Abstract
François’ langur is an Endangered colobine inhabiting limestone habitats in southern China and northern Vietnam. Its global population has been estimated to be just more than 2000 mature individuals. Populations in Vietnam are highly fragmented with reportedly fewer than 200 adults in total and 50 in a single location. Although the François’ langur in Vietnam is highly imperiled as remnant populations persist in only three to four sites, little research has been carried out to provide a reliable estimate of its remaining population. In this study, we conducted field surveys in Lam Binh District, Tuyen Quang Province, northeastern Vietnam. In total, we recorded at least 16 groups of François’ langurs, with 156 individuals, raising the total number of individuals by approximately 10% compared to a previous study. The group structure, group size, activity budget, and density of the Lam Binh population resemble those reported in François’ langurs in China and other limestone langur species. The results show that the behavior ecology of limestone langurs significantly differs from that of forest langurs probably because they occupy separate habitats with distinctly different environmental variables. During our surveys, we detected a number of direct threats to this population, namely illegal logging, hunting, firewood collecting, hydropower development, grazing, and mining. It is recommended that the protection forest be elevated to the nature reserve status to better protect the most important population of the François’ langur in Vietnam.
1. Introduction
Vietnam is home to as many as 27 primate species and subspecies, the highest number among mainland Southeast Asian countries, with several species newly described or resurrected in recent decades [1,2,3,4,5]. At least four taxa are endemic to small areas or islands in Vietnam, namely the Cat Ba langur (Trachypithecus poliocephalus), Con Son long-tailed macaque (Macaca fascicularis condorensis), Delacour’s langur (Trachypithecus delacouri), and Tonkin snub-nosed monkey (Rhinopithecus avunculus) [3,4]. However, due to different direct threats, including habitat loss and poaching, populations of many species, e.g., the Cao-vit Gibbon (Nomascus nasutus), Cat Ba langur, Delacour’s langur, and Tonkin snub-nosed monkey, have been severely declining to lower than a few hundred individuals [6,7,8,9]. The existing threats coupling with the imminent impacts of climate change could bring the species to the brink of extinction [10,11,12,13]. As a result, Vietnam’s primate fauna is considered the most threatened in the region with all but two species, i.e., the Assamese macaque (Macaca assamensis, listed as Near Threatened) and the rhesus macaque (M. mulatta, listed as Least Concern), classified as globally Vulnerable and above [14] (Figure 1).
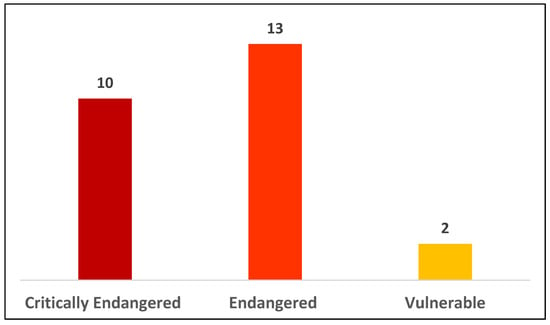
Figure 1.
Conservation status of primates in Vietnam. The numbers of threatened and Critically Endangered species account for approximately 93% and 37% of the country’s primate fauna, respectively [14].
At least seven leaf monkey species of the genus Trachypithecus are found in Vietnam [3,4], and all of them are classified as either Endangered or Critically Endangered by the IUCN Red List of Threatened Species [14]. The François’ langur (Trachypithecus francoisi) is a limestone langur species distributed in southern China and northern Vietnam [15,16]. While the species was more widespread in the past, most populations of the François’ langur now live in highly fragmented limestone forest patches because of the illegal hunting and habitat destruction occurring throughout its former range [17,18,19]. The global population of the François’ langur is estimated to be around 2300–2500 individuals, and the main populations of the langurs are found in China with more than 2000 individuals in total [20].
In Vietnam, it is reported that the total population of the langur may not exceed 200 individuals [17,21]. While the François’ langurs occupied a broader distribution range compared to other limestone langur species in Vietnam, surveys from the early 2010s reported that many known populations, especially in Cao Bang, Lang Son, Vinh Phuc, Lao Cai, and Yen Bai provinces, had been locally extirpated [21,22]. Currently, Cham Chu and Na Hang nature reserves (Tuyen Quang Province), Ba Be National Park and Kim Hy Nature Reserve (Bac Kan Province), Bac Me Nature Reserve (Ha Giang), and Than Sa–Phuong Hoang Nature Reserve (Thai Nguyen) have been considered the last protected forests where Francois’ langurs may still range [21,22]. However, if langurs still persist in Kim Hy, Bac Me, and Cham Chu, their populations may already be too small to survive in the long run [22].
The François’ langur population in Than Sa–Phuong Hoang Nature Reserve was estimated to be about 20 individuals divided in four to five groups. The langurs in Na Hang Nature Reserve were reported to consist of around two to five groups with 4–22 individuals in each group [21,22]. The largest population of the François’ langur in Vietnam is distributed in Lam Binh Protection Forest, a watershed protection forest that is not yet officially recognized as a protected area in Vietnam and located adjacent to Na Hang Nature Reserve [21,22,23]. Together, Lam Binh, Na Hang, Bac Me, and Ba Be form a relatively continuous forested area of approximately 97,000 hectares (Figure 2).
