google Scholar]
- Khalaf, M.A. Fish fauna of the Jordanian coast, Gulf of Aqaba, Red Sea. JKAU Mar. Sci. 2004, 15, 23–50. [Google Scholar] [CrossRef]
- Golani, D.; Lerner, A. A long-term study of the sandy shore ichthyofauna in the Northern Red Sea (Gulf of Aqaba) with reference to adjacent mariculture activity. Raffles Bull. Zool. 2007, 14, 255–264. [Google Scholar]
- Fricke, R. Authorship, availability and validity of fish names described by Peter (Pehr) Simon Forsskål and Johann Christian Fabricius in the ‘Descriptiones animalium’ by Carsten Niebuhr in 1775 (Pisces). Stuttg. Beitraege Naturkunde Ser. A 2008, 1, 1–76. [Google Scholar]
- Golani, D.; Bogorodsky, S.V. The fishes of the Red Sea—Reappraisal and updated checklist. Zootaxa 2010, 2463, 1–100. [Google Scholar] [CrossRef]
- Günther, A. Catalogue of the Fishes in the British Museum. Catalogue of the Acanthopterygian Fishes in the Collection of the British Museum. Gobiidae, Discoboli, Pediculati, Blenniidae, Labyrinthici, Mugilidae, Notacanthi; Wheldon and Wesley: London, UK, 1861; Volume 3, pp. xxv + 586. [Google Scholar]
- Zajonz, U.; Lavergne, E.; Bogorodsky, S.V.; Saeed, F.N.; Aideed, M.S.; Krupp, F. Coastal fish diversity of the Socotra Archipelago, Yemen. Zootaxa 2019, 4636, 1–108. [Google Scholar] [CrossRef] [PubMed]
- Randall, J.E. Coastal Fishes of Oman; University of Hawaii Press: Honolulu, HI, USA, 1995. [Google Scholar]
- Toyama, T.; Fukuchi, I.; Yamazaki, K. Northernmost records of four tropical and subtropical mugilids from Ibaraki Prefecture, Japan. Ichthy Nat. Hist. Fishes Jpn. 2021, 6, 54–65. [Google Scholar]
- Senou, H.; Shinohara, G.; Matsuura, K.; Furuse, K.; Kato, S.; Kikuchi, T. Fishes of Hachijo-jima Island, Izu Islands Group, Tokyo, Japan. Mem. Natl. Sci. Mus. Tokyo 2002, 38, 195–237. [Google Scholar]
- Yoshigou, H. Some field information (mainly inland waters) of the Japanese mullets (Actinopterygii: Mugiliformes: Mugilidae). Hibakagaku 2022, 275, 9–22. [Google Scholar]
- Kwun, H.J.; Myoung, S.H. New record of the Crenimugil crenilabis (Forsskål, 1775) (Mugiliformes: Mugilidae) from Korea, as revealed by mitochondrial DNA barcoding. Mitochondrial DNA Part B 2019, 4, 1947–1950. [Google Scholar] [CrossRef]
- Tortonese, E. Pesci del mar Rosso. In Bollettino dei Musei di Zoologia ed Anatomia Comparata della R. Università di Torino; Series 3; Università di Torino: Torino, Italy, 1937; Volume 45, pp. 153–218. [Google Scholar]
- Rüppell, W.P.E.S. Verzeichniss der in dem Museum der Senckenbergischen Naturforschenden Gesellschaft Aufgestellten Sammlungen. Vierte Abtheilung: Fische und deren Skelette; Gedruckt bei Johann David Sauerländer: Frankfurt am Main, Germany, 1852; pp. iv + 40. [Google Scholar]