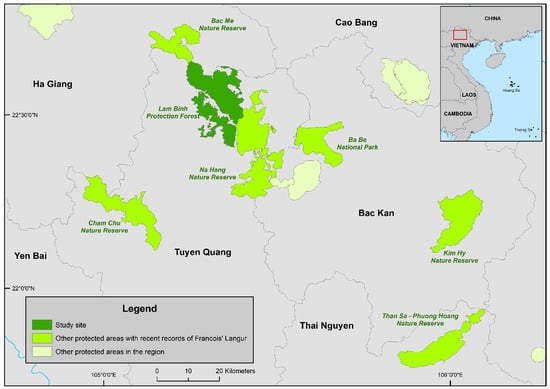
Figure 2.
Lam Binh Protection Forest and other sites that still have recent records of the François’ langur.
The remaining population in Lam Binh Protection Forest, Tuyen Quang Province, has been considered the most viable population of this species in Vietnam, as other populations are thought to be too small for recovery [21,22]. Field surveys in 1992 confirmed the existence of the langurs in this area, and in the early 2000s, it was estimated that Lam Binh might be home to five to seven François’ langur groups, with a total of about 35–60 individuals [15]. In 2011–2012, field surveys recorded five groups of around 28–38 langur individuals in this area, with interview records of up to 10–12 groups [21]. The most recent survey in the Lam Binh area in 2020 revealed 13 groups of about 108–133 individuals of the François’ langur [23]. To fully assess the population status, social organization, behavioral ecology of, and anthropogenic threats to the langur population in the entire protection forest in Lam Binh with a view to better understanding its ecology and developing cost-effective conservation measures, we conducted both interview and field surveys throughout 2021.
2. Methods
The study site is part of the Lam Binh Protection Forest in Lam Binh District, Tuyen Quang Province, northern Vietnam, covering approximately 39,000 hectares of protection forest (Figure 2). It is characterized by dissected limestone karst formations and narrow valleys with fragmented patches of mature secondary forest on ridges and upper slopes and degraded forests with mostly scrubs on lower slopes. The area is currently managed by the Lam Binh Protection Forest Management Authority, and it is classified as a watershed protection forest, a type of low-level protected forest maintained outside the official protected area system designated by the government [22].
Interviews with 96 local community members who were knowledgeable of the site were undertaken prior to the field survey to identify potential areas. We collected information on locations where langurs occurred, dates of the observations, group size, population status and trend, and direct threats to the langur population in Lam Binh. The main methods for field surveying the François’ langur included transect walks and visual observations from fixed points along the transects. Three survey teams, one stationed in Khuon Ha, one in Sinh Long, and one in Thuong Lam Commune and each consisting of two members, were established (Figure 2). During the surveys, each team walked along separate transects at a speed of 1.0–1.5 km/h and observed from independent points to maximize the detection probability [24]. Surveys were carried out every month in 2021 at all three sites with 10 days/month in Sinh Long and 12 days/month in Khuon Ha and Thuong Lam. The surveys took place from 4:00–6:00 in the morning to 6:30–7:00 in the evening with at least 8 h spent on each survey day. For the entire study, 17 transects were set up with the total length of 117.69 km (mean = 6.9; SD = 5.6) (Figure 3).
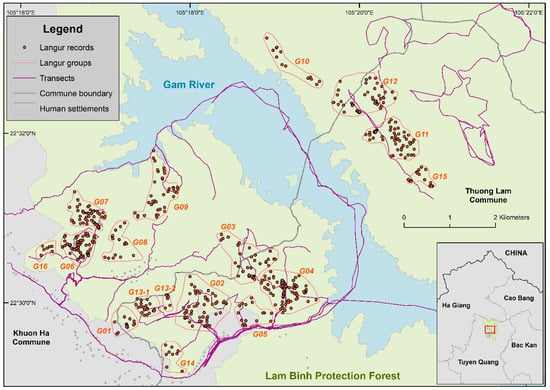
Figure 3.
Survey transects and approximate locations of different groups of the François’ langur (Trachypithecus francoisi) in Lam Binh Protection Forest. G is the abbreviation for Group. (See Table 1 for more information).
When langurs were spotted, we recorded the time and date, coordinates of the team, and direction of the target and estimated the distance to the langurs, their group size, and group structure. The age–sex classification, including adult male, adult female, juvenile, and infant, followed descriptions in previous studies [15,16]. To avoid double counting langur groups that travelled extensively in one day, the teams exchanged and compared obtained data, such as time and location of the records and group size and composition, after each field day. For groups that were not easily separated, we treated them as one single group. In case there were different counts for the same group, both estimations were presented in the final results. Geographic coordinates were recorded using GPS units (Garmin 64 S). Observations were assisted by binoculars (Nikon PROSTAFF 7S 10X42, Nikon, Tokyo, Japan). The home range of each group was estimated using the minimum convex polygon method [25] as implemented in ArcMap v10.2 [26].