- Rajan, R.; Durand, J.-D.; Thomas, L.; Sidharthan, A.; Rahman, M.A.U.; Xavier, B.; Raghavan, R. Barcoding mullets (Mugilidae): Genetic characterization of exploited species in southern peninsular India. Diversity 2023, 15, 1193. [Google Scholar] [CrossRef]
- Bleeker, P. Tweede bijdrage tot de kennis der ichthyologische fauna van Batjan. Natuurkundig Tijdschr. Voor Ned. Indië 1855, 9, 191–202. [Google Scholar]
- Roberts, T.R. An Ichthyological Survey of the Fly River in Papua New Guinea with Descriptions of New Species; Smithsonian Institution Press: Wahington, DC, USA, 1978; pp. i–vi + 1–72. [Google Scholar]
- Séret, B. Les poissons d’eau douce de Nouvelle-Calédonie: Implications biogéographiques de récentes découvertes. Mémoires Du. Muséum Natl. D’histoire Nat. 1997, 171, 369–378. [Google Scholar]
- Li, C.H.; Ortí, G.; Zhang, G.; Lu, G.Q. A practical approach to phylogenomics: The phylogeny of ray-finned fish (Actinopterygii) as a case study. BMC Ecol. Evol. 2007, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.; Martin, A.; Romano, S.; Mcmillan, W.O.; Stice, L.; Grabowski, G. The Simple Fools Guide to PCR; University of Hawaii Press: Honolulu, HI, USA, 2002. [Google Scholar]
- Sevilla, R.G.; Diez, A.; Norén, M.; Mouchel, O.; Jerome, M.; Verrez-Bagnis, V.; Van Pelt, H.; Favre-Krey, L.; Krey, G. Primers and polymerase chain reaction conditions for DNA barcoding teleost fish based on the mitochondrial cytochrome b and nuclear rhodopsin genes. Mol. Ecol. Notes 2007, 7, 730–734. [Google Scholar] [CrossRef]






| Current Species ID (with Photo Marked with *) | In Durand & Borsa 2016 [3] | BOLD Process ID | BIN BOLD: | Sample Identifier | Catalog Museum Number | Locality | COI | 16S | Cytb | RAG | ENC-1 | Myh6 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moolgarda seheli | Crenimugil sp. A | MULID030-23 | ADL4893 | K-14 | n/a | Iran, Chabahar Bay | OR923746 | OR859515 | OR859535 | OR859458 | OR859476 | OR859494 |
| Moolgarda seheli | Crenimugil sp. A | MULID219-23 | ADL4893 | K-15 | n/a | Iran, Chabahar Bay | OR923871 | OR859523 | OR859543 | OR859466 | OR859483 | OR859502 |
| Moolgarda seheli | Crenimugil sp. A | MULID039-23 | ADL4893 | M-1131 | n/a | Yemen (Gulf of Aden), Khor Ambekha | OR923941 | OR859527 | OR859547 | n/a | OR859487 | OR859506 |
| Moolgarda seheli | Crenimugil sp. A | MULID058-23 | ADL4893 | Sudan-10 | n/a | Red Sea, Sudan, Port Sudan | OR923755 | OR859517 | OR859537 | OR859460 | OR859478 | OR859496 |
| Moolgarda seheli | Crenimugil sp. A | MULID040-23 | ADL4893 | M-1132 | n/a | Yemen (Gulf of Aden), Khor Ambekha | OR923923 | OR859525 | OR859545 | OR859468 | OR859485 | OR859504 |
| Moolgarda seheli | Crenimugil sp. A | MULID057-23 | ADL4893 | Sudan-1 | n/a | Red Sea, Sudan, Port Sudan | OR923978 | OR859529 | OR859549 | OR859471 | OR859489 | OR859508 |
| Moolgarda seheli | Crenimugil sp. A | ANGBF5147-12 | ADL4893 | 215 | MNHN ICOS-00266 | Oman, Ra’s al-Hadd | JQ060522 | KF375041 | KF375120 | KF375572 | KF375199 | KF375352 |
| Moolgarda seheli | Crenimugil sp. A | VIMAF024-16 | AAE3562 | LTV_CH_24 | n/a | Cau Hai, Vietnam | OR923810 | OR859520 | OR859540 | OR859463 | OR859481 | OR859499 |
| Moolgarda sp. H * | BIFV006-19 | ADL2086 | PNG10 | NTUM10007 | Papua New Guinea | MT884959 | OR859518 | OR859538 | OR859461 | OR859479 | OR859497 | |
| Moolgarda sp. D * | Crenimugil sp. D | BIFV005-19 | ACC0610 | PNG9 | NTUM10006 | Papua New Guinea | MT885131 | OR859531 | OR859551 | OR859473 | OR859491 | OR859510 |
| Moolgarda sp. D | Crenimugil sp. D | VIMAF097-16 | ACC0610 | LTP_NI_06 | n/a | Negros Isld, Philippines | OR923956 | OR859528 | OR859548 | OR859470 | OR859488 | OR859507 |
| Moolgarda crenilabis * | Crenimugil sp. B | MULID072-23 | AAG6597 | Sudan-5 | n/a | Red Sea, Sudan, Port Sudan | OR923939 | OR859526 | OR859546 | OR859469 | OR859486 | OR859505 |
| Moolgarda crenilabis * | Crenimugil sp. B | MULID065-23 | AAG6597 | Sudan-20 | n/a | Red Sea, Sudan, Port Sudan | OR923754 | OR859516 | OR859536 | OR859459 | OR859477 | OR859495 |
| Moolgarda crenilabis | Crenimugil sp. B | ANGBF5091-12 | AAG6597 | 221a | n/a | Tanzania, Stone Town, Zanzibar | JQ060634 | KF375042 | KF375121 | KF375573 | KF375200 | KF375353 |
| Moolgarda crenilabis * | Crenimugil sp. B | VIMAF541-16 | AAG6597 | 090316-1 | n/a | Con Dao Isld, Vietnam | OR923913 | OR859524 | OR859544 | OR859467 | OR859484 | OR859503 |
| Moolgarda cf. crenilabis * | Crenimugil crenilabis | ANGBF5314-12 | AAC4149 | 190 | MNHN 2008-1002 | French Polynesia, Moorea | JQ060437 | KF375040 | KF375119 | KF375571 | KF375198 | KF375351 |
| Moolgarda cf. crenilabis * | Crenimugil crenilabis | ANGBF5315-12 | AAC4149 | 185 | MNHN 2009-0808 | French Polynesia, Gambier Isl. | JQ060435 | JQ060685 | KF375173 | KF375570 | n/a | KF375350 |
| Moolgarda sp. E | VIMAF191-16 | ADD2265 | TTVT_10_4 | n/a | Bai sau, Kien Giang, Vietnam | KT728938 | OR859521 | OR859541 | OR859464 | n/a | OR859500 | |
| Moolgarda sp. E | VIMAF192-16 | ADD2265 | TTVT_10_5 | n/a | Bai sau, Kien Giang, Vietnam | KT728939 | OR859530 | OR859550 | OR859472 | OR859490 | OR859509 | |
| Moolgarda sp. F | MULID020-23 | AFF1147 | VBNCST696 | n/a | New Caledonia, Nera estuary | OR923967 | KF375043 | KF375122 | KF375574 | KF375201 | KF375354 | |
| Moolgarda sp. C | Crenimugil sp. C | MULID022-23 | AAC4147 | 569a-EP | n/a | beelbi creek, Queensland, Australia | OR924059 | OR859533 | OR859553 | OR859475 | OR859493 | OR859512 |