To estimate the time budget for the population, we selected four representative groups, i.e., Group 1, 2, 6, and 7, as their locations were more accessible, and the groups were easier to observe for extended periods of time compared to the other ones. Behavioral data were collected using methods specified in [27] by scan and focal samplings. Observations were undertaken in March, April, August, and September, when the weather conditions were more favorable for studying langur activities. Each day, each group was watched eight times, from 5:15 a.m. until 6:30 p.m. in the summer and from 6:00 a.m. until 5:30 p.m. in the winter. In total, 768 h were spent in the field to obtain activity budget data from the four groups.
3. Results
Most interviewees reported that the population of François’ langurs in Lam Binh had decreased since the early 1990s. In particular, there was a brief period of steep decline during the completion of the Na Hang hydroelectric dam in 2003/2004. Before the dam’s construction, during the dry seasons, langurs could be spotted occasionally crossing the temporarily dry riverbed. Nevertheless, after 2004 when the dam started raising the water level of the river, such movements were stopped. In addition to the impact of the dam construction, other direct threats, especially poaching and habitat loss and degradation, were considered the main driver of the population decline.
After 352 encounters, our survey recorded a total of 18 groups, including two single-male ones, with around 156 individuals of the François’ langur in the Lam Binh Protection Forest (mean with two single-male groups = 8.21; SD with two single-male groups = 4.48; mean without two single-male groups = 9.06; SD without two single-male groups = 3.91) (Table 1). For Group 13, we observed two groups (13-1 and 13-2) to be quite distinctive in terms of their home range. However, we also spotted the adults of those two groups occasionally interacting with each other, and we could not identify any adult male in the groups. Hence, we used a more conservative approach and still recognized them as one group.

Table 1.
Details of the population structure and group sizes of François’ langurs in Lam Binh Protection Forest. See Figure 3 for additional information of the group locations.
Overall, Group 6 had the highest number of individuals with 17 langurs, and two single-male groups consisted of the lowest number with one individual each. Khuon Ha Commune was home to six groups, i.e., Group 6, Group 7, Group 8, Group 9, Group 10, and Group 16 (a total of 61 individuals), and Thuong Lam Commune was also home to six groups, including Group 2, Group 4, Group 5, Group 11, Group 14, and Group 15 (a total of 56 langurs, including 2 single males). The two communes shared four units, namely Group 1, Group 3, Group 12, and Group 13 (a total of 39 individuals). The two communes are separated by Gam River, and 14 groups, including two single-male groups (a total of 106 individuals), occupied the southwestern side of the river. The remaining four occurred on the northeastern side (a total of 50 individuals).
Based on our field observations and the social characteristics of other Trachypithecus species, all langur groups in Lam Binh, except for Group 13, appeared to be uni-male, even though the number of undetermined adults in most groups was high. In total, 34 young individuals, including 11 infants and 23 juveniles, were detected, accounting for 21.8% of the whole population (Table 1). In addition, most of the small groups, e.g., Group 14 or Group 16, were observed to occur nearer to human settlements, while larger groups, e.g., Group 04, Group 06, and all groups on the western side of the Gam River, appeared to live further away (Figure 3).
François’ langurs began their activity around 5:15 a.m. and 6:00 a.m. in summer and winter, respectively, and ended the day from about 5:30 p.m. to 6:30 p.m. depending on the time of the year. They usually left the sleeping sites later if it rained heavily. As for the activity budget, the langurs spent more than a half of their daily time resting, followed by more than 20% of the time feeding and more than 10% moving. Other activities make up less than 10% of active time (Table 2).

Table 2.
Activity budget of selected Trachypitheticus species.
Home ranges of different langur groups in Lam Binh varied significantly in size, from 11.73 hectares in Group 16 to 96.61 hectares in Group 2 (mean = 46.9, SD = 26.94). Group 13, when combining Group 13-1 and Group 13-2, covered an area of approximately 25.4 hectares. In total, there are six groups whose home ranges are around or greater than 50 hectares (Table 3). Average individual home range reaches the highest size, 16.1 hectares, in Group 2, and the smallest range size, 1.02 hectare, belongs to Group 15 (mean = 5.48, SD = 3.92). Density, a measure of the number of individuals in a hectare, is opposite to the individual home range and attains the greatest value, 0.99, in Group 16 and the lowest one, 0.06, in Group 2 (mean = 0.28, SD = 0.22).

Table 3.
Home ranges of langur groups in Lam Binh Protection Forest.