| Moolgarda sp. C | Crenimugil sp. C | VIMAF036-16 | AAC4147 | LTV_CH_36 | n/a | Cau Hai, Vietnam | OR923858 | OR859522 | OR859542 | OR859465 | OR859482 | OR859501 |
| Moolgarda tade | Crenimugil buchanani | VIMAF160-16 | AAE3561 | LTP_NI_49 | n/a | Negros Island, Philippines | OR923804 | OR859519 | OR859539 | OR859462 | OR859480 | OR859498 |
| Outgroup | ||||||||||||
| Osteomugil robustus | MULID021-23 | AAG6596 | 20110628 port3 | Reunion Isld, Le port | OR974843 | KF375071 | KF375149 | KF375609 | KF375236 | KF375387 | ||
| Osteomugil perusii | MULID096-23 | ACC0061 | RX12 | Taiwan | OR974844 | KF375070 | KF375148 | KF375608 | KF375235 | KF375386 |
| Measurements | KAU11-401 | SMF35158 (1) | SMF35158 (2) | SMF35158 (3) | SMF35158 (4) | Sudan-100 | Sudan-103 | Sudan-105 | Sudan-106 | Sudan-109 | MEAN | MNHN-A-3637 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL (in mm) | 261.0 | 116.8 | 99.8 | 98.0 | 99.8 | 261.6 | 221.0 | 311.0 | 305.0 | 258.4 | 205.0 | |
| Total length | 123.8 | 127.5 | 124.6 | 130.0 | 132.2 | 135.7 | 127.3 | 123.8 | 125.2 | 130.1 | 128.0 ± 3.8 | 130.4 |
| Predorsal length D1 | 52.2 | 49.9 | 49.3 | 50.6 | 53.5 | 53.7 | 49.9 | 48.7 | 47.2 | 50.2 | 50.5 ± 2.0 | 54.0 |
| Predorsal length D2 | 79.4 | 77.6 | 78.4 | 76.2 | 79.2 | 77.7 | 74.8 | 71.7 | 71.2 | 75.1 | 76.1 ± 2.8 | 78.8 |
| Preanal-fin length | 77.6 | 72.7 | 73.7 | 73.3 | 76.4 | 77.7 | 75.6 | 71.7 | 70.3 | 72.1 | 74.1 ± 2.5 | 75.1 |
| Prepelvic length | 40.2 | 41.3 | 40.2 | 40.2 | 42.9 | 41.2 | 40.3 | 38.4 | 37.0 | 36.8 | 39.8 ± 1.8 | 40.6 |
| Body width origin PF | 19.2 | 19.3 | 19.0 | 19.8 | 19.6 | - | - | - | - | - | 19.4 ± 0.3 | 18.3 |
| Body width origin D1 | 19.5 | 18.4 | 17.0 | 16.8 | 17.6 | - | - | - | - | - | 17.8 ± 1.0 | 15.4 |
| Body depth origin D1 | 27.2 | 29.3 | 29.5 | 28.6 | 28.2 | 25.1 | 24.8 | 22.4 | 25.4 | 23.9 | 26.4 ± 2.3 | 25.6 |
| Pectoral-fin length | 21.0 | 22.8 | 22.7 | 24.2 | 22.8 | 21.2 | 21.9 | 18.3 | 19.4 | 20.8 | 21.5 ± 1.7 | 22.1 |
| Pelvic-fin length | 14.4 | 16.6 | 16.0 | 17.1 | 16.0 | 14.5 | 13.6 | 15.1 | 13.2 | 13.7 | 15.0 ± 1.3 | 14.7 |
| Heigth D2 | 15.1 | 15.4 | 14.5 | 14.3 | 14.9 | 13.3 | 14.9 | 13.2 | 12.0 | 13.8 | 14.1 ± 1.0 | 14.1 |
| Heigth D1 | 13.6 | 14.0 | 13.5 | 14.3 | 15.0 | 13.6 | 14.3 | 13.3 | 12.0 | 13.0 | 13.7 ± 0.8 | 13.5 |
| Heigth anal fin | 13.7 | 16.1 | 15.9 | 15.0 | 15.5 | 14.0 | 14.2 | 14.0 | 13.3 | 14.5 | 14.6 ± 0.9 | 16.2 |
| Base D2 | 9.9 | 9.5 | 9.8 | 10.6 | 11.4 | 8.9 | 6.7 | 7.9 | 6.6 | 8.4 | 9.0 ± 1.5 | 9.4 |
| Base anal fin | 11.0 | 11.9 | 10.7 | 12.0 | 12.4 | 8.3 | 8.3 | 9.7 | 9.5 | 9.1 | 10.3 ± 1.5 | 11.2 |
| Pectoral axillary scale length | 9.1 | 7.7 | 6.9 | 8.0 | 7.3 | - | - | - | - | - | 7.8 ± 0.7 | 8.3 |
| Caudal peduncle length | 16.2 | 16.8 | 13.4 | 13.7 | 13.8 | 15.6 | 18.2 | 18.9 | 15.4 | 17.7 | 16.0 ± 1.8 | 13.5 |
| Caudal peduncle depth | 12.2 | 13.9 | 12.6 | 13.4 | 13.0 | 11.1 | 11.7 | 10.3 | 10.3 | 10.3 | 11.9 ± 1.3 | 11.4 |
| Head length | 24.9 | 28.0 | 28.6 | 29.1 | 29.8 | 23.5 | 23.6 | 23.1 | 23.1 | 23.2 | 25.7 ± 2.7 | 24.5 |
| Snout length | 3.3 | 5.0 | 4.3 | 4.1 | 4.5 | 4.9 | 5.5 | 6.7 | 6.5 | 6.2 | 5.1 ± 1.1 | 5.1 |
| Postorbital length | 13.8 | 15.1 | 15.2 | 14.1 | 13.3 | 14.0 | 13.5 | 13.2 | 13.6 | 13.1 | 13.9 ± 0.7 | 13.6 |
| Orbit diameter | 5.9 | 7.2 | 8.3 | 7.5 | 7.6 | 6.4 | 6.7 | 5.6 | 5.8 | 5.3 | 6.6 ± 0.9 | 5.3 |
| Interorbital width | 13.2 | 12.8 | 13.2 | 11.7 | 12.7 | 11.1 | 10.9 | 13.0 | 11.2 | 10.9 | 12.1 ± 0.9 | 11.7 |
| Upper-jaw length | 6.5 | 6.0 | 5.6 | 6.4 | 6.3 | 5.2 | 5.4 | 5.1 | 6.2 | 4.4 | 5.7 ± 0.6 | 6.0 |
| Thickness of upper lip | 2.7 | 3.2 | 3.2 | 2.8 | 3.0 | - | - | - | - | - | 3.0 ± 0.2 | 2.5 |
| Width of mouth | 9.4 | 11.7 | 9.8 | 12.0 | 10.6 | 8.0 | 8.7 | 7.8 | 8.4 | 7.3 | 9.4 ± 1.6 | 10.0 |
| Head depth | 15.6 | 18.0 | 17.9 | 18.7 | 17.7 | 14.3 | 13.2 | 13.4 | 15.2 | 13.5 | 15.8 ± 2.0 | 14.6 |
| Head width | 15.8 | 16.6 | 16.7 | 16.5 | 16.8 | 18.0 | 16.6 | 16.3 | 15.8 | 16.7 | 16.6 ± 0.6 | 15.7 |
| Meristics | Range | |||||||||||
| Longitudinal scale rows | 38 | 37 | 38 | 37 | 37 | 38 | 36 | 39 | 38 | - | 36–39 | 37 |
| TV | 14 | 13 | 13 | 14 | 12 | 13 | 12 | 14 | 14 | - | 12–14 | 13 |
| CP | 20 | 19 | 17 | 19 | 20 | - | - | - | - | - | 17–20 | 20 |
| PD1 | 12 | 11 | 12 | 12 | 12 | - | - | - | - | - | 11–12 | 13 |
| PD2 | 24 | 24 | 25 | 25 | 26 | - | - | - | - | - | 24–26 | 25 |
| Dorsal-fin rays | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 10 | - | - | - | - | - | IV, 10 | IV, 10 |
| Anal-fin rays | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | - | - | - | - | - | III, 10 | III, 10 |
| Pectoral-fin rays | 17 | 17 | 17 | 17 | 16 | - | - | - | - | - | 16–17 | 17 |
| Measurements | KAU13-121 | KAU13-122 | KAU13-461 | KAU13-463 | KAU17-159 | SMF39688 | MEAN | AMS-IB.1568 | Moolgarda pura |
|---|---|---|---|---|---|---|---|---|---|
| SL (in mm) | 257.0 | 308.3 | 105.4 | 107.9 | 250.0 | 112.1 | 92.8 | 390 | |
| Total length | 130.0 | 129.4 | 131.5 | 133.6 | 132.8 | 131.2 | 131.4 ± 1.5 | 125.9 | 125.6 |
| Predorsal length D1 | 55.7 | 52.9 | 52.5 | 52.1 | 51.9 | 48.9 | 52.3 ± 2.0 | 53.8 | - |
| Predorsal length D2 | 75.9 | 77.8 | 76.2 | 76.5 | 79.7 | 76.2 | 77.0 ± 1.3 | 77.0 | - |
| Preanal-fin length | 73.5 | 74.0 | 75.6 | 77.5 | 76.4 | 72.4 | 74.9 ± 1.8 | 75.0 | - |
| Prepelvic length | 41.1 | 39.1 | 40.0 | 42.4 | 40.5 | 42.0 | 40.8 ± 1.1 | 44.8 | - |