4. Discussion
This is the first study that documents several important parameters of any François’ langur population in Vietnam, including group size, population structure, activity budget, home range, and density. With 156 individuals observed in this study, it is clear that the population is growing, as previous study only documented at most 133 langurs [23]. Notably, Group 15 with 14 individuals, the second largest unit only behind Group 6 and located near Group 11, was newly discovered by this study (Figure 3, Table 1). In addition, up to 34 individuals or nearly 22% of the total population were either juveniles or infants, illustrating low infant and juvenile mortality. We also observed two single-male groups of the langurs on the southwestern side of the river. For such groups, we only included them in the total individual counts, but excluded them from the group counts. They generally do not contribute to the reproduction function of any group. There was also one group (Group 13) that seemed to have split into two independent groups (Group 13-1 and Group 13-2). However, our field data did not provide enough evidence for that suggestion, and hence we still counted them as a single group.
The mean group size, 8.21–9.06, of the François’ langur in Lam Binh is comparable with those of other limestone langurs in Vietnam, e.g., 8.11 in the Hatinh langur [35] and 7.27 in the Delacour’s langur [8], but greater than the Laotian langur (Trachypithecus laotum) with mean group size of about 3.88 [36] and the Cat Ba langur (T. poliocephalus) of around 5.27 [37]. While group size in folivores is often determined by several factors, including their behaviors, ecological resources, and predation [38,39,40,41,42,43], they have been shown to differ between limestone and forest langurs, with forest populations usually being larger.
In relatively hunting-free sites, limestone langurs, such as the white-headed langur (T. leucocephalus) and François’ langur, often maintain a mean group size of 9–10 individuals [44,45], whereas that of forest langurs, e.g., T. germaini and T. crepusculus, contains 15–25 individuals [31,46,47]. In the exceptional case, group size in forest langurs can reach close to 100 individuals without exhibiting any sign of ecological constraints [48]. However, limestone langurs living in larger groups of 15 or more individuals show evidence of high competition for food resources, but smaller groups of 5–6 individuals do not exhibit such contention [49]. The difference in group size and the level of competition for food between limestone and forest langurs can be explained by the availability of resources in the ecosystems, as limestone ecosystems are well known for their nutrient deficiency and provide fewer diet options for the folivores [50,51]. Based on the available data, it seems that the mean group size in the Lam Binh population is approaching the equilibrium in limestone langurs, even though poaching still takes place (see more information below). Nonetheless, it is most likely that the small group sizes in other langurs from Laos and Vietnam are attributable to historical and/or current hunting pressures [8,34,35,36].
Our observations reveal that the group composition of langurs in the study area is well structured with typically one adult male and multiple females, except for Group 13 (Table 1). However, the group organization dynamics could change because a large number of adults had an undetermined sex. Multi-male groups have been reported in other species of langurs [47,52]. In the Lam Binh population, the dominant male serves as a group leader and usually sits on a high point to detect any potential danger. The group leader will scream loudly to alert the group when there is any threat. In addition, he also makes calls to signal when to enter the cave to sleep in the evening and when to leave the cave in the morning. The group organization in Lam Binh’s langurs fits well with the typical form found in other species in the genus Trachypithecus [53,54]. This social structure has been shown to be the ancestral trait for all Asian colobines [55].
The langur’s foraging activities peak twice a day, including morning and afternoon sessions, with a break at noon. The morning session starts around 5:15 a.m. and 6:00 a.m. in summer and winter, respectively, and extends until about 10:00 a.m., and the afternoon session occurs from 2:00 p.m. to 4:30 p.m. In the hot season, langurs leave their sleeping places in the early morning, return to their caves in the late hours of the afternoon, and have a long lunch break. In the cold season, they go out looking for food later in the morning and return to their caves earlier in the afternoon. The foraging pattern is similar to that of François’ langurs in Nonggang Nature Reserve, Guangxi Province, China, in the dry season. However, in the rainy season, the langurs only show one peak of feeding activities in the late afternoon [28]. Several hypotheses have been put forward to explain the two feeding peaks and midday resting in langurs and other primates. Midday resting may represent a thermoregulatory strategy to avoid the high temperature at noon in the summer or to warm up by sunbathing during the winter [56,57,58]. On the other hand, the resting time may be used to support fibrous food digestion after the morning meal [28].
The activity budget of Lam Binh’s langurs resembles those of the François’s langurs in Nonggang Nature Reserve and other limestone langurs, including Cat Ba, Delacour’s, and white-headed langurs [28,30,32,52]. Limestone langurs spend most of their daily time resting, followed by feeding, except in while-headed langurs, whose other activities come in second place (Table 2). In forest langurs, including Trachypithecus crepusculus, T. germaini, and T. margarita, however, feeding occupies most of their daily activities while resting takes up a lesser amount of time. As limestone langurs live in an environment with high metal ions, especially calcium [50], their diet contains much higher calcium content as a result of consuming karst plants [59,60,61]. Although all limestone langurs have evolved a genomic mechanism to consume the special diet [62], it might still take them more resting time to digest the plant materials compared to other food items.