| Body width origin PF | 18.3 | 18.3 | 17.7 | 18.9 | 18.4 | 19.4 | 18.5 ± 0.5 | 19.2 | - |
| Body width origin D1 | 18.6 | 17.6 | 13.8 | 14.1 | 15.7 | 17.4 | 16.2 ± 1.8 | 14.1 | - |
| Body depth origin D1 | 27.4 | 23.5 | 25.6 | 28.1 | 28.0 | 28.6 | 26.9 ± 1.8 | 27.4 | 23.6 |
| Pectoral-fin length | 23.9 | 22.9 | 23.9 | 24.3 | 22.9 | 24.4 | 23.7 ± 0.6 | 23.4 | 22.1 |
| Pelvic-fin length | 15.7 | 15.3 | 17.5 | 17.6 | 15.3 | 16.7 | 16.3 ± 1.0 | 16.7 | - |
| Heigth D2 | 15.1 | 14.0 | 16.5 | 15.5 | 13.6 | 14.4 | 14.9 ± 1.0 | 14.8 | - |
| Heigth D1 | 13.8 | 12.2 | 15.8 | 14.4 | 13.4 | 16.1 | 14.3 ± 1.3 | 15.3 | - |
| Heigth anal fin | 15.2 | 13.9 | 17.0 | 15.9 | 14.3 | 15.6 | 15.3 ± 1.0 | 16.9 | - |
| Base D2 | 10.6 | 11.2 | 9.7 | 10.6 | 10.9 | 12.5 | 10.9 ± 0.8 | 10.8 | - |
| Base anal fin | 12.2 | 11.0 | 11.0 | 13.0 | 13.4 | 12.4 | 12.2 ± 0.9 | 11.9 | - |
| Pectoral axillary scale length | 7.9 | 9.6 | 7.0 | 7.0 | 8.8 | 6.9 | 7.9 ± 1.0 | 8.0 | - |
| Caudal peduncle length | 18.4 | 16.7 | - | 15.5 | 17.2 | 16.6 | 16.9 ± 0.9 | 16.4 | - |
| Caudal peduncle depth | 12.3 | 11.6 | 12.5 | 12.5 | 12.9 | 13.9 | 12.6 ± 0.7 | 12.0 | 10.0 |
| Head length | 25.0 | 24.3 | 28.4 | 28.2 | 27.2 | 29.0 | 27.0 ± 1.8 | 29.4 | 25.6 |
| Snout length | 4.0 | 4.1 | 4.6 | 4.5 | 4.1 | 5.5 | 4.4 ± 0.5 | 5.2 | 6.2 |
| Postorbital length | 13.4 | 14.4 | 15.6 | 15.9 | 14.4 | 15.2 | 14.8 ± 0.9 | 15.7 | 14.1 |
| Orbit diameter | 5.6 | 5.4 | 7.5 | 7.0 | 5.7 | 7.6 | 6.5 ± 0.9 | 8.6 | 4.4 |
| Interorbital width | 12.3 | 12.6 | 12.2 | 13.0 | 12.8 | 12.7 | 12.6 ± 0.3 | 12.6 | 12.3 |
| Upper-jaw length | 6.7 | 6.8 | 7.1 | 6.8 | 7.2 | 6.9 | 6.9 ± 0.2 | 7.0 | - |
| Thickness upper lip | 1.7 | 1.3 | - | - | 1.4 | 1.6 | 1.5 ± 0.2 | 2.3 | - |
| Width of mouth | 10.4 | 10.2 | 11.4 | 11.5 | 11.1 | 12.5 | 11.2 ± 0.8 | 9.9 | - |
| Head depth | 14.6 | 14.9 | 17.9 | 17.0 | 14.8 | 16.6 | 15.9 ± 1.3 | 17.1 | - |
| Head width | 14.9 | 14.5 | 18.0 | 18.1 | 15.2 | 17.8 | 16.4 ± 1.6 | - | 17.7 |
| Meristics | Range | ||||||||
| Longitudinal scale rows | 40 | 40 | 38 | 39 | 37 | 38 | 37–40 | 39 | 36 |
| TV | 14 | 12 | 13 | 14 | - | 12 | 12–14 | 12 | 14 |
| CP | 20 | 20 | 20 | 19 | 20 | 20 | 19–20 | 20 | - |
| PD1 | 13 | 12 | 11 | 13 | 12 | 12 | 11–13 | 12 | 14 |
| PD2 | 25 | 24 | 25 | 25 | 24 | 24 | 24–25 | 25 | 26 |
| Dorsal-fin rays | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 10 | IV, 9 * |
| Anal-fin rays | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 10 | III, 9 * |
| Pectoral-fin rays | 18 | 17 | 17 | 17 | 16 | 16 | 16–18 | 17 | 17 |
| Measurements | Sudan-900 | Sudan-901 | Sudan-902 | Sudan-903 | Sudan-904 | Mean |
|---|---|---|---|---|---|---|
| SL (in mm) | 376.0 | 375.0 | 345.0 | 394.0 | 361.0 | |
| Total length | 138.8 | 133.1 | 136.8 | 132.7 | 129.4 | 134.2 ± 3.3 |
| Predorsal length D1 | 51.5 | 48.1 | 48.0 | 49.0 | 49.4 | 49.2 ± 1.3 |
| Predorsal length D2 | 76.6 | 73.4 | 72.6 | 72.7 | 72.0 | 73.5 ± 1.6 |
| Preanal-fin length | 76.4 | 73.7 | 71.0 | 72.5 | 66.7 | 72.1 ± 3.2 |
| Prepelvic length | 38.6 | 37.1 | 37.1 | 36.7 | 35.7 | 37.0 ± 0.9 |
| Body width origin PF | - | - | - | - | - | - |
| Body width origin D1 | - | - | - | - | - | - |
| Body depth origin D1 | 26.4 | 26.2 | 27.7 | 25.7 | 25.1 | 26.2 ± 0.9 |
| Pectoral-fin length | 23.5 | 22.6 | 24.3 | 22.7 | 23.0 | 23.2 ± 0.6 |
| Pelvic-fin length | 17.2 | 16.1 | 19.0 | 15.4 | 15.9 | 16.7 ± 1.3 |
| Heigth D2 | 21.2 | 21.2 | 23.4 | 20.4 | 19.7 | 21.2 ± 1.3 |
| Heigth D1 | 13.8 | 14.1 | 13.2 | 12.7 | 13.8 | 13.5 ± 0.5 |
| Heigth anal fin | 19.9 | 18.8 | 21.8 | 18.0 | 19.7 | 19.7 ± 1.3 |
| Base D2 | 8.6 | 9.3 | 8.9 | 8.7 | 9.3 | 9.0 ± 0.3 |
| Base anal fin | 11.0 | 9.6 | 9.5 | 9.1 | 10.9 | 10.0 ± 0.8 |
| Pectoral axillary scale length | - | - | - | - | - | - |
| Caudal peduncle length | 19.5 | 19.5 | 17.9 | 16.5 | 17.5 | 18.2 ± 1.2 |
| Caudal peduncle depth | 12.3 | 11.9 | 12.4 | 12.6 | 12.3 | 12.3 ± 0.2 |
| Head length | 23.5 | 22.2 | 24.3 | 22.6 | 23.0 | 23.1 ± 0.7 |
| Snout length | 6.3 | 5.9 | 5.7 | 5.5 | 6.2 | 5.9 ± 0.3 |
| Postorbital length | 15.1 | 13.4 | 13.8 | 14.4 | 13.8 | 14.1 ± 0.6 |
| Orbit diameter | 4.9 | 5.5 | 6.0 | 5.3 | 5.4 | 5.4 ± 0.3 |
| Interorbital width | 11.3 | 10.8 | 11.1 | 10.4 | 10.7 | 10.9 ± 0.3 |
| Upper-jaw length | 5.5 | 5.2 | 5.1 | 5.2 | 5.2 | 5.2 ± 0.2 |
| Thickness upper lip | - | - | - | - | - | - |
| Width of mouth | 9.5 | 9.0 | 9.0 | 9.7 | 8.4 | 9.1 ± 0.5 |
| Head depth | 12.4 | 11.6 | 12.2 | 12.7 | 11.9 | 12.1 ± 0.4 |
| Head width | 13.6 | 14.9 | 16.0 | 16.2 | 15.9 | 15.3 ± 1.0 |
| Meristics | Range | |||||
| Longitudinal scale rows | 33 | 34 | 33 | 32 | 36 | 32–36 |
| TV | 11 | 12 | 12 | 13 | 13 | 11–13 |
| CP | 20 | - | - | - | - | - |
| PD1 | - | - | - | - | - | - |
| PD2 | - | - | - | - | - | - |
| Dorsal-fin rays | - | - | - | - | - | - |
| Anal-fin rays | - | - | - | - | - | - |
| Pectoral-fin rays | - | - | - | - | - | - |
| Current Species ID | Proposed Name | Name of Lineage in Durand & Borsa (2015) [3] | Distribution Based on DNA (Including Private Sequences) and Verified Records | Remarks |
|---|---|---|---|---|
| Moolgarda crenilabis (Forsskål, 1775) | - | Crenimugil sp. B | Red Sea, Socotra, Oman (Randall 1995 [52]), Iran (Bushehr), India (Kerala), Seychelles, Reunion, Mauritius, Tanzania, Sri Lanka, Indonesia, Vietnam, New Caledonia | |