On average, the population density of langurs in Lam Binh is approximately 20 individuals/km2, higher than that of white-headed langurs in Guangxi Province, China, with 15 individuals/km2 [45]. Several populations of langurs in Vietnam have a greater population density with 733 individuals/km2 in Trachypethicus germaini in Chua Hang Limestone Outcrop in Kien Giang Province, southern Vietnam [31], or 28 individuals/km2 in T. delacouri in Van Long Nature Reserve, Ninh Binh Province, in northwestern Vietnam [63]. Nevertheless, the suitable habitat in Lam Binh covers an area of nearly 1400 ha, which provides ample opportunities for the population to expand and grow in the future.
The Lam Binh area is indeed home to the largest remaining and viable population of the François’ langur in Vietnam with 122 adult and 34 young individuals. However, the site has received little attention from government authorities as it is not a formally recognized protected area, and as a result, it is unclear if the existing threats could be eliminated or mitigated in the foreseeable future. Interviews with local people and direct observations during the field survey showed that illegal logging and hunting are the two largest direct threats to the François’ langur in Lam Binh. Other anthropogenic pressures include firewood collecting, hydropower development, grazing, and mining. During the field surveys, sporadic gun shots could be heard by the field teams, and local people confirmed that small-scale wildlife trade still takes place at the study site [19]. Furthermore, most of the valley areas in the western part of the Lam Binh Protection Forest have been converted to farmlands and human settlements, including areas inside the protection forest (Figure 3). This land conversion process not only forces the separation of langur groups but also increases the edge effects on the remaining available habitats of T. francoisi. The Na Hang hydroelectric dam just 15 km downstream from Lam Binh is another problem for the François’ langur population in Lam Binh. The dam raises the water level of the Gam River, creating an unpassable barrier between the langur groups on the east and west banks of the river (Figure 3). Finally, the increasing impacts of climate change can negatively affect the suitable habitat of the species, further threatening the survival of this highly threatened primate [11].
According to our results and in comparison with data reported in previous studies, there is a strong possibility that the François’ langur population in Lam Binh can further grow and expand if appropriate conservation measures are implemented immediately. As the species has disappeared from much of its former range, the future of the primate in Vietnam almost entirely depends on this population. Given the population size and the habitat quality in Lam Binh, establishing a protected area at the site may be highly beneficial for the long-term conservation of this endangered species. As a new protected area in Lam Binh is being proposed, our findings are timely to inform the process of gazettement. Moreover, in addition to forming the baseline for the future monitoring of the population, the data from our study can also help design effective conservation measures, such as translocating human settlements at the site, minimizing agriculture expansion, deterring wildlife trade, and enhancing genetic diversity by exchanging animals between two sides of the river.
Author Contributions
Conceptualization, T.A.L. (Tu A. Le), T.S.L. and M.D.L.; methodology, T.A.L. (Tu A. Le), A.T.N. and T.S.L.; validation, T.A.L. (Tu A. Le), A.T.N. and M.D.L.; formal analysis, T.A.L. (Tu A. Le), A.T.N. and M.D.L.; investigation, T.A.L. (Tu A. Le) and T.S.L.; resources, T.A.L. (Tuan A. Le), T.S.L. and M.D.L.; data curation, T.A.L. (Tu A. Le), A.T.N. and M.D.L.; writing—original draft preparation, T.A.L. (Tu A. Le), A.T.N. and M.D.L.; writing—review and editing, all authors; visualization, T.A.L. (Tu A. Le) and A.T.N.; supervision, T.S.L. and M.D.L.; project administration, T.A.L. (Tuan A. Le) and T.S.L.; funding acquisition, T.A.L. (Tu A. Le) and T.S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Rainforest Trust and Critical Ecosystem Partnership Fund.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
As the data in this study collected from the most important population of a highly threatened primate species in Vietnam, and the hunting pressure is still significant at the study site, the authors decided not to release any detailed information. However, the data is available from the corresponding author upon reasonable request.
Acknowledgments
We thank the People Resources and Conservation Foundation for generously supporting the study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nadler, T. A new subspecies of douc langur, Pygathrix nemaeus cinereus ssp. nov. Zool. Gart. 1997, 67, 165–176. [Google Scholar]
- Thinh, V.N.; Mootnick, A.R.; Thanh, V.N.; Nadler, T.; Roos, C. A new species of crested gibbon from the central Annamite mountain range. Vietnam. J. Primatol. 2010, 4, 1–12. [Google Scholar]
- Blair, M.E.; Sterling, E.J.; Hurley, M.M. Taxonomy and conservation of Vietnam’s primates: A review. Am. J. Primatol. 2011, 73, 1093–1106. [Google Scholar] [CrossRef] [PubMed]
- Roos, C.; Boonratana, R.; Supriatna, J.; Fellowes, J.R.; Rylands, A.B.; Mittermeier, R. An updated taxonomy of primates in Vietnam, Laos, Cambodia and China. Vietnam. J. Primatol. 2013, 2, 13–26. [Google Scholar]
- Blair, M.E.; Cao, G.T.H.; Lopez-Nandam, E.; Veronese-Paniagua, A.; Birchette, M.G.; Kenyon, M.; Md-Zain, B.M.; Munds, R.; Nekaris, K.A.I.; Nijman, V.; et al. Molecular phylogenetic relationships and unveiling novel genetic diversity among slow and pygmy lorises, including resurrection of Xanthonycticebus intermedius. Genes 2023, 14, 643. [Google Scholar] [CrossRef] [PubMed]
- Quyet, L.K.; Rawson, B.M.; Duc, H.; Nadler, T.; Covert, H.; Ang, A. Rhinopithecus avunculus; The IUCN Red List of Threatened Species: Gland, Switzerland, 2020; e.T19594A17944213. [Google Scholar]
- Rawson, B.M.; Leonard, N.; Covert, H.; Nadler, T. Trachypithecus poliocephalus; The IUCN Red List of Threatened Species: Gland, Switzerland, 2020; e.T39871A17959804. [Google Scholar]
- Nguyen, A.T.; Trinh, H.D.; Le, M.; Nguyen, H.M. Current status of the second viable population of the critically endangered Delacour’s langur. Oryx 2022, 56, 439–441. [Google Scholar] [CrossRef]
- Wearn, O.R.; Trinh-Dinh, H.; Ma, C.-Y.; Le, Q.K.; Nguyen, P.; Hoang, T.V.; Luong, C.V.; Hua, T.V.; Hoang, Q.V.; Fan, P.-F.; et al. Vocal fingerprinting reveals a substantially smaller global population of the Critically Endangered cao vit gibbon (Nomascus nasutus) than previously thought. Sci. Rep. 2024, 14, 416. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.V.; Vu, T.T.; Tran, B.Q.; Nguyen, M.D.; Vu, P.T.; Tran, T.H.; Nguyen, H.T.; Pham, T.V.; Nguyen, T.C. Modelling the change in the distribution of the black-shanked douc, Pygathrix nigripes (Milne-Edwards) in the context of climate change: Implications for conservation. Raffles Bull. Zool. 2020, 68, 769–778. [Google Scholar]
- Blair, M.E.; Nguyen, A.T.; Le, M.D.; Liu, Z.; Meng, T.; Horning, N.; Sterling, E.J.; Thach, H.M.; Xu, M.; Galante, P.J. Karst as a biotic driver of François’ langur distribution, with predictions for biological communities on karst under climate change. Front. Biogeogr. 2022, 14, e51838. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Man, H.; Nguyen, A. An assessment of potential distribution and climate change impacts on a critically endangered primate, the Delacour’s langur. Raffles Bull. Zool. 2022, 70, 30–38. [Google Scholar]
- Trinh-Dinh, H.; Nguyen, A.T.; Le, M.D.; Li, X.K.; Cao, N.T.H.; Blair, M.E. Assessment of climate change impacts on one of the rarest apes on Earth, the Cao Vit Gibbon Nomascus nasutus. Front. Biogeogr. 2022, 14, e53320. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2023-1. Available online: https://www.iucnredlist.org (accessed on 30 March 2024).
- Nadler, T.; Momberg, F.; Dang, N.X.; Lormee, N. Vietnam Primate Conservation Status Review 2002-Part 2: Leaf Monkeys; Fauna and Flora International-Vietnam Program and Frankfurt Zoological Society: Hanoi, Vietnam, 2003. [Google Scholar]
- Chen, T.; Huang, Z.; Huang, C.; Wei, H.; Zhou, Q. Positional behaviours of François’ langur (Trachypithecus francoisi) in the limestone forest of Nonggang, Guangxi, South-West China. Folia Primatol. 2020, 91, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Nadler, T.; Brockman, D.K. Primates of Vietnam; Endangered Primate Rescue Center: Cuc Phuong, Vietnam, 2014. [Google Scholar]
- Zhou, Q.H.; Huang, Z.H.; Wei, H.; Huang, C.M. Variations in diet composition of sympatric Trachypithecus francoisi and Macaca assamensis in the limestone habitats of Nonggang, China. Zool. Res. 2018, 39, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Le, T.S.; Le, M.D. Human activities affecting the environment of the Trachypithecus francoisi and solutions in Tuyen Quang Province. TNU J. Sci. Technol. 2022, 227, 137–144. [Google Scholar]
- Han, Z.; Hu, G.; Wu, S.; Cao, C.; Dong, X. A census and status review of the Endangered François’ langur Trachypithecus francoisi in Chongqing, China. Oryx 2013, 47, 128–133. [Google Scholar] [CrossRef]
- Insua-Cao, P.; Hoang, T.M.; Dine, M. Conservation Status and Needs of François’ Langur in Vietnam; People Resources and Conservation Foundation: Hanoi, Vietnam, 2012. [Google Scholar]
- Tho, N.D. Species Conservation Action Plan: Local-Based Conservation of François’ Langur at the Lam Binh Forest; Fauna and Flora International: Tuyen Quang, Vietnam, 2012. [Google Scholar]
- Quyet, L.K.; Tu, L.A. Population Survey of François’ Langur (Trachypithecus francoisi) in the Lam Binh-Sinh Long Region of Tuyen Quang Province, Northeastern Vietnam; People Resources and Conservation Foundation: Hanoi, Vietnam, 2020. [Google Scholar]
- Weghorst, J.A. High population density of black-handed spider monkeys (Ateles geoffroyi) in Costa Rican lowland wet forest. Primates 2007, 48, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.O. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 1947, 37, 223–249. [Google Scholar] [CrossRef]
- ArcGIS, version 10.2; Environmental Systems Research Institute: Redlands, CA, USA, 2013.