| Moolgarda cf. crenilabis (Forsskål, 1775) | Moolgarda cirrhostoma (Forster, 1801) | Crenimugil crenilabis | South Africa, Mozambique, Mauritius, Seychelles, Maldives, Coral Sea, Australia (Queensland), Korea (Kwun & Myoung 2019 [56]), Mariana Islands, New Caledonia, Tahiti, Marquesas Islands | The species was described from central Pacific in Cook’s voyage. This is a single species known there; hence, this name can apply to the lineage related to M. crenilabis |
| Moolgarda seheli (Fabricius, 1775) | - | Crenimugil sp. A | Red Sea, Gulf of Aden, Socotra, Oman, Arabian/Persian Gulf, Pakistan, South Africa, Seychelles, Reunion, Maldives, Indonesia, Papua New Guinea, Philippines, Vietnam, Taiwan, Mariana Islands, Australia (Queensland), New Caledonia, Fiji | |
| Moolgarda tade (Forsskål, 1775) | - | Crenimugil buchanani | Red Sea, Iran (Gulf of Oman), Kenya, Madagascar, Seychelles, Mauritius, South Africa, India, Thailand, Indonesia, Philippines, New Caledonia | Mugil buchanani, Mullus malabarica and Mugil pedaraki, described from east coast of India, are junior synonyms. |
| Moolgarda sp. C | Moolgarda delicata (Alleyne & Macleay, 1877) | Crenimugil sp. C | Australia (Queensland, Northern Territory), Malaysia, Indonesia (Bali, Kalimantan), Philippines, Vietnam | Similar to M. tade in having falcate D2 but the fin is slightly shorter than in M. tade |
| Moolgarda sp. D | Moolgarda heterocheilos (Bleeker, 1855) | Crenimugil sp. D | Indonesia, Papua New Guinea, Philippines, Taiwan, New Caledonia (Séret 1997 [62]), Fiji | Upper lip with dermal ridges of papillae |
| Moolgarda sp. E | - | - | Indonesia (Sumatra), Thailand, Malaysia, Vietnam | |
| Moolgarda sp. F | - | - | New Caledonia | |
| Moolgarda sp. H | - | - | eastern Indonesia, Papua New Guinea |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogorodsky, S.V.; Thieme, P.; Senou, H.; Mahmoud, Z.N.; Alpermann, T.J.; Durand, J.-D. Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea. Diversity 2024, 16, 325. https://doi.org/10.3390/d16060325
Bogorodsky SV, Thieme P, Senou H, Mahmoud ZN, Alpermann TJ, Durand J-D. Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea. Diversity. 2024; 16(6):325. https://doi.org/10.3390/d16060325
Chicago/Turabian StyleBogorodsky, Sergey V., Philipp Thieme, Hiroshi Senou, Zuheir N. Mahmoud, Tilman J. Alpermann, and Jean-Dominique Durand. 2024. "Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea" Diversity 16, no. 6: 325. https://doi.org/10.3390/d16060325
APA StyleBogorodsky, S. V., Thieme, P., Senou, H., Mahmoud, Z. N., Alpermann, T. J., & Durand, J.-D. (2024). Contributions to the Taxonomy of the Mugilid Genus Moolgarda Whitley (Teleostei: Mugilidae), with Redescriptions of M. crenilabis, M. seheli and M. tade from the Red Sea. Diversity, 16(6), 325. https://doi.org/10.3390/d16060325