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, F.; Huang, C.; Li, M.; Ren, B.; Luo, B. Seasonal variation in the activity patterns and time budgets of Trachypithecus francoisi in the Nonggang Nature Reserve, China. Int. J. Primatol. 2007, 28, 657–671. [Google Scholar] [CrossRef]
- Nguyen, H.D. Ecological Characteristics and Conservation of the Indochinese Grey Langur Population (Trachypithecus crepusculus) in Xuan Lien Nature Reserve, Thanh Hoa Province. Ph.D. Thesis, National University of Forestry, Hanoi, Vietnam, 2018. [Google Scholar]
- Workman, C. Diet of the Delacour’s langur (Trachypithecus delacouri) in Van Long Nature Reserve, Vietnam. Am. J. Primatol. 2010, 72, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Le, T.H. Investigating Ecological Characteristics of the Indochinese Silver Langur (Trachypithecus germaini, Milne-Edwards, 1876) in Chua Hang Limestone Outcrop, Kien Luong District, Kien Giang Province. Ph.D. Thesis, Institute of Science and Technology, Vietnam Academy of Science and Technology, Ho Chi Minh City, Vietnam, 2019. [Google Scholar]
- Li, Z.; Rogers, E. Habitat Quality and Activity Budgets of White-Headed Langurs in Fusui, China. Int. J. Primatol. 2004, 25, 41–54. [Google Scholar] [CrossRef]
- Tran, B.V. Feeding ecology of Annamese silvered langur (Trachypithecus margarita) at Ta Kou Moutain, Ta Kou Nature Reserve, Binh Thuan Province. Master’s Thesis, Ho Chi Minh University of Science, Ho Chi Minh City, Vietnam, 2013. [Google Scholar]
- Hendershott, R.L. Socioecology of Cat Ba Langurs (Trachypithecus poliocephalus): Implications for Conservation. Ph.D. Thesis, The Australian National University, Canberra, Australia, 2017. [Google Scholar]
- Nguyen, H.M. Some observation on the Hatinh langur, Trachypithecus laotum hatinhensis (Dao, 1970), in North Central Vietnam. Primate Conserv. 2006, 21, 149–154. [Google Scholar]
- Souwideth, J.; Phiapalath, P.; Dong, H.T.; Brakels, P.; Pham, T.V.; Luiselli, L. Ecology and conservation of the Laotian langur Trachypithecus laotum in a protected area of Laos (Southeast Asia). Diversity 2021, 13, 231. [Google Scholar] [CrossRef]
- Lees, C.; Rawson, B.M.; Behie, A.M.; Hendershott, R.; Leonard, N. Preliminary Population Viability Analysis of the Critically Endangered Cat Ba Langur (Trachypithecus poliocephalus); IUCN SSC Conservation Breeding Specialist Group, Fauna & Flora International: Hanoi, Vietnam, 2014. [Google Scholar]
- Anderson, C.M. Predation and primate evolution. Primates 1986, 27, 15–39. [Google Scholar] [CrossRef]
- Janson, C.H.; Goldsmith, M.L. Predicting group size in primates: Foraging costs and predation risks. Behav. Ecol. 1995, 6, 326–336. [Google Scholar] [CrossRef]
- Hill, R.A.; Lee, P.C. Predation risk as an influence on group size in cercopithecoid primates: Implications for social structure. J. Zool. 1998, 245, 447–456. [Google Scholar] [CrossRef]
- Yeager, C.P.; Kirkpatrick, R.C. Asian colobine social structure: Ecological and evolutionary constraints. Primates 1998, 39, 147–155. [Google Scholar] [CrossRef]
- Crockett, C.M.; Janson, C.H. Infanticide in red howlers: Female group size, male membership, and a possible link to folivory. In Infanticide by Males and Its Implications; van Schaik, C.P., Janson, C.H., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 75–98. [Google Scholar]
- Snaith, T.V.; Chapman, C.A. Towards an ecological solution to the folivore paradox: Patch depletion as an indicator of within-group scramble competition in red colobus monkeys (Piliocolobus tephrosceles). Behav. Ecol. Sociobiol. 2005, 59, 185–190. [Google Scholar] [CrossRef]
- Li, Y.; Huang, C.; Ding, P.; Tang, Z.; Wood, C. Dramatic decline of François’ langur Trachypithecus francoisi in Guangxi Province, China. Oryx 2007, 41, 38–43. [Google Scholar] [CrossRef]
- Tang, H.X.; Huang, H.L.; Wang, Z.X.; Wu, J.B.; Wang, A.L.; Nong, D.P.; Garber, P.A.; Zhou, Q.H.; Huang, C.M. Population dynamics and conservation status of the white-headed langur in Chongzuo forest fragments, Guangxi, China. Oryx 2024, 58, 179–182. [Google Scholar] [CrossRef]
- Steinmetz, R.; Timmins, R.J.; Duckworth, J.W. Distribution and conservation status of the Lao leaf monkey (Trachypithecus (francoisi) laotum). Int. J. Primatol. 2011, 32, 587–604. [Google Scholar] [CrossRef]
- Koenig, A.; Borries, C. Social organization and male residence pattern in Phayre’s leaf monkeys. In Long-Term Field Studies of Primates; Kappeler, P.M., Watts, D.P., Eds.; Springer-Verlag: Berlin, Germany, 2012; pp. 215–236. [Google Scholar]
- Fan, P.; Garber, P.; Chi, M.; Ren, G.; Liu, C.; Chen, X.; Yang, J. High dietary diversity supports large group size in Indo-Chinese gray langurs in Wuliangshan, Yunnan, China. Am. J. Primatol. 2015, 77, 479–491. [Google Scholar]
- Zhang, K.; Zhou, Q.; Xu, H.; Huang, Z. Effect of group size on time budgets and ranging behavior of white-headed langurs in limestone forest, Southwest China. Folia Primatol. 2020, 91, 188–201. [Google Scholar] [CrossRef]
- Clements, R.; Sodhi, N.S.; Schilthuizen, M.; Ng, P. Limestone karsts of Southeast Asia: Imperiled arks of biodiversity. BioScience 2006, 56, 733–742. [Google Scholar] [CrossRef]
- Sterling, E.J.; Martha, H.H.; Le, M.D. Vietnam: A Natural History; Yale University Press: New Haven, CT, USA, 2006. [Google Scholar]
- Shil, J.; Biswas, J.; Kumara, H.N. Influence of habitat conditions on group size, social organization, and birth pattern of golden langur (Trachypithecus geei). Primates 2020, 61, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Moore, J. Population density, social pathology, and behavioral ecology. Primates 1999, 40, 1–22. [Google Scholar] [CrossRef]
- Sterck, E.H.M. The behavioral ecology of colobine monkeys. In The Evolution of Primate Societies; Mitani, J.C., Call, J., Kappeler, P.M., Palombit, R.A., Silk, J.B., Eds.; University of Chicago Press: Chicago, IL, USA, 2012; pp. 66–87. [Google Scholar]
- Qi, X.-G.; Wu, J.; Zhao, L.; Wang, L.; Guang, X.; Garber, P.A.; Opie, C.; Yuan, Y.; Diao, R.; Li, G.; et al. Adaptations to a cold climate promoted social evolution in Asian colobine primates. Science 2023, 380, eabl8621. [Google Scholar] [CrossRef]
- Hill, R.A. Ecological and Demographic Determinants of Time Budgets in Baboons: Implications for Cross-Populational Models of Baboon Socioecology. Ph.D. Thesis, University of Liverpool, Liverpool, UK, 1999. [Google Scholar]
- Fashing, P.J. Activity and ranging patterns of guerezas in the Kakamega forest: Intergroup variation and implications for intragroup feeding competition. Int. J. Primatol. 2001, 22, 549–577. [Google Scholar] [CrossRef]
- Huang, C.M.; Wei, F.W.; Li, M.; Li, Y.B.; Sun, R.Y. Sleeping cave selection, activity pattern and time budget of the white-headed langur. Int. J. Primatol. 2003, 24, 825–846. [Google Scholar] [CrossRef]
- Hao, Z.; Kuang, Y.W.; Kang, M. Untangling the influence of phylogeny, soil and climate on leaf element concentrations in a biodiversity hotspot. Funct. Ecol. 2015, 29, 165–176. [Google Scholar] [CrossRef]
- Jin, W.W.; Long, Y.; Fu, C.H.; Zhang, L.B.; Xiang, J.; Wang, B.S.; Li, M.T. Ca2+ imaging and gene expression profiling of Lonicera confusa in response to calcium-rich environment. Sci. Rep. 2018, 8, 7068. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Deng, X.; Xiang, W.; Lei, P.; Ouyang, S.; Wen, H.; Chen, L. Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences 2018, 15, 2991–3002. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Yan, Z.; Ren, Z.; Han, F.; Tan, X.; Xiang, Z.; Dong, F.; Yang, Z.; Liu, G.; et al. Genomic mechanisms of physiological and morphological adaptations of limestone langurs to karst habitats. Mol. Biol. Evol. 2020, 37, 952–968. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.V. Investigating Biological and Ecological Characteristics of the Delacour’s Langur Trachypethicus delacouri (Osgood 1932) in Van Long Nature Reserve and Recommending Conservation Measures. Ph.D. Thesis, Vietnam National University, Hanoi, Vietnam, 2008. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).